Abstract
Aim
To evaluate the accuracy of our new rapid point‐of‐care (POC) test for lung maturity. The method as we describe in an accompanying article was developed with the purpose of improving the outcome from respiratory distress syndrome (RDS). The test enables the delivery of surfactant in infants with immature lungs already at birth and ensures that infants with mature lungs are not treated unnecessarily.
Methods
Fresh gastric aspirate (GAS) was sampled at birth in a cohort of preterm infants with gestational ages ranging between 24 and 31 completed weeks for lung surfactant measurement as lecithin–sphingomyelin ratio (L/S). L/S was prospectively compared with RDS development. The clinical outcome was blinded for the investigators of L/S. The time for analysis was <15 minutes.
Results
GAS was obtained from 72 infants. Forty‐four (61%) developed RDS. The cut‐off for L/S was 3.05; predicting RDS with a sensitivity of 91% and specificity of 79%.
Conclusion
The new improved spectroscopic L/S method of lung maturity on GAS has high sensitivity. The method is designed for use as a POC test at birth, and a spectroscopic prototype has been developed for bedside use. Clinical trials with this new lung maturity test are planned.
Keywords: Gastric aspirate, Lung surfactant, Mid‐infrared spectroscopy, Prematurity, Respiratory distress syndrome
Abbreviations
- CI
Confidence interval
- FiO2
Fraction of oxygen in inspired gas
- FTIR
Fourier transform infrared spectroscopy
- GA
Gestational age
- GAS
Gastric aspirate
- IQR
Interquartile range
- L/S
Lecithin–sphingomyelin ratio
- LUS
Lung ultrasound score
- nCPAP
Nasal continuous positive airway pressure
- POC
Point of care
- RDS
Respiratory distress syndrome
Key Notes.
Respiratory distress syndrome (RDS) is a major cause of mortality and morbidity in premature infants, early targeted surfactant treatment improves outcome.
We have developed a spectroscopic point‐of‐care test to measure lung maturity as lecithin–sphingomyelin ratio (L/S) in gastric aspirate within the first hour of life.
L/S was closely correlated to the development of RDS in 72 very preterm infants, and clinical trials with L/S‐guided surfactant treatment are planned.
Introduction
Treatment of respiratory distress syndrome (RDS) has evolved greatly over the past three decades. Major advances in treatment include antenatal steroids, early nasal continuous positive airway pressure (nCPAP) combined with early rescue surfactant replacement strategies such as Intubation Surfactant Extubation (INSURE) 1, 2, 3 and Less Invasive Surfactant Administration (LISA) 3, 4, 5, together with use of lung protective ventilation and overall reduced use of mechanical ventilation. However, RDS and bronchopulmonary dysplasia (BPD) are still major causes of mortality and morbidity in premature infants 3, 6. To improve the outcome, very early treatment with surfactant is necessary 7. However, only about half of infants with a gestational age (GA) below 30 weeks need surfactant treatment 8, 9 and prophylactic surfactant treatment increases the combined mortality and incidence of BPD contrary to selective rescue surfactant treatment 10. Therefore, there is a need for a rapid test to guide early targeted surfactant treatment 6, 11.
Several invasive prenatal tests based on amniotic fluid such as foam tests, click tests, drop volume methods and thin‐layer chromatography of phospholipids have been developed over time and have been used to asses lung maturity before induced deliveries. For an overview see 12. With the introduction of surfactant treatment for RDS, additional lung maturity tests based on gastric aspirates (GAS) were developed; for example, the microbubble stability test 12, 13, 14, 15 and lamellar body counts 16, 17, 18, 19 and our group have previously published a large randomised trial using lamellar body counts to guide surfactant treatment 19. However, a common problem with all these methods is dilution with foetal urine. Additionally, the methods are time‐consuming laboratory tests and are too slow to be used as a point‐of‐care test (POC) to guide surfactant treatment.
The sphingomyelin concentration in amniotic fluid and accordingly in GAS is relatively constant during the pregnancy, whereas the lecithin concentration increases with the lung maturation. Thus, when measuring lecithin–sphingomyelin ratio (L/S) in GAS, the problem with dilution is avoided 20. The most promising method has been based on mid‐red Fourier Transform Infrared spectroscopy (FTIR) 20. Although this method demonstrated a high sensitivity, it was developed using frozen and thawed GAS. We have now improved this method for use as a POC test, optimising it for fresh GAS. The new method combines dry transmission spectroscopy with concentration of lung surfactant by centrifugation of the lamellar bodies and dilution of the proteins. Thereby, we obtain a spectroscopic amplification of the surfactant signal and omitting interference from proteins and cells. The new method is described in detail in the accompanying article in this number of Acta Paediatrica 21.
In this article, we describe an observational clinical study in premature infants in which the clinical course of RDS is compared with L/S measured at delivery with the new method.
Patients and methods
The study was a multicentre non‐intervention prospective diagnostic cohort study. The trial was approved by the regional research ethics committee. Informed oral and written parental consent was obtained either before or immediately after birth.
Infants with a GA from 24 to 31 completed weeks were eligible. GAS should be sampled as quickly as possible within 45 minutes after birth, as the lamellar body (surfactant) count has been shown to remain stable in GAS up to a maximum of 45 minutes postpartum 18.
Exclusion criteria were lethal malformations, therapeutic infusion into the amniotic cavity, and contamination of GAS by pus or if GAS could not be obtained before surfactant replacement therapy.
Participation in the study did not interfere with routine neonatal care. Monitoring and treatment including surfactant replacement therapy in all cases was done in accordance with the European RDS guidelines 6. Accordingly, rescue treatment with surfactant was done in infants less than 26 weeks’ gestation when the fraction of inspired oxygen (FiO2) requirements increased to more than 0.30 and in infants above 26 weeks’ gestation when FiO2 requirements increased to more than 0.40. Immediate treatment with nCPAP was the primary method of respiratory stabilisation at birth, and intubation was only carried out for resuscitation. To compare the L/S values with the development of RDS, the included infants were observed for the first five days of life, as this is the known period before the surfactant layer is restored after RDS as described by Avery among others 22. Relevant clinical data were secured including FiO2, which was registered at predefined time‐points. This also includes factors which possibly could influence development of RDS and surfactant analyses such as multiple births, asphyxia, antenatal steroid and pre‐existing or gestational diabetes.
RDS was diagnosed when at least two of the four classical symptoms were present: the need for supplemental oxygen, tachypnoea, intercostal retractions and grunting, or chest x‐ray consistent with RDS excluding other causes of respiratory distress. The severity of RDS was graded in four categories as in our previous studies 12, 20 with minor modifications. It was graded as Non‐RDS if the FiO2 was < 0.40 in a maximum of six hours after birth, but with short periods of supplemental oxygen allowed later. Mild RDS was when FiO2 was 0.22–0.40 for more than six hours after birth without need for surfactant or mechanical ventilation. Moderate RDS when there was a need for surfactant or FiO2 > 0.40, but no mechanical ventilation. Severe RDS was if there was need for mechanical ventilation within the study period.
Sampling of GAS and L/S analysis
GAS (0.3–2.5 mL) was collected using a feeding tube attached to a syringe or a suction catheter connected to a tracheal suction set. The feeding tube or suction catheters were placed as routinely done while establishing nCPAP for respiratory stabilisation or intubation for resuscitation.
GAS was stored at 4–5°C for subsequent L/S analysis performed as described in our accompanying article in this number of Acta Paediatrica and without knowledge of the clinical outcome 21.
Statistical analysis
Because the method is new, sample size calculation was not possible. Based on our previous trial 20, we estimated that 70 infants with a GA of 24–31 completed weeks (of which at least 30 would develop RDS) would be sufficient to evaluate the new method.
Data were expressed as medians (interquartile range). A cut‐off value for L/S that maximised the sum of sensitivity and specificity to diagnose RDS was determined 23. The 95% confidence intervals (CI) for sensitivity and specificity were expressed. In addition, the L/S data were stratified based on GA of 24–25, 26–29 and 30–31 weeks. Pearson's chi‐square test was used to compare the accuracy of RDS prediction from L/S versus gestational age and to examine the possible influence on the accuracy of L/S from the mode of delivery, multiple gestation, antenatal steroids, maternal diabetes and asphyxia (Apgar < 8 at 5 minutes).
Analyses were performed in SPSS version 22, and two‐tailed p values of < 0.05 were considered to indicate statistical significance.
Results
Infants were enrolled from September 2017 to April 2018 from seven Danish neonatal units. GAS was obtained from 76 infants. Four infants were excluded because GAS had been frozen, leaving 72 infants in the final cohort.
Clinical characteristics of the included infants are shown in Table 1. Two infants (3%) died before the end of the five‐day study period. Forty‐four (61%) developed RDS, 12 (27%) mild, 14 (32%) moderate and 18 (41%) severe.
Table 1.
Demographic and clinical characteristics of included patients
| Characteristics | N = 72 |
|---|---|
| GA, median (IQR), week | 28.3 (26.9–30.7) |
| Birthweight, median (IQR), grams | 1061 (799–1417) |
| Caesarean section, n (%) | 51 (71) |
| In labour | 36 (71) |
| Not in labour | 15 (29) |
| Apgar score at 5 minutes, median (IQR) | 10 (9–10) |
| Umbilical pH, median (IQR) | 7.29 (7.22–7.33) |
| Antenatal steroid, n (%) | 68 (94) |
| Surfactant, n (%) | 31 (43) |
| Age in minutes when treated, median (IQR) | 240 (90–540) |
| Ventilated during first 5 days of life, n (%) | 18 (25) |
| Age in minutes, when started, median (IQR) | 330 (60–1980) |
| Dead during the first 5 days of life, n (%) | 2 (3) |
| Male gender, n (%) | 41 (57) |
| Singleton, n (%) | 54 (75) |
| Maternal diabetes, n (%) | 7 (10) |
IQR = interquartile range.
L/S from infants with RDS and non‐RDS, and the verification of the cut‐off value to predict RDS are shown in Figure 1. The optimal L/S cut‐off value was 3.05. RDS could be predicted with a sensitivity of 91% (95% CI: 78–97), specificity of 79% (95% CI: 59–92), positive predictive value of 87% (95% CI: 74–95) and negative predictive value of 85% (95% CI: 65–96). Four infants with RDS had L/S values only slightly higher than the cut‐off, from 3.2 to 3.9 (false negative). Six infants without RDS had L/S below the cut‐off value (false positive).
Figure 1.
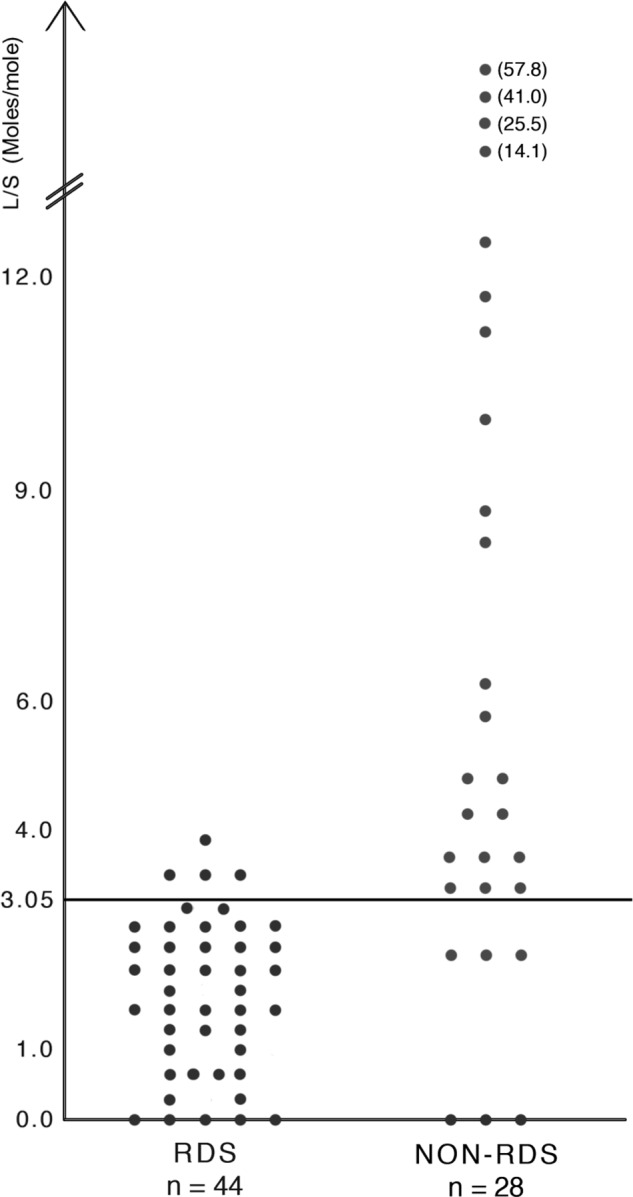
Lecithin/sphingomyelin ratio (L/S) in gastric aspirates determined by mid‐infrared spectroscopy in 44 infants with RDS and 28 infants with non‐RDS. The best cut‐off value is 3.05 moles/mole.
L/S for various degrees of RDS severity and GA are shown in Table 2. Accuracy of RDS prediction from L/S in each of the three GA groups 24–25, 26–29 and 30–31 weeks expressed as sensitivity and specificity was not statistical different, p > 0.05 for the differences. L/S predicted RDS more accurately than the GA (Table 3).
Table 2.
Gestational age and L/S‐ratio in different degrees of RDS severity
| RDS severity | N = 72 | ||
|---|---|---|---|
| n | GA (weeks) | LS‐ratio (moles/mole) | |
| Median (range) | Median(range) | ||
| Non‐RDS | 28 | 29.4 (25.7–31.7) | 4.1 (0–57.8) |
| Mild RDS | 12 | 30.4 (26.4–31.6) | 2.1 (0.6–3.6) |
| Moderate RDS | 14 | 29.0 (25.4–31.3) | 1.0 (0.0–3.0) |
| Severe RDS | 18 | 26.1 (24.3–31.1) | 1.8 (0.0–3.9) |
| RDS (all degrees) | 44 | 28.0 (24.3–31.6) | 1.9 (0.0–3.9) |
Table 3.
Comparison of L/S and gestational age for prediction of RDS
| N = 72 of which 44 developed RDS | ||
|---|---|---|
| L/S in gastric aspirate measured by FTIR | GA (week 24–31) | |
| Best cut‐off | 3.05 moles/mole | 27.6 |
| Sensitivity | 91% (95% CI: 78–97) | 48% (95% CI: 33–63) |
| Specificity | 79% (95% CI: 59–92) | 82% (95% CI: 62–93) |
| Time to diagnosis | <1 hour | At birth |
In four of seven pairs of twins, the lowest L/S was found in the second twin and in two pairs in the first twin, while in one pair L/S was the same in both twins. Prediction of RDS by L/S did not seem to be influenced by multiple gestation, antenatal steroid treatment, diabetes or asphyxia, p > 0.05 for the difference. No samples were contaminated with meconium or pus, and contamination with blood did not influence RDS prediction 21.
In the study, 32 infants with moderate and severe RDS were treated with surfactant. Surfactant was administered before 1 hour of age to six infants; at 1–5 hours of age to nine infants; at 6–9 hours of age to eight infants; at 10–24 hours of age to seven infants; and at 25–48 hours of age to two infants. None of the six infants with false‐positive L/S values (L/S lower than the cut‐off value) were treated with surfactant.
Discussion
In this observational trial, our new improved L/S method predicted the development of RDS with a high sensitivity. The method is based on fresh GAS 21 which is easy and safe to obtain in any preterm infant within minutes from birth and the L/S value takes 10–15 minutes to measure. As our method is fast and based on techniques that can be automated and do not rely on highly trained laboratory personnel, it can be developed into a POC test.
As mentioned, FiO2 was used to guide surfactant administration. Only six infants in our cohort were treated within the first hour of life and 26 from 1 to 48 hours of life. This supports the need for a lung maturity test at birth as very early selective surfactant treatment compared to late selective surfactant treatment leads to improved clinical outcome 7. In accordance with this, the European RDS guidelines also highlights the demand for a fast bedside test to measure the level of endogenous surfactant to guide surfactant treatment 6. Screening of lung maturity at birth is likely to reduce the incidence and severity of both RDS and BPD 3, 19 as it will allow infants with immature lungs to be treated with surfactant in the first hour of life, while at the same time protecting infants with mature lungs from unnecessary surfactant administration and potentially dangerous airway handling. We are currently preparing a paper in which we will describe the development of BPD in the cohort from this study, and if possible identify potential biomarkers for BPD.
De Martino et al. recently evaluated the use of a lung ultrasound score (LUS) to predict the need for surfactant therapy in a cohort of preterm infants admitted to a neonatal unit with extensive experience in lung ultrasound and found high diagnostic accuracy 24. In a still unpublished study performed in the same unit, LUS‐guided surfactant treatment when compared with treatment as per the European RDS guidelines (6) resulted in a significantly but only modestly reduced time to first surfactant administration from a median of 180 minutes to 165 minutes from birth 25. Our L/S‐test can be performed immediately at delivery, before lung damage from progression of RDS. In addition, we speculate if LUS may be less accurate when applied during transition immediately after delivery. Despite being easy to learn and with high inter‐observer agreement in skilled hands, LUS is subject to inter‐observer variability contrary to our L/S‐test and may not be as reliable in prediction of surfactant deficiency in units less proficient in lung ultrasound 26.
Surfactant activity in amniotic fluid obtained at birth measured by a surfactant adsorption test has recently been shown to correlate with lamellar body counts 18 and LUS 24 and to be able to predict CPAP failure 27. However, the method is not a POC test and the analysis takes 60–90 minutes in a laboratory. Furthermore, amniotic fluid is not easy to sample in vaginal deliveries and there is a risk of false‐positive values due to dilution of lung surfactant by foetal urine.
Other clinical factors may influence the development of RDS. For example, L/S‐ratio is often lower in the second twin compared the first‐born twin 22. This tendency was also seen in this and our previous study 20.
Observational studies including preterm infants in all age groups from 24 to 32 weeks gestation, like the one we report here, are planned in Europe and USA and have already started in China. These studies will generate big data sets allowing us to calculate the influence from various factors on RDS prediction.
Similar to our previous study, we found the L/S‐test to have lower specificity than sensitivity 20. All included infants were stabilised on nCPAP at birth which is a well‐known effective treatment of mild‐to‐moderate RDS 6. Consequently, nCPAP may diminish clinical RDS symptoms. Six infants with L/S below the cut‐off value were classified as non‐RDS and three of those needed intermittent supplemental oxygen for short periods in addition to nCPAP. This makes it likely that these infants in fact had subclinical surfactant deficiency. This finding is consistent with what we have found earlier using the microbubble stability test and lamellar body counts for early RDS diagnosis 12, 18 and results from other studies measuring lung maturity at birth 27, 28; all studies in which the included infants were treated with early nCPAP. The cut‐off value for predicting RDS was higher in the present study (3.05) compared to our previous study (2.2) 20. The main reason is most likely due to changes in the new biochemical method 21.
We are continuing our work to develop an effective and easy‐to‐use POC method based on our L/S‐test 21. We have developed a prototype of the spectroscope for bedside use and initiated clinical tests. In the next phase of our studies, we plan to conduct a randomised trial with the aims to assess the possible positive effects of the test on relevant clinical outcomes using the method to guide very early selective surfactant compared to standard treatment based on FiO2.
Our method is not limited to measurements of L/S in GAS, but can be developed further for measurements of L/S and other phospholipids in amniotic fluid, oropharyngeal fluid, tracheal fluid, for plasma measurements 29 and measurements in other human fluids.
Conclusion
Our new FTIR‐based L/S measurement of lung maturity on GAS at birth has a high sensitivity; the method is very fast and is without inter‐user variability. The method can be automated; therefore, it can potentially be developed into a very accurate POC test for use in most neonatal units as a screening test that would enable prediction of RDS in order to guide very early targeted surfactant treatment. A POC test will subsequently be tested in observational and randomised trials of L/S‐guided surfactant treatment vs. standard neonatal approach.
Funding
The project received funding from the European Union's Horizon 2020 research and Innovation programme.
Conflict of interest
Agnar Höskuldsson and Henrik Verder hold part of a patent for spectroscopic analysis of biological samples and Peter Schousboe and Henrik Verder hold part of a patent for a foetal lung maturity test based on lamellar body purification. The authors have no other conflict of interest.
Acknowledgements
We want to thank Holbaek Hospital and Region Zealand, Denmark for help and co‐operation with this project. We also want to thank the nurses and doctors in the participating neonatal departments and Vivi Kuhr Christensen, Holbaek Hospital, Denmark for creating the database in addition to participating families.
References
- 1. Verder H, Robertson B, Greisen G, Ebbesen F, Albertsen P, Lundstrøm K, et al. Surfactant therapy and nasal continuous positive airway pressure for newborns with respiratory distress syndrome. N Engl J Med 1994; 331: 1051–5. [DOI] [PubMed] [Google Scholar]
- 2. Blennow M, Jonsson B, Dahlstrom A, Sarman I, Bohlin K, Robertson B. Lung function in premature infants can be improved. Surfactant therapy and CPAP reduce the need of respiratory support. Läkartidningen 1999; 96: 1571–6. [PubMed] [Google Scholar]
- 3. Isayama T, Iwami H, McDonald S, Beyene J. Association of noninvasive ventilation strategies with mortality and bronchopulmonary dysplasia among preterm infants: a systematic review and meta‐analysis. JAMA 2016; 316: 611–24. [DOI] [PubMed] [Google Scholar]
- 4. Kribs A. Early administration of surfactant in spontaneous breathing with nCPAP through a thin endotracheal catheter—An option in the treatment of RDS in ELBW infants? J Perinatol 2009; 29: 256. [DOI] [PubMed] [Google Scholar]
- 5. Herting E. Less invasive surfactant administration (LISA) ‐ ways to deliver surfactant in spontaneously breathing infants. Early Hum Dev 2013; 89: 875–80. [DOI] [PubMed] [Google Scholar]
- 6. Sweet DG, Carnielli V, Greisen G, Hallman M, Ozek E, Plavka R, et al. European consensus guidelines on the management of respiratory distress syndrome ‐ 2016 update. Neonatology 2017; 111: 107–25. [DOI] [PubMed] [Google Scholar]
- 7. Bahadue FL, Soll R. Early versus delayed selective surfactant treatment for neonatal respiratory distress syndrome. Cochrane Database Syst Rev 2012; 11: CD001456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sandri F, Plavka R, Ancora G, Simeoni U, Stranak Z, Martinelli S, et al. Prophylactic or early selective surfactant combined with nCPAP in very preterm infants. Pediatrics 2010; 125: e1402–9. [DOI] [PubMed] [Google Scholar]
- 9. Bevilacqua G, Parmigiani S, Robertson B. Prophylaxis of respiratory distress syndrome by treatment with modified porcine surfactant at birth: a multicentre prospective randomized trial. J Perinat Med 1996; 24: 609–20. [DOI] [PubMed] [Google Scholar]
- 10. Soll R. Prophylactic versus selective use of surfactant in prevention of morbidity and mortality in preterm infants. Cochrane Rev Update Neonatol 2012; 102: 169–71. [Google Scholar]
- 11. Kearl CR, Young L, Soll R. Surfactant therapy guided by tests for lung maturity in preterm infants at risk of respiratory distress syndrome. Cochrane Database Syst Rev 2018; 11: CD013158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Verder H, Ebbesen F, Linderholm B, Robertson B, Eschen C, Arrøe M, et al. Prediction of respiratory distress syndrome by the microbubble stability test on gastric aspirates in newborns of less than 32 weeks’ gestation. Acta Paediatr 2003; 92: 728–33. [DOI] [PubMed] [Google Scholar]
- 13. Berggren P, Eklind J, Linderholm B, Robertson B. Bubbles and computer‐aided image analysis for evaluation of surfactant inhibition. Biol Neonate 1992; 61(Suppl 1): 15–20. [DOI] [PubMed] [Google Scholar]
- 14. Chida S, Fujiwara T, Konishi M, Takahashi H, Sasaki M. Stable microbubble test for predicting the risk of respiratory distress syndrome: II. prospective evaluation of the test on amniotic fluid and gastric aspirate. Eur J Pediatr 1993; 152: 152–6. [DOI] [PubMed] [Google Scholar]
- 15. Fiori HH, Linderholm B, Fiori RM, Robertson B. Computerized image analysis of bubbles in gastric aspirate for prediction of respiratory distress syndrome. Acta Paediatr 2001; 90: 1402–4. [DOI] [PubMed] [Google Scholar]
- 16. Stucin‐Gantar I, Babnik J. Surfactant inhibitors in amniotic fluid from gastric aspirates in premature infants with respiratory distress syndrome. Biol Neonate 1999; 76(Suppl 1): 43–4. [Google Scholar]
- 17. Daniel IW, Fiori HH, Piva JP, Munhoz TP, Nectoux AV, Fiori RM. Lamellar body count and stable microbubble test on gastric aspirates from preterm infants for the diagnosis of respiratory distress syndrome. Neonatology 2010; 98: 150–5. [DOI] [PubMed] [Google Scholar]
- 18. Verder H, Ebbesen F, Brandt J, Dahl M, Esberg G, Eschen C, et al. Lamellar body counts on gastric aspirates for prediction of respiratory distress syndrome. Acta Paediatr 2011; 100: 175–80. [DOI] [PubMed] [Google Scholar]
- 19. Verder H, Ebbesen F, Fenger‐Grøn J, Henriksen TB, Andreasson B, Bender L, et al. Early surfactant guided by lamellar body counts on gastric aspirate in very preterm infants. Neonatology 2013; 104: 116–22. [DOI] [PubMed] [Google Scholar]
- 20. Verder H, Heiring C, Clark H, Sweet D, Jessen TE, Ebbesen F, et al. Rapid test for lung maturity, based on spectroscopy of gastric aspirate, predicted respiratory distress syndrome with high sensitivity. Acta Paediatr 2017; 106: 430–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Schousboe P, Verder H, Jessen T, Heiring C, Bender L, Dahl M, et al. Predicting respiratory distress syndrome at birth using a fast test based on spectroscopy of gastric aspirates. Acta Paediatr 2019. [DOI] [PubMed] [Google Scholar]
- 22. Avery M. The lung and its disorders in the newborn infant: volume I in the series In Schaffer AJ, editor. Major problems in clinical pediatrics. 2nd ed Philadelphia, London, Toronto: WB Saunders, 1968: 151–4. [PubMed] [Google Scholar]
- 23. Altman DG. Practical statistics for medical research. London, U.K.: Chapman & Hall London, 1991. [Google Scholar]
- 24. De Martino L, Yousef N, Ben‐Ammar R, Raimondi F, Shankar‐Aguilera S, De Luca D. Lung ultrasound score predicts surfactant need in extremely preterm neonates. Pediatrics 2018; 142: e20180463. [DOI] [PubMed] [Google Scholar]
- 25. Raschietti R, V Dell'Oro V, Ben‐Ammar R, Shankar‐Aguilera S, Montané A, Youssef N, et al. Echo‐guided surfactant therapy (ESTHER) in preterm neonates with respiratory distress syndrome: A pilot study. 7th congress of the European Academy of Paediatric Societies 2018.
- 26. Brat R, Yousef N, Klifa R, Reynaud S, Aguilera SS, De Luca D. Lung ultrasonography score to evaluate oxygenation and surfactant need in neonates treated with continuous positive airway pressure. JAMA Pediatr 2015; 169: e151797. [DOI] [PubMed] [Google Scholar]
- 27. Autilio C, Echaide M, Benachi A, Marfaing‐Koka A, Capoluongo ED, Perez‐Gil J, et al. A noninvasive surfactant adsorption test predicting the need for surfactant therapy in preterm infants treated with continuous positive airway pressure. J Pediatr 2017; 182: 66 – 73. [DOI] [PubMed] [Google Scholar]
- 28. Stichtenoth G, Walter G, Lange R, Raith M, Bernhard W, Herting E. Surface tension of airway aspirates withdrawn during neonatal resuscitation reflects lung maturity. Pediatr Pulmonol 2014; 49: 751–6. [DOI] [PubMed] [Google Scholar]
- 29. Jessen TE, Höskuldsson AT, Bjerrum PJ, Verder H, Sørensen L, Bratholm PS, et al. Simultaneous determination of glucose, triglycerides, urea, cholesterol, albumin and total protein in human plasma by fourier transform infrared spectroscopy: direct clinical biochemistry without reagents. Clin Biochem 2014; 47: 1306–12. [DOI] [PubMed] [Google Scholar]


