Abstract
The metabolic basis for observed differences in the yield response of rice to projected carbon dioxide concentrations (CO2) is unclear. In this study, three rice cultivars, differing in their yield response to elevated CO2, were grown under ambient and elevated CO2 conditions, using the free‐air CO2 enrichment technology. Flag leaves of rice were used to determine (1) if manipulative increases in sink strength decreased the soluble sucrose concentration for the ‘weak’ responders and (2), whether the genetic expression of sucrose transporters OsSUT1 and OsSUT2 was associated with an accumulation of soluble sugars and the maintenance of photosynthetic capacity. For the cultivars that showed a weak response to additional CO2, photosynthetic capacity declined under elevated CO2 and was associated with an accumulation of soluble sugars. For these cultivars, increasing sink relative to source strength did not increase photosynthesis and no change in OsSUT1 or OsSUT2 expression was observed. In contrast, the ‘strong’ response cultivar did not show an increase in soluble sugars or a decline in photosynthesis but demonstrated significant increases in OsSUT1 and OsSUT2 expression at elevated CO2. Overall, these data suggest that the expression of the sucrose transport genes OsSUT1 and OsSUT2 may be associated with the maintenance of photosynthetic capacity of the flag leaf during grain fill; and, potentially, greater yield response of rice as atmospheric CO2 increases.
Abbreviations
- FACE
free‐air CO2 enrichment
- RT‐PCR
reverse transcription‐PCR
- WYJ23
Wuyunjing 23
- YD6
Yangdao 6
Introduction
Based on the continual record of atmospheric CO2 measured in Mauna Loa, Hawaii, atmospheric CO2 has increased ∼30% (from 315 to 405 µmol mol−1) since the mid‐1950s (https://www.esrl.noaa.gov/gmd/ccgg/trends/). Although CO2 as a ‘greenhouse gas’ is well recognized, increases in CO2 have also been shown in multiple studies to stimulate photosynthesis, growth, fertility and yield of numerous C3 crop species, including rice (Baker et al. 1990, Zhu et al. 2012).
However, the degree of stimulation varies depending on the functional level studied. For example, leaf photosynthetic rates can be stimulated by elevated CO2, but the extent of photosynthetic stimulation does not necessarily translate into proportional increases in seed yield (Long et al. 2006, Ainsworth et al. 2008, Leakey et al. 2009). This may, in part, be due to a temporal decline in the photosynthetic rate, as the elevated CO2 treatment is extended. This is a common phenomenon within C3 plants that is referred to as photosynthetic acclimation or downregulation (Chen et al. 2005, Kant et al. 2012). The basis for downregulation may be related to CO2‐induced excess photosynthate accumulation in leaves, if sinks for the additional carbon are not available (Stitt 1991, Moore et al. 1999, Haouari et al. 2013, Campany et al. 2017). However, the role of sucrose, the main transporting form of fixed photosynthetic carbon in leaves, is not entirely understood. Specifically, whether the additional photosynthate acquired at elevated CO2 is accumulating not only because of sink limitations, but also because of biochemical limits to transport sucrose out of the leaf.
It is generally recognized that there is significant variation to elevated CO2 and seed yield stimulation among rice genotypes (Hasegawa et al. 2013, Wang et al. 2016a, b). The link between temporal duration of photosynthetic stimulation with elevated CO2 (i.e. lack of downregulation) and the observed stimulation of seed yield is therefore a matter of interest in selecting for greater seed yield responsiveness to rising atmospheric CO2 among rice lines.
In situ assessments of rice to elevated CO2 using free‐air CO2 enrichment (FACE) have demonstrated that genotypes with greater yield response to CO2 also had bigger panicles and additional spikelets relative to genotypes with a smaller yield response, suggesting bigger sink capacity (Hasegawa et al. 2013, Zhu et al. 2015). This indicated a potential increase in the sink:source ratio, and an enhanced capacity to accommodate additional photosynthate and avoid downregulation during grain development under elevated CO2 conditions (Zhu et al. 2014). However, the role of sucrose transport per se under elevated CO2 was not examined.
To determine if a mechanistic link between leaf photosynthetic acclimation and sucrose transportation exists among rice lines differing in yield stimulation to elevated CO2, two ‘weak’ cultivars, Wuyunjing 23 (WYJ23) and Nanjing 9108 (NG9108; both ∼10% increase in seed yield at elevated CO2) were compared to a ‘strong’ cultivar [Yangdao 6, (YD6); ∼30% increase in seed yield at elevated CO2; Zhu et al. 2014]. Our objectives were to determine: (1) if an increase in the sink to source ratio (by removal of source leaves 2 and 3 below the flag leaf) mitigated photosynthetic downregulation in the ‘weak’ cultivars and (2), whether the occurrence of photosynthetic downregulation to elevated CO2 was associated with changes in the expression of genes associated with sucrose transportation.
Materials and methods
Experimental site description
The study was conducted at the FACE platform located in Zongcun village (32°35′5″N, 119°42′0″E), Yangzhou city, Jiangsu province in Eastern China. This location represents a typical rice‐wheat rotation system within a subtropical marine climatic zone (Zhu et al. 2012). The soil is classified as a Shajiang‐Aquic Cambiosol with a sandy loam texture. Operational details for the FACE system at this location have been described previously (Okada et al. 2001). It consists of three identical 17‐m‐diameter octagonal rings with the CO2 at the center of each ring ∼200 µmol mol−1 higher than at ambient conditions (representing elevated CO2 conditions) and three comparison rings without supplemental CO2 (representing ambient CO2 conditions). During the seasons in 2014 and 2015, the average daytime CO2 values were 394 and 590 µmol mol−1 and 395 and 588 µmol mol−1 for the ambient and elevated FACE rings, respectively. The average air temperature from planting to harvest was 22.1 and 24.8°C for 2014 and 2015, respectively.
Rice cultivation and sample pre‐treatment
Based on their relative yield responses to enhanced CO2, three rice cultivars, WYJ23, Nanjing 9108 (NG9108) (Japonica) and YD6 (Indica), were selected. Selection was based on their differential yield responses to elevated CO2, with WYJ23 and NG9108 demonstrating weaker stimulation relative to YD6 (ca 10 vs. 30%, respectively, Table 1; Zhu et al. 2015). Seeds of each variety were sown at ambient CO2 in late May, 2014 and 2015, and seedlings were manually transplanted to ambient and elevated rings on June 21 and June 17 for 2014 and 2015, respectively. Two seedlings per hill with 24 hills per m2 were the planting density for all six rings. Phosphorous (P) and potassium (K) were applied as compound fertilizers at 9 g P2O5 m−2 and 9 g K2O m−2, using a basal dressing 1 day before transplanting. Nitrogen (N, at 22.5 g N m−2 each season) was applied as a basal dressing (40% of the seasonal total), 1 day prior to transplanting and as a top dressing at early tillering (30% of the seasonal total) and again at the panicle initiation stage (30% of the seasonal total).
Table 1.
Effects of FACE on three rice cultivars, WYJ23, NG9108 and YD6 over two growth seasons (2014 and 2015). % Change is relative difference at elevated to ambient CO2. Values are means of three replicates. Values and statistics are from Zhu et al. 2015. ns, not significant.
| Data from 2014 growing season and the data of YD6 combined from Zhu et al. (2015) | ||||||
|---|---|---|---|---|---|---|
| Variety | CO2 | Panicle number (m−2) | Spikelets per panicle | Filled pikelet ratio | Weight per grain | Yield (g m−2) |
| WYJ23 | % Change | 14.5 | −7.1 | 3.4 | 1.8 | 12.4 ns |
| YD6 | % Change | 11.2 | 6.8 | 5.9 | 2.3 | 29.6* |
| Data from 2015 growing season | ||||||
| Variety | CO2 | Panicle number (m−2) | Spikelets per panicle | Filled pikelet ratio | Weight per grain | Yield |
| WYJ23 | % Change | 13.0 | −6.0 | 2.9 | 0.0 | 11.0 ns |
| NG9108 | % Change | 12.3 | −5.4 | 1.2 | 1.4 | 9.1 ns |
| YD6 | % Change | 10.6 | 11.5 | 2.6 | 3.1 | 29.5* |
At the heading stage in each CO2 treatment, two tillers of WYJ23 and NG9108 (i.e. the weaker CO2 response cultivars), were chosen and tagged in all replicates, and the 2nd and 3rd leaves were removed from one of the tillers to increase the sink:source ratio in 2014 and 2015. It has been shown that genotypes with greater response to CO2 have an adequate sink capacity (Hasegawa et al. 2013, Zhu et al. 2015). Therefore, leaves were not removed for YD6, and comparisons were made between flag leaves of the different cultivars.
Photosynthesis gas exchange measurements
Measurements of leaf net photosynthesis were conducted in situ during the grain filling stage for each cultivar, using a portable photosynthesis system equipped with blue and red LED light sources (LI‐6400; LI‐COR, Lincoln). Photosynthesis measurements began at grain fill and continued for a 2‐day period: September 19–21 for WYJ23 and YD6 in 2014, September 13, 14 for NG9108 and September 17, 18 for WYJ23 and September 8, 9 for YD6 in 2015. Measurements were made at a saturating photosynthetic photon flux density of 1800 µmol m−2 s−1. Leaf temperature was set to 30°C and air flow rate was set to 500 µmol s−1.
Sampling and biochemical analyses
Following determination of leaf photosynthesis, during the first 2 days of grain filling, two of the measured flag leaves from all cultivars and experimental treatments were sampled from 9:30–14:30 (Beijing time). Both leaves were stored in liquid nitrogen until analysis. Chosen tillers were divided into panicle and flag leaf for dry weight (at 80°C for 72 h), and then flag leaves were ground to determine soluble sugar and nitrogen content as published in Olano et al. (2006).
An anthrone colorimetric method was used to measure the concentration of soluble sugars (Buysse and Merckx 1993). Leaf tissue nitrogen concentration was measured using an elemental (Carbon‐Hydrogen‐Nitrogen) analyzer (PE2400 series II CHNS/O).
The flag leaves (stored in liquid N) were used to quantify sucrose transport genetics using an established procedure (Lin 2010, Wang et al. 2016a, b): 1 µg of total RNA treated with DNase I (TaKaRa) was used for reverse transcription‐PCR (RT‐PCR). RT was performed using PrimeScript TM RT Master Mix (TaKaRa). PCR was performed at 37°C for 15 min, 85°C for 5 s and cDNA was stored at 4°C. Quantitative RT‐PCR was carried out on a CFX96 real‐time PCR system (Bio‐Rad Laboratories, Hercules) using the SYBR Premix Ex Taq TM (TaKaRa) with 35 cycles of 95°C for 5 s and 60°C for 30 s. Gene expression data analysis included normalizing of OsSUT1 and OsSUT2 Ct values to the housekeeping gene Rac 1 (X16280.1). The expression levels of OsSUT1 and OsSUT2 were calculated as E‐ΔΔCt (analysis in sequence; OsSUT1: F‐5′ CTGTGATTTTCCTGTCCCTG 3′ and R‐5′ AACACTGCTAGTGGACCAGT 3′, OsSUT2: F‐5′ AGGAGGAGAGGTCACCGATAA 3′ and R‐5′ CCAACATCCAATGTACAACAGCA 3′) and the primer sequences mentioned before were used in this PCR study. Quantitative expression of these genes was used to represent sucrose transport capacity in the current study. Housekeeping gene primer sequences were: Rac 1: F‐5′ GTACCCGCATCAGGCATCT 3′ and R‐5′ TCCATCTTGGCATCTCTCAG 3′.
Statistical analysis
Data were analyzed using the SPSS statistical software (SPSS 19.0; SPSS Inc.) and Excel 2016 for Windows 10. The CO2 treatments (ambient and elevated) were analyzed as a randomized complete block, and the sink:source manipulation (removal of leaves) was analyzed as a split‐plot treatment. Each treatment group consisted of three replicates. Analysis of variance (anova) was used to test for significant treatment differences.
Results
Yield components and single‐panicle weight
YD6 showed a consistently greater yield response than WYJ23 and NG9108 at elevated CO2 (Table 1). Among the yield components examined, the effect of elevated CO2 was positive for spikelets per panicle in YD6, while negative in WYJ23 and NG9108. Leaf removal and enhanced sink:source ratio consistently, but not significantly (i.e. P was 0.095 and 0.068 for WYJ23 and NG9108, respectively) lowered single‐panicle dry weight in response to elevated atmospheric CO2 (Table 2).
Table 2.
Effects of sink:source treatment on single‐panicle dry weight for WYJ23 and NG9108 under elevated CO2 in 2014 and 2015. ‘Enhanced’ indicates the increased sink:source ratio through leaf removal, and the unaltered sink:source ratio is represented by ‘Control’. Values are mean of three replicates. P > 0.1; †P ≤ 0.1; *P ≤ 0.05; **P ≤ 0.001.
| Variety | Sink:source | Single‐panicle dry weight (g) | |
|---|---|---|---|
| WYJ23 (2014) | Control | 1.314† | |
| Enhanced | 1.176 | ||
| WYJ23 (2015) | Control | 2.231† | |
| Enhanced | 2.068 | ||
| NG9108 (2015) | Control | 2.733† | |
| Enhanced | 2.573 | ||
| anova result | WYJ23 (2014) | WYJ23 (2015) | NG9108 (2015) |
| P‐Value | 0.088 | 0.095 | 0.068 |
Leaf net photosynthesis and photochemistry
At elevated CO2 conditions, relative to ambient CO2, significant photosynthetic downregulation was observed for WYJ23 and NG9108 (Fig. 1A, C, D). Increasing sink:source ratios through leaf removal did not negate photosynthetic downregulation for these cultivars. In contrast, for YD6, net photosynthetic rate showed no downregulation in response to elevated CO2 (Fig. 1B, E).
Figure 1.
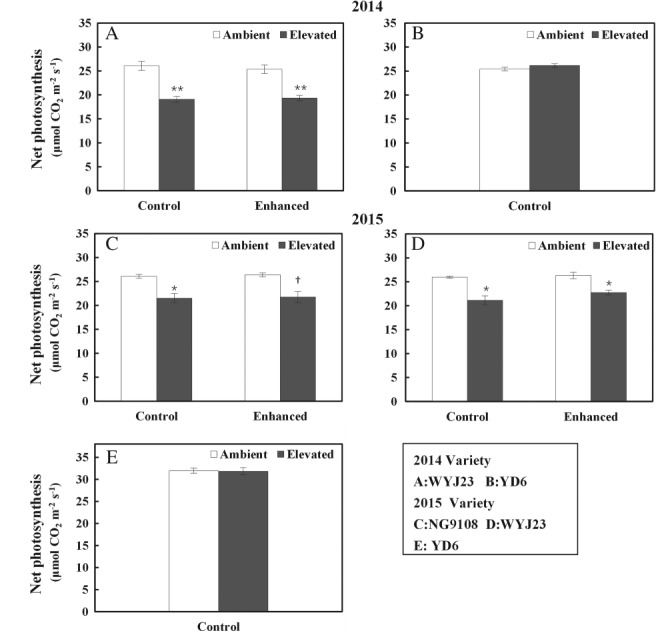
Net photosynthesis rate (µmol CO2 m−2 s−1) of flag leaves for three rice cultivars, WYJ23, NG9108 and YD6 grown at ambient and elevated CO2 in each sink:source treatment. Measurements were made at the same CO2 condition (590 µmol mol−1). ‘Enhanced’ indicates the increased sink:source ratio through leaves removal and the unaltered sink:source ratio is represented by ‘Control’. Bars represent average values of three replicates with standard errors. Symbols indicate significant differences in sink:source treatment for each cultivar as a function of CO2 treatment. ns, not significant. P > 0.1; †P ≤ 0.1; *P ≤ 0.05; **P ≤ 0.001.
Consistent with downregulation, a decrease in the photosynthetic rate of the flag leaf was associated with a significant decline in leaf N concentration at elevated CO2 conditions (Table 3). This decline was observed for WYJ23 and NG9108 and was not altered by sink:source manipulation (Table 3). In contrast, YD6 did not show any significant change in leaf N concentration (Table 3).
Table 3.
Nitrogen content of flag leaves for the rice cultivars, WYJ23, NG9108 and YD6 in each treatment. Values are the average of three replicates of each treatment. ‘Enhanced’ indicates the increased sink:source ratio through leaves removal, and the unaltered sink:source ratio is represented by ‘Control’. Two‐way anova for CO2 and sink:source treatment is used in WYJ23 and NG9108 and one‐way anova for CO2 treatment is used in YD6. E/A, Elevated/Ambient; ‐, no data; ns, not significant. P > 0.1; †P ≤ 0.1; *P ≤ 0.05; **P ≤ 0.001.
| Year | Variety | CO2 | Sink:source | N (%) |
|---|---|---|---|---|
| 2014 | WYJ23 | Ambient | Control | 2.39 |
| Elevated | Control | 2.09 | ||
| Changes (E/A) | −12.7 | |||
| Ambient | Enhanced | 2.47 | ||
| Elevated | Enhanced | 2.28 | ||
| Changes (E/A) | −7.7 | |||
| YD6 | Ambient | Control | 2.56 | |
| Elevated | Control | 2.41 | ||
| Changes (E/A) | −5.9 | |||
| 2015 | WYJ23 | Ambient | Control | 2.24 |
| Elevated | Control | 1.96 | ||
| Changes (E/A) | −12.7 | |||
| Ambient | Enhanced | 2.18 | ||
| Elevated | Enhanced | 1.94 | ||
| Changes (E/A) | −11.0 | |||
| NG9108 | Ambient | Control | 2.19 | |
| Elevated | Control | 1.83 | ||
| Changes (E/A) | −16.6 | |||
| Ambient | Enhanced | 2.19 | ||
| Elevated | Enhanced | 1.86 | ||
| Changes (E/A) | Control | −15.1 | ||
| YD6 | Ambient | Control | 2.35 | |
| Elevated | Control | 2.14 | ||
| Changes (E/A) | −8.6 | |||
| anova result | N (%) | |||
| WYJ23 | NG9108 | YD6 | ||
| 2014 | CO2 | * | ‐ | ns |
| Sink:source | ns | ‐ | ‐ | |
| CO2 × sink:source | ns | ‐ | ‐ | |
| 2015 | CO2 | * | * | ns |
| Sink:source | ns | ns | ‐ | |
| CO2 × sink:source | ns | ns | ‐ | |
Soluble sugars accumulation and OsSUTs expression
At elevated CO2 conditions, a significant increase in leaf soluble sugar concentrations for WYJ23 and NG9108 with and without removal of additional source leaves was measured (Fig. 2). In contrast, no change in soluble sugar concentration in the flag leaf was observed for YD6. No significant differences in leaf soluble sugar concentration were observed for WYJ123 or NG9108 as a function of CO2 concentration (Fig. 2A, C, D).
Figure 2.
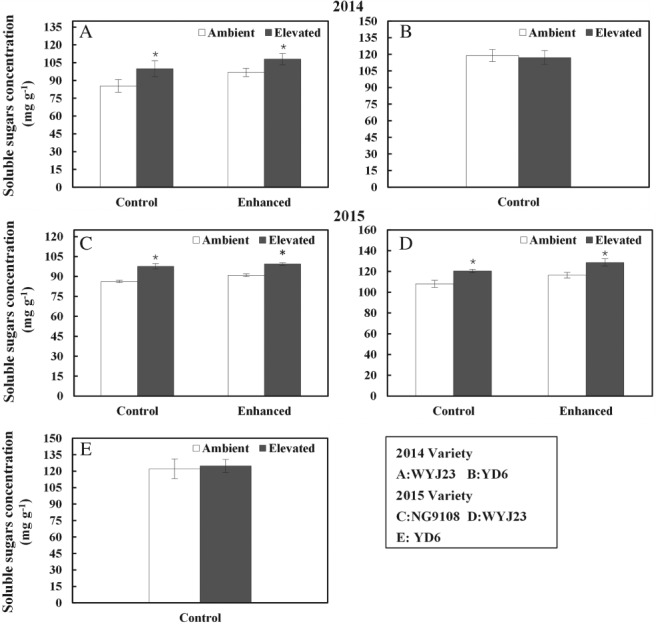
Soluble sugars concentration in flag leaves for three rice cultivars, WYJ23, NG9108 and YD6 grown at ambient and elevated CO2 in combination with sink:source treatments. ‘Enhanced’ indicates the increased sink:source ratio through leaves removal, and the unaltered sink:source ratio is represented by ‘Control’. Bars represent average values of three replicates with standard errors. Symbols indicate the significant difference in a given sink:source treatment for each cultivar as a function of CO2 treatment. ns, not significant. P > 0.1; †P ≤ 0.1; *P ≤ 0.05; **P ≤ 0.001.
OsSUT1 and OsSUT2 represent the sucrose transport genes for rice and are characterized as necessary for sucrose export from source leaves. For YD6, OsSUT1 and OsSUT2 (OsSUTs) expression increased significantly in response to elevated CO2. In contrast, the enhanced source treatment or elevated CO2 had no effect on OsSUTs expression for WYJ23 and NG9108 (Fig. 3).
Figure 3.
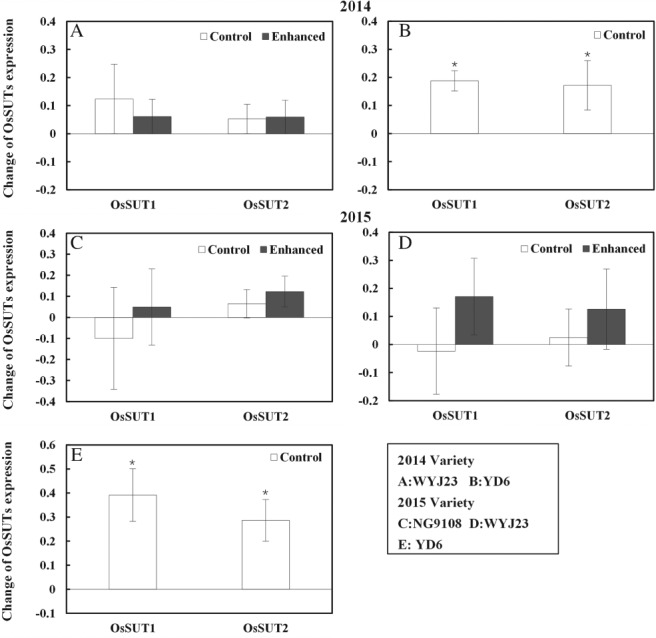
Change of OsSUT1 and OsSUT2 (OsSUTs) expression level of flag leaves under elevated CO2 for three rice cultivars in each sink:source treatment. ‘Enhanced’ indicates the increased sink:source ratio through leaves removal, and the unaltered sink:source ratio is represented by ‘Control’. Bars represent the average (E‐A)/A (relative change at elevated CO2 to those at ambient CO2) of three replicates for OsSUTs expression level with relative standard errors. Symbols indicate the significant difference for the gene expression as a function of CO2 treatment. ns, not significant. P > 0.1; †P ≤ 0.1; *P ≤ 0.05; **P ≤ 0.001.
The elevated/ambient CO2 ratios of soluble sugars and OsSUT expression were analyzed for all cultivars, treatments and years. The soluble sugar ratio was negatively correlated with the OsSUTs expression ratio (Fig. 4, P < 0.001). This suggested that if OsSUT expression was insufficient, soluble sugars would accumulate under elevated CO2.
Figure 4.
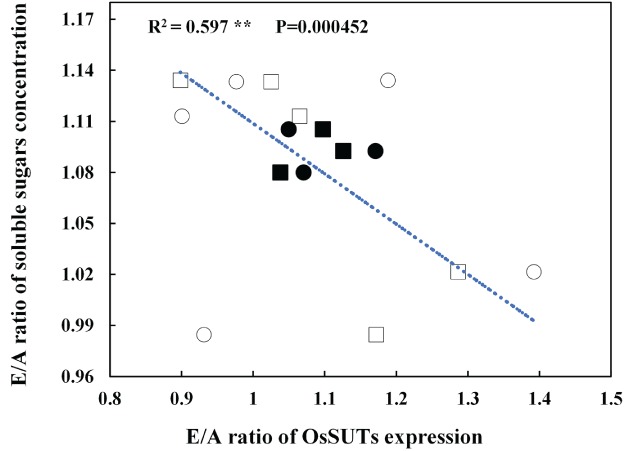
Relationship between E/A (relative values at elevated CO2 to those at ambient CO2) ratio of OsSUTs expression level and E/A ratio of soluble sugars concentration in flag leaves. Values are the average of three replicates. Circles represent the values of OsSUT1 expression level, and boxes represent the values of OsSUT2 expression level. The open symbols indicate the values of control sink:source treatment, and the solid symbols indicate the values of enhanced sink:source treatment. R2 = 0.591, P‐value = 0.000452, **P ≤ 0.001.
Discussion
When grown at projected, elevated levels of CO2, there is consistent intraspecific variation among crop cultivars in growth and yield, some showing a strong stimulation of yield, others little or no stimulation (Ziska et al. 2014, Bishop et al. 2015) Understanding the basis for this variation may be essential in identifying those cultivars that can convert additional CO2 into greater seed yield.
While the basis for intraspecific variation is likely to be multifactorial, photosynthetic capacity over time is of obvious importance. Under elevated CO2 conditions, inadequate sinks for additional carbon may result in a surplus accumulation of photosynthate at the leaf level, with eventual downregulation of photosynthesis (Lin et al. 1997, Shimono and Okada 2013, Ziska et al. 2014, Burnett et al. 2016, Ruiz‐Vera et al. 2017). This had been reported for numerous C3 crop species including rice (Ono et al. 2003, Zhu et al. 2014).
At present, the role of sucrose transport in feedback inhibition of photosynthesis is unclear. Sucrose transport, an essential part in the carbohydrate distribution process, can be sensitive to environmental changes, e.g. cold or heat, with consequences for photoassimilate distribution and photosynthetic downregulation (Takahashi et al. 2017, Zhou et al. 2017). However, it is uncertain whether the capacity of sucrose export from source leaves is related to the overall photosynthetic response to elevated CO2.
Is the extent of downregulation and/or expression of sucrose transport related to a relative yield stimulation among rice cultivars in response to additional CO2? In this study, YD6 had a higher (∼twofold) yield response relative to cultivars WYJ23 and NG9108 at elevated CO2 under field conditions. The yield responses are, in general, in agreement with the observed changes in the source:sink and photosynthetic downregulation for the cultivars WYJ23 and NG9108.
It is interesting to note that when sink limitation was diminished by increasing the ratio of carbon sinks to source for these two cultivars, photosynthetic downregulation was still observed (Table 2, Fig. 1). This suggested that eliminating sink limitation per se did not mitigate photosynthetic downregulation under elevated CO2 conditions. Rather, it suggested that additional factors could be involved, including sucrose transport capacity. For example, sucrose transporter genes OsSUT1 and OsSUT2 have been reported to play an essential role in the sucrose apoplastic loading into the phloem (Aoki et al. 2003, Eom et al. 2011, Braun et al. 2014, Chen et al. 2015).
At elevated CO2 conditions, the enhancement in gene expression of OsSUT1 and OsSUT2 was negatively correlated with soluble sugar accumulation (Fig. 4), consistent with previous research on chilling temperatures (Takahashi et al. 2017). In the current experiment, the relative variation in gene expression among the three lines, relative to yield stimulation, is of interest in the context of CO2. In YD6 e.g. additional photosynthate did not accumulate in the source leaves and photosynthetic downregulation was not observed at elevated CO2 conditions. Conversely, even without the sink restriction, gene expression of OsSUT1 and OsSUT2 was not upregulated significantly under elevated CO2. Overall, the change in gene expression was inversely proportional to the accumulation of photosynthates at elevated CO2 among the examined cultivars.
For this study, the decrease in leaf‐nitrogen concentration of WYJ23 and NG9108 (japonica) was greater than YD6 (indica) at elevated relative to ambient CO2. There are other noted differences between japonica and indica in regard to stomatal conductance, root size and nitrogen distribution (Kant et al. 2012, Shimoda and Maruyama 2014, Muryono et al. 2017). It is possible that insufficient sucrose transport under elevated CO2 may be associated with the relative N shortage. For example, N deficiency could alter the distribution of sucrose across plant organs (Lemoine et al. 2013). In addition, sugar accumulation in functional leaves can inhibit SUT expression and activity (Chiou and Bush 1998, Cordoba et al. 2015). However, additional indica and japonica comparisons would be necessary to validate the role of nitrogen in sucrose gene expression at elevated CO2.
Overall, the relative stimulation of yield at elevated CO2 was correlated with a lack of photosynthetic downregulation that in turn reflected higher expression levels of OsSUT1 and OsSUT2 in this study. While a wider array of rice cultivars needs to be examined to confirm these results, these initial data indicate that stimulation of sucrose transport genes during grain filling could be associated with greater yield sensitivity to rising CO2. Given the global importance of rice in the context of future food security, any mechanism that can enhance the conversion of additional CO2 into seed yield would be of interest in that regard.
Author contributions
J.S.Z., D.F.L. and X.X. performed the experiments and drafted the manuscript. C.W.Z. conceived the study. C.W.Z., L.H.Z, J.G.Z. and G.L. participated in its design. J.S.Z., D.F.L., C.W.Z. and L.H.Z. edited the manuscript. All authors declare that they have no conflict of interest.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (31870423), the Natural Science Foundation of Jiangsu province in China (BK2018402), Youth Innovation Promotion Association CAS (Member No. 2015248) and the frontier projects for the 13th five‐year plan of CAS (Y613890000) to Dr. C.W.Z. The FACE system instruments were supplied by the National Institute of Agro‐Environmental Sciences and the Agricultural Research Center of Tohoku Region (Japan).
References
- Ainsworth EA, Leakey ADB, Ort DR, Long SP (2008) FACE‐ing the facts: inconsistencies and interdependence among field, chamber and modeling studies of elevated CO2 impacts on crop yield and food supply. New Phytol 179: 5–9 [DOI] [PubMed] [Google Scholar]
- Aoki N, Hirose T, Scofield GN, Whitfeld PR, Furbank RT (2003) The sucrose transporter gene family in rice. Plant Cell Physiol 44: 223–232 [DOI] [PubMed] [Google Scholar]
- Baker JT, Allen LH Jr, Boote KJ (1990) Growth and yield responses of rice to carbon dioxide concentration. J Ag Sci 115: 313–320 [Google Scholar]
- Bishop KA, Betzelberger AM, Long SP, Ainsworth EA (2015) Is there potential to adapt soybean (Glycine maxMerr.) to future CO2? An analysis of the yield response of 18 genotypes in free‐air CO2 enrichment. Plant Cell Environ 38: 1765–1774 [DOI] [PubMed] [Google Scholar]
- Braun DM, Wang L, Ruan YL (2014) Understanding and manipulating sucrose phloem loading, unloading, metabolism, and signalling to enhance crop yield and food security. J Exp Bot 65: 1713–1735 [DOI] [PubMed] [Google Scholar]
- Burnett AC, Rogers A, Rees M, Osborne CP (2016) Carbon source‐sink limitations differ between two species with contrasting growth strategies. Plant Cell Environ 39: 2460–2472 [DOI] [PubMed] [Google Scholar]
- Buysse J, Merckx R (1993) A improved colorimetric method to quantify sugar content of of plant tissue. J Exp Bot 44: 1627–1629 [Google Scholar]
- Campany CE, Medlyn BE, Duursma RA (2017) Reduced growth due to belowground sink limitation is not fully explained by reduced photosynthesis. Tree Physiol 37: 1042–1054 [DOI] [PubMed] [Google Scholar]
- Chen GY, Yong ZH, Liao Y, Zhang DY, Chen Y, Zhang HB, Chen J, Zhu JG, Xu DQ (2005) Photosynthetic acclimation in rice leaves to free‐air CO2 enrichment related to both ribulose‐1,5‐bisphosphate carboxylation limitation and ribulose‐1,5‐bisphosphate regeneration limitation. Plant Cell Physiol 46: 1036–1045 [DOI] [PubMed] [Google Scholar]
- Chen L‐Q, Cheung LS, Feng L, Tanner W, Frommer WB (2015) Transport of sugars. Annu Rev Biochem 84: 865–894 [DOI] [PubMed] [Google Scholar]
- Chiou TJ, Bush DR (1998) Sucrose is a signal molecule in assimilate partitioning. Proc Natl Acad Sci USA 95: 4784–4788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordoba E, Aceves‐Zamudio DL, Hernandez‐Bernal AF, Ramos‐Vega M, Leon P (2015) Sugar regulation of SUGAR TRANSPORTER PROTEIN 1 (STP1) expression in Arabidopsis thaliana . J Exp Bot 66: 147–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eom JS, Cho JI, Reinders A, Lee SW, Yoo Y, Tuan PQ, Choi SB, Bang G, Park YI, Cho MH, Bhoo SH (2011) Impaired function of the tonoplast‐localized sucrose transporter in rice, OsSUT2, limits the transport of vacuolar reserve sucrose and affects plant growth. Plant Physiol 157: 109–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haouari A, Van Labeke M‐C, Steppe K, Mariem FB, Braham M, Chaieb M (2013) Fruit thinning affects photosynthetic activity, carbohydrate levels, and shoot and fruit development of olive trees grown under semiarid conditions. Funct Plant Biol 40: 1179–1186 [DOI] [PubMed] [Google Scholar]
- Hasegawa T, Sakai H, Tokida T, Nakamura H, Zhu C, Usui Y, Yoshimoto M, Fukuoka M, Wakatsuki H, Katayanagi N, Matsunami T (2013) Rice cultivar responses to elevated CO2 at two free‐air CO2 enrichment (FACE) sites in Japan. Funct Plant Biol 40: 148–159 [DOI] [PubMed] [Google Scholar]
- Kant S, Seneweera S, Rodin J, Materne M, Burch D, Rothstein SJ, Spangenberg G (2012) Improving yield potential in crops under elevated CO2: integrating the photosynthetic and nitrogen utilization efficiencies. Front Plant Sci 3: 162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leakey ADB, Ainsworth EA, Bernacchi CJ, Rogers A, Long SP, Ort DR (2009) Elevated CO2 effects on plant carbon, nitrogen, and water relations: six important lessons from FACE. J Exp Bot 60: 2859–2876 [DOI] [PubMed] [Google Scholar]
- Lemoine R, La Camera S, Atanassova R, Dédaldéchamp F, Allario T, Pourtau N, Bonnemain JL, Laloi M, Coutos‐Thévenot P, Maurousset L, Faucher M (2013) Source‐to‐sink transport of sugar and regulation by environmental factors. Front Plant Sci 4: 272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin D (2010) The role of sourse tranporters OsSUT2 and OsSUT5 in molecuar regulation of physiological traits during grain filling period in transgenic indica rice (Oryza sativa L.). Master Thesis. Fujian Agriculture and Forestry University, Fuzhou
- Lin W, Ziska LH, Namuco OS, Bai K (1997) The interaction of high temperature and elevated CO2 on photosynthetic acclimation of single leaves of rice in situ. Physiol Plant 99: 178–184 [Google Scholar]
- Long SP, Ainsworth EA, Leakey ADB, Nösberger J, Ort DR (2006) Food for thought: lower‐than‐expected crop yield stimulation with rising CO2 concentrations. Science 312: 1918–1921 [DOI] [PubMed] [Google Scholar]
- Moore BD, Cheng SH, Sims D, Seemann JR (1999) The biochemical and molecular basis for photosynthetic acclimation to elevated atmospheric CO2 . Plant Cell Environ 22: 567–582 [Google Scholar]
- Muryono M, Chen CP, Sakai H, Tokida T, Hasegawa T, Usui Y, Nakamura H, Hikosaka K (2017) Nitrogen distribution in leaf canopies of high‐yielding rice cultivar Takanari. Crop Sci 57: 2080–2088 [Google Scholar]
- Okada M, Lieffering M, Nakamura H, Yoshimoto M, Kim HY, Kobayashi K (2001) Free‐air CO2 enrichment (FACE) using pure CO2 injection: system description. New Phytol 150: 251–260 [Google Scholar]
- Olano JM, Menges ES, Martinez E (2006) Carbohydrate storage in five resprouting Florida scrub plants across a five chronosequence. New Phytol 170: 99–106 [DOI] [PubMed] [Google Scholar]
- Ono K, Sasaki H, Hara T, Kobayashi K, Ishimaru K (2003) Changes in photosynthetic activity and export of carbon by overexpressing a maize sucrose‐phosphate synthase gene under elevated CO2 in transgenic rice. Plant Prod Sci 6: 281–286 [Google Scholar]
- Ruiz‐Vera UM, De Souza AP, Long SP, Ort DR (2017) The role of sink strength and nitrogen availability in the down‐regulation of photosynthetic capacity in field‐grown Nicotiana tabacum L. at elevated CO2 concentration. Front Plant Sci 8: 998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimoda S, Maruyama A (2014) Rice varietal differences in responses of stomatal gas exchange to supplemental nitrogen application. Photosynthetica 52: 397–403 [Google Scholar]
- Shimono H, Okada M (2013) Plasticity of rice tiller production is related to genotypic variation in the biomass response to elevated atmospheric CO2 concentration and low temperatures during vegetative growth. Env Exp Bot 87: 227–234 [Google Scholar]
- Stitt M (1991) Rising CO2 levels and their potential significance for carbon flow in photosynthetic cells. Plant Cell Env 14: 741–762 [Google Scholar]
- Takahashi S, Meguro‐Maoka A, Yoshida M (2017) Analysis of sugar content and expression of sucrose transporter genes (OsSUTs) in rice tissues in response to a chilling temperature. Jpn Agr Res Q 51: 137–146 [Google Scholar]
- Wang DR, Bunce JA, Tomecek MB, Gealy D, McClung A, McCouch SR, Ziska LH (2016a) Evidence for divergence of response in Indica, Japonica, and wild rice to high CO2 x temperature interaction. Globle Change Biol 22: 2620–2632 [DOI] [PubMed] [Google Scholar]
- Wang L, Yang SY, Guo MY, Huang YN, Sentenac H, Very AA, Su YH (2016b) The S1‐S2 linker determines the distinct pH sensitivity between ZmK2.1 and KAT1. Plant J 85: 675–685 [DOI] [PubMed] [Google Scholar]
- Zhou R, Kjaer KH, Rosenqvist E, Yu X, Wu Z, Ottosen CO (2017) Physiological response to heat stress during seedling and anthesis stage in tomato genotypes differing in heat tolerance. J Agron Crop Sci 203: 68–80 [Google Scholar]
- Zhu C, Ziska LH, Zhu J, Zeng Q, Xie Z, Tang H, Jia X, Hasegawa T (2012) The temporal and species dynamics of photosynthetic acclimation in flag leaves of rice (Oryza sativa) and wheat (Triticum aestivum) under elevated carbon dioxide. Physiol Plant 145: 395–405 [DOI] [PubMed] [Google Scholar]
- Zhu C, Zhu J, Cao J, Jiang Q, Liu G, Ziska LH (2014) Biochemical and molecular characteristics of leaf photosynthesis and relative seed yield of two contrasting rice cultivars in response to elevated CO2 . J Exp Bot 65: 6049–6056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu C, Xu X, Wang D, Zhu J, Liu G (2015) An indica rice genotype showed a similar yield enhancement to that of hybrid rice under free air carbon dioxide enrichment. Sci Rep 5: 12719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziska LH, Tomecek MB, Gealy DR (2014) Assessment of cultivated and wild, weedy rice lines to concurrent changes in CO2 concentration and air temperature: determining traits for enhanced seed yield with increasing atmospheric CO2 . Funct Plant Biol 41: 236–241 [DOI] [PubMed] [Google Scholar]


