Abstract
Usutu virus (USUV), a mosquito‐borne flavivirus closely related to West Nile virus, emerged in Austria in 2001, when it caused a considerable mass‐mortality of Eurasian blackbirds. Cases in birds increased until 2003 and quickly declined thereafter, presumably due to developing herd immunity. Since 2006, no further cases were recorded, until two blackbirds were tested positive in 2016. In Hungary, USUV first appeared in 2005 and has caused only sporadic infections since then. Initially, the only genetic USUV lineage found across both countries was Europe 1. This changed in 2015/2016, when Europe 2 emerged, which has since then become the prevalent lineage. Due to dispersal of these strains and introduction of new genetic lineages, USUV infections are now widespread across Europe. In 2009, the first cases of USUV‐related encephalitis were described in humans, and the virus has been frequently detected in blood donations since 2016. To monitor USUV infections among the Austrian wild bird population in 2017/2018, 86 samples were investigated by RT‐PCR. In 67 of them, USUV nucleic acid was detected (17 in 2017, 50 in 2018). The majority of succumbed birds were blackbirds, found in Vienna and Lower Austria. However, the virus also spread westwards to Upper Austria and southwards to Styria and Carinthia. In Hungary, 253 wild birds were examined, but only six of them were infected with USUV (five in 2017, one in 2018). Thus, in contrast to the considerable increase in USUV‐associated bird mortality in Austria, the number of infections in Hungary declined after a peak in 2016. Except for one case of USUV lineage Africa 3 in Austria in 2017, Europe 2 remains the most prevalent genetic lineage in both countries. Since USUV transmission largely depends on temperature, which affects vector populations, climate change may cause more frequent USUV outbreaks in the future.
Keywords: Austria, bird mortality, flavivirus, Hungary, Usutu virus, West Nile virus
1. INTRODUCTION
Usutu virus (USUV) belongs to the mosquito‐borne group within the genus Flavivirus, along with a series of highly relevant human pathogens, such as Japanese encephalitis virus (JEV), Yellow fever virus (YFV), Dengue virus (DENV), West Nile virus (WNV) and Zika virus (ZIKV) (Kuno, Chang, Tsuchiya, Karabatsos, & Cropp, 1998; Poidinger, Hall, & Mackenzie, 1996). USUV was first discovered in South Africa in 1959, in a mosquito feeding mainly on birds (Culex neavei) (McIntosh, 1985; Williams, Simpson, Haddow, & Knight, 1964). The first USUV outbreak outside Africa was observed in eastern Austria in 2001, causing a considerable mass‐mortality of Eurasian blackbirds (Turdus merula) (Weissenböck et al., 2002). Comparison of the whole‐genome sequences of the South African (SA Ar 1776) and the Austrian (Austria 2001) USUV strains revealed 97% nucleotide identity (Bakonyi, Gould, Kolodziejek, Weissenböck, & Nowotny, 2004). A subsequent retrospective analysis of tissues collected from a series of dead wild birds in Italy in 1996, provided evidence that the virus already existed in Europe at least five years earlier (Weissenböck, Bakonyi, Rossi, Mani, & Nowotny, 2013). More recently, a study on the evolutionary history of USUV suggested that the virus was repeatedly introduced into Europe from the African continent over the past 50 years (Engel et al., 2016). However, following the outbreak in 2001, the virus apparently adapted to the new habitat, managed to overwinter and established a local transmission cycle between native bird and mosquito species (Weissenböck et al., 2003). In 2002, only two birds of other species were positive for USUV [one house sparrow (Passer domesticus), and one blue tit (Cyanistes caeruleus)], suggesting that, although several species may be susceptible, very few develop fatal disease (Weissenböck et al., 2003). Moreover, a dead bird surveillance programme executed in Austria from 2003 through 2005 revealed a significant reduction in USUV‐related mortality. While more than half of the birds submitted in 2003 were positive, the proportion drastically declined to 5% and 4% during the subsequent two years, respectively, suggesting the development of herd immunity (Chvala et al., 2007). This hypothesis was confirmed by serological screening, which revealed that the percentage of seropositive birds had indeed increased from below 10% in 2003/2004 to above 50% in 2005/2006 (Meister et al., 2008).
Nevertheless, USUV was able to invade many other European countries, including Hungary (Bakonyi et al., 2007), Switzerland (Steinmetz et al., 2011), Italy (Manarolla et al., 2010), Spain (Bakonyi, Busquets, & Nowotny, 2014; Busquets, Alba, Allepuz, Aranda, & Ignacio Nunez, 2008), Germany (Jost et al., 2011), Greece (Chaintoutis et al., 2014), the Czech Republic (Hubalek et al., 2014), Belgium (Garigliany et al., 2017; Garigliany et al., 2014), France (Lecollinet et al., 2016), the Netherlands (Rijks et al., 2016) and Croatia (Vilibic‐Cavlek et al., 2019). Furthermore, in the UK (Buckley et al., 2003) and Poland (Hubalek et al., 2008) serological indications for the presence of USUV were found. In Hungary, only local outbreaks and sporadic USUV cases were detected from 2005 through 2015; while in 2016, samples of 12 birds tested positive (Bakonyi, Erdelyi, et al., 2017; Bakonyi et al., 2007). Similar to the situation in Hungary, no further cases were identified in Austria since 2006, until two blackbirds tested positive in autumn 2016 (Bakonyi, Erdelyi, et al., 2017).
USUV is clustered into six to eight separate genetic lineages [Europe 1‐3(5) and Africa 1‐3] (Cadar et al., 2017; Engel et al., 2016; Gaibani & Rossini, 2017). During the first years of USUV occurrence in Austria (Bakonyi et al., 2004; Chvala et al., 2007), as well as in Hungary (Bakonyi et al., 2007), the only lineage found was Europe 1. This changed in 2015/2016 with the first detections of USUV lineage Europe 2, which has since then become the prevalent lineage found across both countries (Bakonyi, Erdelyi, et al., 2017). In 2016, USUV lineage Europe 2 was detected for the first time in a human blood donation in Austria, and in six donations in 2017 (Bakonyi, Jungbauer, et al., 2017). In 2018, the number of human blood donors infected with USUV further increased to 18, including two donations infected with USUV lineage Africa 3 (Aberle et al., 2018). Moreover, USUV nucleic acid was found in several Culex pipiens and Aedes japonicus japonicus mosquitoes collected in affected areas in Austria (Camp, Kolodziejek, & Nowotny, 2019). The goal of this study was to continuously monitor USUV infections of the Austrian and Hungarian wild bird populations within the two transmission seasons of 2017 and 2018.
2. MATERIALS AND METHODS
2.1. Samples
In Austria, no regular USUV monitoring programmes were conducted since 2006 (Bakonyi, Erdelyi, et al., 2017). However, in 2017 and particularly in 2018, a high number of bird carcasses was submitted by veterinarians, avian research institutions, animal shelters, but predominantly concerned citizens. Altogether, 86 deceased birds of eight different species were collected, 27 in 2017 and 59 in 2018. Of them, 69 were Eurasian blackbirds (80.2%), five song thrushes (Turdus philomelos, 5.8%), four house sparrows (4.7%), two blue tits (2.3%), two bearded reedlings (Panurus biarmicus, 2.3%), two northern goshawks (Accipiter gentilis, 2.3%), one boreal owl (Aegolius funereus, 1.2%) and one amazon parrot (Amazona sp., 1.2%). All birds were submitted from June through November (three in June, two in July, 60 in August, 16 in September, four in October and one in November). From the 76 samples with known collection places, most were found in Vienna and surrounding Lower Austria (57.9%), followed by Styria (26.3%), Upper Austria (10.6%), Burgenland (2.6%) and Carinthia (2.6%).
In Hungary, a surveillance programme for passive monitoring of avian influenza cases in wild birds has been in place since 2006. Within the scope of this programme, dead bird samples were also tested for flaviviruses, such as WNV and USUV (Bakonyi, Erdelyi, et al., 2017). Altogether, birds of 67 different species were tested for USUV infection, 100 in 2017 and 153 in 2018. Of these birds, 38 were Eurasian blackbirds (15.0%), 33 greenfinches (Chloris chloris, 13.0%), 24 pheasants (Phasianus colchicus, 9.5%), 15 house sparrows (5.9%), seven Eurasian tree sparrows (Passer montanus, 2.8%), seven song thrushes (2.8%) and many other species in lower numbers (51.0%). Samples were submitted throughout the year (seven in January, 13 in February, five in March, 10 in April, 13 in May, 17 in June, 33 in July, 62 in August, 47 in September, 21 in October, 20 in November and five in December). The collection places of 11 samples from 2017 were unknown. From the remaining 242 examined birds, most were collected in the county of Szabolcs‐Szatmár‐Bereg (20.7%), followed by Bács‐Kiskun (19.8%), Győr‐Moson‐Sopron (11.6%), Pest (8.3%), Budapest (7.4%), Jász‐Nagykun‐Szolnok (7.0%), Fejér (5.8%), Hajdú‐Bihar (5.0%), Somogy (2.9%), Zala (2.9%), Borsod‐Abaúj‐Zemplén (2.1%), Heves (1.7%), Komárom‐Esztergom (1.2%), Veszprém (1.2%), Baranya (0.8%), Csongrád (0.8%), Békés (0.4%) and Nógrád (0.4%); from the counties of Tolna and Vas no birds were submitted.
2.2. Map
To show the geographic distribution of USUV‐positive cases detected in Austria in 2017 and 2018, a map was designed as described previously (Kolodziejek et al., 2018).
2.3. Pathological investigations
Species and nutritional status of the animals were determined, assessing the fat storage reserves as well as the development of the pectoral musculature. All carcasses were necropsied, and a macroscopic examination of the inner organs was carried out. Depending on the degree of autolysis, various organ samples were taken for PCR examination (predominantly brain and spleen, in some cases heart, liver, kidney or lung).
2.4. Reverse transcription PCR, sequencing and phylogenetic analysis
Viral nucleic acids were extracted from 140‐µl organ homogenates, either manually by using the QIAamp viral RNA Mini Kit (QIAGEN), or automatically via QIACube (QIAGEN); both according to the manufacturer's instructions.
Extracts derived from Austrian birds from 2018 were initially screened for USUV‐specific RNA by TaqMan real‐time reverse transcription PCR (RT‐qPCR), amplifying a part of the nonstructural protein 5 (NS5) genomic region of USUV, as described previously (Weissenböck et al., 2013). Quanta qScript XLT One‐Step RT‐qPCR ToughMix (Quantabio) was used with primers and probe at concentrations of 0.5 µM each. The Applied Biosystems 7300 Real‐Time PCR System (Thermo Fisher Scientific) was deployed with a thermal profile of 50°C for 15 min, 95°C for 2 min and 45 cycles of 95°C for 15 s and 60°C for 30 s.
All RNA extracts from Hungarian birds, Austrian extracts from 2017, as well as RT‐qPCR‐positive Austrian extracts from 2018, were subjected to conventional RT‐PCR by using QIAGEN OneStep RT‐PCR kit (QIAGEN) and a previously published primer pair, to amplify a 753‐bp PCR product within the NS5 and 3′ untranslated region (3′UTR) of the USUV genome (Bakonyi et al., 2004; Weissenböck et al., 2002). Of note, there is a second, identical binding site for the applied reverse primer (position 10930–10905 of USUV strain Austria 2001, GenBank accession no. AY453411), which may create a second PCR product with a length of 829 bp. Nevertheless, this primer pair is able to detect a broad spectrum of viruses belonging to the JEV complex and is therefore well suitable for screening of mosquito‐borne flaviviruses.
Thereafter, all PCR products were either examined by agarose gel electrophoresis or processed by automatic gel electrophoresis by using QIAxcel Advanced System (QIAGEN). Before sequencing, specific amplicons were either purified via QIAquick Gel Extraction Kit (QIAGEN) or by using PCR Kleen Spin Columns (Bio‐Rad), according to the manufacturer's instructions.
Viral nucleotide sequences were obtained by using Mix2Seq Kits from Eurofins Genomics (Eurofins Scientific) and identified by BLAST search (https://blast.ncbi.nlm.nih.gov/Blast.cgi).
The phylogenetic tree was constructed based on a 690‐nucleotide fragment of the NS5/3′UTR genomic region. In total, 53 sequences were included: 33 identified in this study and 20 from GenBank. The following representative sequences were chosen from GenBank: four of lineage Europe 1, five of Europe 2, four of Europe 3, three of Africa 2 and four of Africa 3. Africa 1 sequences were not included because of the large genetic distance. Of the 33 sequences described in this paper, 13 were generated from birds collected in 2017 (nine blackbirds from Austria, four blackbirds from Hungary), and 20 from birds collected in 2018 (13 blackbirds and six other birds from Austria, one blackbird from Hungary). Although USUV sequences could be determined for all but two Austrian birds, for the phylogenetic tree only representatives were chosen, which were either unique, or—if identical to others—were collected in a different area, at another time point, or obtained from different host species. Before the phylogenetic analysis, ClustalW multiple sequence alignments were conducted by using BioEdit Alignment Editor version 7.0.9.0. The phylogenetic tree was created with MEGA7 programme (Kumar, Stecher, & Tamura, 2016), applying the neighbour‐joining method and Tamura 3‐parameter algorithm employing 1,000 replicates of bootstrap resampling analysis.
2.5. GenBank accession numbers
The 33 newly described NS5‐partial USUV sequences are available in GenBank under the accession nos. MK598442‐MK598474; corresponding sample names are listed in Tables S1 and S2.
3. RESULTS
3.1. Samples
In Austria, altogether 67 out of 86 examined birds (77.9%) tested positive for USUV, 17 out of 27 in 2017 (63.0%) and 50 out of 59 in 2018 (84.7%). These infected birds belonged to five different species, predominantly Eurasian blackbirds (86.5%), followed by song thrushes (4.5%), house sparrows (3.0%), blue tits (3.0%) and bearded reedlings (3.0%). All USUV‐positive birds were submitted during the transmission season of Culex pipiens, which seems to be the major vector transmitting USUV in Austria (Camp et al., 2019), from July through October (one in July, 50 in August, 15 in September and one in October). The majority of infected birds (with known collection places) was found in Vienna and surrounding Lower Austria (52.3%), followed by the federal states of Styria (30.8%), Upper Austria (10.8%), Burgenland (3.1%) and Carinthia (3.1%). The locations where USUV‐positive birds were collected in Austria are indicated in Figure 1.
Figure 1.
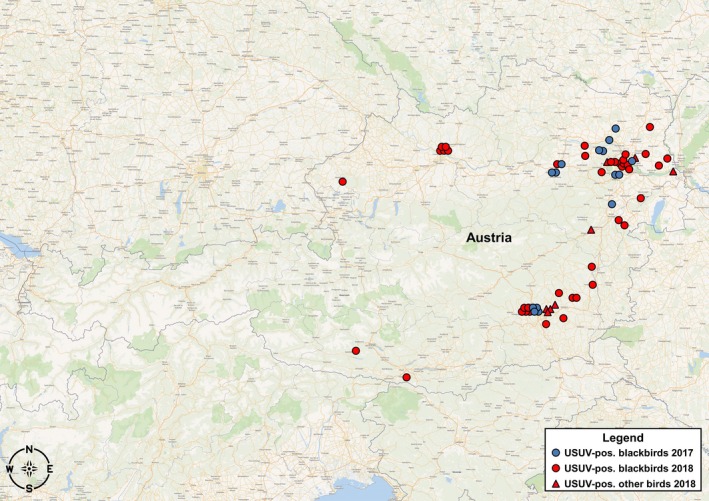
Map of Austria displaying the locations where USUV‐infected birds were found in 2017 and 2018. Colour code indicates years (2017: blue, 2018: red); bird species are indicated by symbols (circle: blackbird, triangle: other birds)
In Hungary, altogether five USUV‐positive birds were found out of 100 that were examined in 2017 (5.0%), and only one out of 153 tested positive in 2018 (0.7%). Of the five infected birds in 2017, four were Eurasian blackbirds and one a Eurasian tree sparrow. The positive case detected in 2018 was a Eurasian blackbird. Also in Hungary, all USUV‐positive birds were submitted during the transmission season (one in July, three in August and two in September). In 2017, the tree sparrow was found in Budapest, and the blackbirds were collected in the counties Bács‐Kiskun, Pest, Somogy and Zala. The 2018 blackbird case was from Budapest. An overview of all birds that were tested for USUV in Austria and Hungary in 2017 and 2018 is shown in Table 1. Details on all USUV‐positive birds are provided in Tables S1 and S2.
Table 1.
Overview of all birds that were tested for USUV in 2017 and 2018 in Austria and Hungary, listed according to species, collection year and country
| Bird species | Number of birds submitted | USUV‐positive | Lineage Europe 2 | Lineage Africa 3 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Collection year | 2017 | 2018 | 2017 | 2018 | 2017 | 2018 | 2017 | 2018 | ||||||||
| Country | A | H | A | H | A | H | A | H | A | H | A | H | A | H | A | H |
| Eurasian blackbird (Turdus merula) | 24 | 19 | 45 | 19 | 17 | 4 | 41 | 1 | 16 | 4 | 39 | 1 | 1 | ‐ | ‐ | ‐ |
| Song thrush (Turdus philomelos) | 2 | 1 | 3 | 6 | ‐ | ‐ | 3 | ‐ | ‐ | ‐ | 3 | ‐ | ‐ | ‐ | ‐ | ‐ |
| House sparrow (Passer domesticus) | ‐ | 2 | 4 | 13 | ‐ | ‐ | 2 | ‐ | ‐ | ‐ | 2 | ‐ | ‐ | ‐ | ‐ | ‐ |
| Blue tit (Cyanistes caeruleus) | ‐ | ‐ | 2 | 1 | ‐ | ‐ | 2 | ‐ | ‐ | ‐ | 2 | ‐ | ‐ | ‐ | ‐ | ‐ |
| Bearded reedling (Panurus biarmicus) | ‐ | ‐ | 2 | ‐ | ‐ | ‐ | 2 | ‐ | ‐ | ‐ | 2 | ‐ | ‐ | ‐ | ‐ | ‐ |
| Eurasian tree sparrow (Passer montanus) | ‐ | 2 | ‐ | 5 | ‐ | 1 | ‐ | ‐ | ‐ | 1 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Other species | 1 | 76 | 3 | 109 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Subtotal | 27 | 100 | 59 | 153 | 17 | 5 | 50 | 1 | 16 | 5 | 48 | 1 | 1 | ‐ | ‐ | ‐ |
| Total/year | 127 | 212 | 22 | 51 | 21 | 49 | 1 | ‐ | ||||||||
A, Austria; H, Hungary.
3.2. Pathological investigations
The nutritional status of Austrian birds ranged from bad to cachectic. A few birds showed signs of autolysis, so that only remnants of organs were available for investigations. The most prominent finding was a severely enlarged spleen. No further macroscopic changes were seen. In Hungary, USUV‐infected birds were in good to moderate body condition, and they all showed signs of splenomegaly. Moreover, varying degrees of hepatomegaly and liver congestion were found in four birds. Generally, the present pathological findings are in accordance with earlier reports (Bakonyi et al., 2007; Chvala, Kolodziejek, Nowotny, & Weissenböck, 2004).
3.3. RT‐PCR, sequencing and phylogenetic analysis
RT‐qPCR‐positive extracts from birds collected in Austria in 2018, as well as all Hungarian and Austrian extracts from 2017, were subjected to conventional RT‐PCR, amplifying a 753‐bp sequence within the NS5/3’UTR genomic region of USUV. For all samples, except two with very low viral loads, partial USUV sequences were achieved and thus the genetic lineage could be determined. In total, 70 out of 71 sequenced samples (98.6%) belonged to the Europe 2 lineage. Another lineage (Africa 3) was identified only in one blackbird found dead in the city of Stockerau in Lower Austria in 2017 (Tables 1 and S1 and S2). Phylogenetic analysis confirmed the genetic relationship among representative USUV sequences and their clustering (Figure 2). All sequences belonging to the Europe 2 lineage cluster together with Italian sequences from 2009 and 2010 (identified in blackbirds and a patient with neurological symptoms), Austrian and Hungarian bird sequences from 2016, as well as an Austrian blood donation sequence from 2017 (Figure 2). The only Africa 3 sequence detected forms a second cluster with representative sequences generated from a man in the Central African Republic in 1981 and from birds in Germany and the Netherlands in 2014 and 2016 (Figure 2). No Austrian or Hungarian sequences were detected belonging to the clusters Europe 1, Europe 3, Africa 1 and Africa 2.
Figure 2.
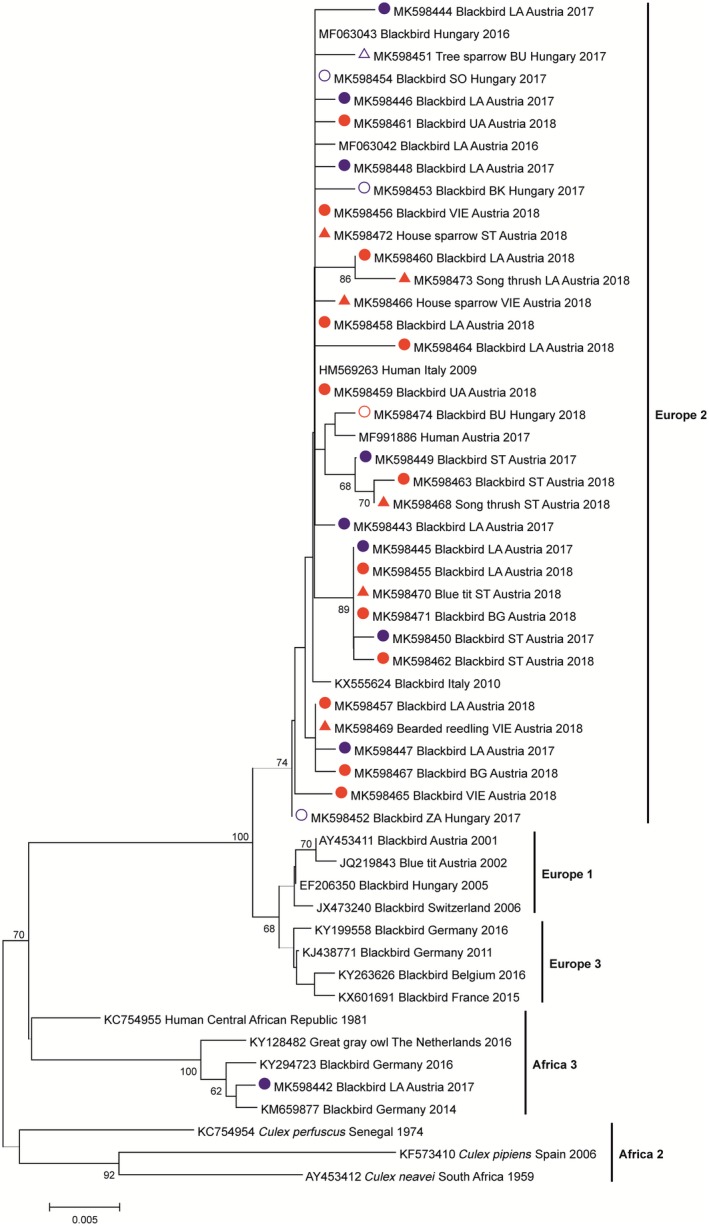
Phylogram indicating the genetic relationships among representative USUV sequences and their clustering. Sequences are labelled by their corresponding GenBank accession number, host species, region of origin (for sequences from this study), country and collection year. Sequences described in this paper are colour‐coded (2017: blue, 2018: red; circle: blackbird, triangle: other birds; filled symbols: Austrian samples, open symbols: Hungarian samples). Abbreviations for federal states of Austria: BG, Burgenland; LA, Lower Austria; ST, Styria; UA, Upper Austria; VIE, Vienna; Abbreviations for counties in Hungary: BK, Bács‐Kiskun; BU, Budapest; SO, Somogy; ZA, Zala. Horizontal lines represent the genetic distance (scale bar = 0.5% nucleotide sequence divergence); genetic lineages are indicated by vertical bars on the right; bootstrap values above 60 are displayed at the nodes
4. DISCUSSION
The sample pool obtained from passive surveillance in 2017 and 2018 indicated a considerable increase in USUV‐related bird mortalities in Austria. Moreover, while the virus had previously been found only in the eastern part of Austria (Vienna, Lower Austria, Burgenland and the city of Graz), it has further spread in 2018 to the west (Upper Austria) and to the south (large parts of Styria as well as Carinthia). As shown in Figure 1, USUV‐positive birds were mainly found in close proximity to larger cities (Vienna, Graz, Linz, Villach, Wiener Neustadt and Sankt Pölten). This might be due to their geographic locations in valleys with natural waters and flood plains, providing ideal breeding grounds for mosquitoes, as well as resting places for migratory birds. Another factor is certainly a higher human population density, leading to a more frequent detection of suspicious bird carcasses. In contrast to the situation in Austria, the number of diagnosed USUV‐infected birds decreased in Hungary after a peak in 2016 according to the avian influenza monitoring programme. However, similar to the geographic distribution of the cases from 2010 through 2016 (Bakonyi, Erdelyi, et al., 2017), all infected birds were found either in the central region (Budapest, Pest and Bács‐Kiskun) or in the western part of the country, close to the Slovenian/Croatian border (Somogy and Zala). USUV‐positive birds in Hungary were similarly collected in urban areas with varied landscapes including rivers or other bodies of water.
Usutu virus is generally clustered into six separate lineages (Europe 1‐3 and Africa 1‐3) (Engel et al., 2016). In other studies, eight different USUV lineages have been proposed (Cadar et al., 2017; Gaibani & Rossini, 2017). During the initial outbreak in 2001 and consecutive years in Austria (Bakonyi et al., 2004; Chvala et al., 2007), as well as during the first years of USUV occurrence in Hungary (Bakonyi et al., 2007), the only lineage found was Europe 1. This changed in 2015 with the first detection of Europe 2, which has since then become the prevalent lineage found in Austria and Hungary (Bakonyi, Erdelyi, et al., 2017). Our results clearly demonstrate that this lineage remains the dominant form of USUV in both countries. Except for one case, all USUV sequences obtained in this study cluster together with other Europe 2‐sequences, independent of species, geographic region or time of collection (Figure 2). A second lineage (Africa 3) was found only in one specimen from 2017 (Figure 2). Apart from its distribution across several African countries, this lineage was found in Europe for the first time in a blackbird in Germany in 2014 (Cadar et al., 2017), in Culex pipiens mosquitoes in South France in 2015 (Eiden et al., 2018) and 2016 in a blackbird in Belgium and a great grey owl in the Netherlands (Cadar et al., 2017). In Austria, lineage Africa 3 was also identified in two blood donations from 2018 (Aberle et al., 2018). Interestingly, these donors live only 30 km north and 10 km east, respectively, from the place where the Africa 3‐infected blackbird was found. Moreover, in an aviary in Vienna (only 21 km apart), there was an outbreak of USUV lineage Africa 3 in 2017, in which five out of eight non‐native azure‐winged magpies (Cyanopica cyanus) were found positive. These cases were not included in the present study, because they will be published separately (D. Thaller, unpub. data). However, as targeted entomological surveillance at specific sites proved to be very effective (Camp et al., 2019), we suggest to trap potential vectors in this area during the upcoming transmission season.
In addition, it is imperative to keep the wild bird population under surveillance, especially migratory birds, which constitute a great reservoir and transport vehicle for zoonotic arboviruses (Michel et al., 2018; Nikolay, 2015). Since the emergence of USUV in Europe, Eurasian blackbirds have been the primary species succumbing to the virus, being equally susceptible to all genetic lineages of USUV.
An overview of the differential USUV dynamics in Austria and Hungary since the initial outbreak in 2001 is shown in Figure 3. Data of the years 2001 through 2016 were obtained from several publications (Bakonyi, Erdelyi, et al., 2017; Bakonyi et al., 2007; Chvala et al., 2007; Weissenböck et al., 2003, 2002). Although sampling methods differed substantially, as active sampling was funded only during the first years after the initial outbreak in Austria, and routine sampling in Hungary is actually targeting avian influenza cases, a rough trend can be recognized. Moreover, the high number of bird carcasses submitted by the public in 2017 and 2018 clearly indicates a remarkable increase in bird mortality during this period of time. Apparently, USUV infections follow a particular cycle, with a few years with a high level of virus circulation followed by several years of low circulation. To a large extent, this dynamics depends on host factors (such as abundance and herd immunity) (Bakonyi, Erdelyi, et al., 2017), and weather conditions that primarily affect the vector population as well as the extrinsic incubation period (the time from virus intake until a mosquito becomes infectious) (Brugger & Rubel, 2009; Nikolay, 2015). According to an epidemic model established to explain the dynamics of USUV in Austria, herd immunity decreases quickly after an epidemic episode, allowing for timely recurring outbreaks (Rubel et al., 2008). Because temperature is one of the major factors determining the development of individual mosquitoes, as well as population size (Becker, 2008), the frequency of epidemics predominantly depends on temperature (Rubel et al., 2008). Other variables, such as flooding, seem to have a minor influence (Rubel et al., 2008). Thus, USUV is mainly found in regions with a temperature of ≥30°C for more than 10 days per year, such as Vienna and surrounding Lower Austria (Brugger & Rubel, 2009). According to a recent risk evaluation for USUV circulation across Europe, also Hungary is highly suitable for further virus propagation (Cheng et al., 2018). Whether the number of cases will increase within the next few years remains to be seen. The peak of USUV infections in Austria in 2003 was probably caused by a remarkably hot summer in that year (Rubel et al., 2008). A simulation of the effect of climate change on USUV dynamics predicted only minor outbreaks thereafter until a major epidemic peak in 2019 (Brugger & Rubel, 2009). According to our results, this peak may have occurred already in 2018. Continued surveillance will reveal whether the highest level of USUV infections has yet to come. However, due to progressing global warming, it can be assumed that USUV outbreaks will be more frequent in the future and the virus will continue to spread across Europe.
Figure 3.
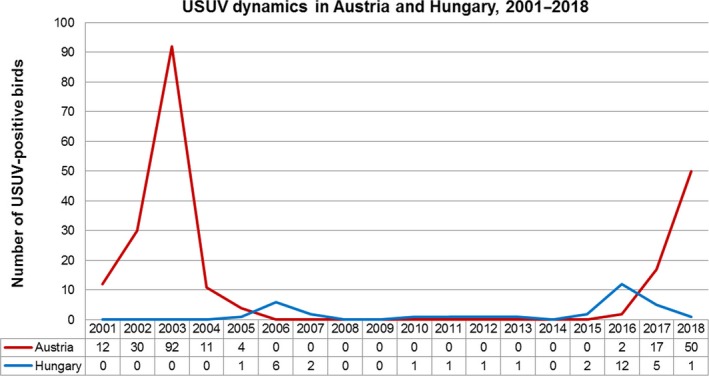
Graph demonstrating the temporal dynamics of avian USUV infections in Austria and Hungary from 2001 through 2018. Austrian USUV cases are indicated by the red line, Hungarian cases by the blue line. Data of 2001–2016 were obtained from several publications (Bakonyi, Erdelyi, et al., 2017; Bakonyi et al., 2007; Chvala et al., 2007; Weissenböck et al., 2003, 2002)
In humans, infections with USUV may cause a transient rash, but are mostly asymptomatic (Weissenböck, Chvala‐Mannsberger, Bakonyi, & Nowotny, 2007). Of all 25 blood donors that were found USUV‐positive in Austria since 2016, only one reported a rash persisting for a few days after the donation (one of the donors infected with the Africa 3 lineage) (Aberle et al., 2018; Bakonyi, Jungbauer, et al., 2017). For decades after the discovery of the virus, only two cases of human infection were reported, in 1981 in the Central African Republic and in 2004 in Burkina Faso (Nikolay, Diallo, Boye, & Sall, 2011). Due to these rare instances, and because USUV was never associated with severe disease in animals or humans, it was not perceived as a potential threat to human health (Vazquez et al., 2011)—until 2009, when the first cases of USUV‐related encephalitis in immunocompromised patients were described in Italy, providing evidence that USUV is capable of triggering severe neurological symptoms (Cavrini et al., 2009; Pecorari et al., 2009). Moreover, in 2016, a patient in France presented with acute USUV infection and possibly associated unilateral facial paralysis (Simonin et al., 2018).
In contrast to USUV, WNV was detected in Europe already in the 1950s and has caused repeated outbreaks of severe neurological disease (Nikolay, 2015), especially since the emergence (Bakonyi et al., 2006) and spread (Bakonyi et al., 2013) of a novel lineage 2 WNV. However, USUV and WNV co‐circulate in at least 10 European countries, including Austria and Hungary, infect a largely overlapping range of host species, and both viruses are transmitted primarily by Culex pipiens mosquitoes (Nikolay, 2015). Moreover, these two flaviviruses are genetically closely related to each other and might induce immunological cross‐reactions with yet unknown implications. Because of their close genetic and antigenic relatedness, routine diagnostic tests are often not able to differentiate between USUV and WNV infections (Aberle et al., 2018; Bakonyi, Jungbauer, et al., 2017; Beck et al., 2013). Therefore, healthcare professionals should be vigilant and consider the possibility of USUV infections, especially in cases of encephalitis (Cavrini et al., 2009; Pecorari et al., 2009) and in transfusion medicine (Aberle et al., 2018; Bakonyi, Jungbauer, et al., 2017; Domanovic et al., 2019).
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ETHICAL STATEMENT
Not applicable because only dead (wild) birds submitted by veterinarians, avian research institutions, animal shelters, but predominantly concerned citizens for diagnostic investigations were included in the study.
Supporting information
ACKNOWLEDGEMENTS
The authors wish to thank Dominika Kapuscinska for the excellent art work, and the many persons who have sent birds to the Universities of Veterinary Medicine in Vienna and Budapest for examination.
Weidinger P, Kolodziejek J, Bakonyi T, et al. Different dynamics of Usutu virus infections in Austria and Hungary, 2017–2018. Transbound Emerg Dis. 2020;67:298–307. 10.1111/tbed.13351
REFERENCES
- Aberle, S. W. , Kolodziejek, J. , Jungbauer, C. , Stiasny, K. , Aberle, J. H. , Zoufaly, A. , Nowotny, N. . (2018). Increase in human West Nile and Usutu virus infections, Austria, 2018. Eurosurveillance, 23(43), 10.2807/1560-7917.ES.2018.23.43.1800545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakonyi, T. , Busquets, N. , & Nowotny, N. (2014). Comparison of complete genome sequences of Usutu virus strains detected in Spain, Central Europe, and Africa. Vector‐Borne and Zoonotic Diseases, 14(5), 324–329. 10.1089/vbz.2013.1510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakonyi, T. , Erdelyi, K. , Brunthaler, R. , Dan, A. , Weissenböck, H. , & Nowotny, N. (2017). Usutu virus, Austria and Hungary, 2010–2016. Emerging Microbes & Infections, 6(10), e85 10.1038/emi.2017.72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakonyi, T. , Erdelyi, K. , Ursu, K. , Ferenczi, E. , Csorgo, T. , Lussy, H. , … Nowotny, N. (2007). Emergence of Usutu virus in Hungary. Journal of Clinical Microbiology, 45(12), 3870–3874. 10.1128/JCM.01390-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakonyi, T. , Ferenczi, E. , Erdelyi, K. , Kutasi, O. , Csorgo, T. , Seidel, B. , … Nowotny, N. (2013). Explosive spread of a neuroinvasive lineage 2 West Nile virus in Central Europe, 2008/2009. Veterinary Microbiology, 165(1–2), 61–70. 10.1016/j.vetmic.2013.03.005 [DOI] [PubMed] [Google Scholar]
- Bakonyi, T. , Gould, E. A. , Kolodziejek, J. , Weissenböck, H. , & Nowotny, N. (2004). Complete genome analysis and molecular characterization of Usutu virus that emerged in Austria in 2001: Comparison with the South African strain SAAR‐1776 and other flaviviruses. Virology, 328(2), 301–310. 10.1016/j.virol.2004.08.005 [DOI] [PubMed] [Google Scholar]
- Bakonyi, T. , Ivanics, E. , Erdelyi, K. , Ursu, K. , Ferenczi, E. , Weissenböck, H. , & Nowotny, N. (2006). Lineage 1 and 2 Strains of Encephalitic West Nile Virus, Central Europe. Emerging Infectious Diseases, 12(4), 618–623. 10.3201/eid1204.051379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakonyi, T. , Jungbauer, C. , Aberle, S. W. , Kolodziejek, J. , Dimmel, K. , Stiasny, K. , Nowotny, N. (2017). Usutu virus infections among blood donors, Austria, July and August 2017 – Raising awareness for diagnostic challenges. Eurosurveillance, 22(41), 10.2807/1560-7917.ES.2017.22.41.17-00644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck, C. , Jimenez‐Clavero, M. A. , Leblond, A. , Durand, B. , Nowotny, N. , Leparc‐Goffart, I. , … Lecollinet, S. (2013). Flaviviruses in Europe: Complex circulation patterns and their consequences for the diagnosis and control of West Nile disease. International Journal of Environmental Research and Public Health, 10(11), 6049–6083. 10.3390/ijerph10116049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker, N. (2008). Influence of climate change on mosquito development and mosquito‐borne diseases in Europe. Parasitology Research, 103(Suppl 1), S19–28. 10.1007/s00436-008-1210-2 [DOI] [PubMed] [Google Scholar]
- Brugger, K. , & Rubel, F. (2009). Simulation of climate‐change scenarios to explain Usutu‐virus dynamics in Austria. Preventive Veterinary Medicine, 88(1), 24–31. 10.1016/j.prevetmed.2008.06.023 [DOI] [PubMed] [Google Scholar]
- Buckley, A. , Dawson, A. , Moss, S. R. , Hinsley, S. A. , Bellamy, P. E. , & Gould, E. A. (2003). Serological evidence of West Nile virus, Usutu virus and Sindbis virus infection of birds in the UK. Journal of General Virology, 84(Pt 10), 2807–2817. 10.1099/vir.0.19341-0 [DOI] [PubMed] [Google Scholar]
- Busquets, N. , Alba, A. , Allepuz, A. , Aranda, C. , & Ignacio Nunez, J. (2008). Usutu virus sequences in Culex pipiens (Diptera: Culicidae), Spain. Emerging Infectious Diseases, 14(5), 861–863. 10.3201/eid1405.071577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadar, D. , Luhken, R. , van der Jeugd, H. , Garigliany, M. , Ziegler, U. , Keller, M. , Schmidt‐Chanasit, J. (2017). Widespread activity of multiple lineages of Usutu virus, western Europe, 2016. Eurosurveillance, 22(4), 10.2807/1560-7917.ES.2017.22.4.30452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camp, J. V. , Kolodziejek, J. , & Nowotny, N. (2019). Targeted surveillance reveals native and invasive mosquito species infected with Usutu virus. Parasites & Vectors, 12(1), 46 10.1186/s13071-019-3316-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavrini, F. , Gaibani, P. , Longo, G. , Pierro, A. M. , Rossini, G. , Bonilauri, P. , Sambri, V. (2009). Usutu virus infection in a patient who underwent orthotropic liver transplantation, Italy, August‐September 2009. Eurosurveillance, 14(50), 19448. [PubMed] [Google Scholar]
- Chaintoutis, S. C. , Dovas, C. I. , Papanastassopoulou, M. , Gewehr, S. , Danis, K. , Beck, C. , … Papadopoulos, O. (2014). Evaluation of a West Nile virus surveillance and early warning system in Greece, based on domestic pigeons. Comparative Immunology, Microbiology and Infectious Diseases, 37(2), 131–141. 10.1016/j.cimid.2014.01.004 [DOI] [PubMed] [Google Scholar]
- Cheng, Y. , Tjaden, N. B. , Jaeschke, A. , Luhken, R. , Ziegler, U. , Thomas, S. M. , & Beierkuhnlein, C. (2018). Evaluating the risk for Usutu virus circulation in Europe: Comparison of environmental niche models and epidemiological models. International Journal of Health Geographics, 17(1), 35 10.1186/s12942-018-0155-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chvala, S. , Bakonyi, T. , Bukovsky, C. , Meister, T. , Brugger, K. , Rubel, F. , … Weissenböck, H. (2007). Monitoring of Usutu virus activity and spread by using dead bird surveillance in Austria, 2003–2005. Veterinary Microbiology, 122(3–4), 237–245. 10.1016/j.vetmic.2007.01.029 [DOI] [PubMed] [Google Scholar]
- Chvala, S. , Kolodziejek, J. , Nowotny, N. , & Weissenböck, H. (2004). Pathology and viral distribution in fatal Usutu virus infections of birds from the 2001 and 2002 outbreaks in Austria. Journal of Comparative Pathology, 131(2–3), 176–185. 10.1016/j.jcpa.2004.03.004 [DOI] [PubMed] [Google Scholar]
- Domanovic, D. , Gossner, C. M. , Lieshout‐Krikke, R. , Mayr, W. , Baroti‐Toth, K. , Dobrota, A. M. , … Nowotny, N. (2019). West Nile and Usutu virus infections and challenges to blood safety in the European Union. Emerging Infectious Diseases, 25(6), 1050–1057. 10.3201/eid2506.181755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiden, M. , Gil, P. , Ziegler, U. , Rakotoarivony, I. , Marie, A. , Frances, B. , … Eloit, M. (2018). Emergence of two Usutu virus lineages in Culex pipiens mosquitoes in the Camargue, France, 2015. Infection, Genetics and Evolution, 61, 151–154. 10.1016/j.meegid.2018.03.020 [DOI] [PubMed] [Google Scholar]
- Engel, D. , Jost, H. , Wink, M. , Borstler, J. , Bosch, S. , Garigliany, M. M. , … Schmidt‐Chanasit, J. (2016). Reconstruction of the evolutionary history and dispersal of Usutu virus, a neglected emerging arbovirus in Europe and Africa. Mbio, 7(1), e01938-15 10.1128/mBio.01938-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaibani, P. , & Rossini, G. (2017). An overview of Usutu virus. Microbes and Infection, 19(7–8), 382–387. 10.1016/j.micinf.2017.05.003 [DOI] [PubMed] [Google Scholar]
- Garigliany, M. , Linden, A. , Gilliau, G. , Levy, E. , Sarlet, M. , Franssen, M. , … Desmecht, D. (2017). Usutu virus, Belgium, 2016. Infection, Genetics and Evolution, 48, 116–119. 10.1016/j.meegid.2016.12.023 [DOI] [PubMed] [Google Scholar]
- Garigliany, M. M. , Marlier, D. , Tenner‐Racz, K. , Eiden, M. , Cassart, D. , Gandar, F. , … Desmecht, D. (2014). Detection of Usutu virus in a bullfinch (Pyrrhula pyrrhula) and a great spotted woodpecker (Dendrocopos major) in north‐west Europe. The Veterinary Journal, 199(1), 191–193. 10.1016/j.tvjl.2013.10.017 [DOI] [PubMed] [Google Scholar]
- Hubalek, Z. , Rudolf, I. , Capek, M. , Bakonyi, T. , Betasova, L. , & Nowotny, N. (2014). Usutu virus in blackbirds (Turdus merula), Czech Republic, 2011–2012. Transboundary and Emerging Diseases, 61(3), 273–276. 10.1111/tbed.12025 [DOI] [PubMed] [Google Scholar]
- Hubalek, Z. , Wegner, E. , Halouzka, J. , Tryjanowski, P. , Jerzak, L. , Sikutova, S. , … Wlodarczyk, R. (2008). Serologic survey of potential vertebrate hosts for West Nile virus in Poland. Viral Immunology, 21(2), 247–253. 10.1089/vim.2007.0111 [DOI] [PubMed] [Google Scholar]
- Jost, H. , Bialonski, A. , Maus, D. , Sambri, V. , Eiden, M. , Groschup, M. H. , … Schmidt‐Chanasit, J. (2011). Isolation of Usutu virus in Germany. The American Journal of Tropical Medicine and Hygiene, 85(3), 551–553. 10.4269/ajtmh.2011.11-0248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolodziejek, J. , Jungbauer, C. , Aberle, S. W. , Allerberger, F. , Bago, Z. , Camp, J. V. , … Nowotny, N. (2018). Integrated analysis of human‐animal‐vector surveillance: West Nile virus infections in Austria, 2015–2016. Emerging Microbes & Infections, 7(1), 25 10.1038/s41426-018-0021-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar, S. , Stecher, G. , & Tamura, K. (2016). MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 for bigger datasets. Molecular Biology and Evolution, 33(7), 1870–1874. 10.1093/molbev/msw054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuno, G. , Chang, G. J. , Tsuchiya, K. R. , Karabatsos, N. , & Cropp, C. B. (1998). Phylogeny of the genus Flavivirus . Journal of Virology, 72(1), 73–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecollinet, S. , Blanchard, Y. , Manson, C. , Lowenski, S. , Laloy, E. , Quenault, H. , … Decors, A. (2016). Dual emergence of Usutu virus in common blackbirds, Eastern France, 2015. Emerging Infectious Diseases, 22(12), 2225 10.3201/eid2212.161272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manarolla, G. , Bakonyi, T. , Gallazzi, D. , Crosta, L. , Weissenböck, H. , Dorrestein, G. M. , & Nowotny, N. (2010). Usutu virus in wild birds in northern Italy. Veterinary Microbiology, 141(1–2), 159–163. 10.1016/j.vetmic.2009.07.036 [DOI] [PubMed] [Google Scholar]
- McIntosh, B. M. (1985). Usutu (SA Ar 1776), nouvel arbovirus du groupe B. [Usutu (SA Ar 1776), novel arbovirus of group B]. The International Catalog of Arboviruses, 3, 1059–1060. [Google Scholar]
- Meister, T. , Lussy, H. , Bakonyi, T. , Sikutova, S. , Rudolf, I. , Vogl, W. , … Weissenböck, H. (2008). Serological evidence of continuing high Usutu virus (Flaviviridae) activity and establishment of herd immunity in wild birds in Austria. Veterinary Microbiology, 127(3–4), 237–248. 10.1016/j.vetmic.2007.08.023 [DOI] [PubMed] [Google Scholar]
- Michel, F. , Fischer, D. , Eiden, M. , Fast, C. , Reuschel, M. , Muller, K. , … Ziegler, U. (2018). West Nile virus and Usutu virus monitoring of wild birds in Germany. International Journal of Environmental Research and Public Health, 15(1), 171 10.3390/ijerph15010171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolay, B. (2015). A review of West Nile and Usutu virus co‐circulation in Europe: How much do transmission cycles overlap? Transactions of the Royal Society of Tropical Medicine and Hygiene, 109(10), 609–618. 10.1093/trstmh/trv066 [DOI] [PubMed] [Google Scholar]
- Nikolay, B. , Diallo, M. , Boye, C. S. , & Sall, A. A. (2011). Usutu virus in Africa. Vector‐Borne and Zoonotic Diseases, 11(11), 1417–1423. 10.1089/vbz.2011.0631 [DOI] [PubMed] [Google Scholar]
- Pecorari, M. , Longo, G. , Gennari, W. , Grottola, A. , Sabbatini, A. , Tagliazucchi, S. , Rumpianesi, F. (2009). First human case of Usutu virus neuroinvasive infection, Italy, August‐September 2009. Eurosurveillance, 14(50), 19446. [PubMed] [Google Scholar]
- Poidinger, M. , Hall, R. A. , & Mackenzie, J. S. (1996). Molecular characterization of the Japanese encephalitis serocomplex of the Flavivirus genus. Virology, 218(2), 417–421. 10.1006/viro.1996.0213 [DOI] [PubMed] [Google Scholar]
- Rijks, J. M. , Kik, M. L. , Slaterus, R. , Foppen, R. , Stroo, A. , IJzer, J. , … Reusken, C. (2016). Widespread Usutu virus outbreak in birds in the Netherlands, 2016. Eurosurveillance, 21(45). 10.2807/1560-7917.ES.2016.21.45.30391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubel, F. , Brugger, K. , Hantel, M. , Chvala‐Mannsberger, S. , Bakonyi, T. , Weissenböck, H. , & Nowotny, N. (2008). Explaining Usutu virus dynamics in Austria: Model development and calibration. Preventive Veterinary Medicine, 85(3–4), 166–186. 10.1016/j.prevetmed.2008.01.006 [DOI] [PubMed] [Google Scholar]
- Simonin, Y. , Sillam, O. , Carles, M. J. , Gutierrez, S. , Gil, P. , Constant, O. , … Foulongne, V. (2018). Human Usutu virus infection with atypical neurologic presentation, Montpellier, France, 2016. Emerging Infectious Diseases, 24(5), 875–878. 10.3201/eid2405.171122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmetz, H. W. , Bakonyi, T. , Weissenböck, H. , Hatt, J. M. , Eulenberger, U. , Robert, N. , … Nowotny, N. (2011). Emergence and establishment of Usutu virus infection in wild and captive avian species in and around Zurich, Switzerland‐Genomic and pathologic comparison to other Central European outbreaks. Veterinary Microbiology, 148(2–4), 207–212. 10.1016/j.vetmic.2010.09.018 [DOI] [PubMed] [Google Scholar]
- Vazquez, A. , Jimenez‐Clavero, M. , Franco, L. , Donoso‐Mantke, O. , Sambri, V. , Niedrig, M. , Tenorio, A. (2011). Usutu virus ‐ potential risk of human disease in Europe. Eurosurveillance, 16(31), 19935. [PubMed] [Google Scholar]
- Vilibic‐Cavlek, T. , Savic, V. , Sabadi, D. , Peric, L. , Barbic, L. , Klobucar, A. , … Savini, G. (2019). Prevalence and molecular epidemiology of West Nile and Usutu virus infections in Croatia in the 'One health' context, 2018. Transboundary and Emerging Diseases, 66(5), 1946–1957. 10.1111/tbed.13225 [DOI] [PubMed] [Google Scholar]
- Weissenböck, H. , Bakonyi, T. , Rossi, G. , Mani, P. , & Nowotny, N. (2013). Usutu virus, Italy, 1996. Emerging Infectious Diseases, 19(2), 274–277. 10.3201/eid1902.121191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissenböck, H. , Chvala‐Mannsberger, S. , Bakonyi, T. , & Nowotny, N. (2007). Emergence of Usutu virus in Central Europe: Diagnosis, surveillance and epizootiology, Vol. 1 Wageningen: Wageningen Academic Publishers. [Google Scholar]
- Weissenböck, H. , Kolodziejek, J. , Fragner, K. , Kuhn, R. , Pfeffer, M. , & Nowotny, N. (2003). Usutu virus activity in Austria, 2001–2002. Microbes and Infection, 5(12), 1132–1136. 10.1016/S1286-4579(03)00204-1 [DOI] [PubMed] [Google Scholar]
- Weissenböck, H. , Kolodziejek, J. , Url, A. , Lussy, H. , Rebel‐Bauder, B. , & Nowotny, N. (2002). Emergence of Usutu virus, an African mosquito‐borne Flavivirus of the Japanese encephalitis virus group, Central Europe. Emerging Infectious Diseases, 8(7), 652–656. 10.3201/eid0807.020094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams, M. C. , Simpson, D. I. , Haddow, A. J. , & Knight, E. M. (1964). The isolation of West Nile virus from man and of Usutu virus from the bird‐biting mosquito Mansonia Aurites (Theobald) in the Entebbe area of Uganda. Annals of Tropical Medicine and Parasitology, 58, 367–374. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


