Summary
Pathogen‐reduced (PR) platelets are routinely used in many countries. Some studies reported changes in platelet and red blood cell (RBC) transfusion requirements in patients who received PR platelets when compared to conventional (CONV) platelets. Over a 28‐month period we retrospectively analysed platelet utilisation, RBC transfusion trends, and transfusion reaction rates data from all transfused adult patients transfused at the Yale‐New Haven Hospital, New Haven, CT, USA. We determined the number of RBC and platelet components administered between 2 and 24, 48, 72 or 96 h. A total of 3767 patients received 21 907 platelet components (CONV = 8912; PR = 12 995); 1,087 patients received only CONV platelets (1578 components) and 1,466 patients received only PR platelets (2604 components). The number of subsequently transfused platelet components was slightly higher following PR platelet components (P < 0·05); however, fewer RBCs were transfused following PR platelet administration (P < 0·05). The mean time‐to‐next platelet component transfusion was slightly shorter following PR platelet transfusion (P = 0·002). The rate of non‐septic transfusion reactions did not differ (all P > 0·05). Septic transfusion reactions (N = 5) were seen only after CONV platelet transfusions (P = 0·011). These results provide evidence for comparable clinical efficacy of PR and CONV platelets. PR platelets eliminated septic transfusion reactions without increased risk of other types of transfusions with only slight increase in platelet utilisation.
Keywords: platelets, pathogen‐reduction, transfusion, efficacy, transfusion reaction
The availability and safety of blood transfusion is a fundamental public health responsibility that is overseen in the United States by the US Food and Drug Administration (FDA). Internationally, the World Health Organization (WHO) recommends adoption of policies to improve the quality, safety and availability of blood (WHO, 2017). While transfusion‐transmitted infections (TTIs) are rare compared with other adverse events (AEs) of transfusion, they remain a source of morbidity and are responsible for 10% of transfusion‐related mortality (Bihl et al, 2007). The search for methods to reduce the risk of TTIs gave rise to a detailed donor selection process and development of serological and nucleic acid tests for known pathogens, with a focus on viral and parasitic infections. Platelet components are additionally cultured to screen for bacterial pathogens, due either to bacteraemia in the donor or to contamination during collection or processing. These efforts are generally considered “reactive” or “passive”, in that the testing is done to detect known pathogens, rather than to remove potential pathogens from the blood supply.
Reactive approaches have been employed multiple times in the past with emerging infections, including human immunodeficiency virus, hepatitis C virus, Babesia and Zika virus, among others. Even for known pathogens, however, test failures and serological window periods can lead to false‐negative results. Current passive approaches to screen for bacterial contamination also presents challenges, especially in platelet units that are stored at room temperature (20–24°C). Published data has shown that the TTI rate was higher (1·95/100 000 platelets) among platelet units than among 1–6°C stored red blood cell (RBC) components (0·53/100 000 RBC) (Haass et al, 2019). To reduce the risk of TTIs and septic reactions, some centres have increased the blood bag incubation time prior to bacterial sampling, increased the volume of the inoculum, added a secondary surveillance culture or implemented point‐of‐release testing (Jacobs et al, 2011). However, each of these approaches adds cost and decreases the amount of time between product availability and expiration. In addition, point‐of‐release testing has been shown to have limited sensitivity (Larsen et al, 2005; Jenkins et al, 2011).
Because of the significant health and economic impacts of TTIs, implementation of a “proactive” approach to mitigate the limitations of the current screening process has the potential to significantly improve transfusion safety. A proactive approach is one that is designed to interdict a blood‐borne pathogen prior to its even being identified as a threat. One such proactive approach involves use of pathogen inactivation (PI) technology to further reduce the risk of TTIs (Klein et al, 2007). The end product of a blood component that has undergone a PI process is referred to as having been pathogen‐reduced (PR) (Lozano et al, 2015). It should be noted that, although these processes result in multiple ‘log reductions’ of the number of pathogens in the end product, no method can provide complete, universal inactivation of all pathogens (Salunkhe et al, 2015).
Given the unique properties of various blood products, each blood component requires a different and specialised PI process. Solvent‐detergent processing, heat and acid pH treatments can be used for the manufacture of plasma and plasma derivatives (Burnouf & Radosevich, 2000). However, other approaches are needed for cellular products. Currently, there are three technologies for producing PR platelets: INTERCEPT Blood System (Cerus Corporation, Concord, CA, USA), MIRASOL PRT System (TerumoBCT, Lakewood, CO, USA), and THERAFLEX UV system (MacoPharma, Mouvaux, France and German Red Cross Service NSTOB, Springe, Germany) (Seltsam, 2017). Each system uses a different approach for PI. For the INTERCEPT system, amotosalen, a photoactive synthetic psoralen compound, is added to the platelet concentrates which then intercalates into nucleic acids, and is activated by exposing the platelets to ultra violet (UV)A light, preventing pathogen replication. The MIRASOL PRT system uses a similar approach with riboflavin (vitamin B2), which is activated by ultraviolet light (UVA + B). The THERAFLEX‐UV system uses UVC light alone, without use of a photoactive compound.
Within the USA, the only currently FDA approved PR technology for platelet products is the INTERCEPT system, which received approval in 2014. This technology has been available in Europe since the early 2000’s, with several large‐scale studies completed regarding the safety and efficacy of these products. Studies comparing conventional (non‐PI treated) apheresis single donor or pooled whole blood‐derived platelets (CONV) to PI treated apheresis single donor platelets, found mixed results regarding haemostatic efficacy, with some finding similar outcomes (van Rhenen et al, 2003; McCullough et al, 2004); others, however, found decreased haemostatic functionality for the PR platelets (Kerkhoffs et al, 2010; Rebulla et al, 2017). A 2017 meta‐analysis based on 12 trials concluded that PR platelets did not affect all‐cause mortality or the risk of significant bleeding, but did result in a lower 1‐h post‐transfusion corrected count increment (CCI) (Estcourt et al, 2017). The CCI is a formula that provides a standardised measure for a post‐platelet transfusion increment. The same meta‐analysis showed that there was no significant difference in transfusion reaction rates following transfusion with PR or CONV platelets (Estcourt et al, 2017). However, there are limited data regarding the safety and efficacy of PR platelets as currently manufactured in the USA. Accordingly, we assessed whether there was a change in the utilisation of platelets or RBCs, or the incidence of transfusion reactions during a transition from an all CONV platelet inventory to a PR platelet inventory at our institution, as part of an ongoing quality assurance review. Supply constraints for PR products did necessitate maintenance of a dual inventory of CONV and PR platelet products and resulted in a prolonged inventory transition period (Fig 1) (Rutter & Snyder, 2019).
Figure 1.
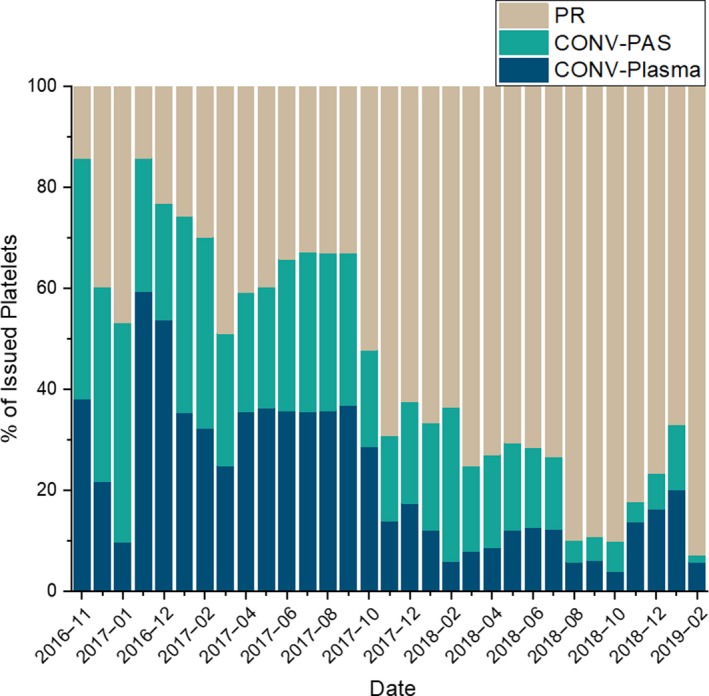
Platelet inventory from 1 November 2016 to 28 February 2019. CONV, conventional platelets; CONV‐PAS, conventional platelets + platelet additive solution; PR, pathogen‐reduced platelets.
Methods
Our institution (Yale‐New Haven Hospital, New Haven, CT, USA) began a transition from CONV platelets to INTERCEPT (Cerus Corporation, Concord, CA, USA) PR platelet products in November 2016. CONV platelets were manufactured by the American Red Cross (ARC, Farmington, CT) or the Rhode Island Blood Center (RIBC, Providence, RI). CONV products were either leucoreduced single donor apheresis platelets or leucoreduced whole‐blood derived platelet pools. Single‐donor PR platelets were manufactured in PAS‐C additive solution by the ARC or collected in plasma by the RIBC. Primary bacterial cultures of CONV platelets were performed at the collecting blood centre. As an additional safety measure, on storage day four or five, CONV platelets were analysed at our institution with a bacterial mitigation assay (PLT PGD test, Verax Biomedical, Marlborough, MA) before being released from our blood bank. All CONV platelets were gamma‐irradiated as per our usual practice; PR platelets were not irradiated. Both PR platelets and CONV platelets, the latter having been screened with a point‐of‐release bacterial detection “safety measure” assay, were considered a standard‐of‐care, as agreed to by our hospital’s Ethics Committee and Department of Risk Management (Rutter & Snyder, 2019). As per policy, either type of platelet, PR or CONV, was dispensed by the blood bank to any recipient, based on inventory availability at the time of the transfusion request.
From 1 November 2016 until 28 February 2019, all transfusion data on adults aged 18 years and older were extracted from our clinical data warehouse and data analysis platform, which captures and merges data from our electronic health record and blood bank management systems, Epic (Epic Corporation, Verona, WI) and SoftBank (SCC Soft Computer, Clearwater, FL), respectively (McPadden et al, 2019). Data were collected as part of an ongoing quality assurance review with no specific inclusion or exclusion criteria, other than patient age. The data included patient demographics, type of transfused platelet product, and time of platelet and RBC transfusion. Transfusion reaction data, if and when a transfusion reaction occurred after a platelet transfusion, were recorded separately. Following transfusion of each index CONV or PR platelet component, the number of subsequent platelet or RBC transfusions in a window from 2 to 24, 48, 72 and 96 h was calculated. All transfusion reactions were passively reported to the blood bank by clinical providers for each service line and the type of reaction classified by a transfusion medicine attending physician based on National Hemovigilance Network Guidelines (CDC, 2018). For patients who received a combination of CONV and PR platelets and had a suspected transfusion reaction reported, the reaction was ascribed to the appropriate product (CONV or PR) based on the timing of the reaction in proximity to the transfusion.
Results were represented as mean ± standard deviation (SD) or median and interquartile range (IQR), as appropriate. Differences in transfusion utilisation are shown with 95% confidence intervals. The Python (version 3.7.0; https://www.python.org), SciPy (version 1.2.0; https://www.scipy.org) and matplotlib (version 3.0.2; https://matplotlib.org) libraries were used to generate confidence intervals and density plots (covariance factor of 0·2). A two‐tailed Student’s t‐test was performed to assess for significance for continuous variables. Comparison of Poisson rates for transfusion reactions was performed using R (version 3.5.1; https://www.r-project.org). Statistical significance was taken at P < 0·05.
Results
Dual‐inventory statistics and patient demographics
Over the 28‐month study period (1 November 2016 to 28 February 2019), the proportion of PR platelets available in our inventory progressively increased (Fig 1). A total of 21 907 platelet components were issued to 3767 patients. Of these, 1,087 patients received only CONV and 1466 received only PR products (Table 1). There was a slightly higher number of platelet transfusions per patient for patients who received only PR platelet products (1·78 platelet components/patient) versus those who received only CONV platelet products (1·45 platelet components/patient) (P < 0·001). Because of our ongoing dual‐inventory, most chronically transfused patients received a combination of CONV and PR products (Table 1), and this group therefore had the highest number of transfusions. For all patients, a total of 8912 CONV and 12 995 PR platelet components were given (Table 2).
Table 1.
Number of platelet components issued for patients who received only conventional platelets (CONV), only pathogen‐reduced platelets (PR), or a combination of both platelet products. Ranges for mean components per patient with 95% confidence interval.
| Product(s) received | Total patients | Total platelet transfusions | Transfusions/patient |
|---|---|---|---|
| CONV only | 1087 | 1578 | 1·45 (1·4–1·5) |
| PR only | 1466 | 2604 | 1·78 (1·7–1·9) |
| Combination (CONV + PR) | 1214 | 17 725 | 14·6 (13·4–15·8) |
Table 2.
Transfusion reaction rates in patients receiving conventional (CONV) or pathogen‐reduced (PR) platelets.
| Reaction type | Conventional (N = 8912) | Pathogen reduced (N = 12 995) | Relative risk (95% CI) | P value |
|---|---|---|---|---|
| Allergic | 34 | 42 | 1·18 (0·73–1·90) | 0·485 |
| FNHTR | 26 | 37 | 1·02 (0·60–1·74) | 1·000 |
| Haemolytic | 0 | 2 | 0·00 (0·00–7·76) | 0·517 |
| Hypotensive | 0 | 1 | 0·00 (0·00–56·9) | 1·000 |
| Septic | 5 | 0 | Inf (1·34–Inf) | 0·011 |
| TACO | 3 | 5 | 0·87 (0·14–4·50) | 1·000 |
| TAD | 2 | 2 | 1·46 (0·11–20·1) | 1·000 |
| TA‐GvHD | 0 | 0 | N/A | N/A |
| TRALI | 0 | 0 | N/A | N/A |
| Total | 70 | 89 | 1·15 (0·83–1·59) | 0·420 |
CI, confidence interval; FNHTR, febrile non‐haemolytic transfusion reaction; Inf, infinite; N/A, not applicable; TA‐GvHD, transfusion‐associated graft‐versus‐host disease; TACO, transfusion associated circulatory overload; TAD, transfusion‐associated dyspnoea; TRALI, transfusion‐related acute lung injury.
Platelet utilisation in patients receiving CONV and/or PR platelets
As patients were not randomised to a single product type for this quality assurance review, most received a combination of CONV and PR platelets. To assess the effectiveness of PR platelets in this group, we calculated subsequent platelet use over multiple time periods following each index transfusion (Fig 2). We determined the number of subsequently transfused platelet components between 2 and 24, 48, 72 or 96 h (Fig 2A). The number of consecutive platelet transfusions in patients receiving PR platelet components, compared to those receiving CONV platelet components, was slightly, but statistically, higher (P < 0·05 for each time period). We also assessed the mean time to the next platelet transfusion for those who received a second platelet transfusion between 2 and 48 h after each index transfusion. This interval was slightly shorter following PR platelet transfusion (20·3 ± 13·5 h) compared to CONV platelet transfusion (21·2 ± 14·2 h) (P = 0·002) (Fig 2B).
Figure 2.
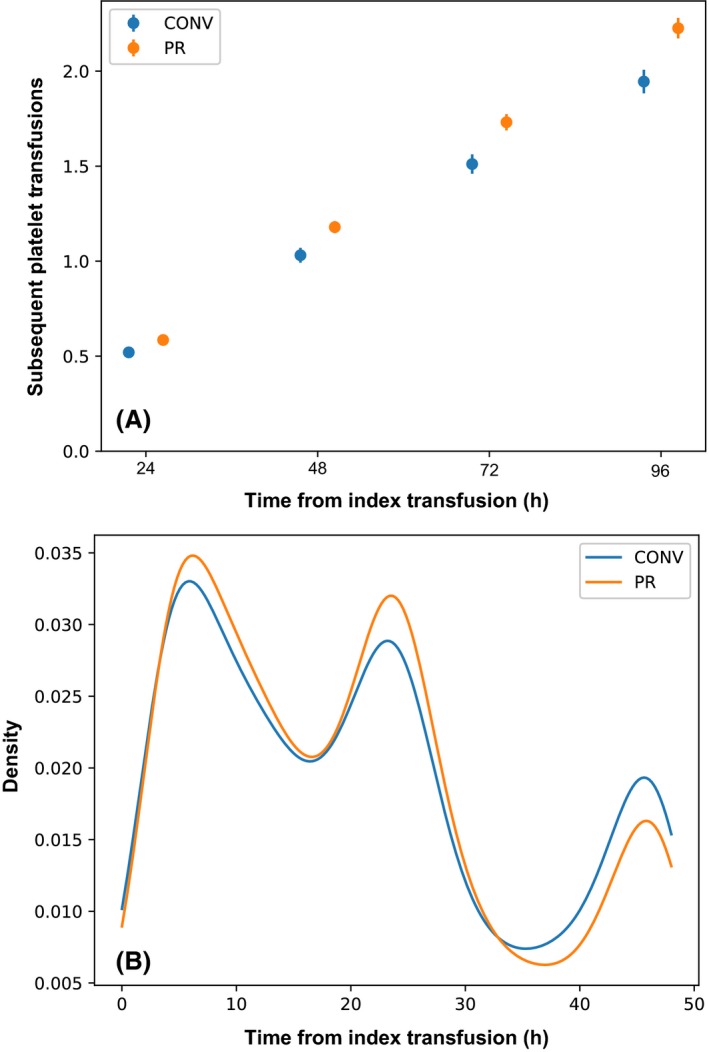
(A) Number of subsequent platelet transfusions between 2 and 24, 48, 72 or 96 h, bars show 95% confidence intervals. (B) Density plot demonstrating time to subsequent platelet transfusion for patients who received a platelet transfusion between 2 and 48 h after each index transfusion. CONV, conventional platelets; PR, pathogen‐reduced platelets.
RBC transfusions in patients receiving CONV and/or PR platelets
To assess the haemostatic efficacy of PR platelets, we determined the number of RBC component transfusions administered between 2 and 24, 48, 72 or 96 h after each index platelet transfusion as a proxy measure of bleeding that required transfusion support, as previously described (Cazenave et al, 2010). At each time interval assessed, there were slightly fewer RBC component transfusions administered following PR platelet transfusion compared to those receiving CONV platelets (P = 0·02 for 96 h; for other time points P < 0·05) (Fig 3).
Figure 3.
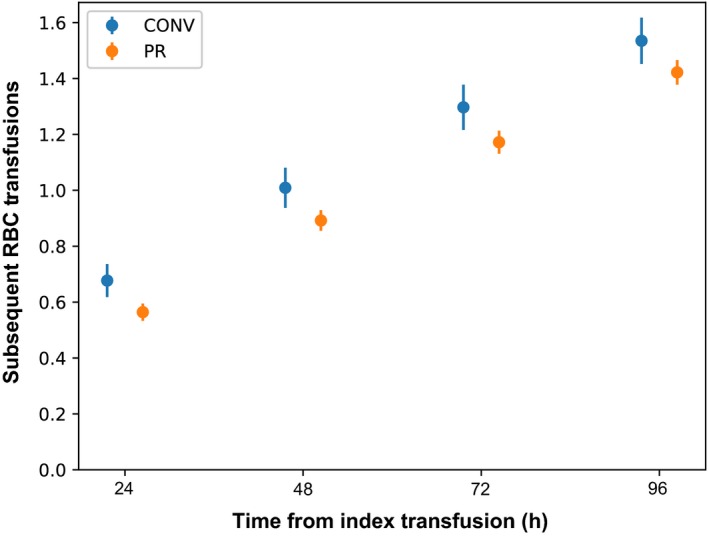
Number of red blood cell transfusions issued between 2 and 24, 48, 72 or 96 h after each index platelet transfusion. Bars represent 95% confidence intervals. CONV, conventional platelets; PR, pathogen‐reduced platelets.
Transfusion reactions
A total of 159 transfusion reactions were identified following 21 907 platelet transfusion episodes (Table 2). Results included all adult patients regardless of admitting diagnosis and without stratification for co‐morbidities. Mean age for the patients who received a CONV platelet component was 55 years (median = 57, IQR = 45–68) and for the patients who received a PR platelet, mean patient age was 58 years (median = 61, IQR = 49–69). In the CONV group, 70 reactions (male = 38, female = 32) were recorded over 8,912 transfusion episodes. In the PR group, a total of 89 reactions (male = 42, female = 47) were identified over 12 995 platelet component transfusions. Overall, the rate and type of reactions were similar between the two groups (P = 0·420) (Table 2), with allergic reactions being the most frequently reported category (n = 76). There were two reports of haemolytic transfusion reactions in the PR group, both of which were due to a minor ABO‐mismatched platelet transfusion, owing to the passive transfusion of anti‐B antibodies. No reports of transfusion‐related acute lung injury (TRALI) were received for any patient, and no cases of transfusion‐associated graft‐versus‐host disease (TA‐GvHD) were reported in any recipient. Importantly, septic transfusion reactions were noted in five patients after receiving CONV platelets while no septic transfusion reactions were seen following PR platelet transfusion (P = 0·011). Two septic reactions were due to Acinetobacter calcoaceticus‐baumanni complex (ACBC) and Staphylococcus saprophyticus (Jones, 2019), and three septic reactions were due to Streptococcus gallolyticus (bovis), with the five units coming from two donors. Within two h of initiation of transfusion, all five patients became febrile and three of them developed hypotension. Post‐transfusion reaction samples and patients’ blood cultures tested positive for the pathogens. Patients were treated with appropriate antibiotic therapy and supportive care in the intensive care unit and they were discharged in stable condition within 5–7 days; no deaths were reported. None of the five contaminated units had bacteria detected by routine culture pre‐transfusion. The three S. gallolyticus contaminated units were 4 days old, and at the time of these transfusions we the units were assayed with the PLT PGD test only on day five of storage. Thus, these CONV units were not tested via the PLT PGD test. The CONV platelet units contaminated with ACBC and S. saprophyticus had point‐of‐release testing performed on storage day five and again on day six after the reactions were reported, all of which were reported as negative.
Discussion
Safety of the blood supply is critical for patient outcomes and impacts the public’s perception of transfusion. Various methods, including donor testing, expanded surveillance cultures and rapid detection assays, aim to increase the safety of blood products. Of these screening methods for bacterial contamination of platelets, delayed large‐volume bacterial culture (DLVBC) implemented by United Kingdom’s National Health System Blood and Transplant and by Northern Ireland Blood Transfusion Service was able to decrease the reported septic fatality to zero cases (Benjamin et al, 2017, SHOT, 2019). A similar experience with DLVBC was also reported by the Belgian Haemovigilance program (FAMHP, 2016). While these strategies have decreased the rate of TTIs, they might be ineffective against emerging pathogens and continue to present a risk for test failures (false negatives) (Benjamin et al, 2014). PI technology has been shown to be effective at reducing TTIs (Jutzi et al, 2018; Haass et al, 2019), particularly in platelet products, which continue to have a relatively high rate of bacterial contamination (FDA, 2017).
An international, open‐label, observational haemovigilance programme on 19 175 transfusions previously showed that adverse events reported after PR platelet transfusions were infrequent and were of low‐grade severity (Knutson et al, 2015). Other large scale studies (>100 patients per group) found either similar (Lozano et al, 2011; Rebulla et al, 2017) or lower (McCullough et al, 2004) reaction rates between CONV and PR platelet products. Importantly, our study revealed that there were no cases of septic transfusion reactions for the 12 995 PR platelet transfusions. In contrast, for the 8912 CONV platelet transfusions, five septic transfusion reactions were identified. These septic reactions occurred despite the use of point‐of‐release testing for two of these platelet units due to false negative results. Multiple year reports have had similar findings, with a persistence of bacterial contamination and septic reactions associated with CONV platelet products. However, no bacterial TTIs were reported in over 200 000 transfusions of PR platelets between 2011 and 2016 (Jutzi et al, 2018). We found no differences in the rate or type of other transfusion reactions following CONV or PR platelet transfusion.
To date, the largest prospective, randomised, controlled, double‐blind trail conducted in the United States comparing therapeutic efficiency and safety of PR to CONV platelets was the SPRINT trial, in which 318 patients received PR and 327 patients received CONV platelets (McCullough et al, 2004). Analysis of the data showed that the two groups were equivalent regarding the incidence of grade II or higher bleeding. PR platelets, however, provided lower mean 1‐h post‐transfusion platelet CCIs and received more total platelet doses over a transfusion period (McCullough et al, 2004). A study by Garban et al (2018) evaluated patients with thrombocytopenia and malignant haematological diseases and demonstrated that PR platelets were non‐inferior to CONV platelets collected in the PAS additive solution, but non‐inferiority was not achieved when compared to CONV platelets collected in plasma. This suggests that some of the observed impact may be due to the PAS rather than PI‐specific processing (Garban et al, 2018). During our 28‐month quality assurance review period, we determined that platelet usage patterns were similar in patients receiving CONV or PR platelet products. Consistent with previously published reports (McCullough et al, 2004; Estcourt et al, 2017; Schulz et al, 2019), we found platelet utilisation to be slightly higher following PR platelet transfusion compared to CONV platelet transfusion. While we did not directly assess haemostatic efficacy, we found that RBC utilisation was slightly lower in patients following PR platelet transfusion compared to CONV platelet transfusion. This is consistent with systematic reviews that found there was no evidence of a difference between CONV and PR platelet products related to the incidence of clinically significant bleeding (Estcourt et al, 2017). We also have noted similar findings in our paediatric population (Schulz et al, 2019).
A 2015 nationwide survey showed that the median price paid by US blood banks per unit of CONV platelet component was $524 (IQR: $495–$560) [£411 (IQR: £389–£440)] (Ellingson et al, 2017). The average cost for a unit of PR platelet component was $749 (SD: $16·30) [£588 (SD: £12·79)] (Kacker et al, 2019). While the acquisition cost of PR platelets is higher than CONV platelets, several factors may offset these costs. First, FDA draft guidance recommends that CONV platelets should undergo a primary culture followed by a secondary culture or secondary rapid testing on day five of storage, which is not required for PR platelet products (FDA, 2018). Additionally, PR products are exempt from Zika testing, are considered equivalent to cytomegalovirus serology testing, and do not require irradiation to prevent the risk of TA‐GVHD. However, the FDA‐approved shelf life for PR platelets is only 5 days, while CONV platelet components’ shelf life could be extended to 7 days with the use of secondary testing methods, such as a bacterial mitigation assay. Taken together with the cost of managing sepsis and other TTIs, economical modelling studies anticipate a small to moderate cost increase in adoption of PR platelet inventory over a CONV inventory, but with the added benefit of an increased transfusion safety profile (McCullough et al, 2015; Benjamin et al, 2017; Prioli et al, 2018). The cost to treat hospital‐related sepsis varies, mostly based on severity of sepsis. The median cost was reported as $32 421 (£25 449) by a 2017 review (Arefian et al, 2017). Costs associated with sepsis probably could be decreased if use of PR platelets was more widely implemented.
Weaknesses of our study include its retrospective, observational nature as a quality assurance review as compared to a randomised clinical trial; it also is a single centre experience. Despite the large number of transfusions assessed, patients often received both CONV and PR platelet products, which prevented direct assessment of utilisation or risk adjustment in the entire population and limited the availability of standardised laboratory results, which could have been used to calculate specific endpoints, such as the CCI. Absence of patient demographics is also a weakness of our study. Accordingly, we could not perform any assessments as to whether a change in patient demographics had co‐occurred with our platelet inventory transition to PR products and caused a shift in transfusion practice, such as decreased transfusion orders. However, our institutional policies for transfusion thresholds were the same during the study period.
This is the first published clinical study on the use of PR platelets manufactured in USA, extending our work on paediatric patients (Schulz et al, 2019). Based on these results, we conclude that platelet and RBC utilisation are comparable in routine clinical practice following either CONV or PR platelet transfusion. While utilisation was statistically higher following PR platelet transfusion, the total increase in platelet transfusion burden was clinically small. Importantly, CONV, but not PR platelets, were associated with septic transfusion reactions and no other differences in transfusion reaction rates were noted. Ongoing evaluation of the benefits and potential drawbacks of PR products is an important public health task and will require ongoing assessment to ensure the safety and efficacy of the blood supply.
Author contributions
BB (conceptualisation/design, data curation, formal analysis, drafting the initial manuscript); WS (conceptualisation/design, data curation, formal analysis, drafting the initial manuscript); AG (data curation, review or editing of the manuscript); BS (conceptualisation/design, review or editing of the manuscript); EG (conceptualisation/design, review or editing of the manuscript); ES (conceptualisation/design, supervision/oversight, review or editing of the manuscript).
Conflicts of Interest
Dr. Snyder receives research support from Cerus Corporation as principal investigator for Phase IV PR Platelet Post Marketing Study (PIPER) and PR RBC Phase III Clinical Trial (ReCePI), no personal remuneration received. Dr. Gehrie receives research funding from: Cerus Corporation as co‐investigator for Phase IV PR Platelet Post Marketing Study (PIPER); from Terumo BCT as principal investigator of the Phase III MiPLATE study; and from Grifols Diagnostics for educational programming and participation on an Immunohematology Advisory Board. Dr. Schulz is a consultant for Hugo Health, a personal health record platform. The other authors declare no conflicts or competing interests.
Previously presented in part at the AABB 2018 Annual Meeting as poster.
References
- Arefian, H. , Heublein, S. , Scherag, A. , Brunkhorst, F.M. , Younis, M.Z. , Moerer, O. , Fischer, D. & Hartmann, M. (2017) Hospital‐related cost of sepsis: a systematic review. Journal of Infection, 74, 107–117. [DOI] [PubMed] [Google Scholar]
- Benjamin, R.J. , McDonald, C.P. ; ISBT Transfusion Transmitted Infectious Disease Bacterial Workgroup . (2014) The international experience of bacterial screen testing of platelet components with an automated microbial detection system: a need for consensus testing and reporting guidelines. Transfusion Medicine Reviews, 28, 61–71. [DOI] [PubMed] [Google Scholar]
- Benjamin, R.J. , Braschler, T. , Weingand, T. & Corash, L.M. (2017) Hemovigilance monitoring of platelet septic reactions with effective bacterial protection systems. Transfusion, 57, 2946–2957. [DOI] [PubMed] [Google Scholar]
- Bihl, F. , Castelli, D. , Marincola, F. , Dodd, R.Y. & Brander, C. (2007) Transfusion‐transmitted infections. Journal of Translational Medicine, 5, 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnouf, T. & Radosevich, M. (2000) Reducing the risk of infection from plasma products: specific preventative strategies. Blood Reviews, 14, 94–110. [DOI] [PubMed] [Google Scholar]
- Cazenave, J.P. , Follea, G. , Bardiaux, L. , Boiron, J.M. , Lafeuillade, B. , Debost, M. , Lioure, B. , Harousseau, J.L. , Tabrizi, R. , Cahn, J.Y. & Michallet, M. (2010) A randomized controlled clinical trial evaluating the performance and safety of platelets treated with MIRASOL pathogen reduction technology. Transfusion, 50, 2362–2375. [DOI] [PubMed] [Google Scholar]
- CDC (2018) National Healthcare Safety Network Biovigilance Component Hemovigilance Module Surveillance Protocol. v2.5.2 Centers for Disease Control and Prevention, Atlanta, GA: Available at: http://www.cdc.gov/nhsn/PDFs/Biovigilance/BV-HV-protocol-current.pdf. (Accessed 18 September 2018). [Google Scholar]
- Ellingson, K.D. , Sapiano, M.R.P. , Haass, K.A. , Savinkina, A.A. , Baker, M.L. , Chung, K.W. , Henry, R.A. , Berger, J.J. , Kuehnert, M.J. & Basavaraju, S.V. (2017) Continued decline in blood collection and transfusion in the United States‐2015. Transfusion, 57, 1588–1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estcourt, L.J. , Malouf, R. , Hopewell, S. , Trivella, M. , Doree, C. , Stanworth, S.J. & Murphy, M.F. (2017) Pathogen‐reduced platelets for the prevention of bleeding. Cochrane Database Systematic Review, 7, CD009072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAMHP (2016) Annual Hemovigilance Annual Reports 2006–2015 Federal Agency for Medicines and Health Product. Available at: https://www.famhp.be/en/human_use/health_products/blood_and_blood_products/haemovigilance/annual_reports. (Accessed Jun 21, 2019).
- FDA (2017) Fatalities Reported to FDA Following Blood Collection and Transfusion Annual Summary for FY2017 US Food and Drug Administration; Available at: https://www.fda.gov/media/124796/download. (Accessed 24 May 2019). [Google Scholar]
- FDA (2018) Bacterial Risk Control Strategies for Blood Collection Establishments and Transfusion Services to Enhance the Safety and Availability of Platelets for Transfusion FDA, Available at: https://www.fda.gov/media/119043/download. (Accessed 24 May 2019). [Google Scholar]
- Garban, F. , Guyard, A. , Labussiere, H. , Bulabois, C.E. , Marchand, T. , Mounier, C. , Caillot, D. , Bay, J.O. , Coiteux, V. , Schmidt‐Tanguy, A. , Le Niger, C. , Robin, C. , Ladaique, P. , Lapusan, S. , Deconinck, E. , Rolland, C. , Foote, A.M. , Francois, A. , Jacquot, C. , Tardivel, R. , Tiberghien, P. & Bosson, J.L. ; Evaluation of the Efficacy of Platelets Treated With Pathogen Reduction Process Study Group . (2018) Comparison of the hemostatic efficacy of pathogen‐reduced platelets vs untreated platelets in patients with thrombocytopenia and malignant hematologic diseases: a randomized clinical trial. JAMA Oncology, 4, 468–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haass, K.A. , Sapiano, M.R. , Savinkina, A. , Kuehnert, M.J. & Basavaraju, S.V. (2019) Transfusion‐transmitted infections reported to the national healthcare safety network hemovigilance module. Transfusion Medicine Reviews, 33, 84–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs, M.R. , Smith, D. , Heaton, W.A. , Zantek, N.D. & Good, C.E. ; PGD Study Group . (2011) Detection of bacterial contamination in prestorage culture‐negative apheresis platelets on day of issue with the Pan Genera Detection test. Transfusion, 51, 2573–2582. [DOI] [PubMed] [Google Scholar]
- Jenkins, C. , Ramirez‐Arcos, S. , Goldman, M. & Devine, D.V. (2011) Bacterial contamination in platelets: incremental improvements drive down but do not eliminate risk. Transfusion, 51, 2555–2565. [DOI] [PubMed] [Google Scholar]
- Jones, S.A. (2019) Sepsis attributed to bacterial contamination of platelets associated with a potential common source—multiple states, 2018. MMWR. Morbidity and Mortality Weekly Report, 68, 519–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jutzi, M. , Mansouri Taleghani, B. , Rueesch, M. , Amsler, L. & Buser, A. (2018) Nationwide implementation of pathogen inactivation for all platelet concentrates in Switzerland. Transfusion Medicine and Hemotherapy, 45, 151–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kacker, S. , Bloch, E.M. , Ness, P.M. , Gehrie, E.A. , Marshall, C.E. , Lokhandwala, P.M. & Tobian, A.A.R. (2019) Financial impact of alternative approaches to reduce bacterial contamination of platelet transfusions. Transfusion, 59, 1291–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerkhoffs, J.L. , van Putten, W.L. , Novotny, V.M. , Te Boekhorst, P.A. , Schipperus, M.R. , Zwaginga, J.J. , van Pampus, L.C. , de Greef, G.E. , Luten, M. , Huijgens, P.C. , Brand, A. , van Rhenen, D.J. ; Dutch ‐ Belgian HOVON Cooperative Group . (2010) Clinical effectiveness of leucoreduced, pooled donor platelet concentrates, stored in plasma or additive solution with and without pathogen reduction. British Journal of Haematology, 150, 209–217. [DOI] [PubMed] [Google Scholar]
- Klein, H.G. , Anderson, D. , Bernardi, M.J. , Cable, R. , Carey, W. , Hoch, J.S. , Robitaille, N. , Sivilotti, M.L. & Smaill, F. (2007) Pathogen inactivation: making decisions about new technologies. Report of a consensus conference. Transfusion, 47, 2338–2347. [DOI] [PubMed] [Google Scholar]
- Knutson, F. , Osselaer, J. , Pierelli, L. , Lozano, M. , Cid, J. , Tardivel, R. , Garraud, O. , Hervig, T. , Domanovic, D. , Cukjati, M. , Gudmundson, S. , Hjalmarsdottir, I.B. , Castrillo, A. , Gonzalez, R. , Brihante, D. , Santos, M. , Schlenke, P. , Elliott, A. , Lin, J.S. , Tappe, D. , Stassinopoulos, A. , Green, J. & Corash, L. (2015) A prospective, active haemovigilance study with combined cohort analysis of 19,175 transfusions of platelet components prepared with amotosalen‐UVA photochemical treatment. Vox Sanguinis, 109, 343–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen, C.P. , Ezligini, F. , Hermansen, N.O. & Kjeldsen‐Kragh, J. (2005) Six years' experience of using the BacT/ALERT system to screen all platelet concentrates, and additional testing of outdated platelet concentrates to estimate the frequency of false‐negative results. Vox Sanguinis, 88, 93–97. [DOI] [PubMed] [Google Scholar]
- Lozano, M. , Knutson, F. , Tardivel, R. , Cid, J. , Maymo, R.M. , Lof, H. , Roddie, H. , Pelly, J. , Docherty, A. , Sherman, C. , Lin, L. , Propst, M. , Corash, L. & Prowse, C. (2011) A multi‐centre study of therapeutic efficacy and safety of platelet components treated with amotosalen and ultraviolet A pathogen inactivation stored for 6 or 7 d prior to transfusion. British Journal of Haematology, 153, 393–401. [DOI] [PubMed] [Google Scholar]
- Lozano, M. , Cid, J. , Prowse, C. , McCullough, J. , Klein, H.G. & Aubuchon, J.P. (2015) Pathogen inactivation or pathogen reduction: proposal for standardization of nomenclature. Transfusion, 55, 690. [DOI] [PubMed] [Google Scholar]
- McCullough, J. , Vesole, D.H. , Benjamin, R.J. , Slichter, S.J. , Pineda, A. , Snyder, E. , Stadtmauer, E.A. , Lopez‐Plaza, I. , Coutre, S. , Strauss, R.G. , Goodnough, L.T. , Fridey, J.L. , Raife, T. , Cable, R. , Murphy, S. , Howard, F.T. , Davis, K. , Lin, J.S. , Metzel, P. , Corash, L. , Koutsoukos, A. , Lin, L. , Buchholz, D.H. & Conlan, M.G. (2004) Therapeutic efficacy and safety of platelets treated with a photochemical process for pathogen inactivation: the SPRINT Trial. Blood, 104, 1534–1541. [DOI] [PubMed] [Google Scholar]
- McCullough, J. , Goldfinger, D. , Gorlin, J. , Riley, W.J. , Sandhu, H. , Stowell, C. , Ward, D. , Clay, M. , Pulkrabek, S. , Chrebtow, V. & Stassinopoulos, A. (2015) Cost implications of implementation of pathogen‐inactivated platelets. Transfusion, 55, 2312–2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPadden, J. , Durant, T.J. , Bunch, D.R. , Coppi, A. , Price, N. , Rodgerson, K. , Torre, C.J. Jr , Byron, W. , Hsiao, A.L. , Krumholz, H.M. & Schulz, W.L. (2019) Health care and precision medicine research: analysis of a scalable data science platform. Journal of Medical Internet Research 21, e13043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prioli, K.M. , Karp, J.K. , Lyons, N.M. , Chrebtow, V. , Herman, J.H. & Pizzi, L.T. (2018) Economic implications of pathogen reduced and bacterially tested platelet components: a US hospital budget impact model. Applied Health Economics and Health Policy, 16, 889–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebulla, P. , Vaglio, S. , Beccaria, F. , Bonfichi, M. , Carella, A. , Chiurazzi, F. , Coluzzi, S. , Cortelezzi, A. , Gandini, G. , Girelli, G. , Graf, M. , Isernia, P. , Marano, G. , Marconi, M. , Montemezzi, R. , Olivero, B. , Rinaldi, M. , Salvaneschi, L. , Scarpato, N. , Strada, P. , Milani, S. & Grazzini, G. (2017) Clinical effectiveness of platelets in additive solution treated with two commercial pathogen‐reduction technologies. Transfusion, 57, 1171–1183. [DOI] [PubMed] [Google Scholar]
- van Rhenen, D. , Gulliksson, H. , Cazenave, J.P. , Pamphilon, D. , Ljungman, P. , Kluter, H. , Vermeij, H. , Kappers‐Klunne, M. , de Greef, G. , Laforet, M. , Lioure, B. , Davis, K. , Marblie, S. , Mayaudon, V. , Flament, J. , Conlan, M. , Lin, L. , Metzel, P. , Buchholz, D. & Corash, L. (2003) Transfusion of pooled buffy coat platelet components prepared with photochemical pathogen inactivation treatment: the euroSPRITE trial. Blood, 101, 2426–2433. [DOI] [PubMed] [Google Scholar]
- Rutter, S. & Snyder, E.L. (2019) How do we … integrate pathogen reduced platelets into our hospital blood bank inventory? Transfusion, 59, 1628–1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salunkhe, V. , van der Meer, P.F. , de Korte, D. , Seghatchian, J. & Gutierrez, L. (2015) Development of blood transfusion product pathogen reduction treatments: a review of methods, current applications and demands. Transfusion and Apheresis Science, 52, 19–34. [DOI] [PubMed] [Google Scholar]
- Schulz, W.L. , McPadden, J. , Gehrie, E.A. , Bahar, B. , Gokhale, A. , Ross, R. , Price, N. , Spencer, B.R. & Snyder, E. (2019) Blood utilization and transfusion reactions in pediatric patients transfused with conventional or pathogen reduced platelets. The Journal of Pediatrics, 209, 220–225. [DOI] [PubMed] [Google Scholar]
- Seltsam, A. (2017) Pathogen inactivation of cellular blood products‐an additional safety layer in transfusion medicine. Frontiers in Medicine 4, 219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHOT . (2019) Serious Hazards of Transfusion annual reports and summaries 1996–2017. Available at: https://www.shotuk.org/shot-reports/. (Accessed 20 June 2019).
- WHO . (2017) Global Status Report on Blood Safety and Availability 2016. World Health Organization; Available at: https://apps.who.int/iris/handle/10665/254987. (Accessed 27 February 2017). [Google Scholar]


