Abstract
Our study used a refined case–control cervical cancer Audit framework to investigate effectiveness of cervical screening, with measures of three screening failures: irregular‐participation, cervical cancer developed after cytological abnormalities and after normal screening results. The register‐based study included 4,254 cervical cancer cases diagnosed in Sweden during 2002–2011, and 30 population‐based controls per case. We used conditional logistic regression models to examine relative risks of cervical cancer in relation to screening participation and screening results in the past two screening rounds from 6 months before cancer diagnosis. We found that women unscreened in past two screening rounds showed four times increased risk of cervical cancer compared to women screened in time (OR = 4.1, 95% CI = 3.8–4.5), and women unscreened in the previous round but screened in the most recent round also showed a statistically significantly elevated risk (OR = 1.6, 95% CI = 1.5–1.8). Women having abnormality in previous two rounds exhibited higher risk of cervical cancer compared to women screened with normal results, while having normal results in the subsequent round after the abnormality also yielded an increased risk (OR = 4.0, 95% CI = 3.2–5.1). Being screened with only normal results was associated with 89% risk reduction for squamous cell cancer, compared to women unscreened, but only 60% reduction for adenocarcinoma. Our findings emphasize the importance of routine participation in cervical screening and suggest that management of abnormalities, as well as sensitivity of the test, warrants improvement especially for preventing cervical adenocarcinoma. The Audit framework serves as routine evaluation model and the findings benchmark for future evaluation of changes in screening practice.
Keywords: cervical screening, cytology, cervical cancer, prevention
Short abstract
What's new?
Systematic review of cancer screening is critical to ensuring high‐quality, effective disease prevention and detection and management. In this study, case–control audit was used to evaluate cervical cancer screening in Sweden. Analyses show that relative to women who undergo routine screening, cervical cancer risk is elevated for women who are screened at irregular intervals. Risk was significantly increased among women with abnormalities detected at screening. The audit further revealed that, overall, management of abnormalities for preventing cervical adenocarcinoma is inferior compared to squamous cell cancer. The findings suggest that case–control auditing is a useful means of evaluating cervical screening programs.
Abbreviations
- AGC
atypical glandular cells
- ASCUS
atypical squamous cells of undetermined significance
- CIN
cervical intraepithelial neoplasia
- FIGO stage
International Federation of Gynaecology and Obstetrics staging system
- HPV
human papillomavirus
- LISA
the longitudinal integration database for health insurance and labor market studies
- NCR
the National Cancer Registry
- NKCx
the Swedish National Cervical Screening Registry
- SNOMED
Systematised Nomenclature of Medicine
Introduction
Cervical cancer has been acknowledged as the first cancer that can be effectively prevented, for which the implementation of cervical screening is meritorious in the past half‐century. Established evidence shows a strong preventive effect from cervical screening, through detecting, managing and treating precursor lesions.1, 2, 3 An organized, population‐based cervical screening program, as recommended by the International Agency for Research on Cancer,4 achieving high coverage and equality, can contribute to a substantial reduction in cervical cancer incidence and mortality.5, 6, 7, 8, 9, 10, 11
The cervical cancer case–control audit can evaluate the effectiveness of the screening program over time,12, 13, 14, 15 and identify potential weaknesses in guidelines or implementation. Therefore, it is a crucial part of the quality assurance of the screening program. Sweden started organized cervical screening in the late 1960s. The screening practice has gradually improved over the years. In 2008, we published the first case–control Audit based on cervical cancer cases in 1999–2001, which demonstrated a strong effectiveness of cervical screening in preventing cervical cancer.12 Due to the limitation of data at the time, assessment of a sufficient length of screening history, adjustment for potential confounding factors, and comparison between histopathological types were not possible. We now present an updated Audit of cervical cancer cases and controls from 2002 to 2011 using more extensive data, as well as refined study design and analysis framework.
This Audit aims to provide an overall evaluation of the three main screening failures in cancer prevention that can be evaluated in an audit framework, that is, cancer development in relation to irregular participation, having an abnormality, or having normal results in screening.16 Findings from the Audit are expected to direct further investigations in detailed aspects for targeted improvement of screening practice. Although cytology‐primary screening was the routine during the period of this Audit, the study will serve as a methodological model and as a benchmark for future comparisons with HPV‐primary screening programs.
Materials and Methods
Study population
In this population‐based nested case–control study, we first identified women diagnosed with invasive cervical cancer or unspecified uterine cancer during 2002–2011 from the Swedish National Cancer Registry (NCR).17 To supply information about FIGO stage (International Federation of Gynaecology and Obstetrics staging system), mode of detection and to verify primary invasive epithelial neoplasia with detailed histopathology classification, all the identified cases underwent thorough clinical review by a senior gynaecologist (BA), who collected and verified information through original medical charts deposited in clinical departments where cancer cases were diagnosed and treated. We were able to retrieve 91% of the sample slides from pathological laboratories all over the country, which were subsequently reviewed by a senior pathologist. After the clinical and histopathological review, cancers not of cervical origin, not epithelial, not invasive cancer, and recurrent cancers were excluded. For each case, 30 controls who were alive, resident in Sweden, and at risk of invasive cervical cancer up to the date the corresponding case was diagnosed, were randomly selected from the Swedish Total Population Register,18 and matched on birth year. Women with a total hysterectomy, as registered in the Swedish National Patient Register,19 were excluded, as they were not at risk of having cervical cancer. This resulted in 4.3% cancer cases having 27–29 controls, and the remaining 95.7% of cancer cases still had 30 controls (Supporting Information Table S1).
Information on cancer cases and cervical screening
Key information on the cases included the date of cancer diagnosis, histopathological type, detailed FIGO stage and mode of detection. Histopathological type included squamous cell cancer, adenocarcinoma and less common types (including adenosquamous cell carcinoma, glassy cell carcinoma, clear cell carcinoma, small cell carcinoma, large cell carcinoma, neuroendocrine carcinoma and undifferentiated carcinoma). Analyses of less common types are addressed in a separate paper.20 FIGO stage was categorized into microinvasive (IA) and frankly invasive (IB+) including localized (IB) and advanced (II+) cancer. Mode of detection recorded in medical charts was categorized as screen‐detected or symptomatic cancer.
For all case and control subjects, we retrieved cervical screening records from the Swedish National Cervical Screening Registry (NKCx),21 which contains cervical screening tests in Sweden since 1970s and reaches full coverage since 1995. Cytological screening using Papanicolaou (Pap) test was the primary test over the study period. Women's screening record in the two screening rounds prior to cancer diagnosis was obtained. Including two screening rounds instead of the full history was done for the following reasons (i) cervical cancer incidence was generally high in the upcoming two screening rounds after an initial abnormality found in screening, according to our previous study.22 This implies that the previous two screening rounds are the critical period for cancer prevention; and (ii) this being an evaluation framework, setting a particular time window for screening history in the Audit is practical for continuous implementation and interpretation. The length of the recommended interval of a screening round differed with age: 3 years for ages 23–50 and 5 years for ages 51–60. Some Swedish counties invited women up to age 65 to make sure that women were screened up to and including age 60. We categorized the screening history for women between age 51 and 65 years into the 5‐year intervals to use a uniform upper‐age limit that reflected what the guidelines intended to recommend. All screening tests within 6 months prior to the cancer diagnosis were disregarded, as they were highly likely directly leading to cancer diagnosis and thus not contributing to prevention of cancer. This time‐frame was verified by linking screening records to the mode of detection in clinical charts (Supporting Information Fig. S1). Accordingly, the time for each round of previous screening was extended for half a year. Therefore, for the cancer cases aged 26–28 and their controls, we obtained screening history of one screening round, that is, 0.5–3.5 years, and for cases aged 29–53, 54–58 and 59–65, we obtained the screening history for two rounds in the past 0.5–6.5, 0.5–8.5 and 0.5–10.5 years, respectively. A sensitivity analysis for defining the screening interval as 3.5 and 5.5 years was performed. For women aged 66 and above, we obtained their screening history at ages 56–65 (representing the last two rounds of organized screening for women aged 66+).
Screening history was categorized into (i) Unscreened, if no test in the past two screening rounds; (ii) Unscreened in the most recent round, if a woman had test(s) in the previous round, but no test in the most recent round; (iii) Unscreened in the previous round, if a woman had no test in the previous round, but had test(s) in the most recent round; and (iv) Screened in time, if she had tests in both rounds. The results of cytological screening in each screening round were further classified as abnormal and normal. Abnormal smears included ASCUS (atypical squamous cells of undetermined significance), mild squamous dysplasia, moderate or severe squamous dysplasia, atypical glandular cells, atypical cells of uncertain origin, adenocarcinoma in situ/invasive adenocarcinoma, cytological implication of squamous cell cancer or malignancy of uncertain origin. SNOMED codes were used to define the Pap test results (Supporting Information Table S2). If any abnormality was found in a screening round, the screening result of this round was defined as abnormal, regardless of whether normal results were also present during the period of the screening interval. For a screening interval to be defined as normal, only normal results were allowed in the interval.
To control for potential confounders, we obtained information on level of education from the longitudinal integration database for health insurance and labor market studies (LISA, Swedish acronym).23 Level of education was classified as (i) low if the highest schooling was primary education 9 years and below, (ii) medium for 2–3 years of secondary schooling (similar to senior high‐school), (iii) high for postsecondary education and above (equivalent to university studies) and (iv) missing data. We used the highest level of education by year of cancer diagnosis for cases and controls. For the sensitivity analysis, we obtained number of children before cancer diagnosis for each woman from the Swedish Multi‐Generation Register for the birth cohorts 1932 and onwards, as an indicator of parity.
Statistical analyses
We presented the distribution of screening history in the past two screening rounds among all cervical cancer cases, and utilized conditional logistic regression models to evaluate the performance of the screening program according to the suggested framework of three potential major screening failures:16 (i) cervical cancer risk associated with nonparticipation, that is (a) the relative risk of invasive cervical cancer in women unscreened or inadequately screened in the past two screening rounds compared to women screened in time, and (b) the relative risk of having a higher FIGO stage of cervical cancer among screen‐detected and symptomatic cases in relation to screening history. This takes into account the possibility that a cancer can be screen‐detected by an overdue screening test in women not participating in previous screening round(s), or it can be symptomatic even if a woman participated in previous screening round(s).13 We utilized ordinal (proportional) logistic regression models to estimate proportional odds ratios for having higher‐stage cervical cancer in relation to screening history by mode of detection of the cancer, among cancer cases only; (ii) risk associated with abnormalities detected in screening, that is, relative risk of cancer in women screened with abnormalities to women screened with normal test results; (iii) risk associated with having normal test results: relative risk of developing cervical cancer in women screened in time with only normal results to women unscreened, comparing across histopathological types.
Analyses were stratified by histopathological type and FIGO stage where applicable and adjusted for level of education. Parity was adjusted for in the sensitivity analysis.
Data manipulation and statistical analyses were conducted in SAS 9.4.
Results
Study population
There were 4,533 women reported with invasive cervical cancer or unspecified uterine cancer to the National Cancer Registry between 2002 and 2011. After the clinical review, 279 of these were recurrent cancers, not cancer of cervical origin, not epithelial, or not invasive cancer and were thus excluded. In total, 4,254 cases with 120,006 controls remained in the study population (Fig. 1). Among all the cases, 20% were microinvasive, 40% were localised, and 40% were advanced cancer. Symptomatic cancer accounted for 71% of all cases. Control subjects were slightly higher educated than case subjects, and had similar number of children as case subjects (Table 1).
Figure 1.
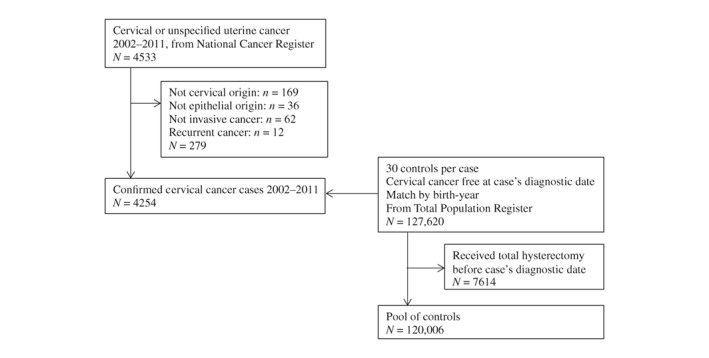
Study population.
Table 1.
Characteristics of study subjects
| Case subjects | Control subjects | |||
|---|---|---|---|---|
| All types (%) | Squamous cell cancer (%) | Adenocarcinoma (%) | (%) | |
| Age of cancer | ||||
| <23 | 8 (0.2) | 5 (0.2) | 1 (0.1) | 240 (0.2) |
| 23–28 | 205 (4.8) | 143 (4.6) | 38 (4.6) | 6,150 (5.0) |
| 29–39 | 999 (23.5) | 710 (23.0) | 223 (27.0) | 29,887 (24.5) |
| 40–49 | 830 (19.5) | 561 (18.2) | 208 (25.2) | 24,314 (19.9) |
| 50–65 | 1,020 (24.0) | 763 (24.7) | 175 (21.2) | 28,395 (23.3) |
| 66+ | 1,192 (28.0) | 906 (29.3) | 181 (21.9) | 33,020 (27.1) |
| FIGO stage | ||||
| IA (microinvasive) | 862 (20.3) | 671 (21.7) | 174 (21.1) | – |
| IB (localized) | 1,685 (39.6) | 1,076 (34.8) | 436 (52.8) | – |
| II+ (advanced) | 1,707 (40.1) | 1,341 (43.5) | 216 (26.1) | |
| Mode of detection | ||||
| Screen‐detected | 1,242 (29.2) | 911 (29.5) | 290 (35.1) | – |
| Symptomatic | 3,005 (70.8) | 2,177 (70.5) | 535 (64.8) | – |
| Missing | 1 (0.0) | 0 | 1 (0.1) | – |
| Education level | ||||
| Low | 1,189 (27.9) | 890 (29.3) | 215 (23.5) | 29,730 (24.4) |
| Medium | 1,870 (44.0) | 1,339 (44.1) | 400 (43.7) | 50,843 (41.7) |
| High | 1,108 (26.1) | 742 (24.4) | 289 (31.6) | 38,096 (31.2) |
| Missing | 87 (2.0) | 67 (2.2) | 11 (1.2) | 3,337 (2.7) |
| Mean number of children1 | 1.80 | 1.82 | 1.76 | 1.81 |
Among women born in 1932 and onwards.
Distribution of screening history of cancer cases
Among all cancer cases aged 29 and above, 42% were not screened in the past two screening rounds, 27% missed one round, 15% had abnormalities and 16% were screened in time and had only normal results. Compared to the distribution for squamous cell cancer, a higher proportion of women with adenocarcinoma had been screened in time with normal results (Table 2).
Table 2.
Distribution of screening history in the past two screening rounds, by histological types of cervical cancer
| Screening history1 | All types | Squamous cell cancer | Adenocarcinoma | Control subjects | ||||
|---|---|---|---|---|---|---|---|---|
| No. of case sub. | % among case sub. | No. of case sub. | % among case sub. | No. of case sub. | % among case sub. | No. of control sub. | % among control sub. | |
| All stages (age 29+) | ||||||||
| Normal–Normal | 651 | 16.1 | 401 | 13.6 | 190 | 24.1 | 48,742 | 42.2 |
| Abnormal–Normal | 85 | 2.1 | 52 | 1.7 | 27 | 3.4 | 1,605 | 1.4 |
| Abnormal–Unscreened | 84 | 2.1 | 62 | 2.1 | 17 | 2.2 | 283 | 0.2 |
| Normal–Abnormal | 237 | 5.9 | 167 | 5.7 | 58 | 7.4 | 1,166 | 1.0 |
| Unscreened–Abnormal | 128 | 3.2 | 105 | 3.6 | 16 | 2.0 | 474 | 0.4 |
| Abnormal–Abnormal | 72 | 1.8 | 45 | 1.5 | 23 | 2.9 | 316 | 0.3 |
| Unscreened–Normal | 374 | 9.3 | 265 | 9.0 | 85 | 10.8 | 15,927 | 13.8 |
| Normal–Unscreened | 699 | 17.3 | 475 | 16.2 | 164 | 20.8 | 17,433 | 15.1 |
| Unscreened–Unscreened | 1,711 | 42.3 | 1,368 | 46.5 | 207 | 26.3 | 29,670 | 25.7 |
Left‐hand side of hyphen represents the previous screening round, and right‐hand side represents the most recent screening round.
Risk from nonparticipating to screening
Compared to women screened in time in the past two screening rounds, women who were completely unscreened had a 4.1 times elevated risk of cervical cancer (95% confidence interval (CI) = 3.8–4.5). The risks for microinvasive (stage IA), frankly invasive (stage IB+), or advanced cancer (stage II+) were 2.6 times (95% CI = 2.1–3.3), 4.5 times (95% CI = 4.1–5.0) and 7.7 times (95% CI = 6.6–9.0) higher, respectively. The increased risk associated with nonparticipation was 5.3 times (95% CI = 4.8–5.9) for squamous cell cancer and 1.7 times (95% CI = 1.4–2.1) for adenocarcinoma (Table 3). Being unscreened in the most recent round of screening was associated with 2.4 times (95% CI = 2.2–2.7) higher risk of cervical cancer, whereas being screened in the most recent round but unscreened in the previous round still yielded 1.6 times (95% CI = 1.5–1.8) increased risk (Table 3). The odds ratios were only marginally affected after further adjusting for parity (Supporting Information Tables S3 and S4), as well as in a sensitivity analysis using 3.5 and 5.5 years for defining the screening interval (Supporting Information Table S5).
Table 3.
Odds ratios of cervical cancer in relation to screening status in the past two screening rounds, by stage and histopathological type of cancer cases
| All types | Squamous cell cancer | Adenocarcinoma | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Screening status1 | No. of case sub. | No. of control sub. | OR | OR adjusted2 | No. of case sub. | No. of control sub. | OR | OR adjusted2 | No. of case sub. | No. of control sub. | OR | OR adjusted2 |
| All stages (age 29+) | ||||||||||||
| Unscr.–Unscr. | 1,711 | 29,670 | 4.03(3.69–4.40) | 4.14(3.79–4.52) | 1,368 | 22,099 | 5.15(4.65–5.72) | 5.28(4.75–5.87) | 207 | 5,103 | 1.61(1.30–1.98) | 1.70(1.38–2.10) |
| Scr.–Unscr. | 783 | 17,716 | 2.41(2.18–2.65) | 2.40(2.18–2.65) | 537 | 12,862 | 2.60(2.31–2.93) | 2.60(2.31–2.93) | 181 | 3,480 | 1.94(1.60–2.35) | 1.95(1.61–2.37) |
| Unscr.–Scr. | 502 | 16,401 | 1.62(1.46–1.81) | 1.63(1.46–1.81) | 370 | 11,854 | 1.89(1.66–2.15) | 1.89(1.66–2.16) | 101 | 3,364 | 1.10(0.87–1.39) | 1.11(0.88–1.40) |
| Scr.–Scr. | 1,045 | 51,829 | Ref. | Ref. | 665 | 37,170 | Ref. | Ref. | 298 | 10,711 | Ref. | Ref. |
| Stage IA (microinvasive) | ||||||||||||
| Unscr.–Unscr. | 165 | 3,280 | 2.51(2.04–3.09) | 2.63(2.13–3.25) | 138 | 2,468 | 2.98(2.36–3.75) | 3.09(2.44–3.91) | 24 | 679 | 1.36(0.83–2.23) | 1.47(0.89–2.42) |
| Scr.–Unscr. | 213 | 3,408 | 3.05(2.52–3.68) | 3.05(2.53–3.69) | 165 | 2,590 | 3.32(2.67–4.13) | 3.33(2.67–4.14) | 43 | 755 | 2.19(1.47–3.27) | 2.21(1.48–3.31) |
| Unscr.–Scr. | 133 | 3,874 | 1.65(1.33–2.04) | 1.65(1.33–2.05) | 100 | 2,966 | 1.72(1.34–2.21) | 1.73(1.35–2.22) | 29 | 843 | 1.31(0.84–2.05) | 1.33(0.85–2.08) |
| Scr.–Scr. | 249 | 11,695 | Ref. | Ref. | 182 | 9,104 | Ref. | Ref. | 64 | 2,418 | Ref. | Ref. |
| Stage IB+ (frankly invasive) | ||||||||||||
| Unscr.–Unscr. | 1,546 | 26,390 | 4.45(4.03–4.90) | 4.54(4.11–5.02) | 1,230 | 19,631 | 5.89(5.23–6.63) | 6.00(5.32–6.76) | 183 | 4,424 | 1.66(1.31–2.09) | 1.75(1.38–2.21) |
| Scr.–Unscr. | 570 | 14,308 | 2.24(2.00–2.50) | 2.23(1.99–2.50) | 372 | 10,272 | 2.39(2.08–2.75) | 2.38(2.07–2.75) | 138 | 2,725 | 1.87(1.50–2.33) | 1.88(1.51–2.35) |
| Unscr.–Scr. | 369 | 12,527 | 1.62(1.43–1.84) | 1.62(1.43–1.84) | 270 | 8,888 | 1.96(1.68–2.29) | 1.96(1.68–2.28) | 72 | 2,521 | 1.04(0.79–1.36) | 1.05(0.80–1.37) |
| Scr.–Scr. | 796 | 40,134 | Ref. | Ref. | 483 | 28,066 | Ref. | Ref. | 234 | 8,293 | Ref. | Ref. |
| Stage II+ (advanced) | ||||||||||||
| Unscr.–Unscr. | 1,066 | 17,466 | 7.76(6.65–9.06) | 7.68(6.57–8.97) | 887 | 13,772 | 9.95(8.30–11.9) | 9.95(8.29–11.9) | 99 | 2,278 | 2.34(1.55–3.54) | 2.30(1.51–3.49) |
| Scr.–Unscr. | 213 | 7,059 | 2.44(2.02–2.96) | 2.41(1.99–2.92) | 150 | 5,567 | 2.58(2.05–3.24) | 2.55(2.03–3.21) | 37 | 880 | 1.84(1.18–2.88) | 1.82(1.16–2.85) |
| Unscr.–Scr. | 148 | 5,632 | 2.12(1.72–2.60) | 2.10(1.71–2.59) | 116 | 4,430 | 2.49(1.95–3.16) | 2.47(1.94–3.15) | 24 | 717 | 1.48(0.90–2.45) | 1.48(0.90–2.45) |
| Scr.–Scr. | 253 | 17,161 | Ref. | Ref. | 171 | 13,512 | Ref. | Ref. | 52 | 2099 | Ref. | Ref. |
Left‐hand side of hyphen represents the previous screening round, and right‐hand side represents the most recent screening round.
Adjusted for education and age group.
Abbreviations: Scr., screened; Unscr., unscreened.
Diagnosing cervical cancer by symptoms exhibited 19 times (95%CI = 15.7–22.8) increased risk of more advanced cervical cancer compared to a diagnosis by screening. The risk increase was 22 times (95%CI = 17.3–26.9) for squamous cell cancer and 10 times (95%CI = 7.2–15.1) for adenocarcinoma (Table 4). Among cancer cases diagnosed by symptoms, being unscreened in the past two screening rounds was associated with a 2.5 times (95%CI = 2.0–3.0) higher risk of advanced cervical cancer compared to those screened in time. The corresponding association was not observed among screen‐detected cancer cases.
Table 4.
Stage distribution of symptomatic and screen‐detected cervical cancer by histological type and screening history
| All types | Squamous cell cancer | Adenocarcinoma | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Stage IA (microinvasive) | Stage IB (localised) | Stage II+ (advanced) | Adjusted PropOR (95%CI)1 | Stage IA (microinvasive) | Stage IB (localised) | Stage II+ (advanced) | Adjusted PropOR (95%CI)1 | Stage IA (microinvasive) | Stage IB (localised) | Stage II+ (advanced) | Adjusted PropOR (95%CI)1 | |
| By mode of detection (all ages) | ||||||||||||
| Symptomatic | 148 (4.9) | 1,197 (39.8) | 1,666 (55.3) | 18.9 (15.7–22.8) | 106 (4.9) | 760 (34.9) | 1,311 (60.2) | 21.6(17.3–26.9) | 38 (7.1) | 291 (54.4) | 206 (38.5) | 10.4(7.2–15.1) |
| Screen‐detected | 714 (57.5) | 488 (39.3) | 40 (3.2) | Ref. | 565 (62.0) | 316 (34.7) | 30 (3.3) | Ref. | 136 (46.9) | 145 (50.0) | 9 (3.1) | Ref. |
| By mode of detection and screening history (age 29+ at cancer) | ||||||||||||
| Symptomatic | ||||||||||||
| Unscr.–Unscr. | 47 (3.1) | 403 (26.7) | 1,058 (70.2) | 2.5 (2.0–3.0) | 34 (2.8) | 283 (23.6) | 883 (73.6) | 2.8 (2.2–3.5) | 11 (6.2) | 71 (39.9) | 96 (53.9) | 1.5 (0.9–2.3) |
| Scr.–Unscr. | 28 (6.2) | 219 (48.4) | 205 (45.4) | 1.2 (0.9–1.5) | 19 (6.3) | 139 (46.3) | 142 (47.3) | 1.2 (0.9–1.6) | 7 (7.4) | 51 (53.7) | 37 (38.9) | 1.5 (0.9–2.5) |
| Unscr.–Scr. | 25 (7.7) | 155 (47.8) | 144 (44.4) | 1.3 (0.9–1.6) | 19 (8.0) | 105 (44.1) | 114 (47.9) | 1.3 (0.9–1.8) | 6 (9.7) | 34 (54.8) | 22 (35.5) | 1.1 (0.6–2.0) |
| Scr.–Scr. | 37 (5.8) | 361 (57.0) | 235 (37.1) | Ref. | 25 (6.4) | 209 (53.4) | 157 (40.5) | Ref. | 12 (6.8) | 117 (66.1) | 48 (27.1) | Ref. |
| Screen‐detected | ||||||||||||
| Unscr.–Unscr. | 118 (58.4) | 77 (38.1) | 7 (3.5) | 0.8 (0.6–1.2) | 104 (61.9) | 60 (35.7) | 4 (2.4) | 0.9 (0.6–1.3) | 13 (46.4) | 13 (46.4) | 2 (7.1) | 1.1 (0.5–2.6) |
| Scr.–Unscr. | 185 (55.9) | 138 (41.7) | 8 (2.4) | 1.0 (0.7–1.3) | 146 (61.6) | 83 (35.0) | 8 (3.4) | 1.0 (0.7–1.4) | 36 (41.9) | 50 (58.1) | 0 (0) | 1.2 (0.7–2.1) |
| Unscr.–Scr. | 108 (60.7) | 66 (37.1) | 4 (2.2) | 0.7 (0.5–1.1) | 81 (61.4) | 49 (37.1) | 2 (1.5) | 0.9 (0.6–1.4) | 23 (59.0) | 14 (35.9) | 2 (5.1) | 0.6 (0.3–1.3) |
| Scr.–Scr. | 212 (51.5) | 182 (44.2) | 18 (4.4) | Ref. | 157 (57.3) | 103 (37.6) | 14 (5.1) | Ref. | 52 (43.0) | 65 (53.7) | 4 (3.3) | Ref. |
Proportional odds ratio, measuring the risk of being diagnosed with progressively higher staged cervical cancer, adjusted for education and age group.
Abbreviations: Scr., screened; Unscr., unscreened.
Risk from having abnormal results in the past two screening rounds
Compared to women screened with only normal results in the past two screening rounds, women with an abnormality in the previous round and no test in the most recent round had the highest relative risk of cervical cancer of any type (OR = 23.7, 95% CI = 18.3–30.7). Women having an abnormality in the previous round and having normal test result in the most recent round presented with a statistically significantly four times elevated risk. Compared to cervical cancer diagnosed in women with normal results in the past two screening rounds, cancer diagnosed in women with abnormalities in the past two screening rounds tended to be of lower FIGO stage, especially in women with abnormal results in the most recent screening round (Table 5).
Table 5.
Odds ratios of cervical cancer (aged 29+) among women having abnormalities compared to women having normal tests in the past two screening rounds, and stage distributions of cases by screening history
| Screening history1 | No. of case sub. | No. of control sub. | OR | OR adjusted2 | Stage distribution of cases | |||
|---|---|---|---|---|---|---|---|---|
| Stage IA (%) | Stage IB (%) | Stage II+ (%) | PropOR (95%CI)3 | |||||
| Nor.–Nor. | 651 | 48,742 | Ref. | Ref. | 116 (17.8) | 343 (52.7) | 192 (29.5) | Ref. |
| Abn.–Nor. | 85 | 1,605 | 4.1 (3.2–5.1) | 4.0 (3.2–5.1) | 19 (22.4) | 45 (52.9) | 21 (24.7) | 0.8 (0.5–1.2) |
| Abn.–Unscr. | 84 | 283 | 24.1 (18.6–31.1) | 23.7 (18.3–30.7) | 21 (25.0) | 34 (40.5) | 29 (34.5) | 1.0 (0.7–1.6) |
| Nor.–Abn. | 237 | 1,166 | 15.4 (13.1–18.0) | 15.3 (13.1–18.0) | 89 (37.6) | 119 (50.2) | 29 (12.2) | 0.4 (0.3–0.6) |
| Unscr.–Abn. | 128 | 474 | 21.3 (17.2–26.3) | 21.1 (17.1–26.1) | 52 (40.6) | 51 (39.8) | 25 (19.5) | 0.5 (0.3–0.7) |
| Abn.–Abn. | 72 | 316 | 17.5(13.4–22.8) | 17.4(13.3–22.8) | 25 (34.7) | 36 (50.0) | 11 (15.3) | 0.5 (0.3–0.8) |
Left‐hand side of hyphen represents the previous screening round, and right‐hand side represents the most recent screening round.
Adjusted for education and age group.
Proportional odds ratio, measuring the risk of being diagnosed with progressively higher staged cervical cancer, adjusted for age group.
Abbreviations: Abn., abnormal; Nor., normal; Unscr., unscreened.
In the supplementary analysis, comparing the risk of squamous cell cancer with adenocarcinoma among women having abnormalities in the past two screening rounds in relation to women unscreened, the odds ratios for adenocarcinoma were mostly significantly higher than that for squamous cell cancer (Supporting Information Table S6).
Risk reduction associated with normal results
Compared to unscreened women, women screened with normal results in the past two screening rounds exhibited a 89% risk reduction of squamous cell cancer (OR = 0.11, 95%CI = 0.10–0.13), but only 60% risk reduction of adenocarcinoma (OR = 0.40, 95%CI = 0.32–0.52) (Table 6).
Table 6.
Odds ratio of cervical cancer (all stages, aged 29+) in women having normal tests compared to women not being screened in the last two screening rounds, by histological type
| All types | Squamous cell cancer | Adenocarcinoma | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Screening history1 | No. of case sub. | No. of control sub. | OR | OR adjusted2 | No. of case sub. | No. of control sub. | OR | OR adjusted2 | No. of case sub. | No. of control sub. | OR | OR adjusted2 |
| Normal–Normal | 651 | 48,742 | 0.16 (0.14–0.17) | 0.15 (0.14–0.17) | 401 | 34,942 | 0.12 (0.10–0.13) | 0.11 (0.10–0.13) | 190 | 10,082 | 0.43 (0.33–0.54) | 0.40 (0.32–0.52) |
| Unscr.–Unscr. | 1,711 | 29,670 | Ref. | Ref. | 1,368 | 22,099 | Ref. | Ref. | 207 | 5,103 | Ref. | Ref. |
Left‐hand side of hyphen represents the previous screening round, and right‐hand side represents the most recent screening round.
Adjusted for education and age group.
Abbreviation: Unscr., unscreened.
Discussion
Main findings and interpretations
Besides reinforcing the association between screening and cervical cancer risk, the present study discovered that irregular screening yielded increased risk; having a normal screening result after previous abnormality did not ensure a sufficiently low risk; the inferior effectiveness of screening for preventing adenocarcinoma was from both lower negative predictivity of normal Pap test results and higher risk after abnormalities.
Risk associated with nonparticipation
An increased risk of cervical cancer associated with screening nonparticipation has been shown in case–control audits from various settings including the first Swedish Audit.12, 14, 15 With this updated Audit, we were also able to adjust for education and parity as potential confounders and exclude women with a total hysterectomy from the control subjects, strengthening the validity and interpretation of the association between screening participation and cervical cancer risk. Furthermore, we assessed longer and more detailed screening history than other audits, and found that the increased risk was not only present in women not participating in the past two screening rounds or in the most recent screening round, but also in women not participating in the previous round although being screened in the most recent round. This implies that missing one scheduled screening test cannot be completely compensated by participating in the next round. Although we did not have the possibility to explore this issue further in the current study, the finding is in line with the general consensus that screening performance improves by repeating tests. Further investigation of this topic may be warranted.
Given the strengthened evidence of the benefit from screening in this updated Audit, routine participation should continuously be emphasized and improved through optimizing the call and recall (invitation and reminding) system,24 targeting groups with low participation such as immigrants25 and certain socioeconomic groups,26 and implementing HPV self‐sampling in nonparticipants.27
Risk associated with abnormal findings in screening
Having a cytological abnormality naturally signifies an increased risk of developing cervical cancer. However, the risk can be largely reduced if sufficient management follows. This is the key procedure that enables cervical screening to prevent cancer. We observed a universally increased risk of cervical cancer in women having abnormalities at any time during the past two screening rounds. It may be due to inadequate execution of the management practice, or that the currently recommended management strategy is not perfectly effective for certain scenarios of risk elevation, which calls for further close investigations. It is worth mentioning that we examined all abnormalities without distinguishing between primary and follow‐up tests in the previous two screening rounds, because we consider both as part of the screening program, and thus they should be included in this overall evaluation. Distinguishing type of tests can be necessary for further close investigations of management for particular abnormalities.
The presence of a fourfold increased risk even if the Pap test in the subsequent screening round was reported normal drew particular attention. This implies that the routine management may not be enough. Furthermore, the supplementary result shows that after abnormal finding(s) in screening, the risk for developing adenocarcinoma was significantly higher than that for developing squamous cell cancer. This suggests that the management following abnormalities is less effective for preventing adenocarcinoma as compared to squamous cell cancer. Therefore, future research can aim for optimizing clinical management to minimize the risk after cervical abnormalities.
For this purpose, more in‐depth studies are needed to evaluate effectiveness of different management strategies, for instance, what is the best treatment and follow‐up strategy for a specific abnormality, how many normal results following a certain abnormality confers a reasonably low risk so that women can go back to regular screening, how can HPV testing add on further assurance, and whether long‐term risk after treatment of an abnormality can be identified by immunity biomarkers. Such studies require a rigorous design for each research question. Several previous studies can serve as examples of such detailed investigations, for example, lifetime risk of cervical cancer after treatment of CIN3,28 long‐term risk after diagnosis of atypical glandular cells (AGC) in screening in relation to the initial biopsy,22 cancer risk after ASCUS/CIN1 at younger ages with and without immediate biopsy,29 risk of recurrent CIN2/3 by treatment type for different grade of CIN lesions.30 It is anticipated that collectively, such studies will help to optimize the management for abnormalities, and consequently improve the effectiveness of the cervical screening program.
Risk reduction associated with normal results
Sixteen percent of the cervical cancer cases occurred in women screened with only normal tests in the past two screening rounds, and this number was as high as 24% for adenocarcinoma. We also found that women screened with normal tests showed a 89% risk reduction for squamous cell cancer compared to women unscreened, while this figure was only 60% for adenocarcinoma. These differences may be due to difficulties in sampling glandular lesions located deep in the endocervical canal,31, 32 judgment when reading slides, or the progression of lesions within the 3‐ to 5‐year interval. Case–control studies from other settings have also shown inferior effectiveness of cervical screening for preventing adenocarcinoma.33, 34, 35, 36 With this audit, we further explained that the inferior effectiveness is due to both suboptimal negative predictivity of repeat normal testing results with a 3‐ to 5‐yearly screening interval and possibly unsatisfactory management of previous abnormalities as discussed above.
Evidence shows that HPV primary screening performs better than cytology for preventing adenocarcinoma.37 This improvement may come from a higher sensitivity of the HPV test, careful slide‐reading by cytologists once the HPV positive result is known, and active clinical assessment after repeat HPV positivity. Future comparison of the risk reduction associated with repeat normal/negative tests between the HPV‐primary and cytology‐primary test will provide deeper understanding of the underlying reasons.
Down‐staging effect of cervical screening
The results unambiguously show that the screen‐detected cervical cancers have considerably lower clinical stage compared to symptomatic cervical cancer cases. Furthermore, our results suggested that the down‐staging may be related to not only the screening test that detects cervical cancer but also the screening history in previous intervals, given the risk gradient for stage IA, IB+ and II+ cervical cancer associated with participation history (Table 3), and the lower FIGO stage of symptomatic cases in those participated in previous screenings (Table 4). It may be due to that once an abnormality is found in screening, women may pay particular attention to symptoms, and be more prone to seek a check‐up immediately when a symptom occurs. This was also supported by our finding that cancers among women who were found with abnormalities in the most recent screening round were at a lower stage as compared to women who were unscreened or had normal results before (Table 5).
Although we found an overall reduction of microinvasive cervical cancer associated with screening history, previous screening was not associated with reduced microinvasive cancer above age 65 and microinvasive adenocarcinoma, but the only reduction of frankly invasive including advanced cancer for the older age group and the specific histopathological type (Table 3 and Supporting Information Table S7). This could be considered as a success, rather than a failure of the screening program since there is evidence that almost all microinvasive cervical cancer can be cured, that is, reach the same level of mortality as the general population,13 and the majority of microinvasive cancers can be treated conservatively. Therefore, it is of value to separate microinvasive and frankly invasive cancer when evaluating the effectiveness of screening, especially for certain histopathological types and age groups.
Application of the case–control Audit and future perspectives
The case–control design and the analysis framework can be used for evaluating future cervical screening programs with different test and follow‐up modalities. Given the upcoming switch to HPV‐primary screening, the relative risk comparing women who participated and who did not participate in screening will indicate the overall effectiveness of HPV primary screening program; the risk associated with abnormal findings will indicate strengths and weaknesses in the clinical management of abnormalities found in HPV‐primary screening, and may highlight needs for optimized management strategies; and the risk reduction associated with normal/negative screening results will provide further evidence on the performance of the HPV testing. Results from the current Audit will give a benchmark of what has been achieved in cytology‐primary screening, and leave record for comparison with newly implemented programs.
Strengths and limitations
Our study has several strengths. The Swedish National Cancer Registry and the National Cervical Screening Registry provided complete records of cervical cancer cases and screening history covering the entire Swedish population, which assured generalizability of our results. The population‐based random control‐selection with birth‐year matching was performed as incidence density sampling, which allowed the odds ratios to be interpreted as incidence rate ratios. By linking with national Swedish databases (LISA and the Multi‐Generation Register), we were able to adjust for education and parity, reducing the chance of confounding the association between screening participation and cervical cancer risk. Furthermore, we evaluated screening history over two screening intervals, which adds important information compared to the first Swedish Audit and audits from other settings.
Limitations of our study are reduced power for the subanalyses of histopathology stratification for some screening history groups and lack of information on treatment of abnormalities in the registers, making us unable to evaluate the effect of treatment methods. Furthermore, we could not adjust for potential confounders as sexual behavior and smoking. If these factors are negatively associated with screening participation, the effectiveness of screening might have been overestimated. However, we believe that adjustment for education may have reduced the confounding effect from behavioral factors.
Conclusions
In this population‐based case–control audit, we reinforced the finding of effectiveness of cervical screening, and raised in‐depth discoveries of suboptimal prevention regarding irregular participation, risk after abnormalities and differences in effectiveness between histopathological types. These findings emphasise the importance of routine participation in cervical screening, and calls for improvement of the management of abnormalities, as well as the sensitivity of the screening test, especially for preventing adenocarcinoma.
Our findings may guide future research projects, inspire screening organizers and practitioners to improve the cervical screening program, and inform the general public. The case–control Audit as a model will be used to continuously evaluate cervical screening with regard to upcoming changes in cervical screening programs internationally, and the results from the presented Audit can be used as a benchmark for comparing the effectiveness of new programs to former programs.
Author Contributions
Analysis plan, conducted the statistical analyses and drafted the article: Wang J. Analysis plan and data interpretation: Elfström KM and Sparén P. Clinical questions and hypotheses and conducted clinical review of cervical cancer cases: Andrae B. Collected and standardized the cervical screening data: Dillner J and Sparén P. Pathology review administration of cancer cases and standardized the data for clinical and pathology review: Nordqvist Kleppe S. Statistical analyses: Ploner A and Lei J. Study design: Dillner J, Sundström K and Sparén P. Article revision: Elfström KM, Andrae B, Nordqvist Kleppe S, Dillner J, Sundström K and Sparén P. Discussion, manuscript revision and data accessibility: all authors. Study guarantor and final version of the article: Sparén P.
Ethical approval
The Regional Ethics Committee in Stockholm, Sweden, granted ethical approval for our study and concluded that informed consent from the study subjects was not required.
Data sharing
All relevant source data are shown in the article and Supporting Information. If access to raw data is required, please contact par.sparen@ki.se. Data may be shared if all ethical and legal requirements are met for such a request.
Supporting information
Table S1: Number of case–control sets with different number of controls after removing women having had total hysterectomy
Table S2: SNOMED codes for cytological diagnoses in the study, defined by the Swedish Association for Clinical Cytology
Table S3: Odds ratios of cervical cancer (all stages, aged 29+) by screening status in the past two screening rounds, by histopathological type, further adjusted for parity
Table S4: Odds ratios of cervical cancer (all stages) by screening status in the past two screening rounds, by age and histopathological type, further adjusted for parity
Table S5: Odds ratios of cervical cancer in relation to screening status in the past two screening rounds, defining screening intervals as 3/5 years and 3.5/5.5 years
Table S6: Odds ratios of cervical cancer (all stages) among women having abnormalities compared to women unscreened in the past two screening rounds, by histological type
Table S7: Odds ratios of cervical cancer (all types) in relation to screening status in the past two screening rounds, by age and stage of cancer cases
Figure S1: Distribution of months between the first abnormality in the past year and cervical cancer diagnosis by mode of detection recorded in the clinical chart.
Acknowledgements
We thank Walter Ryd for histological review of cancer cases, and Pouran Almstedt for data management. Our study was funded by Swedish Foundation for Strategic Research [grant number KF10‐0046]; The Swedish Cancer Society [grant number CAN 2016/840]; The Swedish Research Council [grant number 2017‐02346]; Nordforsk [grant number 62721]; Centre for Research and Development, Uppsala University/Region of Gävleborg.
Conflict of interest: All authors have completed the Unified Competing Interest form and declare that no support from any organisation for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous 3 years; no other relationships or activities that could appear to have influenced the submitted work. Outside of the submitted work, JD has obtained grants to his institution from Merck, Roche and Genomica for research on HPV tests. KS received research grant from MSD for studies of HPV vaccination.
References
- 1. Schiffman M, Castle PE, Jeronimo J, et al. Human papillomavirus and cervical cancer. Lancet 2007;370:890–907. [DOI] [PubMed] [Google Scholar]
- 2. Lynge E, Rygaard C, Baillet MV‐P, et al. Cervical cancer screening at crossroads. APMIS 2014;122:667–73. [DOI] [PubMed] [Google Scholar]
- 3. Lynge E. Screening for cancer of the cervix uteri. World J Surg 1989;13:71–8. [DOI] [PubMed] [Google Scholar]
- 4. International Agency for Research on Cancer . IARC Handbooks of Cancer Prevention: Cervix Cancer Screening. Lyon, France: IARC, 2005. [cited 2019 May 26]; Available from: https://www.iarc.fr/en/publications/pdfs-online/prev/handbook10/HANDBOOK10.pdf. [Google Scholar]
- 5. Vaccarella S, Franceschi S, Engholm G, et al. 50 years of screening in the Nordic countries: quantifying the effects on cervical cancer incidence. Br J Cancer 2014;111:965–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lönnberg S, Hansen BT, Haldorsen T, et al. Cervical cancer prevented by screening: long‐term incidence trends by morphology in Norway. Int J Cancer 2015;137:1758–64. [DOI] [PubMed] [Google Scholar]
- 7. Hakama M. Effect of population screening for carcinoma of the uterine cervix in Finland. Maturitas 1985;7:3–10. [DOI] [PubMed] [Google Scholar]
- 8. Sasieni P, Adams J. Effect of screening on cervical cancer mortality in England and Wales: analysis of trends with an age period cohort model. BMJ 1999;318:1244–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Peto J, Gilham C, Fletcher O, et al. The cervical cancer epidemic that screening has prevented in the UK. Lancet 2004;364:249–56. [DOI] [PubMed] [Google Scholar]
- 10. Bray F, Loos AH, McCarron P, et al. Trends in cervical squamous cell carcinoma incidence in 13 European countries: changing risk and the effects of screening. Cancer Epidemiol Biomarkers Prev 2005;14:677–86. [DOI] [PubMed] [Google Scholar]
- 11. Lăără E, Day NE, Hakama M. Trends in mortality from cervical cancer in the Nordic countries: association with organised screening programmes. Lancet 1987;329:1247–9. [DOI] [PubMed] [Google Scholar]
- 12. Andrae B, Kemetli L, Sparén P, et al. Screening‐preventable cervical cancer risks: evidence from a nationwide audit in Sweden. J Natl Cancer Inst 2008;100:622–9. [DOI] [PubMed] [Google Scholar]
- 13. Andrae B, Andersson TM‐L, Lambert PC, et al. Screening and cervical cancer cure: population based cohort study. BMJ 2012;344:e900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lönnberg S, Anttila A, Luostarinen T, et al. Age‐specific effectiveness of the Finnish cervical cancer screening Programme. Cancer Epidemiol Biomarkers Prev 2012;21:1354–61. [DOI] [PubMed] [Google Scholar]
- 15. Sasieni P, Adams J, Cuzick J. Benefit of cervical screening at different ages: evidence from the UKaudit of screening histories. Br J Cancer 2003;89:88–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cuzick J. Routine audit of large‐scale cervical cancer screening programs. J Natl Cancer Inst 2008;100:605–6. [DOI] [PubMed] [Google Scholar]
- 17. Barlow L, Westergren K, Holmberg L, et al. The completeness of the Swedish cancer register—a sample survey for year 1998. Acta Oncol 2009;48:27–33. [DOI] [PubMed] [Google Scholar]
- 18. Ludvigsson JF, Almqvist C, Bonamy A‐KE, et al. Registers of the Swedish total population and their use in medical research. Eur J Epidemiol 2016;31:125–36. [DOI] [PubMed] [Google Scholar]
- 19. Ludvigsson JF, Andersson E, Ekbom A, et al. External review and validation of the Swedish national inpatient register. BMC Public Health 2011;11:450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lei J, Andrae B, Ploner A, et al. Cervical screening and risk of adenosquamous and rare histological types of invasive cervical carcinoma: population based nested case‐control study. BMJ 2019;365:l1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nationellt Kvalitetsregister för Cervixcancerprevention (NKCx) [Internet]. [cited 2019. May 26]; Available from: http://www.nkcx.se/index_e.htm.
- 22. Wang J, Andrae B, Sundström K, et al. Risk of invasive cervical cancer after atypical glandular cells in cervical screening: nationwide cohort study. BMJ 2016;352:i276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Longitudinal integration database for health insurance and labour market studies (LISA by Swedish acronym) [Internet]. Statistic Sweden. [cited 2019. May 26]; Available from: http://www.scb.se/lisa-en.
- 24. Eaker S, Adami H‐O, Granath F, et al. A large population‐based randomized controlled trial to increase attendance at screening for cervical cancer. Cancer Epidemiol Biomarkers Prev 2004;13:346–54. [PubMed] [Google Scholar]
- 25. Azerkan F, Sparén P, Sandin S, et al. Cervical screening participation and risk among Swedish‐born and immigrant women in Sweden. Int J Cancer 2012;130:937–47. [DOI] [PubMed] [Google Scholar]
- 26. Broberg G, Wang J, Östberg A‐L, et al. Socio‐economic and demographic determinants affecting participation in the Swedish cervical screening program: a population‐based case‐control study. PLoS One 2018;13:e0190171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Broberg G, Gyrd‐Hansen D, Miao Jonasson J, et al. Increasing participation in cervical cancer screening: offering a HPV self‐test to long‐term non‐attendees as part of RACOMIP, a Swedish randomized controlled trial. Int J Cancer 2014;134:2223–30. [DOI] [PubMed] [Google Scholar]
- 28. Strander B, Hällgren J, Sparén P. Effect of ageing on cervical or vaginal cancer in Swedish women previously treated for cervical intraepithelial neoplasia grade 3: population based cohort study of long term incidence and mortality. BMJ 2014;348:f7361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sundström K, Lu D, Elfström KM, et al. Follow‐up of women with cervical cytological abnormalities showing atypical squamous cells of undetermined significance or low‐grade squamous intraepithelial lesion: a nationwide cohort study. Am J Obstet Gynecol 2017;216:48.e1–48.e15. [DOI] [PubMed] [Google Scholar]
- 30. Melnikow J, McGahan C, Sawaya GF, et al. Cervical intraepithelial Neoplasia outcomes after treatment: long‐term follow‐up from the British Columbia cohort study. J Natl Cancer Inst 2009;101:721–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kjaer SK, Brinton LA. Adenocarcinomas of the uterine cervix: the epidemiology of an increasing problem. Epidemiol Rev 1993;15:486–91. [DOI] [PubMed] [Google Scholar]
- 32. Pimenta JM, Galindo C, Jenkins D, et al. Estimate of the global burden of cervical adenocarcinoma and potential impact of prophylactic human papillomavirus vaccination. BMC Cancer 2013;13:553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mitchell H, Medley G, Gordon I, et al. Cervical cytology reported as negative and risk of adenocarcinoma of the cervix: no strong evidence of benefit. Br J Cancer 1995;71:894–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. The International Collaboration of Epidemiological Studies of Cervical Cancer . Comparison of risk factors for invasive squamous cell carcinoma and adenocarcinoma of the cervix: collaborative reanalysis of individual data on 8,097 women with squamous cell carcinoma and 1,374 women with adenocarcinoma from 12 epidemiological studies. Int J Cancer 2007;120:885–91. [DOI] [PubMed] [Google Scholar]
- 35. Zappa M, Visioli CB, Ciatto S, et al. Lower protection of cytological screening for adenocarcinomas and shorter protection for younger women: the results of a case–control study in Florence. Br J Cancer 2004;90:1784–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Castanon A, Landy R, Sasieni PD. Is cervical screening preventing adenocarcinoma and adenosquamous carcinoma of the cervix? Int J Cancer 2016;139:1040–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ronco G, Dillner J, Elfström KM, et al. Efficacy of HPV‐based screening for prevention of invasive cervical cancer: follow‐up of four European randomised controlled trials. Lancet 2014;383:524–32. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: Number of case–control sets with different number of controls after removing women having had total hysterectomy
Table S2: SNOMED codes for cytological diagnoses in the study, defined by the Swedish Association for Clinical Cytology
Table S3: Odds ratios of cervical cancer (all stages, aged 29+) by screening status in the past two screening rounds, by histopathological type, further adjusted for parity
Table S4: Odds ratios of cervical cancer (all stages) by screening status in the past two screening rounds, by age and histopathological type, further adjusted for parity
Table S5: Odds ratios of cervical cancer in relation to screening status in the past two screening rounds, defining screening intervals as 3/5 years and 3.5/5.5 years
Table S6: Odds ratios of cervical cancer (all stages) among women having abnormalities compared to women unscreened in the past two screening rounds, by histological type
Table S7: Odds ratios of cervical cancer (all types) in relation to screening status in the past two screening rounds, by age and stage of cancer cases
Figure S1: Distribution of months between the first abnormality in the past year and cervical cancer diagnosis by mode of detection recorded in the clinical chart.


