Abstract
Painful Hashimoto thyroiditis (pHT) is a rare diagnosis, and optimal treatment remains unclear. To better characterize pHT, PubMed, Embase, Scopus, and Web of Science indexes were searched for case reports or case series reporting pHT, published between 1951 and February 2019. Seventy cases reported in 24 publications were identified. Female predominance (91.4%) and a median age of 39.00 years (interquartile range, 32.50-49.75 years) were observed. Among reported cases, 50.8% had known thyroid disease (including Hashimoto thyroiditis, Graves disease, and seronegative goiters), 83.3% had positive antithyroid peroxidase antibodies, and 71.2% had antithyroglobulin antibodies. Most cases did not have preceding upper respiratory tract symptoms or leukocytosis. Ultrasound features were consistent with Hashimoto thyroiditis. Thyroid function at initial presentation was hypothyroid (35.9%), euthyroid (28.1%), or thyrotoxic (35.9%). Cases evolved into hypothyroidism (55.3%) and euthyroidism (44.7%), whereas none became hyperthyroid after medical treatment. Thyroid size usually decreased after medical treatment. Most cases were empirically treated as subacute thyroiditis with corticosteroids, levothyroxine, or nonsteroidal anti-inflammatory drugs. However, no therapy provided sustained pain resolution. In subgroup analysis, low-dose oral prednisone (<25 mg/d) and intrathyroidal corticosteroid injection showed more favorable outcomes. Total thyroidectomy yielded 100% sustained pain resolution. Diagnosis of pHT is based on clinical evidence of Hashimoto thyroiditis and recurrent thyroid pain after medical treatment. The reference standard of diagnosis is pathology. Total thyroidectomy or intrathyroidal glucocorticoid injection should be considered if low-dose oral prednisone fails to achieve pain control.
Keywords: Hashimoto thyroiditis, Hashimoto disease, subacute thyroiditis, pain
Painful Hashimoto thyroiditis (pHT) is a rare variant of Hashimoto thyroiditis (HT) that mostly affects women. It is also known as acute exacerbation of HT or painful autoimmune thyroiditis. Diagnosis of pHT is established when painful thyroid presents along with HT, which typically features an elevated serum level of thyroid antibodies (antithyroperoxidase [TPO] or antithyroglobulin [Tg]) and a firm, painless goiter (1). According to an earlier hypothesis, capsular stretching, which results in the rapid enlargement of the thyroid and related pain, may be the cause of pHT (2). However, this hypothesis could not be fully supported because various sizes of the thyroid gland were observed in the reported cases (3). Patients with atrophic thyroid glands were still experiencing pain. The epidemiology and pathophysiology of pHT remain unclear given the limited data from the previous publications.
Among the differential diagnoses of pHT, subacute (or de Quervain) thyroiditis, a self-limited disease related to a postviral inflammatory process, is the most common etiology. Other causes include hemorrhagic cyst, suppurative thyroiditis, Riedel thyroiditis, infiltrative disease, trauma, or malignant tumor. The majority of patients were initially diagnosed with subacute thyroiditis. However, most patients had only temporary, partial pain relief or did not benefit from medical treatment, including the administration of corticosteroids and other analgesic medications. A significant number of reported patients experienced repeated relapses that eventually required thyroidectomy to relieve pain effectively (3). However, the appropriate medical treatment for pHT remains unclear.
All the publications regarding pHT are either case reports or case series (4–27), with 1 short review article (3) available to date. To the best of our knowledge, no statistical analysis for disease characteristics and treatment efficacy has been performed. Therefore, we conducted this first comprehensive review including a total of 24 articles and 70 patients from the literature that met our inclusion criteria. The reviewed publications were published between 1951 and early 2019. This study aimed to provide clinicians the basic demographics, features in clinical presentation, laboratory results, and imaging findings of pHT cases that have been reported in the past 70 years, along with treatment options.
1. Materials and Methods
A. Data Sources and Searches
Recommendations from the Preferred Reporting Items for Systematic Reviews and Meta-Analyses were adapted in this review (28). The key words included pain, painful, Hashimoto thyroiditis, Hashimoto disease, and the controlled vocabularies (ie, Medical Subject Heading terms). A medical research informationist assisted us in performing serial electronic literature searches on databases including PubMed, Embase, Scopus, and Web of Science for English-language research published between 1951 and February 2019.
The search terms used in each database are listed as follows: PubMed: (Hashimoto Disease[ Medical Subject Heading] OR hashimoto thyroiditis OR hashimoto disease OR hashimoto’s OR hashimoto [tiab]) AND (pain OR painful). Embase: (Hashimoto disease/exp OR hashimoto thyroiditis OR hashimoto disease OR hashimoto [tiab]) AND (pain OR painful). Scopus: TITLE-ABS-KEY (hashimoto thyroiditis OR hashimoto disease OR hashimoto’s OR hashimoto) AND TITLE-ABS-KEY(pain OR painful). Web of Science: TS = (hashimoto thyroiditis OR hashimoto disease OR hashimoto’s OR hashimoto [tiab]) AND TS = (pain OR painful).
Additional studies also were identified by manual searches of bibliographies from the references in the identified articles. We did not place filters for journal, study design, or subject on the search; however, conference proceedings and abstracts were excluded. The search was last updated February 14, 2019.
B. Study Selection
Two authors (C.C.P. and R.H.C.) independently reviewed the study eligibility, and any conflict was resolved by a third author (H.K.H.). We included the studies that identified clinical cases with the presentation of anterior neck or thyroid pain that eventually was clinically or histologically diagnosed as HT. The following studies were excluded in this study: studies with no full text, studies that are not case reports (eg, original research, discussions, editorials); studies with no final diagnosis of pHT, and studies with cases of typical HT that presented with nontender neck mass.
C. Data Extraction
Two authors (C.C.P. and R.H.C.) independently abstracted data from the included articles into a self-designed template using the Cochrane Handbook for Systematic Reviews of Intervention as a reference (29). Study information and clinical characteristics of reported cases were extracted in all studies.
Authors assessed and categorized thyroid function (classified as hypothyroidism, euthyroidism, and thyrotoxicosis) at the initial presentation and during the recovery phase. The interpretation of the status of thyroid function was based on the values provided along with the normal reference values and the authors’ determination described in the report. If patients appeared euthyroid and were on levothyroxine treatment concurrently, the thyroid function of these patients were classified as hypothyroid. The characteristics of ultrasound and histology were also documented in the original words used in the description.
Medical treatments were classified based on the different mechanisms of action of the medications (corticosteroids, nonsteroidal anti-inflammatory drugs [NSAIDs] including aspirin, and levothyroxine). The status of sustained pain resolution was assessed as the treatment outcome. Only patients who received levothyroxine for pHT without concomitant hypothyroidism at the initial diagnosis were included in this particular analysis. Patients could receive more than 1 medication simultaneously or serially. If multiple medications were administered simultaneously, the pharmacological outcome of each prescription is unlikely to be identified. Therefore, the treatment outcome was recorded as the overall result from the combination of medications. The route of administration, dosage, and duration of corticosteroid use were further classified. Duration of NSAID and levothyroxine use was not evaluated because of insufficient information in the publications reviewed.
The size of the thyroid gland was compared to that before medical treatment. The time length between the onset of neck pain and surgery was calculated, whereas surgical type (total thyroidectomy, near-total thyroidectomy, subtotal thyroidectomy, partial thyroidectomy) and intraoperative findings were documented as described in the original reports. Surgical outcomes were assessed by the status of sustained pain resolution postoperatively, the need for postoperative radioactive iodine ablation, and the relapse rate during the follow-up period. Multiple authors (C.C.P., R.H.C., and H.K.H.) evaluated the abstraction accuracy and agreement. Study authors were contacted for additional data or confirmation when needed.
D. Data Analysis
We collected individual-level data from each study. However, not every patient had complete information for each variable. Most of the variables were established as binary data and coded as 1, 0, or not applicable (N/A), or reported into 3 or more categories. Items that were not mentioned or remained unclear in the case report were assigned N/A and were not considered in the calculation. Only the overall age of each group was coded as continuous data, as shown in Table 1. Age was classified into 4 ranges based on the distribution to compare the proportion difference between groups. Based on the data reported, countries were categorized into Japan, the United States, the United Kingdom, and other countries to highlight geographic differences.
Table 1.
Characteristics of patients and treatments
| No. of Overall Cases | No. of Cases With Reported Treatment Status | Medical Treatment Alone | Surgery | |
|---|---|---|---|---|
| Characteristics | No. of Cases/ Overall Reported No., (%) | No. of Cases/ Total Reported Case No., (%) | No. of Cases/ Total Reported Case No., (%) | |
| Overall | 70 | 60 | 29 | 31 |
| Sex | ||||
| Female | 64/70 (91.4%) | 54 | 27/29 (93.1%) | 27/31 (87.1%) |
| Male | 6/70 (8.6%) | 6 | 2/29 (6.9%) | 4/31 (12.9%) |
| Age, y | ||||
| Median (IQR) | 39.00 (32.50-49.75) | 39 (37-48) | 35 (23-48) | |
| Country | ||||
| Japan | 26/70 (37.1%) | 18 | 11/29 (37.9%) | 7/31 (22.6%) |
| United States | 23/70 (32.9%) | 23 | 6/29 (20.7%) | 17/31 (54.8%) |
| United Kingdom | 9/70 (12.9%) | 8 | 6/29 (20.7%) | 2/31 (6.5%) |
| Other | 12/70 (17.1%) | 11 | 6/29 (20.7%) | 5/31 (16.1%) |
| Known thyroid disease | ||||
| Total | 37/70 (52.8%) | 31 | 11 | 20 |
| Hashimoto thyroiditis | 23/37 (62.2%) | 22 | 9/11 (81.8%) | 13/20 (65.0%) |
| Graves disease | 5/37 (13.5%) | 3 | 0/11 | 3/20 (15.0%) |
| Seronegative goiter | 9/37 (24.3%) | 6 | 2/11 (18.2%) | 4/20 (20.0%) |
| Fever | 17/43 (39.5%) | 11 | 4/19 (21.1%) | 7/15 (46.7%) |
| Recent history of URI | 5/37 (13.5%) | 4 | 2/20 (10.0%) | 2/9 (22.2%) |
| Leukocytosis | 3/27 (11.1%) | 3 | 2/11 (18.2%) | 1/8 (12.5%) |
| Elevated ESR level | 35/57 (61.4%) | 26 | 17/27 (63.0%) | 9/21 (42.9%) |
| Elevated CRP level | 23/29 (79.3%) | 16 | 9/11 (81.8%) | 7/10 (70.0%) |
| Positive anti-TPO at initial presentation | 45/54 (83.3%) | 37 | 16/21 (76.2%) | 21/23 (91.3%) |
| Presence anti-Tg at initial presentation | 37/52 (71.2%) | 31 | 13/21 (61.9%) | 18/21 (85.7%) |
| Initial thyroid functiona | ||||
| Hypothyroidism | 23/64 (35.9%) | 23 | 9/28 (32.1%) | 14/35 (40.0%) |
| Euthyroidism | 18/64 (28.1%) | 17 | 10/28 (35.7%) | 7/35 (20.0%) |
| Thyrotoxicosis | 23/64 (35.9%) | 23 | 9/28 (32.1%) | 14/35 (40.0%) |
| RAIU (uptake at 24 h) | ||||
| < 15% | 19/35 (54.3%) | 11 | 8/14 (57.1%) | 3/13 (23.1%) |
| 15% to 30% | 8/35 (22.9%) | 8 | 3/14 (21.4%) | 5/13 (38.5%) |
| > 30% | 8/35 (22.9%) | 8 | 3/14 (21.4%) | 5/13 (38.5%) |
| Ultrasound showed increased vascularity | 5/6 (83.3%) | 5 | 2/3 (66.7%) | 3/3 (100%) |
| Last thyroid function after treatmenta | ||||
| Hypothyroidism | 21/38 (55.3%) | 21 | 10/19 (52.6%) | 5/10 (50.0%) |
| Euthyroidism | 17/38 (44.7%) | 14 | 9/19 (47.4%) | 5/10 (50.0%) |
| Thyrotoxicosis | 0 | 0 | 0/19 | 0/10 |
| Decreased size of thyroid at end of follow-up | 21/32 (65.6%) | 18 | 8/12 (66.7%) | 10/12 (83.3%) |
Plus–minus values are means ± SD. A total of 100% may not be achieved because values were rounded off.
Abbreviations: anti-Tg, antithyroglobulin; anti-TPO, antithyroid peroxidase; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; IQR, interquartile range; RAIU, radioactive iodine uptake; URI, upper respiratory tract infection.
aIf patients appeared euthyroid but on levothyroxine treatment, they were classified as having hypothyroidism. Patients who were on antithyroid medications were classified as having thyrotoxicosis.
We further specified patients who had reported treatment status (Table 1). The patients who had treatment details, including the type of medication, were divided into the medical group and the surgical group, regardless of their treatment outcome. The medical group comprised patients receiving only medical treatment for pHT. Regardless of the previous history of medical treatment, patients who eventually underwent thyroidectomy were classified into the surgical group. Further analyses were performed to determine the differences in characteristics between the 2 groups. Known thyroid diseases were classified as HT, Graves disease, and seronegative goiter. Radioactive iodine uptake (RAIU) was categorized into the following 3 groups: less than 15%, 15% to 30%, and greater than 30%. The vascularity detected by ultrasound was divided into increased or decreased/absence.
The treatment efficacy has been compared between different medications (corticosteroid, NSAIDs, and levothyroxine) and various surgical procedures (total thyroidectomy, near-total thyroidectomy, subtotal thyroidectomy, and partial thyroidectomy), as shown in Table 2. Subgroup analysis of corticosteroid treatment was performed to identify the differences between administration route, dosage, and therapy duration. Given substantial heterogeneity, limited sample size, and insufficient information due to the nature of case reports among the included studies, formal meta-analyses and inferential analysis were not performed. Categorical variables between the 2 groups were analyzed using the chi-square test.
Table 2.
Treatment efficacy
| Received Therapy | Sustained Pain Resolution | |
|---|---|---|
| Subgroup | No. of Cases/Total No. (%) | No. of Cases/Total No. Received Therapy (%) |
| Drug | ||
| Corticosteroids | 42/58 (72.4%) | 14/42 (33.33%) |
| Route of administration | ||
| Intrathyroidal injectiona | 5/42 (11.9%) | 4/5 (80.0%) |
| Oral | 41/42 (97.6%) | 12/41 (29.3%) |
| Dosage of oral prednisone | ||
| < 25 mg/d | 12/23 (52.2%) | 6/12 (50.0%) |
| 25 to 40 mg/d | 8/23 (34.8%) | 2/8 (25.0%) |
| > 40 mg/d | 3/23 (13.0%) | 0/3 (0.0%) |
| Duration for each episode, mo | ||
| < 1 | 6/29 (20.7%) | 3/6 (50.0%) |
| 1 to 3 | 13/29 (44.8%) | 8/13 (61.5%) |
| > 3 | 10/29 (34.5%) | 1/10 (10.0%) |
| CRP level | ||
| CRP elevated | 13/17 (76.5%) | 4/13 (30.8%) |
| CRP within normal limit | 4/17 (23.5%) | 1/4 (25.0%) |
| ESR level | ||
| ESR elevated | 16/33 (48.5%) | 8/16 (50.0%) |
| ESR within normal limit | 17/33 (51.5%) | 5/17 (29.4%) |
| NSAIDs | 21/58 (36.2%) | 4/21 (19.0%) |
| Levothyroxineb | 26/58 (44.8%) | 9/26 (34.6%) |
| Surgeryc | ||
| Total thyroidectomy | 21/31 (67.7%) | 21/21 (100%) |
| Near total thyroidectomy | 3/31 (9.7%) | 2/3 (66.7%) |
| Subtotal thyroidectomy | 6/31 (19.4%) | 3/6 (50.0%) |
| Partial thyroidectomy | 1/31 (3.2%) | Unknown |
This efficacy analysis shows patients who received treatment that yield sustained pain relief.
Abbreviations: CRP, C-reactive protein; ESR, erythrocyte sedimentation rate, NSAIDs, nonsteroidal anti-inflammatory drugs.
aFour cases had preceding treatment with oral corticosteroids.
bAdministration of levothyroxine in patients without hypothyroidism at the time of initial diagnosis.
cWith or without prior medical treatment.
In addition to the analysis of patient demographics and disease nature, the number of publications and the overall number of patients were grouped into 10-year increments over 7 decades. For each group, we also analyzed the percentage of patients who underwent thyroid surgery (Table 3).
Table 3.
Analysis based on timeline
| Years | No. of Publications | No. of Patients | No. of Thyroid Surgery/Total Patients (%) |
|---|---|---|---|
| 1951 to 1960 | 2 | 7 | 2/7 (28.6) |
| 1961 to 1970 | 0 | 0 | 0/0 (0) |
| 1971 to 1980 | 1 | 2 | 0/2 (0) |
| 1981 to 1990 | 5 | 25 | 3/25 (12.0) |
| 1991 to 2000 | 1 | 1 | 1/1 (100) |
| 2001 to 2010 | 6 | 20 | 15/20 (75) |
| 2011 to 2019 | 9 | 15 | 10/15 (66.7) |
2. Results
A. Study Characteristics
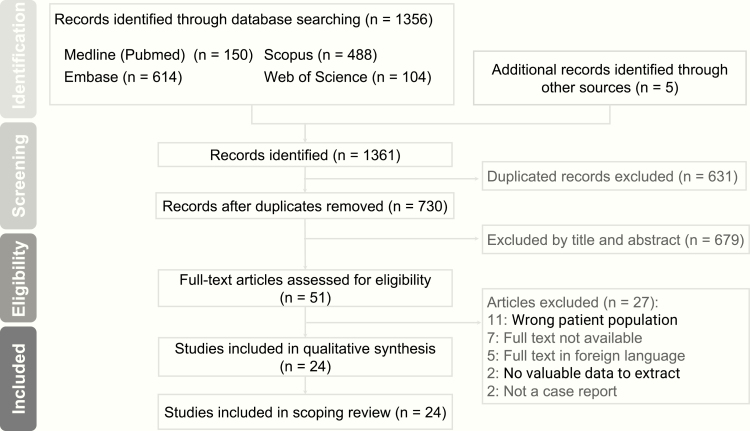
There were 1361 citations identified in our initial database search, and 24 studies eventually met our inclusion criteria (Figure 1). All the included studies were case reports or case series, with the first published in 1957 and the latest one published in 2018. The largest case series consisted of 8 patients. There were 70 patients reported in 24 articles. The group that received medical treatment comprised only 29 patients, and the other group that underwent surgeries comprised 31 patients, regardless of prior medical treatment status.
Figure 1.
Identification of eligible studies for analysis.
B. Demographics
The baseline characteristics of the patients are shown in Table 1. In general, pHT was observed predominantly in women (64 cases, 91.4%). The median age was 39 years (interquartile range, 32.50-49.75 years). Among the 70 patients, most were reported in Japan (26 cases, 37.1%), the United States (23 cases, 32.9%), and the United Kingdom (9 cases, 12.9%). The remaining patients were reported in Asia, Europe, and Canada. Ethnicity data were not available in the majority of published studies.
Among the medical and surgical treatment groups, there was no significant difference in the proportion of sex and the distribution of age. The United States accounted for more than half the surgical cases that failed medical therapy (17 out of 31 cases, 54.8%).
C. Number of Publications, Cases, and Surgeries Reported Based on a Timeline
Table 3 summarizes the number of publications, cases, and surgeries from 1951 to 2019. Most cases were reported during 1981 and 1990, and 2001 and 2010, with 5 articles containing 25 patients and 6 articles containing 20 patients. The majority of thyroid surgeries were performed after 2000, which included 15 cases during 2001 to 2010, and 10 cases during 2011 to 2019. The percentage of patients undergoing surgery was 75% during 2001 to 2010, and 66.7% during 2011 to 2019. From 1951 to 2000, among the 35 patients, there were only 6 (17.1%) who underwent surgeries.
D. Known Thyroid Diseases
There were 37 patients, with 52.8% presenting with known thyroid diseases, including HT, Graves disease, and seronegative goiters. No significant differences were observed between the medical and surgical groups regarding known thyroid diseases.
E. Symptoms
The presence of fever was noted in 17 out of 43 patients (60.5%). Only 5 patients (5/37 cases, 13.5%) reported preceding upper respiratory tract symptoms before developing pHT. The surgical group had a higher percentage of having a fever or a recent history of upper respiratory infection at the first evaluation. Therefore, whether either group had an infection before the thyroiditis is inconclusive in this study.
F. Laboratory Findings
The majority of patients did not have leukocytosis (24 out of 27 patients had normal white blood cell counts, 88.9%). Erythrocyte sedimentation rate (ESR) level was reported in 57 patients, whereas C-reactive protein (CRP) level was reported in 29 patients. Elevated ESR and CRP levels were noted in 35 out of 57 patients (61.4%) and 23 out of 29 patients (79.3%), respectively. The majority of patients had antithyroid peroxidase (anti-TPO) antibodies (45/54 patients, 83.3%) and anti-Tg antibodies (37/52 patients, 71.2%). A total of 7 patients had both negative anti-TPO and anti-Tg levels. Titers of anti-TPO and anti-Tg both were obtained at the patient’s initial presentation.
G. Thyroid Function Tests
There were 64 patients with initial thyroid function defined as hypothyroid (23 cases, 35.9%), euthyroid (18 cases, 28.1%), or thyrotoxic (23 cases, 35.9%). Only 38 patients had reported follow-up thyroid function after medical treatment or before surgical treatment. The last reported thyroid function was still categorized into hypothyroidism (21 cases, 55.3%), euthyroidism (17 cases, 44.7%), or thyrotoxicosis (0 cases). A higher percentage of patients had eventually developed hypothyroidism, whereas a case of thyrotoxicosis was not reported after undergoing both treatments.
H. Radioactive Iodine Uptake
Only one-half of the cases included in this study reported a result on RAIU. The majority of patients (19/35, 54.3%) had a low RAIU, defined as uptake less than 15% at 24 hours. There were 8 patients (8/35, 22.9%) who had an uptake ratio between 15% and 29% or greater than 30%. For those undergoing RAIU, 6 patients did not report thyroid function, 5 patients were hypothyroid, 9 patients were euthyroid, and 15 patients were thyrotoxic.
I. Ultrasound Features
Only one paper mentioned the use of ultrasound to evaluate pHT before 1987. Only 6 cases from a few articles reported vascularity with Doppler ultrasound. Heterogeneous hypoechogenicity with or without intrathyroidal hypoechoic pseudonodules demonstrated in most reported cases is consistent with the characteristics of HT. Increased vascularity was mentioned in 5 cases (83.3%), whereas the absence of vascularity was noted in 1 case (16.7%).
J. Thyroid Gland Size
Small thyroid gland size before surgery was noted in most patients (21 out of 32 patients had available data on thyroid gland size, 65.6%) after receiving medical treatment. Similar percentages of decreased thyroid gland size were shown both for the medical and surgical groups when repeat measurement was performed at the end of follow-up.
K. Treatment Methods and Responses
Only 58 cases reported medical therapy, with details included in the analysis. Among the different medications administered in 58 patients, corticosteroids were most commonly administered (42 patients, 72.4%), followed by levothyroxine (26 patients, 44.8%) and NSAIDs (21 patients, 36.2%) (Table 2). Self-reported pain resolution after the administration of medications was extracted from the articles. Levothyroxine and NSAIDs had less pain relief compared to oral corticosteroids according to the percentages of patients’ subjective pain resolution.
A subgroup analysis focusing on corticosteroids, including its route of administration, dosage range, and treatment duration for each episode, was performed (Table 2). Sustained pain resolution was observed in 50% (6 cases) of patients receiving a prednisone dose of less than 25 mg daily, in 25% (2 patients) of patients receiving a prednisone dose of 25 to 40 mg daily, and in 0% of patients receiving a prednisone dose greater than 40 mg daily. Most patients (13 cases out of all patients reporting the duration of using corticosteroids, 44.8%) received treatment for 1 to 3 months. Regarding sustained pain resolution, 50% (3 cases) of patients in the treatment group of less than 1 month and 61.5% (8 cases) of patients in the treatment group of 1 to 3 months had complete pain resolution.
There were 5 patients who received intrathyroidal corticosteroid injection. In 4 out of 5 patients treated with intrathyroidal injection, triamcinolone acetate 40 mg was administered after oral corticosteroid treatment had failed. In the 4 patients who achieved sustained pain relief, 2 patients experienced pain recurrence. Of these 2 patients, 1 patient received 4 local injections and the other received only 1 local injection before pain permanently resolved.
Besides corticosteroids, NSAIDs, and levothyroxine, Kashyap et al (23) described one case using acetaminophen, amitriptyline, and gabapentin. However, none of these agents was shown to be effective for pain control.
L. Surgery
Time from pain onset to surgery varies, with a median of 1.5 years (range, 7 weeks-12 years). Twenty-one patients underwent total thyroidectomy (67.7%), 3 patients underwent near-total thyroidectomy (9.7%), and 6 patients underwent subtotal thyroidectomy (19.4%). Only one patient, reported by Doniach and Hudson (4), underwent partial thyroidectomy. However, no treatment response was reported about that patient.
Every patient (100%) achieved complete pain resolution postoperatively in the total thyroidectomy group, whereas 66.7% (2 patients) of the patients in the near-total thyroidectomy group and 50% (3 patients) of the patients in the subtotal thyroidectomy group achieved complete pain resolution postoperatively. In the subtotal thyroidectomy group comprising 6 patients, 1 patient had partial pain relief, and 1 patient experienced pain recurrence that was not responsive to postsurgical radioactive iodine ablation for the remnant tissue. Among the 3 patients in the near-total thyroidectomy group, 1 patient developed pain recurrence, which was refractory to radioactive iodine ablation, within 1 year. An eradicative effect of total thyroidectomy in pain relief was observed when comparing subtotal thyroidectomy to total thyroidectomy. No significant difference was noted between total thyroidectomy and near-total thyroidectomy, but comparison between the 2 is limited by the small sample size.
In several articles, the intraoperative findings were described (15, 18, 24). All 3 cases featured atrophic and firm thyroid glands that had severe adhesion to surrounding tissues. No postoperative complications were reported in any patients undergoing surgical treatment.
M. Histopathology
A total of 59 patients (84.3%) had histopathology reports from specimens obtained either through fine-needle aspiration or through surgery. Fine-needle aspiration was performed in 43 patients (61.4%). The results revealed a classic picture of HT. The majority of patients presented with diffuse lymphocytic infiltrate, but a few patients had only focal involvement. Varying degrees of fibrosis, from mild fibrotic change to an extensive degree, were observed, although a greater percentage of patients reported severe fibrosis. Lee and colleagues (25) further specified the presence of immunoglobulin G 4 (IgG4) plasma cells in their case. Two patients had pathology-confirmed seronegative pHT.
N. Graves Disease Transformation
One study reported Graves disease transformation in 4 patients (4 out of 70 patients in total, 5.7%) after being diagnosed with pHT from 2 to 7 years earlier (17). Diagnosis of Graves disease was established with positive antithyrotropin receptor antibody.
3. Discussion
This is a comprehensive review based on case reports and case series to explore the clinical characteristics of pHT, a rare subtype of HT. It may be argued that only cases requiring surgical treatment were reported, whereas the portion of patients with less-severe pain remains unreported. Therefore, by publishing these data with detailed demographic features and analysis of treatment efficacy, we expect that attention will be drawn to pHT by primary care physicians, endocrinologists, and surgeons.
A. History of Reporting Painful Hashimoto Thyroiditis
After 2010, the number of publications increased more compared to that in past decades, although total reported cases were fewer in number compared with the number of cases reported in the previous decades. This is reasonable because pHT has become better recognized after being introduced and emphasized in the articles published previously. However, the time between initial presentation and surgery was not shortened. After Doniach et al (4, 5) reported the first 7 cases of pHT in 1957 and 1960, there was no discussion about pHT for 20 years. It was not until Fui and Jefferys (6) reported 1 case in 1979 followed by multiple case reports and case series starting from 1986 that discussion about pHT returned.
B. Demographics
In our analysis of all the reported patients diagnosed with pHT, the sex ratio of women to men was about 10 to 11:1, which is close to that of HT, with a general sex ratio of 8 to 9:1 (1). pHT can develop in any age group, from teenagers to the elderly, but with a peak between ages 30 and 50 years, similar to HT (1). Most cases were reported by authors in Japan and the United States. However, because of reporting bias, we are unable to conclude that pHT is more prevalent in these 2 countries than in other countries.
C. Clinical Presentation
pHT can present with insidious, progressive pain or acute intolerable pain in one lobe or the whole thyroid. The pain may initially start in one lobe, but it may be felt in the other lobe within days or months (15).
The presence of an atrophic thyroid gland, indicating end-stage thyroid failure, occurs in about 10% of patients with HT (30). Among all the cases that reported thyroid gland size at the end of medical treatment or before surgery, 21 patients (65.5%) reported a decrease in size of the thyroid gland in our analysis. The incidence of thyroid atrophy is much higher in patients with pHT than in those with HT, who present mostly with goiters (1). The rest of the cases demonstrated fluctuating or remitting clinical course in terms of pain and size of the thyroid gland. The pain may or may not respond to medical treatments. Withdrawal from medications may also be difficult despite their initial promising treatment effect.
D. Laboratory Tests
Because of the likelihood of reporting bias, more than half the patients (60.5%) were noted to have a fever and elevated ESR (61.4%) and/or elevated CRP (79.3%) levels at initial presentation. However, leukocytosis was not observed in the majority of patients (88.9%). The febrile episodes and elevated ESR and CRP levels could be an indication of the inflammatory reaction of thyroiditis itself. Therefore, the presence or absence of fever, leukocytosis, and elevation of ESR and CRP levels are insufficient to differentiate pHT from subacute thyroiditis.
In our analysis, most patients had either anti-TPO (83.8%) or anti-Tg (72.1%) at initial presentation. Moreover, 2 patients were diagnosed with seronegative pHT. Presence of antithyroid antibodies is not a diagnostic indicator of TH. In a cohort of 73 patients with painful thyroiditis, 26% of patients had elevated levels of antithyroid antibodies, whereas only 2 patients were diagnosed with pHT based on final pathology (24).
Initial thyroid function in patients with pHT was hypothyroid, euthyroid, or thyrotoxic. Thyroid function often fluctuates over time when measured at various stages of thyroiditis. Thyrotoxic patients were not reported during the final thyroid function testing. However, there was no significant difference in the number of hypothyroid or euthyroid patients. Ipekci et al provided the trajectory of the change of thyroid function test in a patient who was initially euthyroid, but became thyrotoxic within 1 month, followed by a persistent hypothyroid state after another month [19].
E. Ultrasound Findings
The sonographic features of pHT were consistent with HT, which are characterized by a diffuse heterogeneous hypoechoic pattern. A conclusion based on thyroid gland size could not be formulated in 1 cross-sectional result because of insufficient baseline data. In patients with HT, the small size of the thyroid gland at baseline may further complicate the interpretation. Therefore, these assessments should be considered with caution. Onoda and colleagues described the dynamic change of thyroid blood flow on ultrasound during the clinical course of pHT (18). Increased thyroid blood flow to hypoechoic lesions was noted during acute exacerbation, which decreased after medical treatment.
F. Diagnosis
Table 4 lists the comparisons between pHT and subacute thyroiditis (1). We acknowledge that the reference standard of diagnosis is pathology.
Table 4.
Comparison between painful Hashimoto thyroiditis and subacute thyroiditis
| Painful Hashimoto Thyroiditis | Subacute Thyroiditis | |
|---|---|---|
| Age at onset, y | All ages, peak 30 to 50 | 20 to 60 |
| Sex ratio (F:M) | 10 to 11:1 | 5:1 |
| Mechanism | Unknown | Unknown, likely related to viral infection |
| Prior viral infection | Rare | Usual |
| Fever | Usual | Usual |
| ESR/CRP | Usually elevated | Marked elevated |
| Leukocyte count | Usually normal | Normal or slightly elevated |
| Prior thyroid disease | Usual | Rare |
| Antithyroid antibodies | Present | Usually absent |
| 24-h radioactive iodine uptake | Variablea | < 5% |
| Thyroid function at onset | Variablea | Usually thyrotoxicosis |
| Thyroid function in recovery | Variablea | Usually euthyroid |
| Sonography features | Diffuse, heterogeneous, hypoechoic pattern. Increased or absent vascular flow to hypoechoic lesions, if present | Diffusely hypoechoic with decreased blood flow to ill-defined hypoechoic thyroid lesions |
| Pathological findings | Lymphocytic infiltration, germinal centers, Hürthle cells, and variable degree of fibrosis. May be positive for IgG4 | Noncaseating granulomas, neutrophils, and giant cells |
| Respond to corticosteroids or NSAIDs | Poor | Good |
Abbreviations: CRP, C-reactive protein; ESR, erythrocyte sedimentation rate, F, female; IgG4, immunoglobulin G 4; M, male; NSAIDs, nonsteroidal anti-inflammatory drugs.
a Variable stands for any possible results, from low, normal, to high, compared to reference range.
G. Medical Treatment
No oral medications are able to provide good sustained pain resolution. Oral corticosteroids, mainly prednisone, have been widely used for the treatment of pHT. Most cases of subacute thyroiditis were initially treated with oral corticosteroids or NSAIDs. However, pHT responds poorly to oral corticosteroids, regardless of administering a higher dosage of corticosteroids or increasing treatment duration. Corticosteroid treatment did not produce satisfactory pain resolution in either group. Only 25% to 50% of patients reported pain resolution, which indicated that 50% to 75% of patients still experienced neck pain despite the administration of corticosteroids. Development of Cushing syndrome after prolonged high-dose corticosteroid use was occasionally reported (13). NSAIDs also failed to provide sustained pain control in most patients with pHT. Levothyroxine use in the absence of hypothyroidism was reported mostly in cases published before 2010. The response rate with sustained pain relief was 34.6%, similar to oral corticosteroids, which yielded a 33.3% response. Short-term use of levothyroxine at thyrotropin-suppressing doses in patients with HT can decrease the size of the thyroid gland (31). However, thyroid gland size had no effect on the level of pain in patients with pHT.
It is difficult to conclude whether any difference is observed in sustained pain resolution between oral or intrathyroidal injection because of the overall small sample size of the injection group. Intrathyroidal corticosteroid injections were first reported in 1974 (32). Ishihara et al (9) and Paja and Del Cura (27) administered intrathyroidal steroid injections of triamcinolone 40 mg in 5 patients. These resulted in a highly successful rate of pain resolution. However, repeated local injections may be required to completely resolve thyroid pain. From a cytological standpoint, Ishihara and colleagues described a looser arrangement of collagen fibers with edematous inflammation in tender areas of the thyroid that resolved rapidly after local corticosteroid injection (33). Similarly, the administration of local injection with dexamethasone for 3 months along with medical treatment was proven to prevent relapse of Graves disease in a randomized, controlled trial containing 191 patients, indicating the potential role of intrathyroidal corticosteroids in managing inflammation. None of the patients developed Cushing syndrome or overt systemic side effects (34).
H. Surgery
Interestingly, the number of thyroidectomies on patients with pHT increased after the year 2000. Total thyroidectomy was performed in patients with refractory pain after medical treatment. Total thyroidectomy successfully achieved complete pain relief without recurrence, whereas some patients who underwent subtotal or near-total thyroidectomy still had a relapse of pain. Unfortunately, no rationale was provided for patients who did not undergo total thyroidectomy. Recently, a randomized, controlled trial has reported that total thyroidectomy improved the quality of life and fatigue of patients diagnosed with HT (35). This study is applicable only to a subgroup of patients with histologically confirmed HT, with severe symptoms persisting despite being euthyroid on optimal medical treatment. The placebo effect and limited follow-up length of 18 months could possibly confound the study result.
Owing to varying levels of fibrotic tissue, successful complete surgical resection requires experienced and skillful surgeons (15, 18, 24). Despite the challenging nature of surgery for patients with pHT, postoperative complications, including laryngeal nerve injury and parathyroid gland damage, were not noted.
I. Follow-up
Among all 70 patients, only 28 patients (40%) reported duration of follow-up. The relapse of pain occurred within several weeks or up to several years later. Short follow-up of less than 1 year may not accurately detect long-term disease recurrence.
J. Pathophysiology
The cause of pain in patients with pHT was previously proposed as capsular stretching due to rapid enlargement of the thyroid (2). However, the reason why patients still experience pain even if the size of the thyroid gland is the same or even if the thyroid becomes atrophic has not yet been clarified. Thus, the mechanism of developing pain from pHT remains unknown.
A description of IgG4-positive thyroiditis characterized by rapid progression and a higher level of antithyroid antibodies was created by Li and colleagues in 2009 (36). This report was followed by several other accounts reporting this new subtype of autoimmune thyroiditis (25, 37, 38). Li et al retrospectively investigated thyroid specimens from patients who were diagnosed with HT prior to thyroidectomy. Confirmatory diagnosis can be established only by pathology, which was defined as greater than 20 IgG4-positive plasma cells per high-power field and an IgG4:IgG ratio greater than 30%. Histologically, a higher grade of fibrosis, pronounced infiltration of the lymphocytes and plasma cells, and follicular cell degeneration are observed in IgG4-positive and non–IgG4-positive thyroiditis. There was no significant difference in pain observed between patients with IgG4-positive and non–IgG4-positive thyroiditis, but comparisons were limited by a small sample size (37). Although it was reported that IgG4-positive thyroiditis responded well to corticosteroid treatment, there still were patients who underwent thyroidectomy because of pain (25, 37). In our analysis, thyroid specimens revealed various degrees of fibrotic change, but the majority of the patients reported advanced fibrosis. It may be likely that IgG4 prevalence is more common than presumed, but insufficient testing limits the diagnosis.
K. Limitations and Strengths
All the articles included in this systematic review are case reports and case series. There are no randomized, controlled trials or cohort studies of pHT in this study. This review is subject to selection and reporting biases from the observational results reported in the publications evaluated. Although there are a total of 70 reported cases, we were unable to include every case in our subgroup analyses given the lack of data for many of the studied parameters. The period of the included publications was approximately 70 years, which resulted in significant discrepancies in diagnostic tests, treatment methods, and description of cases. However, the limitations could also be considered a strength in our study. By reviewing the historical data of the past 70 years, we were able to learn the history of this rare and mysterious disease. Despite the limitation in data collection, the heterogeneity of reported cases enables the understanding of basic patient characteristics and treatment options for pHT.
L. Implications for Practice and Research
The diagnosis of pHT is based on clinical evidence of HT, including clinical presentation, thyroid function testing, thyroid autoantibodies, ultrasound features, and even histologic findings, along with recurrent or persistent thyroid pain after medical treatment. The reference standard of diagnosis is pathology. Differentiating pHT from subacute thyroiditis remains challenging on the initial presentation of pain, given that both have a high percentage of fever and elevated levels of ESR and CRP. Therefore, empiric treatment for subacute thyroiditis, including NSAIDs or salicylates for mild pain and high-dose oral prednisone at 40 mg daily tapered over 4 to 6 weeks for severe pain, is often initiated (1). If pain is not relieved after initiating prednisone therapy, a diagnosis of pHT or suppurative thyroiditis should be considered. Doppler ultrasound may aid in the diagnosis, but radioiodine or technetium imaging study has limited value in the differential diagnosis.
Table 2 summarizes the different medications and dosages that have been used in treating pHT, along with treatment outcome. Based on the results, treatment options include administering low-dose oral prednisone, less than 25 mg/day, for up to 3 months as first-line treatment for patients with pHT. If pain persists or recurs, an intrathyroidal steroid injection of 40 mg triamcinolone acetate can be considered; however, more studies are needed to determine its overall efficacy. Follow-up duration should be greater than 1 year. Total thyroidectomy should be considered in patients with relapse or insufficient pain relief by medical treatment. Surgery should be performed by experienced surgeons to avoid postoperative complications given the severe adhesion of the thyroid gland to surrounding tissues.
Further trials of intrathyroidal injection of corticosteroids will provide more evidence on its use. If possible, immunostaining of the IgG subclass of the thyroid specimens from patients with pHT may help in classifying HT. Further research in immunology and biomarkers is of paramount importance to help in understanding the pathophysiology of pHT so that targeted therapy may be developed to resolve pain. Publications of cases with less severe pain or with favorable outcomes after medical treatment should be encouraged so that treatment options can be formulated.
Acknowledgments
We thank Katie Lobner, MLIS, for her expert reference search. We also thank Susan Harman for assisting us in obtaining the original full texts of the manuscripts.
Additional Information
Disclosure Summary: No competing financial interests exist, and the authors have nothing to disclose.
References
- 1. Pearce EN, Farwell AP, Braverman LE. Thyroiditis. N Engl J Med. 2003;348(26):2646–2655. [DOI] [PubMed] [Google Scholar]
- 2. Furszyfer J, Kurland LT, Woolner LB, Elveback LR, McConahey WM. Hashimoto’s thyroiditis in Olmsted County, Minnesota, 1935 through 1967. Mayo Clin Proc. 1970;45(8):586–596. [PubMed] [Google Scholar]
- 3. Rotondi M, Capelli V, Locantore P, Pontecorvi A, Chiovato L. Painful Hashimoto’s thyroiditis: myth or reality? J Endocrinol Invest. 2017;40(8):815–818. [DOI] [PubMed] [Google Scholar]
- 4. Doniach D, Hudson RV. Lymphadenoid goitre (Hashimoto’s disease); diagnostic and biochemical aspects. Br Med J. 1957;1(5020):672–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Doniach D, Hudson RV, Roitt IM. Human auto-immune thyroiditis: clinical studies. Br Med J. 1960;1(5170):365–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fui SN, Jefferys DB. Subacute autoimmune thyroiditis simulating De Quervain’s thyroiditis. Lancet. 1979;1(8116):622. [DOI] [PubMed] [Google Scholar]
- 7. Zimmerman RS, Brennan MD, McConahey WM, Goellner JR, Gharib H. Hashimoto’s thyroiditis. An uncommon cause of painful thyroid unresponsive to corticosteroid therapy. Ann Intern Med. 1986;104(3):355–357. [DOI] [PubMed] [Google Scholar]
- 8. Gudbjörnsson B, Kristinsson A, Geirsson G, Hreidarsson AB. Painful autoimmune thyroiditis occurring on amiodarone therapy. Acta Med Scand. 1987;221(2):219–220. [DOI] [PubMed] [Google Scholar]
- 9. Ishihara T, Mori T, Waseda N, Ikekubo K, Akamizu T, Imura H. Histological, clinical and laboratory findings of acute exacerbation of Hashimoto’s thyroiditis—comparison with those of subacute granulomatous thyroiditis. Endocrinol Jpn. 1987;34(6):831–841. [DOI] [PubMed] [Google Scholar]
- 10. Leung AK, Hegde K. Hashimoto’s thyroiditis simulating De Quervain’s thyroiditis. J Adolesc Health Care. 1988;9(5):434–435. [DOI] [PubMed] [Google Scholar]
- 11. Shigemasa C, Ueta Y, Mitani Y, et al. Chronic thyroiditis with painful tender thyroid enlargement and transient thyrotoxicosis. J Clin Endocrinol Metab. 1990;70(2):385–390. [DOI] [PubMed] [Google Scholar]
- 12. Sulimani RA. Lymphocytic thyroiditis presenting as a unilateral painful goitre. East Afr Med J. 1997;74(7):458–459. [PubMed] [Google Scholar]
- 13. Gourgiotis L, Al-Zubaidi N, Skarulis MC, et al. Successful outcome after surgical management in two cases of the “painful variant” of Hashimoto’s thyroiditis. Endocr Pract. 2002;8(4):259–265. [DOI] [PubMed] [Google Scholar]
- 14. Konno S, Konno N, Yokobori M, et al. Autoimmune thyroid disease accompanied by recurring episodes of painful thyroid ameliorated by thyroidectomy. J Endocrinol Invest. 2002;25(11):996–1000. [DOI] [PubMed] [Google Scholar]
- 15. Kon YC, DeGroot LJ. Painful Hashimoto’s thyroiditis as an indication for thyroidectomy: clinical characteristics and outcome in seven patients. J Clin Endocrinol Metab. 2003;88(6):2667–2672. [DOI] [PubMed] [Google Scholar]
- 16. Ohye H, Fukata S, Kubota S, et al. Successful treatment for recurrent painful Hashimoto’s thyroiditis by total thyroidectomy. Thyroid. 2005;15(4):340–345. [DOI] [PubMed] [Google Scholar]
- 17. Ohye H, Nishihara E, Sasaki I, et al. Four cases of Graves’ disease which developed after painful Hashimoto’s thyroiditis. Intern Med. 2006;45(6):385–389. [DOI] [PubMed] [Google Scholar]
- 18. Onoda N, Kato Y, Seki T, et al. Increased thyroid blood flow in the hypoechoic lesions in patients with recurrent, painful Hashimoto’s thyroiditis at the time of acute exacerbation. Endocr J. 2009;56(1):65–72. [DOI] [PubMed] [Google Scholar]
- 19. Ipekci SH, Öztürk K, Cakir M. A difficult decision—Hashimoto’s thyroiditis or subacute thyroiditis? Turkish Journal of Endocrinology and Metabolism. 2011;( 15):125–127. [Google Scholar]
- 20. van Schaik J, Dekkers OM, van der Kleij-Corssmit EP, Romijn JA, Morreau H, van de Velde CJ. Surgical treatment for unexplained severe pain of the thyroid gland: report of three cases and concise review of the literature. Case Rep Med. 2011;2011:349756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Seo HM, Kim M, Bae J, et al. A case of painful hashimoto thyroiditis that mimicked subacute thyroiditis. Chonnam Med J. 2012;48(1):69–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Visser R, de Mast Q, Netea-Maier RT, van der Ven AJ. Hashimoto’s thyroiditis presenting as acute painful thyroiditis and as a manifestation of an immune reconstitution inflammatory syndrome in a human immunodeficiency virus-seropositive patient. Thyroid. 2012;22(8):853–855. [DOI] [PubMed] [Google Scholar]
- 23. Kashyap L, Alsaheel A, Walvekar R, Simon L, Gomez R. A rare case of painful goiter secondary to pediatric Hashimoto’s thyroiditis requiring thyroidectomy for pain control. Pediatr Rep. 2015;7(3):5914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mazza E, Quaglino F, Suriani A, et al. Thyroidectomy for painful thyroiditis resistant to steroid treatment: three new cases with review of the literature. Case Rep Endocrinol. 2015;2015:138327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lee IS, Lee JU, Lee KJ, Jang YS, Lee JM, Kim HS. Painful immunoglobulin G4-related thyroiditis treated by total thyroidectomy. Korean J Intern Med. 2016;31(2):399–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Krakovitz P, Cairns C, Schweiger BM, Burkey B. Surgical management of neck pain and headache associated with pediatric Hashimoto’s thyroiditis. Laryngoscope. 2018;128(9):2213–2217. [DOI] [PubMed] [Google Scholar]
- 27. Paja M, Del Cura JL. Successful treatment of painful Hashimoto’s thyroiditis with intrathyroidal injection of glucocorticoid in two patients. Endocrinol Diabetes Nutr. 2018;65(9):546–547. [DOI] [PubMed] [Google Scholar]
- 28. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Plos Med. 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Higgins JPT, Green Sally, eds. . Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration; 2011. www.handbook.cochrane.org. Accessed May 1, 2019. [Google Scholar]
- 30. Tsuboi K, Yuasa R, Tanaka Y, Ueshiba H, Takeda S, Ito K. Incidence of thyroid atrophy in patients with Hashimoto thyroiditis. In: Nagataki S, Mori T, Torizuka K, eds. 80 Years of Hashimoto Disease. Amsterdam: Elsevier Science; 1993:69–72. [Google Scholar]
- 31. Dayan CM, Daniels GH. Chronic autoimmune thyroiditis. N Engl J Med. 1996;335(2):99–107. [DOI] [PubMed] [Google Scholar]
- 32. Nagata I, Aoki N, Wakisaka G. Treatment of thyroid diseases with intrathyroidal injection of glucocorticoid [in Japanese]. Nihon Naibunpi Gakkai Zasshi. 1974;50(4):774–787. [DOI] [PubMed] [Google Scholar]
- 33. Ishihara T, Mori T, Waseda N, Ikekubo K, Akamizu T, Imura H. Pathological characteristics of acute exacerbation of Hashimoto’s thyroiditis—serial changes in a patient with repeated episodes. Endocrinol Jpn. 1986;33(5):701–712. [DOI] [PubMed] [Google Scholar]
- 34. Mao XM, Li HQ, Li Q, et al. Prevention of relapse of Graves’ disease by treatment with an intrathyroid injection of dexamethasone. J Clin Endocrinol Metab. 2009;94(12):4984–4991. [DOI] [PubMed] [Google Scholar]
- 35. Guldvog I, Reitsma LC, Johnsen L, et al. Thyroidectomy versus medical management for euthyroid patients with Hashimoto disease and persisting symptoms: a randomized trial. Ann Intern Med. 2019;170(7):453–464. [DOI] [PubMed] [Google Scholar]
- 36. Li Y, Bai Y, Liu Z, et al. Immunohistochemistry of IgG4 can help subclassify Hashimoto’s autoimmune thyroiditis. Pathol Int. 2009;59(9):636–641. [DOI] [PubMed] [Google Scholar]
- 37. Li Y, Nishihara E, Hirokawa M, Taniguchi E, Miyauchi A, Kakudo K. Distinct clinical, serological, and sonographic characteristics of Hashimoto’s thyroiditis based with and without IgG4-positive plasma cells. J Clin Endocrinol Metab. 2010;95(3):1309–1317. [DOI] [PubMed] [Google Scholar]
- 38. Zhang J, Zhao L, Gao Y, et al. A classification of Hashimoto’s thyroiditis based on immunohistochemistry for IgG4 and IgG. Thyroid. 2014;24(2):364–370. [DOI] [PubMed] [Google Scholar]