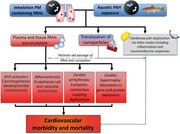
Abstract
Air pollution is associated with detrimental effects on human health, including decreased cardiovascular function. However, the causative mechanisms behind these effects have yet to be fully elucidated. Here we review the current epidemiological, clinical and experimental evidence linking pollution with cardiovascular dysfunction. Our focus is on particulate matter (PM) and the associated low molecular weight polycyclic aromatic hydrocarbons (PAHs) as key mediators of cardiotoxicity. We begin by reviewing the growing epidemiological evidence linking air pollution to cardiovascular dysfunction in humans. We next address the pollution‐based cardiotoxic mechanisms first identified in fish following the release of large quantities of PAHs into the marine environment from point oil spills (e.g. Deepwater Horizon). We finish by discussing the current state of mechanistic knowledge linking PM and PAH exposure to mammalian cardiovascular patho‐physiologies such as atherosclerosis, cardiac hypertrophy, arrhythmias, contractile dysfunction and the underlying alterations in gene regulation. Our aim is to show conservation of toxicant pathways and cellular targets across vertebrate hearts to allow a broad framework of the global problem of cardiotoxic pollution to be established. AhR; Aryl hydrocarbon receptor. Dark lines indicate topics discussed in this review. Grey lines indicate topics reviewed elsewhere.
Keywords: air pollution, cardiotoxicity, cardiovascular dysfunction, heart disease, oil spills, PAH, phenanthrene, particulate matter, PM, PM2.5
Polycyclic aromatic hydrocarbons are common in both air and aquatic pollution. Exposure to these pollutants can lead to PAH accumulation in the body and cause cardiovascular dysfunction via a number of direct and indirect mechanisms. PAH exposure is associated with arrhythmias, hypertrophy, atherosclerosis and developmental toxicity (among others). This review discusses current evidence linking PAH‐based pollution and cardiovascular disease in vertebrates.

Abbreviations
- AA
arachidonic acid
- AhR
aryl hydrocarbon receptor
- AP
action potential
- APD
Action potential duration
- ANP
atrial natriuretic protein
- BaP
Benzo[a]pyrene
- BMP10
bone morphogenetic protein 10
- BNP
brain natriuretic protein
- CdC42
cell division control protein 42 homolog
- CREB
cAMP response element‐binding protein
- CVD/CVS
cardiovascular disease/cardiovascular system
- CYP1A1/1B1
cytochrome P450 1A1/1B1
- DCM
dilated cardiomyopathy
- DEP
diesel exhaust particles
- DWH
deepwater horizon
- EC
excitation‐contraction
- ERG
Ether‐à‐go‐go‐related gene K+ channel
- HCAEC
human coronary artery endothelial cells
- HUVEC
human umbilical vascular endothelial cells
- hiPSC
human induced pluripotent stem cells
- IUPS
international union of physiological sciences
- LTCC
L‐type calcium channels
- MMP‐9
matrix metalloproteinase‐9
- NFAT
nuclear factor of activated T‐cells
- PAH
polycyclic aromatic hydrocarbons
- Phe
phenanthrene
- PLA2
phospholipase A2
- PM
particulate matter
- ROFA
residual oil fly ash
- SERCA
sarcoplasmic reticulum calcium ATPase
- SR
sarcoplasmic reticulum
- Tbx5
T‐box 5
- TCDD
2,3,7,8‐Tetrachlorodibenzo‐p‐dioxin
- TGF‐β
transforming growth factor‐β
Introduction
The disease burden from ambient air pollution is becoming increasingly apparent, with a host of recent studies drawing close association between pollution levels and reduced life expectancy (Hoek et al. 2013; Lelieveld et al. 2019). An estimated 4.2 million premature human deaths worldwide are attributed to ambient air pollution (World Health Organization (WHO), 2018). Over 90% of the population live in areas where air pollution is above the World Health Organization's guidelines and, with air pollution levels still on the rise in many countries, this threat is ever growing (WHO, 2018). Air pollution is derived from a vast range of sources, but combustion of fossil fuels represents a significant proportion of the pollutants known to be detrimental to health. In parallel, industrial pollutant discharge and natural oil seeps in aquatic ecosystems, together with observations from disastrous oil spills, have demonstrated the considerable damage that other forms of fossil fuel pollution can impose on both aquatic life and human livelihood (Smith & Levy, 1990; Beyer et al. 1998; Naes & Oug, 1998; Zakaria et al. 2002; Pampanin & Sydnes, 2013; Stogiannidis & Laane, 2015).
What is becoming increasingly evident is the detrimental effect of fossil fuel‐derived pollution on the cardiovascular system (CVS). Despite the lung being the major entry point into the body and thus the first target of exposure, numerous epidemiological studies have shown strong correlations between air pollution and cardiovascular diseases (CVDs) (Shah et al. 2013; Franklin et al. 2015). Indeed, mortality attributed to air pollution's impact on CVD outweighs mortality due to impact on respiratory diseases (Cohen et al. 2017).
In fish, the cardiotoxicity of exposure to fossil fuels has been recognised for several decades. The negative impact of crude oil exposure on cardiac function was clearly identified in the years following the 1989 Exxon Valdez oil spill (Incardona et al. 2009), and a deeper mechanistic understanding of the cardiotoxic pathways followed the 2010 Deepwater Horizon (DWH) blowout (Brette et al. 2014, 2017). While there are undoubtedly clear differences in air pollutants and aquatic environmental pollution (e.g. physicochemical properties of the pollutants, interaction between pollutants and the environment, the biology of the species exposed and the route of exposure), it has become apparent that parallels exist, especially in terms of the ability of these pollutants to cause cardiovascular toxicity. This review builds on a focus session at the 2017 International Union of Physiological Sciences (IUPS) World Congress (http://www.iups.org/) to outline the current epidemiological, clinical and experimental evidence by which pollution can impact on the heart and other aspects of the CVS. Our focus is on the cardiotoxicity of the lower molecular weight polycyclic aromatic hydrocarbons (PAHs) as key mediators of these effects. This review outlines (1) the growing epidemiological evidence linking air pollution to cardiovascular dysfunction, (2) the importance of particulate matter (PM) and PAHs in cardiotoxicity, (3) the key mechanisms of cardiotoxicity identified in the flurry of fish heart research that followed the Exxon Valdez and DWH oil spills, and (4) the current state of mechanistic knowledge linking PAH exposure to cardiovascular patho‐physiologies such as atherosclerosis, cardiac hypertrophy, arrhythmias, contractile dysfunction and the underlying alterations in gene regulation. In this review we draw on studies from humans, other mammals and fish which collectively form our current understanding of pollution‐based cardiovascular toxicity. In the final section we discuss the implications of interspecies diversity for extrapolating risks identified in aquatic pollution to those of human exposure to air pollution.
Airborne particulate matter and human health risks
Numerous epidemiological studies have linked air pollution to cardiovascular morbidity and mortality. Airborne PM is a key pollutant (in terms of strength, and in many cases magnitude, of associations) driving the cardiovascular effects. PM exposure is linked to cardiac arrhythmias, alterations in heart rate variability, myocardial infarction, arterial vasoconstriction, increased blood coagulability, atherosclerosis (vascular plaques), heart failure and stroke (Mills et al. 2009; Brook et al. 2010; Brook & Brook, 2011; Shah et al. 2013; Franklin et al. 2015; Newby et al. 2015). Atmospheric PM encompasses a complex mixture of particulates and liquid droplets with a wide‐ranging chemical composition (see Fig. 1; Niemann et al. 2017; Environmental Protection Agency, 2018). PM is grouped into three classes based on particle size (Fig. 1B). Course particles (with a diameter between 2.5 and 10 µm) tend to be mechanically derived from construction and demolition, mining, agriculture, tyre fragmentation and other road dust. Fine (diameter of ≤2.5 µm; PM2.5) and ultrafine particles (also known as nanoparticles; diameter ≤ 0.1 µm) have both natural and anthropogenic sources. Ultrafine PM and a substantial proportion of PM2.5, are derived from combustive sources such as motor vehicle exhaust, industrial burning of coal and fuel oil, cigarette smoke and residential wood burning. PM2.5 is widely measured in the environment as an indicator of air quality. This class of PM is particularly concerning due to the high penetration deep into the lungs after inhalation (Lee et al. 2018; and see below) and for the large reactive surface area of these small particles. Ultrafine PM cannot presently be routinely measured in the environment, yet has the potential to be an even greater threat to health due to the markedly greater surface area these particles have and that they may gain access (i.e. translocate) to other organs of the body (Donaldson et al. 2013).
Figure 1. Constituents of air pollution.
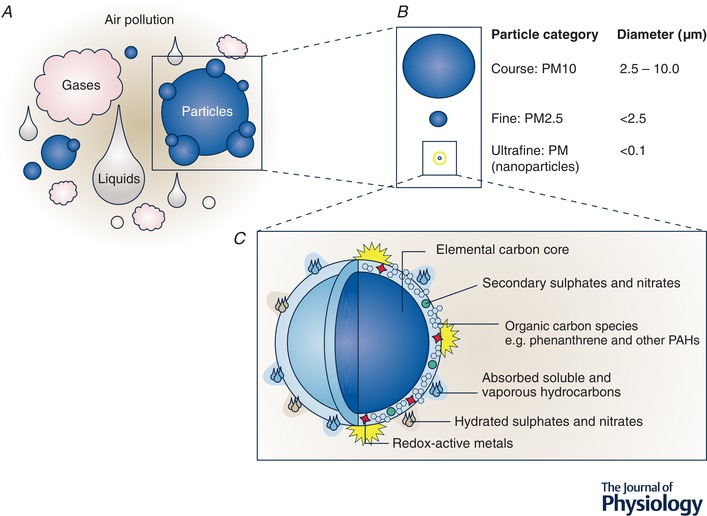
A, air pollution can be broadly characterized into gases, liquids and particles. PAHs can be found in the gaseous phase of air pollution as well as binding to the particle surface. B, particles can be further classified into size categories of particulate matter (PM). For perspective, if a human hair has a width of approximately 50 µm (0.05 mm) then PM10 encompasses particles with a diameter below a fifth of this size (<10 µm). Fine PM (<2.5 µm) would be a quarter of this size, and smaller still, ultrafine (nano) particles a hundredth of the size of PM10 (<0.1 µm). C, schematic diagram providing an example of the complex composition of a combustion derived ultrafine (nano) particle, such as a diesel exhaust particle (DEP), a common PM in urban air. The carbon core of DEP is coated with a diverse range of chemical species including reactive transition metals and polyaromatic hydrocarbons like phenanthrene. Detail of surface chemicals in C is not to scale. Figure reproduced from Environmental Health Perspectives with permission from the authors (Stone et al. 2017).
Positive associations between PM2.5 levels and incidences of stroke are evident in areas with increased industrialization such as India, China and Africa (Shah et al. 2015; Lee et al. 2018). Two long‐term studies based in the USA in 2007 (Miller et al. 2007) and 2011 (Lipsett et al. 2011), identified a significant increase in stroke incidence for every 10 µg m−3 increase in PM2.5 levels (35% and 19%, respectively). Similarly, a ∼20% increase in stroke incidence has been observed in various European countries which met their air quality standards, indicating that current standards may not be sufficient (Lee et al. 2018). Concern for current air quality standards was bolstered by Aung et al. (2018), who showed correlations between UK addresses, ambient air quality data (at levels within current UK air quality standards) and biventricular dilatation that is suggestive of detrimental cardiac remodelling.
Short‐term exposure to air pollution has also been associated with severe cardiac complications. A study in the Greater Boston area found a strong association between the onset of ischaemic stroke and traffic‐related pollutant exposure 12—14 h prior (Wellenius et al. 2012). Associations have even been found between the onset of myocardial infarctions 2 h after high PM exposure associated with traffic (Peters et al. 2001). Daily variations in pollutant levels are also associated with increases in hospital admissions for ischaemic heart disease, particularly in vulnerable subgroups like those with accompanying congestive heart failure and arrhythmias (Mann et al. 2002). It is possible that other vulnerable groups (e.g. newborns, children, the elderly and immunocompromised individuals) may also be particularly susceptible to the impacts of air pollution on the CVS but epidemiological studies are lacking and calls for greater focus in this area have been made (Sram et al. 2013; Wittkopp et al. 2016).
There is growing recognition of the effects of indoor air pollution on health (Lim et al. 2012; WHO, 2014). Indoor air pollution has arguably a greater diversity of sources (e.g. heating, cooking, smoking, moulds, pet dander, mosquito coils, chemical aerosols, cleaning products, ingress of outdoor air pollution, and many others), with marked differences between developed and developing nations. Similar to outdoor/ambient pollution, PM is the pollutant of concern, showing strong associations with CVDs (Mitter et al. 2016). With people spending more than 80% of their time indoors, and with the ready use of unclean/inappropriate fuel sources in developing nations, the true scale of indoor air pollution is likely to come to prominence in the next decade as research in this area grows.
Already, the potential impact of pollution has been substantiated, with Lelieveld et al. recently estimating a new total excess mortality rate of 659,000 per year due to air pollution in the 28 countries of the European Union (Lelieveld et al. 2019), a vast increase from the 400,000 per year currently acknowledged by the European Environment Agency (Agency, 2019).
Inhaled particles and the cardiovascular system
Despite the considerable evidence linking air pollution and CVD, the biological mechanisms linking this association are not fully elucidated. The particulate constituents of air pollution appear to drive the cardiovascular actions. Deposition of PM in the lungs depends on particle size (United States Environmental Protection Agency, 2017). Course particles are largely deposited in the upper airways and are then cleared by ciliary action. Fine and ultrafine particles can reach the lower regions of the lung: the acini, consisting of respiratory bronchioles, alveolar ducts and alveoli where gas exchange occurs. Following deposition on the alveolar walls, fine particles can be cleared with the assistance of alveolar macrophages; however, a proportion of particulates (especially smaller particles) may evade capture or may trigger an inflammatory response within the lungs (Stone et al. 2017). Induction of inflammation with release of inflammatory mediators and activation of sensory afferents are likely to be means by which inhaled PM can induce cardiovascular effects; the former by passage of inflammatory or oxidative mediators into blood, and the latter by changes in autonomic function and neuroendocrine systems (Miller, 2014). Moreover, urban PM and diesel exhaust particles (DEP; a rich source of combustion‐derived nanoparticles in ambient air pollution, see Fig. 1C ) can directly generate free radicals from their surface, and through activation of cellular enzymes induce oxidative stress and inflammation (Donaldson et al. 2005; Miller et al. 2009; Langrish et al. 2012). Both processes are known to impair vascular function in (otherwise) healthy blood vessels to promote disease (Münzel et al. 2018).
The smallest ultrafine nanoparticles (Fig. 1B and C ) appear to be able to pass the alveolar barrier, enter the pulmonary circulation, and be carried to other regions of the body. Due to the very small numbers of ultrafine particles likely to translocate and the difficulty in detecting these particles systemically, it has been challenging to conclusively demonstrate that environmental ultrafine nanoparticles translocate in humans (Nemmar et al. 2002; Mills et al. 2006; Möller et al. 2008). However, experimental studies using model nanoparticles such as radiolabelled carbon or gold nanoparticles have robustly demonstrated that this pathway occurs in rodents (Geiser & Kreyling, 2010) and now recently in humans (Miller et al. 2017a, b ). The translocation pathway is of importance as it provides a biological basis that could account for the widespread effects of inhaled PM across the CVS, and elsewhere in the body (Raftis & Miller, 2019). Thus while changes in autonomic function are likely to play a major role in the arrhythmogenic action of PM (Robertson et al. 2014; Buteau & Goldberg, 2016) and systemic inflammation will most likely exacerbate existing disease processes, the entry of PM into the systemic circulation expands the diversity of routes, targets and time course by which inhaled PM deregulates cardiovascular homeostasis. Access of particles to the blood also provides a means by which inhaled PM and its constituent surface chemicals can directly interact with the CVS.
PAHs in air pollution
Combustion‐derived PM is predominantly carbon based; however, it has a complex composition. A central particle core is composed of both organic and elemental carbon (as well as other substances such as sulphates and nitrates). However, the particle surface is coated with a range of different chemical entities that include organic carbon species such as PAHs, as well as reactive metal species (e.g. iron, copper, nickel; Fig. 1C ). The chemicals within this surface mixture, in particular the PAHs, are believed to greatly influence PM biological activity, and recent evidence links the cardiovascular toxicity of PM2.5 to its organic carbon component (Miller et al. 2012) in humans (Mills et al. 2011).
PAHs are a family of ubiquitous contaminants which can bind to particulates or can form small airborne particles in the gaseous phase of air pollution themselves. Studies in animals show exposure to DEP, but not ultrafine polystyrene particles, caused a pronounced pulmonary inflammatory response (Nemmar et al. 2004). Exposure to residual oil fly ash (ROFA) but not ‘clean’ black carbon, produced electrocardiographic changes (Wellenius et al. 2002), indicating that simply the physical presence of a (relatively inert) particle core is insufficient for a cardiovascular pathophysiological response. These studies are consistent with the bioavailability of PAHs in PM and in vehicle emissions. Indeed, respiratory epithelial cells exposed to PM2.5 take up PAHs (Penn et al. 2005), and in the case of the smallest sized particles in PM2.5, these may be transported across the alveolar epithelium and into the systemic circulation un‐metabolized (Gerde et al. 2001). The transport of PAHs to the heart from the pulmonary circulation would occur before significant hepatic metabolism (Incardona et al. 2005).
Phenanthrene
Each PAH compound contains two or more fused benzene rings (see right hand column in Fig. 2). There are two main sources of PAHs: (1) pyrogenic sources which involve anoxic or low oxygen combustion of fossil fuels and are dominated by the presence of larger 4–6 ring PAHs, and (2) petrogenic sources that include crude oils and associated by‐products and consist primarily of 2–3 ring PAHs. Tricyclic (3‐ringed) PAHs, such as phenanthrene (Phe) and its alkylated homologues (e.g. dimethylphenanthrene; Fig. 2), are naturally enriched in crude oil and refined fuels and they are present in most types of combustive emissions in large quantities (Fig. 2). Excluding 2‐ringed naphthalene, phenanthrenes are the most abundant PAH family in aquatic environments impacted by either oil spills or by urban (non‐point source) pollution (Fig. 2A and B ), and in urban air (Fig. 2C and D ) (Liu et al. 2001; Naumova et al. 2002; Bi et al. 2003; Tsapakis & Stephanou, 2005; Li et al. 2009; Scholz et al. 2011; Incardona et al. 2014). PM contains Phe (Abdel‐Shafy & Mansour, 2016) and significant levels of Phe can be found in the gaseous phase of vehicle exhaust (Schauer et al. 1999, 2002; Möllmann et al. 2006). Levels of Phe in London air were reported at 76–82 ng m−3(Halsall et al. 1994) and ∼10–150 nmol L−1 in the water of the Jiulong River Estuary and Western Xiamen Sea in China (Maskaoui et al. 2002). Therefore, terrestrial animals (including humans) and fishes come into contact with Phe through diet, drinking, breathing, smoking and through other routes of environmental exposure (Laurent et al. 2001; Grova et al. 2005; Zhong et al. 2011). Phe is known to exert direct toxicity in dogs where aerosolized Phe was rapidly transported into the bloodstream following inhalation (Gerde et al. 1993). Particle translocation may also assist the passage of Phe within PM to areas of susceptibility, given that translocated nanoparticles have been shown to preferentially accumulate at sites of vascular disease (Miller et al. 2017a).
Figure 2. Comparison of PAH composition between water and air samples.
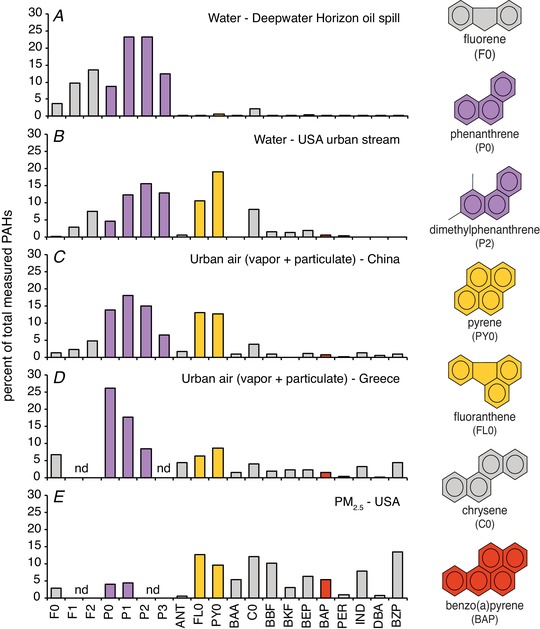
Composition profiles represent the percentage of the indicated PAHs normalized to the sum total of the measured set, with ring number and molecular weight increasing to the right. Parent non‐alkylated compounds are denoted with a 0 (e.g. F0, P0), while alkylated compounds (e.g. those with 1 or 2 additional methyl groups) are indicated by F1, F2, P1, P2, etc. Selected individual structures are shown on the right, with phenanthrene and its alkylated homologues indicated in purple, the 4‐ringed pyrogenic compounds indicated in gold (pyrene and fluoranthene), and the archetypal carcinogenic 5‐ringed PAH benzo(a)pyrene indicated in red. Five sample sources are shown. A, water from the area of the Gulf of Mexico impacted by the 2010 Deepwater Horizon oil spill (Incardona et al. 2014); B, passive samplers placed in an urban stream in Seattle (USA) that receives large quantities of stormwater runoff from nearby roadways (Scholz et al. 2011; J. P. Incardona, unpublished); C, a total high volume air sample from Guangzhou (China) collected July 2001 by combined glass fibre filter (GFF) and polyurethane foam (PUF) plug (Bi et al. 2003); D, the mean of 16 total GFF/PUF samples collected between November 2000 and February 2002 in Heraklion (Greece) (Tsapakis & Stephanou, 2005); E, mean of quarterly samples collected over the year 2004 using a PM2.5 particle composition monitoring system at a heavily trafficked urban site in Atlanta, USA (Li et al. 2009). Abbreviations: F, fluorene; P, phenanthrene; ANT, anthracene; FL, fluoranthene; PY, pyrene; BAA, benz(a)anthracene; C, chrysene; BBF, benzo(b)fluoranthene; BKF, benzo(k)fluoranthene; BEP, benzo(e)pyrene; BAP, benzo(a)pyrene; PER, perylene; IND, indeno(123‐cd)pyrene; DBA, dibenzanthracene; BZP, benzo(ghi)perylene; nd, not determined.
While data on human plasma levels of PAHs are sparse, plasma concentrations of Phe and/or other PAH molecules can be found at the nanomolar level in animals (Zhong et al. 2011; Camacho et al. 2012). A recent study of maternal blood of pregnant (non‐smoker) women and cord blood of newborns reported Phe concentrations of 37.61 and 35.32 µg L−1, respectively; a concentration approximating to 200 nM (Scholz et al. 2011). It is important to note that Phe, like most tricyclic PAHs, is highly lipophilic with a logP (octanol/water) coefficient of ∼4.4, indicating tissue accumulation of Phe at levels higher than those observed in plasma (Carls et al. 1999; Heintz et al. 1999). This was notably shown in studies where tissue concentrations approximating to 3 µM were reported (Jacob & Seidel, 2002; Dhananjayan & Muralidharan, 2013), with levels being exacerbated by smoking (363 ng Phe per cigarette; Severson et al. 1976; Howard et al. 1998).
The aryl hydrocarbon receptor (AhR) and the unique cardiotoxicity of low molecular weight PAHs
For many years the toxicology of PAHs was focused almost exclusively on a single 5‐ringed compound, benzo(a)pyrene (BaP). A dramatic increase in the prevalence of skin cancers among coal‐tar workers during the Industrial Revolution led to the identification of BaP as a metabolically activated chemical carcinogen (Phillips, 1983). Subsequent research showed that BaP activates an orphan transcription factor later described as the aryl hydrocarbon receptor (AhR) (Denison et al. 1998). AhR in turn regulates genes involved in PAH metabolism (Nebert et al. 2004). When activated, AhR migrates into the nucleus and binds to a specific sequence within xenobiotic responsive elements resulting in expression of xenobiotic metabolising enzymes, including cytochrome P450 1A1 (CYP1A1) and cytochrome P450 1B1 (CYP1B1) (Germolec et al. 1996; Korashy & El‐Kadi, 2006). CYPs have the ability to metabolize PAHs such as BaP into reactive metabolites which can alkylate macromolecules and have carcinogenic properties (Denison et al. 1988; Korashy & El‐Kadi, 2006). Interestingly, particles such as 2,3,7,8‐tetrachlorodibenzo‐p‐dioxin (TCDD) which are produced by organic/diesel combustion also interact with CYP pathways, and in mammalian embryos can increase mortality rates and cause severe cardiac hypertrophy (Walker et al. 1997; Dalton et al. 2001; Ivnitski et al. 2001; Lin et al. 2001; Kanzawa et al. 2004). Chronic exposure to TCDD in adults can also increase the incidence of spontaneous cardiomyopathy (Jokinen et al. 2003).
In developing fish, the larger 5‐ringed PAHs cause developmental cardiotoxicity in a manner similar to the very potent AhR ligands of the dioxin family (Incardona, 2017). With these compounds, toxicity is not linked to CYP1A‐mediated metabolism, but results from inappropriate AhR activation within developing cardiomyocytes, leading to reduced cardiomyocyte proliferation and heart malformation (for review, see Incardona, 2017). However, BaP and similar compounds make up a lower proportion of the total PAHs in water and air pollution (see Fig. 2).
Low molecular weight PAHs such as tricyclic Phe have been found to be weak AhR agonists with little to no carcinogenic activity (Barron et al. 2004). Thus, these chemical species were largely ignored through nearly eight decades of PAH research. However, more recent studies from fish indicate that not all PAH toxicity can be attributed to AhR activation (Incardona et al. 2005). In particular, and as detailed further below, Phe and complex PAH mixtures found in air and water pollution cause a functional cardiotoxicity that is independent of the AhR/CYP1A pathway and independent of cardiac developmental abnormalities.
Cardiotoxicity of PAHs in fish
The toxicity of tricyclic (3‐ringed) PAHs became apparent from the effects of the 1989 Exxon Valdez oil spill on Pacific herring (Clupea pallasi) and pink salmon (Oncorhynchus gorbuscha). At the time of the spill, these fish were the basis of the largest commercial fisheries in Alaska, and their near‐shore spawning habitats were contaminated by oil. Field studies identified a malformation syndrome in herring and salmon embryos and larvae that was linked to oiled shoreline exposure. Subsequent laboratory studies associated this syndrome with the uptake of 3‐ringed PAHs (see recent reviews: Incardona & Scholz, 2017, 2018). Briefly, weathering (i.e., water‐washing of oil slicks over time) increased the proportion of 3‐ringed compounds in the water and also the cardiotoxicity (Carls et al. 1999; Heintz et al. 1999). Subsequent work in zebrafish demonstrated several key points: First, individual tricyclic compounds such as fluorene, dibenzothiophene and Phe produced a malformation syndrome (including cardiac) that was overtly similar to that from complex oil‐derived mixtures (Incardona et al. 2004). Second, in contrast to AhR‐mediated developmental cardiotoxicity, defects in cardiac function, observed as abnormalities in heart rate and rhythm, preceded the onset of heart malformation. Finally, the complex malformations observed in non‐cardiac structures (e.g. jaw defects, small eyes, body axis deformations) were all downstream of reduced cardiac output in developing embryos (Incardona et al. 2004). Extensive studies have now demonstrated that PAH mixtures containing nanomolar concentrations of Phe are bioconcentrated to micromolar concentrations in fish embryos (Petersen & Kristensen, 1998). Corresponding cardiotoxicity syndromes range from outright heart failure and larval mortality at the high end to subtle heart malformation with pathological hypertrophy and reduced cardiorespiratory performance at the low end (Incardona et al. 2004, 2005, 2009). Importantly, bradycardia and atrioventricular conduction block observed following Phe exposure in zebrafish embryos were strongly suggestive of molecular targets involving AP generation and excitation‐contraction (EC) coupling (Incardona et al. 2004).
In April 2010, the Deepwater Horizon disaster in the Gulf of Mexico returned global attention to the toxicity of crude oil. The blowout, the largest accidental oil spill in history, released nearly 5 million barrels of complex mixtures of PAHs into the water over a period of 87 days. Many commercially important pelagic fish species were spawning in the Gulf of Mexico during this event, including bluefin (Thunnus thynnus) and yellowfin (Thunnus albacares) tunas, and mahi‐mahi (Coryphaena hippurus) (Hazen et al. 2016). In the wake of the Exxon Valdez oil spill, damage assessment studies following Deepwater Horizon focused on the deleterious effects of oil on fish cardiac function across aquatic species, life stages and levels of biological organization (for review see Incardona & Scholz, 2018). Results showed oil‐exposed pelagic fishes also had compromised swim performance, including reductions in maximal sustained swimming speed and reduced maximal metabolic rate (Mager et al. 2014; Stieglitz et al. 2016; Nelson et al. 2017). Moreover, relatively short‐term whole animal aquatic exposure (24 h) to crude oil was found to reduce cardiac stroke work and cardiac output (Nelson et al. 2016; Cox et al. 2017).
PAHs and EC coupling in fish
A detailed mechanistic understanding of PAH‐induced changes in fish cardiac function came from two papers examining the effects of crude oil (Brette et al. 2014) and its component parts (e.g. Phe; Brette et al. 2017) on action potential (AP) generation and EC coupling in single isolated myocytes from pelagic fish. Consistent with the in vivo whole‐heart effects observed in exposed fishes, these studies demonstrated that complex mixtures of crude oil, or Phe in isolation, altered both cellular Ca2+ cycling and AP waveform (Brette et al. 2014, 2017; see Fig. 3). The studies revealed that other single PAHs (naphthalene, fluorene, carbazole, dibenzothiophene and pyrene) did not alter the amplitude or the time course of the intracellular Ca2+ transient in fish ventricular myocytes (Brette et al. 2017), and that the potency of the oil‐derived mixture was specifically correlated with the content of Phe (Brette et al. 2014). In addition to the depressive effects on Ca2+ cycling, Phe was found to affect membrane excitability by prolonging action potential duration (APD) by inhibiting K+ efflux from the cardiomyocyte via the rapid delayed rectifier K+‐current (I Kr) (Brette et al. 2014, 2017), analogous to the original observations in zebrafish embryos (Incardona et al. 2004). The depression of the Ca2+ transient amplitude and the AP prolongation found in bluefin tuna myocytes following Phe exposure can be seen in Fig. 3B and D . Similar effects were also found in sheep ventricular myocytes following acute Phe exposure, shown in Fig. 3A and C (Unpublished data). Recently, such disruptions to EC coupling in the myocyte have been followed through to contractile failure of cardiac tissue and abnormal contractile rhythm of the isolated whole heart in the freshwater indicator species, the brown trout (Salmo trutta) following acute Phe exposure (Ainerua et al. 2020). A summary of the known and putative effects of PAHs on cardiomyocyte EC coupling is given in Fig. 4A .
Figure 3. The effects of Phe on excitation contraction coupling.
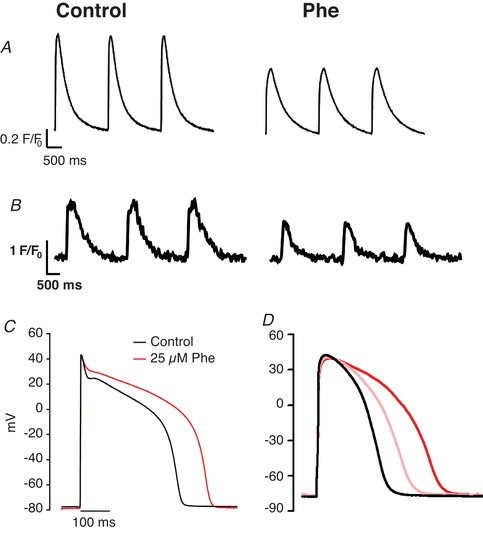
The effect of Phe on the intracellular Ca2+ transient in sheep ventricular myocytes (25 µM) (A) and fish (bluefin tuna, 5 µM) ventricular myocytes (B) loaded with Fluo‐4AM and stimulated to contract at 0.5 Hz. The effect of Phe on the ventricular AP during whole‐cell current clamp in sheep (C) and bluefin tuna (D) ventricular myocytes. The red lines in both traces show data recorded during exposure to 25 µM Phe. The pink line shows the effect of 5 µM Phe exposure in tuna. No discernible effects were seen at 5 µM in sheep (not shown). Tuna data are from Brette et al. 2017, with permission from Scientific Reports, and sheep data are unpublished data of C.R.M., S.N.K. and H.A.S. in myocytes supplied by the members of the laboratories of Dr K. Dibb and Prof. A. Trafford at the University of Manchester.
Figure 4. PAH and EC coupling in cardiac myocytes.
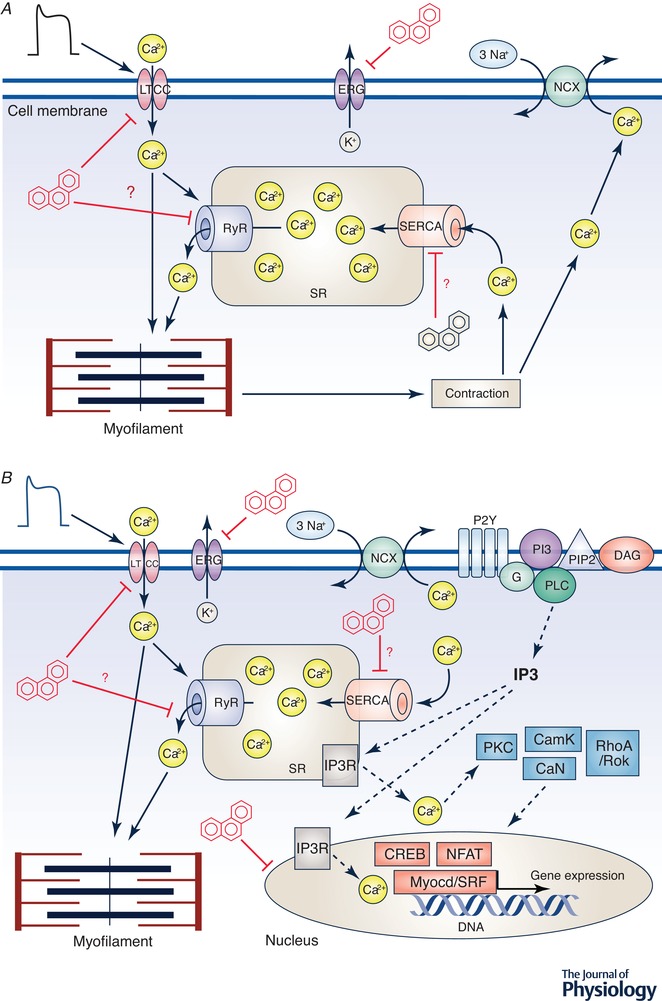
A, the top panel shows a simplified schematic of EC coupling in a cardiac cell, including the main ion channels, pumps, transporters, sarcoplasmic reticulum (SR), mitochondria and contractile proteins. EC coupling proceeds when an action potential (AP) opens L‐type Ca2+ channels (LTCC) in the cell membrane. This Ca2+ entry can directly activate the myofilament and can also initiate Ca2+‐induced Ca2+‐release from Ca2+ channels on SR membrane (ryanodine receptors, RyR). The global increase in the cytosolic [Ca2+]i transient activates contractile proteins leading to contraction. Repolarization of the AP occurs in large part by K+ efflux through ERG (ether‐à‐go‐go‐related gene) channel. Relaxation follows repolarization by removal of Ca2+ from the cytosol primarily via re‐uptake of Ca2+ into the SR (via the sarcoplasmic reticulum calcium ATPase, SERCA) and extrusion of Ca2+ out of the cell via the Na+‐Ca2+ exchanger (NCX). Exposure to PAHs, specifically Phe, impairs EC coupling as indicated by the red inhibition bars at various ionic flux pathways. Ion flux pathways where inhibition has only been measured indirectly have a red question mark beside them. Many pathways have not yet been investigated like direct effect on myofilaments, or the NCX. B, interactions between EC coupling and excitation‐transcription coupling, indicating points of PAH regulation which inhibit Ca2+ cycling and thus transcription. EC coupling is as in A. Excitation‐transcription coupling proceeds when puronergic G‐protein coupled receptors (P2Y) are activated by ATP (not shown), which activates phosphoinositide 3‐kinase (PI3K) via G protein. This causes phospholipase C (PLC) to be recruited to the membrane and produce inositol 1,4,5‐trisphosphate (IP3) and diacyl glycerol (DAG). IP3 receptor‐mediated Ca2+ signals cause Ca2+ to enter the nucleus and through kinases and/or phophatases (PKC, CamK, RhoA/ROK, CaN) activate transcription factors (CREB, NFAT, Myocd/SRF) ultimately leading to gene expression. Tricyclic PAHs disrupt Ca2+ cycling pathways in the cell, reducing Ca2+ stores, and affect Ca2+‐related gene expression pathways. CaN, calcineurin; CamK, calcium–calmodulin‐dependent protein kinase; PKC, protein kinase C; RhoA, Ras homolog gene family, member A; ROK, rho associated kinase; NFAT, nuclear factor of activated T‐cells; SRF, serum response factor; Myocd, myocardin; CREB, cAMP response element‐binding protein.
Mechanistic link between PAHs and cardiovascular disease
Mammalian (including human) studies from urban areas around the world (Shah et al. 2015; Lee et al. 2018) implicate PM2.5 and its associated tricyclic PAHs in the induction of cardiac arrhythmias, the exacerbation of heart failure, the triggering of myocardial infarction and other atherosclerotic/ischaemic complications (Brook et al. 2010). Coupled with the mechanistic understanding of crude oil‐ and PAH‐induced dysfunction in the fish heart, it is certain that PAHs are pollutants of global concern. Below we have reviewed the current literature which mechanistically links PM and/or PAHs and cardiotoxicity in a range of animal models. Our aim is to show conservation of toxicant pathways and cellular targets across vertebrates to allow a broad framework of the problem to be established.
Atherosclerosis
Atherosclerosis is a vascular disease where intraluminal fatty plaque build‐up causes a narrowing of the arteries. Rupture of unstable plaques in specific arteries can lead to thrombotic occlusion of the artery, triggering a cardiovascular event such as a heart attack or stroke. Coronary artery disease is now a leading cause of mortality in the world, affecting 2.3 million people in the UK (British Heart Foundation, 2018). A critical initiating event in the pathogenesis of atherosclerosis is endothelial cell injury. PAH uptake through inhalation puts endothelial cells at the forefront of toxic effects. BaP and other related PAHs have been found to be atherogenic, inducing vascular injury and dysfunction possibly through oxidative stress (Dhalla et al. 2000; Miller & Ramos, 2001). Cigarette smoke contains significant levels of Phe and smoking is a key risk factor of atherosclerosis and linked to increased endothelial cell apoptosis (Severson et al. 1976; Howard et al. 1998). Little work has been carried out on the role of individual PAHs on the apoptotic cascade (Tai et al. 1990) (i.e. arachidonic acid (AA) by phospholipase A2 (PLA2)); however, in 2002, Tithof et al. identified the apoptotic effects of three PAHs – 1‐methylanthracene, Phe and BaP – in human coronary artery endothelial cells (HCAECs) (Tithof et al. 2002). All three PAHs induced time‐ and concentration‐dependent release of AA, an event that precedes the onset of apoptosis. Interestingly, these PAHs activate three distinct PLA2 isoforms, with Phe specifically activating the Group VI and acidic calcium‐independent PLA2 (Tithof et al. 2002). A more recent study (Asweto et al. 2017), showed that individual PAHs could have greater toxicological effects on endothelial cells when combined with silica nanoparticles. BaP was harmless to human umbilical vascular endothelial cells (HUVECs) alone, but caused DNA damage, oxidative stress and apoptosis in the presence of particles (Asweto et al. 2017). These results suggest that the tissue‐specific cytotoxic effects by individual PAHs and PM warrants further study, not only in the initial stages of atherosclerosis, but also in the long‐term development and rupture of atherosclerotic lesions.
Cardiac arrhythmias
Arrhythmias are a broad term for different types of irregular heartbeats, collectively experienced by more than 2 million people a year in the UK (National Health Service, 2018). Although often innocuous, arrhythmias can be indicative of underlying cardiac conditions and can precipitate more hazardous irregularities over time or with various stresses. There is considerable evidence showing exposure to airborne PM can affect cardiac rhythm in humans and mammalian models (Buteau & Goldberg, 2016; COMEAP, 2018). Phe exposure produces severe arrhythmias in fish, including bradycardia and AV conduction block (Incardona et al. 2004, 2005). For example, irregular rhythm has been observed in zebrafish embryos after 72 h of 5 nM Phe exposure (Zhang et al. 2013). In cats, bradycardia has been observed following high‐dose oral administration of Phe (Eddy, 1933). The mechanism of Phe action has been attributed, at least in part, to its direct influence on the multitude of ion channels involved in the AP and EC coupling (Figs 3 and 4; Brette et al. 2017).
Phe's effect on APD is notably detrimental. A prolongation of the APD and reduction in the Ca2+ transient amplitude has been observed in several fish species following Phe exposure (see Fig. 3; Brette et al. 2017). This prolongation of the APD is due, in large part, to an inhibition of the rapid delayer rectifier K+ current (I Kr), slowing K+ efflux during repolarization as exemplified through maximal inhibition of I Kr by 25 µM Phe (Brette et al. 2017; Ainerua et al. 2020). Although this dose is higher than expected in plasma, tissues levels are expected to approach micromolar levels (see discussion above). This is exemplified by bradycardia (50% rate reduction) and serious fibrillation at nanomolar concentrations of Phe (0.8–0.16 µM) in the hearts of herring embryos (Incardona et al. 2009). Moreover, halofantrine, an antimalarial drug that has a similar structure to Phe, also inhibits I Kr and has been associated with acquired long QT syndrome and Torsades de pointes (a specific type of arrhythmia that can lead to sudden death) (Wesche et al. 2000). Interestingly, recent work on rainbow trout (Oncorhynchus mykiss) has shown that Phe and retene (1‐methyl‐7‐isopropyl phenanthrene) shorten APD (Vehniäinen et al. 2019) due to varying potency of the inhibitory effect on K+ versus Ca2+ channels. Although AP shortening can also be arrhythmogenic, these species differences highlight the need to consider multiple species in order to understand overall PAH effects.
EC and excitation‐transcription coupling
The reduced Ca2+ transients in fish myocytes following Phe exposure is attributed to an inhibition of Ca2+ influx through L‐type calcium channels (LTCC; see Fig. 4A ). PAH‐induced changes in intracellular Ca2+ levels not only affect contractility, but may also contribute to changes in gene expression by impinging upon excitation‐transcription coupling (see Fig. 4B ). Excitation‐transcription coupling links gene expression to the physiological state of myocytes through activation of various transcription factors like cAMP response element‐binding protein (CREB), nuclear factor of activated T‐cells (NFAT), and myocardin (Wamhoff et al. 2004, 2006). Perturbation of Ca2+ levels by Phe diminished mRNA and protein expression of the sarcoplasmic reticulum calcium ATPase (SERCA), the Ca2+ sequestering protein on the sarcoplasmic reticulum (SR) and T‐box 5 (Tbx5), a transcription factor regulating SERCA expression in Phe exposed zebrafish embryos and Phe exposed H9C2 cells (rat embryonic cardiac myoblasts) (Zhang et al. 2013).
Crude oil exposure has also been shown to alter Ca2+‐dependent gene expression during embryonic and early larval development in fishes including signalling pathways such as bone morphogenetic protein 10 (BMP10) and myocardin (Sørhus et al. 2017). The initiating event for both EC coupling and excitation‐transcription coupling are the same (Fig. 4). Electrical stimuli depolarize the membrane and trigger the opening of LTCCs and the influx of Ca2+, which in turn induce the cascade leading both to contraction and to Ca2+ regulated induction of gene expression (Fig. 4A ) in the nucleus (Fig. 4B ). Thus, impairment of various ionic influx pathways by PAHs may affect both excitation‐contraction and transcription‐coupling. Interestingly, Ca2+‐induced gene regulation appears to be sensitive to both localized Ca2+ increases near the site of influx and to increases in nuclear Ca2+ (Dolmetsch, 2003). Even though the impact on EC and excitation‐transcription coupling is reversible, downstream effects such as circulatory defects and abnormal gene expression, especially during vulnerable developmental stages, may cause irreversible morphological changes. BMP10's primary function of driving cardiac trabeculation (Grego‐bessa et al. 2007) was found to be up‐regulated (4‐fold) upon dysregulation of the Ca2+ controlled gene myocardin in response to crude oil pollutants (Sørhus et al. 2017). Taken together, these alterations to electrophysiology, EC coupling and gene expression in the myocyte could contribute to contractile failure, abnormal contractile rhythm and the abnormal cardiac phenotype seen in vivo following PAH exposure (Incardona et al. 2004, 2005; Hicken et al. 2011; Mager et al. 2014; Incardona & Scholz, 2016; Sørhus et al. 2016, 2017).
Cardiac hypertrophy
Cardiac hypertrophy is a common pathology in many CVDs and is characterized by increased cell size and protein synthesis, ultimately leading to inefficient contractility of the heart as well as changes in coronary perfusion (Shimizu & Minamino, 2016). Cellular hypertrophy has been observed in H9C2 rat cardiomyoblasts with low Phe exposure, and in excised rat hearts where Phe increased expression of atrial natriuretic protein (ANP), brain natriuretic protein (BNP), and c‐Myc hypertrophic markers (Huang et al. 2016). Furthermore, DNA hypermethylation, another intracellular effect observed upon Phe exposure (Liu et al. 2013) has been implicated in the down‐regulation of microRNA‐133a, leading to increased expression of its target genes such as CdC42 and RhoA (involved in hypertrophic growth response) (Huang et al. 2016). In zebrafish, Zhang et al. (2013) showed that Phe exposure increased matrix metalloproteinase‐9 (MMP‐9) expression and activity. MMP‐9 is an important regulator of cardiac tissue remodelling, disruption of which has been shown to cause cardiac dysfunction such as dilated cardiomyopathy (DCM) via ventricle dilatation and wall thinning. This study also identified up‐regulation of transforming growth factor‐β (TGF‐β), a regulator of collagen synthesis. This increased activity of MMP‐9 and TGF‐β corresponds to degradation of extracellular matrix and disruption of collagen content of cardiac tissue leading to DCM (Pauschinger et al. 1999). Notably, these works support the idea that low levels of Phe exposure are able to change gene and protein expression in animal and cell models (see Fig. 4B ).
Altered cardiac remodelling associated with air pollution has also been recently identified in humans. In a large asymptomatic population, with no prevalent CVD, prior exposure to PM2.5 (specific PAHs were not described) was associated with increased left and right ventricular end‐diastolic volume and end‐systolic volume (Aung et al. 2018). A comprehensive mechanistic study by Wold et al. (2012) further demonstrated that long‐term (9 months) PM exposure was associated with cardiac hypertrophy, changes in cardiac myocyte phenotype and impaired cardiac contractility. These demonstrate a means by which PM could induce/exacerbate heart failure and provide evidence of additional pathways underlying the associations between inhaled PM, hypertrophy, CVD and stroke (Shah et al. 2013).
Interspecies disparity: the need to expand our model systems
The devastating impact of point oil spills on aquatic organisms generated significant interest in uncovering the physiological mechanisms underlying PAH toxicity. Indeed, our mechanistic understanding of PAH cardiotoxicity has been derived largely from work on fish. However, it is now well established that PAHs comprise a significant proportion of air pollution and that PAH cardiotoxicity extends to mammalian and human systems. The core functional and physiological properties of the heart are maintained across vertebrate classes, suggesting that, despite differences in routes of uptake between fish (water/gill/gut) and terrestrial mammals (air/lung/gut) (Incardona & Scholz, 2017), cardiotoxic pathways identified in fishes would be similar in other vertebrates (Shin & Fishman, 2002). Indeed, drugs that have cardiac activity in humans and other mammals have similar effects in fish (Langheinrich, 2003) and individual tricyclic PAHs cause cardiac arrhythmias in zebrafish that mirror those caused by drugs that inhibit the hERG K+ channel subunit (Incardona et al. 2004). This is not surprising given that the region encompassing the canonical binding residues between the human and zebrafish ERG differ by only a single amino acid residue (Langheinrich et al. 2003) (see Fig. 5). Therefore, fish, and in particular zebrafish, provide a convenient model for probing human cardiac safety pharmacology and toxicology at a functional level. Indeed, drugs that are known to prolong the QT interval in humans also modulate adult zebrafish QT interval (Tsai et al. 2011). In this light, it is particularly striking that an analysis of ERG blockade by over 11,000 compounds in a drug development pipeline identified peak potency associated with structures containing three aromatic rings (Ritchie & Macdonald, 2009). Thus, the wide array of Phe‐related and other tricyclic compounds associated with fossil fuel emissions are all potential contributors to acute cardiac impacts of air pollution. However, it should be acknowledged that there are also significant differences between human and (zebra) fish hearts (Table 1), particularly the molecular basis of the cardiac ionic currents which may be produced by non‐orthologous genes in zebrafish and humans (Haverinen et al. 2007, 2018; Hassinen et al. 2015; Vornanen & Hassinen, 2016). Fish also differ from mammals with respect to intracellular Ca2+ cycling (Shiels & Galli, 2014), again highlighting the need to study multiple species in order to understand overall PAH effects.
Figure 5. Homology of ERG channels.
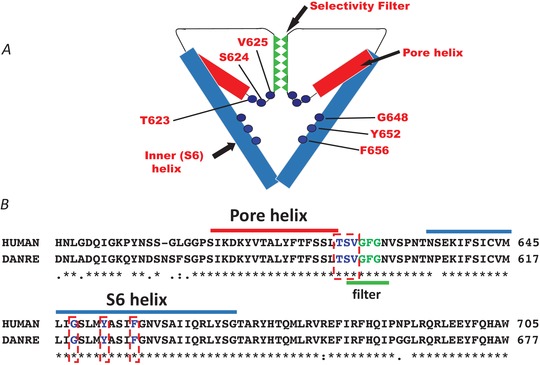
Schematic representation (A) and sequence homology (B) of pore‐helix and S6 helix regions between hERG and zERG channels. Sequence alignments made in UniProt (Q12809 and Q8JH78). The pore‐helix is highlighted in red and S6 helix in blue. The GFG selectivity sequence is highlighted in green. Residues known to contribute to the canonical drug binding site in hERG are highlighted by red dashed boxes. Note that residue numbering in A refers to amino acid position in hERG. hERG:zERG equivalents: T623 = T595; S624 = S596; V625 = V597; G648 = G620; Y652 = Y624; F656 = F628.
Table 1.
Sequence homology in ion channels between human, hiPSC CMs and animal models
| Humana,1,2 | Sheepb | Mouse | Zebrafishc | hiPSC CMs | ||||
|---|---|---|---|---|---|---|---|---|
| Ion Current | Protein (Accession number) | * | Seq. homology to human (Accession no.) | * | Protein; Seq. homology to human (Accession no.) | * | Protein; Seq. homology to human (Accession no.) | * |
| ICaL | Cav1.2 (NP_955630.3) | ✓ 3 | 92% (XP_027823851.1) | ✓ 4 | Cav1.2 91% (NP_033911.2) | ✓ 5 | Cav1.2 76% (NP_571975.1) | ✓ 6,7 |
| IKr | Kv11.1 (BAA37096.1) | ✓ 8 | 95% (XP_027824801.1) | – 9,10 | Kv11.1 96% (NP_038597.2) but proscribed for repolarization assays in ICH S7B guidelines.10 | ✓ 5,11 |
Kv11.2d 60% (NP_998002.1) 99% (canonical binding site) |
✓ 6,7 |
| IKs | Kv7.1 (NP_000209.2) | ✓ 8 | 89% (XP_027815648.1) | – 9,10 | Kv7.1 88% (NP_032460.2) but proscribed for repolarization assays in ICH S7B guidelines.10 | ✓ 12 | Kv7.1 67% (NP_001116714.1) | ✓ 6,7 |
| Ito | ||||||||
|
Ito (slow) Ito (fast) Ito (fast) |
Kv1.4 (NP_002224.1) Kv4.2 (NP_036413.1) Kv4.3 (NP_004971.2) |
✓ 13 |
95% (XP_027835381.1) 99% (XP_011993623.1) 99% (XP_011990111.1) |
✓ 9,14 |
Kv1.4 97% (NP_067250.2) Kv4.2 99% (NP_062671.1) Kv4.3 99% (NP_064315.1) |
– 15,16 |
Kv1.4 63% (XP_005169005.1) zShal2 d 80% (NP_001076336.1) zShal3 d 75% (NP_956096.1) |
✓ 6,7 |
| IK1 |
Kir2.1 (NP_000882.1) Kir2.3 (NP_690607.1) |
✓ 17 |
99% (XP_027829809.1) 98% (XP_004023700.2) |
✓ 18 |
Kir2.1 98% (NP_032451.1) Kir2.3 98% (NP_032453.3) |
✓ 1,2,5 |
Kir2.4 d 67% (NP_001313403.1) Kir2.4 d 61% (NP_001313403.1) |
– 6,7 |
hiPSC CMs, human induced pluripotent stem cell derived cardiomyocytes; * column indicating whether the human ionic current is present in the model organism: ✓ present; – negligible/not present. Numbers in brackets indicate relevant reference. Sequence homologies to humans are given in %.
aIon channel isoforms most expressed in human heart. bNo protein name since REFSEQ is predicted by automated computational analysis. cChannel isoforms most expressed in zebrafish heart and their sequence homology to corresponding human channel isoforms. dIsoforms that are differentially expressed compared to humans.
1(Vornanen & Hassinen, 2016) 2(Vornanen et al., 2018) 3(Dibb et al., 2004) 4(Moosmang et al., 2005) 5(Nemtsas et al., 2010) 6(Ma et al., 2011) 7(Fermini et al., 2016) 8(Noble & Tsien, 1969) 9(Huang, 2017) 10(European Medicines Agency, 2005) 11(Warren et al., 2017) 12(Abramochkin et al., 2018) 13(Boyett, 1981) 14(Bovo et al., 2013) 15(Alday et al., 2014) 16(Nakamura & Coetzee, 2008) 17(Weidmann, 1955) 18(Lopatin & Nichols, 2001)
It is arresting that the most genetically tractable mammalian model (the mouse) has markedly abbreviated ventricular APs and despite the presence of genes for major repolarizing ion channels (see Table 1), it relies on different repolarization currents to those in humans (particularly with respect to the lack of I Kr contribution to adult ventricular repolarization; Nerbonne et al. 2001; Table 1) limiting the use of the mouse for safety assurance pharmacology (European Medicines Agency, 2005). Large mammalian models and human induced pluripotent stem cell (hiPSC) derived cardiomyocytes are important platforms within drug discovery and cardiac safety testing paradigms (Fermini et al. 2016; Chowdhury et al. 2017; Sirenko et al. 2017) but have only very recently been utilized for PAH toxicity testing. High‐throughput in vitro cardiotoxicity of a library of 69 representative environmental chemicals and drugs was successfully assessed using hiPSC‐derived cardiomyocytes (Sirenko et al. 2017). The value of hiPSC‐derived cardiomyocytes for the evaluation of drug‐induced pro‐arrhythmia is further exemplified by the ongoing ‘CiPA’ (comprehensive in vitro pro‐arrhythmia assay) initiative, in which hiPSC‐derived cardiomyocytes comprise a key arm of a multi‐strand paradigm (Fermini et al. 2016). The translational implications of PAH research using hiPSCs in medium and high‐throughput test platforms are therefore far reaching. Our understanding of the cardiovascular effects of air pollution has been greatly expanded by using translational approaches, linking epidemiological results with cellular studies, animal models and controlled exposure in man. The addition of model fish species and high‐throughput cell assays will be valuable in identifying the action of specific PAHs on the CVS, and their interactions with other constituents within pollutants.
Conclusions and future directions
Over the last two decades there has been considerable research into the cardiovascular effects of air pollution and many modes of inducing toxicity have been identified. However, the specific role of PAHs bound to PM requires further elucidation and the aim of this review was to stimulate investigative effort in this area. A comprehensive study of approximately 4800 tricyclic aromatic compounds in the GlaxoSmithKline drug discovery pipeline exhibited IC50 values ranging from 10 nM to 30 µM (mean IC50 ∼3 µM; Ritchie & Macdonald, 2009). Therefore, it is important to note that while Phe has been the focus of this review, other polycyclic aromatic compounds in air can synergistically contribute to cardiotoxicity. Moreover, evidence indicates that other physiological systems in addition to the CVS are affected by PAH/PM exposure, an observation that has clear parallels with the burgeoning list of extrapulmonary effects linked to air pollution over the last decade (Raftis & Miller, 2019; Schraufnagel et al. 2019a, b ). Some of these pathways are acknowledged in the abstract figure which leads this article.
Due to the conserved nature of fundamental biological pathways amongst vertebrates, fish exposed to petroleum‐derived PAH mixtures in the natural environment have served as sentinels, providing significant insights into the potential human health impacts of PAHs and PM pollution. Thus, expanding research into PAH toxicity is important to fill current knowledge gaps, and strengthen the foundation for air and water policy management.
Additional information
Competing interests
The authors declare no competing interests.
Author contributions
C.R.M., S.N.K. and H.A.S. drafted the manuscript with critical intellectual input from M.R.M. and J.P.I. F.B., J.C.H. and E.S. revised the manuscript and supplied specific sections. All authors contributed figures and/or text to the manuscript and approved the final version. All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved and all persons designated as authors qualify for authorship, and all those who qualify for authorship are listed. C.R.M. and S.N.K. contributed equally to this work.
Funding
S.N.K., H.A.S., J.C.H. and F.B. (PG/17/77/33125) are supported by the British Heart Foundation, and C.R.M. is supported by the University of Manchester. E.S. is supported by Research Council of Norway (No. 267820; No. 280511). M.R.M. is supported by the British Heart Foundation (SP/15/8/31575; CH/09/002). J.P.I. is supported by NOAA National Marine Fisheries Service and NOAA National Ocean Service.
Acknowledgements
The authors thank members of the laboratories of Dr K. Dibb and Prof. A. Trafford, University of Manchester, who are supported by British Heart Foundation grants (PG/18/24/33608; FS/17/52/33113), for the provision of sheep ventricular myocytes, and Karen Peck (NOAA) for her review of the manuscript.
Biography
Charlotte R. Marris is a British Heart Foundation PhD student and Shiva N. Kompella is a Postdoctoral Research Associate with an excellent track record in ion channel pharmacology. Holly A. Shiels is a comparative physiologist working together with cardiac physiologists Fabien Brette and Jules C. Hancox to determine the mechanisms underlying PAH cardiotoxicity. By working with colleagues in air pollution (Mark M. Miller) and aquatic toxicology (John P. Incardona and Elin Sørhus) the authors aim to provide novel insights into the potential human health impacts of PAHs and PM pollution.

Edited by: Ian Forsythe & Livia Hool
C. R. Marris and S. N. Kompella contributed equally to this work.
References
- Abdel‐Shafy HI & Mansour MSM (2016). A review on polycyclic aromatic hydrocarbons: Source, environmental impact, effect on human health and remediation. Egypt J Pet 25, 107–123. [Google Scholar]
- Abramochkin D V., Hassinen M & Vornanen M (2018). Transcripts of Kv7.1 and MinK channels and slow delayed rectifier K+ current (I Ks) are expressed in zebrafish (Danio rerio) heart. Pflugers Arch 470, 1753–1764. [DOI] [PubMed] [Google Scholar]
- European Environment Agency (2019). Air Quality in Europe — 2018 Report Available at: http://www.eea.europa.eu/publications/air-quality-in-europe-2012.
- Ainerua MO, Tinwell J, Kompella SN, Sørhus E, White KN, van Dongen BE & Shiels HA (2020). Understanding the cardiac toxicity of the anthropogenic pollutant phenanthrene on the freshwater indicator species (Salmo trutta): From whole heart to cardiomyocytes. Chemosphere 239, 124608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alday A, Alonso H, Gallego M, Urrutia J, Letamendia A, Callol C & Casis O (2014). Ionic channels underlying the ventricular action potential in zebrafish embryo. Pharmacol Res 84, 26–31. [DOI] [PubMed] [Google Scholar]
- Asweto CO, Wu J, Hu H, Feng L, Yang X, Duan J & Sun Z (2017). Combined effect of silica nanoparticles and benzo[a]pyrene on cell cycle arrest induction and apoptosis in human umbilical vein endothelial cells. Int J Environ Res Public Health 14, E298. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Aung N, Sanghvi MM, Zemrak F, Lee AM, Cooper JA, Paiva JM, Thomson RJ, Khanji MY, Lukaschuk E, Carapella V, Jin Kim Y, Munroe PB, Piechnik SK, Neubauer S & Peterson SE (2018). Association between ambient air pollution and cardiac morpho‐functional phenotypes. Circulation 138, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barron MG, Heintz R & Rice SD (2004). Relative potency of PAHs and heterocycles as aryl hydrocarbon receptor agonists in fish. Mar Environ Res 58, 95–100. [DOI] [PubMed] [Google Scholar]
- Beyer J, Aas E, Borgenvik HK & Ravn P (1998). Bioavailability of PAH in effluent water from an aluminium works evaluated by transplant caging and biliary fluorescence measurements of Atlantic cod (Gadus morhua L.). Mar Environ Res 46, 233–236. [Google Scholar]
- Bi X, Sheng G, Peng P, Chen Y, Zhang Z & Fu J (2003). Distribution of particulate‐ and vapor‐phase n‐alkanes and polycyclic aromatic hydrocarbons in urban atmosphere of Guangzhou, China. Atmos Environ 37, 289–298. [Google Scholar]
- Bovo E, Dvornikov AV, Mazurek SR, de Tombe PP & Zima A V (2013). Mechanisms of Ca2+ handling in zebrafish ventricular myocytes. Pflugers Arch 465, 1775–1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyett MR (1981). A study of the effect of the rate of stimulation on the transient outward current in sheep cardiac purkinje fibres. J Physiol 319, 1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brette F, Machado B, Cros C, Incardona JP, Scholz NL & Block BA (2014). Crude oil impairs cardiac excitation‐contraction coupling in fish. Science 343, 772–776. [DOI] [PubMed] [Google Scholar]
- Brette F, Shiels HA, Galli GLJ, Cros C, Incardona JP, Scholz NL & Block BA (2017). A novel cardiotoxic mechanism for a pervasive global pollutant. Sci Rep 7, 41476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- British Heart Foundation (2018). UK CVD Statistics Factsheet.
- Brook RD & Brook JR (2011). A road forward to improve public health. Circulation 123, 1705–1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brook RD, Rajagopalan S, Pope CA, Brook JR, Bhatnagar A, Diez‐Roux AV, Holguin F, Hong Y, Luepker RV, Mittleman MA, Peters A, Siscovick D, Smith SC, Whitsel L & Kaufman JD (2010). Particulate matter air pollution and cardiovascular disease. Circulation 121, 2331–2378. [DOI] [PubMed] [Google Scholar]
- Buteau S & Goldberg MS (2016). A structured review of panel studies used to investigate associations between ambient air pollution and heart rate variability. Environ Res 148, 207–247. [DOI] [PubMed] [Google Scholar]
- Camacho M, Boada LD, Orós J, Calabuig P, Zumbado M & Luzardo OP (2012). Comparative study of polycyclic aromatic hydrocarbons (PAHs) in plasma of Eastern Atlantic juvenile and adult nesting loggerhead sea turtles (Caretta caretta). Mar Pollut Bull 64, 1974–1980. [DOI] [PubMed] [Google Scholar]
- Carls MG, Rice SD & Hose JE (1999). Sensitivity of fish embryos to weathered crude oil: Part I. Low‐level exposure during incubation causes malformations, genetic damage, and mortality in larval pacific herring (Clupea pallasi). Environ Toxicol Chem 18, 481–493. [Google Scholar]
- Chowdhury SK, Liu W, Zi M, Li Y, Wang S, Tsui H, Prehar S, Castro S, Zhang H, Ji Y, Zhang X, Xiao R, Zhang R, Lei M, Cyganek L, Guan K, Millar CB, Liao X, Jain MK, Boyett MR, Cartwright EJ, Shiels HA & Wang X (2017). Stress‐activated kinase mitogen‐activated kinase kinase‐7 governs epigenetics of cardiac repolarization for arrhythmia prevention. Circulation 135, 683–699. [DOI] [PubMed] [Google Scholar]
- Cohen AJ, Brauer M, Burnett R, Anderson HR, Frostad J, Estep K, Balakrishnan K, Brunekreef B, Dandona L, Dandona R, Feigin V, Freedman G, Hubbell B, Jobling A, Kan H, Knibbs L, Liu Y, Martin R, Morawsk L, Pope CA 3rd, Shin H, Straif K, Shaddick G, Thomas M, van Dingenen R, van Donkelaar A, Vos T, Murray CJL & Forouzanfar MH (2017). Estimates and 25‐year trends of the global burden of disease attributable to ambient air pollution: an analysis of data from the Global Burden of Diseases Study 2015. Lancet 389, 1907–1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Committee on the Medical Effects of Air Pollutants (2018). The effects of long‐term exposure to ambient air pollution on cardiovascular morbidity: Mechanistic evidence, pp. 5–14. Available at: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/749657/COMEAP_CV_Mechanisms_Report.pdf.
- Cox GK, Crossley DA, Stieglitz JD, Heuer RM, Benetti DD & Grosell M (2017). Oil exposure impairs in situ cardiac function in response to β‐adrenergic stimulation in cobia (Rachycentron canadum). Environ Sci Technol 51, 14390–14396. [DOI] [PubMed] [Google Scholar]
- Dalton TP, Kerzee JK, Wang B, Miller M, Dieter MZ, Lorenz JN, Shertzer HG, Nebert DW & Puga A (2001). Dioxin exposure is an environmental risk factor for ischemic heart disease. Cardiovasc Toxicol 1, 285–298. [DOI] [PubMed] [Google Scholar]
- Denison MS, Fisher JM & Whitlock JP (1988). Inducible, receptor‐dependent protein‐DNA interactions at a dioxin‐responsive transcriptional enhancer. Proc Natl Acad Sci U S A 85, 2528–2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denison MS, Seidel SD, Rogers WJ, Ziccardi M, Winter GM & Heath‐Pagliuso S (1998). Natural and synthetic ligands for the Ah receptor. In Molecular Biology Approaches to Toxicology, eds Puga A. & Wallace KB, pp. 393–410. Taylor & Francis, Philadelphia, PA, USA.
- Dhalla NS, Temsah RM & Netticadan T (2000). Role of oxidative stress in cardiovascular diseases. J Hypertens 18, 655–673. [DOI] [PubMed] [Google Scholar]
- Dhananjayan V & Muralidharan S (2013). Levels of polycyclic aromatic hydrocarbons, polychlorinated biphenyls, and organochlorine pesticides in various tissues of white‐backed vulture in India. Biomed Res Int 2013, 190353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dibb KM, Rueckschloss U, Eisner DA, Isenberg G & Trafford AW (2004). Mechanisms underlying enhanced cardiac excitation contraction coupling observed in the senescent sheep myocardium. J Mol Cell Cardiol 37, 1171–1181. [DOI] [PubMed] [Google Scholar]
- Dolmetsch R (2003). Excitation‐transcription coupling: signaling by ion channels to the nucleus. Sci STKE 166, pe4. [DOI] [PubMed] [Google Scholar]
- Donaldson K, Duffin R, Langrish JP, Miller MR, Mills NL, Poland CA, Raftis J, Shah A, Shaw CA & Newby DE (2013). Nanoparticles and the cardiovascular system: A critical review. Nanomedicine 8, 403–423. [DOI] [PubMed] [Google Scholar]
- Donaldson K, Tran L, Jimenez L, Duffin R, Newby D, Mills N, MacNee W & Stone V (2005). Combustion‐derived nanoparticles: A review of their toxicology following inhalation exposure. Part Fibre Toxicol 2, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddy NB (1933). Studies of phenanthrene derivatives. I. A comparison of phenanthrene 2‐, 3‐, and 9‐monosubstitution products. J Pharmacol Exp Ther 48, 183–198. [Google Scholar]
- Environmental Protection Agency (2018). Particulate Matter (PM) Pollution. Available at: https://www.epa.gov/pm-pollution [Accessed October 12, 2018].
- European Medicines Agency (2005). ICH Topic S7B The Nonclinical Evaluation of the Potential for Delayed Ventricular Repolarization (QT Interval Prolongation) by Human Pharmaceuticals, pp. 1–9. Available at: https://www.ema.europa.eu/en/documents/scientific-guideline/ich-s-7-b-nonclinical-evaluation-potential-delayed-ventricular-repolarization-qt-interval_en.pdf. [PubMed]
- Fermini B, Hancox JC, Abi‐Gerges N, Bridgland‐Taylor M, Chaudhary KW, Colatsky T, Correll K, Crumb W, Damiano B, Erdemli G, Gintant G, Imredy J, Koerner J, Kramer J, Levesque P, Li Z, Lindqvist A, Obejero‐Paz CA, Rampe D, Sawada K, Strauss DG & Vandenberg JI (2016). A new perspective in the field of cardiac safety testing through the comprehensive in vitro proarrhythmia assay paradigm. J Biomol Screen 21, 1–11. [DOI] [PubMed] [Google Scholar]
- Franklin BA, Brook R & Arden Pope C (2015). Air pollution and cardiovascular disease. Curr Probl Cardiol 40, 207–238. [DOI] [PubMed] [Google Scholar]
- Geiser M & Kreyling W (2010). Deposition and biokinetics of inhaled nanoparticles. Part Fibre Toxicol 7, 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerde P, Muggenburg BA, Hoover MD & Henserson RF (1993). Disposition of polycyclic aromatic hydrocarbons in the respiratory tract of the beagle dog. 1. The alveolar region. Toxicol Appl Pharmacol 121, 313–318. [DOI] [PubMed] [Google Scholar]
- Gerde P, Muggenburg BA, Lundborg M & Dahl AR (2001). The rapid alveolar absorption of diesel soot‐adsorbed benzo a pyrene: bioavailability, metabolism and dosimetry of an inhaled particle‐borne carcinogen. Carcinogenesis 22, 741–749. [DOI] [PubMed] [Google Scholar]
- Germolec DR, Henry EC, Maronpot R, Foley JF, Adams NH, Gasiewicz TA & Luster MI (1996). Induction of CYP1A1 and ALDH‐3 in lymphoid tissues from Fisher 344 rats exposed to 2,3,7,8‐tetrachlorodibenzodioxin (TCDD). Toxicol Appl Pharmacol 137, 57–66. [DOI] [PubMed] [Google Scholar]
- Grego‐bessa J, Luna‐zurita L, Monte G, Bolós V, Arandilla A, Garratt AN, Zang H, Mukouyama Y, Chen H, Shou W, Ballestar E, Esteller M, Rojas A, María J, Luis J & Pompa D (2007). Notch Signalling is essential for ventricular development. Dev Cell 12, 415–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grova N, Monteau F, Le Bizec B, Feidt C, Andre F & Rychen G (2005). Determination of phenanthrene and hydroxyphenanthrenes in various biological matrices at trace levels using gas chromatography‐mass spectrometry. J Anal Toxicol 29, 175–181. [DOI] [PubMed] [Google Scholar]
- Halsall CJ, Coleman PJ, Davis BJ, Burnett V, Waterhouse KS, Harding‐Jones P & Jones KC (1994). Polycyclic aromatic hydrocarbons in U.K. urban air. Environ Sci Technol 28, 2380–2386. [DOI] [PubMed] [Google Scholar]
- Hassinen M, Haverinen J, Hardy ME, Shiels HA & Vornanen M (2015). Inward rectifier potassium current (I K1) and Kir2 composition of the zebrafish (Danio rerio) heart. Pflugers Arch 467, 2437–2446. [DOI] [PubMed] [Google Scholar]
- Haverinen J, Hassinen M, Korajoki H & Vornanen M (2018). Cardiac voltage‐gated sodium channel expression and electrophysiological characterization of the sodium current in the zebrafish (Danio rerio) ventricle. Prog Biophys Mol Biol 138, 59–68. [DOI] [PubMed] [Google Scholar]
- Haverinen J, Hassinen M & Vornanen M (2007). Fish cardiac sodium channels are tetrodotoxin sensitive. Acta Physiol 191, 197–204. [DOI] [PubMed] [Google Scholar]
- Hazen EL, Carlisle AB, Wilson SG, Ganong JE, Castleton MR, Schallert RJ, Stokesbury MJW, Bograd SJ & Block BA (2016). Quantifying overlap between the Deepwater Horizon oil spill and predicted bluefin tuna spawning habitat in the Gulf of Mexico. Sci Rep 6, 33824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heintz RA, Short JW & Rice SD (1999). Sensitivity of fish embryos to weathered crude oil: Part 2. Increased mortality of pink salmon (Oncorhynchus goruscha) embryos incubating downstream from weathered Exxon Valdez crude oil. Environ Toxicol Chem 18, 494–503. [Google Scholar]
- Hicken CE, Linbo TL, Baldwin DH, Willis ML, Myers MS, Holland L, Larsen M, Stekoll MS, Rice SD, Collier TK, Scholz NL & Incardona JP (2011). Sublethal exposure to crude oil during embryonic development alters cardiac morphology and reduces aerobic capacity in adult fish. Proc Natl Acad Sci U S A 108, 7086–7090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoek G, Krishnan R, Beelen R, Peters A, Ostro B, Brunekreef B & Kaufman J (2013). Long‐term air pollution exposure and cardio‐respiratory mortality: A review. Environ Heal 12, 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard G, Wagenknecht LE, Burke GL, Diez‐Roux A, Evans GW, McGovern P, Nieto FJ & Tell GS (1998). Cigarette smoking and progression of atherosclerosis: The Atherosclerosis Risk in Communities (ARIC) Study. JAMA 279, 119–124. [DOI] [PubMed] [Google Scholar]
- Huang CLH (2017). Murine electrophysiological models of cardiac arrhythmogenesis. Physiol Rev 97, 283–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L, Xi Z, Wang C, Zhang Y, Yang Z, Zhang S, Chen Y & Zuo Z (2016). Phenanthrene exposure induces cardiac hypertrophy via reducing miR‐133a expression by DNA methylation. Sci Rep 6, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Incardona JP (2017). Molecular mechanisms of crude oil developmental toxicity in fish. Arch Environ Contam Toxicol 73, 19–32. [DOI] [PubMed] [Google Scholar]
- Incardona JP, Carls MG, Day HL, Catherine A, Bolton JL, Collier TK, Scholz NL & Sloan CA (2009). Cardiac arrhythmia is the primary response of embryonic pacific herring (Clupea pallasi) exposed to crude oil during weathering. Environ Sci Technol 43, 201–207. [DOI] [PubMed] [Google Scholar]
- Incardona JP, Carls MG, Teraoka H, Sloan CA, Collier TK & Scholz NL (2005). Aryl hydrocarbon receptor‐independent toxicity of weathered crude oil during fish development. Environ Health Perspect 113, 1755–1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Incardona JP, Collier TK & Scholz NL (2004). Defects in cardiac function precede morphological abnormalities in fish embryos exposed to polycyclic aromatic hydrocarbons. Toxicol Appl Pharmacol 196, 191–205. [DOI] [PubMed] [Google Scholar]
- Incardona JP, Gardner LD, Linbo TL, Brown TL, Esbaugh AJ, Mager EM, Stieglitz JD, French BL, Labenia JS, Laetz CA, Tagal M, Sloan CA, Elizur A, Benetti DD, Grosell M, Block BA & Scholz NL (2014). Deepwater Horizon crude oil impacts the developing hearts of large predatory pelagic fish. Proc Natl Acad Sci U S A 111, E1510–E1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Incardona JP & Scholz NL (2016). The influence of heart developmental anatomy on cardiotoxicity‐based adverse outcome pathways in fish. Aquat Toxicol 177, 515–525. [DOI] [PubMed] [Google Scholar]
- Incardona JP & Scholz NL (2017). Environmental Pollution and the Fish Heart, 1st edn Elsevier Inc. [Google Scholar]
- Incardona JP & Scholz NL (2018). Case Study: The 2010 Deepwater Horizon oil spill and its environmental developmental impacts. In Development and Environment, pp. 235–283. Springer. [Google Scholar]
- Ivnitski I, Elmaoued R & Walker MK (2001). 2,3,7,8‐Tetrachlorodibenzo‐p‐dioxin (TCDD) inhibition of coronary development is preceded by a decrease in myocyte proliferation and an increase in cardiac apoptosis. Teratology 64, 201–212. [DOI] [PubMed] [Google Scholar]
- Jacob J & Seidel A (2002). Biomonitoring of polycyclic aromatic hydrocarbons in human urine. J Chromatogr B 778, 31–47. [DOI] [PubMed] [Google Scholar]
- Jokinen MP, Walker NJ, Brix AE, Sells DM, Haseman JK & Nyska A (2003). Increase in cardiovascular pathology in female Sprague‐Dawley rats following chronic treatment with 2,3,7,8‐tetrachlorodibenzo‐p‐dioxin and 3,3ʹ,4,4ʹ,5‐pentachlorobiphenyl. Cardiovasc Toxicol 3, 299–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanzawa N, Kondo M, Okushima T, Yamaguchi M, Temmei Y, Honda M & Tsuchiya T (2004). Biochemical and molecular biological analysis of different responses to 2,3,7,8‐tetrachlorodibenzo‐p‐dioxin in chick embryo heart and liver. Arch Biochem Biophys 427, 58–67. [DOI] [PubMed] [Google Scholar]
- Korashy H & El‐Kadi A (2006). The role of aryl hydrocarbon receptor in the pathogenesis of cardiovascular diseases. Drug Metab Rev 38, 411–450. [DOI] [PubMed] [Google Scholar]
- Langheinrich U (2003). Zebrafish: A new model on the pharmaceutical catwalk. BioEssays 25, 904–912. [DOI] [PubMed] [Google Scholar]
- Langheinrich U, Vacun G & Wagner T (2003). Zebrafish embryos express an orthologue of HERG and are sensitive toward a range of QT‐prolonging drugs inducing severe arrhythmia. Toxicol Appl Pharmacol 193, 370–382. [DOI] [PubMed] [Google Scholar]
- Langrish JP, Li X, Wang S, Lee MMY, Barnes GD, Miller MR, Cassee FR, Boon NA, Donaldson K, Li J, Li L, Mills NL, Newby DE & Jiang L (2012). Reducing personal exposure to particulate air pollution improves cardiovascular health in patients with coronary heart disease. Environ Health Perspect 120, 367–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent C, Feidt C, Lichtfouse E, Grova N, Laurent F & Rychen G (2001). Milk–blood transfer of 14C‐tagged polycyclic aromatic hydrocarbons (PAHs) in pigs. J Agric Food Chem 49, 2493–2496. [DOI] [PubMed] [Google Scholar]
- Lee KK, Miller MR & Shah ASV (2018). Air pollution and stroke. J Stroke 20, 2–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lelieveld J, Klingmüller K, Pozzer A, Pöschl U, Fnais M, Daiber A & Münzel T (2019). Cardiovascular disease burden from ambient air pollution in Europe reassessed using novel hazard ratio functions. Eur Heart J 40, 1590–1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Porter EN, Sjödin A, Needham LL, Lee S, Russell AG & Mulholland JA (2009). Characterization of PM2.5‐bound polycyclic aromatic hydrocarbons in Atlanta‐Seasonal variations at urban, suburban, and rural ambient air monitoring sites. Atmos Environ 43, 4187–4193. [Google Scholar]
- Lim SS et al (2012). A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 380, 2224–2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin TM, Ko K, Moore RW, Buchanan DL, Cooke PS & Peterson RE (2001). Role of the aryl hydrocarbon receptor in the development of control and 2,3,7,8‐ tetrachlorodibenzo‐p‐dioxin‐exposed male mice. J Toxicol Environ Health 64, 327–342. [DOI] [PubMed] [Google Scholar]
- Lipsett MJ, Ostro BD, Reynolds P, Goldberg D, Hertz A, Jerrett M, Smith DF, Garcia C, Chang ET & Bernstein L (2011). Long‐term exposure to air pollution and cardiorespiratory disease in the California teachers study cohort. Am J Respir Crit Care Med 184, 828–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Zhang L, Winterroth LC, Garcia M, Weiman S, Wong JW, Sunwoo JB & Nadeau KC (2013). Epigenetically mediated pathogenic effects of phenanthrene on regulatory T cells. J Toxicol 3, 967029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Zhu L & Shen X (2001). Polycyclic aromatic hydrocarbons (PAHs) in indoor and outdoor air of Hangzhou, China. Environ Sci Technol 35, 840–844. [DOI] [PubMed] [Google Scholar]
- Lopatin AN & Nichols CG (2001). Inward rectifiers in the heart: An update on IK1 . J Mol Cell Cardiol 33, 625–638. [DOI] [PubMed] [Google Scholar]
- Ma J, Guo L, Fiene SJ, Anson BD, Thomson JA, Kamp TJ, Kolaja KL, Swanson BJ & January CT (2011). High purity human‐induced pluripotent stem cell‐derived cardiomyocytes: electrophysiological properties of action potentials and ionic currents. Am J Physiol Heart Circ Physiol 301, H2006–H2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mager EM, Esbaugh AJ, Stieglitz JD, Hoenig R, Bodinier C, Incardona JP, Scholz NL, Benetti DD & Grosell M (2014). Acute embryonic or juvenile exposure to deepwater horizon crude oil impairs the swimming performance of mahi‐mahi (Coryphaena hippurus). Environ Sci Technol 48, 7053–7061. [DOI] [PubMed] [Google Scholar]
- Mann JK, Tager IB, Lurmann F, Segal M, Quesenberry CP, Lugg MM, Shan J & Van Den Eeden SK (2002). Air pollution and hospital admissions for ischemic heart disease in persons with congestive heart failure or arrhythmia. Environ Health Perspect 110, 1247–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maskaoui K, Zhou JL, Hong HS & Zhang ZL (2002). Contamination by polycyclic aromatic hydrocarbons in the Jiulong River Estuary and Western Xiamen Sea, China. Environ Pollut 118, 109–122. [DOI] [PubMed] [Google Scholar]
- Miller KA, Siscovick DS, Sheppard L, Shepherd K, Sullivan JH, Anderson GL & Kaufman JD (2007). Long‐term exposure to air pollution and incidence of cardiovascular events in women. N Engl J Med 356, 447–458. [DOI] [PubMed] [Google Scholar]
- Miller KP & Ramos KS (2001). Impact of cellular metabolism on the biological effects of benzo[a]pyrene and related hydrocarbons. Drug Metab Rev 33, 1–35. [DOI] [PubMed] [Google Scholar]
- Miller MR (2014). The role of oxidative stress in the cardiovascular actions of particulate air pollution. Biochem Soc Trans 42, 1006–1011. [DOI] [PubMed] [Google Scholar]
- Miller MR, Borthwick SJ, Shaw CA, McLean SG, McClure D, Mills NL, Duffin R, Donaldson K, Megson IL, Hadoke PWF & Newby DE (2009). Direct impairment of vascular function by diesel exhaust particulate through reduced bioavailability of endothelium‐derived nitric oxide induced by superoxide free radicals. Environ Health Perspect 117, 611–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MR, Raftis JB, Langrish JP, McLean SG, Samutrtai P, Connell SP, Wilson S, Vesey AT, Fokkens PHB, Boere AJF, Krystek P, Campbell CJ, Hadoke PWF, Donaldson K, Cassee FR, Newby DE, Duffin R & Mills NL (2017a). Inhaled nanoparticles accumulate at sites of vascular disease. ACS Nano 11, 4542–4552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MR, Raftis JB, Langrish JP, McLean SG, Samutrtai P, Connell SP, Wilson S, Vesey AT, Fokkens PHB, Boere AJF, Krystek P, Campbell CJ, Hadoke PWF, Donaldson K, Cassee FR, Newby DE, Duffin R & Mills NL (2017b). Correction to: Inhaled Nanoparticles Accumulate at Sites of Vascular Disease. ACS Nano 11, 10623–10624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MR, Shaw CA & Langrish JP (2012). From particles to patients: oxidative stress and the cardiovascular effects of air pollution. Futur Cardiol 8, 577–602. [DOI] [PubMed] [Google Scholar]
- Mills NL, Amin N, Robinson SD, Anand A, Davies J, Patel D, de la Fuente JM, Cassee FR, Boon NA, MacNee W, Millar AM, Donaldson K & Newby DE (2006). Do inhaled carbon nanoparticles translocate directly into the circulation in humans? Am J Respir Crit Care Med 173, 426–431. [DOI] [PubMed] [Google Scholar]
- Mills NL, Donaldson K, Hadoke PW, Boon NA, MacNee W, Cassee FR, Sandström T, Blomberg A & Newby DE (2009). Adverse cardiovascular effects of air pollution. Nat Clin Pract Cardiovasc Med 6, 36–44. [DOI] [PubMed] [Google Scholar]
- Mills NL, Miller MR, Lucking AJ, Beveridge J, Flint L, Boere AJF, Fokkens PH, Boon NA, Sandstrom T, Blomberg A, Duffin R, Donaldson K, Hadoke PWF, Cassee FR & Newby DE (2011). Combustion‐derived nanoparticulate induces the adverse vascular effects of diesel exhaust inhalation. Eur Heart J 32, 2660–2671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitter SS, Rajesh V, Islami F, Pourshams A, Khademi H, Kamangar F, Abnet CC, Dawsey SM, Pharoah PD, Brennan P, Fuster V, Boffetta P & Malekzadeh R (2016). Household fuel use and cardiovascular disease mortality: Golestan Cohort Study Study. Circulation 133, 2360–2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Möller W, Felten K, Sommerer K, Scheuch G, Meyer G, Meyer P, Häussinger K & Kreyling WG (2008). Deposition, retention, and translocation of ultrafine particles from the central airways and lung periphery. Am J Respir Crit Care Med 177, 426–432. [DOI] [PubMed] [Google Scholar]
- Möllmann H, Nef HM, Kostin S, von Kalle C, Pilz I, Weber M, Schaper J, Hamm CW & Elsässer A (2006). Bone marrow‐derived cells contribute to infarct remodelling. Cardiovasc Res 71, 661–671. [DOI] [PubMed] [Google Scholar]
- Moosmang S, Lenhardt P, Haider N, Hofmann F & Wegener JW (2005). Mouse models to study L‐type calcium channel function. Pharmacol Ther 106, 347–355. [DOI] [PubMed] [Google Scholar]
- Münzel T, Gori T, Al‐Kindi S, Deanfield J, Lelieveld J, Daiber A & Rajagopalan S (2018). Effects of gaseous and solid constituents of air pollution on endothelial function. Eur Heart J 39, 3543–3550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naes K & Oug E (1998). The distribution and environmental relationships of polycyclic aromatic hydrocarbons (PAHs) in sediments from Norwegian smelter‐affected fjords. Chemosphere 36, 561–576. [Google Scholar]
- Nakamura TY & Coetzee WA (2008). Functional and pharmacological characterization of a Shal‐related K+ channel subunit in Zebrafish. BMC Physiol 8, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naumova YY, Alimokhtari S, Kwon J, Shendell D, Jones J, Maberti S, Wall SJ, Eisenreich SJ, Turpin BJ, Weisel CP, Morandi MT, Colome SD, Totten LA, Stock TH & Winer AM (2002). Polycyclic aromatic hydrocarbons in the indoor and outdoor air of three cities in the U.S. Environ Sci Technol 36, 2552–2559. [DOI] [PubMed] [Google Scholar]
- Nebert DW, Dalton TP, Okey AB & Gonzalez FJ (2004). Role of aryl hydrocarbon receptor‐mediated induction of the CYP1 enzymes in environmental toxicity and cancer. J Biol Chem 279, 23847–23850. [DOI] [PubMed] [Google Scholar]
- Nelson D, Heuer RM, Cox GK, Stieglitz JD, Hoenig R, Mager EM, Benetti DD, Grosell M & Crossley DA (2016). Effects of crude oil on in situ cardiac function in young adult mahi‐mahi (Coryphaena hippurus). Aquat Toxicol 180, 274–281. [DOI] [PubMed] [Google Scholar]
- Nelson D, Stieglitz JD, Cox GK, Heuer RM, Benetti DD, Grosell M & Crossley DA (2017). Cardio‐respiratory function during exercise in the cobia, Rachycentron canadum: The impact of crude oil exposure. Comp Biochem Physiol C Toxicol Pharmacol 201, 58–65. [DOI] [PubMed] [Google Scholar]
- Nemmar A, Hoet PHM, Vanquickenborne B, Dinsdale D, Thomeer M, Hoylaerts MF, Vanbilloen H, Mortelmans L & Nemery B (2002). Passage of inhaled particles into the blood circulation in humans. Circulation 105, 411–414. [DOI] [PubMed] [Google Scholar]
- Nemmar A, Hoylaerts MF, Hoet PHM & Nemery B (2004). Possible mechanisms of the cardiovascular effects of inhaled particles: Systemic translocation and prothrombotic effects. Toxicol Lett 149, 243–253. [DOI] [PubMed] [Google Scholar]
- Nemtsas P, Wettwer E, Christ T, Weidinger G & Ravens U (2010). Adult zebrafish heart as a model for human heart? An electrophysiological study. J Mol Cell Cardiol 48, 161–171. [DOI] [PubMed] [Google Scholar]
- Nerbonne JM, Nichols CG, Schwarz TL & Escande D (2001). Genetic manipulation of cardiac K+ channel function in mice: What have we learned, and where do we go from here? Circ Res 89, 944–956. [DOI] [PubMed] [Google Scholar]
- Newby DE, Mannucci PM, Tell GS, Baccarelli AA, Brook RD, Donaldson K, Forastiere F, Franchini M, Franco OH, Graham I, Hoek G, Hoffmann B, Hoylaerts MF, Künzli N, Mills N, Pekkanen J, Peters A, Piepoli MF, Rajagopalan S & Storey RF (on behalf of ESC Working Group on Thrombosis , European Association for Cardiovascular Prevention and Rehabilitation , ESC Heart Failure Association ) (2015). Expert position paper on air pollution and cardiovascular disease. Eur Heart J 36, 83–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Health Service (2018). Arrhythmia. Available at: https://www.nhs.uk/conditions/arrhythmia/ [Accessed October 15, 2018].
- Niemann B, Rohrbach S, Miller MR, Newby DE, Fuster V & Kovacic JC (2017). Oxidative stress and cardiovascular risk: Obesity, diabetes, smoking, and pollution. J Am Coll Cardiol 70, 230–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble D & Tsien RW (1969). Outward membrane currents activated in the plateau range of potentials in cardiac Purkinje fibres. J Physiol 200, 205–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pampanin DM & Sydnes MO (2013). Polycyclic aromatic hydrocarbons a constituent of petroleum: Presence and influence in the aquatic environment. In Hydrocarbon, pp. 83–118. IntechOpen.
- Pauschinger M, Knopf D, Petschauer S, Doerner A, Poller W, Schwimmbeck PL, Kühl U & Schultheiss HP (1999). Dilated cardiomyopathy is associated with significant changes in collagen type I/III ratio. Circulation 99, 2750–2756. [DOI] [PubMed] [Google Scholar]
- Penn A, Murphy G, Barker S, Henk W & Penn L (2005). Combustion‐derived ultrafine particles transport organic toxicants to target respiratory cells. Environ Health Perspect 113, 956–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters A, Dockery DW, Muller JE & Mittleman MA (2001). Increased particulate air pollution and the triggering of myocardial infarction. Circulation 103, 2810–2815. [DOI] [PubMed] [Google Scholar]
- Petersen GI & Kristensen P (1998). Bioaccumulation of lipophilic substances in fish early life stages. Environ Toxicol Chem 17, 1385–1395. [Google Scholar]
- Phillips DH (1983). Fifty years of benzo(a)pyrene. Nature 303, 468–472. [DOI] [PubMed] [Google Scholar]
- Raftis JB & Miller MR (2019). Nanoparticle translocation and multi‐organ toxicity: A particularly small problem. Nano Today 26, 8–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie TJ & Macdonald SJF (2009). The impact of aromatic ring count on compound developability – are too many aromatic rings a liability in drug design? Drug Discov Today 14, 1011–1020. [DOI] [PubMed] [Google Scholar]
- Robertson S, Thomson AL, Carter R, Stott HR, Shaw CA, Hadoke PWF, Newby DE, Miller MR & Gray GA (2014). Pulmonary diesel particulate increases susceptibility to myocardial ischemia/reperfusion injury via activation of sensory TRPV1 and β1 adrenoreceptors. Part Fibre Toxicol 11, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauer JJ, Kleeman MJ, Cass GR & Simoneit BRT (1999). Measurement of emissions from air pollution sources. 2. C1 through C30 organic compounds from medium duty diesel trucks. Env Sci Technol 33, 1578–1587. [Google Scholar]
- Schauer JJ, Kleeman MJ, Cass GR & Simoneit BRT (2002). Measurement of emissions from air pollution sources. 5. C1–C32 organic compounds from gasoline‐powered motor vehicles. Environ Sci Technol 36, 1169–1180. [DOI] [PubMed] [Google Scholar]
- Scholz NL, Myers MS, McCarthy SG, Labenia JS, McIntyre JK, Ylitalo GM, Rhodes LD, Laetz CA, Stehr CM, French BL, McMillan B, Wilson D, Reed L, Lynch KD, Damm S, Davis JW & Collier TK (2011). Recurrent die‐offs of adult coho salmon returning to spawn in Puget Sound lowland urban streams. PLoS One 6, e28019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schraufnagel DE, Balmes JR, Cowl CT, De Matteis S, Jung S‐H, Mortimer K, Perez‐Padilla R, Rice MB, Riojas‐Rodriguez H, Sood A, Thurston GD, To T, Vanker A & Wuebbles DJ (2019a). Air pollution and noncommunicable diseases: a review by the Forum of International Respiratory Societies’ Environmental Committee, Part 2: Air pollution and organ systems. Chest 155, 417–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schraufnagel DE, Balmes JR, Cowl CT, De Matteis S, Jung SH, Mortimer K, Perez‐Padilla R, Rice MB, Riojas‐Rodriguez H, Sood A, Thurston GD, To T, Vanker A & Wuebbles DJ (2019b). Air pollution and noncommunicable diseases: a review by the Forum of International Respiratory Societies’ Environmental Committee, Part 1: The damaging effects of air pollution. Chest 155, 409–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severson RF, Snook ME, Arrendale RF & Chortyk OT (1976). Gas chromatographic quantitation of polynuclear aromatic hydrocarbons in tobacco smoke. Anal Chem 48, 1866–1872. [DOI] [PubMed] [Google Scholar]
- Shah AS V, Langrish JP, Nair H, McAllister DA, Hunter AL, Donaldson K, Newby DE & Mills NL (2013). Global association of air pollution and heart failure: A systematic review and meta‐analysis. Lancet 382, 1039–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah ASV, Lee KK, McAllister DA, Hunter A, Nair H, Whiteley W, Langrish JP, Newby DE & Mills NL (2015). Short term exposure to air pollution and stroke: systematic review and meta‐analysis. BMJ 350, h1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiels HA & Galli GLJ (2014). The sarcoplasmic reticulum and the evolution of the vertebrate heart. Physiology 29, 456–469. [DOI] [PubMed] [Google Scholar]
- Shimizu I & Minamino T (2016). Physiological and pathological cardiac hypertrophy. J Mol Cell Cardiol 97, 245–262. [DOI] [PubMed] [Google Scholar]
- Shin JT & Fishman MC (2002). From zebrafish to human: modular medical models. Annu Rev Genomics Hum Genet 3, 311–340. [DOI] [PubMed] [Google Scholar]
- Sirenko O, Grimm FA, R RK, Iwata Y, Chiu WA, Parham F, Wignall JA, Anson B, Cromwell EF, Behl M, Rusyn I & Tice RR (2017). In vitro cardiotoxicity assessment of environmental chemicals using an organotypic human induced pluripotent stem cell‐derived model. Toxicol Appl Pharmacol 322, 60–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JN & Levy EM (1990). Geochronology for polycyclic aromatic hydrocarbon contamination in sediments of the Saguenay Fjord. Environ Sci Technol 24, 874–879. [Google Scholar]
- Sørhus E, Incardona JP, Furmanek T, Goetz GW, Scholz NL, Meier S, Edvardsen RB & Jentoft S (2017). Novel adverse outcome pathways revealed by chemical genetics in a developing marine fish. Elife 6, e20707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sørhus E, Incardona JP, Karlsen Ø, Linbo T, Sørensen L, Nordtug T, Van Der Meeren T, Thorsen A, Thorbjørnsen M, Jentoft S, Edvardsen RB & Meier S (2016). Crude oil exposures reveal roles for intracellular calcium cycling in haddock craniofacial and cardiac development. Sci Rep 6, 1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sram RJ, Binkova B, Dostal M, Merkerova‐Dostalova M, Libalova H, Milcova A, Rossner P, Rossnerova A, Schmuczerova J, Svecova V, Topinka J & Votavova H (2013). Health impact of air pollution to children. Int J Hyg Environ Health 216, 533–540. [DOI] [PubMed] [Google Scholar]
- Stieglitz JD, Mager EM, Hoenig RH, Benetti DD & Grosell M (2016). Impacts of Deepwater Horizon crude oil exposure on adult mahi‐mahi (Coryphaena hippurus) swim performance. Environ Toxicol Chem 35, 2613–2622. [DOI] [PubMed] [Google Scholar]
- Stogiannidis E & Laane R (2015). Source characterization of polycyclic aromatic hydrocarbons by using their molecular indices: an overview of possibilities. Rev Environ Contamin Toxicol 234, 49–139. [DOI] [PubMed] [Google Scholar]
- Stone V, Miller MR, Clift MJD, Elder A, Mills NL, Møller P, Schins RPF, Vogel U, Kreyling WG, Jensen KA, Kuhlbusch TAJ, Schwarze PE & Hoet P (2017). Nanomaterials versus ambient ultrafine particles: an opportunity to exchange toxicology knowledge. Environ Health Perspect 125, 106002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai H, Chang W, Liu Y & Fakuda S (1990). Alterations of arachidonate metabolism in cardiovascular system by cigarette smoking. In Tobacco Smoking and Atherosclerosis, pp. 211–224. Springer. [DOI] [PubMed]
- Tithof P, Elgayyar M, Cho Y, Guan W, Fisher AB & M P‐G (2002). Polycyclic aromatic hydrocarbons present in cigarette smoke cause endothelial cell apoptosis by a phospholipase A2‐dependent mechanism. FASEB J 16, 1463–1464. [DOI] [PubMed] [Google Scholar]
- Tsai CT, Wu CK, Lin JL & Hwang JJ (2011). In‐vitro recording of adult zebrafish heart electrocardiogram – a platform for pharmacological testing. J Arrhythmia 27, 274. [DOI] [PubMed] [Google Scholar]
- Tsapakis M & Stephanou EG (2005). Occurrence of gaseous and particulate polycyclic aromatic hydrocarbons in the urban atmosphere: Study of sources and ambient temperature effect on the gas/particle concentration and distribution. Environ Pollut 133, 147–156. [DOI] [PubMed] [Google Scholar]
- United States Environmental Protection Agency (2017). Particle Pollution Exposure. Available at: https://www.epa.gov/pmcourse/particle-pollution-exposure.
- Vehniäinen E‐R, Haverinen J & Vornanen M (2019). Polycyclic aromatic hydrocarbons phenanthrene and retene modify the action potential via multiple ion currents in rainbow trout Oncorhynchus mykiss cardiac myocytes. Environ Toxicol Chem 38, 2145–2153. [DOI] [PubMed] [Google Scholar]
- Vornanen M & Hassinen M (2016). Zebrafish heart as a model for human cardiac electrophysiology. Channels 10, 101–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vornanen M, Haverinen J & Hassinen M (2018). Excitation and excitation‐contraction coupling of the zebrafish heart: implications for the zebrafish model in drug screening. In Recent Advances in Zebrafish Researches, ed. Y. Bozkurt. Books on Demand.
- Walker MK, Pollenz RS & Smith SM (1997). Expression of the aryl hydrocarbon receptor (AhR) and AhR nuclear translocator during chick cardiogenesis is consistent with 2,3,7,8‐tetrachlorodibenzo‐p‐dioxin‐induced heart defects. Toxicol Appl Pharmacol 143, 407–419. [DOI] [PubMed] [Google Scholar]
- Wamhoff BR, Bowles DK, McDonald OG, Sinha S, Somlyo AP, Somlyo AV & Owens GK (2004). L‐type voltage‐gated Ca2+ channels modulate expression of smooth muscle differentiation marker genes via a rho kinase/myocardin/SRF‐dependent mechanism. Circ Res 95, 406–414. [DOI] [PubMed] [Google Scholar]
- Wamhoff BR, Bowles DK & Owens GK (2006). Excitation‐transcription coupling in arterial smooth muscle. Circ Res 98, 868–878. [DOI] [PubMed] [Google Scholar]
- Warren KS, Baker K & Fishman MC (2017). The slow mo mutation reduces pacemaker current and heart rate in adult zebrafish. Am J Physiol Heart Circ Physiol 281, H1711–H1719. [DOI] [PubMed] [Google Scholar]
- Weidmann S (1955). Rectifier properties of Purkinje fibers. Am J Physiol 183, 671. [Google Scholar]
- Wellenius G, Burger M, Coull B, Schwartz J, Suh H, Koutrakis P, Schlaug G, Gold D & Mittleman M (2012). Ambient air pollution and the risk of acute ischemic stroke. Arch Intern Med 172, 229–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellenius GA, Saldiva PHN, Batalha JRF, Murthy GGK, Coull BA, Verrier RL & Godleski JJ (2002). Electrocardiographic changes during exposure to residual oil fly ash (ROFA) particles in a rat model of myocardial infarction. Toxicol Sci 66, 327–335. [DOI] [PubMed] [Google Scholar]
- Wesche DL, Schuster BG, Wang WX & Woosley RL (2000). Mechanism of cardiotoxicity of halofantrine. Clin Pharmacol Ther 67, 521–529. [DOI] [PubMed] [Google Scholar]
- Wittkopp S, Staimer N, Tjoa T, Stinchcombe T, Daher N, Schauer JJ, Shafer MM, Sioutas C, Gillen DL & Delfino RJ (2016). Nrf2‐related gene expression and exposure to traffic‐related air pollution in elderly subjects with cardiovascular disease: An exploratory panel study. J Expo Sci Env Epidemiol 26, 141–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wold LE, Ying Z, Hutchinson KR, Velten M, Gorr MW, Velten C, Youtz DJ, Wang A, Lucchesi PA, Sun Q & Rajagopalan S (2012). Cardiovascular remodeling in response to long‐term exposure to fine particulate matter air pollution. Circ Hear Fail 5, 452–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (2014). World Health Organization guidelines for indoor air quality: household fuel combustion. World Heal Organ. Available at: http://www.who.int/indoorair/guidelines/hhfc/HHFC_guidelines.pdf. [PubMed] [Google Scholar]
- World Health Organization (2018). Air pollution levels rising in many of the world's poorest cities. Available at: http://www.who.int/news-room/headlines/12-05-2016-air-pollution-levels-rising-in-many-of-the-world-s-poorest-cities [Accessed July 12, 2018].
- Zakaria MP, Takada H, Tsutsumi S, Ohno K, Yamada J, Kouno E & Kumata H (2002). Distribution of polycyclic aromatic hydrocarbons (PAHs) in rivers and estuaries in Malaysia: A widespread input of petrogenic PAHs. Environ Sci Technol 36, 1907–1918. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Huang L, Zuo Z, Chen Y & Wang C (2013). Phenanthrene exposure causes cardiac arrhythmia in embryonic zebrafish via perturbing calcium handling. Aquat Toxicol 142–143, 26–32. [DOI] [PubMed] [Google Scholar]
- Zhong Y, Wang J, Carmella SG, Hochalter JB, Rauch D, Oliver A, Jensen J, Hatsukami DK, Upadhyaya P, Zimmerman C & Hecht SS (2011). Metabolism of [D10]phenanthrene to tetraols in smokers for potential lung cancer susceptibility assessment: comparison of oral and inhalation routes of administration. J Pharmacol Exp Ther 338, 353–361. [DOI] [PMC free article] [PubMed] [Google Scholar]


