Abstract
Background
Breast cancer subtypes are associated with distinct metastatic patterns. Whether germline BRCA1 /BRCA2 mutation status is independently associated with central nervous system (CNS) relapse, controlling for tumor subtype, is unknown.
Methods
Patients who were treated at Dana‐Farber Cancer Institute and diagnosed with a first locoregional recurrence (LRR) or metastasis between 1981 and 2014 were identified using 2 institutional registries: 1) patients treated for recurrent breast cancer and 2) patients who underwent BRCA testing. The frequencies of LRR, sites of metastasis, and breast cancer‐specific survival from LRR or metastasis were calculated, and the factors associated with CNS recurrence were evaluated using multivariable logistic regression models.
Results
The final study cohort included 30 BRCA1 mutation carriers, 32 BRCA2 mutation carriers, and 270 noncarriers. Most BRCA1 carriers (73%) had triple‐negative breast cancer; whereas most BRCA2 carriers (72%) had hormone receptor‐positive tumors. BRCA1 carriers frequently experienced lung and distant lymph node metastasis, whereas BRCA2 carriers and noncarriers most often experienced bone metastasis. Although CNS disease occurred frequently in both BRCA1 and BRCA2 carriers (53% BRCA1, 50% BRCA2, 25% noncarriers; P < .001), only BRCA2 mutation (P = .006) was significantly associated with CNS metastasis in multivariable analysis controlling for tumor subtype. BRCA2 mutation (P = .01), triple‐negative subtype (P < .001), and the involvement of CNS (P < .001) and other non‐CNS distant sites (relative to locoregional recurrence or contralateral disease; P < .001) at presentation of recurrent breast cancer were associated with risk for mortality.
Conclusions
CNS involvement is frequent in women with germline BRCA1/BRCA2 mutations who have metastatic breast cancer. BRCA2 mutation carriers had a significantly higher frequency of CNS metastasis than noncarriers when controlling for breast cancer subtype.
Keywords: brain metastases, BRCA1, BRCA2, recurrence
Short abstract
Germline BRCA1 or BRCA2 alterations are associated with a high frequency (≥50%) of brain metastases in patients with locoregionally recurrent or metastatic breast cancer. In multivariable analysis, only BRCA2 mutation (P = .006) was significantly associated with central nervous system metastasis when controlling for breast cancer subtype.
Introduction
Heritable mutations, a substantial proportion of which occur in the BRCA1 and BRCA2 genes, underlie 5% to 10% of breast cancers.1 BRCA1 and BRCA2 are both involved in homologous, recombination‐mediated DNA repair.2, 3 Given the related functions and direct interactions between the proteins, one of the most provocative observations has been that BRCA1‐associated and BRCA2‐associated breast cancers differ phenotypically. BRCA1‐associated tumors are usually high‐grade carcinomas that do not express estrogen receptor (ER), progesterone receptor (PR), or human epidermal growth factor receptor 2 (HER2), otherwise known as triple‐negative breast cancer (TNBC).4, 5, 6, 7 In contrast, BRCA2 mutation carriers typically develop hormone receptor (HR)‐positive breast cancer that express ER and, less often, PR but are HER2‐nonamplified.4, 5, 6, 7
Breast cancer subtypes exhibit distinct gene expression profiles, biologic behaviors, and patterns of metastasis.8, 9, 10 For example, compared with HR‐positive breast cancer, HER2‐positive and TNBC have an increased propensity to metastasize to the central nervous system (CNS).10, 11, 12, 13, 14, 15, 16
Several studies have asked whether BRCA‐associated breast cancers metastasize to the CNS more frequently than would be predicted by breast cancer subtype, but results have been inconclusive. Albiges et al found a higher incidence of CNS parenchymal metastasis in BRCA1 mutation carriers than in noncarriers but did not control for subtype.17 Analyzing only TNBC, another study showed a statistically nonsignificant trend toward more CNS metastases in BRCA1 mutation carriers than in noncarriers.18 A subsequent study from this group with a larger sample size (N = 59 with distant metastases) found no difference in the frequency of CNS metastasis between BRCA1 mutation carriers and noncarriers with TNBC.19 The study of CNS metastasis in BRCA2 mutation carriers with breast cancer has been limited.
The purpose of this study is to characterize the patterns of recurrence in BRCA1/BRCA2‐associated and nonassociated breast cancers and to determine whether germline BRCA1/BRCA2 mutations are independently associated with CNS metastasis, including both parenchymal and leptomeningeal involvement.
Materials and Methods
Study Population and Data Collection
Approval was obtained from the institutional review boards of the Dana‐Farber/Harvard Cancer Center and Harvard Medical School. Patients with confirmed BRCA1/BRCA2 mutation status and invasive locoregionally recurrent or metastatic breast cancer were retrospectively identified using 2 patient registries at the Dana‐Farber Cancer Institute (DFCI): 1) unselected patients who were treated at DFCI for recurrent breast cancer (N = 1490) and 2) all patients who underwent BRCA genetic testing at DFCI or presented for consultation after testing elsewhere (N = 5939). Males were excluded because of the rarity of sporadic breast cancer in this population. BRCA variants of unknown significance were excluded.
The registries contained 206 overlapping patients who met study criteria (N = 34 mutation carriers, N = 172 noncarriers) (see Supporting Fig. 1). To expand the study cohort, medical records of additional patients in each registry were reviewed for study inclusion. The recurrent disease registry contained 8 additional eligible patients (N = 3 carriers, N = 5 noncarriers). In the BRCA testing registry, 29 of 490 total patients with mutation‐positive breast cancer and 115 of 740 consecutive patients (by medical record number) with mutation‐negative breast cancer met study criteria. Twenty‐six patients were excluded (TP53‐mutation positive: N = 1 noncarrier; incomplete medical records: N = 3 carriers, N = 18 noncarriers; and active, nonbreast malignancies that obscured the source of metastatic disease: N = 1 carrier, N = 3 noncarriers). The final study cohort included 332 patients (BRCA1 carriers, N = 30; BRCA2 carriers, N = 32; noncarriers, N = 270).
Clinical data were abstracted from electronic medical records. Data were collected on patient characteristics, BRCA testing date and result, dates of diagnoses, tumor characteristics, recurrence history, treatment history, last follow‐up, and survival data. ER, PR, and HER2 status were abstracted from pathology reports. No additional central biomarker testing was performed. Chemotherapy was classified as neoadjuvant (yes/no) or adjuvant (yes/no). Receipt or nonreceipt of trastuzumab was not collected. The US Social Security Death Index was used to supplement missing survival data.20
Definitions
Menopausal status was recorded for patients at initial breast cancer diagnosis. Race was self‐reported. Stage was determined according to the American Joint Committee on Cancer (AJCC) system. Breast cancer subtypes were classified as HR‐positive (ER‐positive and/or PR‐positive, HER2‐nonamplified), TNBC (ER‐negative/PR‐negative, HER2‐nonamplified), and HER2‐positive (immunohistochemistry score ≥3 or fluorescence in situ hybridization amplification ≥2.0, and negative or positive for ER and PR). ER and PR were considered positive either if there was >1% staining documented in the pathology report or, if the percentage positivity was missing, if the synoptic report indicated the tumor was ER‐positive or PR‐positive. If missing on the primary tumor, receptor status was abstracted from the biopsy of recurrent disease. Dates of diagnosis were defined by dates of biopsy, when available, or imaging. Locoregional recurrence was defined as involving ipsilateral breast, chest wall, or lymph nodes. Other sites were categorized as contralateral or distant metastases. CNS metastases were subcategorized into parenchymal and leptomeningeal disease (LMD). The presence of parenchymal metastases was defined by pathologic or radiographic evidence. LMD was defined as a clinical diagnosis, integrating radiographic findings and cerebrospinal fluid cytology, and the clinical impression of the treating physician, as documented in the medical record. A positive cerebrospinal fluid cytology was not required for the diagnosis.
For patients diagnosed with more than 1 primary breast cancer, 2 reviewers (Y.S., J.E.G.) identified the primary that most likely recurred: only 1 primary preceded the diagnosis of recurrence (N = 6), receptors and histology similar to the recurrence (N = 10), the same laterality as locoregional recurrence (N = 10), or most recent diagnosis (N = 9). Only the primary that recurred was included in analyses. When patients recurred from multiple primaries, the source of metastatic (not only locoregionally recurrent) disease (N = 2) or the most recent primary (N = 1) and its subsequent recurrences were chosen.
Statistical Methods
Descriptive statistics were used to summarize the characteristics of patients, disease, and treatment in BRCA1 mutation carriers, BRCA2 mutation carriers, and noncarriers. Comparisons were made using the Fisher exact test for discrete variables and the Wilcoxon rank‐sum test for continuous variables. Predictors of CNS disease at recurrence were evaluated using multivariable logistic regression models including all terms, with P < .10 derived from likelihood ratio tests. Significant associations were summarized using odds ratios (ORs) and 95% confidence intervals (CIs). Step‐down tests comparing BRCA1 mutation carriers versus noncarriers and BRCA2 mutation carriers versus noncarriers used Wald‐type tests with a Bonferonni correction for multiple testing. Breast cancer‐specific survival (BCSS) was defined as the interval from the date of locoregional recurrence or metastasis to the date of breast cancer‐related death (ie, caused by disease progression), censoring at death from other or unknown causes or last follow‐up if the patient remained alive. BCSS was summarized using Kaplan‐Meier estimates, and univariable comparisons were made using Bonferonni‐corrected log‐rank tests. A multivariable Cox regression model for BCSS was used to explore the association of BRCA1/BRCA2 mutation status and other covariates and was summarized using hazard ratios (HRs) and 95% CIs. Statistical analyses were performed using R version 3.1.1 1.21
Results
Study Population
Patients were diagnosed with invasive breast cancer between 1979 and 2013 and with their first locoregional recurrence or metastasis between 1981 and 2014. BRCA genetic testing occurred between 1999 and 2014. Demographics, clinicopathologic variables, and treatment characteristics are presented in Table 1. The median patient age at time of diagnosis of first invasive breast cancer was 38 years (range, 27‐57 years) in BRCA1 mutation carriers (P = .005) and 41 years (range, 28‐59 years) in BRCA2 mutation carriers (P = .20) compared with 43 years (range, 22‐80 years) in noncarriers. The cohorts differed in tumor grade (P = .002) and subtype (P < .001). BRCA1 mutation carriers were often diagnosed with high‐grade carcinoma (87%) and TNBC (73%). Most BRCA2 mutation carriers (72%) were diagnosed with HR‐positive breast cancer. Most noncarriers also had HR‐positive breast cancer (52%), but many were diagnosed with HER2‐positive breast cancer (22%) and TNBC (19%). The only statistically significant difference in primary treatment was the use of hormonal therapies (18% of BRCA1 carriers, 74% of BRCA2 carriers, 53% of noncarriers; P < .001). The high rate of chemotherapy use, even among patients with HR‐positive tumors (79% of BRCA2 carriers with HR‐positive/HER2‐negative tumors, 62% of noncarriers with HR‐positive/HER2‐negative tumors), reflects the presenting stage distribution (69% of BRCA2 carriers and 61% of noncarriers presented with stage II or III disease). In addition, mutation carriers were significantly more likely to undergo prophylactic contralateral mastectomy (P < .001) and bilateral salpingo‐oophorectomy (P < .001).
Table 1.
Clinical and Pathologic Characteristics (N = 332) and Primary Breast Cancer Treatment (N = 288 Initially Diagnosed With Stage I‐III Disease)
| Characteristic | No. of Patients (%) | P | ||
|---|---|---|---|---|
| BRCA1 Carriers, N = 30a | BRCA2 Carriers, N = 32 | Noncarriers, N = 270 | ||
| Age at diagnosis of first invasive breast cancer: Median [range], y | 38 [27‐57] | — | 43 [22‐80] | .005 |
| — | 41 [28‐59] | 43 [22‐80] | .20 | |
| Second primary breast cancer | 8 (27) | 1 (3) | 26 (10) | .001 |
| Third primary breast cancer | 2 (7) | 0 (0) | 2 (1) | .053 |
| Race | ||||
| White | 26 (87) | 28 (88) | 248 (92) | |
| Black | 2 (7) | 1 (3) | 5 (2) | .26 |
| Othera | 2 (7) | 3 (9) | 15 (6) | |
| Unknown | 0 (0) | 0 (0) | 2 (1) | |
| Menopausal status | ||||
| Premenopause | 26 (87) | 26 (81) | 203 (75) | |
| Postmenopause | 2 (7) | 3 (9) | 58 (21) | .07 |
| Unknown | 2 (7) | 3 (9) | 9 (3) | |
| Tumor classification | ||||
| T1 | 12 (40) | 11 (34) | 127 (47) | |
| T2 | 7 (23) | 17 (53) | 87 (32) | |
| T3 | 6 (20) | 1 (3) | 26 (10) | .16 |
| T4 | 3 (10) | 2 (6) | 18 (7) | |
| Tx | 2 (7) | 1 (3) | 12 (4) | |
| Lymph node classification | ||||
| N0 | 7 (23) | 10 (31) | 85 (31) | |
| N1 | 10 (33) | 11 (34) | 116 (43) | |
| N2 | 5 (17) | 5 (16) | 34 (13) | .37 |
| N3 | 4 (13) | 5 (16) | 21 (8) | |
| Nx | 4 (13) | 1 (3) | 14 (5) | |
| AJCC stage | ||||
| I | 5 (17) | 5 (16) | 65 (24) | |
| II | 10 (33) | 13 (41) | 108 (40) | |
| III | 12 (40) | 9 (28) | 58 (21) | .36 |
| IV | 2 (7) | 5 (16) | 37 (14) | |
| Unknownb | 1 (3) | 0 (0) | 2 (1) | |
| Tumor grade | ||||
| 1 | 0 (0) | 1 (3) | 18 (7) | |
| 2 | 3 (10) | 10 (31) | 96 (36) | .002 |
| 3 | 26 (87) | 18 (56) | 126 (47) | |
| Unknown | 1 (3) | 3 (9) | 30 (11) | |
| Histology | ||||
| Ductal | 26 (87) | 23 (72) | 208 (77) | |
| Lobular | 0 (0) | 3 (9) | 28 (10) | |
| Mixed | 3 (10) | 5 (16) | 22 (8) | .35 |
| Other | 0 (0) | 0 (0) | 3 (1) | |
| Invasive cancer, not otherwise specified | 1 (3) | 1 (3) | 9 (3) | |
| Breast cancer subtype | ||||
| HR‐positive/HER2‐negative | 6 (20) | 23 (72) | 140 (52) | |
| TNBC | 22 (73) | 5 (16) | 51 (19) | |
| HER2‐positive | 0 (0) | 1 (3) | 59 (22) | <.001 |
| HR‐positive/HER2 unknown | 0 (0) | 3 (9) | 17 (6) | |
| HR‐negative/HER2 unknown | 2 (7) | 0 (0) | 3 (1) | |
| Prophylactic surgeries | ||||
| Bilateral mastectomy | 13 (43) | 14 (44) | 38 (14) | <.001 |
| Bilateral salpingo‐oophorectomy | 16 (53) | 19 (59) | 40 (15) | <.001 |
| Primary breast cancer treatmentc | (N = 28) | (N = 27) | (N = 233) | |
| Surgery | ||||
| Lumpectomy | 8 (29) | 10 (37) | 115 (49) | .07 |
| Mastectomy | 20 (71) | 17 (63) | 118 (51) | |
| Neoadjuvant chemotherapy | 8 (29) | 5 (19) | 43 (18) | .43 |
| Adjuvant chemotherapy | 22 (79) | 19 (70) | 160 (69) | .60 |
| Adjuvant hormone therapy | 5 (18) | 20 (74) | 123 (53) | <.001 |
| Adjuvant radiotherapy | 19 (68) | 17 (63) | 182 (78) | .12 |
Abbreviations: AJCC, American Joint Committee on Cancer; HER2, human epidermal growth factor receptor 2; HR, hormone receptor (estrogen and progesterone receptors); TNBC, triple‐negative breast cancer.
Other race includes Hispanic and Asian. Most patients self‐identified being Hispanic as a race rather than an ethnic group.
Stage was unknown because the size of the tumor was indeterminate.
Primary treatment is only shown for patients who were diagnosed initially with stage I through III breast cancer. Note that, of the BRCA1 carriers, only 3 patients who had germline BRCA1 mutations and presented with stage I through III disease did not receive adjuvant systemic therapy. Their presenting stages were: T1cN0 (n = 1) and T1bN0 (n = 2). Among 23 patients who had HR‐positive/HER2‐negative disease with a BRCA2 mutation, 2 (9%) received chemotherapy, and 16 (70%) received chemotherapy plus endocrine therapy. Among 140 patients who were HR‐positive/HER2‐negative noncarriers, 14 (10%) received chemotherapy, and 73 (52%) received chemotherapy plus endocrine therapy. The overall high rates of chemotherapy receipt in the HR‐positive patients likely reflect the distribution of presenting stage in this cohort.
Locoregional Recurrence and Metastases
Sites of locoregional recurrence and metastases are provided in Table 2 for the first recurrence and for all recurrences. There was not a significant difference in frequencies of locoregional recurrence as a first event (P = .11) or overall (P = .14). Lung and distant lymph node metastases occurred in 50% of BRCA1 mutation carriers and were the most common sites. BRCA2 mutation carriers and noncarriers most frequently had metastases to bone (75% and 53%, respectively), followed by liver (63% and 46%, respectively).
Table 2.
First and All Locoregional Recurrences and Distant Metastases by BRCA Mutation Status
| Variable | No. of Patients (%) | P | ||
|---|---|---|---|---|
| BRCA1 Carriers, N = 30 | BRCA2 Carriers, N = 32 | Noncarriers, N = 270 | ||
| First event | ||||
| Locoregional | 17 (57) | 10 (31) | 129 (48) | .11 |
| Contralateral breast/chest wall | 1 (3) | 0 (0) | 4 (1) | .43 |
| Bone | 8 (27) | 18 (56) | 103 (38) | .053 |
| Liver | 2 (7) | 7 (22) | 57 (21) | .15 |
| Lung | 11 (37) | 4 (13) | 49 (18) | .04 |
| Distant lymph nodes | 11 (37) | 5 (16) | 45 (17) | .04 |
| CNS | 4 (13) | 2 (6) | 8 (3) | .02 |
| Other sitesa | 6 (20) | 6 (19) | 30 (11) | .16 |
| Among all events | ||||
| Locoregional | 18 (60) | 11 (34) | 130 (48) | .14 |
| Contralateral breast/chest wall | 1 (3) | 1 (3) | 8 (3) | .99 |
| Bone | 11 (37) | 24 (75) | 143 (53) | .01 |
| Liver | 9 (30) | 20 (63) | 125 (46) | .04 |
| Lung | 15 (50) | 12 (38) | 94 (35) | .26 |
| Distant lymph nodes | 15 (50) | 17 (53) | 85 (31) | .01 |
| CNS | 16 (53) | 16 (50) | 67 (25) | <.001 |
| Other sitesb | 11 (37) | 12 (38) | 95 (35) | .93 |
Abbreviation: CNS, central nervous system.
These sites include metastases to pleura, pericardium, uterine adnexa, stomach and intestines, orbits, adrenal gland, and subcutaneous/soft tissues.
These sites include metastases to pleura, peritoneum, uterine adnexa, subcutaneous/soft tissue, adrenal gland, stomach and intestines, orbits, uterus, gallbladder, pancreas, spleen, bladder, and epidural space/dura mater.
Seven patients were diagnosed with nonbreast adenocarcinomas, including endometrial (N = 3), bronchoalveolar (N = 1), ampullary (N = 1), colon (N = 1), and both lung and endometrial (N = 1). On medical record review, recurrences in these patients were consistent with their breast primary. Two patients' recurrences preceded the diagnosis of nonbreast adenocarcinomas. In 4 cases, breast cancer recurrence was confirmed on pathology. Biopsy was unable to distinguish origin in only 1 case, but the patient's in‐breast recurrence was clinically consistent with the breast primary.
Because BRCA mutations increase the risk of ovarian cancer, 14 patients (BRCA2 carriers, N = 2; noncarriers, N = 12) with uterine adnexal metastases were reviewed in detail to ensure that they did not represent primary ovarian cancer. Nine cases (BRCA2 carriers, N = 2; noncarriers, N = 7) were confirmed on pathology to be of breast origin. Five cases diagnosed on imaging occurred in noncarriers after the diagnosis of other metastatic sites consistent with the breast primary.
CNS Metastasis
As shown in Table 2, frequencies of CNS disease as a first event (P = .02) and overall (P < .001) were significantly higher in BRCA1 and BRCA2 mutation carriers than in noncarriers. Similar results were seen in the subset of 262 patients who had at least 1 distant recurrence (P = .002). Because subtype is highly correlated with BRCA1/BRCA2 mutation, we further analyzed CNS metastasis by subtype and subcategorized the event into parenchymal metastatic disease and LMD. Once subtype was considered, there was no difference in the frequency of CNS metastasis as a first event between all mutation carriers and noncarriers (see Supporting Table 1). However, when assessing the frequency of CNS disease at any time among the patients who had TNBC with locoregionally recurrent or distant metastatic disease, the overall incidence remained significantly higher in BRCA1 mutation carriers than in noncarriers (64% vs 37%; P = .04), including metastases to both parenchyma and leptomeninges (32% vs 2%; P < .001; Bonferroni‐corrected critical P = .017). Among patients with an HR‐positive subtype, the overall incidence of CNS metastasis also was significantly higher in BRCA2 mutation carriers than in noncarriers (52% vs 14%; P < .001), including parenchyma‐only metastases (26% vs 6%; P = .006; Bonferroni‐corrected critical P = .017).
Only 1 patient who had HER2‐positive disease was a BRCA2 carrier; none were BRCA1 carriers. Among 59 noncarriers with HER2‐positive disease, 44% developed CNS metastases (parenchymal, n = 23; leptomeningeal, n = 1; both parenchymal and leptomeningeal, n = 2) over time.
Predictors of CNS metastasis in patients with locoregional recurrent or metastatic breast cancer were further evaluated using multivariable logistic regression models (Table 3). In the first model, breast cancer subtype was excluded as a variable. On step‐down tests, mutations in both BRCA1 (OR, 3.05; 95% CI, 1.36‐6.89; corrected P = .01) and BRCA2 (OR, 3.11; 95% CI, 1.43‐6.81; corrected P = .008) were significantly associated with CNS disease. A second model included a variable for TNBC versus other subtypes (OR, 2.24; 95% CI, 1.22‐4.09; P = .01). On step‐down tests, only BRCA2 mutation (OR, 3.33; 95% CI, 1.51‐7.38; P = .006), and not BRCA1 mutation (OR, 2.11; 95% CI, 0.89‐4.98; P = .18), was significantly associated with CNS disease. Stage IV disease at initial diagnosis of breast cancer was also significantly associated with CNS involvement.
Table 3.
Multivariable Logistic Regression Models of Central Nervous System Metastasis (N = 314 With Complete Information)
| Comparison | Excluding Breast Cancer Subtype | Including Breast Cancer Subtype | ||
|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | |
| Histology | ||||
| Ductal vs others | 2.01 (1.07‐3.95) | .03 | 1.78 (0.94‐3.55) | .08 |
| Initial stage | ||||
| II vs I | 1.32 (0.66‐2.73) | .02 | 1.24 (0.61‐2.58) | .03 |
| III vs I | 2.26 (1.08‐4.88) | 1.95 (0.91‐4.26) | ||
| IV vs I | 3.17 (1.37‐7.53) | 3.13 (1.34‐7.49) | ||
| BRCA status | ||||
| BRCA1 carrier vs noncarrier | 3.05 (1.36‐6.89) | .001a | 2.11 (0.89‐4.98) | .005b |
| BRCA2 carrier vs noncarrier | 3.11 (1.43‐6.81) | 3.33 (1.51‐7.38) | ||
| Breast cancer subtype | ||||
| TNBC vs others | — | 2.24 (1.22‐4.09) | .01 | |
Abbreviations: OR, odds ratio; TNBC, triple‐negative breast cancer.
Step‐down Wald tests were used with Bonferonni correction (BRCA1 mutation, corrected P = .01; BRCA2 mutation, corrected P = .008).
Step‐down Wald tests were used with Bonferonni correction (BRCA1 mutation, corrected P = .18; BRCA2 mutation, corrected P = .006).
Outcome
In follow‐up, a total of 194 of 332 patients (58%) had died (Fig. 1). Six of 194 deaths (3%) were for unknown reasons (N = 3) or unrelated to breast cancer (N = 3) and were censored for BCSS analysis. Patients who remained alive had a median follow‐up of 64 months (range, 0.5‐357 months). Among the patients who died, the median survival time from locoregional recurrence or metastasis was 27.2 months (95% CI, 18.7‐75.3 months) for BRCA1 mutation carriers (P < .001) and 48.7 months (95% CI, 28.9‐84.5 months) for BRCA2 mutation carriers (P = .08) compared with 76.2 months (95% CI, 64.9‐94.5 months) for noncarriers. In multivariable analysis, BRCA2 mutation (HR, 1.82; 95% CI, 1.14‐2.90; P = .01), but not BRCA1 mutation (HR, 1.21; 95% CI, 0.72‐2.04; P = .48), was associated with an increased risk of death (Table 4). TNBC, compared with other subtypes, also increased the risk of death (HR, 4.22; 95% CI, 2.91‐6.12; P < .001). Furthermore, if the first recurrence event involved the CNS (HR, 10.91; 95% CI, 5.05‐23.58; P < .001) or non‐CNS distant sites (HR, 3.90; 95% CI, 2.62‐5.79; P < .001), compared with locoregional or contralateral disease, the risk of mortality was increased.
Figure 1.
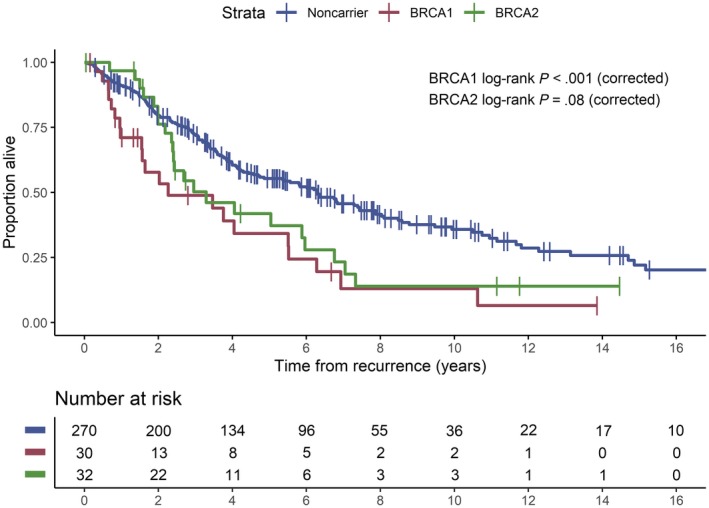
Kaplan‐Meier plot of survival from the time of first locoregional recurrence or metastasis according to BRCA mutation status.
Table 4.
Multivariable Cox regression Model for Breast Cancer‐Related Death
| Comparison | HR (95% CI) | P |
|---|---|---|
| Initial stage | ||
| II vs I | 1.69 (1.07‐2.67) | .02 |
| III vs I | 1.82 (1.11‐2.99) | .02 |
| IV vs I | 1.75 (1.02‐3.00) | .04 |
| Breast cancer subtype | ||
| TNBC vs others | 4.22 (2.91‐6.12) | <.001 |
| BRCA status | ||
| BRCA1 carrier vs noncarrier | 1.21 (0.72‐2.04) | .48 |
| BRCA2 carrier vs noncarrier | 1.82 (1.14‐2.90) | .01 |
| First recurrence sitea | ||
| CNS vs locoregional/contralateral | 10.91 (5.05‐23.58) | <.001 |
| Distant (non‐CNS) vs locoregional/contralateral | 3.90 (2.62‐5.79) | <.001 |
Abbreviations: CNS, central nervous system; HR, hazard ratio; TNBC, triple‐negative breast cancer.
Comparison of CNS involvement versus locoregional/contralateral disease and non‐CNS distant metastases versus locoregional/contralateral disease at first presentation of recurrent or metastatic breast cancer.
Discussion
In this single‐institution, retrospective study, both BRCA1 mutation carriers and BRCA2 mutation carriers experienced high frequencies of CNS metastasis from breast cancer. In multivariable analysis taking into account breast cancer subtype, BRCA2 mutation was independently associated with CNS involvement. To our knowledge, this is the first study to find this association. Albiges et al did not find a high frequency of CNS parenchymal disease in BRCA2 mutation carriers, although their study may have been limited by a small BRCA2 cohort size (N = 12).17 In addition, CNS metastases in BRCA1 and BRCA2 mutation carriers appeared to differ in character, which may be attributable to different breast cancer subtypes. For example, few BRCA2 mutation carriers with HR‐positive breast cancer developed both parenchymal and leptomeningeal disease, whereas nearly one‐third of BRCA1 mutation carriers with TNBC had disease at both sites.
Although 53% of BRCA1 mutation carriers developed CNS metastases, we did not find that BRCA1 mutation was independently associated with CNS disease in multivariable analysis. The high incidence of CNS metastasis observed in BRCA1 mutation carriers may be attributable to the underlying prevalence of TNBC, which is a known risk factor for CNS involvement.13, 14, 15, 16 Our study also demonstrated an association between BRCA2 mutation carrier status and CNS involvement, despite the finding that most BRCA2 mutation carriers had HR‐positive/HER2‐negative tumors, suggesting a true causal relationship between BRCA2 loss and CNS homing and/or growth. If anything, given that our institutional practice is not to obtain routine brain imaging in the absence of symptoms or a clinical trial requirement, our results may even underestimate the true frequency of CNS involvement in BRCA1/BRCA2 mutation carriers.
Several other sites of metastasis also differed by BRCA1/BRCA2 mutation status, although these differences were likely attributable to the clustering of breast cancer subtypes. BRCA1 mutation carriers, the majority of whom had TNBC, frequently developed metastases to lung and distant lymph nodes, consistent with the observations that basal‐like TNBCs often metastasize to the viscera.10, 14, 22 BRCA2 mutation carriers and noncarriers, many of whom had HR‐positive disease, frequently developed bone metastases. One visceral site to which BRCA2 mutation carriers frequently metastasized was the liver, which Albiges et al also found to occur at a high rate (67%).17
Mechanistically, we postulate that deficiencies in DNA repair, including but not limited to homologous recombination repair, may be causally related to the development of brain metastases. Diossy et al have reported the enrichment of mutational signatures indicative of homologous repair deficiency in breast cancer brain metastases compared with matched primary tumors.23 Woditschka et al reported overexpression of BARD1 and RAD51 in breast cancer brain metastases versus matched primary tumors and that forced overexpression in an MDA‐MB‐231‐BR preclinical model increased brain metastatic potential in vivo.24 Ferguson et al reported overexpression of multiple proteins involved in DNA repair in brain metastases across several solid tumor histologies.25 Balendran et al reported a high rate of BRCA1 or BRCA2 mutations detected in the brain metastases of patients with ovarian cancer.26 Of interest, platinum agents have demonstrated efficacy against brain metastases across many tumor types, including breast cancer, suggesting vulnerability of brain metastases to DNA‐damaging agents.27, 28
Notably, our study included patients who received treatment before the regulatory approval of PARP inhibitors for the treatment of metastatic, BRCA1‐associated or BRCA2‐associated breast cancer.29, 30 In the registration trial comparing the PARP inhibitor talazoparib with chemotherapy, subset analyses suggested that the benefit of talazoparib persisted in patients who had stable/treated brain metastases on study entry. None of the phase 3 PARP trials included patients who had active CNS metastases and thus could not speak directly to the presence or absence of CNS activity with these agents. Our data indicate that at least one‐half of patients with BRCA1‐associated or BRCA2‐associated metastatic breast cancer will develop CNS metastases. As discussed above, data from other groups have suggested that homologous recombination deficiency and DNA repair pathways are also more likely to be dysregulated in breast cancer brain metastases overall. The potential for PARP activity in the CNS is highlighted by a single case report in the literature in a patient who had ovarian cancer with leptomeningeal disease and by preclinical evidence of CNS activity in a breast cancer model.31, 32 Of note, an ongoing cooperative group study (clinicaltrials.gov identifier NCT02595905) is testing the value of adding veliparib to a platinum backbone and includes patients with progressive brain metastases.33 We would strongly encourage future trials enrolling patients with BRCA1‐associated/BRCA2‐associated metastatic breast cancer to take the high prevalence of CNS involvement into account and allow patients with both active and stable/treated brain metastases to enroll, in line with recommendations from the American Society of Clinical Oncology‐Friends of Cancer Research Broadening Eligibility Criteria Working Group and the Response Assessment in Neuro‐Oncology guidelines.34, 35
Unlike many breast cancer outcome studies, BCSS in the current study was determined from the date of locoregional recurrence or distant metastasis, rather than the date of initial diagnosis of breast cancer, and was controlled for tumor subtype. Consistent with other studies, we found that the TNBC subtype was associated with significantly shorter BCSS; therefore, when subtype was included in the survival model, patients with BRCA1 mutations did not fare worse than noncarriers. In contrast, we found that BRCA2 carriers did experience substantially shorter metastatic survival compared with noncarriers, even in multivariable analysis that included tumor subtype. In addition, compared with non‐CNS disease, CNS involvement as the first event was associated with an even higher risk of death. This is in accordance with our findings that patients who had TNBC with CNS involvement at first metastatic presentation had shorter survival time than patients with non‐CNS metastases (log‐rank P < .001).13
Previous studies evaluating survival from the time of primary breast cancer diagnosis have shown conflicting results.18, 19, 36, 37, 38, 39, 40, 41, 42, 43 Foulkes et al and Moller et al found decreased survival in BRCA1 mutation carriers versus noncarriers but did not adjust for possible confounders.44, 45 BRCA1 mutation was significantly associated with decreased survival in a few multivariable studies.46, 47 Goodwin et al found that BRCA2 mutation was associated with an increased risk of death on univariate analysis, but BRCA2 mutation was no longer significant once patient age, tumor characteristics, and year of diagnosis were considered.48 Bayraktar et al22 reported shorter metastatic survival time among BRCA1 mutation carriers than noncarriers, but BRCA1 mutation was not significantly associated with outcome in multivariable analysis. However, in contrast to our finding, BRCA2 mutation was not associated with an increased risk of death in their multivariable analysis, possibly because of a smaller BRCA2 cohort size (N = 11).
Finally, in a multivariable model that included tumor subtype, we observed a relationship between presenting stage and the risk of CNS relapse (OR 1.95 [95% CI, 0.91‐4.26] for stage III vs stage I; OR, 3.13 [95% CI, 1.34‐7.49] for stage IV vs stage I). The increased risk for CNS relapse in patients with locally advanced or inflammatory breast cancer has been well described previously in the literature.49, 50, 51, 52 To our knowledge, there are few published data comparing the rate of CNS relapse over time in patients with recurrent metastatic breast cancer versus those with de novo metastatic disease, because most studies that focused on risk factors for CNS relapse restricted the study populations to those presenting with stage I through III breast cancer and/or only reported on CNS involvement at the time of initial metastatic diagnosis. Although we would be cautious with conclusions based on small numbers (only 44 patients in our study presented with stage IV disease), it does raise the possibility that a de novo presentation might be a risk factor for CNS involvement.
We acknowledge several limitations. Noncarriers were defined as patients who tested negative for BRCA1/BRCA2 germline mutations. Patients are often selected for genetic testing because of features that include young age and family history of cancer. The mutation‐negative cohort in this study, on average, is younger than the general breast cancer population. Young age has been associated with CNS metastasis.11, 15 Therefore, using mutation‐negative patients as a control group may have attenuated differences between BRCA1‐associated/BRCA2‐associated and truly sporadic disease. However, using a control cohort with known BRCA1/BRCA2 mutation status allowed us to isolate the effects of BRCA mutations. In addition, CNS screening in the absence of symptoms is not standard at our institution, a practice that may have resulted in incomplete ascertainment of CNS metastases. Also, we used abstraction of existing pathology records for determination of ER, PR, and HER2 status. Given the long timeframe of the study, it is possible that some patients who would now be classified as having ER low‐positive tumors might have been classified in our study as ER‐negative. In addition, we did not repeat central HER2 testing. In defining tumor subtype, we did not always have access to receptor status in both the primary tumor and a metastatic sample, and we cannot speak to the potential impact of subtype switching on outcomes. Other groups have demonstrated differences between BRCA2‐associated HR‐positive tumors versus sporadic HR‐positive tumors, including in the distribution of Oncotype DX (Genomic Health) recurrence scores.53, 54, 55 Because of the timeframe of primary tumor diagnoses encompassed in our study, we chose not to collect information on Oncotype Dx testing, nor did we perform tissue‐based assays to identify other potential molecular differences that might also explain the differences in CNS relapse risk. Methodologic differences in BRCA1/BRCA2 mutation detection may cause some under ascertainment of carriers in this older cohort. In addition, the study of rare mutations in a single institution is often limited by small sample size. Finally, our finding that there is a high incidence of CNS involvement in BRCA1 and BRCA2 carriers with metastatic breast cancer does not necessarily demonstrate that screening of asymptomatic patients would provide clinical benefit. Our group is launching a prospective clinical trial to test the value of magnetic resonance imaging screening in patients with metastatic breast cancer across all subtypes (principal investigator, Ayal Aizer).
Conclusions
Breast cancer metastases to the CNS occurred frequently in women with BRCA1 and BRCA2 germline mutations. BRCA2 mutation was significantly associated with CNS metastasis and an increased risk of death in multivariable analyses controlling for breast cancer subtype. In addition, CNS metastasis and TNBC were strongly associated with increased mortality regardless of BRCA1/BRCA2 mutation status. Future multi‐institutional studies with larger cohort sizes are needed to further confirm these findings.
Funding Support
This research was supported by grants from the Breast Cancer Research Foundation (to Judy E. Garber and Nancy U. Lin).
Conflict of Interest Disclosures
William T. Barry reports institutional research funding from Pfizer and ARMO Biosciences outside the submitted work. Judy E. Garber reports grants from Ambry Genetics, Myriad Genetics, and Invitae Genetics, personal fees from Helix Genetics, Novartis Oncology, and Clinical Care Options, LLC, and research collaboration with AstraZeneca, all outside the submitted work; in addition, her spouse (Dr. Myles Brown) receives sponsored research support from Novartis. He is on the Scientific Advisory Board of Kronos Bio, and is a consultant to H3 Biomedicine, Gtx, Inc. and Aleta Biotherapeutics. Nadine M. Tung reports research funding and personal fees from AstraZeneca outside the submitted work. Nancy U. Lin reports research funding (to institution) from Pfizer, Kadmon, Array Biopharma, and Merck; research funding and personal fees from Roche/Genentech, Seattle Genetics, and Novartis; and personal fees from Shionogi Inc, Kadmon, Puma, and Daichii Sankyo, all outside the submitted work. The remaining authors made no disclosures.
Author Contributions
Yun Song: Design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the article; had full access to all of the data in the study, and takes responsibility for the integrity of the data and the accuracy of the data analysis. William T. Barry: Collection, management, analysis, and interpretation of the data; preparation, review, or approval of the article; had full access to all of the data in the study, and takes responsibility for the integrity of the data and the accuracy of the data analysis. Davinia S. Seah: Design and conduct of the study and preparation, review, or approval of the article. Nadine M. Tung: Preparation, review, or approval of the article. Judy E. Garber: Design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the article; had full access to all of the data in the study, and takes responsibility for the integrity of the data and the accuracy of the data analysis. Nancy U. Lin: Design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the article; had full access to all of the data in the study, and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Supporting information
We are grateful to Elizabeth Root, Lisa Digianni, PhD, and Abbe Janov (all affiliated with Dana‐Farber Cancer Institute) for their assistance in identifying patients with breast cancer who were appropriate for the study. No additional compensation was received for their contribution.
References
- 1. Claus EB, Schildkraut JM, Thompson WD, Risch NJ. The genetic attributable risk of breast and ovarian cancer. Cancer. 1996;77:2318‐2324. [DOI] [PubMed] [Google Scholar]
- 2. Narod SA, Foulkes WD. BRCA1 and BRCA2: 1994 and beyond. Nat Rev Cancer. 2004;4:665‐676. [DOI] [PubMed] [Google Scholar]
- 3. Venkitaraman AR. Cancer susceptibility and the functions of BRCA1 and BRCA2. Cell. 2002;108:171‐182. [DOI] [PubMed] [Google Scholar]
- 4. Honrado E, Benitez J, Palacios J. The molecular pathology of hereditary breast cancer: genetic testing and therapeutic implications. Mod Pathol. 2005;18:1305‐1320. [DOI] [PubMed] [Google Scholar]
- 5. Musolino A, Bella MA, Bortesi B, et al. BRCA mutations, molecular markers, and clinical variables in early‐onset breast cancer: a population‐based study. Breast. 2007;16:280‐292. [DOI] [PubMed] [Google Scholar]
- 6. Atchley DP, Albarracin CT, Lopez A, et al. Clinical and pathologic characteristics of patients with BRCA‐positive and BRCA‐negative breast cancer. J Clin Oncol. 2008;26:4282‐4288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mavaddat N, Barrowdale D, Andrulis IL, et al. Pathology of breast and ovarian cancers among BRCA1 and BRCA2 mutation carriers: results from the Consortium of Investigators of Modifiers of BRCA1/2 (CIMBA). Cancer Epidemiol Biomarkers Prev. 2012;21:134‐147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sorlie T, Perou CM, Tibshirani R, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A. 2001;98:10869‐10874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sorlie T, Tibshirani R, Parker J, et al. Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc Natl Acad Sci U S A. 2003;100:8418‐8423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kennecke H, Yerushalmi R, Woods R, et al. Metastatic behavior of breast cancer subtypes. J Clin Oncol. 2010;28:3271‐3277. [DOI] [PubMed] [Google Scholar]
- 11. Lin NU, Bellon JR, Winer EP. CNS metastases in breast cancer. J Clin Oncol. 2004;22:3608‐3617. [DOI] [PubMed] [Google Scholar]
- 12. Lin NU, Winer EP. Brain metastases: the HER2 paradigm. Clin Cancer Res. 2007;13:1648‐1655. [DOI] [PubMed] [Google Scholar]
- 13. Lin NU, Claus E, Sohl J, Razzak AR, Arnaout A, Winer EP. Sites of distant recurrence and clinical outcomes in patients with metastatic triple‐negative breast cancer: high incidence of central nervous system metastases. Cancer. 2008;113:2638‐2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lin NU, Vanderplas A, Hughes ME, et al. Clinicopathologic features, patterns of recurrence, and survival among women with triple‐negative breast cancer in the National Comprehensive Cancer Network. Cancer. 2012;118:5463‐5472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hung MH, Liu CY, Shiau CY, et al. Effect of age and biological subtype on the risk and timing of brain metastasis in breast cancer patients. PLoS One. 2014;9:e89389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rudat V, El‐Sweilmeen H, Brune‐Erber I, et al. Identification of breast cancer patients with a high risk of developing brain metastases: a single‐institutional retrospective analysis. BMC Cancer. 2014;14:289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Albiges L, Andre F, Balleyguier C, Gomez‐Abuin G, Chompret A, Delaloge S. Spectrum of breast cancer metastasis in BRCA1 mutation carriers: highly increased incidence of brain metastases. Ann Oncol. 2005;16:1846‐1847. [DOI] [PubMed] [Google Scholar]
- 18. Lee LJ, Alexander B, Schnitt SJ, et al. Clinical outcome of triple negative breast cancer in BRCA1 mutation carriers and noncarriers. Cancer. 2011;117:3093‐3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tung N, Gaughan E, Hacker MR, et al. Outcome of triple negative breast cancer: comparison of sporadic and BRCA1‐associated cancers. Breast Cancer Res Treat. 2014;146:175‐182. [DOI] [PubMed] [Google Scholar]
- 20. Ancestry.com . U.S., Social Security Death Index, 1935‐2014. Accessed February 9, 2015. http://www.ancestry.com/search/collections/ssdi/
- 21. The R Core Team . R: A Language and Environment for Statistical Computing. Accessed November 27, 2018. https://www.gbif.org/tool/81287/r-a-language-and-environment-for-statistical-computing
- 22. Bayraktar S, Gutierrez‐Barrera AM, Lin H, et al. Outcome of metastatic breast cancer in selected women with or without deleterious BRCA mutations. Clin Exp Metastasis. 2013;30:631‐642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Diossy M, Reiniger L, Sztupinszki Z, et al. Breast cancer brain metastases show increased levels of genomic aberration‐based homologous recombination deficiency scores relative to their corresponding primary tumors. Ann Oncol. 2018;29:1948‐1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Woditschka S, Evans L, Duchnowska R, et al. DNA double‐strand break repair genes and oxidative damage in brain metastasis of breast cancer. J Natl Cancer Inst. 2014;106:dju145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ferguson SD, Zheng S, Xiu J, et al. Profiles of brain metastases: prioritization of therapeutic targets. Int J Cancer. 2018;143:3019‐3026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Balendran S, Liebmann‐Reindl S, Berghoff AS, et al. Next‐generation sequencing‐based genomic profiling of brain metastases of primary ovarian cancer identifies high number of BRCA‐mutations. J Neurooncol. 2017;133:469‐476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cocconi G, Lottici R, Bisagni G, et al. Combination therapy with platinum and etoposide of brain metastases from breast carcinoma. Cancer Invest. 1990;8:327‐334. [DOI] [PubMed] [Google Scholar]
- 28. Franciosi V, Cocconi G, Michiara M, et al. Front‐line chemotherapy with cisplatin and etoposide for patients with brain metastases from breast carcinoma, nonsmall cell lung carcinoma, or malignant melanoma: a prospective study. Cancer. 1999;85:1599‐1605. [PubMed] [Google Scholar]
- 29. Robson M, Im SA, Senkus E, et al. Olaparib for metastatic breast cancer in patients with a germline BRCA mutation. N Engl J Med. 2017;377:523‐533. [DOI] [PubMed] [Google Scholar]
- 30. Litton JK, Rugo HS, Ettl J, et al. Talazoparib in patients with advanced breast cancer and a germline BRCA mutation. N Engl J Med. 2018;379:753‐763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bangham M, Goldstein R, Walton H, Ledermann JA. Olaparib treatment for BRCA‐mutant ovarian cancer with leptomeningeal disease. Gynecol Oncol Rep. 2016;18:22‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Karginova O, Siegel MB, Van Swearingen AE, et al. Efficacy of carboplatin alone and in combination with ABT888 in intracranial murine models of BRCA‐mutated and BRCA‐wild‐type triple‐negative breast cancer. Mol Cancer Ther. 2015;14:920‐930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. National Cancer Institute . Cisplatin With or Without Veliparib in Treating Patients With Recurrent or Metastatic Triple‐Negative and/or BRCA Mutation‐Associated Breast Cancer With or Without Brain Metastases. ClinicalTrials.gov Identifier: NCT02595905. Accessed November 27, 2018. https://clinicaltrials.gov/ct2/show/NCT02595905
- 34. Lin NU, Prowell T, Tan AR, et al. Modernizing clinical trial eligibility criteria: recommendations of the American Society of Clinical Oncology‐Friends of Cancer Research Brain Metastases Working Group. J Clin Oncol. 2017;35:3760‐3773. [DOI] [PubMed] [Google Scholar]
- 35. Camidge DR, Lee EQ, Lin NU, et al. Clinical trial design for systemic agents in patients with brain metastases from solid tumours: a guideline by the Response Assessment in Neuro‐Oncology Brain Metastases Working Group. Lancet Oncol. 2018;19:e20‐e32. [DOI] [PubMed] [Google Scholar]
- 36. Brekelmans CT, Seynaeve C, Menke‐Pluymers M, et al. Survival and prognostic factors in BRCA1‐associated breast cancer. Ann Oncol. 2006;17:391‐400. [DOI] [PubMed] [Google Scholar]
- 37. Robson M, Gilewski T, Haas B, et al. BRCA‐associated breast cancer in young women. J Clin Oncol. 1998;16:1642‐1649. [DOI] [PubMed] [Google Scholar]
- 38. Robson M, Levin D, Federici M, et al. Breast conservation therapy for invasive breast cancer in Ashkenazi women with BRCA gene founder mutations. J Natl Cancer Inst. 1999;91:2112‐2117. [DOI] [PubMed] [Google Scholar]
- 39. El‐Tamer M, Russo D, Troxel A, et al. Survival and recurrence after breast cancer in BRCA1/2 mutation carriers. Ann Surg Oncol. 2004;11:157‐164. [DOI] [PubMed] [Google Scholar]
- 40. Veronesi A, de Giacomi C, Magri MD, et al. Familial breast cancer: characteristics and outcome of BRCA 1‐2 positive and negative cases. BMC Cancer. 2005;5:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rennert G, Bisland‐Naggan S, Barnett‐Griness O, et al. Clinical outcomes of breast cancer in carriers of BRCA1 and BRCA2 mutations. N Engl J Med. 2007;357:115‐123. [DOI] [PubMed] [Google Scholar]
- 42. Gonzalez‐Angulo AM, Timms KM, Liu S, et al. Incidence and outcome of BRCA mutations in unselected patients with triple receptor‐negative breast cancer. Clin Cancer Res. 2011;17:1082‐1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Budroni M, Cesaraccio R, Coviello V, et al. Role of BRCA2 mutation status on overall survival among breast cancer patients from Sardinia. BMC Cancer. 2009;9:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Foulkes WD, Wong N, Brunet JS, et al. Germ‐line BRCA1 mutation is an adverse prognostic factor in Ashkenazi Jewish women with breast cancer. Clin Cancer Res. 1997;3:2465‐2469. [PubMed] [Google Scholar]
- 45. Moller P, Evans DG, Reis MM, et al. Surveillance for familial breast cancer: differences in outcome according to BRCA mutation status. Int J Cancer. 2007;121:1017‐1020. [DOI] [PubMed] [Google Scholar]
- 46. Stoppa‐Lyonnet D, Ansquer Y, Dreyfus H, et al. Familial invasive breast cancers: worse outcome related to BRCA1 mutations. J Clin Oncol. 2000;18:4053‐4059. [DOI] [PubMed] [Google Scholar]
- 47. Robson ME, Chappuis PO, Satagopan J, et al. A combined analysis of outcome following breast cancer: differences in survival based on BRCA1/BRCA2 mutation status and administration of adjuvant treatment. Breast Cancer Res. 2004;6:R8‐R17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Goodwin PJ, Phillips KA, West DW, et al. Breast cancer prognosis in BRCA1 and BRCA2 mutation carriers: an International Prospective Breast Cancer Family Registry population‐based cohort study. J Clin Oncol. 2012;30:19‐26. [DOI] [PubMed] [Google Scholar]
- 49. Dawood S, Ueno NT, Valero V, et al. Incidence of and survival following brain metastases among women with inflammatory breast cancer. Ann Oncol. 2010;21:2348‐2355. [DOI] [PubMed] [Google Scholar]
- 50. Warren LE, Guo H, Regan MM, et al. Inflammatory breast cancer and development of brain metastases: risk factors and outcomes. Breast Cancer Res Treat. 2015;151:225‐232. [DOI] [PubMed] [Google Scholar]
- 51. Matro JM, Li T, Cristofanilli M, et al. Inflammatory breast cancer management in the National Comprehensive Cancer Network: the disease, recurrence pattern, and outcome. Clin Breast Cancer. 2015;15:1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Gonzalez‐Angulo AM, Cristofanilli M, Strom EA, et al. Central nervous system metastases in patients with high‐risk breast carcinoma after multimodality treatment. Cancer. 2004;101:1760‐1766. [DOI] [PubMed] [Google Scholar]
- 53. Shah PD, Patil S, Dickler MN, Offit K, Hudis CA, Robson ME. Twenty‐one‐gene recurrence score assay in BRCA‐associated versus sporadic breast cancers: differences based on germline mutation status. Cancer. 2016;122:1178‐1184. [DOI] [PubMed] [Google Scholar]
- 54. Lewin R, Sulkes A, Shochat T, et al. Oncotype‐DX recurrence score distribution in breast cancer patients with BRCA1/2 mutations. Breast Cancer Res Treat. 2016;157:511‐516. [DOI] [PubMed] [Google Scholar]
- 55. Halpern N, Sonnenblick A, Uziely B, et al. Oncotype Dx recurrence score among BRCA1/2 germline mutation carriers with hormone receptors positive breast cancer. Int J Cancer. 2017;140:2145‐2149. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


