Abstract
Glucocorticoid hormones are particularly potent with respect to enhancing memory formation. Notably, this occurs in close synergy with arousal (i.e., when norepinephrine levels are enhanced). In the present study, we examined whether glucocorticoid and norepinephrine hormones regulate the number of spines in hippocampal primary neurons. We report that brief administration of corticosterone or the β‐adrenergic receptor agonist isoproterenol alone increases spine number. This effect becomes particularly prominent when corticosterone and isoproterenol are administered together. In parallel, corticosterone and isoproterenol alone increased the amplitude of miniature excitatory postsynaptic currents, an effect that is not amplified when both hormones are administered together. The effects of co‐application of corticosterone and isoproterenol on spines could be prevented by blocking the glucocorticoid receptor antagonist RU486. Taken together, both corticosterone and β‐adrenergic receptor activation increase spine number, and they exert additive effects on spine number for which activation of glucocorticoid receptors is permissive.
Keywords: corticosterone, memory, norepinephrine, plasticity, spines, synapses
1. INTRODUCTION
During stress exposure, norepinephrine (NE) is rapidly released by presynaptic terminals from neurons that originate from the locus coeruleus. Consequently, NE is released in brain regions that are key in memory formation such as the hippocampus.1 More slowly after stress exposure, the hypothalamus‐pituitary‐adrenal axis is activated, which increases circulating levels of glucocorticoids (GCs). As a result of their lipophilic nature, GCs readily enter the brain where they bind to high affinity mineralocorticoid receptors (MRs) and lower affinity glucocorticoid receptors (GRs), which are both present at high levels in the hippocampal formation.1, 2
Activation of MRs and GRs regulates various cellular functions via genomic and non‐genomic actions.3 In this way, stress promotes behavioural adaptation to stressful experiences.2, 4 By enhancing habitual learning strategies, glucocorticoid hormones modulate response selection after stress exposure via MRs.5, 6 Via GRs, glucocorticoid hormones enhance memory consolidation.7, 8, 9, 10 At the cellular level, GC effects involve rapid changes in glutamatergic synaptic transmission, including enhanced neurotransmitter release and alterations in AMPA and NMDA receptor mobility.11, 12, 13, 14 More slowly, corticosterone enhances glutamatergic (AMPA and NMDA receptor‐mediated) synaptic transmission, which underlies enhanced memory formation.11, 15, 16, 17, 18, 19, 20 In addition, various lines of evidence indicate that glucocorticoids also enhance spine formation, which are critical for learning and memory.21, 22, 23, 24, 25, 26, 27, 28
Importantly, glucocorticoids are particularly potent with respect to enhancing memory formation when NE levels, acting via β‐adrenergic receptors, are also enhanced, both in humans and rodents.29, 30 At the cellular level, GCs and NE in concert regulate synaptic transmission by enhancing the frequency of miniature excitatory postsynaptic currents (mEPSCs) and synaptic plasticity.31, 32, 33 Whether and how GCs and NE interact to also regulate the number of spines remains elusive. The present study therefore examined whether GCs and NE regulate the number of spines, both alone, or in an additive mode.
2. MATERIALS AND METHODS
2.1. Rat hippocampal primary cultures
Primary hippocampal cultures were prepared from Wistar rat brains at embryonic day 18 ± 1, as described previously.16, 17, 34 Briefly, hippocampi were dissected and homogenised, and cells were plated on 12 mm coverslips coated with poly‐d‐lysine (0.5 mg mL‐1) at a density of 75 000 neurons/coverslip. Hippocampal cultures were grown in neurobasal medium supplemented with 2% B27, 0.5 mmol L‐1 GlutaMax (ThermoFisher, Waltham, MA, USA), penicillin/streptomycin, 5% foetal bovine serum (FBS) (plating medium) for the first day; from the second day onwards, half of the medium was changed once a week with culturing medium (plating medium without FBS), containing 5‐fluoro‐2′‐deoxyuridine (FUDR) 10 μmol L‐1 to inhibit glial growth. All reagents were obtained from Gibco Invitrogen (Carlsbad, CA, USA), except FUDR (Sigma). All experiments were carried out with permission of the local Animal Committee of the University of Amsterdam.
2.2. Lipofectamine transfection with GFP
Days in vitro (DIV) 13‐17 hippocampal neurons were transfected using Lipofectamine2000 (Invitrogen, Carlsbad, CA, USA) and a total of 1 μg of plasmids, containing a 1:1 ratio of green fluorescent protein (pGW1‐GFP) and empty vector (pGW1). Lipofectamine‐GFP‐empty vector mixture was incubated for 30 minutes before being added to the neuronal cultures for 45 minutes at 37°C and 5% CO2. Next, the neurons were washed and transferred back to their original medium at 37°C, 5% CO2 for 24 hours.
2.3. Experimental design and hormone treatment
After transfection, DIV 14‐18 hippocampal neurons were subjected to either: (a) vehicle (veh) (EtOH, concentration <0.01%), (b) 100 nmol L‐1 corticosterone (CORT) (Sigma); (c) 1 μmol L‐1 isoproterenol (ISO), an NE agonist (Sigma); or (d) both 100 nmol L‐1 CORT and 1 μmol L‐1 isoproterenol. Neurons were then incubated at 37°C, 5% CO2 for either 20 minutes, followed by direct fixation (Experiment 1), or for 20 minutes, after which they were placed back in incubation medium for a remaining 160 minutes (Experiment 2), or for 180 minutes (Experiment 5). In addition, neurons were exposed to the GR antagonist RU486 (500 nmol L‐1, Sigma) for 1 hour prior to the aforementioned treatments with either CORT, or CORT and ISO together (Experiment 3). Colocalisation between the spine heads and the presynaptic marker Bassoon was assessed (Experiment 4). After incubation, neurons were fixed for 15 minutes with 4% formaldehyde/4% sucrose in 0.1 mol L‐1 phosphate‐buffered saline, and washed three times in phosphate buffer (PB) with intervals of 10 minutes. For Experiment 6, neurons underwent a hormone treatment similar to that employed in Experiment 2, and mEPSCs were recorded after 160 minutes.
2.4. Immunohistochemistry
Neurons were incubated in GDB + Triton X‐100 buffer (0.2% bovine serum albumin, 0.8 mol L‐1 NaCl, 30 mmol L‐1 phosphate buffer, 0.6% Triton X‐100, pH 7.4) containing the primary antibody against the presynaptic protein bassoon (bassoon; Enzo Diagnostics, Farmingdale, NY, USA; dilution 1:200; 1 mg mL‐1) for 2 hours at room temperature. After three washes in PB with 10‐minute intervals in between, neurons were incubated with GDB + Triton X‐100 buffer containing the secondary antibody Alexa Fluor goat‐anti‐mouse mA568 (Invitrogen; dilution 1:400; 2 mg mL‐1) for 2 hours at room temperature. Neurons were again washed three times in PB with 10‐minute intervals before mounting using Vectashield mounting medium (Vector Laboratories, Inc., Burlingame, CA, USA).
2.5. Image acquisition
Confocal images were obtained using an LSM 510 microscope (Carl Zeiss, Oberkochen, Germany) with a 63× oil objective with acquisition settings at 1024 × 1024 pixels resolution. lsm software was used to generate Z series projections of approximately six to 10 images, each averaged four times and taken at a fixed 0.4 μm depth interval. For all images, the confocal settings were kept equal.
2.6. Spine density
In each condition, a minimum of three secondary dendrites of ten different GFP transfected neurons were randomly chosen for quantification. For each neurone, a minimum total amount of 120 μm of dendrite was analysed using metamorph image analysis software (Universal Imaging Corporation, Bedford Hills, NY, USA). Single dendrites were selected at random, and protrusion width and length were manually measured (Figure 1A), as well as its possible co‐localisation with bassoon. The width/length ratio of the protrusion was used to classify protrusions into filopodia or spines. If the width/length ratio exceeded 0.5, the protrusion was classified as a spine, protrusions with a ratio below 0.5 were classified as filopodia.35 In case the total length of the protrusion could not be adequately measured or its length was over 5 μm, the protrusions were excluded from analysis. An investigator who was blind to the experimental conditions carried out the morphological analyses.
Figure 1.
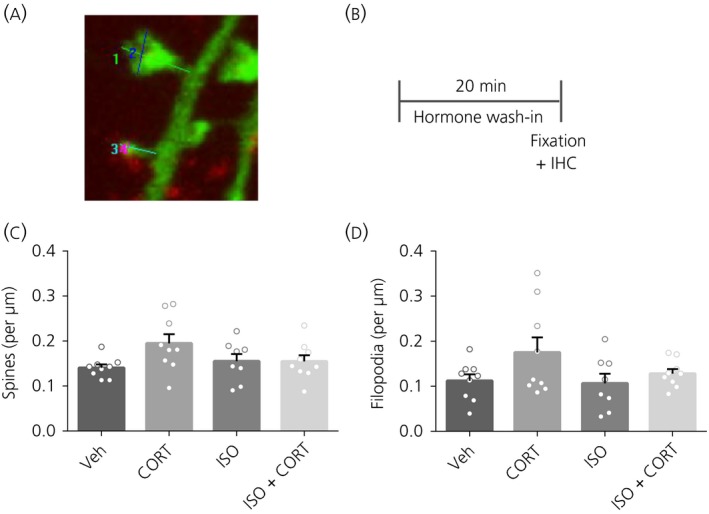
Spine and filopodium density after 20 minutes of hormone treatment. A, Typical example of protrusion measurements. The length of the protrusion is measured from the base to the top (1,3). Maximal protrusion head width is measured from side to side, parallel to the postsynaptic density (2,4). The latter protrusion displays colocalisation with bassoon. B, Time schedule of the experiment. C, No effects on spine density were present in any of the treatments. D, No effects on filopodium density were present in any of the treatments. *P < 0.05. Data are expressed as the mean ± SEM (n = 8‐9 neurons/group). veh, vehicle; IHC, immunohistochemistry
2.7. Electrophysiology
Coverslips with cells attached were placed in a recording chamber mounted on an upright microscope (Axioskop 2FS Plus; Carl Zeiss), which were kept fully submerged with artificial cerebrospinal fluid containing (in mmol L‐1): 145 NaCl, 2.8 KCl, 1.0 MgCl2, 10 Hepes and 10 glucose (pH 7.4). Whole‐cell patch‐clamp recordings were made using an AXOPATCH 200 amplifier (Axon Instruments, Foster City, CA, USA), with electrodes from borosilicate glass (1.5 mm outer diameter; Hilgerberg, Malsfeld, Germany). The electrodes were pulled on a Suttter micropipette puller. The pipette solution contained (in mmol L‐1): 120 Cs methane sulfonate; 17.5 CsCl, 10 Hepes, 5 BAPTA, 2 Mg‐ATP, 0.5 Na‐GTP and 10 QX‐314 (pH 7.4), adjusted with CsOH; pipette resistance was between 3 and 6 MΩ. Under visual control (40× objective and 10× ocular magnification), the electrode was directed towards a neurone with positive pressure. Once sealed on the cell membrane (resistance above 1 GΩ), the membrane patch under the electrode was ruptured by gentle suction and the cell was kept at a holding potential of −70 mV. The liquid junction potential caused a shift of no more than 10 mV, which was compensated for during the mEPSC recordings. Recordings with an uncompensated series resistance of <15 MΩ and <2.5 times of the pipette resistance with a shift of <20% during the recording, were accepted for analysis. Data acquisition was performed with pclamp, version 8.2 (Molecular Devices, Sunnyvale, CA, USA) and analysed offline with mini‐analysis, version 6.0 (Synaptosoft Inc., Fort Lee, NJ, USA).
mEPSCs were recorded at a holding potential of −70 mV.16, 17 Tetrodotoxin (0.25 µmol L‐1; Latoxan, Portes lès Valence, France) and bicuculline methobromide (20 µmol L‐1; Biomol, Hamburg, Germany) were added to the buffer to block action potential induced glutamate release and GABA‐A receptor‐mediated miniature inhibitory postsynaptic currents, respectively. During some recordings the non‐NMDA receptor blocker 6‐cyano‐7‐nitroquinoxaline‐2,3‐dione (10 mol L‐1; Tocris Bioscience, St Louis, MO, USA) was perfused to confirm that the mEPSCs were indeed mediated by AMPA receptors. The events were identified as mEPSCs when the rise time was faster than the decay time. mEPSCs were recorded for 3 minutes in each cell.
2.8. Statistical analysis
Statistical analysis was performed using spss, version 17.0 (SPSS Inc., Chicago, IL, USA). All data are expressed as mean ± SEM. Outliers were removed using Grubb's test. Between‐group comparisons were carried out using a one‐way ANOVA followed by a post‐hoc Sidak test. P < 0.05 was considered statistically significantly.
3. RESULTS
3.1. Experiment 1: Immediate (20 minutes) effects of stress hormones on dendritic spine density
To investigate the immediate effects of CORT or the β‐receptor agonist ISO on the density of spines or filopodia, primary neurons were treated with these hormones for 20 minutes, after which protrusion morphology and density were assessed (Figure 1A, B). No differences were found for total spine or filopodium density immediately after 20 minutes of treatment (spines: F 3,31 = 1.75, P = 0.18); filopodia: F 3,31 = 2.07, P = 0.12; Figure 1C‐D).
3.2. Experiment 2: Later (180 minutes) effects of brief exposure to stress hormones on dendritic spine density
Because the effects of the hormone treatment on spine density may require time to arise, we next investigated effects of 20 minutes of hormone treatment on spine density after a 3‐hour follow‐up (Figure 2A). Spine density was increased after CORT (F 3,33 = 47.71, P < 0.0001, post‐hoc: P = 0.01), ISO (P < 0.0001) and ISO + CORT (P < 0.0001) (Figure 2B). Although ISO increased spine density more than CORT alone (P = 0.0007), the combined ISO + CORT treatment increased spine density even more (P = 0.007).
Figure 2.
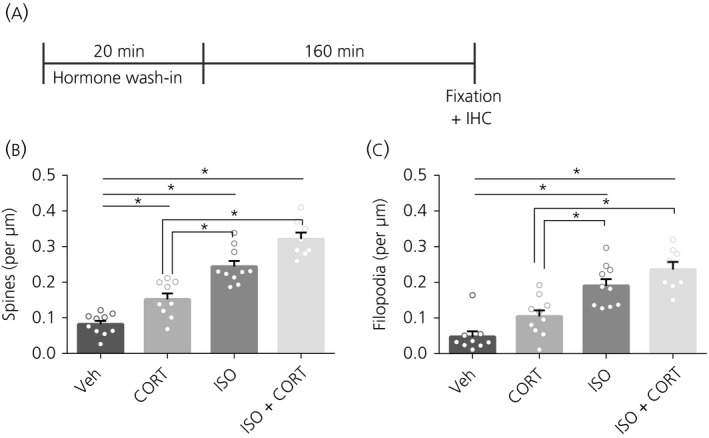
Spine and filopodium density after 3 h with 20 minutes of hormone treatment. A, Time schedule of the experiment. B, Spine density was increased after CORT, ISO and CORT + ISO treatment compared to vehicle (veh). Co‐administration of CORT + ISO resulted in the highest spine density. C, Filopodium density was increased after ISO and CORT + ISO treatment compared to vehicle. Co‐administration of CORT + ISO resulted in the highest spine density. *P < 0.05. Data are expressed as the mean ± SEM (n = 8‐10 neurons/group). IHC, immunohistochemistry
Filopodium density was not affected by CORT treatment alone (F 3,33 = 21.10, P < 0.0001, post‐hoc: P = 0.17), although it was increased by ISO (P < 0.0001) and by CORT + ISO (P < 0.0001) (Figure 2C). CORT also did not further increase the filopodium density following CORT + ISO compared to ISO treatment alone (P = 0.41).
3.3. Experiment 3: Role of GR in CORT and ISO mediated spine density
The GR antagonist RU486 was used to investigate whether the CORT and ISO mediated effects on spine and filopodium density are mediated via the GR (Figure 3A). RU486 pre‐exposure before CORT treatment did not affect spine or filopodium density (spines: F 3,34 = 15.36, P < 0.0001, veh‐CORT vs RU486‐CORT: P = 0.75; filopodia: F 3,35 = 5.13, P = 0.005, veh‐CORT vs RU486‐CORT: P = 0.66) (Figure 3B,C). Yet, pre‐exposure to RU486 completely blocked the enhancing effects of combined CORT + ISO treatment on spine (P < 0.0001) and filopodium density (P = 0.006).
Figure 3.
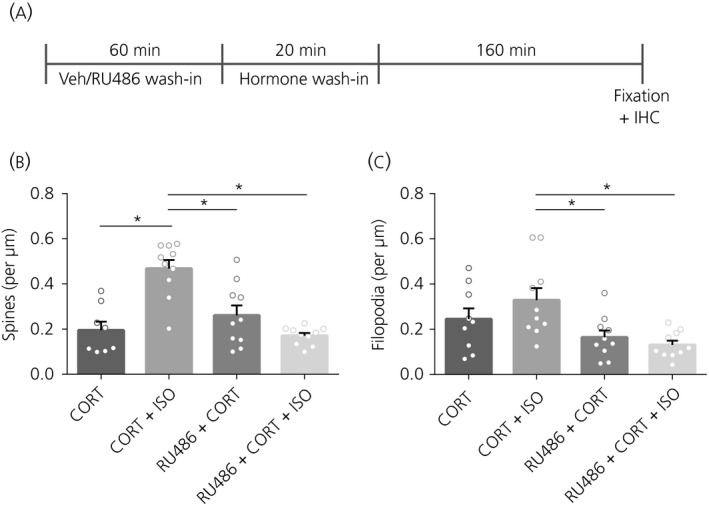
Spine and filopodium density after pre‐treatment with glucocorticoid receptor antagonist RU486. A, Time schedule of the experiment. B, Pre‐treatment with RU486 did not affect spine density after CORT treatment. Vehicle (veh) treatment did not affect the previously observed increased in spine density after CORT + ISO compared to CORT treatment, although it did block the additional effect that CORT + ISO had on spine density. C, Pre‐treatment with RU486 decreased the filopodium density after CORT + ISO treatment. *P < 0.05. Data are expressed as the mean ± SEM (n = 8‐10 neurons/group). IHC, immunohistochemistry
3.4. Experiment 4: Synaptic integration of protrusions
The functional integration of protrusions was assessed by the density of colocalised protrusion heads with the presynaptic protein bassoon.36 Twenty minutes after hormone treatment, there was no difference in the density of colocalised protrusion heads with bassoon between any of the treatment groups (F 3,30 = 2.09, P = 0.12) (Figure 4A). Interestingly, 3 hours after the 20 minutes of hormone treatment, the CORT + ISO protrusions showed higher co‐localisation with bassoon than vehicle‐treated neurons (F 3,32 = 4.43, P = 0.01, post‐hoc: P = 0.007) (Figure 4B).
Figure 4.
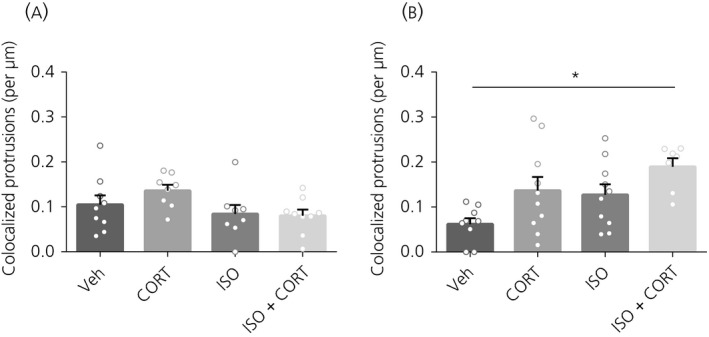
Colocalisation of protrusions with bassoon. A, 20 minutes after hormone treatment, there was no effect on the colocalisation of protrusion heads with bassoon. B, 160 minutes later, there was an increase in the protrusion heads that colocalised with bassoon after CORT + ISO treatment. *P < 0.05. Data are expressed as the mean ± SEM (n = 7‐10 neurons/group). veh, vehicle
3.5. Experiment 5: Effects of long‐term (180 minutes) exposure to stress hormones on dendritic spine density
Because prolonged treatment with CORT may negatively affect spine number, we also investigated whether longer, 180 minutes, treatment with CORT and ISO affected spine formation (Figure 5A). Spine density was increased after ISO (F 3,36 = 6.28, P < 0.01, post‐hoc: P = 0.01) and ISO + CORT (P < 0.01) (Figure 5B). Likewise, filopodium density was affected after ISO (F 3,36 = 6.11, P<0.01, post‐hoc: P < 0.01) and ISO + CORT (P < 0.01) (Figure 5C). Three hours after the 180 minutes treatment, the CORT + ISO and ISO‐treated protrusions showed higher co‐localisation with bassoon than vehicle‐treated neurons (F 3,36 = 4.80, P = 0.01, post‐hoc: P < 0.05 and P < 0.01, respectively) (Figure 5B). We conclude that prolonged treatment with ISO + CORT or CORT alone does not negatively affect spine number, and also that ISO + CORT treatment enhances spine density.
Figure 5.
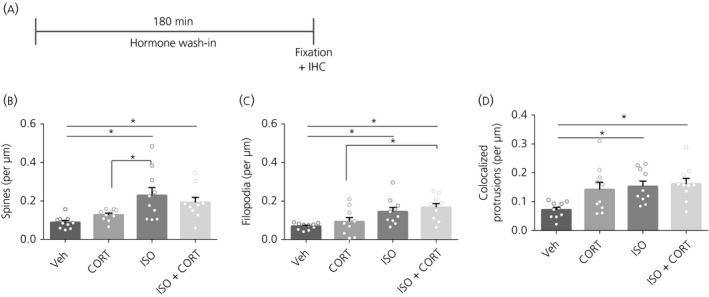
Spine and filopodium density after 3 hours of hormone treatment. A, Time schedule of the experiment. B, Spine density was increased after ISO and CORT + ISO treatment compared to vehicle (veh). C, Filopodium density was increased after ISO and CORT + ISO treatment compared to vehicle. D, Colocalisation of protrusions with bassoon. There was an increase in the protrusion heads that colocalised with bassoon after ISO and CORT + ISO treatment. *P < 0.05. Data are expressed as the mean ± SEM (n = 8‐10 neurons/group). IHC, immunohistochemistry
3.6. Experiment 6: Functional consequences of hormone treatment
To assess the functional consequences of enhanced spine density and synaptic integration, we next measured mEPSCs of primary neurons 3 hours after 20 minutes of hormone treatment (Figure 6A). The mEPSC amplitude was increased following both CORT, ISO and CORT + ISO (F 3,59 = 4.98, P = 0.004, post‐hoc veh‐CORT: P = 0.018; veh‐ISO: P = 0.029; veh‐CORT + ISO: P = 0.04) (Figure 6B). There was no effect of any hormone treatment on the frequency (F 3,57 = 2.51, P = 0.07) (Figure 6C) or the decay time (F 3,59 = 1.78, P = 0.16) (Figure 6D) of the mEPSCs.
Figure 6.
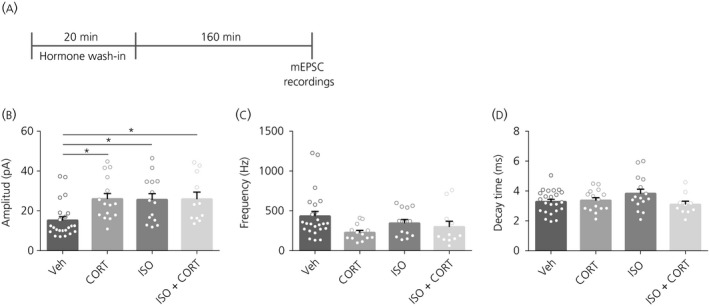
Miniature excitatory postsynaptic currents (mEPSCs) after 3 h with 20 minutes of hormone treatment. A, Time schedule of the experiment. B, mEPSC amplitude was increased after CORT, ISO and CORT + ISO treatment compared to vehicle (veh). C, mEPSC frequency was not affected by any of the treatments. D, The decay time of the mEPSCs was not affected by any of the treatments. *P < 0.05. Data are expressed as the mean ± SEM (n = 11‐24 neurons/group)
4. DISCUSSION
In the present study, we examined whether CORT and β‐adrenergic receptor activation, alone and in concert, regulate spine density. We report that both CORT and the β‐adrenergic receptor agonist ISO increase spine density, an effect that is increased when CORT and ISO are administered together. Interestingly, this effect of co‐application is prevented by blocking the GR with the GR antagonist RU486. These results suggest that both CORT and β‐adrenergic receptor activation increase spine density, and that they exert additive effects that require GR activation. Although CORT and ISO did increase the amplitude of mEPSCs, we did not observe an additive effect of these hormones on mEPSC amplitude.
Various lines of evidence indicate that corticosteroid hormones increase (learning‐evoked) spine formation and spine stabilisation.21, 22, 23, 24, 25, 26, 27, 28 In line with these findings, we report that CORT increases hippocampal spine number in primary cultures. We further report that activation of β‐adrenergic receptors, using the β‐adrenergic receptor agonist ISO, also enhances spine number. Both effects required time, which may suggest that protein synthesis is required.31 Interestingly, combined administration of CORT and ISO further increased the number of spines, which was dependent on GR activation because the GR antagonist RU486 prevented this effect. At present, it still remains unknown why the effects of CORT on spines were not prevented by RU486. One of the possibilities is that MRs might (also) be involved in spine formation.28
At 3 hours after co‐administration, the number of spines that colocalised with the presynaptic marker bassoon was increased. To examine the possible functional consequences in more detail, mEPSCs were recorded after administration of CORT and/or ISO. Both CORT and ISO increased the amplitude of mEPSCs and enhanced synaptic potentiation. Such effects may be linked to increased trafficking and retention of synaptic AMPARs.11, 15, 16, 17, 37, 38, 39, 40 Yet, we found that, 160 minutes after combined CORT and ISO administration, mEPSC frequency and amplitude were not further increased compared to the administration of the drugs alone. These findings suggest that the increase in spine number after co‐administration of ISO and CORT leaves synaptic transmission unaffected at 3 hours. Earlier studies have shown that combined administration of ISO and CORT within minutes increases synaptic transmission by enhancing mEPSC frequency32 and long‐term potentiation.33 Although we observed increases in spine density after 3 hours but not after 20 minutes of co‐application, this is not reflected in the mEPSC amplitude. Although we observed a moderate increase in bassoon colocalisation at 3 hours after co‐administration, the proper functional integration of new spines into the network after exposure to GCs and ISO may require more time. Alternatively, the increase in spines may prepare the capacity of the network for synaptic plasticity.
The formation of presynaptic boutons and the initiation of synaptogenesis, resulting in the formation of spines and their functional integration into the network, is a highly dynamic process, displaying vast ranges of changes in shape over short time.41 Thus, to investigate the stability of our observed changes and their full functional integration, it will be important to investigate in more detail the generation and retraction of spines after combined exposure over longer periods of time.21, 28
In conclusion, behavioural studies indicate that glucocorticoids and NE together promote memory retention, both in rodents29 and humans.30 The results of the present study indicate that these modulators, in an additive fashion, also regulate spine number, although, at the currently examined time points, synaptic transmission was not altered when compared to single administration . It will be important to investigate whether these effects of CORT and ISO on spine number are necessary for their effects on memory consolidation.42 Moreover, because prolonged exposure to GCs reduces spine formation,42 it will be important to understand mechanisms that underlie the transition from increased to reduced spinogenesis.
ACKNOWLEDGEMENTS
This research was supported by the Netherlands Organization for Scientific Research (NWO) grant 820.02.006.
Lesuis SL, Timmermans W, Lucassen PJ, Hoogenraad CC, Krugers HJ. Glucocorticoid and β‐adrenergic regulation of hippocampal dendritic spines. J Neuroendocrinol. 2020;32:e12811 10.1111/jne.12811
Lesuis and Timmermans contributed equally to this work.
DATA AVAILABILITY
The data that support the findings of this study are available from the corresponding authors upon reasonable request.
REFERENCES
- 1. Joëls M, Baram TZ. The neuro‐symphony of stress. Nat Rev Neurosci. 2009;10:459‐466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. de Kloet ER, Joëls M, Holsboer F. Stress and the brain: from adaptation to disease. Nat Rev Neurosci. 2005;6:463‐475. [DOI] [PubMed] [Google Scholar]
- 3. Joëls M, Karst H, Sarabdjitsingh RA. The stressed brain of humans and rodents. Acta Physiol (Oxf). 2018;223:e13066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. de Kloet ER, Oitzl MS, Joëls M. Stress and cognition: are corticosteroids good or bad guys? Trends Neurosci. 1999;22:422‐426. [DOI] [PubMed] [Google Scholar]
- 5. Schwabe L, Schächinger H, de Kloet ER, Oitzl MS. Corticosteroids operate as a switch between memory systems. J Cogn Neurosci. 2010;22:1362‐1372. [DOI] [PubMed] [Google Scholar]
- 6. Schwabe L, Joëls M, Roozendaal B, Wolf OT, Oitzl MS. Stress effects on memory: an update and integration. Neurosci Biobehav Rev. 2012;36:1740‐1749. [DOI] [PubMed] [Google Scholar]
- 7. McGaugh JL, Roozendaal B. Role of adrenal stress hormones in forming lasting memories in the brain. Curr Opin Neurobiol. 2002;12:205‐210. [DOI] [PubMed] [Google Scholar]
- 8. Roozendaal B, McEwen BS, Chattarji S. Stress, memory and the amygdala. Nat Rev Neurosci. 2009;10:423‐433. [DOI] [PubMed] [Google Scholar]
- 9. Oitzl MS, de Kloet ER. Selective corticosteroid antagonists modulate specific aspects of spatial orientation learning. Behav Neurosci. 1992;106:62‐71. [DOI] [PubMed] [Google Scholar]
- 10. Sandi C, Rose SP. Corticosterone enhances long‐term retention in one‐day‐old chicks trained in a weak passive avoidance learning paradigm. Brain Res. 1994;647:106‐112. [DOI] [PubMed] [Google Scholar]
- 11. Groc L, Choquet D, Chaouloff F. The stress hormone corticosterone conditions AMPAR surface trafficking and synaptic potentiation. Nat Neurosci. 2008;11:868‐870. [DOI] [PubMed] [Google Scholar]
- 12. Mikasova L, Xiong H, Kerkhofs A, Bouchet D, Krugers HJ, Groc L. Stress hormone rapidly tunes synaptic NMDA receptor through membrane dynamics and mineralocorticoid signalling. Sci Rep. 2017;7:8053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Popoli M, Yan Z, McEwen BS, Sanacora G. The stressed synapse: the impact of stress and glucocorticoids on glutamate transmission. Nat Rev Neurosci. 2011;13:22‐37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Karst H, Berger S, Turiault M, Tronche F, Schütz G, Joëls M. Mineralocorticoid receptors are indispensable for nongenomic modulation of hippocampal glutamate transmission by corticosterone. Proc Natl Acad Sci USA. 2005;102:19204‐19207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Martin S, Henley JM, Holman D, et al. Corticosterone alters AMPAR mobility and facilitates bidirectional synaptic plasticity. PLoS ONE. 2009;4:e4714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Xiong H, Cassé F, Zhou Y, et al. mTOR is essential for corticosteroid effects on hippocampal AMPA receptor function and fear memory. Learn Mem. 2015;22:577‐583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Xiong H, Cassé F, Zhou M, et al. Interactions between N‐Ethylmaleimide‐sensitive factor and GluA2 contribute to effects of glucocorticoid hormones on AMPA receptor function in the rodent hippocampus. Hippocampus. 2016;26:848‐856. [DOI] [PubMed] [Google Scholar]
- 18. Yuen EY, Liu W, Karatsoreos IN, Feng J, McEwen BS, Yan Z. Acute stress enhances glutamatergic transmission in prefrontal cortex and facilitates working memory. Proc Natl Acad Sci USA. 2009;106:14075‐14079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Liu W, Yuen EY, Yan Z. The stress hormone corticosterone increases synaptic alpha‐amino‐3‐hydroxy‐5‐methyl‐4‐isoxazolepropionic acid (AMPA) receptors via serum‐ and glucocorticoid‐inducible kinase (SGK) regulation of the GDI‐Rab4 complex. J Biol Chem. 2010;285:6101‐6108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yuen EY, Liu W, Karatsoreos IN, et al. Mechanisms for acute stress‐induced enhancement of glutamatergic transmission and working memory. Mol Psychiatry. 2011;16:156‐170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Liston C, Gan WB. Glucocorticoids are critical regulators of dendritic spine development and plasticity in vivo. Proc Natl Acad Sci USA. 2011;108:16074‐16079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chai GS, Wang YY, Zhu D, Yasheng A, Zhao P. Activation of β2‐adrenergic receptor promotes dendrite ramification and spine generation in APP/PS1 mice. Neurosci Lett. 2017;636:158‐164. [DOI] [PubMed] [Google Scholar]
- 23. Murakami G, Hojo Y, Kato A, et al. Rapid nongenomic modulation by neurosteroids of dendritic spines in the hippocampus: androgen, oestrogen and corticosteroid. J Neuroendocrinol. 2018;30:1‐13. 10.1111/jne.12561 [DOI] [PubMed] [Google Scholar]
- 24. Komatsuzaki Y, Hatanaka Y, Murakami G, et al. Corticosterone induces rapid spinogenesis via synaptic glucocorticoid receptors and kinase networks in hippocampus. PLoS ONE. 2012;7:e34124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Anderson RM, Glanz RM, Johnson SB, Miller MM, Romig‐Martin SA, Radley JJ. Prolonged corticosterone exposure induces dendritic spine remodeling and attrition in the rat medial prefrontal cortex. J Comp Neurol. 2016;524:3729‐3746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ikeda M, Hojo Y, Komatsuzaki Y, et al. Hippocampal spine changes across the sleep‐wake cycle: corticosterone and kinases. J Endocrinol. 2015;226:M13‐M27. [DOI] [PubMed] [Google Scholar]
- 27. Yoshiya M, Komatsuzaki Y, Hojo Y, et al. Corticosterone rapidly increases thorns of CA3 neurons via synaptic/extranuclear glucocorticoid receptor in rat hippocampus. Front Neural Circuits. 2013;7:191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Liston C, Cichon JM, Jeanneteau F, Jia Z, Chao MV, Gan WB. Circadian glucocorticoid oscillations promote learning‐dependent synapse formation and maintenance. Nat Neurosci. 2013;16:698‐705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Roozendaal B, Okuda S, Van der Zee EA, McGaugh JL. Glucocorticoid enhancement of memory requires arousal‐induced noradrenergic activation in the basolateral amygdala. Proc Natl Acad Sci USA. 2006;103:6741‐6746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. van Stegeren AH, Roozendaal B, Kindt M, Wolf OT, Joëls M. Interacting noradrenergic and corticosteroid systems shift human brain activation patterns during encoding. Neurobiol Learn Mem. 2010;93:56‐65. [DOI] [PubMed] [Google Scholar]
- 31. Joëls M, Fernandez G, Roozendaal B. Stress and emotional memory: a matter of timing. Trends Cogn Sci. 2011;15:280‐288. [DOI] [PubMed] [Google Scholar]
- 32. Zhou M, Hoogenraad CC, Joëls M, Krugers HJ. Combined β‐adrenergic and corticosteroid receptor activation regulates AMPA receptor function in hippocampal neurons. J Psychopharmacol. 2012;26:516‐524. [DOI] [PubMed] [Google Scholar]
- 33. Pu Z, Krugers HJ, Joëls M. Corticosterone time‐dependently modulates beta‐adrenergic effects on long‐term potentiation in the hippocampal dentate gyrus. Learn Mem. 2007;14:359‐367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hoogenraad CC, Milstein AD, Ethell IM, Henkemeyer M, Sheng M. GRIP1 controls dendrite morphogenesis by regulating EphB receptor trafficking. Nat Neurosci. 2005;8:906‐915. [DOI] [PubMed] [Google Scholar]
- 35. Jaworski J, Kapitein LC, Gouveia SM, et al. Neuron. 2009;61(1):85–100. [DOI] [PubMed] [Google Scholar]
- 36. Dieck S, Sanmartí‐Vila L, Langnaese K, et al. Bassoon, a novel zinc‐finger CAG/glutamine‐repeat protein selectively localized at the active zone of presynaptic nerve terminals. J Cell Biol. 1998;142:499‐509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hu H, Real E, Takamiya K, et al. Emotion enhances learning via norepinephrine regulation of AMPA‐receptor trafficking. Cell. 2007;131:160‐173. [DOI] [PubMed] [Google Scholar]
- 38. Gelinas JN, Nguyen PV. Beta‐adrenergic receptor activation facilitates induction of a protein synthesis‐dependent late phase of long‐term potentiation. J. Neurosci. 2005;25:3294‐3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. O'Dell TJ, Connor SA, Guglietta R, Nguyen PV. β‐Adrenergic receptor signaling and modulation of long‐term potentiation in the mammalian hippocampus. Learn Mem. 2015;22:461‐471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Brandwein NJ, Nguyen PV. A requirement for epigenetic modifications during noradrenergic stabilization of heterosynaptic LTP in the hippocampus. Neurobiol Learn Mem. 2019;161:72‐82. [DOI] [PubMed] [Google Scholar]
- 41. Wong TP, Marchese G, Casu MA, Ribeiro-da-Silva A, Cuello AC, De Koninck Y. J Neurosci. 2000;20(22):8596–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Moda‐Sava RN, Murdock MH, Parekh PK, et al. Sustained rescue of prefrontal circuit dysfunction by antidepressant‐induced spine formation. Science. 2019;364: eaat8078. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding authors upon reasonable request.


