Abstarct
Purpose
Children with Down syndrome (DS) more often have strabismus, refractive errors, accommodative lags and reduced visual acuity (VA) than typically developing children. In this study, we compare the effects of bifocal glasses with those of unifocal glasses in children with DS. Changes in angle of strabismus, accommodation and refractive error were analysed in this paper.
Methods
In a multicentre randomized controlled trial, 119 children with DS, aged 2–16, were randomly allocated for bifocal or unifocal glasses (with full correction of refractive error in cycloplegia). The 15 centres, all in the Netherlands, followed the participants for 1 year. Changes in refractive error, accommodative accuracy, strabismus, binocularity and stereopsis were compared across 4 subsequent visits.
Results
Refractive errors and accommodative errors showed no significant change throughout the course of our study in either intervention group. The manifest angle of strabismus, however, reduced significantly in the bifocal group. This improvement was observed shortly after the children received their new correction (~6 weeks) (linear regression: t = 3.652, p < 0.001) and remained present in the final measurements after 1 year (linear regression: t = 3.604, p < 0.001). The percentage of children with positive binocularity and stereo tests showed no significant differences between the groups.
Conclusion
Bifocals with full correction of refractive error reduce the manifest angle of strabismus within a few weeks. No effects on accommodation, refractive error, stereopsis and binocularity occurred over the course of 1 year.
Keywords: conventional strabismus treatment, esotropia, near addition in children, ocular accommodation, ocular alignment, refractive error
Introduction
In children with Down syndrome (DS), strabismus, accommodative lag, refractive errors and poor visual acuity (VA) are more frequent and severe than in typically developing children. Prevalences mentioned in the literature for children with DS were 15–47%, 50–90%, 40–90% and 80–100%, respectively (de Weger et al. 2018).
The differences in visual development between children with and without DS have to be taken into account when prescribing glasses to children with DS. Major differences exist in accommodation, strabismus and refractive error. Firstly, in children with DS, accurate accommodation often does not develop in the first weeks of life as is the case with typically developing children (Woodhouse et al. 1993, 1996, 2000; Haugen et al. 2001b; Cregg et al. 2001; Al‐Bagdady et al. 2009; Nandakumar & Leat 2009, 2010; Anderson et al. 2011; Doyle et al. 2016, 2017; Candy & Bharadwaj 2007; Horwood et al. 2015).
Secondly, in children with DS, the prevalence of strabismus, usually in the form of acquired esotropia, is higher than in typically developing children. The onset of esotropia is between age 3 and age 6, mostly at age 4, whereas in typically developing children, the onset of acquired esotropia is more often earlier in life, around the age of 2 (da Cunha & Moreira 1996; Haugen & Hovding 2001a; Von Noorden & Campos 2002; Yurdakul et al. 2006; Morton 2011, Watt et al. 2015). In the majority of children with DS, the potential for binocularity could have developed in their early years before the onset of manifest strabismus. However, due to the risk of ocular comorbidities in children with DS, there are many other factors that could have prevented normal visual development and binocularity including uncorrected significant refractive error, anisometropic amblyopia, ocular pathology including congenital cataract, all of which are likely to be present from birth.
Thirdly, in children with DS, the emmetropization process in their first years of life is not the same as in typically developing children. In particular, their refractive errors do not diminish, leaving them, for instance, with hyperopia and oblique astigmatism (Cregg et al. 2003; Haugen et al. 2001b; Ehrlich et al. 1997; Atkinson et al. 2000).
Recent evidence shows that in children who lack the ability to accommodate accurately, bifocals help to improve near visual acuity (NVA) as they produce focused images on the retina for both distance and near vision without requiring accommodation (Nandakumar & Leat 2010; de Weger et al. 2018).
A recent study by Doyle et al. (2017) demonstrated that retinal disparity is the main driver to both the accommodative and vergence systems in DS and furthermore illustrated the diminished influence of retinal blur cues to accommodation and vergence in DS, both indicative of a sensory deficit of the accommodative system (Doyle et al. 2017). This supports the earlier finding that the accommodative system of children with DS may have the physical capacity to respond to a given stimulus, but the neural control of the system is defective (Cregg et al. 2001; Doyle et al. 2016), resulting in a consistent lag of accommodation and a defocused (optically) retinal image at near in most children with DS. Consequently, for near vision without near addition, they attempt to compensate for the accommodative lag by increasing the accommodative effort. The increased effort is accompanied by convergence excess.
Thus, it seems likely that the accommodative lag is a contributing factor in the incidence of esotropia. If alignment and refractive error problems are detected early, latent deviations might be managed before adverse sequelae develop (Watt et al. 2015).
Bifocals diminish the need for accommodation and thereby prevent excessive convergence. Therefore, bifocals could reduce or prevent a manifest angle of strabismus in children with DS. However, thus far there is not enough evidence to support this effect of bifocals. Until now, the only published study on the effect of bifocals on ocular alignment in children with DS is the one by Haugen & Hovding (2001a), who mentioned relief of strabismus with bifocals in four out of five children with DS. Three other studies on strabismus therapy in DS concentrated on surgical methods (Yahalom et al. 2010; Perez et al. 2013; Motley et al. 2012). In relation to surgical methods, the effect of bifocals on strabismus could be relevant because in this vulnerable group, non‐surgical therapy would be preferred.
To analyse the effect of bifocals on the manifest angle of strabismus in a large group of children with DS, we included the assessments of ocular alignment and strabismus in our multicentre randomized controlled trial (de Weger et al. 2018). In the present study, we analysed the effects of bifocals on the angle of strabismus, binocularity, stereopsis, refractive errors and accommodative lags.
Methods and Patients
Study design
We conducted a multicentre randomized controlled trial to compare the effects of bifocals with the effects of unifocals in 119 children with DS, aged 2–16, with accommodative lags. The children from participating institutes were randomly allocated to the two intervention groups: bifocals and unifocals. Randomization, a permuted block randomization schedule, stratified by gender, age and language development (parents report: speaking in 1–3 word sentences and speaking in 4 word or longer sentences). This schedule was used to randomly assign a child with equal probability to one of the two treatment groups. The intervention group to which the child was assigned was always known to the participant, the orthoptist and the investigator, because bifocal glasses are a visually prominent marker.
In both groups, we applied full correction of refractive error measured using cycloplegia. The bifocal segment top of the applied longline (flat‐top or D‐segment) bifocals with addition S + 2.5, used in the bifocal group, was placed at the pupillary centre, as used in previous studies in which good results were achieved in improving near vision and compliance in wearing these glasses (Stewart et al. 2005; Al‐Bagdady et al. 2009). The children were seen on four occasions, T0 (baseline), T1 ~6 weeks, T2 6 months and T3 1 year after inclusion. For further details, see de Weger et al. (2018) and Fig. 1.
Figure 1.
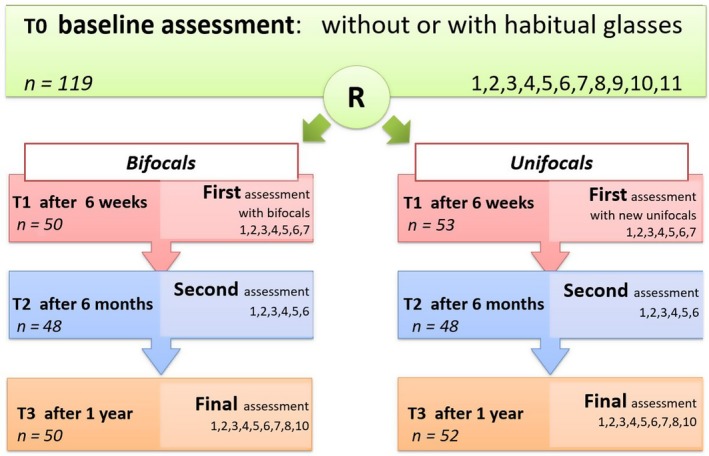
Study design, time line with applied diagnostic procedures at each visit (T0, T1, T2 and T3) and the number of children who were present at that point in time (this figure was duplicated from de Weger et al. 2018). R = age‐ and gender‐matched randomization; Assessments: 1 = anamnesis; 2 = ocular alignment; 3 = binocularity and stereopsis; 4 = Distance Visual Acuity; 5 = Near Visual Acuity, uncrowded and crowded; 6 = dynamic retinoscopy; 7 = Minnesota Executive Function Scale; 8 = objective refractive error in cycloplegia and prescription of glasses, 9 = ophthalmological examination for exclusion of pathology, by the ophthalmologist of the clinic, 10 = questionnaires BRIEF‐P and BRIEF, 11 = questionnaire Vineland‐S.
The project was conducted in accordance with the Declaration of Helsinki and approved by the Dutch medical Ethics Committee of the Isala Hospitals (NL48288.75.14/Metc:14.0333).
Measurement procedures
Compliance in using the bifocals in an appropriate way was assessed in a qualitative manner, by observation and parent report at the start of T1, T2 and T3, and was reaffirmed by the orthoptists during assessment.
In case of (nearly) straight eye position (evaluated with corneal light reflex at the beginning of the assessment), binocularity and stereopsis were assessed. Binocularity was assessed by positive base out 15 dioptre prism test. Stereopsis was tested with Lang Stereotest (no dissociation glasses needed) (Lang‐Stereotest AG, Küsnacht, Switzerland), Titmus Fly (with polarization dissociation glasses) (Stereo Optical Co., Inc., Chicago, IL) or TNO test (red/green dissociation glasses) (Lameris Ootech, Nieuwegein, The Netherlands), chosen by the orthoptist according to the developmental stage of the child.
After that, both manifest and latent strabismus were assessed with the cover test at 30 cm and 5 m. At the first (T0), second (T1) and fourth (T3) visit, the manifest or latent angle was measured using the prism cover test at 30 cm and 5 m. If the prism cover test was not feasible because of lack of co‐operation, the Hirschberg corneal reflex test (Hasebe et al. 1998) at 30 cm and 2.5 m was applied to measure the manifest angle.
Accommodative accuracy was measured at all four visits with dynamic retinoscopy, the ‘modified Nott method’ (Woodhouse et al. 1993; Leat & Gargon 1996; McClelland & Saunders 2003) through unifocals or in the case of bifocals through the distance portion. First, the accommodative accuracy was assessed at a distance of 25 cm (4 dioptres of accommodation). If no accommodative lag was found, the measurement was repeated at 16.7 cm (6 dioptres of accommodation). In previous studies, these distances were found to be useful to elicit accommodative lags (Woodhouse et al. 1996; Al‐Bagdady et al. 2009; Stewart et al. 2007; Nandakumar & Leat 2009).
Refractive errors were assessed at the end of the first (T0) and fourth (T3) visit. A combination of streak retinoscopy and autorefraction was applied (Wübbolt et al. 2006; Marsack et al. 2017). Measurements were taken under cycloplegia (cyclopentolate 0.5% in young children (>3 months < 6 years) and 1.0% from the age of 6 years, as in guidelines for usual care) or if cycloplegia was not possible because of contraindications, under mydriasis (tropicamide 0.5%).
Statistical analysis
Statistical analysis was performed using the statistical package for the social sciences (SPSS version 23, IBM Inc., Chicago, IL). ANCOVA (general linear model, GLM) with baseline performance as the covariate was used to analyse the differences between the two intervention groups. Correction for baseline measurement was applied, because changes were significantly correlated with baseline measurements. Multiple linear regression analysis was applied to analyse the influence of explanatory variables and their interaction.
The difference between the pre‐ and post‐test was determined as the observed change over time: T1‐T0 is the short‐term change and T3‐T0 is the 1‐year change (negative values indicate improvement). For analyses of the manifest angle of strabismus, we used the angles measured with prism test. If these were not available, we used the assessments with Hirschberg corneal reflex test and recalculated the values to prism dioptres. If Hirschberg corneal reflex test data were also not available, we used manifest angles assessed with the cover test, which were converted to prism dioptres as well. For analyses, the refractive errors were expressed in spherical equivalent of the least ametropic eye (SER). For analyses of age dependency of the intervention effect on manifest strabismus, the participants were stratified into age groups, under 6 years and over 6 years, because strabismus is most common onset at the age of 4 (between 3 and 6 years of age).
Influences of the phenomenon of regression to the mean (RTM) and differential treatment effect were analysed as in de Weger et al. (2018).
Results
The population of children with DS that was included in this study proved comparable to DS populations described in the literature as far as ophthalmological findings are concerned (reviews of Watt et al. 2015 and Afifi et al. 2013). Randomization resulted in two intervention groups with no statistical baseline difference in mean age, gender prevalence, strabismus, nystagmus, compliance in wearing glasses, absence of prescription glasses, attendance of mainstream school education, refractive errors (hyperopia, myopia, astigmatism, axis), SER, uncrowded NVA, crowded NVA, DVA, and accommodative lag (de Weger et al. 2018).
Compliance
Nearly all children learned to use their bifocals in the appropriate way. After a few weeks of using their newly prescribed bifocals, parents and orthoptists of only 6 children reported incorrect use; after 6 months, four children did not use their bifocals correctly; and after 1 year, only one child did not always use the bifocal adequately. All children whom were issued bifocals continued to participate in the study.
Refractive errors
The refractive errors were not significantly different between the two intervention groups when measured a second time after 1 year (ANCOVA, F(79) = 1.319, p = 0.254) (Fig. 2A, Table 1).
Figure 2.
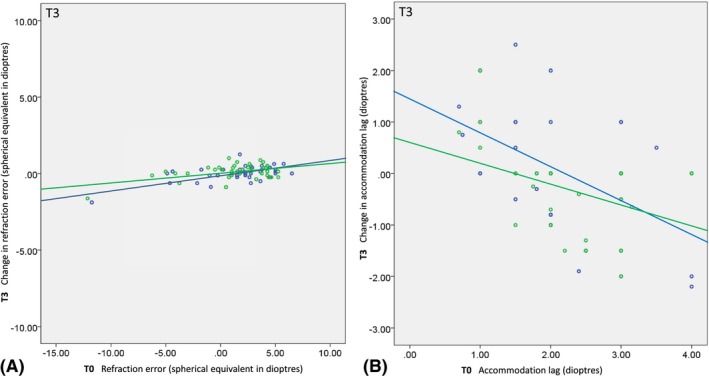
A and B: Scatterplots of the 1‐year change (i.e. the within‐subject difference between T0 and T3) as a function of baseline (T0) for refractive errors (i.e. spherical equivalent of least ametropic eye) (A) and accommodative lags measured through unifocals or the distance part of the bifocals (B) by dynamic retinoscopy ‘modified Nott method’ in the two treatment groups. A: Positive refractive errors indicate hyperopia; negative errors indicate myopias. B: Positive changes in accommodative lag (y‐axis) correspond with increased lags, negative with decreased (improved) lags. Solid lines are regression lines through the data. A: Regression line equations, bifocals Y = 0.12 + 0.09*x, unifocals Y = 0.02 + 0.06*x; B: regression line equations, bifocals Y = 1.39‐0.65*x, unifocals Y = 0.63‐0.42*x. Blue = bifocals; Green = unifocals.
Table 1.
Refractive errors.
| Bifocals | Unifocals | p Value | Test statistic | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | Std dev | Range min | Range max | n | Mean | Std dev | Range min | Range max | n | ||||
| T0 | SER of the least ametropic eye | 1.68 | 3.29 | −11.75 | 6.50 | 50 | 1.32 | 3.14 | −12.13 | 5.25 | 54 | 0.579† | t(102) = 0.556 |
| Hyperopia | 3.14 | 1.35 | 38 | 2.67 | 1.42 | 40 | 0.141† | t(76) = 1.487 | |||||
| Emmetropia | −0.04 | 0.26 | 3 | 0.00 | 0.33 | 7 | 0.847† | t(8) = −0.199 | |||||
| Myopia | −3.93 | 3.33 | 9 | −5.06 | 3.50 | 7 | 0.524† | t(14) = 0.654 | |||||
| T3 | SER of the least ametropic eye | 1.74 | 3.84 | −13.63 | 6.50 | 40 | 1.27 | 3.70 | −13.75 | 5.38 | 42 | 0.253‡ | F(79) = 1.325 |
| Hyperopia | 3.49 | 1.46 | 30 | 2.92 | 1.47 | 31 | 0.772‡ | F(58) = 0.085 | |||||
| Emmetropia | 0.06 | 0.62 | 2 | 0.00 | 0.37 | 4 | 0.899‡ | F(3) = −0.019 | |||||
| Myopia | 4.39 | 4.05 | 8 | −5.32 | 4.10 | 7 | 0.163‡ | F(12) = 2.212 | |||||
Group averages of refractive errors measured in cycloplegia and expressed in spherical equivalents of the least ametropic eye (SER) assessed at T0 (baseline assessment) and at T3 (final assessment after 1 year).
Hyperopia: SER > S + 0.5. Emmetropia: S‐0.5 ≤ SER ≤ S + 0.5. Myopia: SER < S‐0.5.
Max = maximum, Min = minimum, Std dev = standard deviation.
†Student's t‐test.
‡ANCOVA with baseline as covariate.
Accommodative lag
The accommodative lag assessed at 25 cm showed no significant change over time in either intervention group. Neither were any differences found between the two intervention groups at T1, when the children started wearing their newly prescribed glasses, nor at later time‐points T2 and T3 (Fig. 2, Table 2). At T1, the mean change in accommodative lag was ‐0.23 ± 1.09 in the bifocal group and ‐0.32 ± 1.06 in the unifocal group (ANCOVA, F(74) = 0.030, p = 0.862). At T2, the mean change in accommodative lag was ‐0.34 ± 0.93 in the bifocal group and ‐0.20 ± 0.76 in the unifocal group (ANCOVA, F(67) = 0.048, p = 0.828). After 1 year, at T3, the mean change in accommodative lag was ‐0.01 ± 1.20 in the bifocal group and ‐0.36 ± 0.83 in the unifocal group (ANCOVA, F(67) = 1.325, p = 0.254).
Table 2.
Accommodative lag.
| Bifocals | Unifocals | p Value | Test statistic | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | Std dev | Range min | Range max | n | Mean | Std dev | Range min | Range max | n | ||||
| T0 | Accommodation lag (dioptres) | 2.17 | 0.91 | 0.7 | 4.0 | 44 | 2.25 | 0.88 | 0.3 | 4.0 | 50 | 0.673† | t(92) = −0.423 |
| T1 | Accommodation lag (dioptres) | 1.74 | 0.99 | 0.5 | 4.0 | 39 | 2.00 | 1.05 | 0.5 | 4.0 | 40 | 0.313† | t(79) = −1.015 |
| T2 | Accommodation lag (dioptres) | 1.79 | 0.88 | 0.0 | 4.0 | 33 | 1.94 | 0.95 | 0.3 | 4.0 | 40 | 0.499† | t(71) = −0.680 |
| T3 | Accommodation lag (dioptres) | 2.10 | 1.10 | 0.5 | 4.0 | 35 | 1.99 | 0.88 | 0.5 | 4.0 | 37 | 0.570† | t(70) = −0570 |
Accommodative lag in dioptres measured at 25 cm assessed by dynamic retinoscopy ‘modified Nott method’ at T0 through habitual correction and at T1, T2 and T3 through the distance segment of bifocals or through unifocals.
Max = maximum, Min = minimum, Std dev = standard deviation.
†Student's t‐test.
Note that the observed changes in accommodative accuracy showed a significant correlation with the baseline values (correlation bifocals: R = −0.506, p = 0.002; unifocals: R = −0.410, p = 0.013). In both groups, large accommodative lags at baseline tended to decrease while small accommodative lags at baseline tended to increase (Fig. 2B). Further analysis indicated, however, that this significant negative correlation was due to a large percentage of RTM (61%; R (pre,post) = 0.386, p = 0.001, Prm = 1‐0.386). Regression to the mean (RTM) is a statistical phenomenon which is always present in repeated measures, most notably in measures that have considerable uncertainty (Oldham 1962; Barnett et al. 2005; Trochim 2006).
Manifest angle of strabismus
The scatterplots in Fig. 3 illustrate that bifocals had more effect than unifocals in reducing the manifest angles of strabismus. In these plots, full correction of ocular alignment towards straight eye position would result in regression lines with a negative slope of −1 passing through the origin (dotted black lines). Hence, the beneficial effect of bifocals over unifocals is evident from the fact that the slopes of the regression lines were steeper and closer to −1 for the bifocal group (blue) compared with the unifocal group (green). Note that this difference was already present at T1, shortly after the children started wearing their newly prescribed glasses (treatment × baseline interaction: t = 5.913, p < 0.001) (Tables 3 and 4). Further changes between T1 and T3 were not significant (t = 0.857, p = 0.394); the initial improvements remained until the final measurements after 1 year (t = 6.813, p < 0.001). For this analysis, we excluded the two subjects with the largest manifest angles of strabismus (45 prism dioptres), one in the bifocal group and one in the unifocal group (crosses). The slope values of the regression lines in the bifocal group were strongly biased by the one child having a 45 prism dioptres manifest angle of strabismus. However, with all subjects included, the treatment difference was still statistically significant (treatment × baseline interaction at T1: t = 3.652, p < 0.001 and T2: t = 3.604, p < 0.001).
Figure 3.
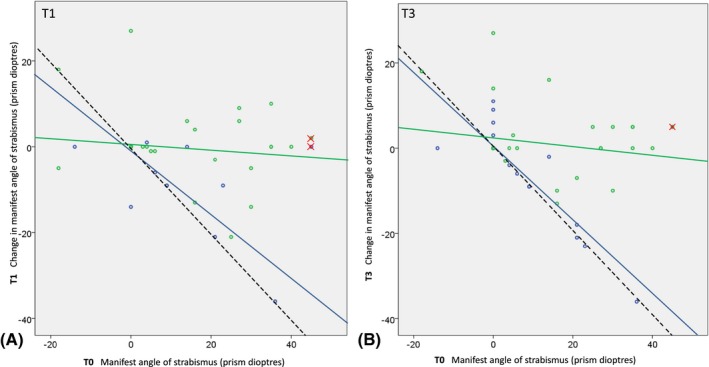
A and B: Scatterplots of change as a function of baseline (T0) manifest angle of strabismus. (A) Short‐term change (i.e. the within‐subject difference between T0 and T1); (B) 1‐year change (i.e. the within‐subject difference between T0 and T3). Positive values in manifest angle of strabismus (x‐axis) indicate esotropias; negative values indicate exotropias. Negative changes in manifest angle of strabismus (y‐axis) indicate decreased (improved) esotropias or increased exotropias, depending on the manifest angle of strabismus at baseline. Solid lines are regression lines through the data excluding the two large esotropias (crosses; one in bifocal and one in unifocal group). Dotted black lines indicate the change in manifest angle of strabismus that is required for perfect correction to ‘straight eyes’. A: Regression line equations, bifocals Y = 0.54‐0.76*x, unifocals Y = 0.91‐0.09*x; B: regression line equations, bifocals Y = 0.30‐0.88*x, unifocals Y = 2.23‐0.12*x. At T1 (i.e. shortly after the children started with their newly prescribed glasses), the slopes of the regression lines of bifocals and unifocals are significantly different (A: t = 5.913, p < 0.001; B: t = 6.813, p < 0.001) representing a significantly different treatment effect of the two interventions. Blue = bifocals; Green = unifocals.
Table 3.
Angle of manifest strabismus.
| Bifocals | Unifocals | p Value | Test statistic | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean | Std dev | Median | Interq 25 | Interq 75 | n | Mean | Std dev | Median | Interq 25 | Interq 75 | ||||
| (A) All participants | |||||||||||||||
| T0 | Absolute angle of manifest strabismus (prism dioptres) | 50 | 4.04 | 9.61 | 0.00 | 0 | 0 | 54 | 8.33 | 13.26 | 0.00 | 0 | 17 | 0.071† | 1125.500 |
| T1 | Absolute angle of manifest strabismus (prism dioptres) | 50 | 2.12 | 7.30 | 0.00 | 0 | 0 | 53 | 8.36 | 14.09 | 0.00 | 0 | 17 | 0.002† | 972.000 |
| T3 | Absolute angle of manifest strabismus (prism dioptres) | 50 | 2.16 | 7.64 | 0.00 | 0 | 0 | 52 | 8.77 | 14.48 | 0.00 | 0 | 14 | 0.010† | 1000.500 |
| (B) Children aged under 6 years | |||||||||||||||
| T0 | Absolute angle of manifest strabismus (prism dioptres) | 15 | 1.67 | 5.45 | 0.00 | 0 | 0 | 15 | 4.87 | 7.18 | 0.00 | 0 | 14 | 0.122† | 83.500 |
| T1 | Absolute angle of manifest strabismus (prism dioptres) | 15 | 0.33 | 1.29 | 0.00 | 0 | 0 | 15 | 5.20 | 9.13 | 0.00 | 0 | 5 | 0.033† | 74.500 |
| T3 | Absolute angle of manifest strabismus (prism dioptres) | 15 | 0.40 | 1.55 | 0.00 | 0 | 0 | 14 | 6.14 | 10.3 | 0.00 | 0 | 8 | 0.023† | 66.000 |
| (C) Children aged over 6 years | |||||||||||||||
| T0 | Absolute angle of manifest strabismus (prism dioptres) | 35 | 5.06 | 10.83 | 0.00 | 0 | 6 | 39 | 9.67 | 14.82 | 0.00 | 0 | 25 | 0.237† | 593.000 |
| T1 | Absolute angle of manifest strabismus (prism dioptres) | 35 | 2.89 | 8.60 | 0.00 | 0 | 0 | 38 | 9.61 | 15.55 | 0.00 | 0 | 19 | 0.022† | 504.500 |
| T3 | Absolute angle of manifest strabismus (prism dioptres) | 35 | 2.91 | 9.01 | 0.00 | 0 | 0 | 38 | 9.74 | 15.75 | 0.00 | 0 | 22 | 0.094† | 546.000 |
Group averages of the absolute manifest angle of strabismus in prism dioptres at T0 (baseline assessment), T1 (when the children started using their new glasses) and T3 (final assessment after 1 year). Participants were stratified into age groups depending on age 6 (B and C) because strabismus is common onset prior to the age of 6. Note the reduction of the size of strabismus angle when using bifocals: A, B and C (ANCOVA, all p < 0.001); in the unifocal group, no changes were found (ANCOVA, all p > 0.087).
Interq = interquartile, Std dev = standard deviation.
†Mann–Whitney U‐test.
Table 4.
Ocular alignment.
| Bifocals | Unifocals | p Value | Test statistic | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Exotropia medium | Orthotropia | Esotropia | n | Exotropia medium | Orthotropia | Esotropia | |||||||||||||
| Micro | Small | Medium | Large | Very large | Micro | Small | Medium | Large | Very large | |||||||||||
| (A) All participants | ||||||||||||||||||||
| T0 | 50 | 1 (2) | 39 (78) | 10 (20) | 2 | 1 | 4 | 1 | 0 | 54 | 2 (4) | 34 (63) | 18 (33) | 4 | 0 | 7 | 7 | 0 | 0.245† | X²(2) = 2.812 |
| T1 | 50 | 2 (4) | 44 (88) | 4 (8) | 1 | 0 | 2 | 1 | 0 | 53 | 1 (2) | 33 (61) | 19 (35) | 6 | 0 | 6 | 7 | 0 | 0.003† | X²(2) = 11.610 |
| T3 | 50 | 1 (2) | 42 (84) | 7 (14) | 3 | 3 | 0 | 1 | 0 | 52 | 0 (0) | 33 (61) | 19 (35) | 4 | 1 | 6 | 8 | 0 | 0.023† | X²(2) = 7.582 |
| Bifocals | Unifocals | p Value | Test statistic | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Exotropia | Orthotropia | Esotropia | n | Exotropia medium | Orthotropia | Esotropia | |||||||||||||
| Micro | Small | Medium | Large | Very large | Micro | Small | Medium | Large | Very large | |||||||||||
| (B) Children aged under 6 years | ||||||||||||||||||||
| T0 | 15 | 0 (0) | 13 (87) | 2 (13) | 1 | 0 | 1 | 0 | 0 | 15 | 1 (7) | 9 (60) | 5 (34) | 2 | 0 | 3 | 0 | 0 | 0.222† | X²(2) = 3.013 |
| T1 | 15 | 0 (0) | 14 (93) | 1 (7) | 1 | 0 | 0 | 0 | 0 | 15 | 0 (0) | 9 (60) | 6 (40) | 3 | 0 | 3 | 0 | 0 | 0.040‡ | X²(1) = 4.658 |
| T3 | 15 | 0 (0) | 14 (93) | 1 (7) | 1 | 0 | 0 | 0 | 0 | 14 | 0(0) | 8 (53) | 6 (40) | 3 | 0 | 2 | 1 | 0 | 0.031‡ | X²(1) = 5.179 |
| Bifocals | Unifocals | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Exotropia medium | Orthotropia | Esotropia | n | Exotropia medium | Orthotropia | Esotropia | p Value | Test statistic | |||||||||||
| Micro | Small | Medium | Large | Very large | Micro | Small | Medium | Large | Very large | |||||||||||
| (C) Children aged over 6 years | ||||||||||||||||||||
| T0 | 35 | 1 (3) | 26 (74) | 8 (23) | 1 | 2 | 3 | 2 | 0 | 39 | 1 (3) | 25 (64) | 13 (33) | 2 | 0 | 3 | 7 | 0 | 0.608† | X²(2) = 0.997 |
| T1 | 35 | 2(6) | 30 (86) | 3 (9) | 0 | 0 | 2 | 1 | 0 | 38 | 1 (3) | 24 (62) | 13 (34) | 3 | 0 | 3 | 7 | 0 | 0.028† | X²(2) = 7.139 |
| T3 | 35 | 1 (3) | 28 (80) | 6 (17) | 2 | 3 | 0 | 1 | 0 | 38 | 0 (0) | 25 (64) | 13 (34) | 1 | 1 | 4 | 7 | 0 | 0.163† | X²(1) = 3.632 |
The number of children in the categories of ocular alignment: exotropia, orthotropia and esotropia, subdivided into size, micro, small, medium, large and very large, assessed at T0 (baseline assessment), T1 (when the children started using their new glasses) and T3 (final assessment after 1 year). Participants were stratified into age groups depending on age 6 (B and C) because strabismus is common onset prior to the age of 6. Note the reduction in proportion of esotropia and prevention of the onset of esotropia when starting with bifocals: A (McNemar, p = 0.030); in the unifocal group, no reduction was found: A(McNemar, p = 0.368).
Large = manifest strabismus angle of 29–57 prism dioptres, Medium = manifest strabismus angle of 14–28 prism dioptres, Micro = manifest strabismus angle of 1–6 prism dioptres, n = number of children assessed, Small = manifest strabismus angle of 7–13 prism dioptres; Very large = manifest strabismus angle of > 58 prism dioptres.
A value between brackets () indicates the percentage.
†Chi‐square test.
‡Fisher's exact test.
Besides baseline angle of manifest strabismus, other factors could have influenced the observed change in the angle of manifest strabismus during the development of the child. We therefore checked, in a multivariate linear regression, for other variables influencing the change of the manifest angle of strabismus (age, accommodative lag, refractive error, nystagmus, distance visual acuity, uncrowded near visual acuity and crowded near visual acuity). No other variables with significant influence on the strabismus angle (all p > 0.155) were found.
When stratifying the participants into age groups under 6 years and over 6 years, we found no age group effect (treatment x age group at T3: t = −0.007, p = 0.994) on the manifest angle of strabismus after 1 year (Tables 3 and 4).
Binocularity and stereopsis
There were no differences in relative frequencies of positive tests between the groups at any time‐point (all p > 0.212) (Tables 1 and 2). However, measures of stereopsis could not be collected in all children, and the choice of test applied varied according to the developmental stage of the child. By combining the results of assessments with different stereo tests in those children in whom we could assess stereopsis, we could only could compare the presence of stereopsis, without being able to grade the stereopsis (Table 5). This analysis showed that the relative frequencies of positive stereo tests were not significantly different between the two intervention groups, neither at T1, nor at T2 or T3 (all p > 0.444).
Table 5.
Binocular functions.
| Bifocals | Unifocals | |||||||
|---|---|---|---|---|---|---|---|---|
| Present at that point in time | Positive test result | Not assessed | Present at that point in time | Positive test result | Not assessed | P Value | ||
| T0 | Stereopsis | 50 | 26 (52) | 9 (18) | 53 | 28 (52) | 14 (26) | 0.530† |
| Binocularity with 15 dioptre prism test | 42 (84) | 4 (8) | 35 (65) | 11 (20) | 0.171† | |||
| T1 | Stereopsis | 50 | 35 (70) | 6 (12) | 53 | 27 (50) | 16 (30) | 0.372† |
| Binocularity with 15 dioptre prism test | 36 (72) | 8 (16) | 30 (56) | 19 (35) | 1.000† | |||
| T2 | Stereopsis | 48 | 32 (64) | 10 (20) | 48 | 31 (57) | 15 (28) | 0.955† |
| Binocularity with 15 dioptre prism test | 38 (76) | 8 (16) | 30 (56) | 16 (30) | 0.149† | |||
| T3 | Stereopsis | 50 | 33 (66) | 8 (16) | 52 | 31 (50) | 15 (28) | 0.919† |
| Binocularity with 15 dioptre prism test | 40 (80) | 4 (8) | 30 (56) | 12 (22) | 0.296† | |||
Number of children with positive test results and children who did not need to be assessed, mainly because of ocular alignment which is incompatible with binocularity or could not be assessed because of poor co‐operation. A value between brackets () indicates percentage.
†chi‐square test.
Discussion
Our results indicate a significant improvement in ocular alignment with bifocals. We found a significant difference in reduction of manifest angle of strabismus between bifocals and unifocals: in the bifocal group, more children with orthotropia were found and in those in whom manifest strabismus remained, the ocular alignment was cosmetically better because of smaller manifest angles of strabismus; in the unifocal group, the manifest angle of strabismus did not change. The improvement in the bifocal group was visible shortly after starting the use of bifocals and persisted up to the following year.
Time frame of changes
We observed a clear difference regarding the time frame in which the effects of bifocals occurred. Our present study did not reveal a change in mean refractive errors in either intervention group over the course of 1 year; apparently this time frame was too short (Esposito Veneruso et al. 2018). Yet, NVA did improve significantly after 1 year in the bifocal group, as we reported in our previous study (de Weger et al. 2018). This agrees with the finding of Atkinson & Braddick (1983) who described the time frame (several years) needed for visual acuity to develop. In contrast, the manifest angle of strabismus showed an almost immediate effect in the bifocal group shortly (~6 weeks) after the start with the new corrections that persisted up to the final measurements after 1 year. During these months, this improved ocular alignment could have supported the development of better near vision. And, vice versa, the improved visual acuity could have supported the relief of strabismus, in line with the finding of Binder et al. (2016). He observed that some patients presenting with CVI and strabismus experience reduction of strabismus concurrently with improvement in their visual acuity, even to the point of spontaneous resolution of strabismus.
Age
Although strabismus is most common onset at the age of 4 (between 3 and 6 years of age), and more improvement of ocular alignment might have been expected in these young children, we found no difference in effect of the bifocals when comparing age groups under 6 years and over 6 years. This implies that we did not find an age limit for treatment of strabismus with bifocals.
Refractive error, accommodation and convergence
At baseline, strabismus occurred with all forms and magnitudes of refractive errors, as previously described by Cregg et al. (2003). After one year, we found better ocular alignment in the bifocal group independent of the children's baseline refractive error. This finding agrees with the findings of Doyle et al. (2016 and) Doyle et al. (2017): (i) the vergence response to disparity is relatively intact and independent of accommodative and pupillary response, that is when eliminating the need for accommodative response, the vergence response is accurate; and (ii), when children with DS change their viewing distance to a nearer target, they are not able to scale their accommodative response accurately. In our study, wearing bifocals with full correction of any refractive error, which diminishes the need to exert accommodation for both distance and for near vision, led to better alignment compared to wearing unifocals. Unifocals only diminish the need to exert accommodation for distance vision, while the problem of not being able to scale the accommodation for near distances persists.
Therefore, if accommodation is not demanded while wearing bifocals, children with accommodative lags (like in DS or CVI (Boot et al. 2010; Hoyt 2013 and Fazzi et al. 2007)) will not be troubled by the associated (excessive) convergence response when changing viewing distance.
Conventional strabismus treatment
We believe that the results of our study, which are in line with Haugen & Hovding (2001a), underline the usefulness of the non‐surgical intervention in DS in convergent strabismus. The more so because the improvement of ocular alignment with bifocals is an additional benefit to the improved near visual acuity with bifocals. A non‐surgical intervention is preferred because children with DS often have comorbidity such as congenital heart defects, and an intervention without anaesthesia is therefore preferred.
Strengths and limitations
The overall strength of our RCT compared to all previous studies is described in our previous publication (de Weger et al. 2018). An important strength relevant for this paper, is the representativeness of the distribution of angles of strabismus for ocular alignment in DS.
However, the scarcity of large esotropias (>40 prism dioptres) or exotropias in a representative population of children with DS led to a very small number of children with exotropia or large esotropia in our study population. As a consequence, our findings regarding the effect of bifocals and unifocals on strabismus may not be generalized to children with exotropias or esotropias with an angle over 40 prism dioptres.
Unfortunately, the manifest angle of strabismus could not be assessed in a uniform way for all participants because of the expected co‐operation problems (Courage et al. 1994, 1997; Woodhouse et al. 1996; McCullough et al. 2014; Doyle et al. 2016, 2017). Our approach to deal with this limitation was to combine data from testing methods, as did Yurdakul et al. (2006), Yahalom et al. (2010) and Perez et al. (2013), and limit the analyses to measurements at short distances.
In the analysis of the quality of binocularity and stereopsis, we encountered substantial variability in co‐operation and communication in children with DS, as did Yahalom et al. (2010) and Haugen & Hovding (2001a). More research is needed to study the development of binocularity and stereopsis after longer follow‐up times in children who ideally start using bifocals at a young age, such as the age of 2.
Conclusion
Bifocals with full correction of refractive errors help relieve the manifest angle of strabismus in children with DS with accommodative lags within a few weeks, whereas unifocals have no effect, not even after a year. In the bifocal group, the angle of strabismus was reduced or orthotropia was achieved. Once the need for an accommodative effort is eliminated with bifocals, ocular alignment can improve. The improvement in ocular alignment indicates that bifocals could be an important solution for children with DS and strabismus, which is preferable to surgical interventions in view of the large number of contraindications for anaesthesia.
We thank all participants in this study and their parents, the research assistants Y. Kras and L. van der Helm and all the orthoptists of the participating locations. Without their co‐operation, we would not have been able to perform this study. Co‐operation parties for this research were Isala Academy, J. Dille, Dr. R.M. Brohet, SDS, TNO, Dr. J.P. van Wouwe, DOC and all the participating locations Isala Klinieken Zwolle, Medisch Centrum Leeuwarden, Ziekenhuis de Tjongerschans Heerenveen, Refaja Ziekenhuis Stadskanaal, Diaconessenhuis Meppel, Ziekenhuis St Jansdal Harderwijk, Diakonessenhuis Utrecht, Flevoziekenhuis Almere, Medisch Centrum Alkmaar, Vlietland Ziekenhuis Schiedam, MCHaaglanden den Haag, Elisabeth Ziekenhuis Tilburg, Twee Steden ziekenhuis Tilburg en Waalwijk, Wilhelmina Ziekenhuis Assen and Royal Dutch Visio. This study was financially supported by ODAS, Oogfonds, Novartis and LSBS (Uitzicht 2013‐23 to FNB and JG, and Bartiméus Institute to FNB). These financial parties had no influence on the design and the progress of the study.
References
- Afifi HH, Abdel Azeem AA, El‐Bassyouni HT, Gheith ME, Rizk A & Bateman JB (2013): Distinct ocular expression in infants and children with Down syndrome in Cairo, Egypt: myopia and heart disease. JAMA Ophthalmol 131: 1057–1066. [DOI] [PubMed] [Google Scholar]
- Al‐Bagdady M, Stewart RE, Watts P, Murphy PJ & Woodhouse JM (2009): Bifocals and Down's syndrome: correction or treatment? Ophthalmic Physiol Opt 29: 416–421. [DOI] [PubMed] [Google Scholar]
- Anderson HA, Manny RE, Glasser A & Stuebing KK (2011): Static and dynamic measurements of accommodation in individuals with down syndrome. Invest Ophthalmol Vis Sci 52: 310–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson J & Braddick O (1983): Assessment of visual acuity in infancy and early childhood. Acta Ophthalmol Suppl 157: 18–26. [DOI] [PubMed] [Google Scholar]
- Atkinson J, Anker S, Bobier W, Braddick O, Durden K, Nardini M & Watson P (2000): Normal emmetropization in infants with spectacle correction for hyperopia. Invest Ophthalmol Vis Sci 41: 3726–3731. [PubMed] [Google Scholar]
- Barnett AG, Van Der Pols JC & Dobson AJ (2005): Regression to the mean: what it is and how to deal with it. Int J Epidemiol 34: 215–220. Review. Erratum (2015): Int J Epidemiol. 44(5):1748. [DOI] [PubMed] [Google Scholar]
- Binder NR, Kruglyakova J & Borchert MS (2016): Strabismus in patients with cortical visual impairment: outcomes of surgery and observations of spontaneous resolution. J AAPOS 20: 121–125. [DOI] [PubMed] [Google Scholar]
- Boot FH, Pel JJ, van der Steen J & Evenhuis HM (2010): Cerebral Visual Impairment: which perceptive visual dysfunctions can be expected in children with brain damage? A systematic review Res Dev Disabil 31: 1149–1159. [DOI] [PubMed] [Google Scholar]
- Candy TR & Bharadwaj SR (2007): The stability of steady state accommodation in human infants. J Vis 7: 4.1‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courage ML, Adams RJ, Reyno S & Kwa PG (1994): Visual acuity in infants and children with Down syndrome. Dev Med Child Neurol 36: 586–593. [DOI] [PubMed] [Google Scholar]
- Courage ML, Adams RJ & Hall EJ (1997): Contrast sensitivity in infants and children with Down syndrome. Vision Res 37: 1545–1555. [DOI] [PubMed] [Google Scholar]
- Cregg M, Woodhouse JM, Pakeman VH, Saunders KJ, Gunter HL, Parker M, Fraser WI & Sastry P. (2001): Accommodation and refractive error in children with Down syndrome: cross‐sectional and longitudinal studies. Invest Ophthalmol Vis Sci 42: 55–63. [PubMed] [Google Scholar]
- Cregg M, Woodhouse JM, Stewart RE, Pakeman VH, Bromham NR, Gunther HL, Trojanowska L, Parker M, Fraser WI (2003): Development of refractive error and strabismus in children with Down syndrome. Invest Ophthalmol Vis Sci 44: 1023–1030. [DOI] [PubMed] [Google Scholar]
- da Cunha RP & Moreira JB (1996): Ocular findings in Down's syndrome. Am J Ophthalmol 122: 236–244. [DOI] [PubMed] [Google Scholar]
- Doyle L, Saunders KJ & Little JA (2016): Trying to see, failing to focus: near visual impairment in Down syndrome. Sci Rep 6: 20444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle L, Saunders KJ & Little JA (2017): Determining the relative contribution of retinal disparity and blur cues to ocular accommodation in Down syndrome. Sci Rep 7: 39860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich DL, Braddick OJ, Atkinson J, Anker S, Weeks F, Hartley T, Wade J & Rudenski A. (1997): Infant emmetropization: longitudinal changes in refraction components from nine to twenty months of age. Optom Vis Sci 74: 822–843. [DOI] [PubMed] [Google Scholar]
- Esposito Veneruso P, Bruzzese D & Magli A (2018): Long‐term development of refractive error in refractive, nonrefractive and partially accommodative esotropia. PLoS ONE 13: e0204396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazzi E, Signorini SG, Bova SM, La Piana R, Ondei P, Bertone C, Misefari W & Bianchi PE (2007): Spectrum of visual disorders in children with cerebral visual impairment. J Child Neurol 22: 294–301. [DOI] [PubMed] [Google Scholar]
- Hasebe S, Ohtsuki H, Kono R & Nakahira Y (1998): Biometric confirmation of the Hirschberg ratio in strabismic children. Invest Ophthalmol Vis Sci 39: 2782–2785. [PubMed] [Google Scholar]
- Haugen OH & Hovding G (2001a): Strabismus and binocular function in children with Down syndrome. A population based, longitudinal study. Acta Ophthalmol Scand 79: 133–139. [DOI] [PubMed] [Google Scholar]
- Haugen OH, Hovding G & Lundstrom I (2001b): Refractive development in children with Down's syndrome: a population based, longitudinal study. Br J Ophthalmol 85: 714–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwood AM, Toor SS & Riddell PM (2015): Convergence and Accommodation Development is Preprogramed in Premature Infants. Invest Ophthalmol Vis Sci 56: 5370–5380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyt CS (2013). Taylor & Hoyt's Systematic pediatric ophthalmology, Section 4, Part 7, Neural Visual Systems, Chapter 60, The brain and cerebral visual impairment: 629–638.
- Leat SJ & Gargon JL (1996): Accommodative response in children and young adults using dynamic retinoscopy. Ophthalmic Physiol Opt 16: 375–384. [PubMed] [Google Scholar]
- Marsack JD, Ravikumar A, Benoit JS & Anderson HA (2017): Variability in objective refraction for persons with down syndrome. Optom Vis Sci 94: 574–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClelland JF & Saunders KJ (2003): The repeatability and validity of dynamic retinoscopy in assessing the accommodative response. Ophthalmic Physiol Opt 23: 243–250. [DOI] [PubMed] [Google Scholar]
- McCullough SJ, Little JA & Saunders KJ (2014): Higher order aberrations in children with Down syndrome. Invest Ophthalmol Vis Sci 54: 1527–1535. Erratum (2014): Invest Ophthalmol Vis Sci. 55(4):2055‐6. [DOI] [PubMed] [Google Scholar]
- Morton GV (2011). Why do children with down syndrome have subnormal vision? Am Orthopt J 61: 60–70. [DOI] [PubMed] [Google Scholar]
- Motley WW 3rd, Melson AT, Gray ME & Salisbury SR (2012): Outcomes of strabismus surgery for esotropia in children with Down syndrome compared with matched controls. J Pediatr Ophthalmol Strabismus 49: 211–214. [DOI] [PubMed] [Google Scholar]
- Nandakumar K & Leat SJ (2009): Bifocals in Down Syndrome Study (BiDS): design and baseline visual function. Optom Vis Sci 86: 196–207. [DOI] [PubMed] [Google Scholar]
- Nandakumar K & Leat SJ (2010): Bifocals in children with Down syndrome (BiDS) – visual acuity, accommodation and early literacy skills. Acta Ophthalmol 88: e196–e204. [DOI] [PubMed] [Google Scholar]
- Oldham PD (1962): A note on the analysis of repeated measurements of the same subjects. J Chronic Dis 15: 969–977. [DOI] [PubMed] [Google Scholar]
- Perez CI, Zuazo F, Zanolli MT, Guerra JP, Acuña O & Iturriaga H (2013): Esotropia surgery in children with Down syndrome. J AAPOS 17: 477–479. [DOI] [PubMed] [Google Scholar]
- Stewart RE, Margaret WJ & Trojanowska LD (2005): In focus: the use of bifocal spectacles with children with Down's syndrome. Ophthalmic Physiol Opt 25: 514–522. [DOI] [PubMed] [Google Scholar]
- Stewart RE, Woodhouse JM, Cregg M & Pakeman VH (2007): Association between accommodative accuracy, hypermetropia, and strabismus in children with Down's syndrome. Optom Vis Sci 84: 149–155. [DOI] [PubMed] [Google Scholar]
- Trochim WMK (2006). https://www.socialresearchmethods.net/kb/regrmean.php
- Von Noorden GK & Campos EC (2002). Esodeviations In: Lambert R, ed. Binocular Vision and Ocular Motility: theory and Management of Strabismus, 6th ed St. louis: CV Mosby; 311–355. [Google Scholar]
- Watt T, Robertson K & Jacobs RJ (2015): Refractive error, binocular vision and accommodation of children with Down syndrome. Clin Exp Optom 98: 3–11. [DOI] [PubMed] [Google Scholar]
- de Weger C, Boonstra N & Goossens J (2018): Effects of bifocals on visual acuity in children with Down syndrome: a randomized controlled trial. Acta Ophthalmol 97: 378–393. 10.1111/aos.13944. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodhouse JM, Meades JS, Leat J & Saunders KJ (1993): Reduced accommodation in Children with Down Syndrome. Invest Ophthalmol Vis Sci 34: 2382–2387. [PubMed] [Google Scholar]
- Woodhouse JM, Pakeman VH, Saunders KJ, Parker M, Fraser WI, Lobo S & Sastry P (1996): Visual acuity and accommodation in infants and young children with Down's syndrome. J Intellect Disabil Res 40(Pt 1): 49–55. [DOI] [PubMed] [Google Scholar]
- Woodhouse JM, Cregg M, Gunter HL Sanders DP, Saunders KJ, Pakeman VH, Parker M, Fraser WI, Sastry P (2000): The effect of age, size of target, and cognitive factors on accommodative responses of children with Down syndrome. Invest Ophthalmol Vis Sci 41: 2479–2485. [PubMed] [Google Scholar]
- Wübbolt IS, von Alven S, Hülssner O & Erb C (2006): Vergleich der manuellen und automatischen Refraktionsbestimmung mit dem subjektiven Abgleich. Klin Monbl Augenheilkd 223: 904–907. [DOI] [PubMed] [Google Scholar]
- Yahalom C, Mechoulam H, Cohen E & Anteby I (2010): Strabismus surgery outcome among children and young adults with Down syndrome. J AAPOS 14: 117–119. [DOI] [PubMed] [Google Scholar]
- Yurdakul NS, Ugurlu S & Maden A (2006): Strabismus in Down syndrome. J Pediatr Ophthalmol Strabismus 43: 27–30. [DOI] [PubMed] [Google Scholar]


