Abstract
This systematic review and meta‐analysis investigated ω‐3 fatty‐acid enriched parenteral nutrition (PN) vs standard (non‐ω‐3 fatty‐acid enriched) PN in adult hospitalized patients (PROSPERO 2018 CRD42018110179). We included 49 randomized controlled trials (RCTs) with intervention and control groups given ω‐3 fatty acids and standard lipid emulsions, respectively, as part of PN covering ≥70% energy provision. The relative risk (RR) of infection (primary outcome; 24 RCTs) was 40% lower with ω‐3 fatty‐acid enriched PN than standard PN (RR 0.60, 95% confidence interval [CI] 0.49‐0.72; P < 0.00001). Patients given ω‐3 fatty‐acid enriched PN had reduced mean length of intensive care unit (ICU) stay (10 RCTs; 1.95 days, 95% CI 0.42‐3.49; P = 0.01) and reduced length of hospital stay (26 RCTs; 2.14 days, 95% CI 1.36‐2.93; P < 0.00001). Risk of sepsis (9 RCTs) was reduced by 56% in those given ω‐3 fatty‐acid enriched PN (RR 0.44, 95% CI 0.28‐0.70; P = 0.0004). Mortality rate (co‐primary outcome; 20 RCTs) showed a nonsignificant 16% reduction (RR 0.84, 95% CI 0.65‐1.07; P = 0.15) for the ω‐3 fatty‐acid enriched group. In summary, ω‐3 fatty‐acid enriched PN is beneficial, reducing risk of infection and sepsis by 40% and 56%, respectively, and length of both ICU and hospital stay by about 2 days. Provision of ω‐3‐enriched lipid emulsions should be preferred over standard lipid emulsions in patients with an indication for PN.
Keywords: fish oil, intensive care, lipid emulsion, meta‐analysis, omega‐3, parenteral nutrition, surgery, systematic review
Introduction
Lipid emulsions are a key component of parenteral nutrition (PN) and are used as an energy‐dense source of calories, reducing the glycemic load, supplying essential fatty acids, and lowering osmolarity.1, 2 The first generation of lipid emulsions was based on soybean oil or soybean/safflower oil and characterized by high concentrations of long‐chain triglycerides providing high levels of ω‐6 polyunsaturated fatty acids (PUFAs).1, 3 However, concerns arose that soybean oil lipid emulsions could promote inflammation and suppress immune function, thought to be related partly to an excess of ω‐6 PUFAs and a low concentration of ω‐3 PUFAs.3, 4, 5
The idea that ω‐6 PUFAs might be “proinflammatory and immunosuppressive” led to the development of alternative lipid emulsions, including the partial replacement of soybean oil with medium‐chain triglycerides, olive oil, and by the inclusion of fish oil.3, 4, 5 Fish oil has been shown to have anti‐inflammatory and immunomodulatory effects, most likely because of fish oil's ω‐3 PUFA content, consisting of docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA) as they influence diverse inflammatory processes – from signal transduction to protein expression.6 EPA and DHA are now known to be direct precursors of potent specialized proresolution mediators (ie, resolvins, protectins, and maresins) that improve outcomes in many animal disease models.7 Fish oil may oppose the actions of ω‐6 PUFAs, improve hepatic metabolism and liver function, and exert anticoagulatory and antiarrhythmic effects.3 Thus, many trials have compared PN with or without fish oil to explore potential benefits for certain clinical conditions, in particular those characterized by an inflammatory over‐response (eg, sepsis, pancreatitis, acute respiratory distress syndrome, and following major abdominal surgery).
The use of systematic reviews and meta‐analyses is crucial to the formulation of guidelines, as they are the most powerful methods to inform healthcare decisions and form the highest level of the evidence‐based medicine hierarchy,8 summarizing evidence to allow judgement of risks and benefits.9 In our previous meta‐analysis we found significant clinical benefits for ω‐3 fatty‐acid enriched PN in hospitalized patients.10 The rationale for an update is that many new clinical trials have been published, and though other recent meta‐analyses have been performed, these do not have as broad a scope as our 2012 publication.10 Furthermore, the update will adapt (1) the inclusion criteria to more closely match clinical practice and (2) the methodology to reflect the latest meta‐analyses requirements. Thus, the objective for this new systematic review and meta‐analysis was to investigate potential benefits of ω‐3 fatty‐acid enriched PN vs standard PN in adult hospitalized patients.
Methods
Registration and Overview
The protocol was published prospectively (PROSPERO 2018 CRD42018110179).11 The systematic review and meta‐analysis covered ω‐3 fatty‐acid enriched PN vs standard (non‐ω‐3 fatty‐acid enriched) PN in adult hospitalized patients regarding clinical efficacy and laboratory parameter outcomes. The methods can be summarized as follows: (a) defining the eligibility criteria, (b) identification of databases and search strategy, (c) performing a structured literature search to identify publications followed by study selection based on title, abstract, and full text, progressively, and (d) data extraction and synthesis of the results.
Eligibility Criteria
Eligibility criteria for included studies are shown according to participants, interventions, comparisons, outcomes, and study designs (PICOS).12, 13
Participants
Publications included human studies of adult hospitalized patients (later assigned as being within an intensive care unit [ICU] or non‐ICU setting, as defined by the authors using the criteria that ICU studies should have a mean of at least 48 hours in an ICU) who were eligible to receive PN covering at least 70% of their total energy provision. This excluded nontarget populations (ie, pediatric or neonatal patients), or enteral nutrition studies.
Interventions and comparisons
Interventions and comparators included were ω‐3 fatty‐acid enriched PN and standard (non‐ω‐3 fatty‐acid enriched) PN, respectively. This excluded “off‐label” interventions (specifically in which fish oil was used as the sole source of parenteral lipids), and studies in which enteral nutrition accounted for >30% of the daily caloric provision.
Outcomes
Clinical outcomes were infection rate (primary outcome), mortality rate (co‐primary outcome), length of hospital stay, length of ICU stay, sepsis rate, hospital readmissions, ICU‐free days until day 30 or day 60, and ventilation‐free days until day 30 (note: sepsis included events defined by publication authors as septic or systemic inflammatory response syndrome; see Table S1). Other outcomes were transfused blood units and oxygenation index, fatty‐acid composition of plasma phospholipids and lipid profile (α‐tocopherol, EPA, DHA, arachidonic acid, plasma triglycerides), markers of inflammation and antioxidant status (interleukin‐6, leukotriene [LT] B5, LTB4, LTB5:LTB4 ratio, C‐reactive protein, tumor necrosis factor [TNF]‐α), and routine laboratory parameters (lactate; urea; serum creatinine; creatinine clearance; platelets; prothrombin time; partial thromboplastin time [PTT]; international normalized ratio; bleeding time; liver enzymes aspartate [AST], alanine aminotransferase [ALT], and γ‐glutamyl transferase [GGT]; and total bilirubin).
Study design
Randomized controlled trials (RCTs) published in English in peer‐review journals containing at least 1 predefined outcome were included.
Information Sources and Search Methods
Keywords for the search were “parenteral nutrition,” “fish oil,” “omega‐3,” “lipids,” “emulsion,” and “randomized controlled trial.” The search strategy was formulated a priori in a structured manner using the PICOS criteria.11, 12, 13 No restrictions or filters were used, and exclusions were based on the selection process defined in the eligibility criteria. The time interval of inclusion was from any date to present (September 28, 2018). MEDLINE (PubMed interface), EMBASE (Elsevier interface), and the Cochrane Central Register of Controlled Trials (Wiley interface) were searched. The search string was modified according to each database's requirements.11 Results were combined to eliminate duplicates using an Excel‐based algorithm, constituting the core systematic review database. Manual searches were performed of reference lists of included studies, plus reviews and meta‐analyses on the subject. Extra RCTs identified were integrated into the core database.
Study Selection, Data Collection, and Data Items
Two review authors independently screened titles and abstracts of all publications in the core database against the eligibility criteria. The full text of eligible papers was then checked against the inclusion criteria and to ensure no exclusion criteria were present. Conflicting opinions were discussed with a third review author, and original publication authors were consulted for clarification if necessary. Two authors independently extracted data from each trial using a predefined standardized collection grid. Disagreements were resolved in consultation with the principal investigator. If outcomes were only shown in a graphical format, then numerical values were extrapolated using Engauge digitizer software version 10.11.14 Outcomes were reported as SI units or those prevalent in clinical practice. Standard error of the mean (SEM) values were transformed into standard deviations (SD) using standard formulas. Data reported as median and interquartile range were converted into estimated mean and SD using the formulas suggested in Wan et al.15 When dispersion data (SD/SEM) were missing, the original authors of the study were contacted. If these data could not be obtained, an imputation based on the coefficient of variation (SD/mean) of all available data was performed.
Risk of Bias in Individual Studies
Included trials were assessed by 2 reviewers working independently using the Cochrane Collaboration tool for assessing the risk of bias.12 If there was insufficient detail reported in the study, the risk of bias was judged as “unclear,” and the original study investigators were contacted for more information.
Summary Measures
For continuous outcomes, the summary measure was the weighted mean difference (with 95% confidence interval [CI]), although standardized mean difference was used in the case of different measurement scales. For dichotomous outcomes, the summary measure was relative risk (RR) with 95% CI. The proportional odds ratio was used as a summary measure for categorical outcomes on an ordinal scale.
Synthesis of Results (Meta‐Analysis) and Trial Sequential Analysis
Data from included studies was statistically combined through meta‐analysis using Review Manager (RevMan5.3; Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014). All methods applied are thoroughly detailed in the Cochrane Handbook.12 As per Cochrane Handbook recommendations,12 analyses were performed first via fixed effect models, based on which heterogeneity was analyzed. Trial sequential analyses were performed for all primary and secondary outcomes with a significant pooled effect using TSA 0.9.5.10 beta (Copenhagen Trial Unit, Centre for Clinical Intervention Research, Copenhagen, Denmark, 2011) as detailed in the published protocol.11 This explored whether the pooled analyses were adequately powered to evaluate treatment effect on outcomes.
Subgroup Analyses and Meta‐Regression
For highly heterogeneous outcomes (I 2 > 50%), data were included in random effects models. Any source of heterogeneity, or outcomes with ≥10 studies, underwent subgroup analyses and meta‐regression, stratifying data by patient characteristics, intervention, study characteristics, and clinical setting. Mantel–Haenszel study weighting was performed for dichotomous outcomes, and inverse variance was used for continuous data. The DerSimonian and Laird inverse‐variance approach was used for random effects meta‐analysis, adjusting study weights by heterogeneity among intervention effects. The between‐study variation was estimated by comparing each study's intervention effect with the pooled estimate of the corresponding fixed effects analysis. Note: a 0‐cell correction was applied for meta‐analyses of dichotomous and count of events data in studies in which there were no events in 1 or both groups, requiring STATA statistical software (STATA 14.2, StataCorp LLC, College Station, TX, USA).
Risk of Bias Across Studies (Meta Bias) and Confidence in Cumulative Estimate
Risk of bias that could affect the cumulative evidence (eg, publication bias, selective reporting within studies) was assessed by checking whether a protocol for each RCT was published before the RCT was conducted and by evaluating whether selective reporting of outcomes was present. Reporting bias was further explored by funnel plots if ≥10 studies were available. Confidence in cumulative estimates for all statistically significant outcomes was judged using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) working group methodology using GRADEpro v.3.6.1 (GradePro.org).16
Results
Study Selection and Characteristics
A total of 49 studies with 3641 patients were included in the review and meta‐analysis (Figure 1 and Table 1).10, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65
Figure 1.
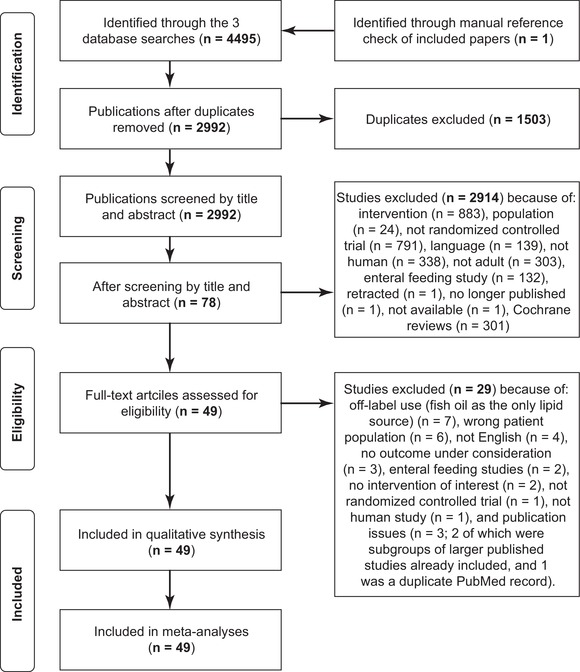
Study selection and screening.
Table 1.
Characteristics of the Randomized Controlled Trials Included (n = 49), Showing Extracted Outcomes
| Randomized Control Trial | Patient Type (Number Randomized)a | ω‐3‐Enriched Lipid Emulsion | Standard Lipid Emulsion | Primary and Secondary Clinical Outcomesb | Laboratory Outcomes |
|---|---|---|---|---|---|
| ICU patients | |||||
| Antebi et al, 200417 | Major surgery (n = 20) | SO/MCT/OO/FO | SO | Alpha‐T, ALT, AST, CRP, GGT, TG | |
| Barbosa et al, 201018 | SIRS or sepsis (n = 23, received study treatments) | SO/MCT/FO | SO/MCT | Mortality, H LOS, ICU LOS | AA, ALT, AST, bilirubin, CRP, DHA, EPA, GGT, IL‐6, Lac, LTB4, OI, PTT, Plt, TNF |
| Berger et al, 200819 | Abdominal aortic aneurism surgery (n = 24, completed trial) | SO/MCT/FO | SO/MCT | Mortality, H LOS, ICU LOS | AA, alpha‐T, CRP, DHA, EPA, TG |
| Chen et al, 201720 | Severe sepsis with Grade III acute gastrointestinal injury (n = 78) | SO/FO | SO | Mortality | CRP |
| Chen et al, 201721 | Patients with septicaemia and intestinal dysfunction (n = 48) | Standard TPN/FO | Standard TPN | Mortality, ICU LOS | CRP |
| Friesecke et al, 200822 | Critically ill medical (n = 165) | SO/MCT/FO | SO/MCT | Mortality, infections, H LOS, ICU LOS, bleeding events | IL‐6, TBU |
| Grau‐Carmona et al, 201523 | Medical and surgical ICU patients (n = 175) | SO/MCT/FO | SO/MCT | Mortality, infections, H LOS, ICU LOS | |
| Gultekin et al, 201424 | ICU patients with sepsis (n = 32) | SO/OO/FO | SO/OO | Mortality, H LOS | CRP, IL‐6, LTB4, TG, TNF |
| Han et al, 201225 | Major surgery (n = 38) | SO/MCT/FO | SO/MCT | Infections | IL‐6, TNF |
| Heller et al, 200226 | Cancer, major abdominal surgery (n = 44) | SO/FO | SO | Plt, PT, PTT | |
| Heller et al, 200427 | Cancer, major abdominal surgery (n = 44) | SO/FO | SO | H LOS, ICU LOS | ALT, AST, bilirubin, CRP |
| Morlion et al, 199628 | Major abdominal surgery (n = 20) | SO/FO | SO | AA, DHA, EPA, LTB4, LTB5 | |
| Piper et al, 200929 | Major abdominal or craniomaxillofacial surgery (n = 44) | SO/MCT/OO/FO | SO/OO | ALT, AST, Plt, TG | |
| Roulet et al, 199730 | Cancer, esophagectomy (n = 19, completed trial) | SO/FO | SO | AA, DHA, EPA, BT | |
| Sabater et al, 201131 | ARDS (n = 16) | SO/MCT/FO | SO | Mortality | LTB4 |
| Stephenson et al, 201332 | Surgery for hepatic colorectal metastasis (n = 20) | SO/MCT/FO | SO/MCT | AA, DHA, EPA | |
| Wachtler et al, 199733 | Cancer, major intestinal surgery (n = 40) | SO/MCT/FO | SO/MCT | Infections, H LOS, ICU LOS | IL‐6, LTB4, LTB5, LTB ratio, TNF |
| Wang et al, 200834 | Severe acute pancreatitis (n = 40) | SO/FO | SO | Mortality, infections, H LOS, ICU LOS, sepsis | CRP, EPA, IL‐6, OI |
| Wang et al, 200935 | Severe acute pancreatitis (n = 56) | SO/FO | SO | Mortality, infections | |
| Weiss et al, 200236 | Gastrointestinal surgery (n = 24) | SO/FO | SO | Mortality, infections, H LOS, ICU LOS | IL‐6, TNF |
| Wendel et al, 200737 | Cancer, major abdominal surgery (n = 44) | SO/FO | SO | TG | |
| Wichmann et al, 200738 | Major intestinal surgery (n = 256) | SO/MCT/FO | SO | Mortality, infections, H LOS, ICU LOS, sepsis | Alpha‐T, AST, bilirubin, Cr, CRP, EPA, GGT, LTB5, LTB ratio, Plt, PT, TG |
| Surgical patients | |||||
| Aliyazicioglu et al, 201339 | Colorectal cancer surgery (n = 36) | Standard TPN/FO | Standard TPN | H LOS | |
| Badia‐Tahull et al, 201040 | Major intestinal surgery (n = 29) | SO/FO | SO/OO | Mortality, infections, H LOS, sepsis | ALT, Cr, CRP, GGT, PU, TBU |
| Chen et al, 201741 | Gastric cancer surgery (n = 120) | SO/MCT/OO/FO | SO | Infections, H LOS | ALT, bilirubin, CRP, IL‐6 |
| Demirer et al, 201642 | Major abdominal surgery (n = 52) | SO/OO/FO | SO/OO or SO/MCT | CRP, IL‐6, TNF | |
| Grimm et al, 200643 | Major abdominal surgery (n = 33) | SO/MCT/OO/FO | SO | H LOS | AA, alpha‐T, DHA, EPA, LTB4, LTB5, LTB ratio |
| Hallay et al, 201044 | Gastrointestinal surgery (n = 41) | SO/MCT/OO/FO | SO/MCT | ALT, AST, bilirubin, GGT | |
| Jiang et al, 201045 | Gastrointestinal cancer surgery (n = 206) | SO/FO | SO | Infections, H LOS, sepsis | Cr, IL‐6, TNF |
| Klek et al, 200546 | Gastric cancer surgery (n = 105, enrolled) | SO/MCT/FO | SO/MCT | Infections, H LOS | ALT, AST, Cr, PU |
| Klek et al, 200847 | Gastrectomy or pancreaticoduodenectomy (n = 205) | SO/MCT/FO (plus glutamine) | SO/MCT | Mortality, infections, H LOS, sepsis | |
| Klek et al, 201148 | Gastrectomy or pancreaticoduodenectomy (n = 167) | SO/MCT/FO (plus glutamine) | SO/MCT | Mortality, infections, sepsis | |
| Koller et al, 200349 | Major abdominal surgery (n = 30) | SO/MCT/FO | SO | LTB4, LTB5, LTB ratio | |
| Liang et al, 200850 | Radical colorectal cancer resection (n = 41) | SO/FO | SO | Mortality, infection, H LOS | GGT, IL‐6, Plt, TNF |
| Linseisen et al, 200051 | Major abdominal surgery (n = 33) | SO/MCT/FO | SO | AA, alpha‐T, DHA, EPA | |
| Ma et al, 201252 | Gastrointestinal tumor surgery (n = 40) | SO/MCT/OO/FO | SO/MCT | H LOS | ALT, AST, bilirubin, Cr, CRP, IL‐6, PU, TG, TNF |
| Ma et al, 201553 | Gastric and colorectal cancer surgery (n = 99) | SO/MCT/FO | SO/MCT | Infections | ALT, AST, bilirubin, CRP, GGT, IL‐6, TG, TNF |
| Makay et al, 201154 | Major gastric cancer surgery (n = 26) | SO/FO | SO | Mortality, infections, H LOS | ALT, AST, Cr, Lac, PU |
| Mertes et al, 200655 | Abdominal or thoracic surgery (n = 249) | SO/MCT/OO/FO | SO | Mortality, H LOS | ALT, AST, bilirubin, GGT, TG |
| Schauder et al, 200256 | Large bowel surgery (n = 60) | SO/FO | SO | TNF | |
| Senkal et al, 200757 | Colorectal surgery (n = 40, received study treatments) | SO/MCT/FO | SO/MCT | Infections | AA, DHA, EPA |
| Wang et al, 201258 | Gastrointestinal surgery (n = 64) | SO/MCT/FO | SO/MCT | Infections, sepsis | ALT, AST, bilirubin, CRP, GGT, IL‐6, LTB ratio, Plt, PT, PTT, TG, TNF |
| Wei et al, 201459 | Surgical resection of gastric tumors (n = 52) | SO/FO | SO | Infections | CRP, IL‐6, TNF |
| Wu et al, 201460 | Gastrointestinal surgery (n = 40) | SO/MCT/OO/FO | SO/MCT | Infections, H LOS | ALT, AST, bilirubin, Cr, CRP, GGT, IL‐6, PU, TG, TNF |
| Zhang et al, 201761 | Hepatectomy (n = 320) | SO/MCT/FO | SO/MCT | Mortality, infections, H LOS, sepsis | ALT, bilirubin, Cr, CRP, TG, Plt, PU, PTT |
| Zhixue et al, 201862 | Liver cancer surgery (n = 75) | SO/MCT/FO | SO/MCT | IL‐6, TNF | |
| Zhu et al, 201263 | Liver transplant (n = 66) | SO/MCT/FO | SO/MCT | Mortality, infection, H LOS | ALT, AST, bilirubin, PT |
| Zhu et al, 201264 | Colorectal cancer surgery (n = 57, completed trial) | SO/FO | SO | Infection, H LOS, sepsis | IL‐6, TNF |
| Zhu et al, 201365 | Pancreaticoduodenectomy (n = 76) | SO/MCT/FO | SO/MCT | Mortality, infection, H LOS, hospital readmission | ALT, AST, bilirubin |
AA, (%) content of arachidonic acid in serum/cellular membranes; alpha‐T, alpha‐tocopherol; ALT, alanine aminotransferase; ARDS, acute respiratory distress syndrome; AST, aspartate aminotransferase; BT, bleeding time; Cr, serum creatinine; CRP, C‐reactive protein; DHA, (%) docosahexaenoic acid content in serum/cellular membranes; EPA, (%) eicosapentaenoic acid content in serum/cellular membranes; FO, fish oil emulsion; Lac, lactate; GGT, γ‐glutamyl transferase; (H) LOS, (hospital) length of stay; ICU, intensive care unit; LTB, leukotriene B; LTB5:LTB4, LTB ratio; MCT, medium‐chain triglycerides; OI, oxygenation index; OO, olive oil emulsion; PU, plasma urea; Plt, Platelet; PT, prothrombin time; PTT, partial thromboplastin time; SIRS, systemic inflammatory response syndrome; SO, soybean oil emulsion; TBU, transfused blood unit; TGs, triglycerides; TNF, tumor necrosis factor.
Number of patients randomized was listed if available, but if not available an alternative descriptor was used for the patient population/number.
An outcome of sepsis included events defined by publication authors as septic or as systemic inflammatory response syndrome.
Clinical Outcomes
For the primary outcome, infection rate, 24 studies (2154 patients) were included that reported any nosocomial infections: 7 studies for ICU patients and 17 for non‐ICU patients. Compared with standard lipid emulsions, ω‐3 fatty‐acid enriched PN resulted in a significant 40% reduction of infection rates (RR 0.60, 95% CI 0.49‐0.72; P < 0.00001) (Figure 2). No subgroup analysis was performed, as heterogeneity was low (I 2: 0%).
Figure 2.
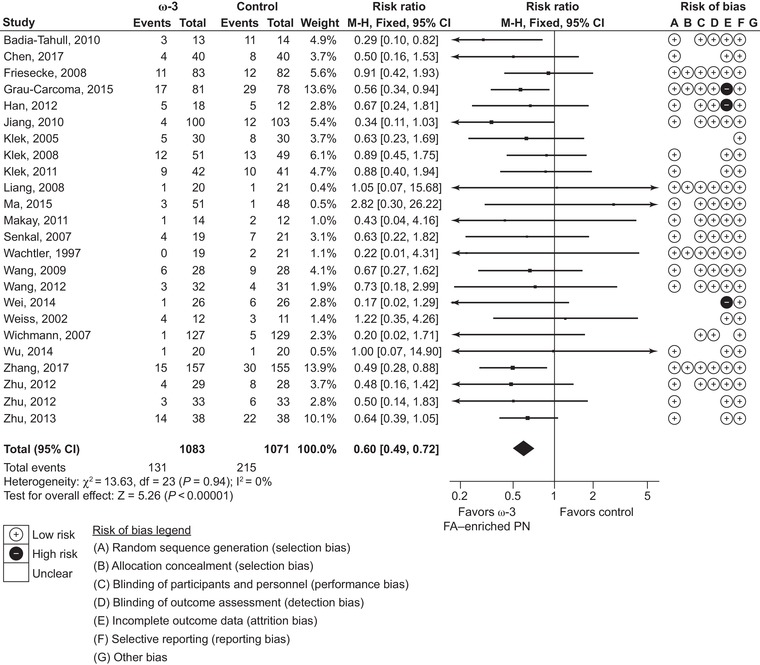
Infection rates. Forest plot of fixed effects meta‐analysis showing individual study means, pooled estimates, and risk of bias for individual studies (Cochrane tool). CI, confidence interval; FA, fatty acid; PN, parenteral nutrition.
The 30‐day mortality rate was reported by 20 studies (1839 patients): 9 studies of ICU patients and 11 for non‐ICU patients (note: in this study, 30‐day mortality was defined as any deaths occurring up to 30 days after receiving at least 1 dose of study treatment or prior to hospital discharge, whichever was reported). There was a nonsignificant 16% reduction in mortality rate (RR 0.84, 95% CI 0.65‐1.07; P = 0.15) (Figure 3).
Figure 3.
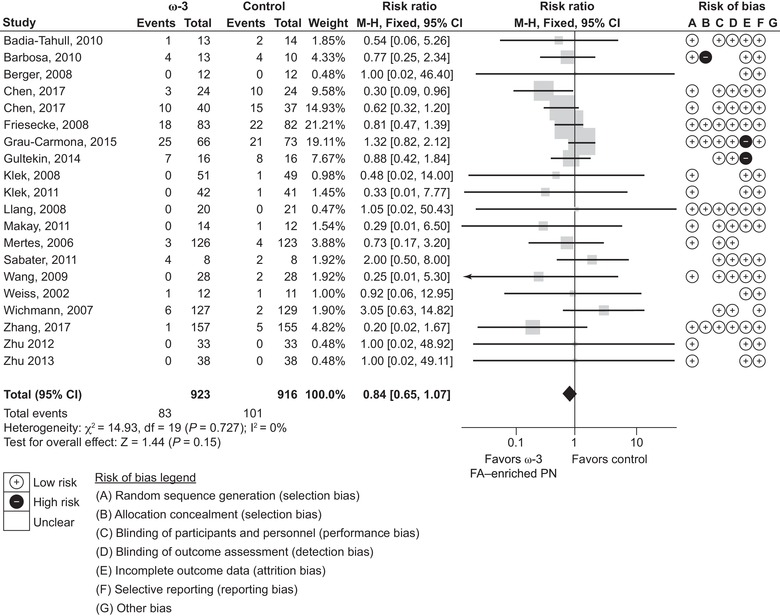
Thirty‐day mortality rates. Forest plot of fixed effects meta‐analysis showing individual study means, pooled estimates, and risk of bias for individual studies (Cochrane tool). Note: to correct for the 0 event studies as per the protocol (to add 0.5 events in both arms), this meta‐analysis was performed using STATA software, as it is difficult to use RevMan for this correction. CI, confidence interval; FA, fatty acid; PN, parenteral nutrition.
Length of hospital stay was reported by 26 studies (2182 patients), of which 10 were ICU studies and 16 non‐ICU studies, and length of ICU stay was reported by 10 studies (822 patients). Results showed a reduction in ICU stay of 1.95 days (95% CI 0.42‐3.49; P = 0.01) and reduction in length of hospital stay of 2.14 days (95% CI 1.36‐2.93; P < 0.00001) (Figures 4 and 5, respectively). As data for both length of stay outcomes were classed as highly heterogeneous (I 2 > 50%), subgroup analyses were considered. Although no subgroup analyses were performed for length of ICU stay (<10 studies were available for each subgroup analysis), length of hospital stay data were analyzed further. These subgroup analyses showed significantly greater effect with no heterogeneity (I 2 = 0%) in total PN vs PN groups, and comparable but less heterogeneous effects in oncological studies vs non‐oncological studies, and in non‐ICU vs ICU studies. Thus, effects on length of stay were more consistent in more homogenous groups of patients such as these.
Figure 4.
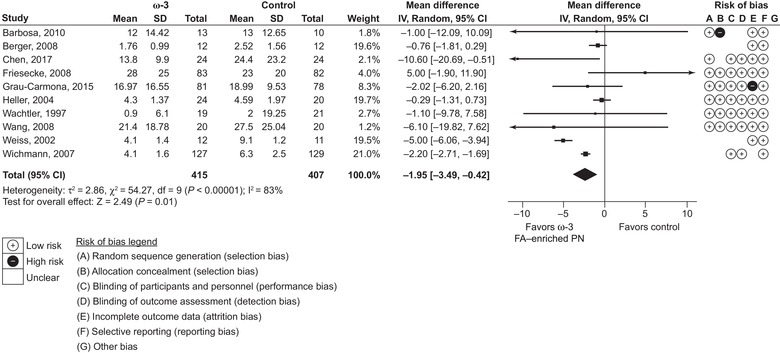
Length of intensive care unit stay. Forest plot of random effects meta‐analysis showing individual study means, pooled estimates, and risk of bias for individual studies (Cochrane tool). CI, confidence interval; FA, fatty acid; IV, inverse variance; PN, parenteral nutrition; SD, standard deviation.
Figure 5.
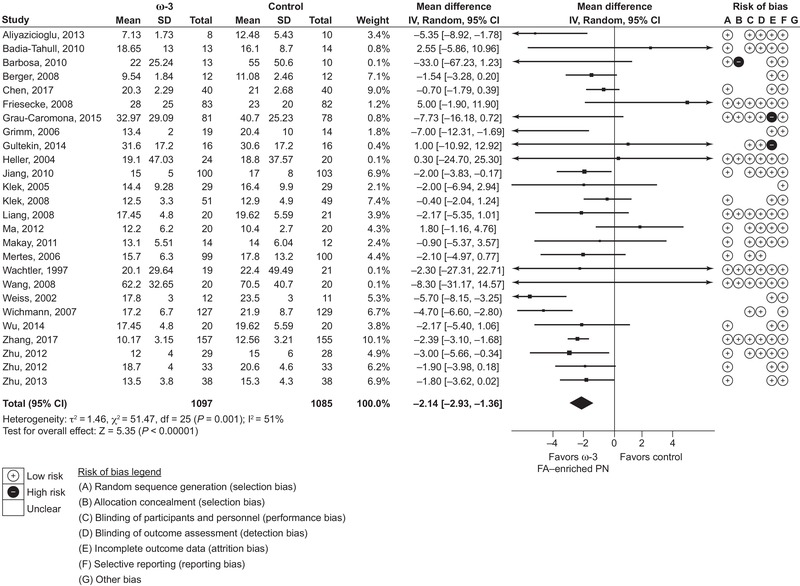
Length of hospital stay. Forest plot of random effects meta‐analysis showing individual study means, pooled estimates, and risk of bias for individual studies (Cochrane tool). CI, confidence interval; FA, fatty acid; IV, inverse variance; PN, parenteral nutrition; SD, standard deviation.
Sepsis was reported in 9 studies (1141 patients), of which 2 were ICU studies and 7 non‐ICU studies. Compared with standard lipid emulsions, ω‐3 fatty‐acid enriched PN resulted in a significant 56% reduction in the risk of sepsis (RR 0.44, 95% CI 0.28‐0.70; P = 0.0004) (Figure 6). No meta‐analyses were performed on hospital readmissions, ICU‐free days, or ventilation‐free days, as only 1 or no studies reported each of these outcomes.
Figure 6.
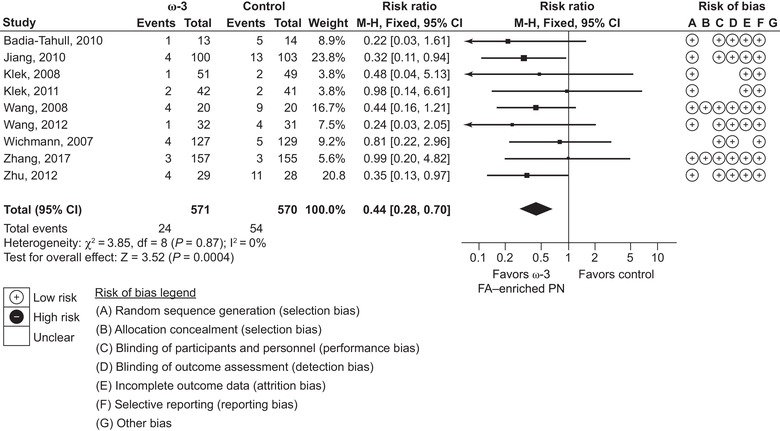
Sepsis. Forest plot of fixed effects meta‐analysis showing individual study means, pooled estimates, and risk of bias for individual studies (Cochrane tool). CI, confidence interval; FA, fatty acid; PN, parenteral nutrition.
Trial sequential analysis for all significant clinical outcomes (infection rate, length of hospital stay, length of ICU stay, and sepsis) showed adequate power (Figures S1–S4), and thus these estimates can be considered conclusive.
Nonclinical Outcomes
Significant benefits were found in 10 of the 24 laboratory parameters analyzed (Table S2). These were significant benefits in marker liver enzyme levels (AST, ALT, and GGT), higher levels of the antioxidant α‐tocopherol, as well as lower levels for markers of inflammation such as TNF‐α. A significant benefit was observed in fatty‐acid profiles, with increases in levels of the ω‐3 fatty acids, DHA, and EPA. A positive influence was also observed on LT levels, with a significant increase in LTB5 levels as well as on the LTB5:LTB4 ratio. PTT also increased significantly.
Confidence in Cumulative Estimate and Meta‐Bias and Meta‐Regression Results
Confidence in cumulative estimates for clinical outcomes was high for infection and sepsis rates and moderate for both length of hospital and ICU stays (Table S3). Confidence in cumulative estimates for laboratory parameters was either high or moderate, except TNF‐α, which was judged as low.
The potential for meta‐bias (reporting bias) was explored by funnel plots for clinical outcomes. These appeared symmetrical, and there was no evidence of significant bias on the weighted regression using either Begg's or Egger's tests (Figure S5). Funnel plots were also performed for all other outcomes, and none showed evidence of bias.
Univariate and multivariate meta‐regression were performed for length of hospital stay. Univariate meta‐regression found no potential associations between length of hospital stay and the exclusiveness of parenteral administration (P = 0.1868), reason for PN (P = 0.6406), nutrition status of malnourished or non‐malnourished (P = 0.2281), ICU or non‐ICU setting (P = 0.0956), medical vs surgical ICU setting (P = 0.8161), or patients’ oncological status (P = 0.7452), infection rate difference between the treatment and control groups (P = 0.1485), or mean age (P = 0.3710). However, univariate meta‐regression found a significant association between treatment effect estimate and the mortality difference between the treatment and the control group (P = 0.0255). Nevertheless, there were more deaths in the control group, excluding any hypothesis that saved hospital days could be because of excess mortality in the fish‐oil group. Multivariate regression results (P = 0.0405) were consistent with these, indicating a potential association between the mortality difference and treatment effect estimate on the length of hospital stay.
Discussion
ω‐3 Fatty‐acid enriched PN significantly reduces the risk of infections and length of both ICU and hospital stays compared with standard PN. Furthermore, ω‐3 fatty‐acid enriched PN had potentially beneficial effects on liver chemistry, antioxidant status, markers of inflammation, coagulation, and fatty‐acid profile.
The validity and robustness of results from our previous publication that encompassed 23 RCTs10 have been confirmed and extended by the present study using a much larger and current dataset and the addition of trial sequential analysis. Moreover, this update was needed, as the Cochrane Collaboration recommends that systematic reviews and meta‐analyses are updated at least every 2 years, if possible.12 When comparing the results of the previous meta‐analysis10 and this update, there is a great degree of similarity, but an increased number of patients have resulted in greater precision (narrower CIs) (Table S4). The current results also include sepsis, demonstrating a significant (approximately 56%) reduction in sepsis associated with the use of PN including fish oils (P = 0.0004). The only clinical outcome that was not statistically significant was mortality, as shown previously.
To the best of our knowledge, the current systematic review and meta‐analysis is the largest conducted to date on this subject. A number of other meta‐analyses have compared clinical outcomes for PN enriched with ω‐3 fatty acids vs standard PN in surgical patients,66, 67, 68, 69, 70 ICU and/or critically ill patients,71, 72, 73 ICU and non‐ICU patients,10, 74 or patients with gastrointestinal cancer.75 Only 2 of these 11 meta‐analyses failed to find 1 or more significant clinical benefits in favor of ω‐3 fatty‐acid enriched PN,68, 72 though both were probably underpowered, as each only included 6 RCTs, 1 with a total of 306 patients68 and the other 390 patients.72 To our knowledge, no meta‐analyses have found any significant clinical benefits in favor of standard PN.
There is considerable confidence in the effect estimates of the current study as assessed using GRADE and trial sequential analysis. This is necessary for the result to be relevant to clinical practice.76 The quality of evidence for clinical outcomes and all laboratory parameters (except TNF‐α) were rated as high or moderate. Moreover, there was no evidence of meta‐bias (reporting bias) from funnel plots. Although we have a high level of confidence in the meta‐analysis estimates, especially infection and sepsis reduction estimates, ideally it would be useful to confirm these evaluations by performing further large‐scale RCTs. In particular, large, properly designed trials are required to prove or reject any effect on mortality rates. Finally, we adhered to best practices, such as prospective registration of methods and following the PRISMA statement for reporting systematic reviews and meta‐analyses.
In summary, this meta‐analysis confirms and extends previous results in greater numbers of patients and clinical trials, providing greater precision. It provides clear evidence that omega‐3 fatty‐acid enriched PN provides significant clinical and nonclinical benefits over standard non‐ω‐3 fatty‐acid enriched PN in adult hospitalized patients.
Supporting information
Supporting information
Acknowledgments
The authors thank Dr. Martina Sintzel (mcs medical communication services, Erlenbach, Switzerland) for valuable consultation services.
Statement of Authorship
L. Pradelli, K. Mayer, S. Klek, A. J. O. Alsaleh, M. D. Rosenthal, A. R. Heller, and M. Muscaritoli contributed to the conception and design of the research; L. Pradelli and A. J. O. Alsaleh contributed to the acquisition and analysis of the data; L. Pradelli, K. Mayer, S. Klek, A. J. O. Alsaleh, R. A. C. Clark, M. D. Rosenthal, A. R. Heller, and M. Muscaritoli contributed to the interpretation of the data; and R. A. C. Clark drafted the manuscript. All authors critically revised the manuscript, agree to be fully accountable for ensuring the integrity and accuracy of the work, and read and approved the final manuscript.
Financial disclosure: This work was supported by Fresenius Kabi GmbH. R. A. C. Clark (freelance medical writer, Dunchurch, Warwickshire, UK) drafted the manuscript and Dr. Martina Sintzel (mcs medical communication services, Kusnacht, Switzerland) provided consultancy services, both funded by Fresenius Kabi GmbH. These services complied with international guidelines for Good Publication Practice (GPP3).
Conflicts of interest: L. Pradelli is a director and employee of AdRes, which has received project funding from Fresenius Kabi. K. Mayer has received fees from Abbott, AstraZeneca, Baxter, BBraun, Fresenius Kabi, MSD, Nestlé, Novartis, and Pfizer. S. Klek has received speaker's honoraria from Baxter, Braun, Fresenius Kabi, Nestlé, Nutricia, Shire, and Vipharm and acted as an advisory board member for Fresenius Kabi, Shire, and Tracheron. A. J. O. Alsaleh is an employee of AdRes, which has received project funding from Fresenius Kabi. R. A. C. Clark has received project funding from Fresenius Kabi for medical writing services. M. D. Rosenthal declares no conflicts of interest. A. R. Heller has received project funding from Fresenius Kabi and speaker honoraria from CSL Behring. M. Muscaritoli has received speaker's fees from Fresenius Kabi.
[This article was modified on August 12, 2019, after initial online publication to correct one sentence in the Results, Nonclinical Outcomes section.]
References
- 1. Raman M, Almutairdi A, Mulesa L, Alberda C, Beattie C, Gramlich L. Parenteral nutrition and lipids. Nutrients. 2017;9(4):388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gura KM. Is there still a role for peripheral parenteral nutrition? Nutr Clin Pract. 2009;24(6):709‐717. [DOI] [PubMed] [Google Scholar]
- 3. Calder PC, Adolph M, Deutz NE, et al. Lipids in the intensive care unit: recommendations from the ESPEN Expert Group. Clin Nutr. 2018;37(1):1‐18. [DOI] [PubMed] [Google Scholar]
- 4. Waitzberg DL, Torrinhas RS, Jacintho TM. New parenteral lipid emulsions for clinical use. JPEN J Parenter Enteral Nutr. 2006;30(4):351‐367. [DOI] [PubMed] [Google Scholar]
- 5. Calder PC. Use of fish oil in parenteral nutrition: rationale and reality. Proc Nutr Soc. 2006;65(3):264‐277. [DOI] [PubMed] [Google Scholar]
- 6. Grimm H, Kraus A. Immunonutrition – supplementary amino acids and fatty acids ameliorate immune deficiency in critically ill patients. Langenbecks Arch Surg. 2001;386(5):369‐376. [DOI] [PubMed] [Google Scholar]
- 7. Serhan CN. Pro‐resolving lipid mediators are leads for resolution physiology. Nature. 2014;510(7503):92‐101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Haidich AB. Meta‐analysis in medical research. Hippokratia. 2010;14(suppl 10):29‐37. [PMC free article] [PubMed] [Google Scholar]
- 9. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate healthcare interventions: explanation and elaboration. PLoS Med. 2009;6(7):e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pradelli L, Mayer K, Muscaritoli M, Heller AR. n‐3 fatty acid‐enriched parenteral nutrition regimens in elective surgical and ICU patients: a meta‐analysis. Crit Care. 2012;16(5):R184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. PROSPERO . International register of prospective systematic reviews. PROSPERO 2018 CRD42018110179. Available at: http://www.crd.york.ac.uk/PROSPERO/display_record.php?ID=CRD42018110179. Accessed March 13, 2019.
- 12. Higgins JPT, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available at: http://handbook.cochrane.org. [Google Scholar]
- 13. Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta‐analysis protocols (PRISMA‐P) 2015 statement. Syst Rev. 2015;4:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mitchell M, Muftakhidinov B, Winchen T, et al. Engauge Digitizer Software. Available at: http://markummitchell.github.io/engauge-digitizer. Accessed March 13, 2019.
- 15. Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Andrews JC, Schunemann HJ, Oxman AD, et al. GRADE guidelines: 15. Going from evidence to recommendation—determinants of a recommendation's direction and strength. J Clin Epidemiol. 2013;66(7):726‐735. [DOI] [PubMed] [Google Scholar]
- 17. Antebi H, Mansoor O, Ferrier C, et al. Liver function and plasma antioxidant status in intensive care unit patients requiring total parenteral nutrition: comparison of 2 fat emulsions. JPEN J Parenter Enteral Nutr. 2004;28(3):142‐148. [DOI] [PubMed] [Google Scholar]
- 18. Barbosa VM, Miles EA, Calhau C, Lafuente E, Calder PC. Effects of a fish oil containing lipid emulsion on plasma phospholipid fatty acids, inflammatory markers, and clinical outcomes in septic patients: a randomized, controlled clinical trial. Crit Care. 2010;14(1):R5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Berger MM, Tappy L, Revelly JP, et al. Fish oil after abdominal aorta aneurysm surgery. Eur J Clin Nutr. 2008;62(9):1116‐1122. [DOI] [PubMed] [Google Scholar]
- 20. Chen H, Wang W, Hong C, et al. Omega‐3 fish oil reduces mortality due to severe sepsis with acute gastrointestinal injury grade III. Pharmacogn Mag. 2017;13(51):407‐412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chen H, Wang W, Hong Y, Zhang H, Hong C, Liu X. Single‐blinded, randomized, and controlled clinical trial evaluating the effects of omega‐3 fatty acids among septic patients with intestinal dysfunction: a pilot study. Exp Ther Med. 2017;14(2):1505‐1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Friesecke S, Lotze C, Kohler J, Heinrich A, Felix SB, Abel P. Fish oil supplementation in the parenteral nutrition of critically ill medical patients: a randomised controlled trial. Intensive Care Med. 2008;34(8):1411‐1420. [DOI] [PubMed] [Google Scholar]
- 23. Grau‐Carmona T, Bonet‐Saris A, Garcia‐de‐Lorenzo A, et al. Influence of n‐3 polyunsaturated fatty acids enriched lipid emulsions on nosocomial infections and clinical outcomes in critically ill patients: ICU lipids study. Crit Care Med. 2015;43(1):31‐39. [DOI] [PubMed] [Google Scholar]
- 24. Gultekin G, Sahin H, Inanc N, Uyanik F, Ok E. Impact of omega‐3 and omega‐9 fatty acids enriched total parenteral nutrition on blood chemistry and inflammatory markers in septic patients. Pak J Med Sci. 2014;30(2):299‐304. [PMC free article] [PubMed] [Google Scholar]
- 25. Han YY, Lai SL, Ko WJ, Chou CH, Lai HS. Effects of fish oil on inflammatory modulation in surgical intensive care unit patients. Nutr Clin Pract. 2012;27(1):91‐98. [DOI] [PubMed] [Google Scholar]
- 26. Heller AR, Fischer S, Rossel T, Geiger S, Siegert G, Ragaller M, et al. Impact of n‐3 fatty acid supplemented parenteral nutrition on haemostasis patterns after major abdominal surgery. Br J Nutr. 2002;87(suppl 1):S95‐101. [DOI] [PubMed] [Google Scholar]
- 27. Heller AR, Rossel T, Gottschlich B, et al. Omega‐3 fatty acids improve liver and pancreas function in postoperative cancer patients. Int J Cancer. 2004;111(4):611‐616. [DOI] [PubMed] [Google Scholar]
- 28. Morlion BJ, Torwesten E, Lessire H, et al. The effect of parenteral fish oil on leukocyte membrane fatty acid composition and leukotriene‐synthesizing capacity in patients with postoperative trauma. Metabolism. 1996;45(10):1208‐1213. [DOI] [PubMed] [Google Scholar]
- 29. Piper SN, Schade I, Beschmann RB, Maleck WH, Boldt J, Rohm KD. Hepatocellular integrity after parenteral nutrition: comparison of a fish‐oil‐containing lipid emulsion with an olive‐soybean oil‐based lipid emulsion. Eur J Anaesthesiol. 2009;26(12):1076‐1082. [DOI] [PubMed] [Google Scholar]
- 30. Roulet M, Frascarolo P, Pilet M, Chapuis G. Effects of intravenously infused fish oil on platelet fatty acid phospholipid composition and on platelet function in postoperative trauma. JPEN J Parenter Enteral Nutr.1997;21(5):296‐301. [DOI] [PubMed] [Google Scholar]
- 31. Sabater J, Masclans JR, Sacanell J, Chacon P, Sabin P, Planas M. Effects of an omega‐3 fatty acid‐enriched lipid emulsion on eicosanoid synthesis in acute respiratory distress syndrome (ARDS): a prospective, randomized, double‐blind, parallel group study. Nutr Metab (Lond). 2011;8(1):22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Stephenson JA, Al‐Taan O, Arshad A, et al. Unsaturated fatty acids differ between hepatic colorectal metastases and liver tissue without tumour in humans: results from a randomised controlled trial of intravenous eicosapentaenoic and docosahexaenoic acids. Prostaglandins Leukot Essent Fatty Acids. 2013;88(6):405‐410. [DOI] [PubMed] [Google Scholar]
- 33. Wachtler P, Konig W, Senkal M, Kemen M, Koller M. Influence of a total parenteral nutrition enriched with omega‐3 fatty acids on leukotriene synthesis of peripheral leukocytes and systemic cytokine levels in patients with major surgery. J Trauma.1997;42(2):191‐198. [DOI] [PubMed] [Google Scholar]
- 34. Wang X, Li W, Li N, Li J. Omega‐3 fatty acids‐supplemented parenteral nutrition decreases hyperinflammatory response and attenuates systemic disease sequelae in severe acute pancreatitis: a randomized and controlled study. JPEN J Parenter Enteral Nutr. 2008;32(3):236‐241. [DOI] [PubMed] [Google Scholar]
- 35. Wang X, Li W, Zhang F, Pan L, Li N, Li J. Fish oil‐supplemented parenteral nutrition in severe acute pancreatitis patients and effects on immune function and infectious risk: a randomized controlled trial. Inflammation. 2009;32(5):304‐309. [DOI] [PubMed] [Google Scholar]
- 36. Weiss G, Meyer F, Matthies B, Pross M, Koenig W, Lippert H. Immunomodulation by perioperative administration of n‐3 fatty acids. Br J Nutr. 2002;87(suppl 1):S89‐94. [DOI] [PubMed] [Google Scholar]
- 37. Wendel M, Rossel T, Bergmann S, et al. Impact of total parenteral nutrition including omega‐3 fatty acids on the regulation of plasma lipoproteins and glycemic control after major abdominal surgery. E Spen Eur E J Clin Nutr Metab. 2007;2(5):e103‐110. [Google Scholar]
- 38. Wichmann MW, Thul P, Czarnetzki HD, Morlion BJ, Kemen M, Jauch KW. Evaluation of clinical safety and beneficial effects of a fish oil containing lipid emulsion (Lipoplus, MLF541): data from a prospective, randomized, multicenter trial. Crit Care Med. 2007;35(3):700‐706. [DOI] [PubMed] [Google Scholar]
- 39. Aliyazicioglu T, Canturk NZ, Simsek T, Kolayli F, Cekmen M. Effects of standard and/or glutamine dipeptide and/or omega‐3 fatty acid‐supplemented parenteral nutrition on neutrophil functions, interleukin‐8 level and length of stay—a double blind, controlled, randomised study. East Afr Med J. 2013;90(2):59‐66. [PubMed] [Google Scholar]
- 40. Badia‐Tahull MB, Llop‐Talaveron JM, Leiva‐Badosa E, et al. A randomised study on the clinical progress of high‐risk elective major gastrointestinal surgery patients treated with olive oil‐based parenteral nutrition with or without a fish oil supplement. Br J Nutr. 2010;104(5):737‐741. [DOI] [PubMed] [Google Scholar]
- 41. Chen H, Pan D, Li L. The effects of multi‐oil fat emulsion on older patients with gastric cancer. Biomed Res India. 2017;28(10):4270‐4276. [Google Scholar]
- 42. Demirer S, Sapmaz A, Karaca AS, et al. Effects of postoperative parenteral nutrition with different lipid emulsions in patients undergoing major abdominal surgery. Ann Surg Treat Res. 2016;91(6):309‐315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Grimm H, Mertes N, Goeters C, et al. Improved fatty acid and leukotriene pattern with a novel lipid emulsion in surgical patients. Eur J Nutr. 2006;45(1):55‐60. [DOI] [PubMed] [Google Scholar]
- 44. Hallay J, Olah AV, Fulesdi B, et al. Hepatobiliary response in postoperative lipid therapy in gastrointestinal surgery. Hepatogastroenterology. 2010;57(102–103):1069‐1073. [PubMed] [Google Scholar]
- 45. Jiang ZM, Wilmore DW, Wang XR, et al. Randomized clinical trial of intravenous soybean oil alone versus soybean oil plus fish oil emulsion after gastrointestinal cancer surgery. Br J Surg. 2010;97(6):804‐809. [DOI] [PubMed] [Google Scholar]
- 46. Klek S, Kulig J, Szczepanik AM, Jedrys J, Kolodziejczyk P. The clinical value of parenteral immunonutrition in surgical patients. Acta Chir Belg. 2005;105(2):175‐179. [PubMed] [Google Scholar]
- 47. Klek S, Kulig J, Sierzega M, et al. The impact of immunostimulating nutrition on infectious complications after upper gastrointestinal surgery: a prospective, randomized, clinical trial. Ann Surg. 2008;248(2):212‐220. [DOI] [PubMed] [Google Scholar]
- 48. Klek S, Sierzega M, Szybinski P, et al. Perioperative nutrition in malnourished surgical cancer patients—a prospective, randomized, controlled clinical trial. Clin Nutr. 2011;30(6):708‐713. [DOI] [PubMed] [Google Scholar]
- 49. Koller M, Senkal M, Kemen M, Konig W, Zumtobel V, Muhr G. Impact of omega‐3 fatty acid enriched TPN on leukotriene synthesis by leukocytes after major surgery. Clin Nutr. 2003;22(1):59‐64. [DOI] [PubMed] [Google Scholar]
- 50. Liang B, Wang S, Ye YJ, et al. Impact of postoperative omega‐3 fatty acid‐supplemented parenteral nutrition on clinical outcomes and immunomodulations in colorectal cancer patients. World J Gastroenterol. 2008;14(15):2434‐2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Linseisen J, Hoffmann J, Lienhard S, Jauch KW, Wolfram G. Antioxidant status of surgical patients receiving TPN with an omega‐3‐fatty acid‐containing lipid emulsion supplemented with alpha‐tocopherol. Clin Nutr. 2000;19(3):177‐184. [DOI] [PubMed] [Google Scholar]
- 52. Ma CJ, Sun LC, Chen FM, et al. A double‐blind randomized study comparing the efficacy and safety of a composite vs a conventional intravenous fat emulsion in postsurgical gastrointestinal tumor patients. Nutr Clin Pract. 2012;27(3):410‐415. [DOI] [PubMed] [Google Scholar]
- 53. Ma CJ, Wu JM, Tsai HL, et al. Prospective double‐blind randomized study on the efficacy and safety of an n‐3 fatty acid enriched intravenous fat emulsion in postsurgical gastric and colorectal cancer patients. Nutr J. 2015;14:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Makay O, Kaya T, Firat O, et al. Omega‐3 fatty acids have no impact on serum lactate levels after major gastric cancer surgery. JPEN J Parenter Enteral Nutr. 2011;35(4):488‐492. [DOI] [PubMed] [Google Scholar]
- 55. Mertes N, Grimm H, Furst P, Stehle P. Safety and efficacy of a new parenteral lipid emulsion (SMOFlipid) in surgical patients: a randomized, double‐blind, multicenter study. Ann Nutr Metab. 2006;50(3):253‐259. [DOI] [PubMed] [Google Scholar]
- 56. Schauder P, Rohn U, Schafer G, Korff G, Schenk HD. Impact of fish oil enriched total parenteral nutrition on DNA synthesis, cytokine release and receptor expression by lymphocytes in the postoperative period. Br J Nutr. 2002;87(suppl 1):S103‐S110. [DOI] [PubMed] [Google Scholar]
- 57. Senkal M, Geier B, Hannemann M, et al. Supplementation of omega‐3 fatty acids in parenteral nutrition beneficially alters phospholipid fatty acid pattern. JPEN J Parenter Enteral Nutr. 2007;31(1):12‐17. [DOI] [PubMed] [Google Scholar]
- 58. Wang J, Yu JC, Kang WM, Ma ZQ. Superiority of a fish oil‐enriched emulsion to medium‐chain triacylglycerols/long‐chain triacylglycerols in gastrointestinal surgery patients: a randomized clinical trial. Nutrition. 2012;28(6):623‐629. [DOI] [PubMed] [Google Scholar]
- 59. Wei Z, Wang W, Chen J, Yang D, Yan R, Cai Q. A prospective, randomized, controlled study of omega‐3 fish oil fat emulsion‐based parenteral nutrition for patients following surgical resection of gastric tumors. Nutr J. 2014;13:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Wu MH, Wang MY, Yang CY, Kuo ML, Lin MT. Randomized clinical trial of new intravenous lipid (SMOFlipid 20%) versus medium‐chain triglycerides/long‐chain triglycerides in adult patients undergoing gastrointestinal surgery. JPEN J Parenter Enteral Nutr. 2014;38(7):800‐808. [DOI] [PubMed] [Google Scholar]
- 61. Zhang B, Wei G, Li R, et al. n‐3 fatty acid‐based parenteral nutrition improves postoperative recovery for cirrhotic patients with liver cancer: a randomized controlled clinical trial. Clin Nutr. 2017;36(5):1239‐1244. [DOI] [PubMed] [Google Scholar]
- 62. Zhixue G, Changqing G, Bing H, et al. Effects of parenteral nutrition of omega‐3 polyunsaturated fatty acid, arginine and glutamine on cellular immune status of patients following liver cancer surgery. Trop J Pharm Res. 2018;17(3):507‐511. [Google Scholar]
- 63. Zhu XH, Wu YF, Qiu YD, Jiang CP, Ding YT. Liver‐protecting effects of omega‐3 fish oil lipid emulsion in liver transplantation. World J Gastroenterol. 2012;18(42):6141‐6147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Zhu MW, Tang DN, Hou J, et al. Impact of fish oil enriched total parenteral nutrition on elderly patients after colorectal cancer surgery. Chin Med J (Engl). 2012;125(2):178‐181. [PubMed] [Google Scholar]
- 65. Zhu X, Wu Y, Qiu Y, Jiang C, Ding Y. Effect of parenteral fish oil lipid emulsion in parenteral nutrition supplementation combined with enteral nutrition support in patients undergoing pancreaticoduodenectomy. JPEN J Parenter Enteral Nutr. 2013;37(2):236‐242. [DOI] [PubMed] [Google Scholar]
- 66. Wei C, Hua J, Bin C, Klassen K. Impact of lipid emulsion containing fish oil on outcomes of surgical patients: systematic review of randomized controlled trials from Europe and Asia. Nutrition. 2010;26(5):474‐481. [DOI] [PubMed] [Google Scholar]
- 67. Chen B, Zhou Y, Yang P, Wan HW, Wu XT. Safety and efficacy of fish oil‐enriched parenteral nutrition regimen on postoperative patients undergoing major abdominal surgery: a meta‐analysis of randomized controlled trials. JPEN J Parenter Enteral Nutr. 2010;34(4):387‐394. [DOI] [PubMed] [Google Scholar]
- 68. Tian H, Yao X, Zeng R, et al. Safety and efficacy of a new parenteral lipid emulsion (SMOF) for surgical patients: a systematic review and meta‐analysis of randomized controlled trials. Nutr Rev. 2013;71(12):815‐821. [DOI] [PubMed] [Google Scholar]
- 69. Li NN, Zhou Y, Qin XP, et al. Does intravenous fish oil benefit patients post‐surgery? A meta‐analysis of randomised controlled trials. Clin Nutr. 2014;33(2):226‐239. [DOI] [PubMed] [Google Scholar]
- 70. Bae HJ, Lee GY, Seong JM, Gwak HS. Outcomes with perioperative fat emulsions containing omega‐3 fatty acid: a meta‐analysis of randomized controlled trials. Am J Health Syst Pharm. 2017;74(12):904‐918. [DOI] [PubMed] [Google Scholar]
- 71. Palmer AJ, Ho CKM, Ajibola O, Avenell A. The role of omega‐3 fatty acid supplemented parenteral nutrition in critical illness in adults: a systematic review and meta‐analysis. Crit Care Med. 2013;41(1):307‐316. [DOI] [PubMed] [Google Scholar]
- 72. Manzanares W, Dhaliwal R, Jurewitsch B, Stapleton RD, Jeejeebhoy KN, Heyland DK. Parenteral fish oil lipid emulsions in the critically ill: a systematic review and meta‐analysis. JPEN J Parenter Enteral Nutr. 2014;38(1):20‐28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Manzanares W, Langlois PL, Dhaliwal R, Lemieux M, Heyland DK. Intravenous fish oil lipid emulsions in critically ill patients: an updated systematic review and meta‐analysis. Critical Care. 2015;19(1):167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Kreymann KG, Heyland DK, de Heer G, Elke G. Intravenous fish oil in critically ill and surgical patients—historical remarks and critical appraisal. Clin Nutr. 2018;37(3):1075‐1081. [DOI] [PubMed] [Google Scholar]
- 75. Bai H, Li Z, Meng Y, et al. Effects of parenteral omega‐3 fatty acid supplementation in postoperative gastrointestinal cancer on immune function and length of hospital stay: a systematic review and meta‐analysis. Asia Pac J Clin Nutr. 2018;27(1):121‐128. [DOI] [PubMed] [Google Scholar]
- 76. Murad MH, Montori VM, Ioannidis JP, et al. How to read a systematic review and meta‐analysis and apply the results to patient care: users' guides to the medical literature. JAMA. 2014;312(2):171‐179. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information


