 Alcoholism is a multifactorial disease with high risk for dependence determined by genetic background, environmental factors and neuroadaptations.
Alcoholism is a multifactorial disease with high risk for dependence determined by genetic background, environmental factors and neuroadaptations.
Abstract
Alcoholism is a multifactorial disease with high risk for dependence determined by genetic background, environmental factors and neuroadaptations. The excessive consumption of this substance is related to psychiatric problems, epilepsy, cardiovascular disease, cirrhosis and cancers. Caffeine is one of the most popular psychostimulants currently consumed in the world. The combination of ethanol and caffeine ingested by consuming “energy drinks” is becoming increasingly popular among young people. We analyzed the effect of simultaneous consumption of ethanol and caffeine on the serum profile of miRNAs differentially expressed in the ethanol-drinking rat model (UChB strain). Adult rats were divided into three groups (n = 5 per group): UChB group (rats fed with 1 : 10 (v/v) ethanol ad libitum); UChB + caffeine group (rats fed with 1 : 10 (v/v) ethanol ad libitum + 3 g L–1 of caffeine); control group (rats drinking water used as the control for UChB). The treatment with caffeine occurred from day 95 to 150 days old, totalizing 55 days of ethanol + caffeine ingestion. The expressions of microRNAs (miR) -9-3p, -15b-5p, -16-5p, -21-5p, -200a-3p and -222-3p were detected by Real Time-PCR (RT-PCR). The expressions of miR-9-3p, -15b-5p, -16-5p and -222-3p were upregulated in the UChB group. Conversely, simultaneous ingestion of ethanol and caffeine significantly reversed these expressions to similar levels to control animals, thus emphasizing that caffeine had a protective effect in the presence of ethanol. In addition, miR-21-5p was downregulated with ethanol consumption whereas miR-222-3p was unchanged. Ethanol and caffeine consumption was capable of altering serum miRNAs, which are potential biomarkers for the systemic effects of these addictive substances.
1. Introduction
Indiscriminate ethanol consumption is one of the main risk factors to health, responsible for 5.9% of the total amount of deaths worldwide.1 Alcoholism is a multifactorial disease, ethanol dependence being a neuropsychiatric disorder in which genetic and environmental factors are combined.2 Clinically, alcoholism appears as an amorphous entity, with diagnostics and prognostics not well understood.3
Caffeine is one of the most popular psychostimulants currently consumed worldwide and it is associated with alertness, arousal and energy.4,5 Also, caffeine ingestion has been related to changes in cognitive performance and mood.6 On the other hand, caffeine has excitatory effects on the central nervous system and chronic intake can develop into a dependence syndrome.7 The combination of ethanol and caffeine ingested by consuming “energy drinks” is becoming increasingly popular among young people with intense multiplicative social problems. Alcohol mixed with energy drink (AMED) ingestion might increase an individual's interest in using illicit drugs.8 Neurobiological evidence supports the possibility that frequent AMED consumption contributes to an increased risk for substance use disorders due to caffeine's ability to potentiate the addictive properties of other drugs.9 Energy drinks are associated with perceived changes in physiological stimulants and sedation side effects of alcohol.10
Considering the factors that explain the etiology of alcohol dependence, genetic factors are really considered once ethanol induces epigenetic changes especially acetylation, histone methylation and hypo and hypermethylation of DNA. One of the possible reasons for the deregulation of gene expression could be a genetic variation in the biogenesis pathway of miRNA genes.11
MicroRNAs have recently been identified as master regulators of the transcriptome and cellular proteome and were discovered during the development of mutants in C. elegans in the early 1990s.12 They are non-coding RNAs composed of 19 to 25 nucleotides originating from 60 to 110 pre-miRNAs from the enzyme RNA polymerase II.13 MiRs are strongly involved in the formation, differentiation and function of tissues in both health and disease with critical roles in the broad range of fundamental biological processes, including cell cycle regulation, ontogenic transformation, regeneration of stem cells, differentiation of immune cells, development of metazoa and organogenesis.14 They act as potent post-transcriptional regulators of gene expression, performing the signaling function in the different gene silencing pathways.15 A single miRNA can potentially reach hundreds of mRNAs transcribed by repression in translation or in degradation. Thus, it is possible that small RNAs serve as “masters” regulating cell function.14
The finding that miRNAs play central roles in the disease has provided a new perspective on the understanding of pathophysiological mechanisms and offers innovative therapeutic modality. The ability to modulate miRNA activity through the systemic distribution of non-toxic inhibitors offers unique opportunities to counteract in disease processes.16 Therefore, identifying molecular markers involved in the different pathologies, including chronic alcoholism,17 would facilitate the diagnosis and development of future therapeutic targets.18
The relationship between miRNAs and alcoholism is still poorly addressed, but it is possible that miRNAs can mediate the effects of ethanol consumption.19 As a consequence, it opens a new area of interest in ethanol research by providing new approaches to its actions at the nucleosome level in relation to gene expression and its pathophysiological consequences.20
Rossetto and colleagues have demonstrated influences of chronic consumption of ethanol increasing serum miRNAs related to inflammation as miR-145-5p and miR-146-5p.21 In the same study, the concomitant consumption of caffeine was able to reduce the expression of these miRNAs systemically. A better understanding of the relationship between AMED and its underlying mechanisms is crucial for effective and preventive intervention strategies.10 Considering all current evidence on AMED use, it would be premature and erroneous to conclude that AMED use is safe. From a public health perspective, it is important that researchers continue to understand the negative outcomes associated with high-risk alcohol use behaviors, including AMED use.22 Therefore, we aimed to determine which miRNAs are significantly altered in the serum of ethanol-consuming rats after long-term simultaneous exposure to ethanol and caffeine.
2. Materials and methods
2.1. Animals and selection of ethanol consumption
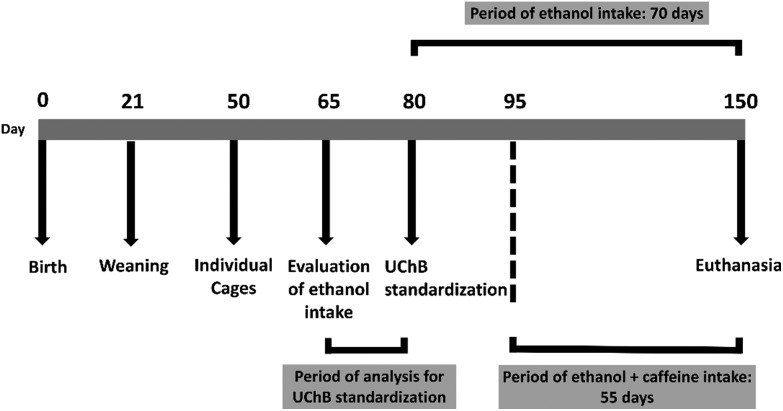
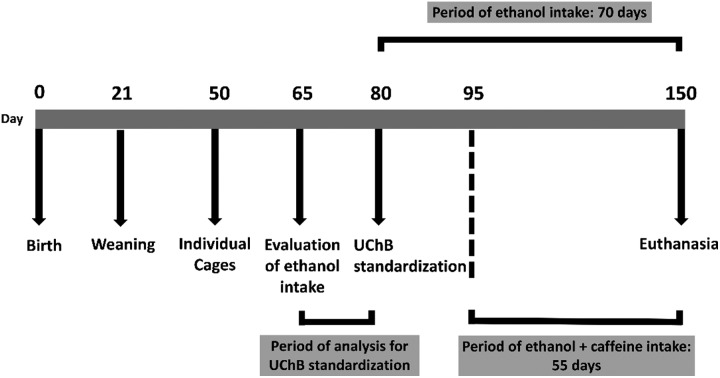
Ten adult male UChB rats (20-week-old) were obtained from the Department of Anatomy, Institute of Biosciences, UNESP – Campus de Botucatu-SP, and five adult male Wistar rats (20-week-old) came from the UFSCar Vivarium – Campus de São Carlos-SP. All animals were housed in individual polyethylene boxes of 40 × 30 × 15 cm with a solid bottom coated with a wood shavings substratum under controlled conditions of luminosity (12 h light and 12 h dark) and temperature (20 to 25 °C) receiving filtered water and animal food (Nuvital®). The UChB strain is genetically predisposed to ethanol consumption at 10%.23,24 During the entire experiment, the UChB animals received both a flask of water ad libitum, and a flask containing 10% (v/v) ethanol solution, both being often changed by position. Initially, two experimental groups were formed: UChB group (UChB rats fed with 1 : 10 v/v ethanol ad libitum, free choice for water or ethanol, drinking from 2.0 to 6.0 g kg–1 day–1 of ethanol); UChB + caffeine group: (UChB rats fed with 1 : 10 v/v ethanol + 3 g L–1 caffeine ad libitum, drinking from 2.0 to 6.0 g kg–1 day–1 of ethanol). A group of Wistar rats were used as the control for the UChB strain (drinking water only). The UChB + caffeine group received ethanol + caffeine solution during 55 consecutive days (from 95 to 150 days of age) (Fig. 1), according to Martinez and colleagues.25 The experimental protocol follows the ethical principles in animal research adopted by the Brazilian School of Animal Experimentation (SBCAL/COBEA) and Ethics Committee on Animal Use of UFSCar (CEUA no. 9895280815).
Fig. 1. Selection for ethanol intake by the UChB rats and the experimental schedule.
2.2. RT-PCR for the expression of miRNAs
Animals were anesthetized via intraperitoneal injection of 90 mg kg–1 ketamine chloride (Ketalar/laboratory: Parke-Davis) and 10 mg kg–1 of xilazin (Rompum/laboratory: Bayer). Later, 1 ml of blood from each rat's lateral tail vein were collected and processed for RNA extraction. The expression profiles of the miRNAs-9-3p (assay ID: 002231), -15b-5p (assay ID: 000390), -16-5p (assay ID: 000391), -21-5p (assay ID: 000397), -200a-3p (assay ID: 000502) and -222-3p (assay ID: 002276) were analyzed in whole blood from each animal. Total cellular RNA was extracted using Trizol Reagent (Invitrogen, USA) and RNA was reverse transcribed to single-stranded cDNA, using a High Capacity Kit (Applied Biosystems, USA) with specific primers provided from the Taqman microRNA assays according to the manufacturer's protocol. For quantitative analysis of the miRNAs-9-3p, -15b-5p, -16-5p, -21-5p, -200a-3p and -222-3p, we used the commercially available system TaqMan Assay-on demand (Applied Biosystems). Reverse transcription was performed using 5 ng total RNA for each sample in 7.5 μL of the total reaction mixture. The cDNA obtained was diluted 1 : 4 and 4.5 μL was used for each 10 μL of the quantitative real-time polymerase chain reaction mixture using the TaqMan Master Mix (Applied Biosystems). All reactions were carried out in duplicate and analyzed with the 7500 Sequence Detection System apparatus (Applied Biosystems). Data were analyzed using the ABI-7500 SDS software. The total RNA absorbed was normalized based on the Ct value for U6 (000391). The variation in expression among samples was calculated by the 2–ΔΔCt method, with the mean ΔCt value for a group of miRNAs-9-3p, -15b-5p, -16-5p, -21-5p, -200a-3p and -222-3p samples from control rats used as a calibrator. For the studied miRNAs, the expression evaluation and statistical analysis were carried out by using the Shapiro–Wilk normality test and one-way ANOVA. Statistical significance was set at P < 0.05. Graphical analysis was performed using GraphPad Prism version 4.0 go Windows, (GraphPad Software, San Diego – California USA).
3. Results
3.1. Ethanol increases the expression of miRNAs after chronic exposure
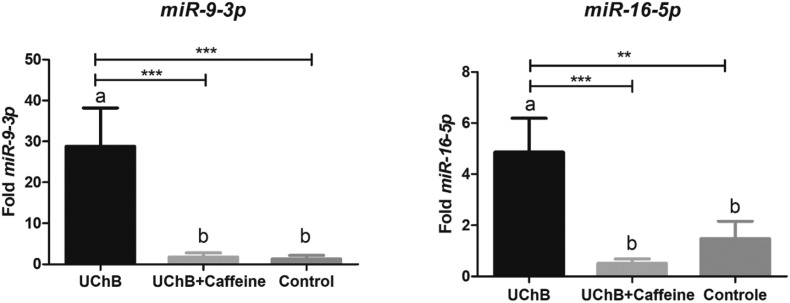
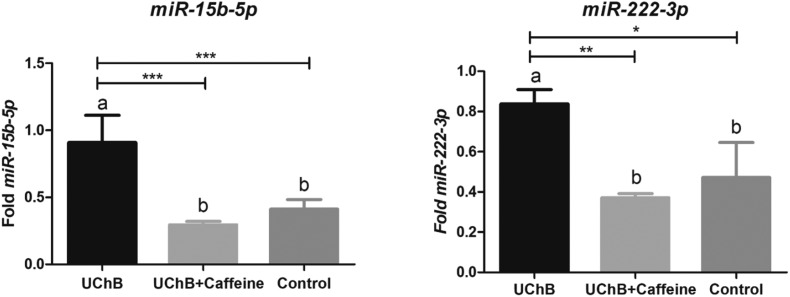
The expressions of miR-9-3p, -15b-5p, -16-5p and -222-3p were upregulated in UChB groups after chronic ethanol consumption. The most evident difference of expression was found for miR-9-3p. The level of this miRNA was around twenty-one times higher in the UChB group than in the control group. For miR-16-5p a considerable difference was also observed: the expression of this miRNA was three and nine times bigger in the UChB group when compared to the control group. The results for miR-15b-5p showed expression two times increased compared to the control group and for miR-222-3p, one and a half times bigger (Fig. 2 and 3).
Fig. 2. Fold-change analysis of miR-9-3p and miR-16-5p expressions in the serum of animals. Data are expressed as the mean ± SD. Five animal samples were considered per group. Different letters indicate the statistical difference among the groups. ** p < 0.01, *** p < 0.001.
Fig. 3. Fold-change analysis of miR-15b-5p and miR-222-3p expressions in the serum of animals. Data are expressed as the mean ± SD. Five animal samples were considered per group. Different letters indicate the statistical difference among the groups. * p < 0.05, ** p < 0.01, *** p < 0.001.
3.2. Caffeine attenuates the expression of miRNAs despite the ethanol consumption
The ingestion of caffeine downregulated the miR-9-3p, -15b-5p, -16-5p and -222-3p expressions to levels similar to those observed in the control group despite the ethanol consumption. This reduction was most evident for miR-9-3p: the UChB + caffeine group had around sixteen times less fold expression than the UChB group. For miR-16-5p, the expression for the UChB + caffeine group was nine times smaller than the levels for the UChB group and around three times less expressive than the control group. The results for miR-15b-5p showed expression three times smaller than the UChB + caffeine group and for miR-222-3p, two times lesser (Fig. 2 and 3).
3.3. Ethanol attenuates the expression of miR-21-5p
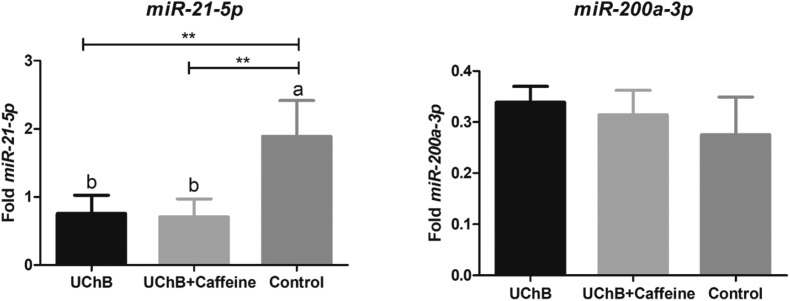
The expression of miR-21-5p was downregulated in alcoholic rats and caffeine was not capable of elevating this expression to normal levels, comparable to the control group. The level of this miRNA for the control group was two and a half times higher than the level for UChB and UChB + caffeine groups. The expression of miR-200a-3p was unchanged after treatments (Fig. 4).
Fig. 4. Fold-change analysis of miR-21-5p and miR-200a-3p expressions in the serum of animals. Data are expressed as the mean ± SD. Five animal samples were considered per group. Different letters indicate the statistical difference among the groups. ** p < 0.01.
4. Discussion
Ethanol and other abuse drugs are capable of altering levels of specific miRNAs which in turn deregulate target genes and, in general, suppress the translation interfering with normal cell function. Miranda and colleagues have documented alterations in 3% percent of miRNAs in models of hepatic disease induced by ethanol consumption.14 MicroRNAs are usually present in the bloodstream within extracellular vesicles such as exosomes. When cell lines, animal models and patients with alcoholic hepatitis are exposed to ethanol, the number of circulating exosomes has been reported to increase with concomitant changes in their microRNA cargo.26,27
Ten Berg and colleagues documented the capability of serum miRNA alteration after unrestricted social alcohol consumption by young men.28 A total of 1305 miRNAs were identified in serum and RNA-sequencing, which demonstrates a significant increase in 265 miRNA species. The hsa-miR-4433a-3p and hsa-miR-676-3p were particularly augmented after ethanol exposure. Gardiner et al. performed a prospective study with pregnant women recruited from substance abuse and general maternity clinics; they reported alterations in serum miRNA expression associated with alcohol use during human pregnancy.29 Importantly, the analysis revealed 55 altered miRNAs between the group of mothers who had related drinking episodes during pregnancy and the abstemious group.
Once they are present in the bloodstream, the miRNAs have systemic actions, being addressed to organs and tissues. In general, miRNAs have the ability to regulate a wide range of targets, and some of these targets appear to have greater functional relationships and clinical relevance, being more reported in the literature.30,31 The ethanol consumption modulating the levels of these circulating miRNAs can be considered as another pathway for systemic alterations caused by this substance abuse. Martinez and colleagues showed the capability of ethanol and caffeine consumption in altering the gene expression of caspase-3, XIAP, and IGF-1R in the cerebellum of UChB rats after ethanol and caffeine exposure, evidencing the action of these substances related to apoptosis.25
The present study showed that the circulating miRNAs-9-3p, -15b-5p, -222-3p and -16-5p were increased in the serum of the UChB group when compared with UChB + caffeine and control groups. While ethanol consumption was responsible for elevating these expressions in the UChB group, the ingestion of caffeine was capable of reducing the levels of serum miRNAs to values comparable to those in the control group. Thus, combination of caffeine and ethanol was able to affect and modulate these expressions systemically.
In clinical studies, the elevated serum expression of miRNA-9-3p appears as an indication for noninvasive detection of Alzheimer's disease, this miRNA being considered as an important biomarker.32 This miRNA seems to be related to the control of neurogenesis when measured at the brain tissue.33 Prospective research has shown miR-9-3p as a biomarker for acute concussion and alterations of cognitive functions.34 Ogata and colleagues found miR-9-3p and miR-384-5p elevated in the serum of rats with the evolution of neural cell death, considering them as possible novel indicators of neurotoxicity.35 Other clinical issues are related to the augment of this miRNA in serum and not just neural disorders. MiRNA-9-3p is increased in the serum of patients with osteosarcoma36 and other cancers, such as hepatocellular carcinoma.37
The expressions of miRNA-9-3p and -15b-5p were increased in maternal and neonatal plasma in an ovine model after ethanol exposure and shared profiles between pregnant dam and neonate, suggesting possible maternal-fetal miRNA transfer.38 Pappalardo-Carter and colleagues reported that ethanol exerts regulatory control at multiple levels of miR-9 biogenesis.39 Moreover, early embryonic loss of the miR-9 function recapitulates the severe range of teratology associated with ethanol exposure during human development. MiRNA-9 seems to play a pivotal role in CNS mediating the deleterious effects of ethanol through important targets of neuronal physiology such as excitability, genic expressions and lipid metabolism. Levels of miRNA-9 were increased in supraoptic nuclei cells and striatum in the brains of adult mammals after ethanol consumption.15
Wang et al. have demonstrated elevated expression of miRNA-9, -10, -145 and -152 in mice embryos after prenatal exposure to ethanol.40 A study by Lewohl et al. reported that the prefrontal cortex of alcoholic human brains presented 35 miRNAs with higher expressions than the control group of healthy individuals.41 The miRNA-15b had an expression 18% higher in alcoholic brains than in healthy brains. Otherwise, the exposure of neuronal culture to ethanol for 24 and 48 hours diminished the levels of miRNAs-9, -29a, -29b and -133.42
The augment of miR-15b-5p in the bloodstream is considered a signature of some malignancies such as lung,43 bladder,44 liver45 and colon cancer.46 In addition, this miRNA is related to cellular senescence and seems to promote the proinflammatory status of aging.47 The elevation of miR-15b-5p in serum has been investigated as an Alzheimer's disease indicator.32
The present results have shown lower expression of circulating miR-21-5p in the animals of UChB and UChB + caffeine groups. Park and colleagues also quantified lower levels of miRNAs-21-5p and 338-3p during the neuronal stem-cell differentiation at diencephalons of mice embryos exposed to ethanol.48 The most part of these miRNA targets was related to cellular processes such as apoptosis, neuronal differentiation, cellular cycle, metabolism, cellular signaling and development. Balaraman et al. examined the simultaneous effects of ethanol and nicotine on mice and showed that the isolated ethanol consumption suppressed the level of miRNAs-9, -21, -153 and -335.38 In addition, Olivieri et al. reported ingestion of high doses of ethanol, comparable to alcoholic individuals.49 The collective suppression of miRNAs is consistent with the presence of ethanol affecting the cellular cycle and neuroepithelial maturation in the absence of apoptosis. Variable expressions of miR-21-5p have been described in different organic systems and diseases. MiRNA-21-5p seems to be involved in the extrinsic apoptotic pathway in the alcoholic hepatic lesion in mice treated with ethanol and in cultures of human hepatic cells.50
Ignacio, Mooney & Midletton found alterations in miR-222-3p after fetal alcohol exposure in rats, and showed that miRNA expression was ameliorated by social enrichment.51 The miR-16-5p was reported to be present in easily measurable and similar amounts in plasma and in stable amounts in the presence of cardiac injury.52 Therefore, our results of miRNA could suggest the systemic modulation of tissues, ethanol and caffeine being capable of influencing the processes even in cardiac tissue.
Caffeine promotes a myriad of physiological effects in organic systems, especially in CNS. These are attributed to the competitive binding of caffeine with the adenosinergic receptors into presynaptic terminals due to caffeine structural similarity to adenosine. Once these adenosinergic receptors are occupied by adenosine, they limit the amount of calcium translocation into the cell's calcium channels. The blockage of these receptors with caffeine minimizes the limitation and increases the cytosolic levels of calcium.53 The elevated levels of calcium facilitate the neurotransmitter vesiculation and its release at the synaptic cleft. So, the release of neurotransmitters, including acetylcholine, norepinephrine and dopamine, is responsible for augmenting the neural transduction.54
Kaster et al. analyzed the relationship between the caffeine consumption and the prevention of chemical depression and loss of memory.55 They concluded that caffeine ingestion is inversely correlated with the development of depression and memory deterioration. Moreover, the antagonistic role of caffeine in the involvement of the A2a receptor is evident, being extremely relevant to create the ability of the brain in controlling glutamatergic transmission, plasticity based on apoptotic events and neuroinflammation.55 Varma & Kovtun analyzed the caffeine protector action in mouse retinas against the transcription of some miRNAs induced after high levels of sugar consumption.56 The level of 19 miRNAs was higher in galactose-treated groups compared with the control group and the miRNA-9 and -16 were significantly reverted after caffeine consumption. Our results also showed that caffeine reduced the expression of miRNA-9-3p and -16-5p even in the presence of ethanol consumption.
Caffeine can be associated with the regulation of miRNA expression.57 This substance is recognized as a xanthine and a potent antioxidant able to inhibit alterations caused by oxidative stress at the miRNA transcription. The abnormal augment of miRNA expression silences a considerable range of genes and, consequently, deactivates the translation of multiple proteins; in line with this, caffeine seems to act negatively, thus suppressing the silencing.57
The alternative splicing (AS) of pre-miRNA is a fundamental cellular process which links selectively alternated exons in a group producing variants of mRNA of a single gene.58 More than 90% of human genes undergo alternative splicing.58,59 Studies about the genome scale reveal that splicing regulators employ a two-fold mechanism adding alternative splicing with the mechanism of nonsense-mediated mRNA decay (AS-NMD) in order to limit their own expression and prevent their excessive accumulation that could damagingly affect the cells.60 Caffeine blocks the nonsense-mediated decay (NMD) and induces the 3′UTR alternative splicing; this process downregulates miRNA targets of SRSF2 interrupting the negative feedback to increase SRSF2. Therefore, caffeine modulates post-transcriptional processes in order to increase SRSF2.57 Fang et al. demonstrated that caffeine exposure altered gene expression, transcription factors and miRNAs.61 The analysis revealed that 124 genes, 849 transcriptions and 590 exons were modified after prenatal exposure to caffeine.61
The miRNAs are opening a new era in systemic and tissue-specific fields as potential biomarkers, performing even therapeutic strategies. Recent evidence suggests that some miRNAs modulate pathways linked to cellular senescence, inflammation and angiogenesis. Then, they can be considered related to the development of a broad range of diseases, including cancers. The best understanding of its origin and regulation, recognition and validation of target genes is of significant value to potentiate the therapeutic role of these noncoding RNAs.49 Moreover, the miRNA composition of human serum is dynamic and environmental factors can have an important impact. Given the variability within subjects, the fold change of a putative microRNA biomarker must imply accurate patient stratification to be achievable in later stages of the biomarker qualification process.28
As presented in this study, circulating miRNAs are sensitive to ethanol and to caffeine consumption. So, these substances are able to act systemically and one of their mechanisms of actions includes miRNA expression. In fact, the miRNAs sensitive to ethanol are master key regulators and they can control the development of tolerance to alcohol, a crucial component of alcoholism. As evidenced by Miranda et al. the miRNAs serve as molecular points of convergence that ground the tolerance and dependence on drugs.14 Chemical exposures in early life are increasingly associated with permanent effects in developmental and neurobehavioral functions.62 Studies indicate that miRNAs can decrease or mediate behavioral effects associated with drugs and highlight the relevance of miRNA during the development of behavior in consequence to fetal exposure.63,64
We conclude that the ingestion of caffeine and ethanol can produce antagonistic effects with implications of different expressions of miRNAs which may have important actions on the CNS. Although caffeine shows significant neuroprotector effects, the ingestion must be controlled since there is no consensus around boundaries and safe doses for humans. The best comprehension of chronic consumption of ethanol and caffeine simultaneously is far from being settled, providing broad directions in this research line. The presented results can motivate new analysis with genetic focus, searching for the susceptibility of the organisms to the ethanol and caffeine interaction.
Funding
This work was supported by a grant from the Fundação de Amparo à Pesquisa do Estado de São Paulo – FAPESP (grants numbers: 2013/11095-2; 2014/06240-6).
Abbreviations
- miR
microRNAs
- RT-PCR
Real time-PCR
- AMED
Alcohol mixed with energy drinks
- XIAP
X-linked IAP
- UChB rats
University of Chile B rats
- SBCAL/COBEA
Brazilian School of Animal Experimentation
- CEUA
Ethics Committee on Animal Use
- UFSCar
Federal University of São Carlos
Conflicts of interest
The authors declare that they have no conflict of interest.
Acknowledgments
We would like to thank Mr Wanderley Thiago da Silva for animal care and Mr Gelson Rodrigues for technical support.
References
- WHO, World Health Organization, Global status report on alcohol and health, Geneva, Switzerland, 2018. [Google Scholar]
- Edwards G., Gross M. M. Br. Med. J. 1976;1(6017):1058–1061. doi: 10.1136/bmj.1.6017.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beresford T. P., Wongngamnit N. and Temple B. A., Alcoholism: diagnosis, prognosis, epidemiology, and burden of the disease, in Handbooks of Neurology, 2014, vol. 125, pp. 3–13. [DOI] [PubMed] [Google Scholar]
- Malinauskas B. M., Aeby V. G., Overton R. F. Nutr. J. 2007;6:35. doi: 10.1186/1475-2891-6-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaascht F., Dicato M., Diederich M. Genes Nutr. 2015;10:51. doi: 10.1007/s12263-015-0501-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasman W. J., Boessen R., Donner Y. JMIR Res. Protoc. 2017;6(9):e169. doi: 10.2196/resprot.6727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappelletti S., Piacentino D., Sani G., Aromatario M. Curr. Neuropharmacol. 2015;13:71–88. doi: 10.2174/1570159X13666141210215655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reissig C. J., Strain E. C., Griffiths R. R. Drug Alcohol Depend. 2009;99:1–10. doi: 10.1016/j.drugalcdep.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferré S. Psychopharmacology. 2016;233:1963–1979. doi: 10.1007/s00213-016-4212-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roemer A., Stockwell T. J. Stud. Alcohol Drugs. 2017;78(2):175–183. doi: 10.15288/jsad.2017.78.175. [DOI] [PubMed] [Google Scholar]
- Gedik H., Erdal M. E., Yilmaz S. G. DNA Cell Biol. 2015;34:220–226. doi: 10.1089/dna.2014.2549. [DOI] [PubMed] [Google Scholar]
- Lee R. C., Feinbaum R. L., Ambros V. Cell. 1993;75:843–854. doi: 10.1016/0092-8674(93)90529-y. [DOI] [PubMed] [Google Scholar]
- Nass D., Rosenwald S., Meiri E. Brain Pathol. 2009;19:375–383. doi: 10.1111/j.1750-3639.2008.00184.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda R. C., Pietrzykowski A. Z., Tang Y. Alcohol Clin. Exp. Res. 2010;34:575–587. doi: 10.1111/j.1530-0277.2009.01126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietrzykowski A. Z., Friesen R. M., Martin G. E. Neuron. 2008;59:274–287. doi: 10.1016/j.neuron.2008.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendell J. T., Olson E. N. Cell. 2012;148:1172–1187. doi: 10.1016/j.cell.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinirlioglu Z. A., Coskunpinar E., Akbas F. Cell. Mol. Biol. 2017;63(2):49–56. doi: 10.14715/cmb/2017.63.2.7. [DOI] [PubMed] [Google Scholar]
- Moustafa A. A., Kim H., Albeltagy R. S. Exp. Biol. Med. 2018;243:817–825. doi: 10.1177/1535370218775657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuo K., Katada R., Okazaki S. Jpn. J. Alcohol Stud. Depend. 2012;47:155–163. [PubMed] [Google Scholar]
- McDaniel K., Herrera L., Zhou T. J. Cell. Mol. Med. 2014;18:197–207. doi: 10.1111/jcmm.12223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossetto I. M. U., Cagnon V. H. A., Lizarte F. S. N., Tirapelli L. F., Tirapelli D. P. C., Arantes R. M. S., Chuffa L. G. A., Martinez F. E., Martinez M. Life Sci. 2019;15(229):180–186. doi: 10.1016/j.lfs.2019.05.016. [DOI] [PubMed] [Google Scholar]
- Linden-Carmichael A. N., Stamates A. L., Marczinski C. A., Lau-Barraco C. Hum. Psychopharmacol. 2018;33(4):e2664. doi: 10.1002/hup.2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mardones J., Segovia-Riquelme N. Neurobehav. Toxicol. Teratol. 1993;5:171–178. [PubMed] [Google Scholar]
- Quintanilla M. E., Israel Y., Sapag A., Tampier L. Addict. Biol. 2006;11:310–323. doi: 10.1111/j.1369-1600.2006.00030.x. [DOI] [PubMed] [Google Scholar]
- Martinez M., Rossetto I. M. U., Neto F. S. L. Cell Biol. Int. 2018;42:1575–1583. doi: 10.1002/cbin.11054. [DOI] [PubMed] [Google Scholar]
- Momen-Heravi F., Bala S., Kodys K., Szabo G. Sci. Rep. 2015;5:9991. doi: 10.1038/srep09991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Momen-Heravi F., Saha B., Kodys K. J. Transl. Med. 2015;13:261. doi: 10.1186/s12967-015-0623-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ten Berg P. W., Shaffer J., Vliegenthart A. D. B. Biomarkers. 2018;23:781–786. doi: 10.1080/1354750X.2018.1499128. [DOI] [PubMed] [Google Scholar]
- Gardiner A. S., Gutierrez H. L., Luo L. Alcohol.: Clin. Exp. Res. 2016;40:826–837. doi: 10.1111/acer.13026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartel D. P. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- Benes V., Castoldi M. Methods. 2010;50:244–249. doi: 10.1016/j.ymeth.2010.01.026. [DOI] [PubMed] [Google Scholar]
- Kumar S., Reddy H. Biochim. Biophys. Acta. 2016;1862:1617–1627. doi: 10.1016/j.bbadis.2016.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coolen M., Katz S., Bally-Cuif L. Front. Cell. Neurosci. 2013;7:220. doi: 10.3389/fncel.2013.00220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papa L., Slobounov S., Breiter H. J. Neurotrauma. 2018 doi: 10.1089/neu.2018.6072. [DOI] [Google Scholar]
- Ogata K., Sumida K., Miyata K. Toxicol. Pathol. 2015;43:198–208. doi: 10.1177/0192623314530533. [DOI] [PubMed] [Google Scholar]
- Fei D., Li Y., Zhao D. J. Int. Med. Res. 2014;42:932–937. doi: 10.1177/0300060514534643. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Wei C., Guo C. C. Oncotarget. 2017;8(63):107237–107257. doi: 10.18632/oncotarget.20883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balaraman S., Lunde E. R., Sawant O. Alcohol.: Clin. Exp. Res. 2014;38:1390–1400. doi: 10.1111/acer.12378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappalardo-Carter D. L., Balaraman S., Sathyan P. Alcohol.: Clin. Exp. Res. 2013;10:1657–1667. doi: 10.1111/acer.12139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L. L., Zhang Z., Li Q. Hum. Reprod. 2009;24:562–579. doi: 10.1093/humrep/den439. [DOI] [PubMed] [Google Scholar]
- Lewohl J. M., Nunez Y. O., Dodd P. R. Alcohol.: Clin. Exp. Res. 2011;35:1928–1937. doi: 10.1111/j.1530-0277.2011.01544.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi Y., Zhang M., Li H., Frank J. A., Dai L., Liu H., Chen G. J. Biol. Chem. 2014 doi: 10.1074/jbc.M113.535195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin X., Chen Y., Chen H. Clin. Cancer Res. 2017;23:5311–5319. doi: 10.1158/1078-0432.CCR-17-0577. [DOI] [PubMed] [Google Scholar]
- Jiang X., Du L., Wang L. Int. J. Cancer. 2015;136:854–862. doi: 10.1002/ijc.29041. [DOI] [PubMed] [Google Scholar]
- Chen Y., Chen J., Liu Y., Li S., Huang P. Med. Sci. Monit. 2015;21:1864–1871. doi: 10.12659/MSM.893082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C., Zhao Q., Zhang C. Sci. Rep. 2017;7:4194. doi: 10.1038/s41598-017-04172-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terlecki-Zaniewicz L., Lämmermann I., Latreille J. Aging. 2018;10:1103–1132. doi: 10.18632/aging.101452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J. H., Debasish H., Mi Ran C. BioChip J. 2012;6(1):73–83. [Google Scholar]
- Olivieri F., Rippo M. R., Monsurrò V. Ageing Res. Rev. 2013;12:1056–1068. doi: 10.1016/j.arr.2013.05.001. [DOI] [PubMed] [Google Scholar]
- Francis H., McDaniel K., Han Y. J. Biol. Chem. 2014;289:27526–27539. doi: 10.1074/jbc.M114.602383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignacio C., Mooney S. M., Midletton F. A. Front. Pediatr. 2014;2:103. doi: 10.3389/fped.2014.00103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Cai J. Biochim. Biophys. Acta, Mol. Basis Dis. 2017;1863:2019–2030. doi: 10.1016/j.bbadis.2016.11.034. [DOI] [PubMed] [Google Scholar]
- Pedata F., Giovannelli L., De Sarno P., Pepeu G. J. Neurosci. 2002;22:6321–6324. [Google Scholar]
- Dobmeyer D. J., Stine R. A., Leier C. V., Greenberg R., Schaal S. F. N. Engl. J. Med. 1983;308:814–816. doi: 10.1056/NEJM198304073081405. [DOI] [PubMed] [Google Scholar]
- Kaster M. P., Machado N. J., Silva H. B. Proc. Natl. Acad. Sci. U. S. A. 2015;112(25):7833–7888. doi: 10.1073/pnas.1423088112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varma S. D., Kovtun S. Mol. Vision. 2013;19:493–500. [PMC free article] [PubMed] [Google Scholar]
- Shi J., Pabon K., Scotto K. W. J. Biol. Chem. 2015;290:14986–15003. doi: 10.1074/jbc.M114.624254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda J., Suzuki Y., Sakate R. Nucleic Acids Res. 2010;38:86–90. doi: 10.1093/nar/gkp984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauman J. A., Kole R. Bioengineered Bugs. 2011;2:125–128. doi: 10.4161/bbug.2.3.15165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIlwain D. R., Pan Q., Reilly P. T. Proc. Natl. Acad. Sci. U. S. A. 2010;107:12186–12191. doi: 10.1073/pnas.1007336107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang X., Mei W., Barbazuk W. B., Rivkees S. A., Wendler C. C. Am. J. Physiol.: Regul., Integr. Comp. Physiol. 2014;307:1471–1487. doi: 10.1152/ajpregu.00307.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korosi A., Naninck E. F., Oomen C. A. Behav. Brain Res. 2012;227:400–409. doi: 10.1016/j.bbr.2011.07.037. [DOI] [PubMed] [Google Scholar]
- Sathyan P., Golden H. B., Miranda R. C. J. Neurosci. 2007;27:8546–8557. doi: 10.1523/JNEUROSCI.1269-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tal T., Tanguay R. L. NeuroToxicology. 2012;33:530–544. doi: 10.1016/j.neuro.2012.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]