Abstract
Background
Several studies evidenced significant increase of cortisol is the consequence of UV or emotional stress and leads to various deleterious effects in the skin.
Aim
The well‐aging, a new concept of lifestyle, procures an alternative to the anti‐aging strategy. We demonstrated that Tephrosia purpurea extract is able to stimulate well‐being hormones while reducing cortisol release. Furthermore, we hypothesized that the extract could positively influence the global skin homeostasis.
Method
We evaluated the impact of the extract on cortisol, β‐endorphin, and dopamine, released by normal human epidermal keratinocytes (NHEKs). A gene expression study was realized on NHEKs and NHDFs. The protein over‐expression of HMOX1 and NQO1 was evidenced at cellular and tissue level. Finally, we conducted a clinical study on 21 women living in a polluted environment in order to observe the impact of the active on global skin improvement.
Results
The extract is able to reduce significantly the cortisol release while inducing the production of β‐endorphin and dopamine. The gene expression study revealed that Tephrosia purpurea extract up‐regulated the genes involved in antioxidant response and skin renewal. Moreover, the induction of HMOX and NQO1 expression was confirmed on NHDFs, NHEKs and in RHE. We clinically demonstrated that the extract improved significantly the skin by reducing dark circles, represented by an improvement of L*, a*, and ITA parameters.
Conclusion
Tephrosia purpurea extract has beneficial effects on skin homeostasis through control of the well‐being state and antioxidant defenses leading to an improvement of dark circles, a clinical features particularly impacted by emotional and environmental stress.
Keywords: antioxidant, beta‐endorphin, cortisol, dopamine, healthy aging, Tephrosia purpurea extract, well‐aging
1. INTRODUCTION
The skin is a complex organ that has the ability to produce and respond to neurotransmitters, neuropeptides, and other factors. Its neuroendocrine activities include the local synthesis of hypothalamo‐pituitary‐adrenal (HPA) axis elements.1 Because of its complexity, the skin has to be considered as a whole organ when we want to protect it from deleterious effects. Exogenous factors such as UV radiation and pollution, but also endogenous factors such as emotional stress, can trigger harmful effects, impacting both skin health and beauty.2, 3
One of the major elements of the HPA axis is the corticosteroids family. Among its members, cortisol, which is a secreted hormone, has a large number of glucocorticoid receptors on the skin cell surface.4 This ubiquitous hormone has a major impact when its homeostasis is imbalanced.5 Indeed, recent studies have demonstrated that prolonged exposure to glucocorticoids could have a direct impact on skin barrier recovery kinetics and immune response.2, 4
It has also been shown that UV exposure and emotional stress increase the level of active cortisol in skin by stimulating the expression of 11beta‐hydroxysteroid dehydrogenase type 1 (11β‐HSD1), the enzyme that converts inactive cortisone to cortisol.6, 7 This activation leads to delayed wound healing, a decrease in collagen production and the activation of Nf‐kB, provoking the release of pro‐inflammatory cytokines and thus the production of reactive oxygen species (ROS). Moreover, it has been shown that 11β‐HSD1 increases with aging 8 and in photo‐exposed skin.1, 7
Furthermore, environmental factors such as UV radiation, pollution, and tobacco smoking also induce molecular modifications, which lead to biological consequences in skin.9 Among these modifications, there is ROS generation in cells, which has a negative impact on stress response and protein oxidation, and by activating the inflammatory process through the MAPK/NF‐kB pathway. ROS also induce activation of some proteins involved in extracellular matrix degradation, such as matrix metalloproteinases.3, 10
Skin cells have developed the ability to fight against ROS production through the activation of the nuclear factor erythroid 2‐related factor (Nrf2) pathway responsible for the redox balance.11 Nrf2 activation leads to the expression of detoxification markers such as heme oxygenase‐1 (HMOX‐1), which is an enzyme inducing a cascade of degradation leading to bilirubin production, which has anti‐inflammatory, anti‐oxidative, and anti‐apoptotic properties.11 Another important detoxification marker activated by the Nrf2 pathway is NAD(P)H‐quinone oxidoreductase (NQO‐1), a cytoprotective protein that reduces quinones, which are cytotoxic, immunotoxic, and carcinogenic molecules.12 NQO‐1 also has a direct antioxidant activity by scavenging superoxides.13
The accumulation of these biological consequences of endogenous and exogenous stress leads to the premature aging of skin and to the appearance of visible signs such as wrinkles, pigment spots, and dark circles under the eyes, among others, albeit that the mechanisms remain to be clearly defined.3, 4 Indeed, ROS production induces the activation of matrix metalloproteinases, which are in part responsible for wrinkle formation.14, 15 On the other hand, ROS are also responsible for their appearance by oxidizing proteins which will be transported in blood and accumulated under the eyes, giving a dark coloration to the skin.16, 17, 18, 19
A recent report by Great Britain's Royal Society for Public Health revealed that the term “anti‐aging” is now being rejected by mature women and these women are more prone to a lifestyle promoting well‐being and a healthier way of living. This new behavior is now extended to “healthy aging” or “well‐aging.” This lifestyle, characterized by exercise, both physical and mental, such as yoga practice, is a well‐known contributor to healthier aging.8, 20 The mental aspect can be played by peptides involved in the skin‐HPA axis and known to be associated with a “feel‐good” factor, through their pain‐relieving properties.2 Indeed, endogenous peptides such as beta‐endorphin and encephalin, two peptides derived from pro‐opiomelanocortin (POMC) polypeptides and expressed by epidermal and dermal cells, can restore skin homeostasis. These allow retro‐regulation of immune responses, tissue integrity, and repair.2, 21, 22
Moreover, dopamine, a neurotransmitter derived from L‐DOPA in the catecholamine family, could have a link with a healthy lifestyle and seems to have the same beneficial properties as beta‐endorphin.23 Indeed, dopamine is known to play a role in the motivation and pleasure induced by rewarding.24 In a recent study, it was shown that the dopamine precursor was altered by oxidative stress and pro‐inflammatory cytokines, so we could hypothesize that its synthesis could be modified with age.25 Its production may be restored through a healthy lifestyle since it has been demonstrated to be up‐regulated following yoga practice.8
Tephrosia purpurea is an Indian plant recognized by Ayurvedic traditional medicine. Extracts of T purpurea have pleiotropic positive effects on health: hepatoprotective activity, anti‐ulcer, antidiabetic, antiallergic,26 and seed extract enter the composition of many skin care composition. The ethanolic seed extract studied in this article was previously chemically described.27 Among the major compounds, stachyose, ciceritol, caffeic acid, quercetin‐3‐O‐rutinoside, ethyl galactoside, saccharose, and citric acid were identified. Stachyose and ciceritol as well as T purpurea seed extract were demonstrated to stimulate beta‐endorphin secretion in normal human keratinocytes of the skin in a dose‐dependent manner (Patent US20030157200A1), and the presence of polyphenols suggests a potential antioxidant activity. Exploring the positive effects of T purpurea seed extract on skin from the in vitro to the clinical level would help to understand the mode of action of a renewed plant cosmetic active.
In this study, we first determined the influence of T purpurea extract on the well‐being sensation through the analysis of beta‐endorphin, dopamine, and cortisol release, which are associated with comfort and stress. To complete our results on the potential protection of cells against exogenous stress, we then evaluated the antioxidant activity of T purpurea extract through its ability to modulate NQO‐1 and HMOX‐1 gene expression, as well as their protein expression on the cellular level and tissue level, using reconstructed human epidermis. Following these in vitro studies, we carried out a clinical investigation on 21 women living in a polluted environment to evaluate the beneficial effects of T purpurea extract on global skin improvement.
2. MATERIALS AND METHODS
2.1. Tephrosia purpurea extract
Tephrosia purpurea seeds were collected in India, dried, and grounded. Hydroalcoholic extraction was realized in 70% ethanol with plant/solvent ratio at 8.3%. Following filtration, the extract was concentrated to remove ethanol and diluted in propanediol to obtain a final dry matter upper than 4%. Stachyose and ciceritol were quantified (≥0.4%) to assert extract quality.
2.2. Cell culture
Normal human epidermal keratinocytes (NHEKs) isolated from foreskin epidermis were used for the cortisol release study, the gene expression study and for the HMOX‐1 and NQO‐1 assays. Normal human dermal fibroblasts (NHDFs) isolated from foreskin dermis were also used for the gene expression study and for the HMOX‐1 and NQO‐1 assays. Normal human epidermal keratinocytes (NHEKs) freshly isolated from abdominoplasty were used for a beta‐endorphin release study. Normal human epidermal keratinocytes (NHEKs) freshly isolated from a 51‐year‐old donor were used for the dopamine release study.
2.3. Cortisol release study
Normal human epidermal keratinocytes were seeded in 12 well‐plates coated with collagen I until confluence, then rinsed and incubated in medium without hydrocortisone for 24 hours. Cells were then treated with 1% T purpurea extract for 2 hours at 37°C. All wells were then incubated with 1 μmol/L cortisone (Sigma‐Aldrich) for 24 hours. NHEKs were also treated for 24 hours with 1% T purpurea extract and without cortisone treatment. Supernatants were collected and centrifuged at 1000 g for 10 minutes at 4°C and stored at −20°C. An ELISA kit named Parameter Cortisol Assay was purchased from R&D Systems (reference KGE008). Cortisol present in the supernatant was dosed according to the kit manufacturer's instructions. The results were expressed as the concentration of cortisol released in pg/mL. This study has been done in triplicate.
2.4. Beta‐endorphin release study
NHEKs in confluent monolayers were incubated for 24 hours with T purpurea extract at 0.0025% (1 μg/mL), 0.0125% (5 μg/mL), or 0.0625% (25 μg/mL). As beta‐endorphin is a protein, a cocktail of protease inhibitors (aprotinin 5 μg/mL, leupeptin 1 μg/mL, and PMSF 1mM; Sigma‐Aldrich) was added to the incubation medium. Dibutyryl AMPc (Sigma‐Aldrich) was used as a positive control to stimulate beta‐endorphin release. The amount of beta‐endorphin released by the cells was quantified using the ELISA test (Peninsula laboratories kit). This study has been done in triplicate.
2.5. Dopamine release study
Normal human epidermal keratinocytes in confluent monolayers were incubated for 24 hours without or with T purpurea extract at 1% and 2%. They were also incubated with amantadine hydrochloride at 0.1 µmol/L (Sigma‐Aldrich), used as a positive reference of dopamine production and release. At the end of the incubation period, the dopamine released into the culture medium was quantified using a sensitive and specific ELISA kit (MyBioSource, ref#MBS2504780). The proteins present in the cell lysates were quantified using the spectro‐colorimetric Bradford method. Results are expressed as pg of dopamine per mg of total protein. This study has been done in triplicate.
2.6. Gene expression studies
This study was conducted on NHDFs and NHEKs cultured in monolayers in three independent replicate. T purpurea extract at 1% was added to the culture media for 24 hours prior to RNA extraction for gene expression analyses through qRT‐PCR. Total RNA was extracted using the RNeasy mini kit (Qiagen). After 24 hours of treatment, cells were rinsed with PBS and lysed in the appropriate buffer. Extraction and purification of RNA were performed according to the manufacturer's instructions. Total RNA was then stored at −80°C. Reverse transcription was performed with the high capacity RNA‐to‐cDNA kit (Applied Biosystems, 438706) according to the manufacturer's instructions, from 2 μg of total RNA. This reaction was carried out at 37°C for 1 hour, then 5 minutes at 95°C. The cDNA was then stored at −20°C. The target sequences of the genes of interest were amplified by PCR using "TaqMan gene Expression Assays"(Applied Biosystems). For standardization purposes, the reaction mixtures with probes and primers corresponding to glyceraldehyde‐3‐phosphate (GAPDH) for NHDFs or to β2‐microglobulin (B2M) for NHEKs were also prepared with the same cDNA samples. A control without cDNA served as the negative control of amplification. The thermal cycler was programmed with an incubation step at 50°C for 2 minutes, followed by a first denaturation step at 95°C for 10 minutes. The PCR amplification protocol was then set to 40 cycles of 15 seconds at 95°C, followed by 1 minute at 60°C. Gene expression levels were calculated using the comparative Ct (ΔΔCt) method (Pfaffl, 2001; Livak and Schmittgen, 2001).
2.7. Heme oxygenase HMOX‐1 and NQO‐1 expressions at the cellular level
Cells (NHDFs purchased from ATCC, reference CRL‐2522, and NHEKs purchased from Lonza, reference 00192906) were seeded in T25 flasks for 24 hours before treatment with 1% Tephrosia purpurea extract. Celastrol at 500 or 750 nmol/L (Sigma‐Aldrich, reference C0869) and sulforaphane at 3 μmol/L (Sigma‐Aldrich, reference S6317) were used as reference molecules to increase the protein levels of HMOX‐1 and NQO‐1 in both cell types. Each condition was performed in triplicate (n = 3). After 24 and 48 hours of treatment, the protein concentrations of HMOX‐1 and NQO‐1 in cell lysates were quantified by ELISA according to the kit manufacturer's instructions. Briefly, cells were rinsed with phosphate buffer solution before lysis in the appropriate buffer. After 30 minutes of incubation on ice, lysates were harvested and centrifuged to remove cell debris and collect the supernatants. The lysates were then used for ELISA tests specific for HMOX‐1 (Enzo Life Sciences, reference ADI‐800) and NQO‐1 (RayBiotech, reference 68EHL‐NQO1).
2.8. Heme oxygenase HMOX‐1 and NQO‐1 expression at the tissue level
The study was conducted on three independent human epidermis reconstructed from primary keratinocytes in culture (StratiCELL® EPI/001, batch CB0715/1). Tissues were cultured for 14 days at the air‐liquid interface in an appropriate culture medium and kept in a humid atmosphere at 37°C with 5% CO2. T purpurea extract formulated at 10 mg/mL (1%) and the placebo were then applied topically on the corneal layer of the reconstructed epidermis at a dose of 2 mg/cm2 for 48 hours. Celastrol (Sigma‐Aldrich, c0869l) and sulforaphane (Sigma‐Aldrich S6317) were placed in the culture medium of the epidermis, as reference molecules to induce HMOX‐1 and NQO‐1 expression. At the end of culture, tissues were placed in a fixative solution (4% formaldehyde) and dried by successive passages in baths of ethanol and isopropanol before being embedded in paraffin. For immunostaining, 6 μm sections of each paraffin‐embedded sample were placed on glass slides. The first step was to remove the paraffin and rehydrate the tissue. The epitope was retrieved using citrate buffer 0.01 mol/L at pH 6. After PBS washes and saturation in the presence of serum, slides were placed in the presence of primary antibodies specific for HMOX‐1 (Abcam, reference Ab13248) and NQO1 (Abcam, reference Ab28947). Fluorescein‐conjugated secondary antibody (Molecular Probes, reference A11001) was then used to allow detection of target proteins. DAPI or 4 ',6'‐diamidino‐2‐phenylindole (Invitrogen, reference D1306), a fluorescent molecule capable of binding to the adenine and thymine bases of DNA, was used to detect the nuclei of keratinocytes. Slides were then mounted with Mowiol (Sigma, reference 32,459‐0) and stored at 4°C in the dark until microscopy analysis. For immunofluorescence, in both cases, 3 photographs were taken from tissue triplicates, using a Leica microscope (DM200040x) and a Leica camera (DFC420C). Analysis software (QWin3, Leica) was then used for quantification. The detection limits were determined beforehand, to avoid saturation. For each picture, the marked surface and the average intensity were obtained within the area of interest (viable layers of the epidermis). The surface of the area of interest was also obtained (in pixels). The final value was obtained for each picture by multiplying the average intensity by the "marked surface/area of interest” ratio.
INCI description of cream compositions used in this study:
AQUA/WATER, CAPRIC/CAPRYLIC TRIGLYCERIDE, CETEARYL WHEAT STRAW GLYCOSIDES, CETEARYL ALCOHOL, ±BETAPHROLINE PROPANEDIOL, PHENOXYETHANOL, METHYL PARABEN, PROPYL, PRABEN, ETHYL PARABEN, DIMETHICONE, FRAGRANCE,HEXYL CINNAMAL, BUTYLPHENYL METHYLPROPIONAL, CITRONELLOL, ALPHA ISOMETHYL IONONE, HYDROXYISOHEXYL 3‐CYCLOHEXENE CARBOXALDEHYDE.
2.9. Statistical analysis
In vitro statistical analyses were performed in Excel® using Student t test. For the ex vivo study, a mix model of ANOVA was performed in XLstat® followed by a Fisher LSD test.
2.10. Clinical investigation
2.10.1. Cream compositions (INCI composition)
AQUA/WATER, CAPRIC/CAPRYLIC TRIGLYCERIDE, CETEARYL WHEAT STRAW GLYCOSIDES, CETEARYL ALCOHOL, ±BETAPHROLINE PROPANEDIOL, PHENOXYETHANOL, METHYL PARABEN, PROPYL, PRABEN, ETHYL PARABEN, DIMETHICONE, FRAGRANCE,HEXYL CINNAMAL, BUTYLPHENYL METHYLPROPIONAL, CITRONELLOL, ALPHA ISOMETHYL IONONE, HYDROXYISOHEXYL 3‐CYCLOHEXENE CARBOXALDEHYDE.
2.10.2. Description of the panel and study conditions
A simple blind and placebo‐controlled clinical evaluation was carried out on 21 women (aged between 40 and 67 years old) showing clinical signs of tiredness (under eye redness). All of the subjects participating in the study gave their informed consent, signed at the beginning of the study. The clinical test was run during the summer, under skin‐stressing conditions, as the volunteers were living in a geographical area known to have significant atmospheric pollution (measured in ten air quality control stations around the location of the clinical test during the testing period, see Figure 7). Pollution levels from PM10 (Particulate Matter at 10 µm) and ozone were always very close to the recommended limits and exceeded them at times. Therefore, the pollution was very high during the testing period. Twice a day, the volunteers applied the placebo or the cream containing 2% T purpurea extract on the most sensitive part of their face: the skin under their eyes. The clinical assessment was run for one month. The zones of application of the products were randomized. This was an open, intra‐individual study; each subject was her own control.
Figure 7.
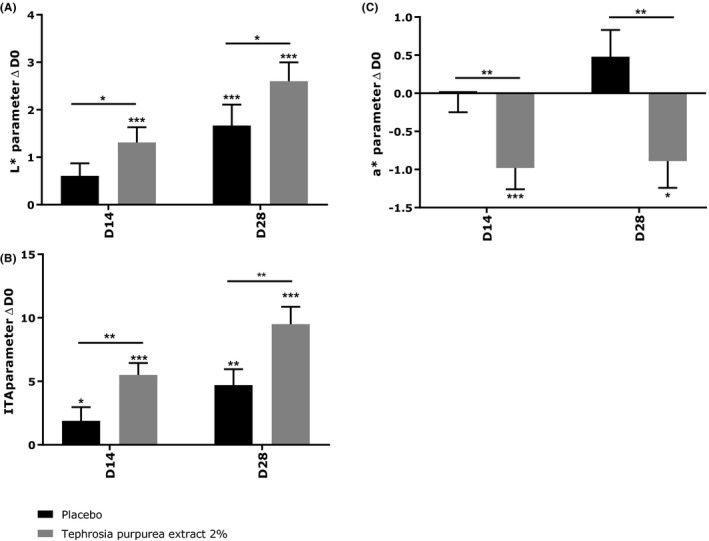
Effect of Tephrosia purpurea extract at 2% after 14 and 28 d of treatment on L* parameter (a), ITA (b) and a* parameter (c). Mixed ANOVA model *** P < 0.001, ** P < 0.01, and * P < 0.05
2.10.3. Skin colorimetric measurements
Skin colorimetric measurements were done on the dark circles with a MINOLTA® CM700‐d Spectrocolorimeter® equipped with a 3 mm diameter head. A spectrocolorimeter® converts colors perceived by man to a digital code composed of three parameters:
L*: for clarity (from dark to light),
a*: for green‐to‐red region,
b*: for the blue‐to‐yellow part of spectrum.
a* and b* are chrominance parameters and L* is a luminance parameter. It is therefore possible to express down to the smallest detail the differences between two cutaneous zones that appear to have the same color. After a calibration phase, measurements are done directly on the skin using a pulsed Xenon light source and a dual beam system designed to measure the light transmitted and to correct any slight deviation. This instrument is commonly used in cosmetics and medicine to measure skin color. The parameter a* (redness) was also studied during a study on redness under eyes.
The parameters studied were: L*, a*, b*, and ITA (Individual Typological Angle).
L* and b* parameters were exploited through the calculation of the ITA, which defines the skin pigmentation degree of a subject according to the following formula:
ITA° = [Arc tan((L*‐50)/b*)] x 180/ p. The higher the ITA°, the lighter the skin.
2.10.4. Illustrative images acquisition
The device used was the VISIA® CR from CANFIELD® imaging systems. This allows high‐resolution pictures to be taken with multiple lighting modes and a very rapid capture of images. Control of the repositioning takes place directly on the data‐processing screen using an overlay visualization of the images at each time of acquisition.
A series of photographs taken under multi‐spectral imaging and analysis (crossed polarized light) allows to capture visual information.
2.11. Statistical analysis
For each parameter (L*, a* and b*), descriptive statistics for quantitative data were computed and tabulated (number of valid values, number of missing values, mean, median, standard deviation, standard error of the mean, minimum, and maximum) for each time point (Day 0, Day 14, and Day 28) and by‐product (cream containing Tephrosia purpurea extract and placebo). A mixed ANOVA model was fitted on the raw data (Di) with product factor, time and the interaction product * time as fixed factors, and subject factor as blocking factor.
The specific contrasts of interest were set up:
To assess the change on each (Di) from baseline (D0), within each product level (cream containing Tephrosia purpurea extract and placebo)
To test whether cream containing Tephrosia purpurea extract and placebo differed significantly in terms of change from baseline (Di‐D0).
2.12. Ethics statement
The European Directive 2001/20/EC and regulations issued by the Minister of Health (Order of the Minister of Health of May 2, 2012 regarding Good Clinical Practice, Dz.U. 2012, item 491) are not applicable for cosmetic products. The study 14E1822 “Evaluation of the anti‐dark circles effect of a cosmetic product versus placebo” was conducted on healthy subjects with noninvasive measurements methods, to compare the cosmetics products “CPL 14.1 Batch #14.178A” and “CBE 14.2 Batch #14.178 D.” The study was therefore considered as noninterventional and did not require the Ethics Committee Approval and the Competent Authority Authorization.
3. RESULTS
3.1. Well‐being: impact of Tephrosia purpurea on the main hormones involved in stress and comfort
It is now globally described that the emotional stress represented by cortisol has deleterious impact on the skin. The “healthy aging” way of life, associated with beta‐endorphin and dopamine, has been demonstrated as having a beneficial effect on body. In this context, we developed several models to study the impact of Tephrosia purpurea on the release of these hormones.
We developed a specific in vitro model using cortisone in order reproduce in vitro its over‐expression found during emotional stress. In this study, we measured the ability to T purpurea extract to reduce this type of stress after 24 hours of treatment in presence of cortisone at 1 µmol/L. We validated our model as observed by the significant increase of cortisol level after cortisone stimulation. These first results demonstrated that NHEKs were able to convert cortisone to the cortisol active form.
We then confirmed that T purpurea extract was not able to stimulate cortisol release at the basal condition.
Under stress condition induced by the presence of cortisone at 1 µmol/L, we observed that a 2 hours prestimulation with T purpurea extract at 1% promoted an significant reduction of cortisol release by 70%, (P < 0.01) (Figure 1).
Figure 1.
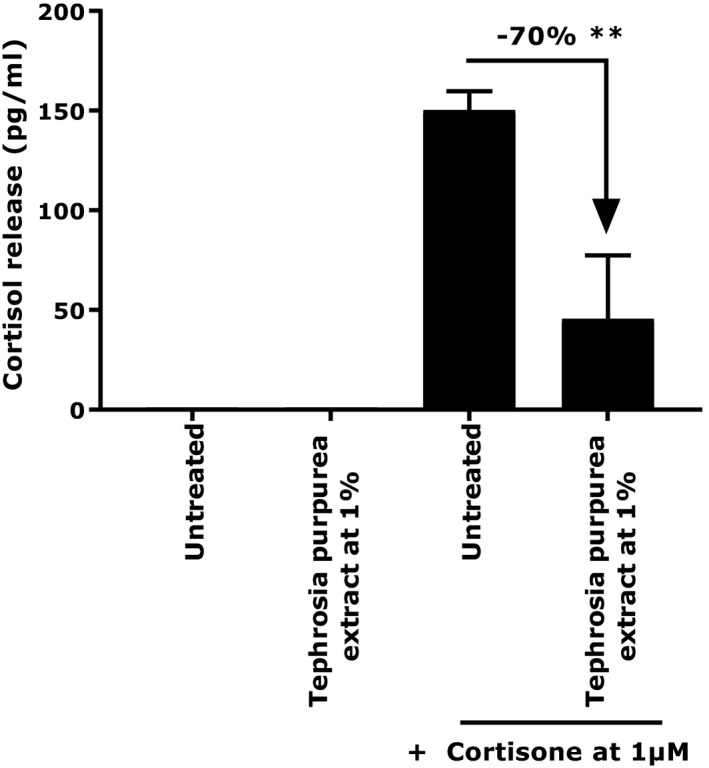
Cortisol release by NHEKs under basal or cortisone‐treated conditions in the presence or not of Tephrosia purpurea extract at 1%. Student's t test in comparison with untreated + cortisone at 1 µmol/L with **P < 0.01
Regarding the mediators of comfort, we first evaluated the release of beta‐endorphin and dopamine release at the basal level. Indeed, NHEKs naturally produce beta‐endorphins. We demonstrated that after 24 hours of treatment, T purpurea extract was able to enhance significantly beta‐endorphin production at 0.0125% and 0.0625% by 220%(P < 0.01) and 197.5% (P < 0.05), respectively (Figure 2). Similar results were obtained with the positive reference (Dibutyryl AMP cyclic at 2 mmol/L) showing a significant increase of beta‐endorphins production.
Figure 2.
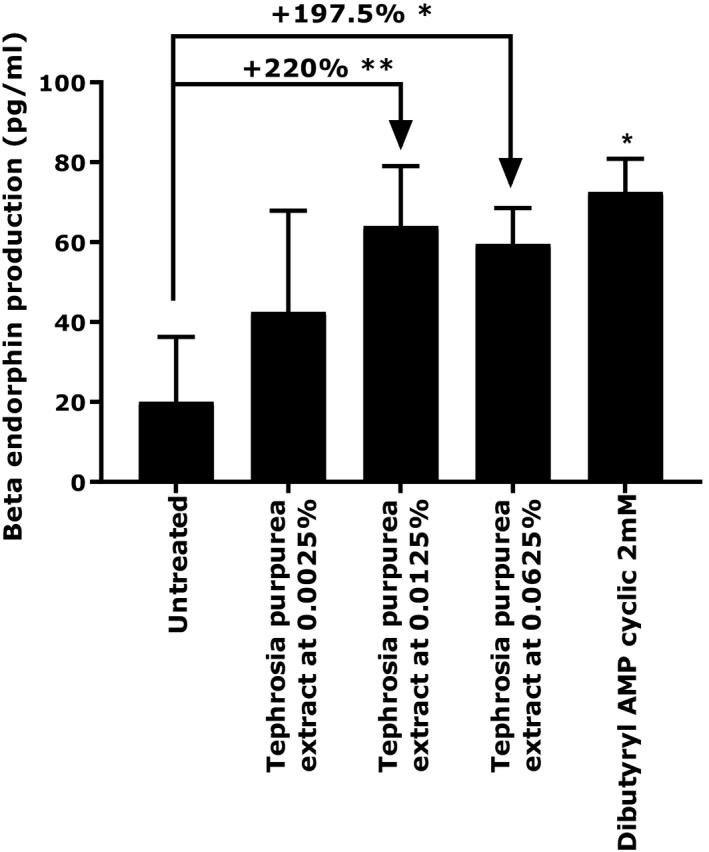
Beta‐endorphin production by NHEKs under basal condition or treated with Tephrosia purpurea extract at 0.0025%, 0.0125%, and 0.0625%. Dibutyryl cyclic AMP at 2 mmol/L was used as a positive reference. Student's t test in comparison to untreated condition, with ** P < 0.01, * P < 0.05
Finally, after the confirmation that NHEKs were able to produce dopamine, we then applied T purpurea extract at 1% for 24 hours in a comparison to a positive reference, amantadine hydrochloride at 0.1 µmol/L. We observed that the active ingredient was able to significantly increase the release of dopamine by 21%, P < 0.01 (Figure 3).
Figure 3.
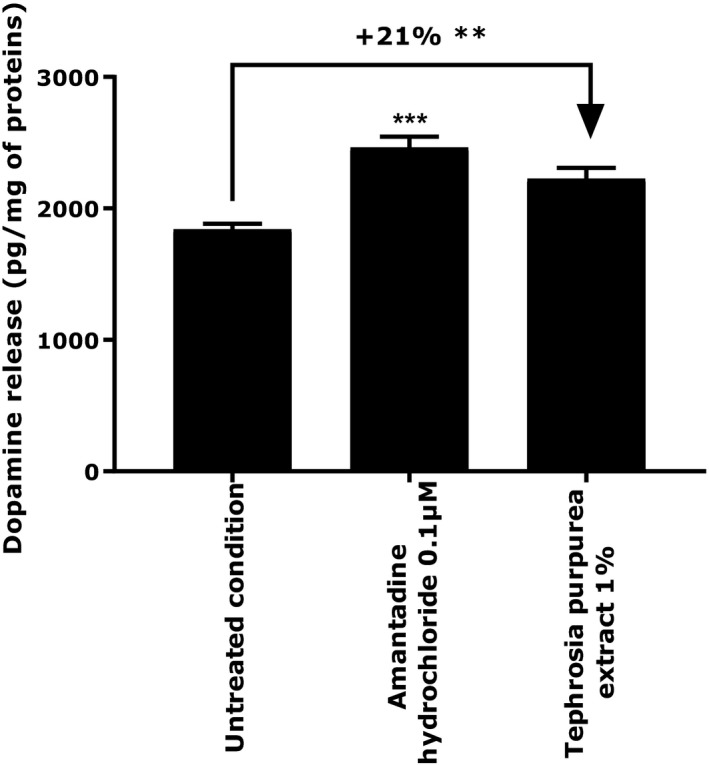
Evaluation of the extracellular content in dopamine release by NHEKs under basal condition and treated with Tephrosia purpurea extract at 1%. Amantadine hydrochloride at 0.1 µmol/L was used as positive reference. Student's t test in comparison to untreated condition, with ** P < 0.01
To conclude, T purpurea extract is a global actor in skin homeostasis by reducing the deleterious impact of emotional stress represented by the cortisol and by activating the release of “healthy aging” molecules such as beta‐endorphin and dopamine.
3.2. Gene expression studies
We performed gene expression study in order to identify the potential bioactivity of T purpurea extract on NHEKs and NHDFs. After 24 hours of incubation with T purpurea extract at 1%, the analysis of the transcriptomic study revealed that the active ingredient was able to significantly activate several genes involved in skin homeostasis in both cell types. We evidenced a significant increase of NQO‐1, NQO‐2, FTL, and MT2A gene expression, responsible for detoxification showing an induction of x3.14, x2.72, x3.21, and x3.66, respectively. The main actors of antioxidant defense, HMOX‐1 and TRXNRD, were also significantly up‐regulated in fibroblasts by x17.57 and x2.76, respectively. Finally, the extract stimulated the anti‐inflammatory defenses as observed by the significant induction of FTL and TRXNRD genes expression. These effects suggested that T purpurea extract have a potent detoxification, antioxidant, and anti‐inflammatory properties.
In NHEKs, we also observed a significant up‐regulation of HMOX‐1 and NQO‐1 gene expression by an induction of x9.8 and x4, respectively. Moreover, the active ingredient significantly improved the antimicrobial defense by increasing the expression of the antimicrobial peptide cathelicidin LL‐37 by x2.7. Finally, T purpurea extract induced renewal of the skin barrier by up‐regulation of gene expression of the serine protease KLK‐5 by x2.4 and the lamellar granule formation biomarker ABCA12 by x3 (Figure 4). These results evidenced that T purpurea extract seems to play an active role on defense mechanisms involving oxidation and microbial responses associated with an improvement of epidermal renewal process.
Figure 4.
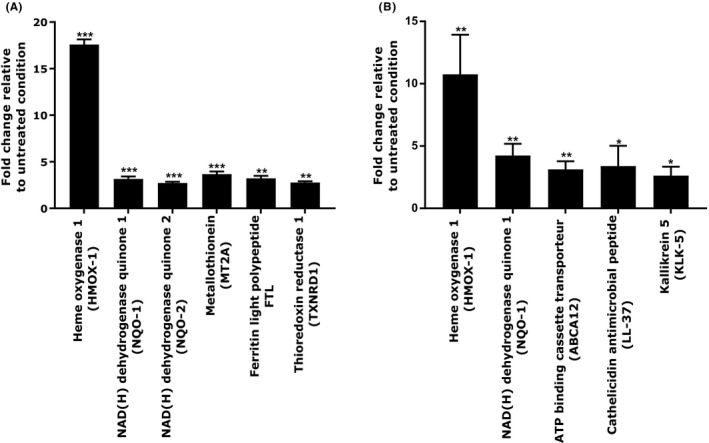
Genes over‐regulated after 24 h of treatment with Tephrosia purpurea extract at 1% in NHDFs (A) and NHEKs (B). Student's t test in comparison to untreated condition, with *** P < 0.001, ** P < 0.01, and * P < 0.05
Overall, the transcriptomic analysis permits to clearly identify that T purpurea extract possesses a potent antioxidant activity on fibroblasts and keratinocytes.
3.3. Impact on HMOX‐1 and NQO‐1
We previously found that T purpurea extract was able to up‐regulate, at the gene level, the expression of strong actors in antioxidant defense: HMOX‐1 and NQO‐1. We then wanted to confirm these results at the protein level.
We evaluated the effect of the active ingredient at 1% in both cell types for 24 hours and 48 hours of treatment. The treatments were performed in comparison to two positive references: celastrol (500 nmol/L) and sulforaphane (3 µmol/L).
After 24 hours of treatment, T purpurea extract strongly and significantly increased the level of HMOX‐1 in NHEKs (+3224% relative to untreated, P < 0.001) and NHDFs (+291% relative to untreated, P < 0.001). Its effect was better than the positive reference in keratinocytes and quite similar to the positive reference in fibroblasts. After 48 hours of treatment, the active ingredient still significantly raised the expression of HMOX‐1 protein, showing + 3116% (P < 0.001) and + 419% (P < 0.001) relative to untreated condition, on fibroblasts and keratinocytes, respectively.
Regarding NQO‐1 expression, after 24 hours, T purpurea extract significantly over‐expressed its expression in both cells types with an induction equal to the positive reference (+80%, P < 0.001, relative to untreated in NHEKs and + 40%, P < 0.001, relative to untreated in NHDFs). After 48 hours of treatment, the expression of NQO‐1 was still significantly higher (+113%, P < 0.001, relative to untreated in NHEKs and + 104%, P < 0.001, relative to untreated in NHDFs) (Figure 5).
Figure 5.
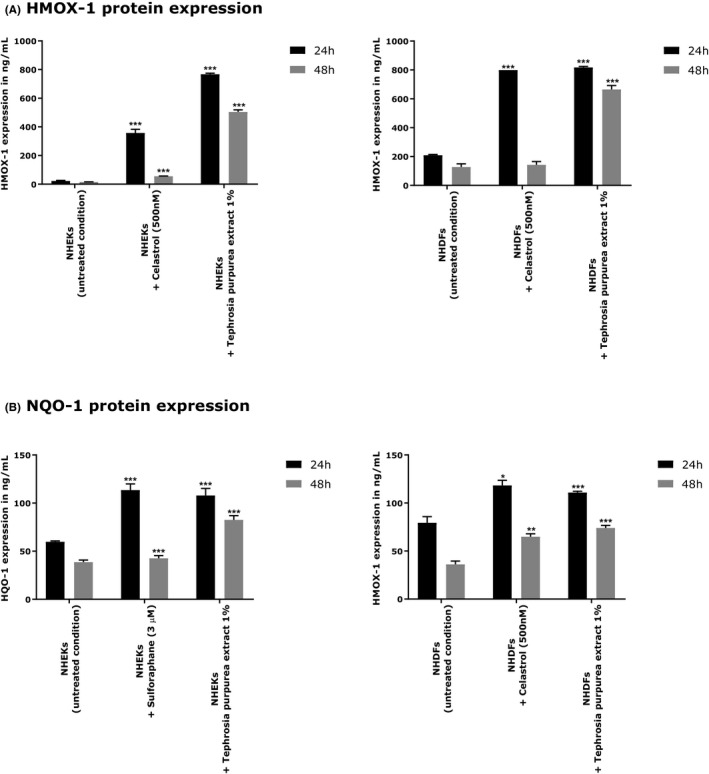
HMOX‐1(A) and NQO‐1(B) protein expressions using NHEKs and NHDFs treated with Tephrosia purpurea extract at 1% after 24 and 48 h of treatment, respectively. ANOVA analysis of variance and Dunnett's comparison test comparing treated conditions to their respective control *** P < 0.001, ** P < 0.01, and * P < 0.05
These results were then confirmed on a more complex model: human reconstructed epidermis. Before performing the immunolabeling, the tissue morphology was checked after topical treatment with the formulated active ingredient and the placebo formula for 24 and 48 hours, to ensure their noncytotoxicity. There was no morphological alteration reflecting cytotoxicity observed and no difference regarding the thickness and shape, so the immunolabelings were then made from the same samples (data not shown). Two positive controls were used to validate the stimulation of the two major proteins HMOX‐1 and NQO‐1 involved in cell homeostasis: celastrol at 750 nmol/L for HMOX‐1 and sulforaphane at 3 µmol/L for NQO‐1. They both increased the expression of each protein by 695% (P < 0.01) and 324% (P < 0.05), respectively.
After 48 hours of topical application of T purpurea extract formulated in cream at 1% on RHE, the expression of HMOX‐1 and NQO‐1 was considerably and significantly increased by + 888% (P < 0.05) and + 528%(P < 0.05), respectively, compared to the placebo. These results demonstrated that the active ingredient was also able to improve the expression of these antioxidant proteins at the tissue level (Figure 6).
Figure 6.
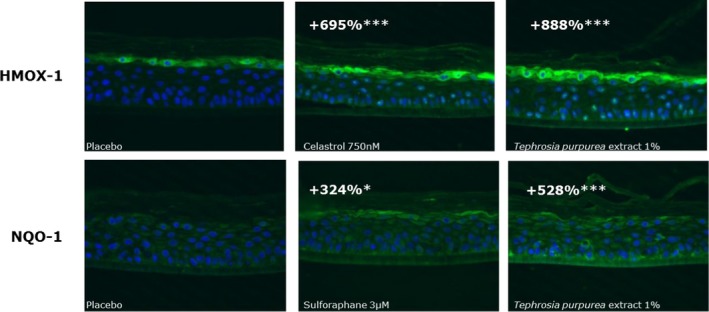
HMOX‐1 and NQO‐1 protein expressions in reconstructed human epidermis (RHE) topically treated for 48 h with Tephrosia purpurea extract formulated in cream at 1%. The quantification of protein expression is proportional to immunofluorescence intensity (green staining) measured using image analysis and converted in arbitrary unit. The percentage of improvement is obtained in comparison to the untreated condition. The significance is calculated with a mix model of ANOVA followed by Fisher statistical test in comparison to placebo for the active and in comparison to DMSO for both positive references with *** P < 0.001 and * P < 0.05
3.4. Clinical investigation
We carried out a clinical investigation in order to evaluate the beneficial improvement of the skin treated with T purpurea extract.
The clinical investigation was performed in a simple‐blinded intra‐individual study on a panel of 21 women living in a polluted area and having dark circles under their eyes. During the clinical study, the air quality was measured in the area and pollution by PM10 and ozone was very close to the recommended limits, even exceeding them at times. In a polluted environment, the skin is sensitive, especially the thin skin under the eyes.3 The stress triggered by pollution leads to dark circles and redness. These parameters were evaluated after 14 and 28 days of a twice daily application of Tephrosia purpurea extract at 2% in a cream, compared to its placebo and represented by the L*, a*, and ITA parameters.
After 14 days of twice daily application, the cream containing the active ingredient significantly increased the L* parameter compared to D0 reflecting the luminance of the skin (+1.31, P < 0.001). The placebo cream did not induce any significant variation compared to D0. In comparison to the placebo, T purpurea extract at 2% improved significantly the L* parameter. After 28 days of twice daily application, the placebo cream induced a significant modulation of the L* parameter (+1.67, P < 0.001) but the improvement was more pronounced using the active ingredient. Indeed, it induced a variation of + 2.60 (P < 0.001) compared to D0 and this improvement was significant compared to the placebo (P < 0.05).
Regarding the a* parameter reflecting the redness, the placebo cream did not modify the value after 14 and 28 days. Contrariwise, the cream containing Tephrosia purpurea extract at 2% induced a significant reduction in the a* parameter after 14 days (−0.98, P < 0.001) and 28 days (−0.89, P < 0.05), compared to D0, revealing an important anti‐redness effect. These results were both significant compared to the placebo (P < 0.01).
Finally, after 14 and 28 days of application, we observed significant modulation of the ITA parameter. The placebo induced a significant increase in the ITA parameter at 14 days (+1.9, P < 0.05) and at 28 days (+4.7, P < 0.01), but this effect was higher with the cream containing T purpurea extract at 2%. Indeed, after 14 days of application, the ITA parameter was significantly increased giving a variation of + 5.5 (P < 0.001 relative to D0 and P < 0.01 relative to placebo). After 28 days of application, the active increased by + 9.5 (P < 0.001) the ITA parameter and this effect was also significant compared to the placebo (P < 0.01). These results demonstrated a lightening effect of T purpurea extract (Figure 7). Representative images of two volunteers showing the average efficacy of the active are presented in Figure 8.
Figure 8.
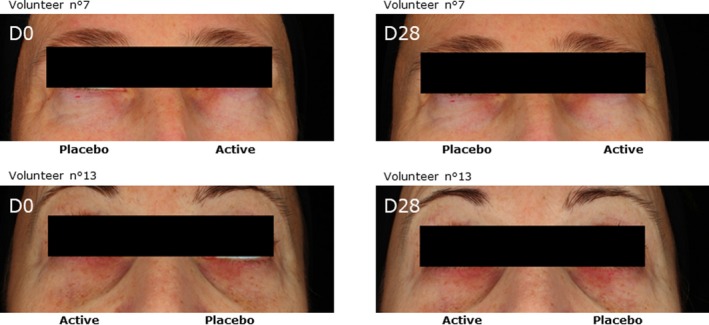
Illustrative macrophotographie were acquired by Visia CR under crossed polarized light after 28 d of cream application containing Tephrosia purpurea extract at 2% in comparison to a placebo
4. DISCUSSION
Skin homeostasis is a sensitive and complex state that can be modulated by various stimuli, including endogenous and exogenous factors. Among the endogenous factors, some are correlated to the sensation of well‐being. The secreted hormone cortisol, a member of the corticosteroid family and major element of the HPA axis, has been described as having a negative impact on skin homeostasis.28
The main effects include delayed skin barrier recovery and disturbance of the immune response.2 We developed an in vitro model to observe cortisol release after cortisone stimulation on NHEKs. We then evaluated the impact of T purpurea extract on this cortisol release. We demonstrated that after 24 hours of treatment in the presence of cortisone at 1 µmol/L, 1% T purpurea extract was able to significantly reduce cortisol release by 70% (Figure 1). This result shows that the extract has a direct impact on skin homeostasis by reducing one major component of the HPA axis involved in different skin disorders. Meanwhile, we used two supplementary in vitro models allowing the confirmation that our active ingredient also has the ability to have a positive effect on skin homeostasis and well‐being. Indeed, it has been shown that beta‐endorphin and dopamine, two peptides associated with a healthy lifestyle and known for their pain relief and reward properties, have beneficial effects on skin homeostasis and the retro‐regulation of immune response.2, 23 The release of these two peptides was evaluated on NHEKs after 24 hours of treatment. We observed that T purpurea extract induced a significant increase in beta‐endorphin production by 220% (P < 0.01) and 197.5% (P < 0.05) at 0.015% and 0.0625%, respectively (Figure 2). The extract also increased dopamine release by 21% (P < 0.01) compared to the untreated condition (Figure 3). In light of these results, we can confirm that our active ingredient has not only the ability to reduce a negative component in well‐being but also to induce the production of two well‐known peptides associated with skin comfort and homeostasis.23
Regarding the exogenous factors, some of these are involved in the disturbance of skin homeostasis through the generation of ROS. It is now generally considered that environmental factors such as UV radiation and pollution have a negative impact in stress response and activate various pro‐inflammatory pathways leading to skin aging.3 We investigated the activity of T purpurea extract on these parameters by means of several studies from gene to tissue level. As a result of the gene expression study on the two main cell types of the skin (NHDFs and NHEKs), we observed that T purpurea extract at 1% after 24 hours of treatment was able to stimulate significantly the key actors of the antioxidant response: HMOX‐1, NQO‐1, NQO‐2, MT2A, FTL29, 30, 31 and TXNRD1, HMOX‐1 and NQO‐1 being the major components of the Nrf2 pathway11, 13, 32, 33, 34 (Figure 4). The active ingredient was also able to stimulate the expression of proteins involved in antimicrobial defense (LL‐37) and skin renewal (KLK5 and ABCA12)35, 36 We then focused on the protein expression of HMOX‐1 and NQO‐1, and their levels were measured after 24 and 48 hours of treatment with T purpurea extract at 1% on NHEKs and NHDFs. We observed that the active ingredient increased significantly the expression of each protein (Figure 5). We then confirmed these results at the tissue level on RHE after 48h of treatment (Figure 6). All these data allow us to claim that the extract is a major inducer of the antioxidant response leading to an improvement in skin homeostasis.10, 29 We can suppose here that T purpurea extract could act as a hormetic molecule.37, 38 Indeed, the extract may fortify cellular defense capacities, rendering cells adaptive to oxidant challenges. The in vitro and ex vivo experimental studies led to the demonstration of the activity of T purpurea extract on global skin homeostasis by re‐balancing the consequences of emotional stress and exogenous factors. These stress, as described in the introduction, are expressed by the apparition of dark circles at the clinical level.3, 4
Finally, we carried out a clinical investigation on 21 healthy women living in a polluted environment. As pollution leads to premature aging and darkening of the skin, especially the area under the eye,2 we evaluated the impact of T purpurea extract on three parameters: L* parameter reflecting skin clarity, a* parameter representing redness of the skin, and the ITA parameter, quantifying the color of the skin. After 14 and 28 days, T purpurea extract formulated at 2% in a cream showed an improvement concerning the visible signs of aging by increasing the lightening of the dark circles and by reducing skin redness (Figures 7 and 8).
5. CONCLUSION
Skin homeostasis is a delicate balance that can be disrupted by endogenous stress such as emotions and external factors such as UV radiation and pollution. A state of well‐being, represented in our study by the secretion of beta‐endorphin and dopamine, demonstrated the beneficial impact on this balance and suggests a healthier aging for mature women. T purpurea extract showed a counteracting effect on cortisol release and a stimulating effect on dopamine and beta‐endorphin production. These positive effects are complemented by an improvement of the antioxidant defenses. All these data lead to restoration of skin homeostasis by reaching a state of well‐being. These data are backed up by a clinical study demonstrating the global improvement of skin appearance through a lightening of the dark circles, the most visible sign of aging of women living in a polluted environment.
6. LIMITATIONS
In this study, we demonstrated that our active ingredient is able to control the stress by a strong antioxidant activity and promotes well‐being. The apparition of dark circles is one of the visible consequences of the psycho‐emotional stress. Indeed, it is well‐known now that the external or internal stress such as pollution, UV, cigarette smoke, or emotions could influence our level of stress by cortisol release and reduction of well‐being state. Using in vitro models, we confirmed that the active ingredient allows the control of cortisol release correlated to an increase of well‐being hormones. Consequently, this active is specifically dedicated to people who undergo environmental and emotional stress.
ACKNOWLEDGEMENTS
The authors wish to thank Dr Sandre's team from Polyclinique Courlancy (Reims, France) for their support in providing skin biopsies. The authors would like also to thank their partner Straticell (Les Isnes, Belgium) and Ephyscience (Nantes, France) for their help in conducting these studies. Finally, the authors thank Hanane Chajra for its scientific support in this project.
De Tollenaere M, Meunier M, Scandolera A, et al. Well‐aging: A new strategy for skin homeostasis under multi‐stressed conditions. J Cosmet Dermatol. 2019;19:444–455. 10.1111/jocd.13047
The copyright line for this article was changed on 19 August 2019 after original online publication.
REFERENCES
- 1. Terao M, Katayama I. Local cortisol/corticosterone activation in skin physiology and pathology. J Dermatol Sci. 2016;84:11‐16. [DOI] [PubMed] [Google Scholar]
- 2. Hunter H, Momen SE, Kleyn CE. The impact of psychosocial stress on healthy skin. Clin Exp Dermatol. 2015;40:540‐546. [DOI] [PubMed] [Google Scholar]
- 3. Krutmann J, Bouloc A, Sore G, Bernard BA, Passeron T. The skin aging exposome. J Dermatol Sci. 2017;85:152‐161. [DOI] [PubMed] [Google Scholar]
- 4. Chen Y, Lyga J Brain‐skin connection: stress, inflammation and skin aging. Inflamm Allergy Drug Targets. 2014;13:177‐190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schäcke H. Mechanisms involved in the side effects of glucocorticoids. Pharmacol Ther. 2002;96:23‐43. [DOI] [PubMed] [Google Scholar]
- 6. Boudon SM, Vuorinen A, Geotti‐Bianchini P, et al. 11β‐hydroxysteroid dehydrogenase 1 inhibitors reduce cortisol levels in keratinocytes and improve dermal collagen content in human ex vivo skin after exposure to cortisone and UV. PLoS ONE. 2017;12:e0171079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nam J‐J, Min J‐E, Son M‐H, Oh J‐H, Kang S. Ultraviolet‐ and infrared‐induced 11 beta‐hydroxysteroid dehydrogenase type 1 activating skin photoaging is inhibited by red ginseng extract containing high concentration of ginsenoside Rg3(S). Photodermatol Photoimmunol Photomed. 2017;33:311‐320. [DOI] [PubMed] [Google Scholar]
- 8. Pal R, Singh SN, Chatterjee A, Saha M. Age‐related changes in cardiovascular system, autonomic functions, and levels of BDNF of healthy active males: role of yogic practice. Age (Dordr). 2014;36:9683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Portugal‐Cohen M, Oron M, Cohen D, Ma’or Z. Antipollution skin protection ‐ a new paradigm and its demonstration on two active compounds. Clin Cosmet Investig Dermatol. 2017;10:185‐193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mittal M, Siddiqui MR, Tran K, Reddy SP, Malik AB. Reactive oxygen species in inflammation and tissue injury. Antioxid Redox Signal. 2014;20:1126‐1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Loboda A, Damulewicz M, Pyza E, Jozkowicz A, Dulak J. Role of Nrf2/HO‐1 system in development, oxidative stress response and diseases: an evolutionarily conserved mechanism. Cell Mol Life Sci. 2016;73:3221‐3247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bolton JL, Trush MA, Penning TM, Dryhurst G, Monks TJ. Role of quinones in toxicology. Chem Res Toxicol. 2000;13:135‐160. [DOI] [PubMed] [Google Scholar]
- 13. Dinkova‐Kostova AT, Talalay P. NAD(P)H:quinone acceptor oxidoreductase 1 (NQO1), a multifunctional antioxidant enzyme and exceptionally versatile cytoprotector. Arch Biochem Biophys. 2010;501:116‐123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pittayapruek P, Meephansan J, Prapapan O, Komine M, Ohtsuki M. Role of matrix metalloproteinases in photoaging and photocarcinogenesis. Int J Mol Sci. 2016;17:868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zegarska B, Pietkun K, Zegarski W, et al. Air pollution, UV irradiation and skin carcinogenesis: what we know, where we stand and what is likely to happen in the future? Adv Dermatol Allergol. 2017;1:6‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chavan M, Sun Y, Litchauer J, Denis A. Fucus extract: cosmetic treatment for under‐eye dark circles. J Cosmet Science. 2014;103‐113. [PubMed] [Google Scholar]
- 17. Freitag FM, Cestari TF. What causes dark circles under the eyes? J Cosmet Dermatol. 2007;6:211‐215. [DOI] [PubMed] [Google Scholar]
- 18. Huang Y‐L, Chang S‐L, Ma L, Lee M‐C, Hu S. Clinical analysis and classification of dark eye circle. Int J Dermatol. 2014;53:164‐170. [DOI] [PubMed] [Google Scholar]
- 19. Puca AA, Carrizzo A, Villa F, et al. Vascular ageing: the role of oxidative stress. Int J Biochem Cell Biol. 2013;45:556‐559. [DOI] [PubMed] [Google Scholar]
- 20. Rea IM. Towards ageing well: Use it or lose it: Exercise, epigenetics and cognition. Biogerontology. 2017;18:679‐691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dieamant GDC, Velazquez Pereda MDC, Eberlin S, Nogueira C, Werka RM, Queiroz MLdS. Neuroimmunomodulatory compound for sensitive skin care: in vitro and clinical assessment. J Cosmet Dermatol. 2008;7:112‐119. [DOI] [PubMed] [Google Scholar]
- 22. Slominski AT, Manna PR, Tuckey RC. On the role of skin in the regulation of local and systemic steroidogenic activities. Steroids. 2015;103:72‐88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Slominski AT, Zmijewski MA, Skobowiat C, Zbytek B, Slominski M, Steketee JD Sensing the Environment: Regulation of Local and Global Homeostasis by the Skin Neuroendocrine System; 2013:98 Memphis, TN: Adv Anat Embryol Cell Biol; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Del Río JP, Alliende MI, Molina N, Serrano FG, Molina S, Steroid VP. Hormones and their action in women’s brains: the importance of hormonal balance. Front Public Health. 2018;6:141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Vumma R, Johansson J, Venizelos N. Proinflammatory cytokines and oxidative stress decrease the transport of dopamine precursor tyrosine in human fibroblasts. Neuropsychobiology. 2017;75(4):178‐184. [DOI] [PubMed] [Google Scholar]
- 26. Palbag S, Dey BK, Singh NK. Ethnopharmacology, phytochemistry and pharmacology of Tephrosia purpurea. Chin J Nat Med. 2014;12(1):1‐7. [DOI] [PubMed] [Google Scholar]
- 27. Hubert J, Chollet S, Purson S, et al. Exploiting the complementarity between dereplication and computer‐assisted structure elucidation for the chemical profiling of natural cosmetic ingredients: Tephrosia purpurea as a case study. J Nat Prod. 2015;78(7):1609‐1617. [DOI] [PubMed] [Google Scholar]
- 28. Schacke H. Mechanisms involved in the side effects of glucocorticoids. Pharmacol Ther. 2002;96(1):23‐43. [DOI] [PubMed] [Google Scholar]
- 29. Bock KW. Ah receptor‐ and Nrf2‐gene battery members: Modulators of quinone‐mediated oxidative and endoplasmic reticulum stress. Biochem Pharmacol. 2012;83(7):833‐838. [DOI] [PubMed] [Google Scholar]
- 30. Ishii T, Itoh K, Takahashi S, et al. Transcription factor Nrf2 coordinately regulates a group of oxidative stress‐inducible genes in macrophages. J Biol Chem. 2000;275(21):16023‐16029. [DOI] [PubMed] [Google Scholar]
- 31. Simon BR, Wilson MJ, Wickliffe JK. The RPTEC/TERT1 cell line models key renal cell responses to the environmental toxicants, benzo[a]pyrene and cadmium. Toxicol Rep. 2014;1:231‐242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bolton JL, Trush MA, Penning TM, Dryhurst G, Monks TJ. Role of quinones in toxicology † . Chem Res Toxicol. 2000;13(3):135‐160. [DOI] [PubMed] [Google Scholar]
- 33. Kozakowska M, Dulak J, Kozakowska JA. Heme oxygenase‐1 – more than the cytoprotection. Postepy Biochem. 2015;61(2):147‐158. [PubMed] [Google Scholar]
- 34. Tian H, Matsuo Y, Fukunaga A, Ono R, Nishigori C, Yodoi J. Thioredoxin ameliorates cutaneous inflammation by regulating the epithelial production and release of pro‐inflammatory cytokines. Front Immunol. 2013;4:269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Borgono CA, Michael IP, Komatsu N, et al. A potential role for multiple tissue kallikrein serine proteases in epidermal desquamation. J Biol Chem. 2006;282(6):3640‐3652. [DOI] [PubMed] [Google Scholar]
- 36. Sakai K, Akiyama M, Sugiyama‐Nakagiri Y, McMillan JR, Sawamura D, Shimizu H. Localization of ABCA12 from Golgi apparatus to lamellar granules in human upper epidermal keratinocytes. Exp Dermatol. 2007;16(11):920‐926. [DOI] [PubMed] [Google Scholar]
- 37. Oliveira MF, Geihs MA, França T, Moreira DC, Is H‐L. “Preparation for oxidative stress” a case of physiological conditioning hormesis? Front Physiol. 2018;9:945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sthijns M, Weseler A, Bast A, Haenen G. Time in redox adaptation processes: from evolution to hormesis. Int J Mol Sci. 2016;17(10):1649. [DOI] [PMC free article] [PubMed] [Google Scholar]


