Abstract
Background
The short‐form Headache Impact Test (HIT‐6) is a widely used patient‐reported outcome measure that assesses the negative effects of headaches on normal activity. It was developed using the general headache population and prior to the establishment of the now well‐accepted FDA patient‐reported guidance.
Objective
The objective of this narrative review was to examine existing qualitative research in patients with migraine and headache, providing insight into the relevance and meaningfulness of HIT‐6 items to the lives of migraine patients.
Methods
Articles were identified through database searches (National Library of Medicine and Google Scholar) and review of reference lists of candidate articles.
Results
A total of 3227 articles were identified through database and hand searching. Of these, 12 contained patient‐ or expert‐generated qualitative information regarding headache patients’ experience (8 specific to migraine [episodic and chronic] patients and 4 citing general headache patients). The combined publications described a total of 283 patient interviews. Overarching themes and specific information were identified that provide support of the relevance of content for each HIT‐6 item to migraine patients’ lives. Identified effects of headaches on patients with migraine included limitations in daily activities, needing to lie down during headaches, feeling tired, being irritated by headaches, difficulty concentrating, and the experience of pain. Further, previous research specific to the HIT‐6 indicated that patients understood the instructions, items, and response scales as intended by the instrument authors.
Conclusions
This narrative literature review demonstrates qualitative research support for the relevance of the items of the HIT‐6 in migraine patients, supporting its ongoing use in clinical migraine research and practice.
Keywords: short‐form Headache Impact Test, chronic migraine, outcomes research, epidemiology
Introduction
Rationale
Migraine is a common disorder with an estimated global prevalence exceeding 1 billion.1 In 2016, migraine was identified as the second leading cause of disability worldwide1 and is the most disabling disease in individuals aged 15‐49 years.2 Migraine symptoms often interfere with family, education, and work, and are associated with comorbidities such as cardiovascular disease, depression, and anxiety.3, 4, 5, 6, 7, 8, 9 Clinical trials evaluating migraine preventive therapies typically include outcome measures designed to quantify changes in headache and migraine frequency.10, 11, 12, 13, 14, 15 Whereas reductions in migraine days and prolongation of migraine‐free periods would be expected to improve a patient's ability to function in daily life, the use of measures designed to identify and quantify these changes in migraine impact has been inconsistent. The inclusion of patient‐reported outcome measures (PROMs), which specifically ask patients about symptoms, functional status, health‐related quality of life (QoL), care received, health‐related behaviors, and/or other associated burdens, could provide insight into patient perceptions of migraine impact and the effects of treatment.16 Numerous generic, headache‐specific, and migraine‐specific PROMs have been evaluated to assess headache impact, of which few have demonstrated strong evidence of content validity sufficient for use in clinical research or in routine clinical practice.17 The US Food and Drug Administration (FDA) has, however, recognized the potential benefits of including PROMs in clinical trial design and has published guidelines for identifying measures appropriate for this use.18
The short‐form Headache Impact Test (HIT‐6; Fig. 1) is a widely used PROM that assesses the negative impact of headaches on normal daily activity.19 It was developed for use in a general headache population and comprises 6 items that measure how often recent headaches resulted in severe pain, how often they impacted daily activities, and how often they resulted in the desire to lie down, headache‐related fatigue, irritability, or difficulty concentrating.19, 20 Each item is rated using 5 response categories (Never, Rarely, Sometimes, Very often, or Always), each category of which is associated with a numerical value (6, 8, 10, 11, and 13, respectively), resulting in a range of possible total summed scores of 36‐78. Despite being identified by the American Headache Society as one of 3 clinically relevant tools for assessing the benefit of preventive treatment,21 questions remain regarding its validity for use in migraine‐specific clinical research.
Figure 1.

The 6‐Item Headache Impact Test™ (HIT‐6™); (© 2001, 2015 QualityMetric Incorporated and the GlaxoSmithKline Group of Companies. All rights reserved. HIT‐6™ United States [English] Version. Reprinted with permission).
Objective
The original reporting of the larger HIT item bank22 states it was created from 4 existing measures of headache impact, and the HIT‐6 items were selected from this bank and from 35 additional clinician‐suggested items.19 Given the age of the scales used as the basis of the HIT item bank and based on the available reporting, it appears that at no point was qualitative work with people with migraine conducted during the development to ensure that the content of the HIT‐6 items (selected to the scale based solely on statistical properties) was important to those with migraine. This narrative literature review was performed to examine the extent to which the HIT‐6 could be considered “patient‐centered” and content‐valid in the migraine population, using existing qualitative research.
Methods
A comprehensive research protocol for this literature review was developed. Publications were identified through searches of the National Library of Medicine (primary data source, peer‐reviewed literature only) and Google Scholar (secondary data source and peer‐reviewed publications) from database inception through October 15, 2018. Studies were eligible for inclusion if the data collection (questionnaires, qualitative interviews, etc) was administered in English (when language was noted) and the results were published in English in a peer‐reviewed journal or chapter in an edited volume. Peer‐reviewed, stand‐alone abstracts and conference proceedings were not considered, given the limited information and difficulty in obtaining full documents for such references.
Search
Search terms, strings, and phrases used to identify relevant publications are listed in Table 1. Terms used to identify PROMs other than HIT‐6 were included to provide a comprehensive evaluation of the HIT‐6 in relation to other established measures and to identify any overlap among measures. We selected a variety of established measures to provide a broad perspective for comparison, including Migraine Physical Function Impact Diary (MPFID),23 Migraine‐Specific Questionnaire (MSQ),24 Migraine Disability Assessment (MIDAS) questionnaire,25 and Migraine Treatment Optimization Questionnaire (M‐TOQ).26 Additional PROMs located in the course of the literature search were also reviewed, again focusing on content validation information. These PROMs included Headache Activities of Daily Living Index,27 Completeness of Response Survey,28 Impact of Migraine on Partners and Adolescent Children scale,29 Functional Assessment in Migraine questionnaire,30 Migraine‐Specific QoL instrument,31 Patient Perceptions of Migraine questionnaire32 (and its revised version33), and 24‐hour Migraine QoL Questionnaire.34
Table 1.
Search Terms
| “Headache Impact Test” |
| HIT‐6 |
| HIT6 |
| HIT‐6 “Chronic Migraine” |
| HIT‐6 “Content Validity” |
| HIT‐6 Validation |
| HIT‐6 Validity |
| HIT‐6 Reliability |
| HIT‐6 “Fit for Purpose” |
| HIT‐6 MID |
| HIT‐6 MCID |
| HIT‐6 “Important Difference” |
| HIT‐6 “Responder Definition” |
| HIT‐6 Responsiveness |
| HIT‐6 “Sensitivity to Change” |
| HIT‐6 “Focus Groups” |
| HIT‐6 Interviews |
| HIT‐6 “Cognitive Interviews” |
| “Chronic Migraine” PRO |
| “Chronic Migraine” “Patient Interview” |
| “Chronic Migraine” “Focus Group” |
| “Migraine Physical Function Impact Diary” |
| “Migraine Physical Function Impact Diary” “Content Validity” |
| MPFID “Content Validity” |
| MPFID “Focus Groups” |
| MPFID Interviews |
| MPFID “Cognitive Interviews” |
| “Migraine‐Specific Quality‐of‐Life Questionnaire” |
| “Migraine‐Specific Quality‐of‐Life Questionnaire” “Content Validity” |
| MSQ “Content Validity” |
| MSQ “Focus Groups” |
| MSQ Interviews |
| MSQ “Cognitive Interviews” |
| “Migraine Disability Assessment” |
| “Migraine Disability Assessment” “Content Validity” |
| MIDAS “Content Validity” |
| MIDAS “Focus Groups” |
| MIDAS Interviews |
| MIDAS “Cognitive Interviews” |
| “Migraine Treatment Optimization Questionnaire” |
| “Migraine Treatment Optimization Questionnaire” “Content Validity” |
| MTOQ “Content Validity” |
| MTOQ “Focus Groups” |
| MTOQ Interviews |
| MTOQ “Cognitive Interviews” |
The basic search function of the National Library of Medicine, via PubMed (with minimal use of filters or specific field identifiers), was used (eg, “headache impact test” [All Fields]). For Google Scholar, results were displayed using the “sort by relevance” option, and the first 6 pages (12 references per page) of each search were reviewed to identify candidate publications. When using abbreviations in Google Scholar, the word “migraine” was included in the search term as well, to eliminate irrelevant similarly named scales from other fields. Reference lists of the located candidate publications were reviewed to identify any additional previously undiscovered citations.
Study Selection
A list of key candidate references, including those with general headache/migraine qualitative research, was compiled via title and abstract review. Publications passed screening based on: (1) the inclusion of a qualitative research identifier or related descriptors (eg, “mixed methods,” “focus groups,” “qualitative,” and “cognitive interview”) in the title, abstract, or key words; AND/OR (2) the inclusion of “patient experience,” “patient voice,” or similar descriptors in the publication title or abstract. Two methodologists subsequently performed brief reviews of each full publication that passed screening to confirm relevance. Candidate publications were considered excluded if no qualitative work was conducted or reported the publication mentioned qualitative research (eg, “focus groups were conducted”) but failed to significantly report patient input, such as direct interviewee quotes or results from patient interview exercises (eg, importance rankings of symptoms), etc. A third methodologist reviewed any publications for which there was disagreement between the initial reviewers with respect to relevance. The majority decision (2 out of 3 reviewers) was adhered to for any disputed publications.
Data extraction from the selected publications included information related to the overarching item content and item‐level support of the HIT‐6. Specifically, we extracted (1) overarching topics identified/used to classify the interview results by the original authors and (2) when qualitative exercises were reported – such as the ranking or other categorization of symptoms or impacts by patients – the reported information (eg, frequency of endorsement, average importance ratings) was reviewed and data extracted for content relevant to any of the HIT‐6 items. Item‐level supportive information was extracted in the form of reported interviewee quotes or author‐supplied summarizations of specific content; extracted quotes/text were reviewed for content and assigned to relevant HIT‐6 items or to an “other” category.
Results
Study Selection/Characteristics
A total of 3193 articles were identified through database searching (Fig. 2). Of these, 52 were identified as appropriate for potential inclusion. An additional 34 references were identified as possible candidates through review of the reference sections of these 52 articles; 5 of these articles were retained for more in‐depth review. The final preliminary list for the qualitative‐specific portion of the review comprised 57 unique citations, 26 of which, upon brief review of the full article, were deemed likely to include relevant qualitative information. Upon close reading of each of the 26 articles, 12 contained qualitative evidence related to the experiences of people with migraine (Table 2). Of these 12 publications, 8 were specific to migraine patients (both episodic and chronic)23, 31, 32, 34, 35, 36, 37, 38 and 4 used more general headache patients.39, 40, 41, 42 In total, these 12 studies gathered information from 283 headache patients, 214 of whom were patients with migraine and 16 of whom were explicitly labeled as patients with CM.
Figure 2.
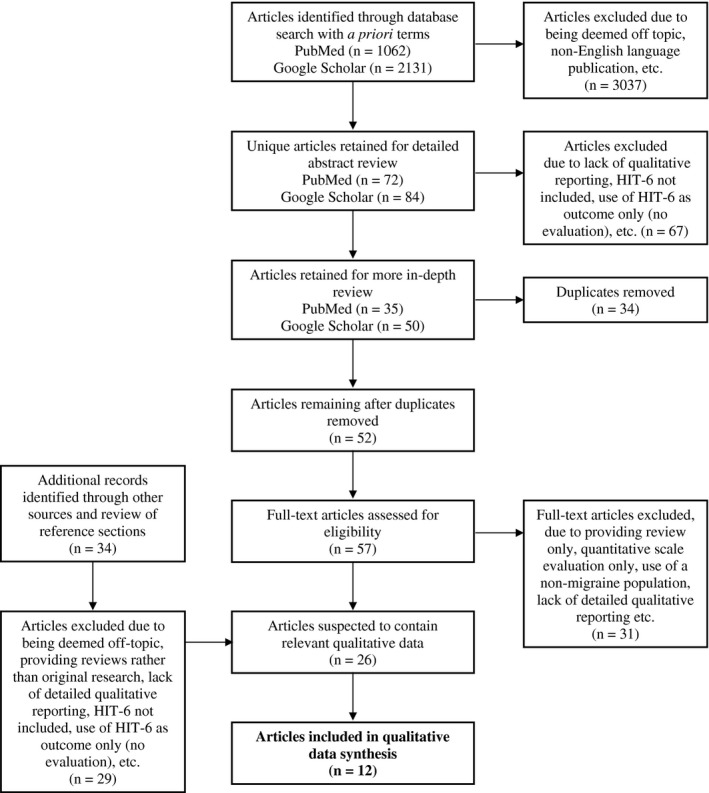
Flow Diagram.
Table 2.
Characteristics of Included Studies (n = 12)
| Author/Year | Design | Instrument(s) | Study Population | N |
|---|---|---|---|---|
| Coeytaux et al, 200739 | Focus group | HIT‐6 | Frequent headaches | 19, including 13 patients with CM and 6 patients with CTTH |
| MIDAS | ||||
| SF‐36 | ||||
| Cottrell et al, 200235 | Focus group | None | Migraine (ICHD‐1) | 24 |
| Davis et al, 200232 | Open‐label, randomized, parallel‐group clinical trial | PPMQ | Migraine (ICHD‐1) | 793 |
| Hareendran et al, 201723 | Cognitive interview | HIT‐6 | Migraine (ICHD‐2) | 17 |
| MPFID | ||||
| Hartmaier et al, 199534 | Questionnaire | MQoLQ | Migraine | 76 |
| Leiper et al, 200640 | Semi‐structured interview | None | Chronic headache | 19 |
| Mannix et al, 201636 | Concept elicitation interview | HIT‐6 | Migraine (ICHD‐2) | 32, including 11 patients with CM |
| MIDAS | ||||
| Migraine‐ACT | ||||
| MSQ | ||||
| PPMQR | ||||
| McKenna et al, 199831 | Qualitative interview; postal survey | MSQOL | Migraine | 137, including 50 interviews and 87 postal survey participants |
| Peters et al, 200537 | Semi‐structured interview | MIDAS | Migraine (ICHD‐1) | 13 |
| Ruiz de Velasco et al, 200338 | Focus groups | None | Migraine (ICHD‐1) | 29 |
| Individual interviews | ||||
| Tenhunen and Elander, 200541 | Semi‐structured interviews | None | Chronic daily headache | 9 |
| Turner‐Bowker et al, 201242 | Semi‐structured cognitive interview | HIT | Headache | 9 |
CM = chronic migraine; CDH = chronic daily headache; CTTH = chronic tension‐type headache; DynHA HIT = Headache Impact Test total item pool; HIT‐6 = short‐form Headache Impact Test; MIDAS = Migraine Disability Assessment questionnaire; Migraine‐ACT = Migraine‒Assessment of Current Therapy; MPFID = Migraine Physical Function Impact Diary; MSQ = Migraine‐Specific Questionnaire; MSQOL = Migraine‐Specific Quality‐of‐Life Instrument; MQoLQ = Migraine‐Specific Quality of Life Questionnaire; PPMQ = Patient Perception of Migraine Questionnaire; SF‐36 = Medical Outcomes Study Short Form 36.
Item Content
In these 12 studies, overarching themes were identified supporting the relevance of the HIT‐6 item content to patients living with migraine. In their report of focus‐group discussions with 24 migraine patients, Cottrell and colleagues35 identified 6 major discussion themes, 4 of which were related to HIT‐6 content (social function, effect on family functioning, effect on work, and effect on relationships); the remaining 2 themes were reported to be issues related to primary care and problems with insurance and drug companies. In their report of 13 semi‐structured interviews with migraine patients, Peters and colleagues37 described similar patient concerns, including negative impact on work, family, and social life. The patients participating in these interviews also identified migraine severity and frequency as factors affecting their experienced disability/functioning.
In a separate report of focus‐group discussions with 29 patients with migraine by Ruiz de Velasco and colleagues,38 patients indicated that migraine pain and discomfort led to tiredness and poor memory, which, in turn, negatively impacted school and work. Patients described negative effects on family and social relationships arising from limitations on activity and ability to engage in relationships. They also described emotional effects such as mood swings, unhappiness, anger, and hopelessness. These findings were consistent with the primary themes recognized by McKenna and colleagues in an earlier report of focus‐group discussion with 30 migraine patients,31 which included lost time due to migraine, limitations to physical activity, difficulty planning, negative psychological impacts due to migraine, the need for routines, avoidance of certain foods and drinks, and lack of understanding from others.
In another report of one‐on‐one interviews with 32 patients with migraine, Mannix and colleagues36 noted that 78% of interviewees identified effects on everyday activities (eg, difficulty completing household chores and errands outside the home, inability to concentrate, inability to keep a schedule), 59% reported emotional impact, 100% reported the need to lie down or rest, and 97% reported being frustrated or irritated (when specifically asked). They also observed that migraine intensity influenced impact.
Item‐Level Support
The concepts queried in the HIT‐6 are assessing important aspects of the migraine patient's experience. Qualitative support for the individual HIT‐6 items is summarized in Tables 3, 4, 5, 6, 7, 8.
Table 3.
Qualitative Support for HIT‐6 Item 1 (Pain Severity)
| Author/Year | Population (n) | Qualitative Support |
|---|---|---|
| Coeytaux et al, 200739 | Headache (19) | Patients report pain is the most important symptom |
| Davis et al, 200232 | Migraine (793) | At least 75% of focus group participants (3 focus groups, n = 10‐15 per group) listed pain relief as important or very important |
| Hartmaier et al, 199534 | Migraine (82) | Migraine impairments were generated from 6 one‐on‐one interviews with migraine patients. Presented to 76 new patients to indicate if they experienced an impairment or not and to rate impairments for importance, “throbbing head pain” was an impairment experienced by 91% and has a mean importance of 4.47 on a 1‐5 scale |
| Mannix et al, 201636 | Migraine (literature review/9 articles) | “… intensity of the migraine often had a direct and immediate impact on their ability to function …” |
| Peters et al, 200537 | Migraine (13) | “Impact was a relative concept that was influenced by pain severity and headache frequency” |
| Ruiz de Velasco et al, 200338 | Migraine (29) | “And when the pain is very, very severe there are times when I can't get to sleep because of the pain” |
| “The pain is excruciating, unbearable” | ||
| “… because your whole body hurts, and you feel pain when there is any kind of noise, light, anything at all …” | ||
| “In my case, for example, I was in pain every Sunday …” |
Italics indicate direct interviewee quote reported in publication.
Table 4.
Qualitative Support for HIT‐6 Item 2 (Limits to Daily Activities)
| Author/Year | Population (n) | Qualitative Support |
|---|---|---|
| Cottrell et al, 200235 | Migraine (24) | “All aspects of social and recreational activities were hampered by migraines” |
| “Being unable to participate in social activities because of migraines … was especially troublesome” | ||
| “Inability to prepare meals, help with homework, or complete other routine household chores” | ||
| Hartmaier et al, 199534 | Migraine (82) | Migraine impairments were generated from 6 one‐on‐one interviews with migraine patients. Presented to 76 new patients to indicate if they experienced an impairment or not and to rate impairments for importance, “general activities” was an impairment experienced by 93% and had a mean importance of 4.13 on a 1‐5 scale; “ability to accomplish tasks” was experienced by 88% and had a mean importance rating of 3.91 |
| Leiper et al, 200640 | Chronic headache (59) | “Most participants mentioned that it was difficult to carry out daily activities …” |
| “… many spoke of incidents where their headaches had caused them to miss out on something …” | ||
| “It was a common example for participants to have been sent home from work because a headache had developed” | ||
| McKenna et al, 199831 | Migraine (30) | “The main issues raised by the interviewees were: time lost due to migraine, … the need to limit the extent of their physical activity, difficulty with planning …” |
| Mannix et al, 201636 | Migraine (literature review/9 articles) | “A total of 25 (78%) subjects spontaneously reported that migraine in some way affected their ability to do everyday activities” |
| Peters et al, 200537 | Migraine (13) | “Headache impact was mainly described in terms of disability, ie, the participants’ inability to carry out their everyday tasks” |
| “Disability was seen as personal to the patients and referred to limitations to the participants’ everyday activities …” | ||
| Ruiz de Velasco et al, 200338 | Migraine (29) | “… made reference to the difficulties in performing one's duties at work …” |
| “… terrible for housewives who suffer from migraines to have to think about cleaning, the chores …” | ||
| Tenhunen and Elander, 200541 | Chronic daily headache (9) | “… all [the] participants described how headaches affected their ability to perform daily activities …” |
Italics indicate direct interviewee quote reported in publication.
Table 5.
Qualitative Support for HIT‐6 Item 3 (Limits to Concentration)
| Author/Year | Population (n) | Qualitative Support |
|---|---|---|
| Hareendran et al, 201723 | Migraine (17) | “… because, uh, when I have a migraine, when I had the last migraine, I still had to do activities or maybe I was in church and I needed to concentrate – I could, but if I'm having a migraine it's very difficult to sit there – and I don't even want to hear anyone talk, I just want to be in a dark room, lying down” |
| Hartmaier et al, 199534 | Migraine (82) | Migraine impairments were generated from 6 one‐on‐one interviews with migraine patients. Presented to 76 new patients to indicate if they experienced an impairment or not and to rate impairments for importance, “concentration” was experienced by 92% and had a mean importance of 4.12 on a 1‐5 scale |
| Leiper et al, 200640 | Chronic headache (59) | “Several people found it hard to concentrate when suffering headaches …” |
| “… you can't think clearly and you look at a sheet of paper and try and read it and you're not taking it in …” | ||
| Mannix et al, 201636 | Migraine (literature review/9 articles) | “… being unable to do activities requiring concentration (n = 25, 78%) or clear thinking (n = 23, 72%) …” |
| Ruiz de Velasco et al, 200338 | Migraine (29) | “At school, you can't study, you're in class but you can't concentrate, you're reading but you can't focus on what you're reading …” |
| Tenhunen and Elander, 200541 | Chronic daily headache (9) | “I'm lacking concentration and I'm so tired that I can't speak” |
| “… it's difficult to concentrate on reading, I'm falling asleep all the time and things don't seem to stay in my memory” |
Italics indicate direct interviewee quote reported in publication.
Table 6.
Qualitative Support for HIT‐6 Item 4 (Too Tired to Work)
| Author/Year | Population (n) | Qualitative Support |
|---|---|---|
| Cottrell et al, 200235 | Migraine (24) | “… feeling … physically drained for days after a migraine” |
| Hartmaier et al, 199534 | Migraine (82) | Migraine impairments were generated from 6 one‐on‐one interviews with migraine patients. Presented to 76 new patients to indicate if they experienced an impairment or not and to rate impairments for importance, “energy level” was an impairment experienced by 92% and had a mean importance of 3.95 on a 1‐5 scale |
| Ruiz de Velasco et al, 200338 | Migraine (29) | “I feel awful, tired” |
| Tenhunen and Elander, 200541 | Chronic daily headache (9) | “… I'm falling asleep all the time and things don't seem to stay in my memory” |
| “… recovering from a very severe headache attack and I need to stay in bed” | ||
| “Nowadays I'm so worn out that I'm just watching TV or taking naps … Somehow I'm lacking energy …” |
Italics indicate direct interviewee quote reported in publication.
Table 7.
Qualitative Support for HIT‐6 Item 5 (Wish to Lie Down)
| Author/Year | Population (n) | Qualitative support |
|---|---|---|
| Hareendran et al, 201723 | Migraine (17) | “… I just want to be in a dark room, lying down” |
| Mannix et al, 201636 | Migraine (literature review/9 articles) | “Impact on physical ability mentioned included ‐ needing to rest or lie down …” |
| Peters et al, 200537 | Migraine (13) | “… because I just can't do anything … I just have to lie down” |
| Tenhunen and Elander, 200541 | Chronic daily headache (9) | “… recovering from a very severe headache attack and I need to stay in bed” |
Italics indicate direct interviewee quote reported in publication.
Table 8.
Qualitative Support for HIT‐6 Item 6 (Fed Up/Irritated)
| Author/Year | Population (n) | Qualitative Support |
|---|---|---|
| Cottrell et al, 200235 | Migraine (24) | “Others’ reactions to migraines are associated with shame, anger, and frustration” |
| Leiper et al, 200640 | Chronic headache (59) | “Descriptions of the effects headaches had included feeling depressed or down, self‐pity, aggression, and embarrassment” |
| Mannix et al, 201636 | Migraine (literature review/9 articles) | “Emotional impact included feeling frustrated or irritated (n = 31, 97%) …” |
| Peters et al, 200537 | Migraine (13) | “… because you feel like that all the time and you just get sick of it …” |
| Ruiz de Velasco et al, 200338 | Migraine (29) | “They refer to a great sense of frustration and impotence …” |
| Tenhunen and Elander, 200541 | Chronic daily headache (9) | “I feel quite trapped … and frustrated and angry” |
| “… frustrated and very annoyed …” |
Italics indicate direct interviewee quote reported in publication.
The HIT‐6 contains items similar to those found in the MPFID23 and the MSQ.24 All 3 measures include items related to limitations in daily activities (limited daily activities, difficulty in keeping daily routine or schedule, and difficulty in performing work or daily activities, respectively) and concentration (limited concentration, difficulty in doing activities requiring clear thinking, and limited ability to concentrate on work or daily activities, respectively). Both the HIT‐6 and the MSQ also include items related to tiredness (too tired for activities and too tired to do work or daily activities, respectively) and irritability (fed up/irritable and fed up or frustrated, respectively) and both also include an item regarding the desire/need to lie down. Among these 3 measures, the frequency of severe headache pain item is unique to the HIT‐6.20
Response Options/Recall Period
The appropriateness of the HIT‐6 response options was examined in a qualitative examination of the full HIT item bank.42 Through cognitive interviews with 9 patients with headache, some of whom attributed their headaches to migraine, the researchers determined that current response options (Never, Rarely, Sometimes, Very often, and Always) were well‐understood by respondents and that the addition of a “Not applicable” response could hinder the accuracy of responses. For the 3 items for which a 4‐week recall period is specified, patients felt they were able to accurately recall their headache experiences over that time frame. The 4‐week recall period matches that of the MSQ.24 For the 3 HIT‐6 items for which no recall period is specified, patients tended to use an expanded recall period (longer than 4 weeks, but exact length unspecified in the publication).
Discussion
The HIT‐6 was initially developed as a practical tool for measuring the impact of headache in the clinical setting;19 however, it has also been used as a measure of impact in clinical trials investigating headache and migraine treatments.43, 44, 45, 46, 47, 48 In these trials, HIT‐6 scores were found to be responsive to change produced by an efficacious treatment, but the role of the HIT‐6 was predominantly supportive, providing additional information beyond the primary outcome measures. Additionally, the HIT‐6 has been validated in the chronic migraine trial population49 using data from the PREEMPT trial,43, 44, 45 and other research has proposed within‐person change thresholds for the HIT‐6 total scores to define responders.20, 49, 50, 51, 52
In comparison to other existing migraine‐related PROMs, the MSQ, MPFID, and HIT‐6 all include items that assess limitations to daily activities. Additionally, both the MSQ and HIT‐6 have items related to concentration, tiredness, and frustration/irritability; the HIT‐6 additionally assesses headache pain severity and the desire to lie down.20, 24, 53 In comparison, the MPFID and HIT‐6 have in common items related to concentration and the desire to lie down; the HIT‐6 additionally assesses headache pain severity, tiredness, and frustration/irritability. These additional HIT‐6 items measure effects that are important to patients, particularly the first HIT‐6 item, which assesses frequency of severe headache pain, and, relative to the other noted measures, the HIT‐6 provides a somewhat unique understanding of the impact of migraine therapies on daily life, based on item content.
A sample of patients with headache, including migraine patients, reportedly understood the response scales and was able to recall impact for periods of 4 weeks or more. The qualitative evidence demonstrates that each item of the HIT‐6 measures a concept that is relevant to migraine patients.
Limitations
This was a narrative review of the qualitative literature relevant to the HIT‐6. However, these findings must be interpreted in light of the limitations inherent in this type of research, including reliance on second‐hand reports, as well as the need to synthesize information from sources using varied data collection and reporting methods. Descriptions of patients varied, as did descriptions of questions, recall timeframes, and other study methods. The exclusion of unpublished manuscripts, abstracts/conference presentations, and other materials not readily available may have resulted in the failure to include all relevant information. While the provided evidence cannot demonstrate that every aspect of the migraine patient's experience is addressed by the HIT‐6, it does support the contention that the recurring primary themes of impact and disability in migraine patients’ lives, reported across multiple independent qualitative research projects, are present in the HIT‐6.
Conclusions
The recurring primary themes of impact and disability in migraine patients’ lives, reported across multiple independent qualitative research projects, are present in the HIT‐6. The results of this narrative review of published literature suggest that the HIT‐6 is an appropriate and patient‐centered measure for assessing the impact of migraines on the daily lives of patients with migraine.
Statement of Authorship
Category 1
(a) Conception and Design
Eric Kassel, Steven Snapinn, Roger Cady
(b) Acquisition of Data
Carrie R. Houts, R.J. Wirth, James S. McGinley, Chad Gwaltney
(c) Analysis and Interpretation of Data
Carrie R. Houts, R.J. Wirth, James S. McGinley, Chad Gwaltney, Eric Kassel, Steven Snapinn, Roger Cady
Category 2
(a) Drafting the Manuscript
Carrie R. Houts, R.J. Wirth, James S. McGinley
(b) Revising It for Intellectual Content
Carrie R. Houts, R.J. Wirth, James S. McGinley, Chad Gwaltney, Eric Kassel, Steven Snapinn, Roger Cady
Category 3
(a) Final Approval of the Completed Manuscript
Carrie R. Houts, R.J. Wirth, James S. McGinley, Chad Gwaltney, Eric Kassel, Steven Snapinn, Roger Cady
Acknowledgments
The authors thank Mary Tom, PharmD, Nicole Coolbaugh, CMPP, and Philip Sjostedt, BPharm, of The Medicine Group (New Hope, PA, United States) for providing medical writing support, which was funded by Lundbeck Seattle (Bothell, WA, United States) in accordance with Good Publication Practice guidelines.
Conflict of Interest: C.R. Houts, R.J. Wirth, J.S. McGinley, and C. Gwaltney are contracted service providers for Lundbeck Seattle BioPharmaceuticals. E. Kassel was employed by Lundbeck Seattle BioPharmaceuticals (formerly known as Alder BioPharmaceuticals) at the time of manuscript preparation. S. Snapinn was employed by Lundbeck Seattle BioPharmaceuticals (formerly known as Alder BioPharmaceuticals) at the time of manuscript preparation and is currently contracting with Lundbeck Seattle Biopharmaceuticals. R. Cady is a full‐time employee and stockholder of Lundbeck Seattle BioPharmaceuticals.
Funding: This study was funded by Lundbeck Seattle BioPharmaceuticals, Inc. (formerly known as Alder BioPharmaceuticals, Inc.), Bothell, WA, USA.
References
- 1. GBD 2016 Disease and Injury Incidence and Prevalence Collaborators . Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990‐2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390:1211‐1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Steiner TJ, Stovner LJ, Vos T, Jensen R, Katsarava Z. Migraine is first cause of disability in under 50s: Will health politicians now take notice? J Headache Pain. 2018;19:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bigal ME, Serrano D, Reed M, Lipton RB. Chronic migraine in the population: Burden, diagnosis, and satisfaction with treatment. Neurology. 2008;71:559‐566. [DOI] [PubMed] [Google Scholar]
- 4. McLean G, Mercer SW. Chronic migraine, comorbidity, and socioeconomic deprivation: Cross‐sectional analysis of a large nationally representative primary care database. J Comorb. 2017;7:89‐95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Minen MT, Begasse De Dhaem O, Kroon Van Diest A, et al. Migraine and its psychiatric comorbidities. J Neurol Neurosurg Psychiatry. 2016;87:741‐749. [DOI] [PubMed] [Google Scholar]
- 6. Adams AM, Serrano D, Buse DC, et al. The impact of chronic migraine: The Chronic Migraine Epidemiology and Outcomes (CaMEO) Study methods and baseline results. Cephalalgia. 2015;35:563‐578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Adelborg K, Szepligeti SK, Holland‐Bill L, et al. Migraine and risk of cardiovascular diseases: Danish population based matched cohort study. BMJ. 2018;360:k96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kurth T, Winter AC, Eliassen AH, et al. Migraine and risk of cardiovascular disease in women: Prospective cohort study. BMJ. 2016;353:i2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Schurks M, Rist PM, Bigal ME, Buring JE, Lipton RB, Kurth T. Migraine and cardiovascular disease: Systematic review and meta‐analysis. BMJ. 2009;339:b3914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dodick DW, Ashina M, Brandes JL, et al. ARISE: A phase 3 randomized trial of erenumab for episodic migraine. Cephalalgia. 2018;38:1026‐1037. [DOI] [PubMed] [Google Scholar]
- 11. Detke HC, Goadsby PJ, Wang S, Friedman DI, Selzler KJ, Aurora SK. Galcanezumab in chronic migraine: The randomized, double‐blind, placebo‐controlled REGAIN study. Neurology. 2018;91:e2211‐e2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Goadsby PJ, Reuter U, Hallstrom Y, et al. A controlled trial of erenumab for episodic migraine. N Engl J Med. 2017;377:2123‐2132. [DOI] [PubMed] [Google Scholar]
- 13. Dodick DW, Silberstein SD, Bigal ME, et al. Effect of fremanezumab compared with placebo for prevention of episodic migraine: A randomized clinical trial. JAMA. 2018;319:1999‐2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Reuter U, Goadsby PJ, Lanteri‐Minet M, et al. Efficacy and tolerability of erenumab in patients with episodic migraine in whom two‐to‐four previous preventive treatments were unsuccessful: A randomised, double‐blind, placebo‐controlled, phase 3b study. Lancet. 2018;392:2280‐2287. [DOI] [PubMed] [Google Scholar]
- 15. Skljarevski V, Matharu M, Millen BA, Ossipov MH, Kim BK, Yang JY. Efficacy and safety of galcanezumab for the prevention of episodic migraine: Results of the EVOLVE‐2 Phase 3 randomized controlled clinical trial. Cephalalgia. 2018;38:1442‐1454. [DOI] [PubMed] [Google Scholar]
- 16. Weldring T, Smith SM. Patient‐reported outcomes (PROs) and patient‐reported outcome measures (PROMs). Health Serv Insights. 2013;6:61‐68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Haywood KL, Mars TS, Potter R, Patel S, Matharu M, Underwood M. Assessing the impact of headaches and the outcomes of treatment: A systematic review of patient‐reported outcome measures (PROMs). Cephalalgia. 2018;38:1374‐1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. US Food and Drug Administration . Guidance for Industry. Patient‐Reported Outcome Measures: Use in Medical Product Development to Support Labeling Claims. 2009. Available at: http://www.fda.gov/downloads/Drugs/.../Guidances/UCM193282.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kosinski M, Bayliss MS, Bjorner JB, et al. A six‐item short‐form survey for measuring headache impact: The HIT‐6. Qual Life Res. 2003;12:963‐974. [DOI] [PubMed] [Google Scholar]
- 20. Bayliss MS, Batenhorst AS. The HIT‐6™: A User's Guide. Lincoln, RI: QualityMetric Incorporated; 2002. [Google Scholar]
- 21. American Headache Society . The American Headache Society position statement on integrating new migraine treatments into clinical practice. Headache. 2019;59:1‐18. [DOI] [PubMed] [Google Scholar]
- 22. Ware JE Jr, Kosinski M, Bjorner JB, et al. Applications of computerized adaptive testing (CAT) to the assessment of headache impact. Qual Life Res. 2003;12:935‐952. [DOI] [PubMed] [Google Scholar]
- 23. Hareendran A, Mannix S, Skalicky A, et al. Development and exploration of the content validity of a patient‐reported outcome measure to evaluate the impact of migraine‐ the migraine physical function impact diary (MPFID). Health Qual Life Outcomes. 2017;15:224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jhingran P, Osterhaus JT, Miller DW, Lee JT, Kirchdoerfer L. Development and validation of the Migraine‐Specific Quality of Life Questionnaire. Headache. 1998;38:295‐302. [DOI] [PubMed] [Google Scholar]
- 25. Stewart WF, Lipton RB, Kolodner K, Liberman J, Sawyer J. Reliability of the migraine disability assessment score in a population‐based sample of headache sufferers. Cephalalgia. 1999;19:107‐114; discussion 174. [DOI] [PubMed] [Google Scholar]
- 26. Lipton RB, Kolodner K, Bigal ME, et al. Validity and reliability of the Migraine‐Treatment Optimization Questionnaire. Cephalalgia. 2009;29:751‐759. [DOI] [PubMed] [Google Scholar]
- 27. Vernon H, Lawson G. Development of the headache activities of daily living index: Initial validity study. J Manipulative Physiol Ther. 2015;38:102‐111. [DOI] [PubMed] [Google Scholar]
- 28. Coon CD, Fehnel SE, Davis KH, Runken MC, Beach ME, Cady RK. The development of a survey to measure completeness of response to migraine therapy. Headache. 2012;52:550‐572. [DOI] [PubMed] [Google Scholar]
- 29. Lipton RB, Buse DC, Adams AM, Varon SF, Fanning KM, Reed ML. Family impact of migraine: Development of the Impact of Migraine on Partners and Adolescent Children (IMPAC) scale. Headache. 2017;57:570‐585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pathak DS, Chisolm DJ, Weis KA. Functional Assessment in Migraine (FAIM) questionnaire: Development of an instrument based upon the WHO's international classification of functioning, disability, and health. Value Health. 2005;8:591‐600. [DOI] [PubMed] [Google Scholar]
- 31. McKenna SP, Doward LC, Davey KM. The development and psychometric properties of the MSQOL: A migraine‐specific quality‐of‐life instrument. Clin Drug Investig. 1998;15:413‐423. [DOI] [PubMed] [Google Scholar]
- 32. Davis KH, Black L, Sleath B. Validation of the Patient Perception of Migraine Questionnaire. Health. 2002;5:422‐430. [DOI] [PubMed] [Google Scholar]
- 33. Revicki DA, Kimel M, Beusterien K, et al. Validation of the revised Patient Perception of Migraine Questionnaire: Measuring satisfaction with acute migraine treatment. Headache. 2006;46:240‐252. [DOI] [PubMed] [Google Scholar]
- 34. Hartmaier SL, Santanello NC, Epstein RS, Silberstein SD. Development of a brief 24‐hour Migraine‐Specific Quality of Life Questionnaire. Headache. 1995;35:320‐329. [DOI] [PubMed] [Google Scholar]
- 35. Cottrell CK, Drew JB, Waller SE, Holroyd KA, Brose JA, O'Donnell FJ. Perceptions and needs of patients with migraine: A focus group study. J Fam Pract. 2002;51:142‐147. [PMC free article] [PubMed] [Google Scholar]
- 36. Mannix S, Skalicky A, Buse DC, et al. Measuring the impact of migraine for evaluating outcomes of preventive treatments for migraine headaches. Health Qual Life Outcomes. 2016;14:143‐143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Peters M, Huijer Abu‐Saad H, Vydelingum V, Dowson A, Murphy M. The patients' perceptions of migraine and chronic daily headache: A qualitative study. J Headache Pain. 2005;6:40‐47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ruiz de Velasco I, Gonzalez N, Etxeberria Y, Garcia‐Monco JC. Quality of life in migraine patients: A qualitative study. Cephalalgia. 2003;23:892‐900. [DOI] [PubMed] [Google Scholar]
- 39. Coeytaux RR, Frasier PY, Reid A. Patient‐centered outcomes for frequent headaches. Headache. 2007;47:480‐485. [DOI] [PubMed] [Google Scholar]
- 40. Leiper DA, Elliott AM, Hannaford PC. Experiences and perceptions of people with headache: A qualitative study. BMC Fam Pract. 2006;7:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tenhunen K, Elander J. A qualitative analysis of psychological processes mediating quality of life impairments in chronic daily headache. J Health Psychol. 2005;10:397‐407. [DOI] [PubMed] [Google Scholar]
- 42. Turner‐Bowker DM, Saris‐Baglama RN, Derosa MA, Paulsen CA. Cognitive testing and readability of an item bank for measuring the impact of headache on health‐related quality of life. Patient. 2012;5:89‐99. [DOI] [PubMed] [Google Scholar]
- 43. Aurora SK, Dodick DW, Diener HC, et al. OnabotulinumtoxinA for chronic migraine: Efficacy, safety, and tolerability in patients who received all five treatment cycles in the PREEMPT clinical program. Acta Neurol Scand. 2014;129:61‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lipton RB, Rosen NL, Ailani J, DeGryse RE, Gillard PJ, Varon SF. OnabotulinumtoxinA improves quality of life and reduces impact of chronic migraine over one year of treatment: Pooled results from the PREEMPT randomized clinical trial program. Cephalalgia. 2016;36:899‐908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Matharu M, Halker R, Pozo‐Rosich P, DeGryse R, Manack Adams A, Aurora SK. The impact of onabotulinumtoxinA on severe headache days: PREEMPT 56‐week pooled analysis. J Headache Pain. 2017;18:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Blumenfeld AM, Stark RJ, Freeman MC, Orejudos A, Manack AA. Long‐term study of the efficacy and safety of OnabotulinumtoxinA for the prevention of chronic migraine: COMPEL study. J Headache Pain. 2018;19:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ashina M, Dodick D, Goadsby PJ, et al. Erenumab (AMG 334) in episodic migraine: Interim analysis of an ongoing open‐label study. Neurology. 2017;89:1237‐1243. [DOI] [PubMed] [Google Scholar]
- 48. Gaul C, Diener HC, Danesch U. Improvement of migraine symptoms with a proprietary supplement containing riboflavin, magnesium and Q10: A randomized, placebo‐controlled, double‐blind, multicenter trial. J Headache Pain. 2015;16:516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Rendas‐Baum R, Yang M, Varon SF, Bloudek LM, DeGryse RE, Kosinski M. Validation of the Headache Impact Test (HIT‐6) in patients with chronic migraine. Health Qual Life Outcomes. 2014;12:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Smelt AF, Assendelft WJ, Terwee CB, Ferrari MD, Blom JW. What is a clinically relevant change on the HIT‐6 questionnaire? An estimation in a primary‐care population of migraine patients. Cephalalgia. 2014;34:29‐36. [DOI] [PubMed] [Google Scholar]
- 51. Coeytaux RR, Kaufman JS, Chao R, Mann JD, DeVellis RF. Four methods of estimating the minimal important difference score were compared to establish a clinically significant change in Headache Impact Test. J Clin Epidemiol. 2006;59:374‐380. [DOI] [PubMed] [Google Scholar]
- 52. Castien RF, Blankenstein AH, Windt DA, Dekker J. Minimal clinically important change on the Headache Impact Test‐6 questionnaire in patients with chronic tension‐type headache. Cephalalgia. 2012;32:710‐714. [DOI] [PubMed] [Google Scholar]
- 53. Rendas‐Baum R, Bloudek LM, Maglinte GA, Varon SF. The psychometric properties of the Migraine‐Specific Quality of Life Questionnaire version 2.1 (MSQ) in chronic migraine patients. Qual Life Res. 2013;22:1123‐1133. [DOI] [PMC free article] [PubMed] [Google Scholar]


