Abstract
In the 20th Nationwide Follow‐up Survey of Primary Liver Cancer in Japan, data from 21 075 new patients and 40 769 previously followed patients were compiled from 544 institutions over a 2‐year period from 1 January 2008 to 31 December 2009. Compared with the previous 19th survey, the population of patients with hepatocellular carcinoma (HCC) was older at the time of clinical diagnosis, included more female patients, included more patients with non‐B non‐C HCC, had smaller tumor diameters and more frequently received radiofrequency ablation as local ablation therapy. Cumulative survival rates were calculated for HCC, intrahepatic cholangiocarcinoma, and combined hepatocellular cholangiocarcinoma (combined HCC and intrahepatic cholangiocarcinoma) by treatment type and by background characteristics for patients newly registered between 1998 and 2009 whose final outcome was survival or death. Cumulative survival rates for HCC were calculated by dividing patients by combinations of background factors (number of tumors, tumor diameter, and Child–Pugh grade) and by treatment types (hepatectomy, local ablation therapy, and transcatheter arterial chemoembolization). Cumulative survival rates and median overall survival in patients treated by resection, transcatheter arterial chemoembolization, and local ablation therapy were calculated. The same values were also calculated by the registration date by dividing patients newly registered between 1978 and 2009 into four time period groups . The results of the analysis show that the prognosis of HCC is improving dramatically. It is expected that the data obtained from this nationwide follow‐up survey will contribute to advancing clinical research, including the design of clinical trials, as well as the treatment strategy of primary liver cancer in the clinical practice setting.
Keywords: combined hepatocellular cholangiocarcinoma, cumulative survival rate, hepatocellular carcinoma, intrahepatic cholangiocarcinoma, Liver Cancer Study Group of Japan, nationwide follow‐up survey
Introduction
The Liver Cancer Study Group of Japan has worked to advance the study and treatment of liver cancer since 1969, carrying out 19 national surveys on primary liver cancer with institutional members and collaborating institutions across Japan based on its General Rules for the Clinical and Pathological Study of Primary Liver Cancer,1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11 and publishing the official results of those surveys12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41 and original article using this database.42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70 The group also reports on the Response Evaluation Criteria in Cancer of the Liver.71, 72, 73, 74, 75
This report presents the results of the 20th Nationwide Follow‐up Survey of Primary Liver Cancer in Japan, with data obtained for 21 075 newly registered patients attending 544 institutions across Japan over the 2‐year period from 1 January 2008 to 31 December 2009. The valid response rate for the 40 769 previously followed patients was 90.4%. Epidemiological, clinicopathological, diagnostic, and treatment‐related data were compiled for newly registered patients. Cumulative survival rates by histological type, background characteristics, and treatments were also calculated for patients newly registered in the 15th to 20th surveys during 1978 to 2009. This special report is a concise version of the original full‐version report published in Japanese.41, 76
Methods
Basic statistics
The participants of this survey were patients who were hospitalized, underwent outpatient treatment, or underwent autopsy for primary liver cancer at each of 544 collaborating institutions across Japan during the 2‐year period from 1 January 2008 to 31 December 2009, and whose treating institutions had entered patient data for survey items created by the Follow‐up Survey Committee of the Liver Cancer Study Group of Japan (Chair: Masatoshi Kudo) into the National Clinical Database. A total of 21 075 patients were newly registered during this period. When clinical diagnosis, histopathological diagnosis, and histopathological diagnosis determined by autopsy were not consistent, the autopsy result was given precedence when available, and the histopathological diagnosis otherwise. The histological type was hepatocellular carcinoma (HCC) in 94.3% of patients, intrahepatic cholangiocarcinoma (ICC) in 4.8%, and combined hepatocellular cholangiocarcinoma in 0.7% (Table 1). The results for patients newly registered in this survey were tabulated. Patients with unknown data for a given parameter were excluded from tabulation for that parameter. The abbreviations used in the table are based on the Fifth Revised Edition of the General Rules for the Clinical and Pathological Study of Primary Liver Cancer.6
Table 1.
Primary liver cancer diagnosed clinically or histopathologically
| Histological type | Men | Women |
Total n (%) |
|---|---|---|---|
| n = 14 512 | n = 6563 | ||
| Hepatocellular carcinoma | 13 626 | 6043 | 19 669 (93.33) |
| Intrahepatic cholangiocarcinoma | 626 | 379 | 1005 (4.77) |
| Cholangiolocellular carcinoma | 29 | 13 | 42 (0.20) |
| Biliary cystadenocarcinoma | 14 | 12 | 26 (0.12) |
| Combined hepatocellular cholangiocarcinoma | 113 | 42 | 155 (0.74) |
| Hepatoblastoma | 6 | 6 | 12 (0.06) |
| Undifferentiated carcinoma | 11 | 5 | 16 (0.08) |
| Other | 87 | 63 | 150 (0.71) |
| Total | 21 075 |
Cumulative survival
Cumulative survival rates were calculated for HCC, ICC, and combined HCC and ICC by treatments and by background characteristics for patients newly registered between 1998 and 2009 whose final outcome was survival or death (excluding unknown). Cumulative survival rates for HCC were calculated by treatments (hepatectomy, local ablation therapy, and transcatheter arterial chemoembolization [TACE]). Cumulative survival rates were also calculated by the registration date by dividing patients newly registered between 1978 and 2009 into four time period groups. These cumulative survival rate calculations were made without censoring any deaths, including those in the “Other” category.
Results
Basic statistics
Causes of death of newly enrolled patients during the survey period
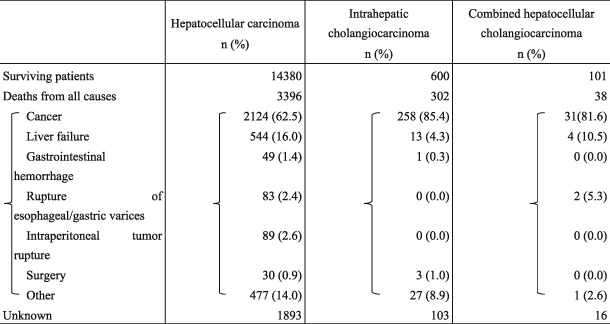
The mortality rate during the survey period for newly enrolled patients with HCC was 17.2% (3396 patients). The cause of death was cancer for 62.5%, liver failure for 16.0%, gastrointestinal hemorrhage for 1.4%, and rupture of esophageal or gastric varices for 2.4%. The mortality rate from surgery among the patients who underwent surgery was 0.9% (30 patients). The mortality rate for newly enrolled patients with ICC was 30.0% (302 patients). The cause of death was cancer for 85.4% and liver failure for 4.3% (Table 2).
Table 2.
Prognosis
|
Past medical history
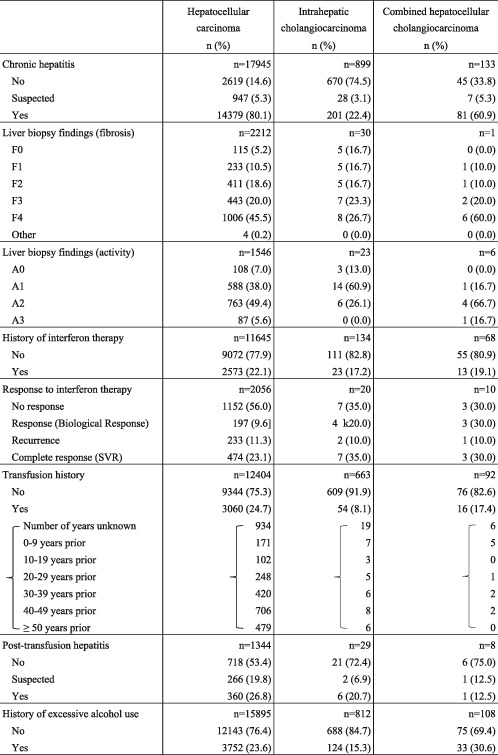
The proportion of patients with a history of chronic hepatitis and cirrhosis was 80.1% for HCC and 22.4% for ICC. The proportion for patients with HCC who had received interferon therapy for chronic hepatitis was 22.1%. For patients with a history of transfusion and excessive alcohol use, the figures were 24.7% and 23.6% for HCC, and 8.1% and 15.3% for ICC, respectively (Table 3).
Table 3.
Past medical history
|
For all parameters, n is the total number of patients, excluding those in the “unknown” category, and (%) is the percentage of n.
F0, no fibrosis; F1, fibrous expansion of portal tract; F2, fibrous septa formation, usually incomplete; F3, bridging fibrous formation accompanying lobular distortion A0, no necroinflammatory reaction; A1, mild necroinflammatory reaction; A2, moderate necroinflammatory reaction; A3, severe necroinflammatory reaction; SVR; sustained viral response.
Clinical diagnosis
Mean age of men and women at clinical diagnosis of primary liver cancer was 67.8 and 71.2 years for HCC, and 67.4 and 68.2 years for ICC, respectively. The ratio of male to female patients was 2.26:1 for HCC and 1.68:1 for ICC.
The Child–Pugh grade was A for 76.8%, B for 19.4%, and C for 3.8% of patients (Table 4). In HCC, serum α‐fetoprotein was <15 ng/mL in 46.4%, 15–199 ng/mL in 31.1%, and ≥ 200 ng/mL in 22.5% of patients. lectin‐reactive α‐fetoprotein was <10% in 68.3%, 10–14.9% in 4.1%, and ≥15% in 27.6% of patients. Protein induced by vitamin K absence or antagonist‐II was <40 mAU/mL in 39.5%, 40–99 mAU/mL in 15.4%, and ≥100 mAU/mL in 45.1% of patients. In ICC, CEA was <5.0 ng/mL in 63.5%, 5.0–9.9 ng/mL in 14.8%, and ≥10 ng/mL in 21.7% of patients. CA19–9 was <37 U/mL in 37.9%, 37–99 U/mL in 13.3%, and ≥ 100 U/mL in 48.8% of patients (Table 4).
Table 4.
Clinical diagnosis
|
Hepatocellular carcinoma n (%) |
Intrahepatic cholangiocarcinoma n (%) |
Combined hepatocellular cholangiocarcinoma n (%) |
|
|---|---|---|---|
| Evidence for diagnosis | n = 33 125 | n = 1822 | n = 255 |
| CT | 15 306 | 822 | 118 |
| MRI | 4666 | 324 | 38 |
| Ultrasound | 6889 | 342 | 46 |
| Contrast ultrasound | 822 | 23 | 4 |
| Angiography | 3984 | 90 | 25 |
| Pathology | 1213 | 165 | 22 |
| Other | 245 | 56 | 2 |
| Percentages not calculated as multiple responses were allowed | |||
| Performance status | n = 17 307 | n = 887 | n = 138 |
| PS0 | 13 618 (78.7) | 633 (71.4) | 111 (80.4) |
| PS1 | 2685 (15.5) | 185 (20.9) | 16 (11.6) |
| PS2 | 616 (3.6) | 42 (4.7) | 9 (6.5) |
| PS3 | 274 (1.6) | 19 (2.1) | 2 (1.4) |
| PS4 | 114 (0.7) | 8 (0.9) | 0 (0.0) |
| Encephalopathy | n = 18 488 | n = 913 | n = 143 |
| No | 18 021 (97.5) | 907 (99.3) | 143 (100.0) |
| Mild | 359 (1.9) | 4 (0.4) | 0 (0.0) |
| Moderate‐to‐severe | 108 (0.6) | 2 (0.2) | 0 (0.0) |
| Ascites | n = 18 863 | n = 947 | n = 147 |
| No | 16 707 (88.6) | 878 (92.7) | 130 (88.4) |
| Responded to treatment | 1434 (7.6) | 31 (3.3) | 8 (5.4) |
| Refractory to treatment | 722 (3.8) | 38 (4.0) | 9 (6.1) |
| Serum bilirubin | n = 19 053 | n = 949 | n = 150 |
| 0.0–0.9 | 11 422 (59.9) | 626 (66.0) | 100 (66.7) |
| 1.0–1.9 | 6097 (32.0) | 214 (22.6) | 42 (28.0) |
| 2.0–3.0 | 983 (5.2) | 26 (2.7) | 3 (2.0) |
| ≥3.1 mg/dL | 551 (2.9) | 83 (8.7) | 5 (3.3) |
| Serum albumin | n = 18 997 | n = 946 | n = 148 |
| <2.8 | 1438 (7.6) | 52 (5.5) | 4 (2.7) |
| 2.8–2.9 | 878 (4.6) | 27 (2.9) | 5 (3.4) |
| 3.0–3.5 | 5085 (26.8) | 146 (15.4) | 34 (23.0) |
| >3.5 g/dL | 11 596 (61.0) | 721 (76.2) | 105 (70.9) |
| ICG R15 | n = 10 619 | n = 622 | n = 106 |
| ≤14 | 4508 (42.5) | 466 (74.9) | 65 (61.3) |
| 15–24 | 3105 (29.2) | 117 (18.8) | 31 (29.2) |
| 25–40 | 2021 (19.0) | 35 (5.6) | 7 (6.6) |
| >40% | 985 (9.3) | 4 (0.6) | 3 (2.8) |
| Prothrombin activity | n = 18 174 | n = 877 | n = 148 |
| < 40 | 287 (1.6) | 25 (2.9) | 2 (1.4) |
| 40–49 | 296 (1.6) | 14 (1.6) | 2 (1.4) |
| 50–70 | 3012 (16.6) | 65 (7.4) | 8 (5.4) |
| 71–80 | 3641 (20.0) | 108 (12.3) | 28 (18.9) |
| > 80% | 10 938 (60.2) | 665 (75.8) | 108 (73.0) |
| Prothrombin time (INR) | n = 9175 | n = 436 | n = 67 |
| ≤1.20 | 6840 (74.6) | 363 (83.3) | 59 (88.1) |
| 1.21–1.30 | 1140 (12.4) | 37 (8.5) | 5 (7.5) |
| 1.31–1.50 | 826 (9.0) | 19 (4.4) | 2 (3.0) |
| 1.51–1.80 | 253 (2.8) | 11 (2.5) | 1 (1.5) |
| ≥1.81 | 116 (1.3) | 6 (1.4) | 0 (0.0) |
| Platelets | n = 18 875 | n = 942 | n = 150 |
| <3.0 | 133 (0.7) | 2 (0.2) | 0 (0.0) |
| 3.0–4.9 | 807 (4.3) | 8 (0.8) | 5 (3.3) |
| 5.0–9.9 | 5541 (29.4) | 54 (5.7) | 20 (13.3) |
| 10.0–14.9 | 5768 (30.6) | 151 (16.0) | 36 (24.0) |
| 15.0–19.9 | 3513 (18.6) | 236 (25.1) | 42 (28.0) |
| 20.0–99.9 | 2980 (15.8) | 479 (50.8) | 45 (30.0) |
| ≥100 × 103/mm3 | 133 (0.7) | 12 (1.3) | 2 (1.3) |
| Liver damage grade by LCSGJ | n = 15 137 | n = 807 | n = 128 |
| A | 10 388 (68.6) | 700 (86.7) | 96 (75.0) |
| B | 4007 (26.5) | 77 (9.5) | 29 (22.7) |
| C | 742 (4.9) | 30 (3.7) | 3 (2.3) |
| Child–Pugh grade | n = 18 314 | n = 890 | n = 145 |
| A | 14 068 (76.8) | 775 (87.1) | 122 (84.1) |
| B | 3545 (19.4) | 85 (9.6) | 20 (13.8) |
| C | 701 (3.8) | 30 (3.4) | 3 (2.1) |
| AFP | n = 18 438 | n = 675 | n = 143 |
| <15 | 8551 (46.4) | 571 (84.6) | 57 (39.9) |
| ≤199 | 5732 (31.1) | 69 (10.2) | 40 (28.0) |
| ≤399 | 835 (4.5) | 12 (1.8) | 4 (2.8) |
| ≤999 | 912 (4.9) | 7 (1.0) | 15 (10.5) |
| ≤9999 | 1390 (7.5) | 10 (1.5) | 21 (14.7) |
| ≤99 999 | 670 (3.6) | 4 (0.6) | 5 (3.5) |
| ≥100 000 ng/mL | 348 (1.9) | 2 (0.3) | 1 (0.7) |
| AFPL3 | n = 8619 | n = 195 | n = 81 |
| Below detectable levels | 3063 (35.5) | 124 (63.6) | 25 (30.9) |
| <5.0 | 2129 (24.7) | 30 (15.4) | 14 (17.3) |
| ≤9.9 | 699 (8.1) | 7 (3.6) | 6 (7.4) |
| ≤14.9 | 354 (4.1) | 2 (1.0) | 2 (2.5) |
| ≤19.9 | 256 (3.0) | 3 (1.5) | 1 (1.2) |
| ≥20.0% | 2118 (24.6) | 29 (14.9) | 33 (40.7) |
| PIVKA‐II | n = 17 540 | n = 565 | n = 137 |
| <40 | 6927 (39.5) | 449 (79.5) | 61 (44.5) |
| ≤99 | 2700 (15.4) | 46 (8.1) | 12 (8.8) |
| ≤299 | 2303 (13.1) | 26 (4.6) | 17 (12.4) |
| ≤499 | 823 (4.7) | 4 (0.7) | 5 (3.6) |
| ≤999 | 919 (5.2) | 4 (0.7) | 6 (4.4) |
| ≤2999 | 1237 (7.1) | 14 (2.5) | 16 (11.7) |
| ≤9999 | 1036 (5.9) | 7 (1.2) | 8 (5.8) |
| ≥10 000 mAU/mL | 1595 (9.1) | 15 (2.7) | 12 (8.8) |
| CEA | n = 7294 | n = 858 | n = 117 |
| <2.5 | 2803 (38.4) | 295 (34.4) | 53 (45.3) |
| ≤4.9 | 2826 (38.7) | 250 (29.1) | 31 (26.5) |
| ≤9.9 | 1351 (18.5) | 127 (14.8) | 17 (14.5) |
| ≤19.9 | 215 (2.9) | 60 (7.0) | 5 (4.3) |
| ≤49.9 | 55 (0.8) | 47 (5.5) | 4 (3.4) |
| ≤99.9 | 17 (0.2) | 26 (3.0) | 1 (0.9) |
| ≥100 ng/mL | 27 (0.4) | 53 (6.2) | 6 (5.1) |
| CA19–9 | n = 6311 | n = 837 | n = 109 |
| <37 | 4541 (72.0) | 317 (37.9) | 54 (49.5) |
| ≤99 | 1303 (20.6) | 111 (13.3) | 24 (22.0) |
| ≤299 | 338 (5.4) | 110 (13.1) | 11 (10.1) |
| ≤999 | 78 (1.2) | 80 (9.6) | 7 (6.4) |
| ≤2999 | 28 (0.4) | 76 (9.1) | 9 (8.3) |
| ≤9999 | 8 (0.1) | 59 (7.0) | 2 (1.8) |
| ≥10 000 U/mL | 15 (0.2) | 84 (10.0) | 2 (1.8) |
For all parameters, n is the total number of patients, excluding those in the “unknown” category, and (%) is the percentage of n.
AFP, α‐fetoprotein; AFPL3, lectin‐reactive α‐fetoprotein; CA 19–9, carbohydrate antigen 19–9; CEA, carcinoembryonic antigen; ICG R15, indocyanine green retention rate at 15 min; INR, international normalized ratio; LCSGJ, Liver Cancer Study Group of Japan; PIVKA‐II, protein induced by vitamin K absence or antagonist‐II.
The hepatitis B surface antigen‐positive rate was 15.2% for HCC, 8.2% for ICC, and 16.3% for combined HCC and ICC. The hepatitis C virus antibody‐positive rate was 60.7% for HCC, 14.9% for ICC, and 35.3% for combined HCC and ICC (Table 5).
Table 5.
Hepatitis B virus antigen/antibody and hepatitis C virus antibody
|
Hepatocellular carcinoma n (%) |
Intrahepatic cholangiocarcinoma n (%) |
Combined hepatocellular cholangiocarcinoma n (%) |
|
|---|---|---|---|
| HBsAg | n = 18 219 | n = 934 | n = 147 |
| Negative | 15 449 (84.8) | 857 (91.8) | 123 (83.7) |
| Positive | 2768 (15.2) | 77 (8.2) | 24 (16.3) |
| Equivocal | 2 (0.0) | 0 (0.0) | 0 (0.0) |
| HBsAb | n = 5670 | n = 251 | n = 45 |
| Negative | 4362 (76.9) | 190 (75.7) | 34 (75.6) |
| Positive | 1267 (22.3) | 61 (24.3) | 10 (22.2) |
| Equivocal | 41 (0.7) | 0 (0.0) | 1 (2.2) |
| HBcAb | n = 6033 | n = 229 | n = 62 |
| Negative | 2850 (47.2) | 129 (56.3) | 33 (53.2) |
| Positive | 3141 (52.1) | 100 (43.7) | 29 (46.8) |
| Equivocal | 42 (0.7) | 0 (0.0) | 0 (0.0) |
| HBeAg | n = 4030 | n = 152 | n = 42 |
| Negative | 3456 (85.8) | 141 (92.8) | 39 (92.9) |
| Positive | 569 (14.1) | 11 (7.2) | 3 (7.1) |
| Equivocal | 5 (0.1) | 0 (0.0) | 0 (0.0) |
| HBeAb | n = 4006 | n = 151 | n = 46 |
| Negative | 2155 (53.8) | 90 (59.6) | 22 (47.8) |
| Positive | 1837 (45.9) | 61 (40.4) | 24 (52.2) |
| Equivocal | 14 (0.3) | 0 (0.0) | 0 (0.0) |
| HBV‐DNA load | n = 1379 | n = 20 | n = 9 |
| <3.7 LGE | 65 (4.7) | 0 (0.0) | 0 (0.0) |
| 3.7–3.9 LGE | 24 (1.7) | 0 (0.0) | 0 (0.0 |
| 4.0–4.9 LGE | 39 (2.8) | 0 (0.0) | 0 (0.0) |
| 5.0–5.9 LGE | 27 (2.0) | 0 (0.0) | 0 (0.0) |
| 6.0–6.9 LGE | 34 (2.5) | 0 (0.0) | 0 (0.0) |
| 7.0–7.9 LGE | 25 (1.8) | 0 (0.0) | 0 (0.0) |
| 8.0–8.7 LGE | 9 (0.7) | 0 (0.0) | 0 (0.0) |
| >8.7 LGE | 2 (0.1) | 0 (0.0) | 0 (0.0) |
| <2.1 Logcopy | 90 (6.5) | 2 (10.0) | 0 (0.0) |
| 2.1–2.9 Logcopy | 269 (19.5) | 7 (35.0) | 1 (11.1) |
| 3.0–3.9 Logcopy | 176 (12.8) | 3 (15.0) | 3 (33.3) |
| 4.0–4.9 Logcopy | 145 (10.5) | 1 (5.0) | 0 (0.0) |
| 5.0–5.9 Logcopy | 131 (9.5) | 2 (10.0) | 1 (11.1) |
| 6.0–6.9 Logcopy | 201 (14.6) | 3 (15.0) | 3 (33.3 |
| 7.0–7.9 Logcopy | 114 (8.3) | 2 (10.0) | 1 (11.1) |
| 8.0–8.8 Logcopy | 26 (1.9) | 0 (0.0) | 0 (0.0) |
| >8.8 Logcopy | 2 (0.1) | 0 (0.0) | 0 (0.0) |
| HCV‐Ab | n = 18 097 | n = 931 | n = 13 |
| Negative | 7094 (39.2) | 790 (84.9) | 86 (64.7) |
| Positive | 10 976 (60.7) | 139 (14.9) | 47 (35.3) |
| Equivocal | 27 (0.1) | 2 (0.2) | 0 (0.0) |
| HCV‐RNA | n = 3761 | n = 69 | n = 22 |
| Negative | 885 (23.5) | 38 (55.1) | 10 (45.5) |
| <1.2 | 41 (1.1) | 1 (1.4) | 0 (0.0) |
| 1.2–2.9 | 98 (2.6) | 1 (1.4) | 2 (9.1) |
| 3.0–4.9 | 428 (11.4) | 5 (7.2) | 2 (9.1) |
| 5.0–6.9 | 2006 (53.3) | 18 (26.1) | 6 (27.3) |
| 7.0– logIU/mL | 303 (8.1) | 6 (8.7) | 2 (9.1) |
For all parameters, n is the total number of patients, excluding those in the “unknown” category, and (%) is the percentage of n.
HBcAb, antibody to hepatitis B core antigen; HBeAb, antibody to hepatitis B e antigen; HBeAg, hepatitis B e antigen; HBsAb, antibody to hepatitis B surface antigen; HBsAg, hepatitis B surface antigen; HCC, hepatocellular carcinoma; HCV, hepatitis C virus.
Tumor diameter on imaging at diagnosis was ≤2 cm in 34.4% and 2.1–5.0 cm in 43.7% of patients with HCC. For ICC, these figures were 13.7% and 47.2%. The proportion of patients with unifocal disease was 61.3% for HCC and 74.8% for ICC (Table 6). Tumor staining was observed in 93.1%, tumor rupture in 0.9%, and F2 or larger/red color sign (+) esophageal or gastric varices in 35.8% of patients.
Table 6.
Imaging diagnosis
|
Hepatocellular carcinoma n (%) |
Intrahepatic cholangiocarcinoma n (%) |
Combined hepatocellular cholangiocarcinoma n (%) |
|
|---|---|---|---|
| Maximum diameter of primary tumor | n = 18 524 | n = 901 | n = 142 |
| ≤1 cm | 995 (5.4) | 22 (2.4) | 1 (0.7) |
| >1 cm to ≤2 cm | 5367 (29.0) | 102 (11.3) | 13 (9.2) |
| >2 cm to ≤3 cm | 4354 (23.5) | 179 (19.9) | 27 (19.0) |
| >3 cm to ≤5 cm | 3733 (20.2) | 246 (27.3) | 41 (28.9) |
| >5 cm to ≤10 cm | 2841 (15.3) | 279 (31.0) | 42 (29.6) |
| >10 cm to ≤15 cm | 898 (4.8) | 60 (6.7) | 14 (9.9) |
| >15 cm to ≤20 cm | 232 (1.3) | 12 (1.3) | 4 (2.8) |
| >20 cm to ≤25 cm | 73 (0.4) | 1 (0.1) | 0 (0.0) |
| >25 cm | 31 (0.2) | 0 (0.0) | 0 (0.0) |
| No. tumors | n = 18 629 | n = 923 | n = 139 |
| 1 | 11 424 (61.3) | 690 (74.8) | 84 (60.4) |
| 2 | 3150 (16.9) | 69 (7.5) | 20 (14.4) |
| 3 | 1236 (6.6) | 27 (2.9) | 7 (5.0) |
| 4 | 524 (2.8) | 18 (2.0) | 3 (2.2) |
| 5 | 276 (1.5) | 4 (0.4) | 2 (1.4) |
| ≥6 | 2019 (10.8) | 115 (12.5) | 23 (16.5) |
| Multifocal disease | n = 14 184 | n = 669 | n = 121 |
| Single lobe | 10 222 (72.1) | 534 (79.8) | 89 (73.6) |
| Both lobes | 3962 (27.9) | 135 (20.2) | 32 (26.4) |
|
Hepatocellular carcinoma Morphological classification of primary tumor on imaging |
n = 17 276 | ||
| Nodular | 15 307 (88.6) | ||
| Massive | 1308 (7.6) | ||
| Diffuse | 599 (3.5) | ||
| Other | 62 (0.4) | ||
| Arterial phase enhancement | n = 17 642 | n = 823 | n = 135 |
| No | 1214 (6.9) | 440 (53.5) | 16 (11.9) |
| Yes | 16 428 (93.1) | 383 (46.5) | 119 (88.1) |
| Venous phase washout | n = 16 180 | n = 729 | n = 113 |
| No | 1489 (9.2) | 543 (74.5) | 26 (23.0) |
| Yes | 14 691 (90.8) | 186 (25.5) | 87 (77.0) |
| Internal component of tumor | n = 16 941 | n = 761 | n = 137 |
| Solid | 16 793 (99.1) | 738 (97.0) | 136 (99.3) |
| Cystic | 148 (0.9) | 23 (3.0) | 1 (0.7) |
| Portal vein invasion by imaging | n = 17 855 | n = 854 | n = 137 |
| Vp0 | 15 669 (87.8) | 580 (67.9) | 97 (70.8) |
| Vp1 | 579 (3.2) | 61 (7.1) | 10 (7.3) |
| Vp2 | 463 (2.6) | 78 (9.1) | 9 (6.6) |
| Vp3 | 616 (3.5) | 102 (11.9) | 15 (10.9) |
| Vp4 | 528 (3.0) | 33 (3.9) | 6 (4.4) |
| Hepatic vein invasion by imaging | n = 17 263 | n = 808 | n = 133 |
| Vv0 | 16 401 (95.0) | 683 (84.5) | 119 (89.5) |
| Vv1 | 305 (1.8) | 45 (5.6) | 6 (4.5) |
| Vv2 | 295 (1.7) | 60 (7.4) | 4 (3.0) |
| Vv3 | 262 (1.5) | 20 (2.5) | 4 (3.0) |
| Bile duct invasion by imaging | n = 17 039 | n = 801 | n = 133 |
| B0 | 16 569 (97.2) | 488 (60.9) | 113 (85.0) |
| B1 | 195 (1.1) | 76 (9.5) | 6 (4.5) |
| B2 | 121 (0.7) | 99 (12.4) | 6 (4.5) |
| B3 | 96 (0.6) | 91 (11.4) | 5 (3.8) |
| B4 | 58 (0.3) | 47 (5.9) | 3 (2.3) |
| Tumor rupture | n = 18 337 | n = 759 | n = 144 |
| No rupture | 17 853 (97.4) | 753 (99.2) | 144 (100.0) |
| Suspected rupture | 316 (1.7) | 3 (0.4) | 0 (0.0) |
| Rupture | 168 (0.9) | 3 (0.4) | 0 (0.0) |
| Extrahepatic spread | n = 1091 | n = 264 | n = 41 |
| Lung | 319 | 48 | 6 |
| Bone | 204 | 22 | 2 |
| Adrenal gland | 67 | 6 | 2 |
| Lymph node | 410 | 148 | 27 |
| Brain | 11 | 1 | 0 |
| Peritoneum | 44 | 21 | 3 |
| Other | 36 | 18 | 1 |
| Percentages not calculated as multiple sites were allowed | |||
| Esophageal/gastric varices | n = 4195 | n = 30 | n = 15 |
| ≤F1, RC (−) | 2428 (57.9) | 21 (70.0) | 9 (60.0) |
| ≥F2, RC (+) | 1503 (35.8) | 8 (26.7) | 5 (33.3) |
| Rupture | 264 (6.3) | 1 (3.3) | 1 (6.7) |
| TNM Stage by LCSGJ | n = 18 207 | n = 882 | n = 138 |
| Stage I | 4583 (25.2) | 78 (8.8) | 24 (17.4) |
| Stage II | 7280 (40.0) | 268 (30.4) | 36 (26.1) |
| Stage III | 4162 (22.9) | 206 (23.4) | 32 (23.2) |
| Stage IVA | 1504 (8.3) | 118 (13.4) | 21 (15.2) |
| Stage IVB | 678 (3.7) | 212 (24.0) | 25 (18.1) |
For all parameters, n is the total number of patients excluding those in the “unknown” category, and (%) is the percentage of n.
B0, absence of invasion of the bile ducts; B1, invasion of (or tumor thrombus in) the third order or more peripheral branches of the bile duct, but not of second order branches; B2, invasion of (or tumor thrombus in) the second order branches of the bile duct; B3, invasion of (or tumor thrombus in) the first order branches of the bile duct; B4, invasion of (or tumor thrombus in) the common hepatic duct.; F1, small varices; F2, moderate varices; RC, red color sign; Vp0, absence of invasion of (or tumor thrombus in) the portal vein; Vp1, invasion of (or tumor thrombus in) distal to the second order branches of the portal vein, but not of the second order branches; Vp2, invasion of (or tumor thrombus in) second order branches of the portal vein; Vp3, invasion of (or tumor thrombus in) first order branches of the portal vein; Vp4, invasion of (or tumor thrombus in) the main trunk of the portal vein and/or contra‐lateral portal vein branch to the primarily involved lobe; Vv0, absence of invasion of (or tumor thrombus in) the hepatic vein; Vv1, invasion of (or tumor thrombus in) peripheral branches of the hepatic vein; Vv2, invasion of (or tumor thrombus in) the right, middle, or left hepatic vein, the inferior right hepatic vein, or the short hepatic vein; Vv3, invasion of (or tumor thrombus in) the inferior vena cava.
Initial treatments
The initial treatment method for HCC was surgical intervention (resection or transplantation) in 37.7%, local ablation therapy in 28.4%, and TACE in 27.5% of patients. For ICC, these figures were 72.9% for surgery (resection only) and 16.6% for systemic chemotherapy, and for combined HCC and ICC, they were 71.8% for surgery (hepatectomy only) and 4.2% for systemic chemotherapy (Table 7). The distribution of Child–Pugh grades (A/B/C) was 78.4%/20.3%/1.3% for those who underwent surgery, 69.2%/27.4%/3.3% for those who underwent local ablation therapy, and 59.7%/35.8%/4.4% for those who underwent TACE.
Table 7.
Initial treatment
|
Hepatocellular carcinoma n (%) |
Intrahepatic cholangiocarcinoma n (%) |
Combined hepatocellular cholangiocarcinoma n (%) |
|
|---|---|---|---|
| n = 18 458 | n = 872 | n = 142 | |
| Surgery | 6960 (37.7) | 636 (72.9) | 102 (71.8) |
| Local ablation therapy | 5249 (28.4) | 31 (3.6) | 5 (3.5) |
| Transcatheter arterial chemoembolization | 5083 (27.5) | 15 (1.7) | 16 (11.3) |
| Hepatic arterial infusion chemotherapy | 829 (4.5) | 25 (2.9) | 11 (7.7) |
| Systemic chemotherapy | 166 (0.9) | 145 (16.6) | 6 (4.2) |
| Other therapy | 171 (0.9) | 20 (2.3) | 2 (1.4) |
| No therapy (BSC) | 1099 | 118 | 11 |
BSC. best supportive care.
Surgery
A total of 6940 patients with HCC underwent hepatectomy and 122 underwent liver transplantation. The most common macroscopic classification of resected specimens was simple nodular type for HCC at 59.9% and mass‐forming type for ICC at 76.4% (Tables 8, 9).
Table 8.
Macroscopic classification of hepatocellular carcinoma
| Macroscopic classification | Hepatocellular carcinoma | ||||
|---|---|---|---|---|---|
| Hepatectomy | Liver transplantation | Total | |||
| n = 4961 | n = 71 | 5032 | |||
| Small nodular type with indistinct margin | 95 | (1.9) | 1 | (1.4) | 96 |
| Simple nodular type | 2970 | (59.9) | 55 | (77.5) | 3025 |
| Simple nodular type with extranodular growth | 1039 | (20.9) | 5 | (7.0) | 1044 |
| Confluent multinodular type | 793 | (16.0) | 10 | (14.1) | 803 |
| Infiltrative type | 64 | (1.3) | 0 | (0.0) | 64 |
Table 9.
Macroscopic classification of intrahepatic cholangiocarcinoma
| Macroscopic classification | Intrahepatic cholangiocarcinoma | |
|---|---|---|
| n = 521 | ||
| Mass‐forming type | 398 | (76.4) |
| Periductal infiltrating type | 23 | (4.4) |
| Intraductal growth type | 14 | (2.7) |
| Mix of mass‐forming type and periductal infiltrating type | 71 | (13.6) |
| Mix of periductal infiltrating type and intraductal growth type | 6 | (1.2) |
| Mix of mass‐forming type and intraductal growth type | 8 | (1.5) |
| Other | 1 | (0.2) |
Tumor diameter among patients who underwent hepatectomy for HCC was ≤2 cm in 20.0%, 2–5 cm in 52.0%, and 5–10 cm in 20.3%. The percentage with unifocal disease was 75.9%. Vascular invasion was observed in the portal vein in 16.1%, hepatic veins in 6.8%, and bile duct in 2.8% of patients. The non‐cancerous part of the liver was normal in 10.6%, showed chronic hepatitis or fibrosis in 50.9%, and showed cirrhosis in 38.4% of patients. The type of surgery was Hr0 (limited resection) in 28.0%, HrS (1 subsegmentectomy) in 25.1%, Hr1 (1 segmentectomy) in 24.1%, Hr2 (2 segmentectomy) in 20.6%, and Hr3 (3 segmentectomy) in 2.2% of patients (Table 10).
Table 10.
Macroscopic findings in resected specimen and surgery‐related factors
|
Hepatocellular carcinoma n (%) |
Intrahepatic cholangiocarcinoma n (%) |
Combined hepatocellular cholangiocarcinoma n (%) |
|
|---|---|---|---|
| Maximum diameter of resected primary tumor | n = 6586 | n = 597 | n = 100 |
| ≤1 cm | 169 (2.6) | 6 (1.0) | 1 (1.0) |
| >1 cm to ≤2 cm | 1143 (17.4) | 69 (11.6) | 11 (11.0) |
| >2 cm to ≤3 cm | 1619 (24.6) | 125 (20.9) | 25 (25.0) |
| >3 cm to ≤5 cm | 1806 (27.4) | 194 (32.5) | 30 (30.0) |
| >5 cm to ≤10 cm | 1339 (20.3) | 168 (28.1) | 27 (27.0) |
| >10 cm to ≤15 cm | 380 (5.8) | 30 (5.0) | 5 (5.0) |
| >15 cm to ≤20 cm | 98 (1.5) | 5 (0.8) | 1 (1.0) |
| >20 cm to ≤25 cm | 24 (0.4) | 0 (0.0) | 0 (0.0) |
| >25 cm | 8 (0.1) | 0 (0.0) | 0 (0.0) |
| No. tumors resected | n = 6616 | n = 609 | n = 98 |
| 1 | 5021 (75.9) | 517 (84.9) | 72 (73.5) |
| 2 | 907 (13.7) | 42 (6.9) | 12 (12.2) |
| 3 | 272 (4.1) | 13 (2.1) | 6 (6.1) |
| 4 | 112 (1.7) | 10 (1.6) | 2 (2.0) |
| 5 | 57 (0.9) | 3 (0.5) | 1 (1.0) |
| ≥ 6 | 247 (3.7) | 24 (3.9) | 5 (5.1) |
| Tumor distribution1 | n = 6456 | n = 591 | n = 97 |
| HS | 2648 (41.0) | 123 (20.8) | 37 (38.1) |
| H1 | 1877 (29.1) | 182 (30.8) | 23 (23.7) |
| H2 | 1514 (23.5) | 240 (40.6) | 23 (23.7) |
| H3 | 309 (4.8) | 33 (5.6) | 10 (10.3) |
| H4 | 108 (1.7) | 13 (2.2) | 4 (4.1) |
| Tumor distribution2 | n = 6383 | n = 589 | n = 96 |
| Localized to one lobe | 5582 (87.5) | 517 (87.8) | 78 (81.3) |
| Both lobes | 801 (12.5) | 72 (12.2) | 18 (18.8) |
| Growth pattern | n = 6288 | n = 536 | n = 92 |
| Eg | 5843 (92.9) | 267 (49.8) | 67 (72.8) |
| Ig | 445 (7.1) | 269 (50.2) | 25 (27.2) |
| Capsule formation | n = 6317 | n = 540 | n = 94 |
| Fc (−) | 1477 (23.4) | 474 (87.8) | 48 (51.1) |
| Fc (+) | 4840 (76.6) | 66 (12.2) | 46 (48.9) |
| Capsule invasion | n = 4718 | n = 66 | n = 43 |
| Fc‐Inf (−) | 2385 (50.6) | 30 (45.5) | 20 (46.5) |
| Fc‐Inf (+) | 2333 (49.4) | 36 (54.5) | 23 (53.5) |
| Septum formation | n = 6037 | n = 530 | n = 79 |
| Sf (−) | 2682 (44.4) | 477 (90.0) | 43 (54.4) |
| Sf (+) | 3355 (55.6) | 53 (10.0) | 36 (45.6) |
| Serosal invasion | n = 6307 | n = 552 | n = 92 |
| S0 (no serosal invasion) | 5398 (85.6) | 363 (65.8) | 68 (73.9) |
| S1 (invasion +) | 697 (11.1) | 138 (25.0) | 18 (19.6) |
| S2 (invasion to adjacent organ) | 110 (1.7) | 47 (8.5) | 4 (4.3) |
| S3 (intraperitoneal rupture) | 102 (1.6) | 4 (0.7) | 2 (2.2) |
| Lymph node metastasis | n = 6234 | n = 581 | n = 91 |
| N0 | 6172 (99.0) | 434 (74.7) | 81 (89.0) |
| N1 | 62 (1.0) | 147 (25.3) | 10 (11.0) |
| Portal vein invasion | n = 6468 | n = 586 | n = 97 |
| Vp0 | 5429 (83.9) | 378 (64.5) | 68 (70.1) |
| Vp1 | 618 (9.6) | 90 (15.4) | 16 (16.5) |
| Vp2 | 188 (2.9) | 58 (9.9) | 8 (8.2) |
| Vp3 | 158 (2.4) | 51 (8.7) | 5 (5.2) |
| Vp4 | 75 (1.2) | 9 (1.5) | 0 (0.0) |
| Hepatic vein invasion | n = 6463 | n = 580 | n = 97 |
| Vv0 | 6022 (93.2) | 457 (78.8) | 88 (90.7) |
| Vv1 | 289 (4.5) | 72 (12.4) | 7 (7.2) |
| Vv2 | 96 (1.5) | 42 (7.2) | 2 (2.1) |
| Vv3 | 56 (0.9) | 9 (1.6) | 0 (0.0) |
| Hepatic artery invasion | n = 6191 | n = 543 | n = 83 |
| Va0 | 6132 (99.0) | 483 (89.0) | 81 (97.6) |
| Va1 | 46 (0.7) | 28 (5.2) | 2 (2.4) |
| Va2 | 13 (0.2) | 18 (3.3) | 0 (0.0) |
| Va3 | 0 (0.0) | 14 (2.6) | 0 (0.0) |
| Bile duct invasion | n = 6440 | n = 567 | n = 95 |
| B0 | 6258 (97.2) | 296 (52.2) | 87 (91.6) |
| B1 | 89 (1.4) | 83 (14.6) | 5 (5.3) |
| B2 | 40 (0.6) | 78 (13.8) | 1 (1.1) |
| B3 | 38 (0.6) | 74 (13.1) | 1 (1.1) |
| B4 | 15 (0.2) | 36 (6.3) | 1 (1.1) |
| Intrahepatic metastasis | n = 6228 | n = 561 | n = 93 |
| IM0 (no metastasis) | 5375 (86.3) | 483 (86.1) | 76 (81.7) |
| IMS (within subsegment) | 167 (2.7) | 14 (2.5) | 3 (3.2) |
| IM1 (within 1 segment) | 314 (5.0) | 31 (5.5) | 7 (7.5) |
| IM2 (within 2 segment) | 249 (4.0) | 23 (4.1) | 2 (2.2) |
| IM3 (within 3 segment) | 123 (2.0) | 10 (1.8) | 5 (5.4) |
| Peritoneal metastasis | n = 6417 | n = 597 | n = 97 |
| P0 (no metastasis) | 6382 (99.5) | 584 (97.8) | 97 (100.0) |
| P1 (proximal peritoneum) | 27 (0.4) | 9 (1.5) | 0 (0.0) |
| P2 (distal peritoneum) | 8 (0.1) | 4 (0.7) | 0 (0.0) |
| Invasion of surgical margin | n = 6298 | n = 568 | n = 93 |
| SM (+) with exposure of cancer | 298 (4.7) | 51 (9.0) | 9 (9.7) |
| SM (−) 0 mm | 693 (11.0) | 47 (8.3) | 8 (8.6) |
| SM (−) ≤5 mm | 1255 (19.9) | 91 (16.0) | 16 (17.2) |
| SM (−) ≤10 mm | 676 (10.7) | 53 (9.3) | 9 (9.7) |
| SM (−) >10 mm | 548 (8.7) | 57 (10.0) | 3 (3.2) |
| SM (−) distance unknown | 2828 (44.9) | 269 (47.4) | 48 (51.6) |
| Findings in non‐cancerous liver parenchyma | n = 6237 | n = 549 | n = 89 |
| Normal liver | 664 (10.6) | 366 (66.7) | 13 (14.6) |
| Chronic hepatitis, liver fibrosis | 3177 (50.9) | 132 (24.0) | 48 (53.9) |
| Liver cirrhosis | 2396 (38.4) | 51 (9.3) | 28 (31.5) |
| Hepatectomy | n = 6296 | n = 594 | n = 97 |
| Hr0 (<subsegmentectomy) | 1766 (28.0) | 54 (9.1) | 16 (16.5) |
| HrS (<1 segmentectomy) | 1579 (25.1) | 58 (9.8) | 26 (26.8) |
| Hr1 (1segmentectomy) | 1515 (24.1) | 97 (16.3) | 19 (19.6) |
| Hr2 (2 segmentectomy) | 1297 (20.6) | 338 (56.9) | 28 (28.9) |
| Hr3 (3 segmentectomy) | 138 (2.2) | 46 (7.7) | 8 (8.2) |
| Total hepatectomy | 1 (0.0) | 1 (0.2) | 0 (0.0) |
| Lymph node dissection | n = 6116 | n = 575 | n = 89 |
| D (−) | 5983 (97.8) | 285 (49.6) | 76 (85.4) |
| D (+) | 133 (2.2) | 290 (50.4) | 13 (14.6) |
| Residual cancer | n = 6277 | n = 587 | n = 93 |
| No | 6006 (95.7) | 557 (94.9) | 87 (93.5) |
| Yes | 271 (4.3) | 30 (5.1) | 6 (6.5) |
| Extrahepatic metastasis | n = 6299 | n = 605 | n = 86 |
| M0 | 6227 (98.9) | 583 (96.4) | 84 (97.7) |
| M1 | 72 (1.1) | 22 (3.6) | 2 (2.3) |
| TNM stage by LCSGJ | n = 6407 | n = 590 | n = 82 |
| Stage I | 945 (14.7) | 33 (5.6) | 8 (9.8) |
| Stage II | 3294 (51.4) | 197 (33.4) | 30 (36.6) |
| Stage III | 1534 (23.9) | 171 (29.0) | 30 (36.6) |
| Stage IVA | 543 (8.5) | 67 (11.4) | 10 (12.2) |
| Stage IVB | 91 (1.4) | 122 (20.7) | 4 (4.9) |
For all parameters, n is the total number of patients, excluding those in the “unknown” category, and (%) is the percentage of n.
B0, absence of invasion of the bile ducts; B1, invasion of (or tumor thrombus in) the third order or more peripheral branches of the bile duct, but not of second order branches; B2, invasion of (or tumor thrombus in) the second order branches of the bile duct; B3, invasion of (or tumor thrombus in) the first order branches of the bile duct; B4, invasion of (or tumor thrombus in) the common hepatic duct; Eg, expansive growth, well‐demarcated border; Fc (−), absence of capsule formation; Fc (+), presence of capsule formation; Fc‐Inf (−), absence of cancerous infiltration of the tumor capsule; Fc‐Inf (+), presence of cancerous infiltration of the tumor capsule; H1, cancer limited to one segment; H2, cancer limited to two segments; H3, cancer limited to three segments; H4, cancer involving more than three segments; Hr0, resection of less than one subsegment (Couinaud's segment); HrS, resection of one subsegment (Couinaud's segment); Hr1, resection of one segment (anterior, posterior, medial, or left lateral segmentectomy); Hr2, resection of two segments (right or left bisegmentectomy or central bisegmentectomy); Hr3, resection of three segments (right or left trisegmentectomy); Hs, cancer limited to one subsegment, poorly demarcated border; Ig, infiltrative growth; IM0, absence of intrahepatic metastasis; IM1, intrahepatic metastasis within the subsegment in which the principal tumor is located; IM2, intrahepatic metastasis in two segments; IM3, intrahepatic metastasis to three or more segments; IMs, intrahepatic metastasis within the subsegment in which the principal tumor is located; LCSGJ, Liver Cancer Study Group of Japan; S0, absence of invasion of the serosa; S1, tumor invasion of the serosa; S2, tumor invasion of adjacent organs; S3, tumor rupture with intraperitoneal bleeding; Sf (−), absence of formation of a fibrous septum within the tumor; Sf (+), presence of fibrous septum within the tumor; TNM, tumor–node–metastasis; Va0, absence of invasion of the hepatic artery; Va1, invasion distal to the second order branches of the hepatic artery, but not of the second order branches; Va2, invasion to the second order branches of the hepatic artery; Va3, invasion to the left or right hepatic artery, or the proper hepatic artery; Vp0, absence of invasion of (or tumor thrombus in) the portal vein; Vp1, invasion of (or tumor thrombus in) distal to the second order branches of the portal vein, but not of the second order branches; Vp2, invasion of (or tumor thrombus in) second order branches of the portal vein; Vp3, invasion of (or tumor thrombus in) first order branches of the portal vein; Vp4, invasion of (or tumor thrombus in) the main trunk of the portal vein and/or contra‐lateral portal vein branch to the primarily involved lobe; Vv0, absence of invasion of (or tumor thrombus in) the hepatic vein; Vv1, invasion of (or tumor thrombus in) peripheral branches of the hepatic vein; Vv2, invasion of (or tumor thrombus in) the right, middle, or left hepatic vein, the inferior right hepatic vein, or the short hepatic vein; Vv3, invasion of (or tumor thrombus in) the inferior vena cava.
Among patients with ICC, tumor diameter was ≤2 cm in 12.6%, 2–5 cm in 53.4%, and 5–10 cm in 28.1% of patients, and 84.9% had unifocal disease.
Local ablation therapy
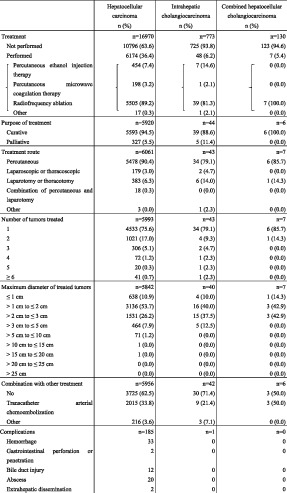
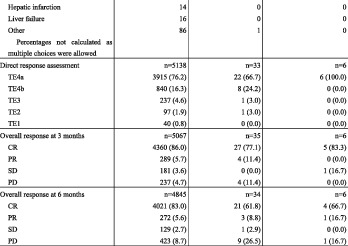
Local ablation therapy was performed in 6174 patients with HCC. Percutaneous ethanol injection therapy was performed in 7.4%, percutaneous microwave coagulation therapy in 3.2%, and radiofrequency ablation in 81.5% (Table 11). The treatment route was percutaneous for 88.8%. The percentage with unifocal disease was 89.2%. Tumor diameter was ≤2 cm in 64.6% and 2–3 cm in 26.2% of patients. The response assessed at 3 months after treatment initiation was complete response (CR) in 86.0%, partial response (PR) in 5.7%, stable disease (SD) in 3.6%, and progressive disease (PD) in 4.7% of patients. The corresponding response assessed at 6 months after treatment initiation was 83.0%, 5.0%, 2.7%, and 8.7%.
Table 11.
Local ablation therapy
|
|
For all parameters, n is the total number of patients, excluding those in the “unknown” category, and (%) is the percentage of n.
CR, complete response; PD, progressive disease; PR, partial response; SD, stable disease; TE2, treatment effect in target lesion (partial response); TE1, treatment effect in target lesion (progressive disease)TE3, treatment effect in target lesion (partial response); TE4a; treatment effect in target lesion (complete response with ablative margin); TE4b, treatment effect in target lesion (complete response without ablative margin).
TACE
TACE was carried out in 8334 patients with HCC. Lipiodol alone was used in 16.0%, gelatin sponge alone in 2.2%, and lipiodol plus gelatin sponge particles in 78.7% of patients (Table 12). In addition, 93.6% of patients were also being treated together with anticancer cytotoxic agents. The scope of embolization was less than one segment for 36.6%, at least one segment but less than one lobe for 40.3%, one lobe or more for 16.6%, and the entire liver for 6.5%. The response assessed at 3 months after treatment initiation was CR in 41.0%, PR in 21.8%, SD in 15.7%, and PD in 21.6% of patients. The corresponding response assessed at 6 months after treatment initiation was 40.1%, 17.3%, 12.1%, and 30.5%.
Table 12.
Transcatheter arterial chemoembolization
|
Hepatocellular carcinoma n (%) |
Intrahepatic cholangiocarcinoma n (%) |
Combined hepatocellular cholangiocarcinoma n (%) |
|
|---|---|---|---|
| Transarterial therapy | n = 16 920 | n = 76 | n = 115 |
| Not performed | 8586 (50.7) | 697 (91.6) | 74 (64.3) |
| Performed | 8334 (49.3) | 64 (8.4) | 41 (35.7) |
| Transcatheter arterial chemoembolization | n = 8283 | n = 63 | n = 41 |
| Not performed | 729 (8.8) | 24 (38.1) | 11 (26.8) |
| Performed | 7554 (91.2) | 39 (61.9) | 30 (73.2) |
| Therapy through implanted catheter system | n = 7102 | n = 56 | n = 41 |
| Not performed | 6488 (91.4) | 42 (75.0) | 32 (78.0) |
| Performed | 614 (8.6) | 14 (25.0) | 9 (22.0) |
| Embolic agent | n = 7012 | n = 33 | n = 30 |
| Lipiodol alone | 1125 (16.0) | 8 (24.2) | 10 (33.3) |
| Gelatin sponge alone | 157 (2.2) | 1 (3.0) | 2 (6.7) |
| Lipiodol + gelatin sponge | 5518 (78.7) | 22 (66.7) | 17 (56.7) |
| Other | 212 (3.0) | 2 (6.1) | 1 (3.3) |
| Lipiodol dose | n = 5250 | n = 18 | n = 20 |
| Not used | 2 (0.0) | 0 (0.0) | 0 (0.0) |
| 0.1–1.0 | 440 (8.4) | 0 (0.0) | 1 (5.0) |
| 1.1–3.0 | 2002 (38.1) | 5 (27.8) | 4 (20.0) |
| 3.1–5.0 | 1439 (27.4) | 7 (38.9) | 10 (50.0) |
| 5.1–7.0 | 559 (10.6) | 3 (16.7) | 2 (10.0) |
| 7.1–10.0 | 662 (12.6) | 3 (16.7) | 3 (15.0) |
| >10 | 146 (2.8) | 0 (0.0) | 0 (0.0) |
| Combination with chemotherapy agents | n = 7804 | n = 38 | n = 30 |
| Doxorubicin | 399 | 2 | 0 |
| Epirubicin | 4281 | 16 | 19 |
| Mitomycin | 1008 | 4 | 3 |
| Cisplatin | 1416 | 10 | 6 |
| SMANCS | 203 | 3 | 1 |
| Miriplatin | 131 | 0 | 0 |
| 5FU | 97 | 1 | 1 |
| Interferon | 17 | 1 | 0 |
| Other | 252 | 1 | 0 |
| Percentages not calculated as multiple choices were allowed | |||
| Area of embolization | n = 6385 | n = 33 | n = 24 |
| <1 segment | 2339 (36.6) | 10 (30.3) | 3 (12.5) |
| ≥1 segment to <1 lobe | 2574 (40.3) | 12 (36.4) | 10 (41.7) |
| ≥1 lobe to < entire liver | 1058 (16.6) | 9 (27.3) | 8 (33.3) |
| Entire liver | 414 (6.5) | 2 (6.1) | 3 (12.5) |
| Complications | n = 241 | n = 2 | n = 0 |
| Acute cholecystitis | 26 | 1 | 0 |
| Biloma | 10 | 0 | 0 |
| Hepatic abscess | 27 | 0 | 0 |
| Hepatic infarction | 11 | 0 | 0 |
| Liver failure | 48 | 0 | 0 |
| Tumor rupture | 9 | 0 | 0 |
| Gastrointestinal hemorrhage | 10 | 0 | 0 |
| Pulmonary infarction | 0 | 0 | 0 |
| Spinal cord injury | 0 | 0 | 0 |
| Other | 100 | 1 | 0 |
| Percentages not calculated as multiple choices were allowed | |||
| Direct response assessment | n = 5602 | n = 28 | n = 22 |
| TE4a | 1134 (20.2) | 5 (17.9) | 1 (4.5) |
| TE4b | 1494 (26.7) | 3 (10.7) | 4 (18.2) |
| TE3 | 1459 (26.0) | 9 (32.1) | 6 (27.3) |
| TE2 | 1053 (18.8) | 6 (21.4 | 7 (31.8) |
| TE1 | 325 (5.8) | 3 (10.7) | 2 (9.1) |
| Overall response at 3 months | n = 5128 | n = 23 | n = 19 |
| CR | 2100 (41.0) | 7 (30.4) | 3 (15.8) |
| PR | 1118 (21.8) | 5 (21.7) | 4 (21.1) |
| SD | 803 (15.7) | 5 (21.7) | 6 (31.6) |
| PD | 1107 (21.6) | 6 (26.1) | 6 (31.6) |
| Overall response at 6 months | n = 4505 | n = 21 | n = 16 |
| CR | 1806 (40.1) | 5 (23.8) | 2 (12.5) |
| PR | 781 (17.3) | 5 (23.8) | 3 (18.8) |
| SD | 545 (12.1) | 2 (9.5) | 3 (18.8) |
| PD | 1373 (30.5) | 9 (42.9) | 8 (50.0) |
For all parameters, n is the total number of patients, excluding those in the “unknown” category, and (%) is the percentage of n.
5FU, fluorouracil; CR, complete response; PD, progressive disease; PR, partial response; SD, stable disease; SMANCS, styrene maleic acid neocarzinostatin; TE1, treatment effect in target lesion (progressive disease); TE2, treatment effect in target lesion (partial response); TE3, treatment effect in target lesion (partial response); TE4a; treatment effect in target lesion (complete response with ablative margin); TE4b, treatment effect in target lesion (complete response without ablative margin).
Systemic chemotherapy
Systemic chemotherapy was carried out in 372 patients with HCC. The response assessed at 3 months after treatment initiation was CR in 3.3%, PR in 13.5%, SD in 21.4%, and PD in 61.9%. Systemic chemotherapy was carried out for 196 patients with ICC. The route of administration was intravenous for 80.6% and oral for 17.9%. The response assessed at 3 months after treatment initiation was CR in 4.2%, PR in 15.0%, SD in 30.8%, and PD in 50.0% of patients (Table 13).
Table 13.
Systemic chemotherapy
|
5FU, fluorouracil; CR, complete response; PD, progressive disease; PR, partial response; SD, stable disease; SMANCS, styrene maleic acid neocarzinostatin; TE1, treatment effect in target lesion (progressive disease); TE2, treatment effect in target lesion (partial response); TE3, treatment effect in target lesion (partial response); TE4a; treatment effect in target lesion (complete response with ablative margin); TE4b, treatment effect in target lesion (complete response without ablative margin).
Pathology
A pathological diagnosis was obtained for 44.7% of patients with HCC. Of these, 17.6% were made from a biopsy alone, 79.6% from a resected specimen alone, and 2.8% from a biopsy and resected specimen. In addition, 55.3% of patients had no pathological diagnosis, and the rate of diagnosis by biopsy had decreased since previous surveys, and the number of patients without a pathological diagnosis had increased. The histological grade of HCC was well differentiated in 24.9%, moderately differentiated in 62.8%, and poorly differentiated in 11.7% of patients (Table 14). ICC was well differentiated in 18.5%, moderately differentiated in 58.0%, and poorly differentiated in 20.3% of patients (Table 15). Table 16 shows details of pathological diagnosis. The non‐cancerous part of the liver in patients with HCC was normal in 4.6%, showed chronic hepatitis or fibrosis in 30.8%, and showed cirrhosis in 64.6%; in patients with ICC, it was normal in 66.7%, showed chronic hepatitis or fibrosis in 33.8%, and showed cirrhosis in 0.0%.
Table 14.
Histological grade of hepatocellular carcinoma
|
Well differentiated n (%) |
Moderately differentiated n (%) |
Poorly differentiated n (%) |
Undifferentiated n (%) |
Fibrolamellar carcinoma n (%) |
Sarcomatous n (%) |
|
|---|---|---|---|---|---|---|
| n = 7620 | 1900 | 4787 | 890 | 34 | 3 | 6 |
| (24.9) | (62.8) | (11.7) | (0.4) | (0.0) | (0.1) |
Table 15.
Histological grade of intrahepatic cholangiocarcinoma
|
Well differentiated adenocarcinoma n (%) |
Moderately differentiated adenocarcinoma n (%) |
Poorly differentiated adenocarcinoma n (%) |
Special type n (%) |
|
|---|---|---|---|---|
| n = 612 | 113 | 355 | 124 | 20 |
| (18.5) | (58.0) | (20.3) | (3.3) |
Table 16.
Pathological findings from resected specimen or biopsy specimen
|
Hepatocellular carcinoma n (%) |
Intrahepatic cholangiocarcinoma n (%) |
Combined hepatocellular cholangiocarcinoma n (%) |
||
|---|---|---|---|---|
| Capsule formation | n = 6398 | n = 553 | n = 93 | |
| fc (−) | 1640 (25.6) | 512 (92.6) | 54 (58.1) | |
| fc (+) | 4758 (74.4) | 41 (7.4) | 39 (41.9) | |
| Capsule invasion | n = 4641 | n = 40 | n = 35 | |
| fc‐inf (−) | 1323 (28.5) | 15 (37.5) | 8 (22.9) | |
| fc‐inf (+) | 3318 (71.5) | 25 (62.5) | 27 (77.1) | |
| Septum formation | n = 6177 | n = 525 | n = 80 | |
| sf (−) | 2174 (35.2) | 463 (88.2) | 29 (36.3) | |
| sf (+) | 4003 (64.8) | 62 (11.8) | 51 (63.8) | |
| Serosal invasion | n = 6279 | n = 554 | n = 79 | |
| s0 | 5509 (87.7) | 373 (67.3) | 63 (79.7) | |
| s1 | 605 (9.6) | 133 (24.0) | 13 (16.5) | |
| s2 | 84 (1.3) | 44 (7.9) | 2 (2.5) | |
| s3 (rupture) | 81 (1.3) | 4 (0.7) | 1 (1.3) | |
| Lymph node metastasis | n = 5441 | n = 546 | n = 84 | |
| n0 | 5384 (99.0) | 384 (70.3) | 73 (86.9) | |
| n1 | 57 (1.0) | 162 (29.7) | 11 (13.1) | |
| Portal vein invasion | n = 6560 | n = 578 | n = 95 | |
| vp0 | 4795 (73.1) | 307 (53.1) | 54 (56.8) | |
| vp1 | 1341 (20.4) | 169 (29.2) | 28 (29.5) | |
| vp2 | 190 (2.9) | 41 (7.1) | 8 (8.4) | |
| vp3 | 168 (2.6) | 50 (8.7) | 5 (5.3) | |
| vp4 | 66 (1.0) | 11 (1.9) | 0 (0.0) | |
| Hepatic vein invasion | n = 6560 | n = 573 | n = 94 | |
| vv0 | 5812 (88.6) | 406 (70.9) | 76 (80.9) | |
| vv1 | 607 (9.3) | 134 (23.4) | 14 (14.9) | |
| vv2 | 88 (1.3) | 25 (4.4) | 4 (4.3) | |
| vv3 | 53 (0.8) | 8 (1.4) | 0 (0.0) | |
| Hepatic artery invasion | n = 6474 | n = 549 | n = 81 | |
| va0 | 6413 (99.1) | 505 (92.0) | 81 (100.0) | |
| va1 | 54 (0.8) | 27 (4.9) | 0 (0.0) | |
| va2 | 4 (0.1) | 6 (1.1) | 0 (0.0) | |
| va3 | 3 (0.0) | 11 (2.0) | 0 (0.0) | |
| Bile duct invasion | n = 6507 | n = 550 | n = 93 | |
| b0 | 6284 (96.6) | 272 (49.5) | 80 (86.0) | |
| b1 | 137 (2.1) | 107 (19.5) | 8 (8.6) | |
| b2 | 39 (0.6) | 64 (11.6) | 2 (2.2) | |
| b3 | 33 (0.5) | 76 (13.8) | 2 (2.2) | |
| b4 | 14 (0.2) | 31 (5.6) | 1 (1.1) | |
| Intrahepatic metastasis | n = 6265 | n = 555 | n = 88 | |
| im0 (no metastasis) | 5394 (86.1) | 465 (83.8) | 69 (78.4) | |
| ims (within subsegment) | 170 (2.7) | 14 (2.5) | 3 (3.4) | |
| im1 (within 1 segment) | 394 (6.3) | 41 (7.4) | 8 (9.1) | |
| im2 (within 2 segment) | 205 (3.3) | 23 (4.1) | 5 (5.7) | |
| im3 (within 3 segment) | 102 (1.6) | 12 (2.2) | 3 3.4) | |
| Invasion of surgical margins | n = 6334 | n = 570 | n = 91 | |
| sm (+) with tumor exposure | 467 (7.4) | 93 (16.3) | 14 (15.4) | |
| sm (−) 0 mm | 566 (8.9) | 38 (6.7) | 5 (5.5) | |
| sm (−) ≤5 mm | 1340 (21.2) | 89 (15.6) | 17 (18.7) | |
| sm (−) ≤10 mm | 565 (8.9) | 44 (7.7) | 5 (5.5) | |
| sm (−) >10 mm | 485 (7.7) | 44 (7.7) | 4 (4.4) | |
| sm (−) distance unknown | 2911 (46.0) | 262 (46.0) | 46 (50.5) | |
| Findings in non‐cancerous liver parenchyma | n = 6283 | n = 531 | n = 92 | |
| Normal | 521 (8.3) | 315 (59.3) | 13 (14.1) | |
| Chronic hepatitis, liver fibrosis | 3106 (49.4) | 167 (31.5) | 49 (53.3) | |
| Liver cirrhosis | 2656 (42.3) | 49 (9.2) | 30 (32.6) | |
| Fibrosis (new Inuyama classification) | n = 5020 | n = 329 | n = 70 | |
| F0 (normal) | 411 (8.2) | 176 (53.5) | 8 (11.4) | |
| F1 | 763 (15.2) | 68 (20.7) | 11 (15.7) | |
| F2 | 939 (18.7) | 32 (9.7) | 13 (18.6) | |
| F3 | 852 (17.0) | 19 (5.8) | 15 (21.4) | |
| F4 (liver cirrhosis) | 2055 (40.9) | 34 (10.3) | 23 (32.9) | |
| Activity (new Inuyama classification) | n = 3300 | n = 231 | n = 46 | |
| A0 | 482 (14.6) | 130 (56.3) | 11 (23.9) | |
| A1 | 1585 (48.0) | 74 (32.0) | 20 (43.5) | |
| A2 | 1097 (33.2) | 24 (10.4) | 14 (30.4) | |
| A3 | 136 (4.1) | 3 (1.3) | 1 (2.2) | |
For all parameters, n is the total number of patients, excluding those in the “unknown” category, and (%) is the percentage of n.
A0, no necroinflammatory reaction; A1, mild necroinflammatory reaction; A2, moderate necroinflammatory reaction; A3, severe necroinflammatory reaction; proper hepatic artery; b0, no invasion of bile duct, b1, branches of the bile duct, but not of second order branches; b2, invasion of (or tumor thrombus in) the second order branches of the bile duct; b3, invasion of (or tumor thrombus in) the first order branches of the bile duct; b4, invasion of (or tumor thrombus in) the common hepatic duct; F1, fibrous expansion of portal tract; F2, fibrous septa formation, usually incomplete; F3, bridging fibrous formation accompanying lobular distortion; fc (−), absence of capsule formation; fc (+), presence of capsule formation; b0, absence of invasion of the bile ducts; b1, invasion of (or tumor thrombus in) the third order or more peripheral; fc‐inf (−), absence of cancerous infiltration of the tumor capsule; fc‐inf (+), presence of cancerous infiltration of the tumor capsule; im1, intrahepatic metastasis within the subsegment in which the principal tumor is located; im2, intrahepatic metastasis in two segments; im3, intrahepatic metastasis to three or more segments; ims, intrahepatic metastasis within the subsegment in which the principal tumor is located; sf (−), absence of formation of a fibrous septum within the tumor; sf (+), presence of fibrous septum within the tumor; s0, absence of invasion of the serosa; s1, tumor invasion of the serosa; s2, tumor invasion of adjacent organs; s3, tumor rupture with intraperitoneal bleeding; va0, absence of invasion of the hepatic artery; va1, invasion distal to the second order branches of the hepatic artery, but not of the second order branches; va2, invasion to the second order branches of the hepatic artery; va3, invasion to the left or right hepatic artery, or the im0, absence of intrahepatic metastasis; vp0, absence of invasion of (or tumor thrombus in) the portal vein; vp1, invasion of (or tumor thrombus in) distal to the second order branches of the portal vein, but not of the second order branches; vp2, invasion of (or tumor thrombus in) second order branches of the portal vein; vp3, invasion of (or tumor thrombus in) first order branches of the portal vein; vp4, invasion of (or tumor thrombus in) the main trunk of the portal vein and/or contra‐lateral portal vein branch to the primarily involved lobe; vv0, absence of invasion of (or tumor thrombus in) the hepatic vein; vv1, invasion of (or tumor thrombus in) peripheral branches of the hepatic vein; vv2, invasion of (or tumor thrombus in) the right, middle, or left hepatic vein, the inferior right hepatic vein, or the short hepatic vein; vv3, invasion of (or tumor thrombus in) the inferior vena cava.
Recurrence
Recurrence during the survey period (within 2 years of diagnosis) was reported in 34.3% of patients with HCC. The most frequently performed treatment for intrahepatic recurrence was TACE at 40.2%, followed by local ablation therapy at 24.9%. The most common sites of extrahepatic recurrence were the lungs, bone, and lymph nodes. The most frequently performed treatments for distant recurrence were systemic chemotherapy (total includes both cytotoxic chemotherapy agents and molecularly targeted agents), radiotherapy, and surgery, in that order.
Autopsy
Autopsies were carried out for a total of 91 patients with primary liver cancer. HCC was found in 85 patients. The rate of cirrhosis among autopsied patients with HCC was 64.6%, and rates of invasion of the portal vein, hepatic veins, and bile duct were 54.5%, 40.4%, and 18.7%, respectively. Extrahepatic spread was most frequently detected in the lungs (31.5%), lymph nodes (19.0%), and bone (16.7%). For ICC, the most common sites of extrahepatic spread were the intraperitoneal organs and the lungs, and the rate of lymph node metastasis was 75.0% (Table 17).
Table 17.
Pathological findings on autopsy
|
Hepatocellular carcinoma n (%) |
Intrahepatic cholangiocarcinoma n (%) |
Combined hepatocellular cholangiocarcinoma n (%) |
|
|---|---|---|---|
| Autopsy | n = 2855 | n = 248 | n = 35 |
| No | 2770 (97.0) | 242 (97.6) | 35 (100.0) |
| Yes | 85 (3.0) | 6 (2.4) | 0 (0.0) |
| Liver weight | n = 56 | n = 5 | n = 0 |
| Not measured | 7 (12.5) | 2 (40.0) | 0 (0.0) |
| 400–499 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| ≤599 | 1 (1.8) | 0 (0.0) | 0 (0.0) |
| ≤699 | 2 (3.6) | 1 (20.0) | 0 (0.0) |
| ≤799 | 4 (7.1) | 0 (0.0) | 0 (0.0) |
| ≤899 | 5 (8.9) | 0 (0.0) | 0 (0.0) |
| ≤999 | 6 (10.7) | 0 (0.0) | 0 (0.0) |
| ≤1099 | 2 (3.6) | 0 (0.0) | 0 (0.0) |
| ≤1199 | 1 (1.8) | 0 (0.0) | 0 (0.0) |
| ≤1299 | 1 (1.8) | 0 (0.0) | 0 (0.0) |
| ≤1399 | 7 (12.5) | 0 (0.0) | 0 (0.0) |
| ≤1499 | 1 (1.8) | 0 (0.0) | 0 (0.0) |
| ≤1599 | 4 (7.1) | 0 (0.0) | 0 (0.0) |
| ≤1699 | 2 (3.6) | 0 (0.0) | 0 (0.0) |
| ≤1799 | 1 (1.8) | 0 (0.0) | 0 (0.0) |
| ≤1899 | 2 (3.6) | 0 (0.0) | 0 (0.0) |
| ≤1999 | 3 (5.4) | 0 (0.0) | 0 (0.0) |
| ≥2000 | 7 (12.5) | 2 (40) | 0 (0.0) |
| Maximum tumor diameter | n = 54 | n = 3 | n = 0 |
| ≤1 cm | 2 (3.7) | 0 (0.0) | 0 (0.0) |
| ≤2 cm | 5 (9.3) | 0 (0.0) | 0 (0.0) |
| ≤3 cm | 5 (9.3) | 0 (0.0) | 0 (0.0) |
| ≤5 cm | 12 (22.2) | 0 (0.0) | 0 (0.0) |
| ≤10 cm | 18 (33.3) | 1 (33.3) | 0 (0.0) |
| ≤15 cm | 6 (11.1) | 2 (66.7) | 0 (0.0) |
| ≤20 cm | 5 (9.3) | 0 (0.0) | 0 (0.0) |
| ≤25 cm | 1 (1.9) | 0 (0.0) | 0 (0.0) |
| >25 cm | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Capsule formation | n = 34 | n = 2 | n = 0 |
| fc (−) | 11 (32.4) | 2 (100.0) | 0 (0.0) |
| fc (+) | 23 (67.6) | 0 (0.0) | 0 (0.0) |
| Portal vein invasion | n = 5 | n = 3 | n = 0 |
| vp0 | 25 (45.5) | 3 (100.0) | 0 (0.0) |
| vp1 | 9 (16.4) | 0 (0.0) | 0 (0.0) |
| vp2 | 4 (7.3) | 0 (0.0) | 0 (0.0) |
| vp3 | 6 (10.9) | 0 (0.0) | 0 (0.0) |
| vp4 | 11 (20.0) | 0 (0.0) | 0 (0.0) |
| Hepatic vein invasion | n = 52 | n = 3 | n = 0 |
| vv0 | 31 (59.6) | 3 (100.0) | 0 (0.0) |
| vv1 | 6 (11.5) | 0 (0.0) | 0 (0.0) |
| vv2 | 5 (9.6) | 0 (0.0) | 0 (0.0) |
| vv3 | 10 (19.2) | 0 (0.0) | 0 (0.0) |
| Hepatic artery invasion | n = 42 | n = 3 | n = 0 |
| va0 | 38 (90.5) | 3 (100.0) | 0 (0.0) |
| va1 | 3 (7.1) | 0 (0.0) | 0 (0.0) |
| va2 | 1 (2.4) | 0 (0.0) | 0 (0.0) |
| va3 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Bile duct invasion | n = 48 | n = 3 | n = 0 |
| b0 | 39 (81.3) | 3 (100.0) | 0 (0.0) |
| b1 | 1 (2.1) | 0 (0.0) | 0 (0.0) |
| b2 | 3 (6.3) | 0 (0.0) | 0 (0.0) |
| b3 | 4 (8.3) | 0 (0.0) | 0 (0.0) |
| b4 | 1 (2.1) | 0 (0.0) | 0 (0.0) |
| Intrahepatic metastasis | n = 54 | n = 3 | n = 0 |
| im0 | 22 (40.7) | 1 (33.3) | 0 (0.0) |
| ims | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| im1 | 5 (9.3) | 1 (33.3) | 0 (0.0) |
| im2 | 8 (14.8) | 0 (0.0) | 0 (0.0) |
| im3 | 19 (35.2) | 1 (33.3) | 0 (0.0) |
| Serosal invasion | n = 47 | n = 3 | n = 0 |
| s0 | 31 (66.0) | 3 (100.0) | 0 (0.0) |
| s1 | 6 (12.8) | 0 (0.0) | 0 (0.0) |
| s2 | 4 (8.5) | 0 (0.0) | 0 (0.0) |
| s3 (rupture) | 6 (12.8) | 0 (0.0) | 0 (0.0) |
| Peritoneal dissemination | n = 61 | n = 4 | n = 0 |
| No | 51 (83.6) | 2 (50.0) | 0 (0.0) |
| Yes | 10 (16.4) | 2 (50.0) | 0 (0.0) |
| Ascites | n = 72 | n = 4 | n = 0 |
| No | 13 (18.1) | 1 (25.0) | 0 (0.0) |
| Yes | 59 (81.9) | 3 (75.0) | 0 (0.0) |
| Findings in non‐cancerous liver parenchyma | n = 65 | n = 3 | n = 0 |
| Normal | 3 (4.6) | 2 (66.7) | 0 (0.0) |
| Chronic hepatitis, liver fibrosis | 20 (30.8) | 1 (33.3) | 0 (0.0) |
| Liver cirrhosis | 42 (64.6) | 0 (0.0) | 0 (0.0) |
| Extrahepatic metastasis | n = 53 | n = 6 | n = 0 |
| Lung | 23 (43.4) | 3 (50.0) | 0 (0.0) |
| Bone | 11 (20.8) | 0 (0.0) | 0 (0.0) |
| Brain | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Intraperitoneal organs | 7 (13.2) | 3 (50.0) | 0 (0.0) |
| Adrenal gland | 6 (11.3) | 0 (0.0) | 0 (0.0) |
| Skin | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Other | 6 (11.3) | 0 (0.0) | 0 (0.0) |
| Lymph node metastasis | n = 63 | n = 4 | n = 0 |
| n0 | 51 (81.0) | 1 (25.0) | 0 (0.0) |
| n1 | 12 (19.0) | 3 (75.0) | 0 (0.0) |
| Esophagus/gastric varices | n = 63 | n = 4 | n = 0 |
| No | 29 (46.0) | 4 (100.0) | 0 (0.0) |
| Yes | 34 (54.0) | 0 (0.0) | 0 (0.0) |
| Splenomegaly | n = 63 | n = 4 | n = 0 |
| No | 31 (49.2) | 3 (75.0) | 0 (0.0) |
| Yes | 32 (50.8) | 1 (25.0) | 0 (0.0) |
For all parameters, n is the total number of patients, excluding those in the “unknown” category, and (%) is the percentage of n.
b0, no invasion of bile duct, b1, branches of the bile duct, but not of second order branches; b2, invasion of (or tumor thrombus in) the second order branches of the bile duct; b3, invasion of (or tumor thrombus in) the first order branches of the bile duct; b4, invasion of (or tumor thrombus in) the common hepatic duct; fc (−), absence of capsule formation; fc (+), presence of capsule formation; fc‐inf (−), absence of cancerous infiltration of the tumor capsule; fc‐inf (+), presence of cancerous infiltration of the tumor capsule; im0, absence of intrahepatic metastasis; im1, intrahepatic metastasis within the subsegment in which the principal tumor is located; im2, intrahepatic metastasis in two segments; im3, intrahepatic metastasis to three or more segments; ims, intrahepatic metastasis within the subsegment in which the principal tumor is located; sf (−), absence of formation of a fibrous septum within the tumor; sf (+), presence of fibrous septum within the tumor; s0, absence of invasion of the serosa; s1, tumor invasion of the serosa; s2, tumor invasion of adjacent organs; s3,tumor rupture with intraperitoneal bleeding; va0, absence of invasion of the hepatic artery; va1, invasion distal to the second order branches of the hepatic artery, but not of the second order branches; va2, invasion to the second order branches of the hepatic artery; va3, invasion to the left or right hepatic artery, or the proper hepatic artery; vp0, absence of invasion of (or tumor thrombus in) the portal vein; vp1, invasion of (or tumor thrombus in) distal to the second order branches of the portal vein, but not of the second order branches; vp2, invasion of (or tumor thrombus in) second order branches of the portal vein; vp3, invasion of (or tumor thrombus in) first order branches of the portal vein; vp4, invasion of (or tumor thrombus in) the main trunk of the portal vein and/or contra‐lateral portal vein branch to the primarily involved lobe; vv0, absence of invasion of (or tumor thrombus in) the hepatic vein; vv1, invasion of (or tumor thrombus in) peripheral branches of the hepatic vein; vv2, invasion of (or tumor thrombus in) the right, middle, or left hepatic vein, the inferior right hepatic vein, or the short hepatic vein; vv3, invasion of (or tumor thrombus in) the inferior vena cava.
Cumulative survival rates
Cumulative survival rates from the 15th to 20th surveys (1998–2009)
Cumulative survival rates were calculated for patients with HCC, ICC, and combined HCC and ICC who were newly registered in the surveys from 1998 to 2009, and whose final outcome was survival or death (excluding unknown).
Cumulative survival rates for hepatocellular carcinoma
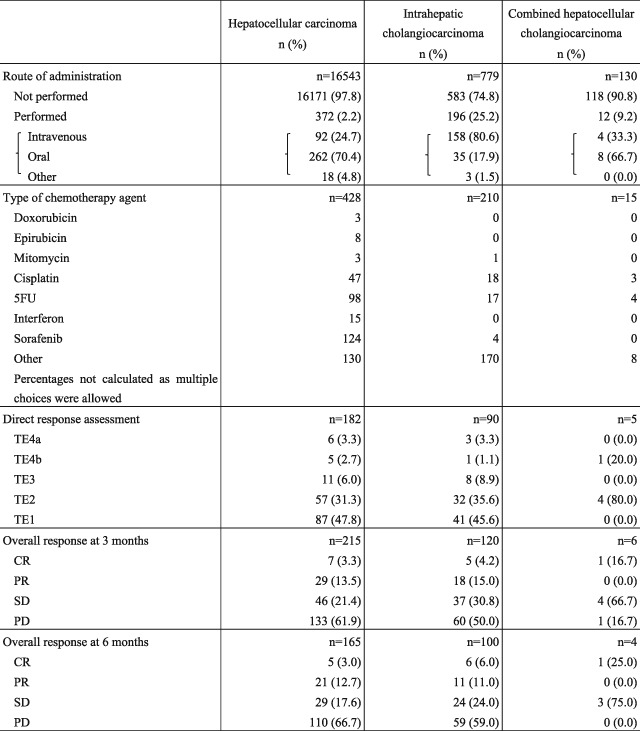
Table 18 shows cumulative survival rates among the 100 394 patients with HCC registered during 1998 and 2009. Median survival time was 53.95 months, and 5‐ and 10‐year survival rates were 46.6% and 24.7% (Table 18; Fig. 1).
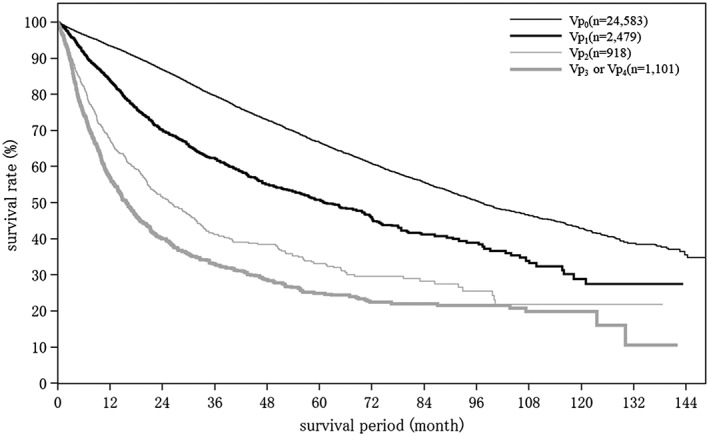
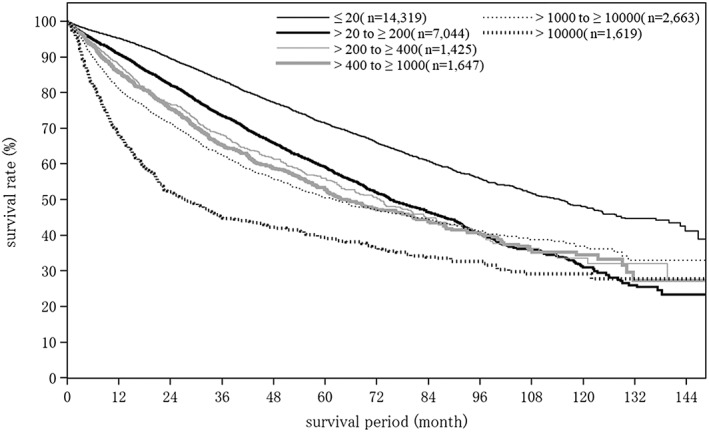
Cumulative survival rates for resected HCC: median overall survival (OS) for all patients with a Child–Pugh grade A who underwent resection was 96.89 months, and 5‐ and 10‐year survival rates were 65.8% and 44.0% (Table 19; Fig. 2). The TNM stage was well correlated with survival (Table 19; Fig. 3). Maximum tumor diameter, number of tumors, and extent of portal invasion (Fig. 4), as well as α‐fetoprotein (Fig. 5), were also well correlated with survival (Table 19).
Cumulative survival rates for HCC treated with local ablation therapy: median OS for patients with a Child–Pugh grade A who underwent local ablation therapy was 81.41 months, and 5‐ and 10‐year survival rates were 63.8% and 23.2% (Fig. 6). The number of tumors and tumor diameter were well correlated with cumulative survival rate (Table 20).
Cumulative survival rates for HCC treated with TACE: median OS for patients with a Child–Pugh grade A who underwent TACE was 46.06 months, and 5‐ and 10‐year survival rates were 40.0% and 11.3% (Table 21; Fig. 7). The number of tumors was well correlated with survival rate after TACE (Table 21; Fig. 8).
Table 18.
Cumulative overall survival rates in patients with hepatocellular carcinoma, who were registered between 1998 and 2009
| Fig. No. | Title | Category name | n | Median OS (months) | Survival rate (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 year | 2 years | 3 years | 4 years | 5 years | 6 years | 7 years | 8 years | 9 years | 10 years | |||||
| Fig. 1 | All patients | 100 394 | 53.95 | 82.8 | 71.5 | 61.9 | 53.6 | 46.6 | 40.7 | 35.7 | 31.3 | 27.9 | 24.7 | |
Figure 1.
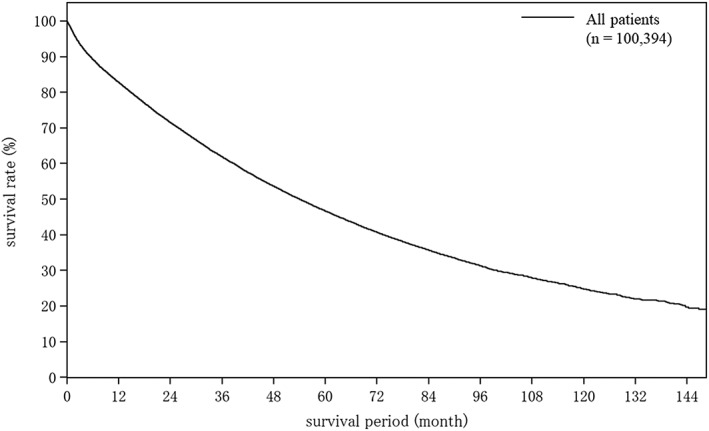
Overall survival among all 100 394 registered patients with hepatocellular carcinoma during 1998–2009. The median overall survival was 53.95 months, and 5‐ and 10‐year survival rates were 46.6% and 24.7%.
Table 19.
Cumulative survival rates for resected hepatocellular carcinoma (in patients registered between 1998 and 2009)
| Fig. No. | Title | Category name | n | Median OS (months) | Survival rate (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 year | 2 years | 3 years | 4 years | 5 years | 6 years | 7 years | 8 years | 9 years | 10 years | |||||
| All patients | 30 040 | 90.18 | 90.4 | 82.5 | 75.3 | 68.8 | 62.8 | 57.3 | 52.4 | 47.7 | 43.8 | 40.3 | ||
| Fig. 2 | Child–Pugh grade | Child–Pugh A | 22 258 | 96.89 | 91.7 | 84.3 | 77.6 | 71.6 | 65.8 | 60.1 | 55.5 | 50.3 | 46.6 | 44.0 |
| Child–Pugh B | 2136 | 60.19 | 81.6 | 72.7 | 62.4 | 55.0 | 50.0 | 42.7 | 36.4 | 33.7 | 23.2 | – | ||
| Child–Pugh C | 74 | 39.26 | 67.4 | 53.3 | 51.1 | 46.5 | 43.1 | 43.1 | 32.4 | 24.3 | 24.3 | 24.3 | ||
| Fig. 3 | TNM stage by LCSGJ | Stage I | 2810 | 124.42 | 97.2 | 94.6 | 90.0 | 84.9 | 79.3 | 74.9 | 69.3 | 64.3 | 59.5 | 51.0 |
| Stage II | 13 859 | 106.91 | 94.9 | 89.0 | 82.5 | 76.3 | 70.5 | 64.2 | 59.1 | 53.6 | 49.6 | 47.4 | ||
| Stage III | 6681 | 66.07 | 87.2 | 76.5 | 67.3 | 59.1 | 53.1 | 47.5 | 42.6 | 38.2 | 34.8 | 30.9 | ||
| Stage IVA | 2186 | 28.45 | 70.5 | 53.6 | 43.8 | 37.8 | 32.2 | 28.3 | 25.7 | 23.0 | 20.3 | 18.9 | ||
| Stage IVB | 333 | 15.34 | 57.1 | 40.5 | 31.7 | 30.2 | 25.8 | 23.7 | 19.3 | 19.3 | 19.3 | 19.3 | ||
| Maximum tumor diameter | ≤2 cm | 5478 | 118.05 | 96.1 | 92.4 | 87.0 | 80.8 | 74.3 | 69.2 | 64.0 | 58.3 | 54.5 | 47.5 | |
| >2 cm to ≤3 cm | 7247 | 99.48 | 94.9 | 88.9 | 82.0 | 75.7 | 69.5 | 63.2 | 57.1 | 51.9 | 46.2 | 42.1 | ||
| >3 cm to ≤5 cm | 8253 | 87.23 | 92.1 | 84.2 | 76.2 | 69.1 | 62.9 | 56.6 | 51.6 | 47.3 | 44.4 | 41.7 | ||
| >5 cm to ≤10 cm | 5858 | 68.37 | 85.2 | 73.9 | 65.3 | 58.6 | 53.2 | 48.2 | 45.1 | 40.4 | 37.7 | 36.1 | ||
| >10 cm | 2352 | 32.79 | 70.6 | 56.2 | 48.5 | 42.7 | 39.2 | 35.8 | 32.8 | 29.8 | 27.1 | 26.4 | ||
| No. tumors | 1 | 21 676 | 105.07 | 92.6 | 86.2 | 79.8 | 73.8 | 68.3 | 62.7 | 57.9 | 53.1 | 49.1 | 45.7 | |
| 2 | 4302 | 71.72 | 88.9 | 79.1 | 70.7 | 63.0 | 55.6 | 49.7 | 43.9 | 38.4 | 34.5 | 30.4 | ||
| ≥3 | 3317 | 40.54 | 78.7 | 64.7 | 53.5 | 44.9 | 38.7 | 33.5 | 30.3 | 26.4 | 23.9 | 20.2 | ||
| Fig. 4 | Portal vein invasion | Vp0 | 24 583 | 97.02 | 93.4 | 86.8 | 79.6 | 72.9 | 66.7 | 60.8 | 55.6 | 50.4 | 46.5 | 42.8 |
| Vp1 | 2479 | 61.21 | 83.8 | 70.1 | 62.3 | 55.1 | 50.7 | 45.9 | 41.1 | 38.9 | 33.9 | 28.9 | ||
| Vp2 | 918 | 25.86 | 67.3 | 51.5 | 41.2 | 38.5 | 33.1 | 29.6 | 28.2 | 25.4 | 21.8 | 21.8 | ||
| ≥Vp3 | 1101 | 15.70 | 56.8 | 40.0 | 33.1 | 28.6 | 24.9 | 22.4 | 22.0 | 21.5 | 19.9 | 19.9 | ||
| Fig. 5 | AFP (ng/mL) | ≤20 | 14 319 | 114.43 | 95.2 | 89.6 | 83.5 | 77.3 | 71.5 | 65.9 | 60.9 | 55.8 | 51.9 | 48.1 |
| >20 to ≤200 | 7044 | 75.17 | 90.8 | 82.1 | 73.6 | 65.9 | 59.1 | 52.0 | 46.5 | 40.3 | 36.1 | 30.9 | ||
| >200 to ≤400 | 1425 | 72.71 | 87.8 | 76.8 | 68.1 | 61.4 | 55.9 | 50.6 | 44.8 | 41.0 | 36.3 | 33.5 | ||
| >400 to ≤1000 | 1647 | 65.02 | 85.7 | 75.5 | 65.3 | 58.7 | 52.8 | 47.1 | 43.8 | 40.3 | 35.9 | 34.4 | ||
| >1000 to ≤10, 000 | 2663 | 62.23 | 81.0 | 71.5 | 62.6 | 55.8 | 50.5 | 47.2 | 44.4 | 41.2 | 38.8 | 36.9 | ||
| >10 000 | 1619 | 27.04 | 67.8 | 52.2 | 45.2 | 42.2 | 39.1 | 36.3 | 33.9 | 32.6 | 29.2 | 29.2 | ||
AFP, α‐fetoprotein; LCSGJ, Liver Cancer Study Group of Japan; TNM, tumor–node–metastasis; Vp0, absence of invasion of (or tumor thrombus in) the portal vein; Vp1, invasion of (or tumor thrombus in) distal to the second order branches of the portal vein, but not of the second order branches; Vp2, invasion of (or tumor thrombus in) second order branches of the portal vein; Vp3, invasion of (or tumor thrombus in) first order branches of the portal vein; vp4, invasion of (or tumor thrombus in) the main trunk of the portal vein and/or contra‐lateral portal vein branch to the primarily involved lobe.
Figure 2.
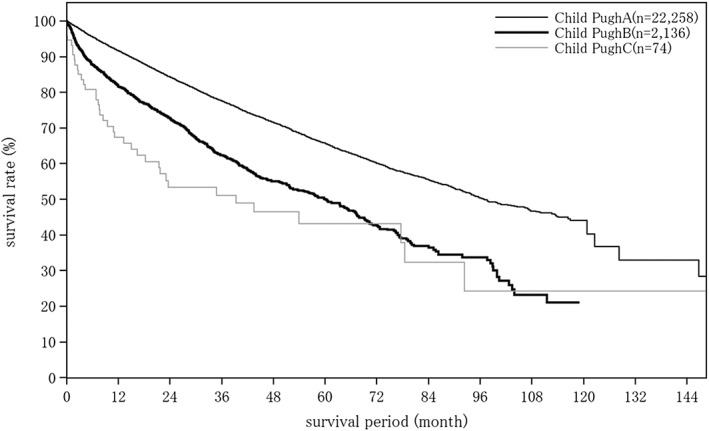
Overall survival by Child–Pugh grade in patients with hepatocellular carcinoma treated with resection (n = 30 040). The median overall survival for patients with a Child–Pugh grade A who underwent resection was 96.89 months, and 5‐ and 10‐year survival rates were 65.8% and 44.0%.
Figure 3.
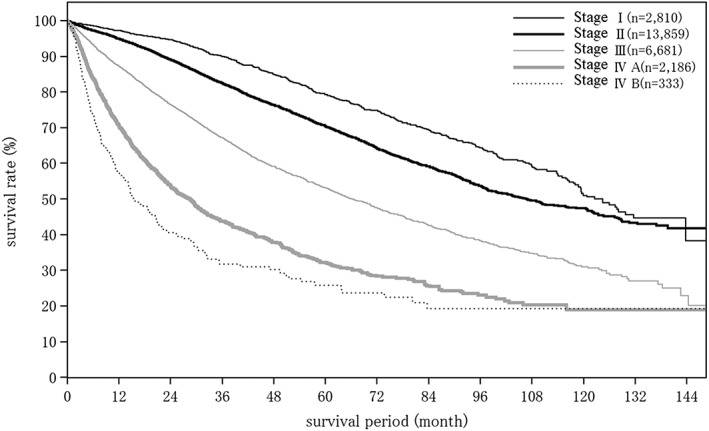
Overall survival in patients with hepatocellular carcinoma treated with resection according to the TNM stage by the Liver Cancer Study Group of Japan.
Figure 4.
Overall survival according to portal vein invasion in patients with hepatocellular carcinoma treated with resection.
Figure 5.
Overall survival by serum α‐fetoprotein level in patients with hepatocellular carcinoma treated with resection.
Figure 6.
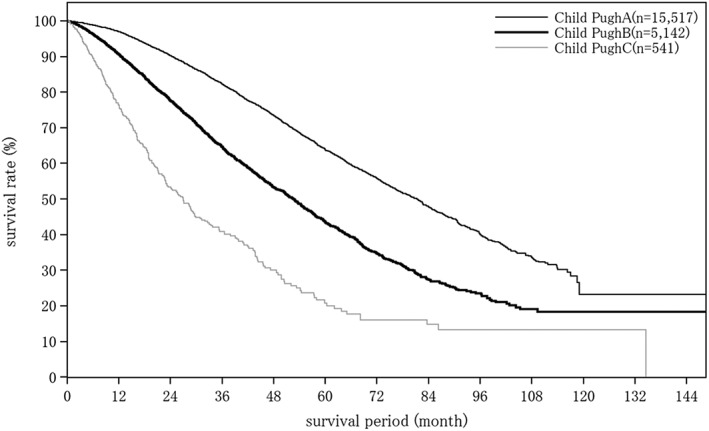
Overall survival by Child–Pugh grade in patients with hepatocellular carcinoma treated with local ablation therapy. The median overall survival for patients with a Child–Pugh grade A who underwent local ablation therapy was 81.41 months, and 5‐ and 10‐year survival rates were 63.8% and 23.2%.
Table 20.
Cumulative survival rates for hepatocellular carcinoma treated with local ablation therapy (in patients registered between 1998 and 2009)
| Fig. No. | Title | Category name | n | Median OS (months) | Survival rate (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 year | 2 years | 3 years | 4 years | 5 years | 6 years | 7 years | 8 years | 9 years | 10 years | |||||
| All patients | 27 181 | 67.19 | 94.3 | 85.2 | 75.0 | 64.6 | 55.0 | 47.1 | 39.8 | 33.8 | 28.9 | 24.3 | ||
| Fig. 6 | Child–Pugh grade | Child–Pugh A | 15 517 | 81.41 | 97.0 | 90.2 | 82.3 | 73.5 | 63.8 | 55.8 | 47.8 | 40.0 | 33.5 | 23.2 |
| Child–Pugh B | 5142 | 52.63 | 90.7 | 77.6 | 64.7 | 53.2 | 43.6 | 34.7 | 27.3 | 23.5 | 19.0 | 18.3 | ||
| Child–Pugh C | 541 | 26.94 | 76.3 | 53.4 | 40.9 | 30.0 | 20.8 | 16.0 | 14.8 | 13.3 | 13.3 | 13.3 | ||
| No. tumors | 1 | 17 986 | 74.68 | 95.3 | 87.2 | 78.2 | 68.6 | 59.6 | 51.7 | 44.0 | 38.8 | 33.4 | 28.2 | |
| 2 | 5309 | 60.12 | 93.4 | 83.5 | 72.5 | 61.0 | 50.1 | 42.2 | 35.5 | 28.3 | 22.8 | 19.9 | ||
| 3 | 1986 | 51.48 | 92.8 | 80.8 | 66.8 | 53.0 | 43.3 | 35.0 | 28.4 | 21.6 | 18.7 | 15.1 | ||
| 4 | 688 | 50.40 | 90.8 | 78.3 | 64.7 | 52.0 | 44.1 | 32.9 | 28.4 | 21.0 | 18.5 | 12.7 | ||
| ≥5 | 818 | 40.80 | 87.4 | 69.8 | 54.9 | 44.8 | 33.1 | 27.0 | 23.1 | 17.8 | 17.0 | 15.0 | ||
| Maximum tumor diameter | ≤1 cm | 2034 | 86.67 | 96.8 | 90.8 | 82.6 | 75.2 | 65.9 | 57.5 | 51.6 | 44.5 | 38.2 | 29.9 | |
| >1 cm to ≤2 cm | 13 151 | 74.09 | 95.8 | 88.2 | 79.1 | 69.1 | 60.1 | 51.5 | 43.3 | 36.9 | 31.4 | 26.1 | ||
| >2 cm to ≤3 cm | 7387 | 63.18 | 94.2 | 84.2 | 72.7 | 61.6 | 51.6 | 43.7 | 36.4 | 31.0 | 26.7 | 22.8 | ||
| >3 cm to ≤5 cm | 2732 | 48.89 | 89.8 | 75.3 | 63.1 | 50.5 | 40.9 | 35.1 | 30.2 | 25.6 | 20.2 | 18.2 | ||
| >5 cm | 572 | 46.39 | 83.4 | 68.5 | 57.8 | 48.9 | 38.5 | 30.3 | 27.9 | 21.6 | 20.7 | 14.3 | ||
OS, overall survival.
Table 21.
Cumulative survival rates for hepatocellular carcinoma treated with transcatheter arterial chemoembolization (in patients registered between 1998 and 2009)
| Fig. No. | Title | Category name | n | Survival rate (%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Median OS (months) | 1 year | 2 years | 3 years | 4 years | 5 years | 6 years | 7 years | 8 years | 9 years | 10 years | ||||
| All patients | 22 695 | 37.62 | 82.3 | 65.2 | 51.7 | 40.1 | 32.0 | 25.3 | 20.5 | 16.9 | 15.1 | 11.8 | ||
| Fig. 7 | Child–Pugh grade | Child–Pugh A | 12 134 | 46.06 | 87.2 | 72.6 | 59.9 | 48.5 | 40.0 | 32.2 | 26.0 | 21.3 | 19.0 | 11.3 |
| Child–Pugh B | 4803 | 27.83 | 77.0 | 55.8 | 39.2 | 26.2 | 19.2 | 14.5 | 10.4 | 8.9 | 7.5 | – | ||
| Child–Pugh C | 686 | 16.16 | 59.6 | 35.5 | 23.8 | 16.6 | 12.2 | 9.2 | 7.0 | 7.0 | 7.0 | – | ||
| Fig. 8 | No. tumors | 1 | 9406 | 47.34 | 87.1 | 73.6 | 61.4 | 49.4 | 41.1 | 33.4 | 27.6 | 23.5 | 20.6 | 15.3 |
| 2 | 4347 | 41.07 | 86.3 | 69.5 | 55.5 | 41.7 | 31.3 | 24.0 | 18.6 | 13.5 | 12.4 | 10.4 | ||
| 3 | 2541 | 33.48 | 84.6 | 64.5 | 47.4 | 34.4 | 26.1 | 20.3 | 14.9 | 13.4 | 12.4 | 11.0 | ||
| 4 | 1232 | 31.47 | 84.5 | 61.5 | 42.9 | 32.3 | 24.2 | 17.6 | 14.0 | 9.3 | 9.3 | 8.0 | ||
| ≥5 | 4565 | 21.19 | 68.1 | 46.1 | 32.8 | 23.8 | 18.2 | 14.7 | 12.6 | 10.3 | 8.6 | 6.6 | ||
OS, overall survival.
Figure 7.
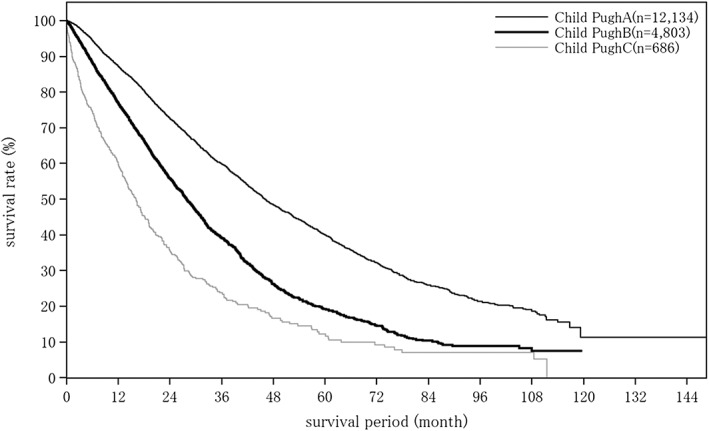
Overall survival by Child–Pugh grade in patients with hepatocellular carcinoma treated with transcatheter arterial chemoembolization. Cumulative survival rate by Child–Pugh score among patients with hepatocellular carcinoma treated with transcatheter arterial chemoembolization. The median overall survival for patients with a Child–Pugh grade A who underwent transcatheter arterial chemoembolization was 46.06 months, and 5‐ and 10‐year survival rates were 40.0% and 11.3%.
Figure 8.
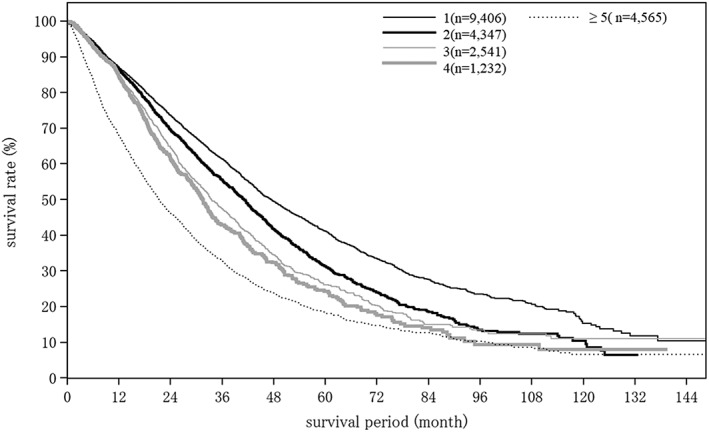
Overall survival by number of tumors in patients with hepatocellular carcinoma treated with transcatheter arterial chemoembolization.
Cumulative survival rates for intrahepatic cholangiocarcinoma and combined hepatocellular cholangiocarcinoma
Tables 22 and 23 show cumulative survival rates for patients with ICC (all patients and by patient factors) and combined HCC and ICC (all patients).
Table 22.
Cumulative survival rates for intrahepatic cholangiocarcinoma (in patients registered between 1998 and 2009)
| Fig. No. | Title | Category name | n | Median OS (months) | Survival rate (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 year | 2 years | 3 years | 4 years | 5 years | 6 years | 7 years | 8 years | 9 years | 10 years | |||||
| All patients | 4436 | 18.14 | 59.6 | 43.4 | 37.4 | 32.9 | 28.9 | 26.2 | 24.2 | 21.8 | 20.2 | 18.2 | ||
| Hepatectomy | Yes | 2412 | 43.27 | 78.5 | 61.0 | 53.8 | 47.5 | 41.9 | 38.8 | 35.9 | 32.9 | 30.7 | 28.6 | |
| No | 263 | 33.31 | 71.5 | 53.4 | 44.6 | 39.6 | 37.1 | 31.1 | 31.1 | 25.4 | 25.4 | 12.7 | ||
| Hepatectomy: maximum tumor diameter | ≤2 cm | 244 | – | 91.4 | 80.5 | 73.0 | 68.3 | 63.4 | 61.5 | 61.5 | 50.9 | 50.9 | 50.9 | |
| >2 cm to ≤5 cm | 1144 | 53.72 | 84.3 | 68.2 | 60.3 | 53.0 | 45.9 | 42.0 | 38.2 | 35.0 | 33.2 | 33.2 | ||
| >5 cm to ≤10 cm | 739 | 22.67 | 68.1 | 47.4 | 40.4 | 34.9 | 30.7 | 27.7 | 25.9 | 25.9 | 23.9 | 20.5 | ||
| >10 cm | 149 | 17.41 | 62.8 | 42.4 | 40.9 | 37.3 | 32.3 | 29.8 | 29.8 | 25.6 | 17.0 | 17.0 | ||
| Hepatectomy: No. tumors | 1 | 1, 925 | 54.28 | 81.8 | 66.0 | 59.3 | 52.9 | 46.8 | 43.4 | 40.9 | 36.9 | 35.1 | 32.2 | |
| 2 | 143 | 27.50 | 78.8 | 57.0 | 45.5 | 36.2 | 33.6 | 33.6 | 33.6 | 33.6 | 33.6 | 33.6 | ||
| ≥3 | 241 | 14.00 | 56.1 | 26.9 | 19.4 | 13.7 | 11.1 | 9.7 | 7.3 | 7.3 | 3.6 | – | ||
| Hepatectomy: curability | Curability A or B | 372 | 64.13 | 84.2 | 69.4 | 61.2 | 57.6 | 51.7 | 47.4 | 43.1 | 39.0 | 35.8 | 33.9 | |
| Curability C | 892 | 25.36 | 71.8 | 51.1 | 44.1 | 37.1 | 31.5 | 29.0 | 27.1 | 22.9 | 22.9 | 22.9 | ||
| Hepatectomy: lymph node metastasis | N0 | 1612 | 64.26 | 85.6 | 70.2 | 62.7 | 56.0 | 51.0 | 48.3 | 45.4 | 41.4 | 40.4 | 40.4 | |
| N1 | 645 | 16.07 | 61.2 | 36.1 | 30.6 | 25.0 | 19.4 | 16.3 | 13.2 | 11.3 | 8.5 | – | ||
OS, overall survival.
Table 23.
Cumulative survival rates for combined hepatocellular cholangiocarcinoma (in patients registered between 1998 and 2009)
| Fig. No. | Title | Category name | n | Median OS (months) | Survival rate (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 year | 2 years | 3 years | 4 years | 5 years | 6 years | 7 years | 8 years | 9 years | 10 years | |||||
| All patients | 708 | 19.78 | 60.8 | 44.8 | 37.9 | 34.0 | 30.2 | 28.4 | 24.9 | 22.4 | 20.7 | 17.7 | ||
| Hepatectomy | Yes | 441 | 36.63 | 75.4 | 58.7 | 51.0 | 45.6 | 41.2 | 38.4 | 32.8 | 28.7 | 25.8 | 20.7 | |
| No | 108 | 17.91 | 60.5 | 34.2 | 28.3 | 25.1 | 16.8 | 16.8 | 16.8 | 16.8 | 16.8 | 16.8 | ||
OS, overall survival.
Changes in cumulative survival rate over time
Changes in survival over time for hepatocellular carcinoma
Cumulative survival rates were calculated by registration date for newly registered patients with HCC whose final outcome was survival or death (excluding unknown). Patients were grouped by 8‐year periods into four time period groups. Period 1 consisted of those registered in the fifth to eighth surveys (1978–1985), period 2 of those registered in the ninth to 12th surveys (1986–1993), period 3 of those registered in the 13th to 16th surveys, and period 4 of those registered in the 17th to 20th surveys (2002–2009; Table 24; Fig. 9). Cumulative survival rates (5‐year/10‐year) and median OS were 11.9%/5.0% and 4.99 months for period 1 (1978–1985, n = 5551), 31.9%/10.1% and 25.63 months for period 2 (1986–1993), 39.7%/20.6% and 42.97 months for period 3 (1994–2001, n = 53 775), and 50.4%/24.0% and 60.81 months for period 4 (2002–2009, n = 65 711). The number of new registrations has been increasing over time, and the prognosis of HCC is improving dramatically (Table 24; Fig. 9).
Table 24.
Cumulative survival rates from the 5th to 20th surveys (patients registered between 1978 and 2009)
| Fig. No. | Title | Category name | n | Median OS (months) | Survival rate (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 year | 2 years | 3 years | 4 years | 5 years | 6 years | 7 years | 8 years | 9 years | 10 years | |||||
| Fig. 9 | All patients with hepatocellular carcinoma | Registered in 1978–1985 | 5551 | 4.99 | 33.9 | 23.4 | 18.1 | 14.9 | 11.9 | 9.6 | 8.3 | 7.1 | 5.9 | 5.0 |
| Registered in 1986–1993 | 32 721 | 25.63 | 66.9 | 51.7 | 40.5 | 31.9 | 25.5 | 20.5 | 16.9 | 14.1 | 11.7 | 10.1 | ||
| Registered in 1994–2001 | 53 775 | 42.97 | 78.4 | 65.6 | 55.3 | 46.7 | 39.7 | 34.2 | 29.8 | 26.1 | 23.0 | 20.6 | ||
| Registered in 2002–2009 | 65 711 | 60.81 | 84.4 | 73.8 | 64.8 | 57.0 | 50.4 | 44.3 | 39.1 | 32.7 | 28.4 | 24.0 | ||
| Fig. 10 | All patients with intrahepatic cholangiocarcinoma | Registered in 1978–1985 | 338 | 3.71 | 21.2 | 14.9 | 11.5 | 11.5 | 11.0 | 9.1 | 9.1 | 9.1 | 8.2 | 8.2 |
| Registered in 1986–1993 | 1056 | 7.56 | 38.2 | 25.5 | 20.6 | 16.8 | 14.1 | 11.7 | 10.3 | 9.6 | 8.0 | 7.7 | ||
| Registered in 1994–2001 | 4552 | 17.02 | 57.7 | 41.4 | 33.0 | 27.1 | 22.6 | 19.6 | 17.4 | 15.2 | 13.4 | 12.2 | ||
| Registered in 2002–2009 | 3184 | 20.60 | 62.4 | 46.6 | 40.4 | 35.8 | 30.9 | 28.0 | 23.7 | 21.4 | 21.4 | 10.7 | ||
| All patients with combined hepatocellular cholangiocarcinoma | Registered in 1978–1985 | 69 | 2.86 | 15.5 | 7.7 | 7.7 | 6.2 | 6.2 | 3.1 | 3.1 | 3.1 | 3.1 | 3.1 | |
| Registered in 1986–1993 | 126 | 19.55 | 61.9 | 35.8 | 29.0 | 23.7 | 22.6 | 21.4 | 18.7 | 17.3 | 17.3 | 17.3 | ||
| Registered in 1994–2001 | 330 | 16.82 | 55.5 | 39.6 | 29.8 | 25.1 | 23.2 | 21.7 | 18.8 | 16.9 | 15.5 | 12.9 | ||
| Registered in 2002–2009 | 501 | 24.02 | 65.5 | 50.1 | 43.0 | 39.2 | 33.6 | 31.0 | 28.8 | – | – | – | ||
OS, overall survival.
Figure 9.
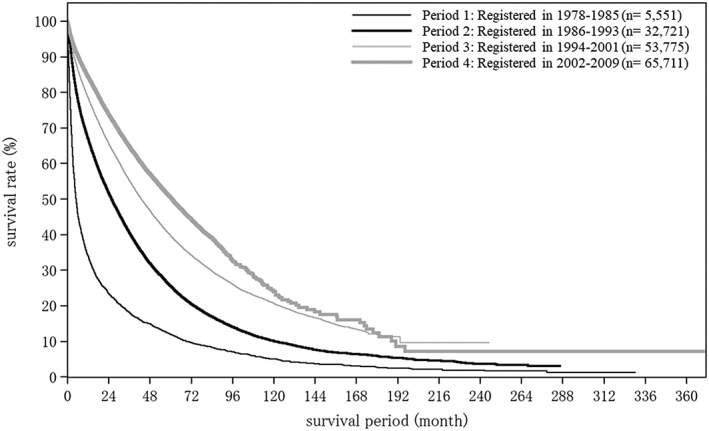
Improvement of overall survival among all patients with hepatocellular carcinoma according to the registration period. A comparison of cumulative overall survival among patients newly registered between 1978 and 2009 (n = 157 758) divided into four groups by registration date. The median overall survival and cumulative survival rates (5‐year/10‐year) were 4.99 months and 11.9%/5.0% for group 1 (1978–1985, n = 5551), 25.63 months and 31.9%/10.1% for group 2 (1986–1993), 42.97 months and 39.7%/20.6% for group 3 (1994–2001, n = 53 775), and 60.81 months and 50.4%/24.0% for group 4 (2002–2009, n = 65 711).
Changes in survival over time for intrahepatic cholangiocarcinoma
Cumulative survival rate and median OS were calculated to determine how survival for ICC has changed over time. Median OS and survival rates (5‐year/10‐year) were 3.71 and 11.0%/8.2% for period 1 (n = 338), 7.56 months and 14.1%/7.7% for period 2 (n = 1056), 17.02 months and 22.6%/12.2% for period 3 (n = 4552), and 20.6 months and 30.9%/10.7% for period 4 (n = 3184). Survival has gradually improved over the years, although not as dramatically as for HCC (Table 24; Fig. 10).
Figure 10.
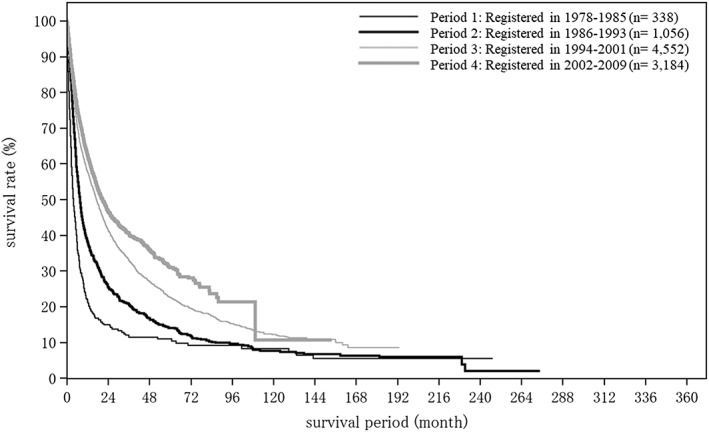
Improvement of overall survival among all patients with intrahepatic cholangiocarcinoma according to the registration period. A comparison of cumulative survival rates among patients newly registered between 1978 and 2009 (n = 9130). The median overall survival and survival rates (5‐year/10‐year) were 3.71 and 11.0%/8.2% for group 1 (n = 338), 7.56 months and 14.1%/7.7% for group 2 (n = 1056), 17.02 months and 22.6%/12.2% for group 3 (n = 4552), and 20.6 months and 30.9%/10.7% for group 4 (n = 3184).
Conclusion
Primary liver cancer is the fifth most common cause of cancer death in Japan after lung cancer, colorectal cancer, gastric cancer, and pancreatic cancer.77 The number of deaths from HCC peaked at 34 510 in 2004, and has continued to decrease gradually every year since. Nevertheless, it is reported that >28 000 people still die from HCC each year. Approximately 37.5% of the patients with primary liver cancer registered in the 20th Nationwide Follow‐up Survey of Primary Liver Cancer in Japan were newly registered.
Compared with the 19th survey, the population of patients with HCC in this survey was older at the time of clinical diagnosis, included more female patients, had smaller tumor diameters, and more frequently received radiofrequency ablation as local ablation therapy. Calculation of the median OS and cumulative survival rates for patients newly registered in the four time periods between 1978 and 2009 revealed marked improvement in survival rates for HCC, which can be attributed to advances in early diagnosis and treatment. The OS of HCC in Japan is number 1 in the world according to the comparison with the published data from other countries worldwide.62, 78 We believe that the large set of data from registered patients analyzed in this follow‐up survey will advance the research and treatment outcome of primary liver cancer.
Acknowledgments
The authors express sincere gratitude to the all doctors at the 544 institutions across Japan who contributed to this Nationwide Survey Study by Liver Cancer Study Group of Japan. We also thank Ms T Tamura and N Kozuma for compiling the data.
Kudo, M. , Izumi, N. , Kubo, S. , Kokudo, N. , Sakamoto, M. , Shiina, S. , Tateishi, R. , Nakashima, O. , Murakami, T. , Matsuyama, Y. , Takahashi, A. , Miyata, H. , and Takayama, T. (2020) Report of the 20th Nationwide follow‐up survey of primary liver cancer in Japan. Hepatol Res, 50: 15–46. 10.1111/hepr.13438.
Conflict of interest: All authors have no conflicts of interest to declare.
Footnote: This article is based on a study and short version first reported in the “Report of the 20th Nationwide Follow‐up Survey of Primary Liver Cancer (2008–2009). Liver Cancer Study Group of Japan, Osaka, 2019 (in Japanese) (reference #41)” and English version was published as “Kudo M, Izumi N, Kubo S, et al. Report of the 20th Nationwide Follow‐up Survey of Primary Liver Cancer (2008‐2009). Kanzo 60:258‐293, 2019 (in Japanese) (reference #76).
References
- 1. Liver Cancer Study Group of Japan . General rules for the clinical and pathological study of primary liver cancer, 1st edn. : Tokyo: Kanehara & Co., Ltd, 1983. (in Japanese). [Google Scholar]
- 2. Liver Cancer Study Group of Japan . General rules for the clinical and pathological study of primary liver cancer, 2nd edn. : Tokyo: Kanehara & Co., Ltd, 1987. (in Japanese). [Google Scholar]
- 3. Liver Cancer Study Group of Japan . General rules for the clinical and pathological study of primary liver cancer, 3rd edn. : Tokyo: Kanehara & Co., Ltd, 1992. (in Japanese). [Google Scholar]
- 4. Liver Cancer Study Group of Japan . General rules for the clinical and pathological study of primary liver cancer, 4th edn. : Tokyo: Kanehara & Co., Ltd, 2000. (in Japanese). [Google Scholar]
- 5. Liver Cancer Study Group of Japan . General rules for the clinical and pathological study of primary liver cancer, 5th edn. : Tokyo: Kanehara & Co., Ltd, 2008. (in Japanese). [Google Scholar]
- 6. Liver Cancer Study Group of Japan . General rules for the clinical and pathological study of primary liver cancer, 5th revised edn. : Tokyo: Kanehara & Co., Ltd, 2009. (in Japanese). [Google Scholar]
- 7. Liver Cancer Study Group of Japan . General rules for the clinical and pathological study of primary liver cancer, 6th edn. : Tokyo: Kanehara & Co., Ltd, 2015. (in Japanese). [Google Scholar]
- 8. Liver Cancer Study Group of Japan . General rules for the clinical and pathological study of primary liver cancer, 6th revised edn. : Tokyo: Kanehara & Co., Ltd, 2019. (in Japanese). [Google Scholar]
- 9. Liver Cancer Study Group of Japan . Classification of primary liver cancer, 1st English edn. : Tokyo: Kanehara & Co., Ltd, 1997. [Google Scholar]
- 10. Liver Cancer Study Group of Japan . General rules for the clinical and pathological study of primary liver cancer, 2nd English edn. : Tokyo: Kanehara & Co., Ltd, 2003. [Google Scholar]
- 11. Liver Cancer Study Group of Japan . General rules for the clinical and pathological study of primary liver cancer, 3rd English edn. : Tokyo: Kanehara & Co., Ltd, 2010. [Google Scholar]
- 12. Okuda K, The Liver Cancer Study Group of Japan . Primary liver cancers in Japan. Cancer 1980; 45: 2663–2669.6155197 [Google Scholar]
- 13. Liver Cancer Study Group of Japan . Report of the 5th Nationwide Follow‐up Survey of Primary Liver Cancer (1978‐1979). Liver Cancer Study Group of Japan, Kyoto, 1982. (in Japanese).
- 14. The Liver Cancer Study Group of Japan . Primary liver cancer in Japan. Cancer 1984; 54: 1747–1755. [DOI] [PubMed] [Google Scholar]
- 15. Liver Cancer Study Group of Japan . Report of the 6th Nationwide Follow‐up Survey of Primary Liver Cancer (1980‐1981). Liver Cancer Study Group of Japan, Kyoto, 1984. (in Japanese).
- 16. Liver Cancer Study Group of Japan . Report of the 7th Nationwide Follow‐up Survey of Primary Liver Cancer (1982‐1983). Liver Cancer Study Group of Japan, Kyoto, 1986. (in Japanese).
- 17. Liver Cancer Study Group of Japan . Report of the 8th Nationwide Follow‐up Survey of Primary Liver Cancer (1984‐1985). Liver Cancer Study Group of Japan, Kyoto, 1988. (in Japanese).
- 18. The Liver Cancer Study Group of Japan . Primary liver cancer in Japan. Sixth report. Cancer 1987; 60: 1400–1411. [DOI] [PubMed] [Google Scholar]
- 19. The Liver Cancer Study Group of Japan . The general rules for the clinical and pathological study of primary liver cancer. Japanese Journal of Surgery 1989; 19: 98–129. [DOI] [PubMed] [Google Scholar]
- 20. The Liver Cancer Study Group of Japan . Primary liver cancer in Japan. Ann Surg 1990; 211: 277–287. [PMC free article] [PubMed] [Google Scholar]
- 21. Liver Cancer Study Group of Japan . Report of the 9th Nationwide Follow‐up Survey of Primary Liver Cancer (1986‐1987). Liver Cancer Study Group of Japan, Kyoto, 1990. (in Japanese).
- 22. Primary Liver Cancer in Japan . Eds. Tobe T. et al. Springer‐Verlag Tokyo, Berlin, Heidelberg, New York, London, Paris, Hong Kong, Barcelona 1992.
- 23. Liver Cancer Study Group of Japan . Report of the 10th Nationwide Follow‐up Survey of Primary Liver Cancer (1988‐1989). Liver Cancer Study Group of Japan, Kyoto, 1992. (in Japanese).
- 24. The Liver Cancer Study Group of Japan . Predictive factors for long term prognosis after partial hepatectomy for patients with hepatocellular carcinoma in Japan. Cancer 1994; 74: 2772–2780. [DOI] [PubMed] [Google Scholar]
- 25. Liver Cancer Study Group of Japan . Report of the 11th Nationwide Follow‐up Survey of Primary Liver Cancer (1990‐1991). Liver Cancer Study Group of Japan, Kyoto, 1994. (in Japanese).
- 26. Liver Cancer Study Group of Japan . Report of the 12th Nationwide Follow‐up Survey of Primary Liver Cancer (1992‐1993). Liver Cancer Study Group of Japan, Kyoto, 1996. (in Japanese).
- 27. Liver Cancer Study Group of Japan . Report of the 13th Nationwide Follow‐up Survey of Primary Liver Cancer (1994‐1995). Liver Cancer Study Group of Japan, Kyoto, 1998. (in Japanese).
- 28. Liver Cancer Study Group of Japan . Report of the 14th Nationwide Follow‐up Survey of Primary Liver Cancer (1996‐1997). Liver Cancer Study Group of Japan, Kyoto, 2000. (in Japanese).
- 29. Arii S, Yamaoka Y, Futagawa S et al Results of surgical and nonsurgical treatment for small‐sized hepatocellular carcinomas: a retrospective and nationwide survey in Japan. The Liver Cancer Study Group of Japan. Hepatology 2000; 32: 1224–1229. [DOI] [PubMed] [Google Scholar]
- 30. Liver Cancer Study Group of Japan . Report of the 15th Nationwide Follow‐up Survey of Primary Liver Cancer (1998‐1999). Liver Cancer Study Group of Japan, Kyoto, 2002. (in Japanese).
- 31. Ikai I, Itai Y, Okita K et al Report of the 15th follow‐up survey of primary liver cancer. Hepatol Res 2004; 28: 21–29. [DOI] [PubMed] [Google Scholar]
- 32. Ikai I, Arii S, Kojiro M et al Reevaluation of prognostic factors for survival after liver resection in patients with hepatocellular carcinoma in a Japanese nationwide survey. Cancer 2004; 101: 796–802. [DOI] [PubMed] [Google Scholar]
- 33. Liver Cancer Study Group of Japan . Report of the 16th Nationwide Follow‐up Survey of Primary Liver Cancer (2000‐2001). Liver Cancer Study Group of Japan, Kyoto, 2004. (in Japanese).
- 34. Ikai I, Arii S, Ichida T et al Report of the 16th follow‐up survey of primary liver cancer. Hepatol Res 2005; 32: 163–172. [DOI] [PubMed] [Google Scholar]
- 35. Liver Cancer Study Group of Japan . Report of the 17th Nationwide Follow‐up Survey of Primary Liver Cancer (2002‐2003). Liver Cancer Study Group of Japan, Kyoto, 2006. (in Japanese).
- 36. Ikai I, Arii S, Okazaki M et al Report of the 17th Nationwide follow‐up survey of primary liver cancer in Japan. Hepatol Res 2007; 37: 676–691. [DOI] [PubMed] [Google Scholar]
- 37. Liver Cancer Study Group of Japan . Report of the 18th Nationwide Follow‐up Survey of Primary Liver Cancer (2004‐2005). Liver Cancer Study Group of Japan, Kyoto, 2009. (in Japanese).
- 38. Ikai I, Kudo M, Arii S et al Report of the 18th follow‐up survey of primary liver cancer in Japan. Hepatol Res 2010; 40: 1043–1059. [DOI] [PubMed] [Google Scholar]
- 39. Liver Cancer Study Group of Japan . Report of the 19th Nationwide Follow‐up Survey of Primary Liver Cancer (2006‐2007). Liver Cancer Study Group of Japan, Osaka, 2014. (in Japanese).
- 40. Kudo M, Izumi N, Ichida T et al Report of the 19th follow‐up survey of primary liver cancer in Japan. Hepatol Res 2016; 46: 372–390. [DOI] [PubMed] [Google Scholar]
- 41. Liver Cancer Study Group of Japan . Report of the 20th Nationwide Follow‐up Survey of Primary Liver Cancer (2008‐2009). Liver Cancer Study Group of Japan, Osaka, 2019. (in Japanese).
- 42. Takayasu K, Arii S, Ikai I et al Prospective cohort Study of transarterial chemoembolization for unresectable hepatocellular carcinoma in 8510 patients. Gastroenterology 2006; 131: 461–469. [DOI] [PubMed] [Google Scholar]
- 43. Ikai I, Takayasu K, Omata M, , Okita K., Nakanuma Y., Matsuyama Y., Makuuchi M., Kojiro M., Ichida T., Arii S., Yamaoka Y., for the Liver Cancer Study Group of Japan . A modified Japan Integrated Stage score for prognostic assessment in patients with hepatocellular carcinoma. J Gastroenterol 2006; 41: 884―892. [DOI] [PubMed] [Google Scholar]
- 44. Minagawa M, Ikai I, Matsuyama Y, Yamaoka Y, Makuuchi M. Staging of Hepatocellular Carcinoma:assessment of the Japanese TNM and AJCC/UICC TNM systems in a cohort of 13,772 patients in Japan. Ann Surg 2007; 245: 909–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Eguchi S, Kanematsu T, Arii S et al Comparison of the outcomes between an anatomical subsegmentectomy and a non‐anatomical minor hepatectomy for single hepatocellular carcinomas based on a Japanese nationwide survey. Surgery 2008; 143: 469–475. [DOI] [PubMed] [Google Scholar]
- 46. Hasegawa K, Makuuchi M, Takayama T et al Surgical resection vs. percutaneous ablation for hepatocellular carcinoma: A preliminary report of the Japanese nationwide survey. J Hepatol 2008; 49: 589–594. [DOI] [PubMed] [Google Scholar]
- 47. Hasegawa K, Makuuchi M, Takayama T et al Hepatectomy versus radiofrequency ablation for early hepatocellular carcinoma: Reply. J Hepatol 2009; 50: 1051–1056. [DOI] [PubMed] [Google Scholar]
- 48. Takayasu K, Arii S, Ikai I et al Overall survival after transarterial lipiodol infusion chemotherapy with or without embolization for unresectable hepatocellular carcinoma: Propensity score analysis. AJR 2010; 194: 830–837. [DOI] [PubMed] [Google Scholar]
- 49. Eguchi S, Kanematsu T, Arii S et al Recurrence‐free survival more than 10 years after liver resection for hepatocellular carcinoma. Brit J Surg 2011; 98: 552–557. [DOI] [PubMed] [Google Scholar]
- 50. Higashi T, Hasegawa K, Kokudo N et al Demonstration of quality of care measurement using the Japanese liver cancer registry. Hepatol Res 2011; 41: 1208–1215. [DOI] [PubMed] [Google Scholar]
- 51. Takayasu K, Arii S, Kudo M et al Superselective transarterial chemoembolization for hepatocellular carcinoma. Validation of treatment algorithm proposed by Japanese guidelines. J Hepatol 2012; 56: 886–892. [DOI] [PubMed] [Google Scholar]
- 52. Takayasu K, Arii S, Sakamoto M et al Clinical implication of hypovascular hepatocellular carcinoma studied in 4,474 patients with solitary tumour equal or less than 3 cm. Liver Int 2013; 33: 762–770. [DOI] [PubMed] [Google Scholar]
- 53. Hasegawa K, Kokudo N, Makuuchi M et al Comparison of resection and ablation for hepatocellular carcinoma: a cohort study based on a Japanese nationwide survey. J Hepatol 2013; 58: 724–729. [DOI] [PubMed] [Google Scholar]
- 54. Nouso K, Miyahara K, Uchida D et al Effect of hepatic arterial infusion chemotherapy of 5‐fluorouracil and cisplatin for advanced hepatocellular carcinoma in the Nationwide Survey of Primary Liver Cancer in Japan. BJC 2013; 542: 1904–1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Aoki T, Kokudo N, Matsuyama Y et al Prognostic impact of spontaneous tumor rupture in patients with hepatocellular carcinoma: an analysis of 1,160 cases from a nationwide survey. Annals of Surgery Ann Surg 2014; 259: 532–542. [DOI] [PubMed] [Google Scholar]
- 56. Utsunomiya T, Shimada M, Kudo M et al Nationwide study of 4741 patients with non‐B non‐C hepatocellular carcinoma with special reference to therapeutic impact. Ann Surg 2014; 259: 336–345. [DOI] [PubMed] [Google Scholar]
- 57. Hasegawa K, Makuuchi M, Kokudo N et al Impact of histologically confirmed lymph node metastases on patient survival after surgical resection for hepatocellular carcinoma: a report of a Japanese nationwide survey. Ann Surg 2014; 259: 166–170. [DOI] [PubMed] [Google Scholar]
- 58. Kitai S, Kudo M, Izumi N et al Validation of three staging systems for hepatocellular carcinoma (JIS score, biomarker‐combined JIS score and BCLC system) in 4,649 cases from a Japanese nationwide survey. Dig Dis 2014; 32: 717–724. [DOI] [PubMed] [Google Scholar]
- 59. Utsunomiya T, Shimada M, Kudo M et al A comparison of the surgical outcomes among patients with HBV‐positive, HCV‐positive, and non‐B non‐C hepatocellular carcinoma: A nationwide study of 11,950 patients. Ann Surg 2015; 261: 513–520. [DOI] [PubMed] [Google Scholar]
- 60. Sakamoto Y, Kokudo N, Matsuyama Y et al Proposal of a new staging system for intrahepatic cholangiocarcinoma: Analysis of surgical patients from a nationwide survey of the Liver Cancer Study Group of Japan. Cancer 2016; 122: 61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kitai S, Kudo M, Nishida N et al Survival Benefit of Locoregional Treatment for Hepatocellular Carcinoma with Advanced Liver Cirrhosis. Liver Cancer 2016; 5: 175–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Kudo M, Izumi N, Sakamoto M et al Survival analysis over 28 years of 173,378 patients with hepatocellular carcinoma in Japan. Liver Cancer 2016; 5: 190–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Kokudo T, Hasegawa K, Matsuyama Y et al Survival benefit of liver resection for hepatocellular carcinoma associated with portal vein invasion. J Hepatol 2016; 65: 938–943. [DOI] [PubMed] [Google Scholar]
- 64. Toyoda H, Tada T, Johnson PJ et al Validation of serological models for staging and prognostication of HCC in patients from a Japanese nationwide survey. J Gastroenterol 2017; 52: 1112–1121. [DOI] [PubMed] [Google Scholar]
- 65. Kokudo T, Hasegawa K, Matsuyama Y et al Liver resection for hepatocellular carcinoma associated with hepatic vein invasion: A Japanese nationwide survey. Hepatology 2017; 66: 510–517. [DOI] [PubMed] [Google Scholar]
- 66. Hiraoka A, Michitaka K, Kumada T et al Validation and potential of albumin‐bilirubin grade and prognostication in a nationwide survey of 46,681 hepatocellular carcinoma patients in Japan: the need for a more detailed evaluation of hepatic function. Liver Cancer 2017; 6: 325–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Kaibori M, Yoshii K, Yokota I et al Impact of advanced age on survival in patients undergoing resection of hepatocellular carcinoma: report of a Japanese nationwide survey. Ann Surg 2019; 269: 692–699. [DOI] [PubMed] [Google Scholar]
- 68. Takayasu K, Arii S, Sakamoto M et al Impact of resection and ablation for single hypovascular hepatocellular carcinoma ≤2 cm analyzed with propensity score weighting. Liver Int 2018; 38: 484–493. [DOI] [PubMed] [Google Scholar]
- 69. Kaibori M, Yoshii K, Hasegawa K et al Treatment optimization for hepatocellular carcinoma in elderly patients in a Japanese nationwide cohort. Ann Surg 2019; 270: 121–130. [DOI] [PubMed] [Google Scholar]
- 70. Fukami Y, Kaneoka Y, Maeda A et al Liver Resection for Multiple Hepatocellular Carcinomas: A Japanese Nationwide Survey. Ann Surg 2019. epub ahead of print; 1. [DOI] [PubMed] [Google Scholar]
- 71. Liver Cancer Study Group of Japan . Response Evaluation Criteria in Cancer of the Liver (RECICL Version 1). Kanzo 1994; 35: 193–205 (in Japanese). [Google Scholar]
- 72. Liver Cancer Study Group of Japan . Response Evaluation Criteria in Cancer of the Liver (RECICL Version 2). Kanzo 2004; 45: 380–385 (in Japanese). [Google Scholar]
- 73. Kudo M, Kubo S, Takayasu K et al Response evaluation criteria in cancer of the liver (RECICL) proposed by the Liver Cancer Study Group of Japan (2009 Revised Version). Hepatol Res 2010; 40: 686–692. [DOI] [PubMed] [Google Scholar]
- 74. Kudo M, Ueshima K, Kubo S et al Response evaluation criteria in cancer of the liver (RECICL) (2015 Revised Version). Hepatol Res 2016; 46: 3–9. [DOI] [PubMed] [Google Scholar]
- 75. Kudo M, Ikeda M, Ueshima K et al Response evaluation criteria in cancer of the Liver Version 5(2019 revised version). Hepatol Res 2019; 49: 981–989. [DOI] [PubMed] [Google Scholar]
- 76. Kudo M, Izumi N, Kubo S et al Report of the 20th Nationwide Follow‐up Survey of Primary Liver Cancer (2008‐2009). Kanzo 2019; 60: 258–293 (in Japanese). [Google Scholar]
- 77. Cancer Registry and Statistics . Cancer Information Service, National Cancer Center, Japan. https://ganjoho.jp/reg_stat/statistics/dl/index.html
- 78. Kudo M. Management of hepatocellular carcinoma in Japan as a world‐leading model. Liver Cancer 2018; 7: 134–147. [DOI] [PMC free article] [PubMed] [Google Scholar]


