Abstract
The late endosomes/endo‐lysosomes of vertebrates contain an atypical phospholipid, lysobisphosphatidic acid (LBPA) (also termed bis[monoacylglycero]phosphate [BMP]), which is not detected elsewhere in the cell. LBPA is abundant in the membrane system present in the lumen of this compartment, including intralumenal vesicles (ILVs). In this review, the current knowledge on LBPA and LBPA‐containing membranes will be summarized, and their role in the control of endosomal cholesterol will be outlined. Some speculations will also be made on how this system may be overwhelmed in the cholesterol storage disorder Niemann‐Pick C. Then, the roles of intralumenal membranes in endo‐lysosomal dynamics and functions will be discussed in broader terms. Likewise, the mechanisms that drive the biogenesis of intralumenal membranes, including ESCRTs, will also be discussed, as well as their diverse composition and fate, including degradation in lysosomes and secretion as exosomes. This review will also discuss how intralumenal membranes are hijacked by pathogenic agents during intoxication and infection, and what is the biochemical composition and function of the intra‐endosomal lumenal milieu. Finally, this review will allude to the size limitations imposed on intralumenal vesicle functions and speculate on the possible role of LBPA as calcium chelator in the acidic calcium stores of endo‐lysosomes.
Keywords: ALIX, anthrax, bis(monoacylglycero)phosphate BMP, calcium store, cholesterol, enveloped virus, ESCRTs, exosome, intralumenal vesicle ILV, lipidomics, lysobisphosphatidic acid, lysosome, lysosome storage disease, multivesicular endosome, Niemann‐pick C, pathogen, penetration, toxin
Multivesicular late endosomes contain the atypical phospholipid LBPA, which is abundant in intralumenal membranes/vesicles (ILVs). The role of LBPA will be discussed, as well as ILV functions and mechanisms that drive ILV biogenesis in light of their diverse composition and fate. The review will also explore how ILVs are hijacked by pathogens, and discuss the biochemical composition of the lumenal milieu, the size limitations imposed on ILV functions and possible roles of LBPA as calcium chelator in acidic calcium stores.
1. SETTING THE STAGE: THE ENDOSOMAL SYSTEM
The endosomes of eukaryotic cells are at center stage in controlling the reutilization vs degradation of membrane components, and thus regulate fundamental cellular processes in nutrient uptake, immunity, signaling, adhesion, membrane turnover and development. Components that have been endocytosed by several pathways are delivered to a common early endosome, from where some lipids and proteins, including housekeeping receptors, are recycled back to the plasma membrane (Figure 1), and others are routed by retrograde transport to the trans‐Golgi network (TGN).1, 2, 3 By contrast, molecules that are destined for late endosomes and lysosomes, including activated signaling receptors, are selectively sorted into lumenal invaginations, which are pinched off as free cargo‐containing intralumenal vesicles (ILVs). These multivesicular regions detach or mature from early endosomes and become multivesicular endosomes (or multivesicular bodies) that transport cargoes toward late endosomes and lysosomes.1, 2, 4
Figure 1.

Outline of the endocytic pathway. Organization of the endosomal pathway in mammalian cells, but not in yeast or plant cells.2 Endocytosed components are delivered to a common early endosome, from where some proteins and lipids are recycled back to the plasma membrane, or routed by retrograde transport to the trans‐Golgi network. Molecules destined for late endosomes are sorted into ILVs forming on early endosomal membranes, giving rise to multivesicular endosomes. These detach (or mature) from early endosomes and transports cargoes toward late endosomes and lysosomes. Eventually, some ILVs are delivered to lysosomes where they are degraded together with their protein cargo. Late endosomes and lysosomes exchange membrane components and solutes, forming a transient hybrid endo‐lysosome, which is then re‐converted into secondary lysosomes, where hydrolases are stored. Endosomes and lysosomes can also undergo fusion with the plasma membrane as secretory endo‐lysosomes, and ILVs can also be released extracellularly as exosomes. The endosomal pathway also serves as an input or output for other membrane trafficking pathways, as indicated. In particular, endosomes and lysosomes also function at a crossroad with the autophagy pathway, and engage in physical contacts via membrane contact sites with other organelles, including the endoplasmic reticulum
Late endosomes and lysosomes rapidly exchange membrane components and solutes in vivo leading to the prevailing notion that, upon fusion, they form a transient hybrid endo‐lysosome, which is then re‐converted into secondary lysosomes, where hydrolases are stored1, 2, 5, 6 (Figure 1). As a result, in this network, the net distinction between late endosomes, endo‐lysosomes and lysosomes is often blurred.2 Late endosomes also function at a crossroad with the autophagy pathway, which, in addition to endocytosis and TGN‐derived traffic, provides an additional entry point in the endocytic pathway for the degradation of cytoplasmic material, including organelles.7, 8, 9 In addition, endosomes engage in physical contacts with other organelles, including in particular the endoplasmic reticulum, via membrane contact sites that play a key role in lipid movement, calcium exchange and endosome dynamics.10, 11, 12, 13, 14
Endosomes and lysosomes can also acquire the capacity to fuse with the plasma membrane as secretory endo‐lysosomes—a process reminiscent of the regulated exocytosis of lysosome‐related organelles in specialized cell types.15, 16, 17 As a consequence, ILVs not only mediate protein and lipid transport to lysosomes for degradation, but can also be released extracellularly as exosomes, which package cellular molecules that, upon delivery to target cells, regulate a wide range of functions at a distance from the exosome‐secreting cell.18, 19, 20, 21 ILVs may also meet additional fates in specialized cell types,22 and contribute to the biogenesis of melanosomes in melanocytes,23, 24 or harbor MHC class II molecules loaded with peptides for presentation at the plasma membrane in antigen‐presenting cells.25, 26, 27 They may also undergo back‐fusion with the endosome limiting membrane28, 29, 30—as do exosomes after endocytosis by the target cell.31 Well‐integrated with the above functions, late endosomes serve as key sensing/signaling platforms that inform the cell about the cell nutrient situation.2, 32, 33
2. AN ATYPICAL LIPID WITH TWO NAMES
Lysobisphosphatidic acid (LBPA) was discovered as a structural isomer of phosphatidyl glycerol (PG) in 1967 by Body and Gray34 (Figure 2), close to a decade after PG,35 and a century after the description of the first phospholipid (lecithin or phosphatidyl choline).36, 37 Soon after its discovery, it was found that LBPA accumulates in the lysosomal storage disease Niemann‐Pick at a time when a precise diagnosis of this lipidosis was uncertain,38 and later that the lipid is enriched in rat liver lysosomes.39, 40 In the early 70s, LBPA was re‐named bis(monoacylglycero)phosphate (bis[MAG]P or today BMP)39 —a name unfortunately easily confused with the cognate bone morphogenetic factor (≈ 17.000 citations in PubMed).
Figure 2.
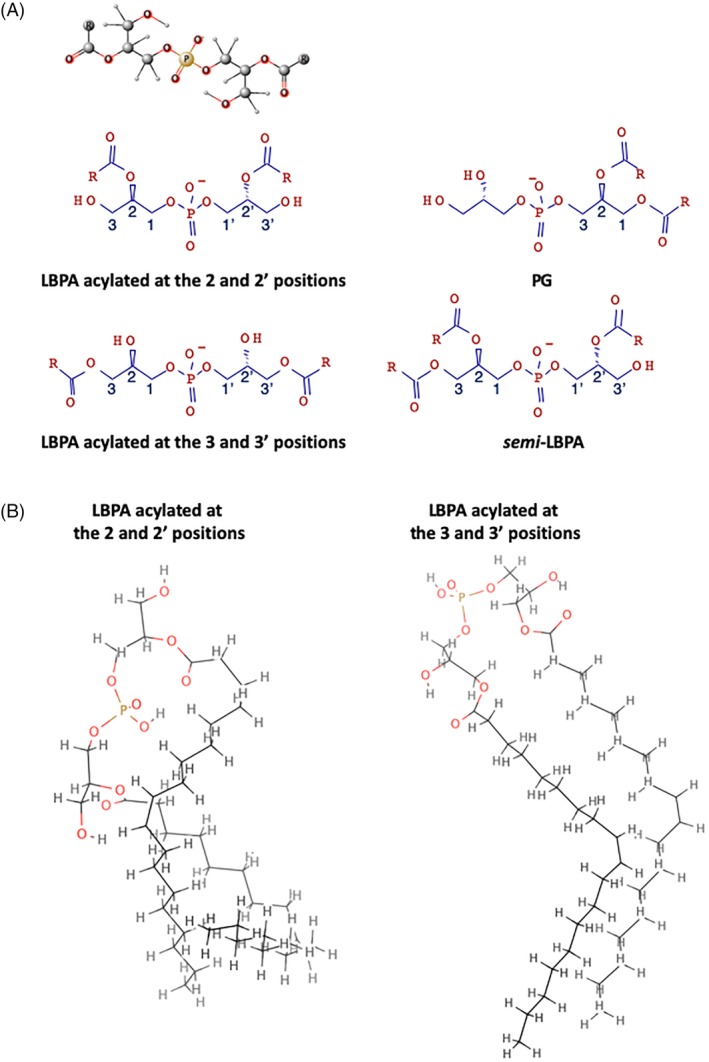
LBPA and isoforms. A, LBPA vs PG and LBPA isoforms. The ball‐and‐stick model of LBPA acylated at the 2 and 2′ positions is shown on top of the figure, above the schematic representations of the same isoform, as well as LBPA acylated at the 3 and 3′ positions, PG and semi‐LBPA. B, LBPA acylated at the 2 and 2′ positions vs LBPA acylated at the 3 and 3′ positions. The outlines show the atomistic description by molecular dynamics at the quantum mechanical level of two of the lowest energy conformers for both 2,2’‐LBPA and 3,3’‐LBPA83
LBPA seems to be ubiquitously distributed in all mammalian cells and tissues of high eukaryotic cells. However, with the possible exception of Dictyostelium,41 the lipid has not been detected in lower eukaryotes, including yeast. Prokaryotes42 and perhaps plants,43 however, contain the related lipid, acyl‐PG. Immunofluorescence and immunogold labeling of cryosections using a monoclonal antibody against LBPA revealed that the lipid is present exclusively in multivesicular regions of late endosomes and abundant in intralumenal membranes (Figure 3), a distribution further confirmed by subcellular fractionation.44, 45, 46 This distribution is consistent with the original finding—before endosomes had been characterized47—that LBPA is present in lysosomes.39, 40 While LBPA is a minor cellular lipid, it is abundant in these late endocytic compartments (late endosomes or endo‐lysosomes), where it may account for 15‐20 mol% of total phospholipids.39, 40, 44 This distribution is unique, because other phospholipids, in contrast to phosphoinositides,48 are not restricted to a subset of membranes of endocytic and secretory organelles, even though their relative abundance varies between organelles or membrane domains.49, 50, 51
Figure 3.
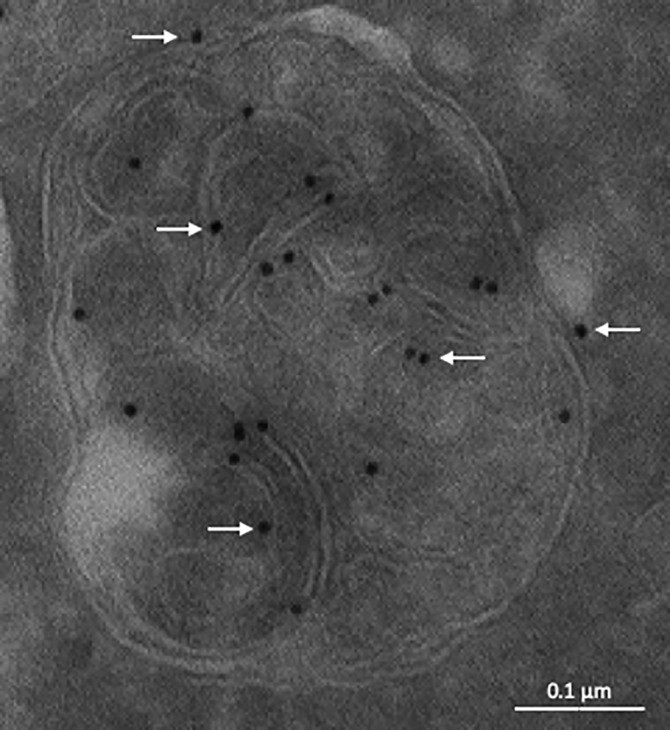
Distribution of LBPA in late endosomes illustrated by immunogold labeling of cryosections. The electron micrograph shows a late endosome of HeLa cells labeled with the anti‐LBPA monoclonal antibody 6C4, followed by 10 nm protein A‐gold (arrows). Bar: 0.1 μm. [Courtesy of Robert G. Parton, Brisbane, Australia]
2.1. Stereo‐configuration and biosynthesis
LBPA is an unconventional phospholipid not only because of its restricted distribution, but also because it exhibits a unique sn‐1‐glycerophosphate‐sn‐1′‐glycerol (sn‐1:sn‐1′) stereo‐configuration52, 53, 54 (Figure 2). LBPA is thus a poor substrate for most phospholipases,46, 55 and a perfect candidate to reside in the degradative environment of late endocytic compartments. However, despite its unusual headgroup and acyl chain organization, LBPA does not act like a detergent and has properties similar to other phospholipids.56
Both the unconventional stereo‐configuration and sub‐cellular distribution raise the issue of LBPA metabolism. In contrast to other phospholipids of the vacuolar apparatus that are synthesized in the early secretory pathway, LBPA is believed to be synthesized in late endocytic compartments from a phospholipid precursor. In vitro57 and in vivo58 observations have led to the notion that LBPA may be synthesized from phosphatidyl glycerol (PG), and that sn‐3‐PG could be converted into sn‐1:sn‐1′‐LBPA through a complex series of enzymatic reactions.59, 60 Since then, it has been shown that PG, but not cardiolipin, is indeed an LBPA precursor, but the biosynthetic pathway remains unclear.61 PG is synthesized in and confined to mitochondria—like cardiolipin that is synthesized from PG.49, 62 This raises the interesting possibility that mitochondrial PG may become available as LBPA precursor in late endocytic compartments through mitophagy.63
2.2. Trans‐bilayer distribution
The enzymes that mediate LBPA biosynthesis—or conversion from PG—should be present in the endosome lumen, a situation that may contribute to explain the restricted distribution of LBPA to late endocytic compartments. Newly synthesized LBPA is thus expected to be asymmetrically inserted into the exoplasmic leaflet of late endosomal membranes, including presumably ILV and limiting membranes. The presence of LBPA in the exoplasmic leaflet of the bilayer is consistent with observations that it binds endocytosed function‐blocking anti‐LBPA antibodies.44, 64, 65, 66, 67, 68, 69, 70, 71 Similarly, LBPA‐rich membranes may also serve as antigen for endocytosed antibodies associated with the antiphospholipid syndrome44, 65, 70 perhaps via beta(2)‐glycoprotein 1.72, 73
While LBPA is present in the exoplasmic leaflet of the bilayer, translocation across the bilayer to the cytoplasmic leaflet must occur because the lipid also interacts with the cytosolic ESCRT‐protein ALIX.74, 75 So far, no LBPA flippase has been identified. However, like other negatively‐charged phospholipids, LBPA may rapidly flip across the membrane if the charge were neutralized at low pH.76 The close proximity of the headgroups because of LBPA self‐assembly or clustering46 may cause partial protonation of proximal LBPA phosphate groups and transbilayer redistribution of the protonated form.77, 78 In turn, this may drive membrane shape changes, consistent with the capacity of LBPA to deform the bilayer in a pH‐dependent fashion74—keeping in mind that the redistribution of a very small fraction of phospholipids (< 0.1%) can induce significant shape changes.78 The unique features of LBPA are also illustrated by observations that, at the acidic late endosome pH, LBPA promotes liposome and virus fusion in vitro.46, 79 LBPA is thus present in both leaflets of the bilayer and on both ILVs and limiting membranes (Figure 3)—albeit more abundant in intralumenal membranes—and yet it is restricted to the multivesicular regions of late endosomes. Presumably, LBPA is preferentially incorporated into forming ILVs and may in fact play a direct role in ILV biogenesis74 (see also below), preventing LBPA redistribution to other membranes and ensuring replenishment of the lumenal content.
2.3. Acyl chain composition
In several cell‐types, LBPA is predominantly present as dioleoyl isoform (50%‐80%),46, 80 but the acyl chain composition of LBPA in rat liver and brain is more complex, including long polyunsaturated acyl chains.81 In vivo, acyl chains are predominantly present on the 2 and 2′ positions of the LBPA glycerol backbone, but these positions are unstable and the acyl chains can migrate to the 3,3′ positions46, 82 (Figure 2). It is not known to what extent acyl chain remodeling occurs in vivo and may accompany changes occurring in the intralumenal membrane organization. However, given the fact that the structures of these isomers are significantly different83 (Figure 2), it is likely that, in addition to the composition, the position of the acyl chains on the glycerol backbone determine LBPA shape and functions, and thus endosomal membrane dynamics. In fact, the peculiar structure of LBPA combined with its organization in LBPA‐rich membrane domains likely explain LBPA antigenicity.44, 65, 70
3. LBPA‐CONTAINING MEMBRANES CONTROL ENDOSOMAL LIPIDS
LBPA‐membrane play a crucial role in controlling the fate of other lipids, in particular sphingolipids and cholesterol, which are functionally linked in health84 and in sphingolipid and cholesterol storage disorders.85, 86, 87
3.1. Glycosphingolipid and ceramide degradation
Elegant biochemical studies have shown that LBPA‐rich membranes play a crucial role in the degradation of sphingolipids. This role has been discussed in comprehensive reviews,88, 89 and will only be briefly summarized below. In this process, sphingolipids are degraded in a stepwise manner by lysosomal enzymes with the help of saposins (Sap‐A, ‐B, ‐C, ‐D and GM2‐AP) in the presence of anionic phospholipids including LBPA.90, 91, 92 In vitro experiments showed that the degradation of the ganglioside GM2 can be stimulated 100‐fold by 20 mol% LBPA in the presence of GM2‐AP93—a concentration well in the range of LBPA levels in endosomes.44
3.2. Cholesterol transport
LBPA‐rich membranes also play a crucial role in controlling the fate of endosomal cholesterol. Most cells acquire cholesterol from circulating LDL endocytosed by the LDL receptor.94 Once in late endosomes, cholesteryl esters are de‐esterified and free cholesterol is rapidly incorporated into nearby membranes,95 including LBPA‐containing membrane. Cholesterol then reaches the endosome limiting membrane and becomes available for further export to the endoplasmic reticulum for cholesterol‐sensing,96 and to other organelles including the plasma membrane.50, 97 LBPA‐membranes also regulate the flux of cholesterol through endosomes during lipid droplet biogenesis induced by Wnt.98, 99 Cholesterol transfer from endosomes to the endoplasmic reticulum may be direct12, 13 or indirect via the plasma membrane,100, 101 and likely involves nonvesicular transport routes at membrane contact sites.12, 13, 50, 95
Within endosomes, cholesterol transfer to the limiting membrane depends on the proteins Niemann‐Pick type C1 and C2, and loss‐of‐function mutations in either of these proteins result in a cholesterol storage disease.102, 103 NPC1 is a multi‐spanning protein of the limiting membrane and NPC2 a globular protein present in the lumen,104, 105 and both proteins bind cholesterol.106, 107 Structural and mutagenesis evidence indicate that cholesterol is transferred from NPC2 to NPC1, thereby facilitating export from endosomes,108, 109, 110, 111, 112, 113, 114 and atomistic simulations indicate that LBPA is required for NPC2‐membrane interactions.115 Recent studies showed that NPC2 interacts directly with LBPA and that these interactions are necessary for cholesterol trafficking from endo‐lysosomes.116, 117 In addition, endocytosed antibodies against LBPA phenocopy NPC at the cellular level.64, 66, 118 Conversely, knockdown of the LBPA partner ALIX74, 75 decreases LBPA levels and endosomal cholesterol, suggesting that LBPA becomes limiting in NPC cells.119 Consistent with this view, a high‐content drug screen revealed that the small compound thioperamide raises LBPA levels, without affecting other endosomal functions, and concomitantly reduces the cholesterol overload in cells from Niemann‐Pick type C patients and in Npc1−/− mice.81 This compound is an inverse agonist of the histamine receptors H3/H4 and accordingly LBPA levels are inversely correlated with histamine receptor expression levels, but it is not known how this receptor controls LBPA levels.81 LBPA‐membranes may thus serve as platform to accommodate endosomal cholesterol, controlling both the cholesterol storage capacity of late endosomes and the flux of cholesterol through these organelles.
3.3. LBPA in NPC cells
Elevated levels of LBPA have been found in NPC38 and other lysosomal storage diseases.120, 121, 122 This increase may reflect some specific need for LBPA, for example in sphingolipid degradation.89 Alternatively, this increase may reflect the general expansion of the endo‐lysosomal compartment in storage disorders, upon upregulation of endo‐lysosomal gene expression by the transcription factor TFEB.123, 124 Consistent with the latter view, the increase in LBPA levels in NPC cells are correlated with the general expansion of late endosome volume, protein and lipid.125 Similarly, the elevated levels of LBPA in macrophages80 may reflect the higher degradative capacity of these cells.
Eventually, the cellular attempt to compensate for the accumulation of storage materials by an increase in the endosomal system collapses under the excess load in NPC cells and presumably in other storage disorders, leading to a traffic jam and a breakdown of endosomal membrane dynamics.85, 86 Given its role in endosomal cholesterol transport,64, 98, 119 LBPA may then become limiting119—and its capacity to accommodate or buffer excess cholesterol may be overwhelmed in NPC endosomes. Moreover, a lipidomic analysis revealed that, in addition to LBPA, the amounts of the LBPA‐related, minor lipid sLBPA (semi‐lysobisphosphatidic acid)126 (Figure 2) increases dramatically in the liver of Npc1−/− mice, up to the physiological levels of LBPA itself in WT mice.81 This analysis also revealed a profound and highly selective remodeling of the acyl chain composition of both LBPA and sLBPA in NPC mice, but not of any other phospholipid81—confirming the notion that a metabolic relationship exists between LBPA and sLBPA.126 One may thus speculate that such changes reflect some additional adjustment in LBPA‐membrane chemical and physical properties to better accommodate the changes caused by cholesterol accumulation.127, 128, 129
There is no approved treatment against NPC except for Miglustat, which delays but does not arrest the progression of the disease.130 Cyclodextrins clear cholesterol storage and restore cholesterol feedback regulation in NPC mice,131, 132, 133, 134, 135 improve symptoms and survival in NPC animal models,136, 137 and decrease the neurological progression of the disease in phase 1‐2 trials in NPC patients,138 suggesting that cyclodextrins may emerge as therapeutical strategy. However, the mechanism of action is being debated.139, 140 Recent studies indicate that hydroxypropyl‐cyclodextrin acts by promoting the secretion of the endo‐lysosome content, including LBPA, via a mechanism that requires the lysosomal cation channel mucolipin‐1 (MCOLN1 or TRPML1)141 (see Figure 6), which is itself responsible for the lysosome storage disease (LSD) mucolipidosis type 4 when mutated.142 Interestingly, endo‐lysosome secretion elicited by cyclodextrin in NPC cells decreases endosomal cholesterol but not total cell cholesterol, indicating that the secreted cholesterol is presumably incorporated into the plasma membrane or released and recaptured by cells, and eventually redistributed intracellularly.141 On the whole, these data fit nicely with observations that secretory endosomes or lysosomes15 mediate the secretion of storage material in lysosome storage disorders via activation of TFEB‐family transcription factors,143, 144, 145 and that the secretion of endo/lysosome storage materials depends on MCOLN1 activation.146, 147
Figure 6.
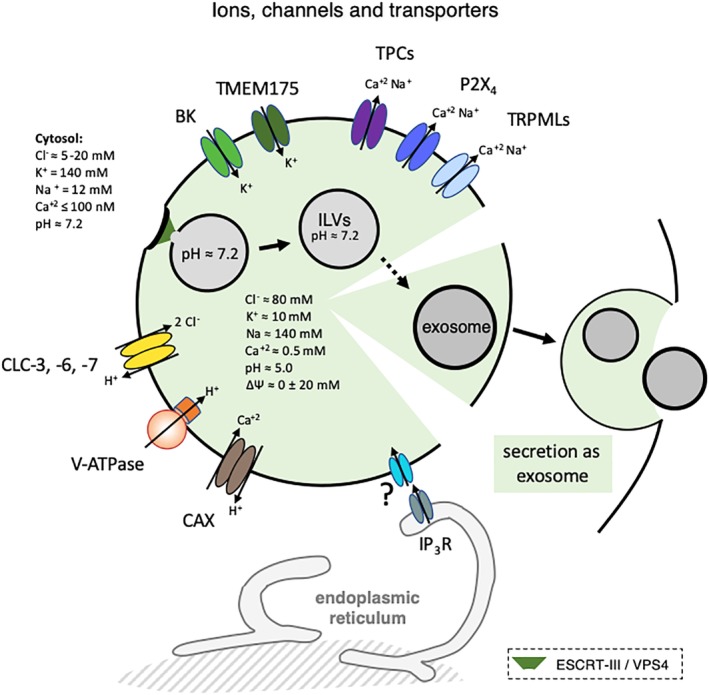
Ions, channels and transporters. The figure outlines the major ion channels and transporters present in endo‐lysosome, as well as the estimated ion concentration in the lumen of endo‐lysosomes and in the cytoplasm. The intralumenal concentration of Cl− was estimated using a DNA‐based, fluorescent chloride reporter271 and see also.272 The lumenal concentration of Na is estimated to be around 140‐150 mM.245 Li and collaborators recently proposed that ΔΨ of resting lysosomes is around 0 (±20 mV).235 Essentially nothing is known about the ionic situation within ILVs or exosomes, except for the observation that ILVs remain neutral until at least 20 minutes after formation.266 At ER‐lysosome membrane contact sites, the ER may sequester lysosomal Ca2+,273 and ER Ca2+ may refill lysosomal Ca2+ stores.274 Ca2+ is released from ER stores via Ins(1,4,5)P3 receptor (IP3R) and calcium refilling of the endosomes may be driven by the proton gradient via a vertebrate Ca2+/H+ exchanger (CAX),275 or depend directly on the ER in a pH‐independent fashion.276 Membranes shown in the black color imply that it is not known whether the corresponding processes involve LBPA‐containing membranes. V‐ATPase: the vacuolar ATPase240; CLC‐3, ‐6, ‐7: the 2Cl—/H+‐exchangers CLC‐3, ‐6, ‐7 (chloride channels) that distribute in endo‐lysosomes238; CAX, a putative endo‐lysosomal Ca2+/H+ exchanger involved in Ca2* uptake into endo‐lysosomes275; P2X4, purinergic P2X receptor subtype 4; TPC, two‐pore channels; TRPMLs, transient receptor potential channels; BK, big conductance Ca2+‐activated potassium channel274; TMEM175: K+‐selective channel235
4. BIOGENESIS OF INTRALUMENAL MEMBRANES
4.1. ILV and exosome biogenesis
Downregulated signaling receptors, and other proteins destined for late endosomes and lysosomes, are selectively sorted into ILVs, in a process that begins in early endosomes.2, 148 (Figure 1). Protein sorting into ILVs and ILV formation depend on endosomal sorting complexes required for transport (ESCRT)‐0, ‐I, ‐II and ‐III. In yeast an alternative intralumenal fragment pathway149 may also mediate the ESCRT‐independent downregulation of surface transporters delivered to the vacuole limiting membrane.150
The current view is that ESCRT‐0 initiates the process by binding both PtdIns3P on the membrane and ubiquitin conjugated to cargo molecules, and recruits ESCRT‐I, which in turn recruits ESCRT‐II as nucleator for ESCRT‐III filaments151, 152 (Figure 4). In addition to ESCRT‐0, ‐I and ‐II, the filaments of ESCRT‐III can also be nucleated by other factors, including the LBPA partner ALIX,30, 153, 154 and perhaps HD‐PTP, which shares a Bro‐1 domain with ALIX.155, 156, 157 ALIX mediates the ESCRT sorting of the GPCRs PAR1 and P2Y1,158, 159, 160 while HD‐PTP is required for the downregulation of the EGF receptor,155 PDGF receptor,161 α5β1 integrin,162 and virally ubiquitinated MHC class I.156
Figure 4.
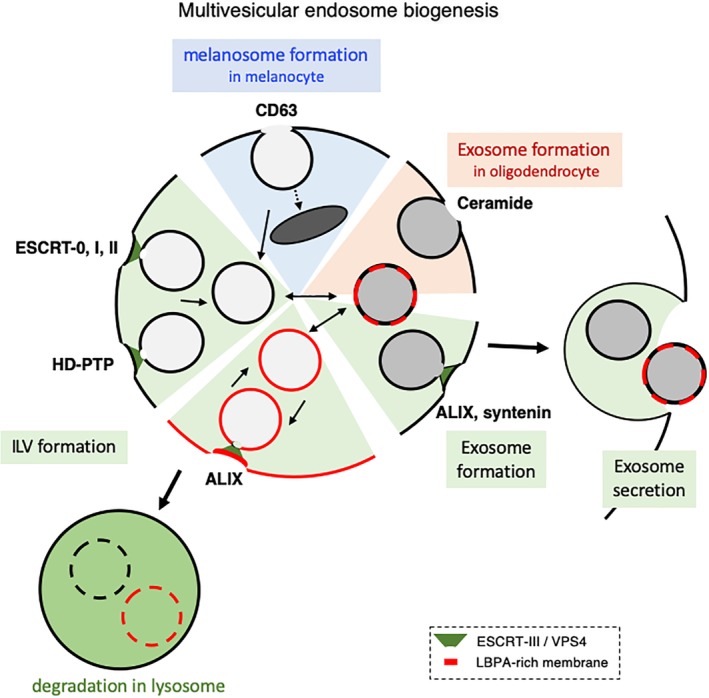
Multivesicular endosome biogenesis. The figure outlines the proposed mechanisms driving the formation of ILVs and exosomes in most cell types (green), exosomes in oligodendrocytes (brown) and melanosomes in melanocytes (blue). In most cell types, sorting into ILVs is mediated by ESCRT‐0, ‐I and ‐II, HD‐PTP or ALIX, as is presumably the nucleation of ESCRT‐III filaments, which drive the membrane deformation process. However, ILVs may also be formed in a CD63‐dependent and ESCRT‐independent manner—a process presumably akin to the biogenesis of melanosomes in melanocytes. ILVs formed in early endosomes presumably lack LBPA, because the lipid is only found in late endosomes. The biogenesis of exosomes may require ALIX and ESCRTs, as well as syntenin presumably, but not in oligodendrocytes where the process seems to depend on ceramides and to be ALIX‐ and ESCRT‐independent. Once formed, ILVs and exosomes follow different pathways. ILVs can be targeted to lysosomes for degradation, or undergo back‐fusion with the limiting membrane. Exosomes by contrast are secreted upon endosome fusion with the plasma membrane. The relationship between ILVs and exosomes are not clear. Neither are the mechanisms that discriminate their selective fates. The factors that have been reported to control each process are indicated. Membranes shown in the black color imply that it is not known whether the corresponding processes involve LBPA‐containing membranes
In vivo and in vitro observations show that ESCRT‐III filaments drive the membrane deformation process that leads to ILV formation,163, 164, 165 presumably in conjunction with the triple A ATPase VPS4.166, 167 LBPA itself may also play a direct role in this process.74 In addition, ESCRT‐III drives other membrane deformation processes that share the same topology, including cytokinetic abscission, viral budding, nuclear envelope reformation,168, 169, 170, 171 as well as plasma membrane 172, 173 and endo‐lysosome membrane repair.153, 154, 174 Hence, ESCRT‐III functions as the general membrane deformation and fission machinery with an orientation opposite to endocytosis, away from the cytoplasm.
In addition to ESCRT‐dependent mechanisms, ILVs may also form via ESCRT‐independent pathways.175 In melanocytes, the melanosomal protein PMEL is sorted into ILVs in an ESCRT‐independent176 but CD63‐dependent manner177 (Figure 4). Similarly, different ILV populations may be formed in a Hrs‐ or CD63‐dependent manner in HeLa cells.178 It should be noted that EGF, which triggers EGF receptor endocytosis and sorting into ILVs, also increases multivesicular endosomes biogenesis and ILV formation179 in an ESCRT‐dependent manner.175 However, the mechanism driving the increase in ILV formation is not known, perhaps dependent on annexin 1179 and SCAMP3.180 In addition, stress exposure triggers the ligand‐independent internalization of EGF receptor via a route that diverts from the canonical pathway and that depends on WASH and Tsg101‐ALIX, leading to EGF receptor accumulation in a subset of LBPA‐rich multivesicular endosomes.181
4.2. Microautophagy and exosome biogenesis
In a process clearly reminiscent of ILV biogenesis, cytosolic components can be engulfed within the lumen of nascent ILVs via microautophagy, and then delivered to lysosomes.7, 182 Microautophagy may be mediated via more than one pathway, dependent or not on autophagy‐related (ATG) genes. In budding yeast, the NPC orthologs, Ncr1p and Ncr2p, promote microautophagy presumably by increasing sterol in the vacuole limiting membrane.183 In fission yeast, Nbr1 was identified as autophagy receptor for the ESCRT‐dependent targeting of soluble cargos to the vacuole.184 Accumulating evidence support the notion that the ESCRT machinery is required for microautophagy.185, 186, 187, 188, 189, 190 In addition, evidence also suggests that proteins encoded by ATG genes have pleiotropic effects on exosome biogenesis and release.9 In particular, the ATG3‐ATG12 conjugate was reported to interact with ALIX in order to promote autophagy and exosome biogenesis.191
Exosomes correspond to a sub‐population of extracellular vesicles that originate from ILVs and are released outside cells upon endosome fusion with the plasma membrane 31, 192, 193 (Figure 1). Consistently, exosome biogenesis depends on ESCRT‐III,194 and ALIX71, 195, 196—although exosomes secreted by oligodendrocytes may form in a ceramide‐dependent but ALIX‐ and ESCRT‐independent manner197 (Figure 4). In addition, LBPA is present in exosomes198 and ALIX is considered as one of the best‐established exosome markers,31, 199, 200 which is surprising given the fact that ESCRTs remain cytosolic and are typically excluded from ILVs.201, 202
Essentially nothing is known about the mechanisms that control the alternative fates of ILVs—degradation in lysosomes, back‐fusion or secretion as exosomes. Neither is anything known about the principles responsible for the lysosomal targeting of ILV cargoes or retrieval to other destinations, including exosomes.
4.3. Biochemically‐distinct populations of ILVs
The sub‐cellular distribution of LBPA clearly demonstrates that biochemically‐distinct populations of ILVs co‐exist within endosomes. Indeed, the lipid cannot be detected in early endosomes,44 where ILV biogenesis begins.148 Neither is the lipid detected in canonical multivesicular endosomes/bodies, which serve as intermediate between early and late endosomes (Figure 1). LBPA is found, and thus likely synthesized, in late endosomes or endo‐lysosomes,44 which are filled with internal membranes of various origins, including exosomes in the making, ILVs destined for lysosomes, as well as remnants of organelles delivered by autophagy (see tomogram of late endosomes in Cos cells—Movie S1). LBPA itself seems to be enriched in one sub‐population of these intralumenal membranes.46 Consistent with this notion, PtdIns3P and LBPA localize to different ILV populations within endosomes.203
The notion than more than one population of ILVs co‐exist in endosomes204 is clearly further supported by observations that, in addition to ESCRT‐dependent mechanisms, ILVs may also form via ESCRT‐independent pathways, as discussed above. One of the future challenges will be to establish what are the overlapping vs unique mechanisms, dependent or not on ESCRT subunits or ESCRT‐associated proteins, which may drive the biogenesis of functionally‐distinct populations of ILVs, microautophagosomes or exosomes. Interestingly, disruption of the class III PI3‐kinase Vps34 in neurons, which is required for both autophagy and ILV formation, triggers the secretion of unique exosomes enriched for undigested lysosomal substrates, specific sphingolipids, and LBPA.205
4.4. ILVs hijacked by pathogens
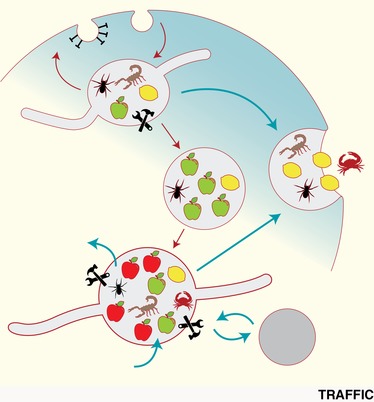
Pathogens use all tricks in the book to overcome cellular defenses, and not surprisingly, they also exploit the multivesicular endosome pathway206 (Figure 5). The anthrax toxin penetrates the target cell in a process that depends on LBPA, ALIX and other ESCRTs.71, 207 Similarly, during vesicular stomatitis virus (VSV) infection, the release of viral RNA into the cytosol depends on LBPA, ALIX and ESCRTs,67, 68, 75 as do Lassa virus and lymphocytic choriomeningitis virus69—Lassa virus was also shown to depend on LAMP1.208 Crimean‐Congo hemorrhagic fever virus (CCHFV) infection may also depend on ALIX and ESCRTs,209 while Human Papillomavirus (HPV) infection may rely on CD63, syntenin‐1 and ALIX,210 and Ebola virus on NPC1 and cation two‐pore channels (TPC)211, 212, 213 (Figures 5 and 6). Influenza A virus (IAV) infection depends on VPS4, as well as ubiquitination,214 the SPOPL/Cullin‐3 ubiquitin ligase complex and its target EPS15.215, 216 Although the precise role of LBPA, ALIX and ESCRTs in infection or intoxication remains to be elucidated, it has been proposed that anthrax,71, 207 VSV67, 68 and Japanese encephalitis and yellow fever flaviviruses217 may hijack ILVs so that toxin or nucleic acid be delivered to the cytoplasm by ILV back‐fusion with the limiting membrane.29, 30, 218 Interestingly, in this context, the fusion of dengue virus219 and VSV 79, 220 during infection depends on anionic phospholipids including LBPA—as does the cytoplasmic entry of the non‐enveloped Bluetongue Virus capsid.221
Figure 5.

Viruses, toxin and ILV‐membrane dynamics. The left side of the figure (penetration) outlines the pathways used by some endocytosed pathogenic agents that enter the host‐cell cytoplasm through endosomes, in a process that depends on proteins/lipids involved in ILV membrane dynamics. VSV, Lassa virus, LCMV, and Flaviviruses may penetrate cells in a two‐step process. First, the viral enveloped undergoes fusion with the ILV membrane (eg, in early endosomes) so that the capsid be delivered into the protected environment of the ILV lumen. Then, the capsid is released into the host‐cell cytoplasm upon fusion of the ILV membrane with the late endosome limiting membrane (so‐called back‐fusion). Similarly, the anthrax toxin is first translocated across the ILV membrane and then delivered to the cytoplasm upon ILV back‐fusion. Other endocytosed viruses may penetrate cells upon direct fusion of the viral envelope with the late endosome membrane.206 The lower part of the figure outlines the role of ESCRT‐III and other ESCRT sub‐units in repairing damage to vacuoles containing the indicated bacteria. The right side of the figure outlines the inclusion of some viruses and viral particles into exosomes (enclosure) in a process that depends on ESCRT components, and their release as exosomes. The endocytosed anthrax toxin can also be released as exosomes, rather than being delivered to the cytoplasm of the target cell. Membranes shown in the black color imply that it is not known whether the corresponding processes involve LBPA‐containing membranes. CCHFV, Crimean‐Congo hemorrhagic fever virus; HAV, hepatitis A virus; HCV, hepatitis C virus; HPV, human papillomaviruses; IAV, influenza A virus; LCMV, lymphocytic choriomeningitis; VSV, vesicular stomatitis virus; M marinum, Mycobacterium marinum (in Dictyostelium discoideum cells); M tuberculosis, Mycobacterium tuberculosis); C burnetii, Coxiella burnetii
The ESCRT machinery was also recently shown to play additional roles during bacterial infection, in light with a general role for ESCRTs in repairing endo‐lysosome membranes153, 154 and other membranes.222 Vacuoles containing the intracellular pathogen Coxiella burnetii recruit ESCRTs to maintain an intact vacuole, which presumably provides the bacterium with a replication advantage.154 Similarly, ESCRTs are required to repair small membrane damage in the vacuole containing Mycobacterium marinum in Dictyostelium discoideum 174 or Mycobacterium tuberculosis in macrophage,223 presumably to ensure that the pathogen remains contained within intact compartments.
ILVs as exosomes have also been proposed to mediate the spreading of pathogens or pathogenic agents from cell to cell (Figure 5). In fact, it is being discussed whether viruses and exosomes (or other types of extracellular vesicles) share similarities and may be related.224 It has been reported that exosomes may mediate the transmission of hepatitis C virus225 in a process that depends on the ESCRT subunit HRS.226 Similarly, exosomes have also been proposed to transfer hepatitis C viral RNA.227, 228 as well as nucleic acids from other viruses including HIV.229, 230 The non‐enveloped hepatitis A virus was also shown to be released after inclusion within a host‐derived exosomal‐like membrane generated in a process that depends on the ESCRTs, VPS4B and ALIX231, 232—an observation that blurs the classic distinction between enveloped and non‐enveloped viruses. In addition, uropathogenic Escherichia coli (UPEC), which targets lysosomes but avoids degradation by pH neutralization, can be expelled in exosomes by bladder epithelial cells, upon pH sensing via the calcium channel TRPML3 (TRP channel 3 or mucolipin 3)233 (see Figure 6). Finally, in addition to delivering their toxin cargo to the cytoplasm by back‐fusion, ILVs containing anthrax toxin may also be released as exosomes so that the toxin can be transmitted to naïve cells.71 Interestingly, however, anthrax toxin containing ILVs fail to be targeted to lysosomes for degradation.71 It thus appears that that the machinery controlling ILV formation and dynamics has been hijacked to mediate viral RNA or toxin release to the cytoplasm during infection/intoxication, or secretion to the extracellular medium as exosomes in order to propagate the infection or to spread the toxin to naïve cells.
5. LIFE IN THE LUMEN
5.1. Protons, anions and cations
In the late endosome lumen, where LBPA is found, ILVs and other intralumenal membranes are packed within a highly crowded environment (Movie S1). Beyond the diversity of membranes already discussed above, relatively little is known about the biochemical and biophysical properties of the lumenal milieu,234 although much progress has been made in the characterization of endo‐lysosomal ion channels and in the description of the ionic situation within the endo‐lysosomal milieu (for recent reviews, see235, 236, 237, 238, 239). It is well‐established that endo‐lysosome acidification depends on the V‐ATPase, with early endosomes having a mildly acidic pH ≈ 6.2 and late endosomes/lysosomes a more acidic pH ≈ 5.0234, 240, 241 (Figure 6). Numerous physiological processes, including ligand‐receptor uncoupling, lysosomal enzyme activity and membrane traffic are controlled by the acidification properties of endo‐lysosomes. The low endo‐lysosomal pH is also used by enveloped viruses to trigger fusion of the viral envelope with the endosomal membrane and by some toxins to cross the endo‐lysosomal membrane so that the viral nucleic acid or the toxin can reach the host‐cell cytoplasm.206, 242 In addition to protons, cations and anions also play important roles in the regulation of the endo‐lysosomal lumenal environment. Chloride controls ion homeostasis and endo‐lysosome acidification, and is regulated in endo‐lysosomes by 2Cl−/H+‐exchangers of the CLC anion transporter family (ClC‐3 through ClC‐7), which are responsible for several disorders when mutated238 (Figure 6).
In mammalian cells, endo‐lysosomes, in addition to the ER and mitochondria, also serve calcium storage functions—referred to as acidic calcium stores—presumably regulated via ER‐endosome membrane contact sites 12, 237, 243, 244 (Figure 6). Cation channels, including in particular the mucolipin subfamily of TRPML (transient receptor potential) channels or the distantly related TPCs (two‐pore channels) maintain endosomal calcium homeostasis,237, 243, 244, 245 and may also function as key regulators of endo‐lysosomal trafficking and autophagy‐related processes.246, 247 Calcium is indeed believed to play an important role in the regulation of endo‐lysosome and autophagosome membrane dynamics.5, 246, 247, 248 As already mentioned, mutations in TRPML1 (or mucolipin‐1, MCOLN1) are responsible for the LSD mucolipidosis type 4,142 and dysfunction of endo‐lysosomal calcium is observed in various LSDs.249, 250, 251 In addition, TPCs are involved in Ebola virus penetration from endo‐lysomes into host‐cells,211 while delivery of the viral core to the cytoplasm depends on the NPC1 protein 212, 213 (Figure 5). Finally, efflux of calcium from damaged endosomes serves as a signal to trigger an ESCRT‐mediated repair process.153
Much like in the ER,252 the free Ca+2 in endosomes is estimated to 0.4‐0.6 mM.249, 253 In the ER, most Ca+2 is buffered by abundant lumenal Ca+2‐binding proteins. 254, 255 However, these proteins or their functional homologs are not found in endosomes and lysosomes, and the nature of the Ca+2‐binding molecules that play similar roles in the acidic calcium stores is unknown. Yet, it can be estimated that ≈99.9% of Ca+2 in acidic stores is chelated, supporting the notion that buffer molecules or matrix must exist.256 It is appealing to propose that the abundant, negatively‐charged lipid LBPA serves as calcium buffer in the lumen of late endosome/endo‐lysosomes. Indeed, the capacity of calcium to bind negatively‐charged lipids is a universal principle, which is best illustrated by the active translocation of the negatively‐charged lipid PS from the outer leaflet of the plasma membrane (high calcium environment of the blood) to the inner leaflet (low calcium environment of the cell).257 Moreover, calcium exhibits a substantial capacity to bind membrane phospholipids258, 259, 260, 261 and to alter the properties of the bilayer.262 In fact, accumulation of the divalent cation Zn+2 in the LBPA‐containing late endosomes of cells expressing the ZnT2 zinc transporter caused cholesterol accumulation much like in NPC cells.64 It can be anticipated that calcium association to LBPA‐rich membranes in the endosome lumen may not only control the fate and dynamics of ILVs, but may also play a key‐role in the late endosome/endo‐lysosome capacity to modulate calcium‐dependent processes, including in lysosomal signaling.246
5.2. The lumen in the lumen: Size matters
In mammalian cells, typical ILVs form one or more fairly homogenous populations of vesicles with a mean diameter around 50 nm,263, 264 while ILVs in yeast are smaller with a diameter of ≈ 25 nm.265 Essentially nothing is known about the chemical conditions that exist within the lumen of ILVs and exosomes, beyond the observations that the pH of newly‐formed ILVs is neutral.266 One should keep in mind that the volume of a 50 nm diameter ILV is exceedingly small, corresponding to ≈ 65 × 10−3 aL, implying that a fraction of a proton only suffices to reduce the pH by two units, from 7 to 5. Whatever the fate of ILVs, degradation, secretion or retrieval, one may consider these vesicles as unit containers packaging quantum amounts of cargo in the membrane or in the lumen.
This notion becomes important when considering some of the ILV or exosome functions. For example, exosomes presumably transport miRNAs from donor to acceptor cells,20, 21, 267, 268 and thus regulate gene expression in target cells, by repressing translation of target mRNAs and/or by inducing their degradation.269 One miRNA targets a single RNA molecule, in contrast to enzymes that are regenerated during the catalytic cycle and can process many substrates. Thus, if incorporation into exosome was strictly passive, one would need 2‐16 × 106 exosomes of 50‐100 nm diameter to transfer one miRNA species from one typical donor cell with a volume ≈ 1000 fL,270 to a target cell of the same volume in order to achieve the same miRNA concentration as in the donor—irrespective of what the concentration is—hence, a volume equivalent to the total volume of the donor cell. Thus, a highly efficient mechanism must exist to produce, sort and package miRNAs into exosomes, and to target these exosomes to the recipient cells, for such a transfer mechanism to operate in a physiologically‐relevant manner—miRNAs and RNAs associated to extracellular vesicles are reported to be enriched in certain sorting motifs.224
Using an assay that measures the biogenesis of ILVs into late endosomes in vitro, the ILV lumenal pH was found to be neutral for a relatively long time, up to 20 minutes after ILV formation.266 However, given the fact that an ATP‐dependent mechanism is unlikely to maintain the pH gradient across the ILV membrane inside endosomes, it is not known whether the pH gradient persists until digestion in the lysosomes, or whether proton permeation across the bilayer eventually acidify the lumen, prior to degradation. In any case, the asymmetry across the ILV membrane driven by pH and ion gradients, as well as the asymmetric protein and lipid composition of the ILV bilayer likely contribute to regulate the fate of ILVs.
6. CONCLUSION
Late endosomes/endo‐lysosomes are unique organelles of the vertebrate vacuolar apparatus in that they contain membrane vesicles within their lumenal environment, which is topologically equivalent to the extracellular space. These vesicles are highly specialized, in particular because some are rich in LBPA—an atypical lipid that is not found elsewhere in the cell. LBPA not only has an unconventional biosynthetic pathway and stereochemistry, but also has a unique shape and acyl chain migration capacity, likely to influence its impact on membrane organization and dynamics.
A fully unanswered and outstanding question is the nature of the mechanism that drive the sorting of ILVs toward one of their possible fates—degradation in lysosomes, secretion as exosomes, or recycling to the limiting membrane via back‐fusion. The privileged and secluded environment of ILVs, bathed into the late endosome/endo‐lysosome lumen, is fully disconnected from all cytosolic machineries that drive signaling or protein and lipid sorting, and therefore the fate of ILVs cannot rely on these established mechanisms. Future work will be needed to address this issue. However, some speculations are already possible. LBPA‐rich membranes are involved in the regulation of several features of the endo‐lysosome intralumenal membrane system, including cholesterol transport, sphingolipid degradation, and membrane dynamics, as well as perhaps endosomal Ca+2. LBPA also exhibits a rare capacity for adaptive shape changes, via acyl chain remodeling, because of its unique structure. It is therefore attractive to believe that LBPA‐rich membranes play a crucial role in modulating trafficking within the endosome and the fate and dynamics of intralumenal membranes. In particular, given the fact that the LBPA partner ALIX is involved in the biogenesis of at least some exosome populations and is itself found in exosome, LBPA‐rich endosomal membrane domains may ultimately control the biogenesis of exosomes.
Supporting information
Supplementary Movie S1 The Movie shows the tomographic reconstruction of a late endosome/endolysosome in Cos cells, which illustrates the complexity of the intralumenal membrane system, including multilamellar regions with a Russian Doll‐like organization. In this tomogram, ILVs are clearly visible (and definitely bilayered), as well as other intralumenal membranes, including presumably remnants of organelles delivered by autophagy. Cells were fast frozen, and then processed for rapid freeze substitution. Images represent a 300 nm section of a typical endosome. [Courtesy of Robert G. Parton, Brisbane, Australia]
ACKNOWLEDGMENTS
I wish to thank warmly Vincent Mercier (University of Geneva), Oksana Sergeeva (EPFL, Lausanne), Gisou van der Goot (EPFL, Lausanne), Cameron Scott (University of Geneva) and Ueli Schibler (University of Geneva) for useful comments and critical reading of the manuscript. Support was from the Swiss National Science Foundation, the NCCR in Chemical Biology and LipidX from the Swiss SystemsX.ch Initiative, evaluated by the Swiss National Science Foundation.
Gruenberg J. Life in the lumen: The multivesicular endosome. Traffic. 2020;21:76–93. 10.1111/tra.12715
Funding information LipidX from SystemsX.ch; National Center of Competence in Research Chemical Biology; Schweizerischer Nationalfonds zur Förderung der Wissenschaftlichen Forschung, Grant/Award Number: 31003A_159479
Peer Review The peer review history for this article is available at https://publons.com/publon/10.1111/tra.12715/
REFERENCES
- 1. Huotari J, Helenius A. Endosome maturation. EMBO J. 2011;30(17):3481‐3500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Scott CC, Vacca F, Gruenberg J. Endosome maturation, transport and functions. Semin Cell Dev Biol. 2014;31:2‐10. [DOI] [PubMed] [Google Scholar]
- 3. Simonetti B, Cullen PJ. Endosomal sorting: architecture of the Retromer coat. Curr Biol. 2018;28(23):R1350‐R1352. [DOI] [PubMed] [Google Scholar]
- 4. Hanson PI, Cashikar A. Multivesicular body morphogenesis. Annu Rev Cell Dev Biol. 2012;28:337‐362. [DOI] [PubMed] [Google Scholar]
- 5. Luzio JP, Pryor PR, Bright NA. Lysosomes: fusion and function. Nat Rev Mol Cell Biol. 2007;8(8):622‐632. [DOI] [PubMed] [Google Scholar]
- 6. Saffi GT, Botelho RJ. Lysosome fission: planning for an exit. Trends Cell Biol. 2019;29(8):635‐646. [DOI] [PubMed] [Google Scholar]
- 7. Mizushima N, Yoshimori T, Ohsumi Y. The role of Atg proteins in autophagosome formation. Annu Rev Cell Dev Biol. 2011;27:107‐132. [DOI] [PubMed] [Google Scholar]
- 8. Lamb CA, Yoshimori T, Tooze SA. The autophagosome: origins unknown, biogenesis complex. Nat Rev Mol Cell Biol. 2013;14(12):759‐774. [DOI] [PubMed] [Google Scholar]
- 9. Levine B, Kroemer G. Biological functions of autophagy genes: a disease perspective. Cell. 2019;176(1‐2):11‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wong LH, Eden ER, Futter CE. Roles for ER: endosome membrane contact sites in ligand‐stimulated intraluminal vesicle formation. Biochem Soc Trans. 2018;46(5):1055‐1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Raiborg C, Wenzel EM, Pedersen NM, Stenmark H. ER‐endosome contact sites in endosome positioning and protrusion outgrowth. Biochem Soc Trans. 2016;44(2):441‐446. [DOI] [PubMed] [Google Scholar]
- 12. Phillips MJ, Voeltz GK. Structure and function of ER membrane contact sites with other organelles. Nat Rev Mol Cell Biol. 2016;17(2):69‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ridgway ND, Zhao K. Cholesterol transfer at endosomal‐organelle membrane contact sites. Curr Opin Lipidol. 2018;29(3):212‐217. [DOI] [PubMed] [Google Scholar]
- 14. Valm AM, Cohen S, Legant WR, et al. Applying systems‐level spectral imaging and analysis to reveal the organelle interactome. Nature. 2017;546(7656):162‐167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Marks MS, Heijnen HF, Raposo G. Lysosome‐related organelles: unusual compartments become mainstream. Curr Opin Cell Biol. 2013;25(4):495‐505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Griffiths GM. Secretion from myeloid cells: secretory lysosomes. Microbiol Spectr. 2016;4(4). [DOI] [PubMed] [Google Scholar]
- 17. Luzio JP, Hackmann Y, Dieckmann NM, Griffiths GM. The biogenesis of lysosomes and lysosome‐related organelles. Cold Spring Harb Perspect Biol. 2014;6(9):a016840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kowal J, Tkach M, Thery C. Biogenesis and secretion of exosomes. Curr Opin Cell Biol. 2014;29:116‐125. [DOI] [PubMed] [Google Scholar]
- 19. Bissig C, Gruenberg J. Lipid sorting and multivesicular endosome biogenesis. Cold Spring Harb Perspect Biol. 2013;5(10):a016816 10.1101/cshperspect.a016816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 2013;200(4):373‐383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Latifkar A, Hur YH, Sanchez JC, Cerione RA, Antonyak MA. New insights into extracellular vesicle biogenesis and function. J Cell Sci. 2019;132(13):222406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Delevoye C, Marks MS, Raposo G. Lysosome‐related organelles as functional adaptations of the endolysosomal system. Curr Opin Cell Biol. 2019;59:147‐158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Berson JF, Harper DC, Tenza D, Raposo G, Marks MS. Pmel17 initiates premelanosome morphogenesis within multivesicular bodies. Mol Biol Cell. 2001;12(11):3451‐3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hurbain I, Geerts WJ, Boudier T, et al. Electron tomography of early melanosomes: implications for melanogenesis and the generation of fibrillar amyloid sheets. Proc Natl Acad Sci U S A. 2008;105(50):19726‐19731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kleijmeer M, Ramm G, Schuurhuis D, et al. Reorganization of multivesicular bodies regulates MHC class II antigen presentation by dendritic cells. J Cell Biol. 2001;155(1):53‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Peters PJ, Neefjes JJ, Oorschot V, Ploegh HL, Geuze HJ. Segregation of MHC class II molecules from MHC class I molecules in the Golgi complex for transport to lysosomal compartments. Nature. 1991;349(6311):669‐676. [DOI] [PubMed] [Google Scholar]
- 27. Zwart W, Griekspoor A, Kuijl C, et al. Spatial separation of HLA‐DM/HLA‐DR interactions within MIIC and phagosome‐induced immune escape. Immunity. 2005;22(2):221‐233. [DOI] [PubMed] [Google Scholar]
- 28. van der Goot FG, Gruenberg J. Intra‐endosomal membrane traffic. Trends Cell Biol. 2006;16(10):514‐521. [DOI] [PubMed] [Google Scholar]
- 29. Nour A, Modis Y. Endosomal vesicles as vehicles for viral genomes. Trends Cell Biol. 2014;24:449‐454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bissig C, Gruenberg J. ALIX and the multivesicular endosome: ALIX in wonderland. Trends Cell Biol. 2014;24:19‐25. [DOI] [PubMed] [Google Scholar]
- 31. van Niel G, D'Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol. 2018;19(4):213‐228. [DOI] [PubMed] [Google Scholar]
- 32. Settembre C, Fraldi A, Medina DL, Ballabio A. Signals from the lysosome: a control Centre for cellular clearance and energy metabolism. Nat Rev Mol Cell Biol. 2013;14(5):283‐296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Huber LA, Teis D. Lysosomal signaling in control of degradation pathways. Curr Opin Cell Biol. 2016;39:8‐14. [DOI] [PubMed] [Google Scholar]
- 34. Body DR, Gray GM. The isolation and characterisation of phosphatidylglycerol and a structural isomer from pig lung. Chem Phys Lipids. 1967;1:254‐263. [Google Scholar]
- 35. Benson AA, Maruo B. Piant phospholipids. I. Identification of the phosphatidyl glycerols. Biochim Biophys Acta. 1958;27:189‐195. [DOI] [PubMed] [Google Scholar]
- 36. Gobley TN. Recherches Chimiques sur les Oeufs de Carpe. J Pharm Chim. 1850;17:401‐430. [Google Scholar]
- 37. Sourkes TL. The discovery of lecithin, the first phospholipid. Bull Hist Chem. 2004;29:9‐15. [Google Scholar]
- 38. Rouser G, Kritchevsky G, Knudson AG Jr, Simon G. Accumulation of a glycerolphospholipid in classical niemann‐pick disease. Lipids. 1968;3(3):287‐290. [DOI] [PubMed] [Google Scholar]
- 39. Wherrett JR, Huterer S. Enrichment of bis‐(monoacylglyceryl) phosphate in lysosomes from rat liver. J Biol Chem. 1972;247(13):4114‐4120. [PubMed] [Google Scholar]
- 40. Brotherus J, Renkonen O. Subcellular distributions of lipids in cultured BHK cells: evidence for the enrichment of lysobisphosphatidic acid and neutral lipids in lysosomes. J Lipid Res. 1977;18(2):191‐202. [PubMed] [Google Scholar]
- 41. Rodriguez‐Paris JM, Nolta KV, Steck TL. Characterization of lysosomes isolated from Dictyostelium discoideum by magnetic fractionation. J Biol Chem. 1993;268(12):9110‐9116. [PubMed] [Google Scholar]
- 42. Kobayashi T, Nishijima M, Tamori Y, Nojima S, Seyama Y, Yamakawa T. Acyl phosphatidylglycerol of Escherichia coli . Biochim Biophys Acta. 1980;620(3):356‐363. [PubMed] [Google Scholar]
- 43. Holmback J, Karlsson AA, Arnoldsson KC. Characterization of N‐acylphosphatidylethanolamine and acylphosphatidylglycerol in oats. Lipids. 2001;36(2):153‐165. [DOI] [PubMed] [Google Scholar]
- 44. Kobayashi T, Stang E, Fang KS, de Moerloose P, Parton RG, Gruenberg J. A lipid associated with the antiphospholipid syndrome regulates endosome structure and function. Nature. 1998;392(6672):193‐197. [DOI] [PubMed] [Google Scholar]
- 45. Brankatschk B, Pons V, Parton RG, Gruenberg J. Role of SNX16 in the dynamics of tubulo‐cisternal membrane domains of late endosomes. PLoS ONE. 2011;6(7):e21771 10.1371/journal.pone.0021771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kobayashi T, Beuchat MH, Chevallier J, et al. Separation and characterization of late endosomal membrane domains. J Biol Chem. 2002;277(35):32157‐32164. [DOI] [PubMed] [Google Scholar]
- 47. Helenius A, Mellman I, Wall D, Hubbard A. Endosomes. Trends Biochem Sci. 1983;8:245‐250. [Google Scholar]
- 48. Schink KO, Tan KW, Stenmark H. Phosphoinositides in control of membrane dynamics. Annu Rev Cell Dev Biol. 2016;32:143‐171. [DOI] [PubMed] [Google Scholar]
- 49. van Meer G, de Kroon AI. Lipid map of the mammalian cell. J Cell Sci. 2011;124(1):5‐8. [DOI] [PubMed] [Google Scholar]
- 50. Holthuis JC, Menon AK. Lipid landscapes and pipelines in membrane homeostasis. Nature. 2014;510(7503):48‐57. [DOI] [PubMed] [Google Scholar]
- 51. Bohdanowicz M, Grinstein S. Role of phospholipids in endocytosis, phagocytosis, and macropinocytosis. Physiol Rev. 2013;93(1):69‐106. [DOI] [PubMed] [Google Scholar]
- 52. Brotherus J, Renkonen O, Herrmann J, Fisher W. Novel stereochemical configuration in lysobisphosphatidic acid of cultured BHK cells. Chem Phys Lipids. 1974;13:178‐182. [DOI] [PubMed] [Google Scholar]
- 53. Joutti A, Brotherus J, Renkonen O, Laine R, Fischer W. The stereochemical configuration of lysobisphosphatidic acid from rat liver, rabbit lung and pig lung. Biochim Biophys Acta. 1976;450(2):206‐209. [DOI] [PubMed] [Google Scholar]
- 54. Tan HH, Makino A, Sudesh K, Greimel P, Kobayashi T. Spectroscopic evidence for the unusual stereochemical configuration of an endosome‐specific lipid. Angew Chem. 2012;51(2):533‐535. [DOI] [PubMed] [Google Scholar]
- 55. Matsuzawa Y, Hostetler KY. Degradation of bis(monoacylglycero)phosphate by an acid phosphodiesterase in rat liver lysosomes. J Biol Chem. 1979;254(13):5997‐6001. [PubMed] [Google Scholar]
- 56. Frederick TE, Goff PC, Mair CE, Farver RS, Long JR, Fanucci GE. Effects of the endosomal lipid bis(monoacylglycero)phosphate on the thermotropic properties of DPPC: a 2H NMR and spin label EPR study. Chem Phys Lipids. 2010;163(7):703‐711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Poorthuis BJ, Hostetler KY. Studies on the subcellular localization and properties of bis(monoacylglyceryl)phosphate biosynthesis in rat liver. J Biol Chem. 1976;251(15):4596‐4602. [PubMed] [Google Scholar]
- 58. Somerharju P, Renkonen O. Conversion of phosphatidylglycerol lipids to bis(monoacylglycero)phosphate in vivo. Biochim Biophys Acta. 1980;618(3):407‐419. [DOI] [PubMed] [Google Scholar]
- 59. Amidon B, Schmitt JD, Thuren T, King L, Waite M. Biosynthetic conversion of phosphatidylglycerol to sn‐1:sn‐1' bis(monoacylglycerol) phosphate in a macrophage‐like cell line. Biochemistry. 1995;34(16):5554‐5560. [DOI] [PubMed] [Google Scholar]
- 60. Amidon B, Brown A, Waite M. Transacylase and phospholipases in the synthesis of bis(monoacylglycero)phosphate. Biochemistry. 1996;35:13995‐14002. [DOI] [PubMed] [Google Scholar]
- 61. Hullin‐Matsuda F, Kawasaki K, Delton‐Vandenbroucke I, et al. De novo biosynthesis of the late endosome lipid, bis(monoacylglycero)phosphate. J Lipid Res. 2007;48(9):1997‐2008. [DOI] [PubMed] [Google Scholar]
- 62. Mayr JA. Lipid metabolism in mitochondrial membranes. J Inherit Metab Dis. 2015;38(1):137‐144. [DOI] [PubMed] [Google Scholar]
- 63. Mijaljica D, Prescott M, Devenish RJ. Different fates of mitochondria: alternative ways for degradation? Autophagy. 2007;3(1):4‐9. [DOI] [PubMed] [Google Scholar]
- 64. Kobayashi T, Beuchat MH, Lindsay M, et al. Late endosomal membranes rich in lysobisphosphatidic acid regulate cholesterol transport. Nat Cell Biol. 1999;1(2):113‐118. [DOI] [PubMed] [Google Scholar]
- 65. Galve‐de Rochemonteix B, Kobayashi T, Rosnoblet C, et al. Interaction of anti‐phospholipid antibodies with late endosomes of human endothelial cells. Arterioscler Thromb Vasc Biol. 2000;20(2):563‐574. [DOI] [PubMed] [Google Scholar]
- 66. Lebrand C, Corti M, Goodson H, et al. Late endosome motility depends on lipids via the small GTPase Rab7. EMBO J. 2002;21(6):1289‐1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Le Blanc I, Luyet PP, Pons V, et al. Endosome‐to‐cytosol transport of viral nucleocapsids. Nat Cell Biol. 2005;7(7):653‐664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Luyet PP, Falguières T, Pons V, Pattnaik AK, Gruenberg J. The ESCRT‐I subunit Tsg101 controls endosome‐to‐cytosol release of viral RNA. Traffic. 2008;9:2279‐2290. [DOI] [PubMed] [Google Scholar]
- 69. Pasqual G, Rojek JM, Masin M, Chatton JY, Kunz S. Old world arenaviruses enter the host cell via the multivesicular body and depend on the endosomal sorting complex required for transport. PLoS Pathog. 2011;7(9):e1002232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Sorice M, Ferro D, Misasi R, et al. Evidence for anticoagulant activity and beta2‐GPI accumulation in late endosomes of endothelial cells induced by anti‐LBPA antibodies. Thromb Haemost. 2002;87(4):735‐741. [PubMed] [Google Scholar]
- 71. Abrami L, Brandi L, Moayeri M, et al. Hijacking multivesicular bodies enables long‐term and exosome‐mediated long‐distance action of anthrax toxin. Cell Rep. 2013;5:986‐996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Dunoyer‐Geindre S, Kruithof EK, Galve‐de Rochemonteix B, et al. Localization of beta2‐glycoprotein 1 in late endosomes of human endothelial cells. Thromb Haemost. 2001;85(5):903‐907. [PubMed] [Google Scholar]
- 73. Alessandri C, Bombardieri M, Di Prospero L, et al. Anti‐lysobisphosphatidic acid antibodies in patients with antiphospholipid syndrome and systemic lupus erythematosus. Clin Exp Immunol. 2005;140(1):173‐180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Matsuo H, Chevallier J, Mayran N, et al. Role of LBPA and Alix in multivesicular liposome formation and endosome organization. Science (New York, NY). 2004;303:531‐534. [DOI] [PubMed] [Google Scholar]
- 75. Bissig C, Lenoir M, Velluz MC, et al. Viral infection controlled by a calcium‐dependent lipid‐binding module in ALIX. Dev Cell. 2013;25(4):364‐373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. van Meer G, Voelker DR, Feigenson GW. Membrane lipids: where they are and how they behave. Nat Rev Mol Cell Biol. 2008;9(2):112‐124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Cullis PR, Hope MJ, Bally MB, Madden TD, Mayer LD, Fenske DB. Influence of pH gradients on the transbilayer transport of drugs, lipids, peptides and metal ions into large unilamellar vesicles. Biochim Biophys Acta. 1997;1331(2):187‐211. [DOI] [PubMed] [Google Scholar]
- 78. Farge E, Devaux PF. Shape changes of giant liposomes induced by an asymmetric transmembrane distribution of phospholipids. Biophys J. 1992;61(2):347‐357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Matos PM, Marin M, Ahn B, Lam W, Santos NC, Melikyan GB. Anionic lipids are required for vesicular stomatitis virus G protein‐mediated single particle fusion with supported lipid bilayers. J Biol Chem. 2013;288(18):12416‐12425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Akgoc Z, Iosim S, Seyfried TN. Bis(monoacylglycero)phosphate as a macrophage enriched phospholipid. Lipids. 2015;50(9):907‐912. [DOI] [PubMed] [Google Scholar]
- 81. Moreau D, Vacca F, Vossio S, et al. Drug‐induced increase in lysobisphosphatidic acid reduces the cholesterol overload in Niemann‐pick type C cells and mice. EMBO Rep. 2019;20(7):e47055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Chevallier J, Sakai N, Robert F, Kobayashi T, Gruenberg J, Matile S. Rapid access to synthetic lysobisphosphatidic acids using P(III) chemistry. Org Lett. 2000;2(13):1859‐1861. [DOI] [PubMed] [Google Scholar]
- 83. Goursot A, Mineva T, Bissig C, Gruenberg J, Salahub DR. Structure, dynamics, and energetics of lysobisphosphatidic acid (LBPA) isomers. J Phys Chem B. 2010;114(47):15712‐15720. [DOI] [PubMed] [Google Scholar]
- 84. Hannich JT, Umebayashi K, Riezman H. Distribution and functions of sterols and sphingolipids. Cold Spring Harb Perspect Biol. 2011;3(5):a004762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Liscum L. Niemann‐pick type C mutations cause lipid traffic jam. Traffic. 2000;1(3):218‐225. [DOI] [PubMed] [Google Scholar]
- 86. Simons K, Gruenberg J. Jamming the endosomal system: lipid rafts and lysosomal storage diseases. Trends Cell Biol. 2000;10(11):459‐462. [DOI] [PubMed] [Google Scholar]
- 87. Breiden B, Sandhoff K. Lysosomal glycosphingolipid storage diseases. Annu Rev Biochem. 2019;88:461‐485. [DOI] [PubMed] [Google Scholar]
- 88. Gallala HD, Sandhoff K. Biological function of the cellular lipid BMP‐BMP as a key activator for cholesterol sorting and membrane digestion. Neurochem Res. 2011;36(9):1594‐1600. [DOI] [PubMed] [Google Scholar]
- 89. Schulze H, Sandhoff K. Sphingolipids and lysosomal pathologies. Biochim Biophys Acta. 2014;1841(5):799‐810. [DOI] [PubMed] [Google Scholar]
- 90. Wilkening G, Linke T, Sandhoff K. Lysosomal degradation on vesicular membrane surfaces. Enhanced glucosylceramide degradation by lysosomal anionic lipids and activators. J Biol Chem. 1998;273(46):30271‐30278. [DOI] [PubMed] [Google Scholar]
- 91. Wilkening G, Linke T, Uhlhorn‐Dierks G, Sandhoff K. Degradation of membrane‐bound ganglioside GM1. Stimulation by bis(monoacylglycero)phosphate and the activator proteins SAP‐B and GM2‐AP. J Biol Chem. 2000;275(46):35814‐35819. [DOI] [PubMed] [Google Scholar]
- 92. Linke T, Wilkening G, Sadeghlar F, et al. Interfacial regulation of acid ceramidase activity. Stimulation of ceramide degradation by lysosomal lipids and sphingolipid activator proteins. J Biol Chem. 2001;276(8):5760‐5768. [DOI] [PubMed] [Google Scholar]
- 93. Werth N, Schuette CG, Wilkening G, Lemm T, Sandhoff K. Degradation of membrane‐bound ganglioside GM2 by beta ‐hexosaminidase a. stimulation by GM2 activator protein and lysosomal lipids. J Biol Chem. 2001;276(16):12685‐12690. [DOI] [PubMed] [Google Scholar]
- 94. Goldstein JL, Brown MS. The low‐density lipoprotein pathway and its relation to atherosclerosis. Annu Rev Biochem. 1977;46:897‐930. [DOI] [PubMed] [Google Scholar]
- 95. Antonny B, Bigay J, Mesmin B. The oxysterol‐binding protein cycle: burning off PI(4)P to transport cholesterol. Annu Rev Biochem. 2018;87:809‐837. [DOI] [PubMed] [Google Scholar]
- 96. Brown MS, Goldstein JL. Cholesterol feedback: from Schoenheimer's bottle to Scap's MELADL. J Lipid Res. 2009;50(Suppl):S15‐S27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Maxfield FR, Menon AK. Intracellular sterol transport and distribution. Curr Opin Cell Biol. 2006;18(4):379‐385. [DOI] [PubMed] [Google Scholar]
- 98. Scott CC, Vossio S, Vacca F, et al. Wnt directs the endosomal flux of LDL‐derived cholesterol and lipid droplet homeostasis. EMBO Rep. 2015;16(6):741‐752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Scott CC, Vossio S, Rougemont J, Gruenberg J. TFAP2 transcription factors are regulators of lipid droplet biogenesis. elife. 2018;7:e36330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Lange Y, Ye J, Steck TL. How cholesterol homeostasis is regulated by plasma membrane cholesterol in excess of phospholipids. Proc Natl Acad Sci U S A. 2004;101(32):11664‐11667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Infante RE, Radhakrishnan A. Continuous transport of a small fraction of plasma membrane cholesterol to endoplasmic reticulum regulates total cellular cholesterol. elife. 2017;6:e25466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Ikonen E, Holtta‐Vuori M. Cellular pathology of Niemann‐pick type C disease. Semin Cell Dev Biol. 2004;15(4):445‐454. [DOI] [PubMed] [Google Scholar]
- 103. Schulze H, Sandhoff K. Lysosomal lipid storage diseases. Cold Spring Harb Perspect Biol. 2011;3(6):a009704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Carstea ED, Morris JA, Coleman KG, et al. Niemann‐pick C1 disease gene: homology to mediators of cholesterol homeostasis. Science (New York, NY). 1997;277(5323):228‐231. [DOI] [PubMed] [Google Scholar]
- 105. Naureckiene S, Sleat DE, Lackland H, et al. Identification of HE1 as the second gene of Niemann‐pick C disease. Science (New York, NY). 2000;290(5500):2298‐2301. [DOI] [PubMed] [Google Scholar]
- 106. Infante RE, Radhakrishnan A, Abi‐Mosleh L, et al. Purified NPC1 protein: II. Localization of sterol binding to a 240‐amino acid soluble luminal loop. J Biol Chem. 2008;283(2):1064‐1075. [DOI] [PubMed] [Google Scholar]
- 107. Xu S, Benoff B, Liou HL, Lobel P, Stock AM. Structural basis of sterol binding by NPC2, a lysosomal protein deficient in Niemann‐pick type C2 disease. J Biol Chem. 2007;282(32):23525‐23531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Kwon HJ, Abi‐Mosleh L, Wang ML, et al. Structure of N‐terminal domain of NPC1 reveals distinct subdomains for binding and transfer of cholesterol. Cell. 2009;137(7):1213‐1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Li X, Saha P, Li J, Blobel G, Pfeffer SR. Clues to the mechanism of cholesterol transfer from the structure of NPC1 middle lumenal domain bound to NPC2. Proc Natl Acad Sci U S A. 2016;113(36):10079‐10084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Wang ML, Motamed M, Infante RE, et al. Identification of surface residues on Niemann‐pick C2 essential for hydrophobic handoff of cholesterol to NPC1 in lysosomes. Cell Metab. 2010;12(2):166‐173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Gong X, Qian H, Zhou X, et al. Structural insights into the Niemann‐pick C1 (NPC1)‐mediated cholesterol transfer and Ebola infection. Cell. 2016;165(6):1467‐1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Infante RE, Wang ML, Radhakrishnan A, Kwon HJ, Brown MS, Goldstein JL. NPC2 facilitates bidirectional transfer of cholesterol between NPC1 and lipid bilayers, a step in cholesterol egress from lysosomes. Proc Natl Acad Sci U S A. 2008;105(40):15287‐15292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Trinh MN, Brown MS, Seemann J, Goldstein JL, Lu F. Lysosomal cholesterol export reconstituted from fragments of Niemann‐pick C1. elife. 2018;7:e38564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Pfeffer SR. NPC intracellular cholesterol transporter 1 (NPC1)‐mediated cholesterol export from lysosomes. J Biol Chem. 2019;294(5):1706‐1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Enkavi G, Mikkolainen H, Gungor B, Ikonen E, Vattulainen I. Concerted regulation of npc2 binding to endosomal/lysosomal membranes by bis(monoacylglycero)phosphate and sphingomyelin. PLoS Comput Biol. 2017;13(10):e1005831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Cheruku SR, Xu Z, Dutia R, Lobel P, Storch J. Mechanism of cholesterol transfer from the Niemann‐pick type C2 protein to model membranes supports a role in lysosomal cholesterol transport. J Biol Chem. 2006;281(42):31594‐31604. [DOI] [PubMed] [Google Scholar]
- 117. McCauliff LA, Langan A, Li R, et al. Intracellular cholesterol trafficking is dependent upon NPC2 interaction with lysobisphosphatidic acid. elife. 2019;8:e50832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Delton‐Vandenbroucke I, Bouvier J, Makino A, et al. Anti‐bis(monoacylglycero)phosphate antibody accumulates acetylated LDL‐derived cholesterol in cultured macrophages. J Lipid Res. 2007;48(3):543‐552. [DOI] [PubMed] [Google Scholar]
- 119. Chevallier J, Chamoun Z, Jiang G, et al. Lysobisphosphatidic acid controls endosomal cholesterol levels. J Biol Chem. 2008;283:27871‐27880. [DOI] [PubMed] [Google Scholar]
- 120. Walkley SU, Vanier MT. Secondary lipid accumulation in lysosomal disease. Biochim Biophys Acta. 2009;1793(4):726‐736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Meikle PJ, Duplock S, Blacklock D, et al. Effect of lysosomal storage on bis(monoacylglycero)phosphate. Biochem J. 2008;411(1):71‐78. [DOI] [PubMed] [Google Scholar]
- 122. Akgoc Z, Sena‐Esteves M, Martin DR, Han X, d'Azzo A, Seyfried TN. Bis(monoacylglycero)phosphate: a secondary storage lipid in the gangliosidoses. J Lipid Res. 2015;56(5):1006‐1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Sardiello M, Palmieri M, di Ronza A, et al. A gene network regulating lysosomal biogenesis and function. Science (New York, NY). 2009;325(5939):473‐477. [DOI] [PubMed] [Google Scholar]
- 124. Napolitano G, Ballabio A. TFEB at a glance. J Cell Sci. 2016;129(13):2475‐2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Sobo K, Le Blanc I, Luyet PP, et al. Late endosomal cholesterol accumulation leads to impaired intra‐endosomal trafficking. PLoS ONE. 2007;2(9):e851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Brotherus J, Niinioja T, Sandelin K, Renkonen O. Experimentally caused proliferation of lysosomes in cultured BHK cells involving an increase of biphosphatidic acids and triglycerides. J Lipid Res. 1977;18(3):379‐388. [PubMed] [Google Scholar]
- 127. Barelli H, Antonny B. Lipid unsaturation and organelle dynamics. Curr Opin Cell Biol. 2016;41:25‐32. [DOI] [PubMed] [Google Scholar]
- 128. Harroun TA, Katsaras J, Wassall SR. Cholesterol hydroxyl group is found to reside in the center of a polyunsaturated lipid membrane. Biochemistry. 2006;45(4):1227‐1233. [DOI] [PubMed] [Google Scholar]
- 129. van Meer G. Dynamic transbilayer lipid asymmetry. Cold Spring Harb Perspect Biol. 2011;3(5):a004671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Patterson MC, Mengel E, Vanier MT, et al. Stable or improved neurological manifestations during miglustat therapy in patients from the international disease registry for Niemann‐pick disease type C: an observational cohort study. Orphanet J Rare Dis. 2015;10:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Liu B, Ramirez CM, Miller AM, Repa JJ, Turley SD, Dietschy JM. Cyclodextrin overcomes the transport defect in nearly every organ of NPC1 mice leading to excretion of sequestered cholesterol as bile acid. J Lipid Res. 2010;51(5):933‐944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Rosenbaum AI, Zhang G, Warren JD, Maxfield FR. Endocytosis of beta‐cyclodextrins is responsible for cholesterol reduction in Niemann‐pick type C mutant cells. Proc Natl Acad Sci U S A. 2010;107(12):5477‐5482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Abi‐Mosleh L, Infante RE, Radhakrishnan A, Goldstein JL, Brown MS. Cyclodextrin overcomes deficient lysosome‐to‐endoplasmic reticulum transport of cholesterol in Niemann‐pick type C cells. Proc Natl Acad Sci U S A. 2009;106(46):19316‐19321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Davidson CD, Ali NF, Micsenyi MC, et al. Chronic cyclodextrin treatment of murine Niemann‐pick C disease ameliorates neuronal cholesterol and glycosphingolipid storage and disease progression. PLoS ONE. 2009;4(9):e6951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Camargo F, Erickson RP, Garver WS, et al. Cyclodextrins in the treatment of a mouse model of Niemann‐pick C disease. Life Sci. 2001;70(2):131‐142. [DOI] [PubMed] [Google Scholar]
- 136. Vite CH, Bagel JH, Swain GP, et al. Intracisternal cyclodextrin prevents cerebellar dysfunction and Purkinje cell death in feline Niemann‐pick type C1 disease. Sci Transl Med. 2015;7(276):276ra226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Liu B, Turley SD, Burns DK, Miller AM, Repa JJ, Dietschy JM. Reversal of defective lysosomal transport in NPC disease ameliorates liver dysfunction and neurodegeneration in the npc1‐/‐ mouse. Proc Natl Acad Sci U S A. 2009;106(7):2377‐2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Ory DS, Ottinger EA, Farhat NY, et al. Intrathecal 2‐hydroxypropyl‐beta‐cyclodextrin decreases neurological disease progression in Niemann‐pick disease, type C1: a non‐randomised, open‐label, phase 1‐2 trial. Lancet. 2017;390(10104):1758‐1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Vance JE, Karten B. Niemann‐pick C disease and mobilization of lysosomal cholesterol by cyclodextrin. J Lipid Res. 2014;55(8):1609‐1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Hammond N, Munkacsi AB, Sturley SL. The complexity of a monogenic neurodegenerative disease: more than two decades of therapeutic driven research into Niemann‐pick type C disease. Biochim Biophys Acta Mol Cell Biol Lipids. 2019;1864(8):1109‐1123. [DOI] [PubMed] [Google Scholar]
- 141. Vacca F, Vossio S, Mercier V, et al. Cyclodextrin triggers MCOLN1‐dependent endo‐lysosome secretion in Niemann‐pick type C cells. J Lipid Res. 2019;60(4):832‐843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Boudewyn LC, Walkley SU. Current concepts in the neuropathogenesis of mucolipidosis type IV. J Neurochem. 2019;148(5):669‐689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Medina DL, Fraldi A, Bouche V, et al. Transcriptional activation of lysosomal exocytosis promotes cellular clearance. Dev Cell. 2011;21(3):421‐430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Samie MA, Xu H. Lysosomal exocytosis and lipid storage disorders. J Lipid Res. 2014;55(6):995‐1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Martina JA, Diab HI, Lishu L, et al. The nutrient‐responsive transcription factor TFE3 promotes autophagy, lysosomal biogenesis, and clearance of cellular debris. Sci Signal. 2014;7(309):ra9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. LaPlante JM, Sun M, Falardeau J, et al. Lysosomal exocytosis is impaired in mucolipidosis type IV. Mol Genet Metab. 2006;89(4):339‐348. [DOI] [PubMed] [Google Scholar]
- 147. Dong XP, Wang X, Shen D, et al. Activating mutations of the TRPML1 channel revealed by proline‐scanning mutagenesis. J Biol Chem. 2009;284(46):32040‐32052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Wenzel EM, Schultz SW, Schink KO, et al. Concerted ESCRT and clathrin recruitment waves define the timing and morphology of intraluminal vesicle formation. Nat Commun. 2018;9(1):2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Wang L, Seeley ES, Wickner W, Merz AJ. Vacuole fusion at a ring of vertex docking sites leaves membrane fragments within the organelle. Cell. 2002;108(3):357‐369. [DOI] [PubMed] [Google Scholar]
- 150. McNally EK, Brett CL. The intralumenal fragment pathway mediates ESCRT‐independent surface transporter down‐regulation. Nat Commun. 2018;9(1):5358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Raiborg C, Stenmark H. The ESCRT machinery in endosomal sorting of ubiquitylated membrane proteins. Nature. 2009;458(7237):445‐452. [DOI] [PubMed] [Google Scholar]
- 152. Gatta AT, Carlton JG. The ESCRT‐machinery: closing holes and expanding roles. Curr Opin Cell Biol. 2019;59:121‐132. [DOI] [PubMed] [Google Scholar]
- 153. Skowyra ML, Schlesinger PH, Naismith TV, Hanson PI. Triggered recruitment of ESCRT machinery promotes endolysosomal repair. Science (New York, NY). 2018;360(6384):eaar5078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Radulovic M, Schink KO, Wenzel EM, et al. ESCRT‐mediated lysosome repair precedes lysophagy and promotes cell survival. EMBO J. 2018;37(21):e99753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Doyotte A, Mironov A, McKenzie E, Woodman P. The Bro1‐related protein HD‐PTP/PTPN23 is required for endosomal cargo sorting and multivesicular body morphogenesis. Proc Natl Acad Sci U S A. 2008;105(17):6308‐6313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Parkinson MD, Piper SC, Bright NA, et al. A non‐canonical ESCRT pathway, including histidine domain phosphotyrosine phosphatase (HD‐PTP), is used for down‐regulation of virally ubiquitinated MHC class I. Biochem J. 2015;471(1):79‐88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Tabernero L, Woodman P. Dissecting the role of his domain protein tyrosine phosphatase/PTPN23 and ESCRTs in sorting activated epidermal growth factor receptor to the multivesicular body. Biochem Soc Trans. 2018;46(5):1037‐1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Dores MR, Chen B, Lin H, et al. ALIX binds a YPX3L motif of the GPCR PAR1 and mediates ubiquitin‐independent ESCRT‐III/MVB sorting. J Cell Biol. 2012;197(3):407‐419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Dores MR, Grimsey NJ, Mendez F, Trejo J. ALIX regulates the ubiquitin‐independent Lysosomal sorting of the P2Y1 Purinergic receptor via a YPX3L motif. PLoS ONE. 2016;11(6):e0157587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Dores MR, Trejo J. Endo‐lysosomal sorting of G‐protein‐coupled receptors by ubiquitin: diverse pathways for G‐protein‐coupled receptor destruction and beyond. Traffic. 2019;20(2):101‐109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Ma H, Wardega P, Mazaud D, et al. Histidine‐domain‐containing protein tyrosine phosphatase regulates platelet‐derived growth factor receptor intracellular sorting and degradation. Cell Signal. 2015;27(11):2209‐2219. [DOI] [PubMed] [Google Scholar]
- 162. Kharitidi D, Apaja PM, Manteghi S, et al. Interplay of Endosomal pH and ligand occupancy in integrin alpha5beta1 Ubiquitination, Endocytic sorting, and cell migration. Cell Rep. 2015;13(3):599‐609. [DOI] [PubMed] [Google Scholar]
- 163. Alonso YAM, Migliano SM, Teis D. ESCRT‐III and Vps4: a dynamic multipurpose tool for membrane budding and scission. FEBS J. 2016;283(18):3288‐3302. [DOI] [PubMed] [Google Scholar]
- 164. Chiaruttini N, Roux A. Dynamic and elastic shape transitions in curved ESCRT‐III filaments. Curr Opin Cell Biol. 2017;47:126‐135. [DOI] [PubMed] [Google Scholar]
- 165. Caillat C, Maity S, Miguet N, Roos WH, Weissenhorn W. The role of VPS4 in ESCRT‐III polymer remodeling. Biochem Soc Trans. 2019;47(1):441‐448. [DOI] [PubMed] [Google Scholar]
- 166. Mierzwa BE, Chiaruttini N, Redondo‐Morata L, et al. Dynamic subunit turnover in ESCRT‐III assemblies is regulated by Vps4 to mediate membrane remodelling during cytokinesis. Nat Cell Biol. 2017;19(7):787‐798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Adell MAY, Migliano SM, Upadhyayula S, et al. Recruitment dynamics of ESCRT‐III and Vps4 to endosomes and implications for reverse membrane budding. elife. 2017;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Vietri M, Schink KO, Campsteijn C, et al. Spastin and ESCRT‐III coordinate mitotic spindle disassembly and nuclear envelope sealing. Nature. 2015;522(7555):231‐235. [DOI] [PubMed] [Google Scholar]
- 169. Olmos Y, Hodgson L, Mantell J, Verkade P, Carlton JG. ESCRT‐III controls nuclear envelope reformation. Nature. 2015;522(7555):236‐239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Denais CM, Gilbert RM, Isermann P, et al. Nuclear envelope rupture and repair during cancer cell migration. Science (New York, NY). 2016;352(6283):353‐358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Raab M, Gentili M, de Belly H, et al. ESCRT III repairs nuclear envelope ruptures during cell migration to limit DNA damage and cell death. Science (New York, NY). 2016;352(6283):359‐362. [DOI] [PubMed] [Google Scholar]
- 172. Jimenez AJ, Maiuri P, Lafaurie‐Janvore J, Divoux S, Piel M. Perez F. ESCRT machinery is required for plasma membrane repair. Science (New York, NY). 2014;343(6174):1247136. [DOI] [PubMed] [Google Scholar]
- 173. Scheffer LL, Sreetama SC, Sharma N, et al. Mechanism of Ca(2)(+)‐triggered ESCRT assembly and regulation of cell membrane repair. Nat Commun. 2014;5:5646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Lopez‐Jimenez AT, Cardenal‐Munoz E, Leuba F, et al. The ESCRT and autophagy machineries cooperate to repair ESX‐1‐dependent damage at the mycobacterium‐containing vacuole but have opposite impact on containing the infection. PLoS Pathog. 2018;14(12):e1007501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Stuffers S, Sem Wegner C, Stenmark H, Brech A. Multivesicular endosome biogenesis in the absence of ESCRTs. Traffic. 2009;10(7):925‐937. [DOI] [PubMed] [Google Scholar]
- 176. Theos AC, Truschel ST, Tenza D, et al. A lumenal domain‐dependent pathway for sorting to intralumenal vesicles of multivesicular endosomes involved in organelle morphogenesis. Dev Cell. 2006;10(3):343‐354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. van Niel G, Charrin S, Simoes S, et al. The tetraspanin CD63 regulates ESCRT‐independent and ‐dependent endosomal sorting during melanogenesis. Dev Cell. 2011;21(4):708‐721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Edgar JR, Eden ER, Futter CE. Hrs‐ and CD63‐dependent competing mechanisms make different sized endosomal intraluminal vesicles. Traffic. 2014;15(2):197‐211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. White IJ, Bailey LM, Aghakhani MR, Moss SE, Futter CE. EGF stimulates annexin 1‐dependent inward vesiculation in a multivesicular endosome subpopulation. EMBO J. 2006;25(1):1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Falguières T, Castle JD, Gruenberg J. Regulation of the MVB pathway by SCAMP3. Traffic. 2012;13:131‐142. [DOI] [PubMed] [Google Scholar]
- 181. Tomas A, Vaughan SO, Burgoyne T, et al. WASH and Tsg101/ALIX‐dependent diversion of stress‐internalized EGFR from the canonical endocytic pathway. Nat Commun. 2015;6:7324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Mizushima N. A brief history of autophagy from cell biology to physiology and disease. Nat Cell Biol. 2018;20(5):521‐527. [DOI] [PubMed] [Google Scholar]
- 183. Tsuji T, Fujimoto M, Tatematsu T, et al. Niemann‐pick type C proteins promote microautophagy by expanding raft‐like membrane domains in the yeast vacuole. elife. 2017;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Liu XM, Sun LL, Hu W, Ding YH, Dong MQ, Du LL. ESCRTs cooperate with a selective autophagy receptor to mediate Vacuolar targeting of soluble cargos. Mol Cell. 2015;59(6):1035‐1042. [DOI] [PubMed] [Google Scholar]
- 185. Sahu R, Kaushik S, Clement CC, et al. Microautophagy of cytosolic proteins by late endosomes. Dev Cell. 2011;20(1):131‐139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Oku M, Maeda Y, Kagohashi Y, et al. Evidence for ESCRT‐ and clathrin‐dependent microautophagy. J Cell Biol. 2017;216(10):3263‐3274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Mejlvang J, Olsvik H, Svenning S, et al. Starvation induces rapid degradation of selective autophagy receptors by endosomal microautophagy. J Cell Biol. 2018;217(10):3640‐3655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Hatakeyama R, De Virgilio C. TORC1 specifically inhibits microautophagy through ESCRT‐0. Curr Genet. 2019;65(5):1243‐1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Lefebvre C, Legouis R, Culetto E. ESCRT and autophagies: Endosomal functions and beyond. Semin Cell Dev Biol. 2018;74:21‐28. [DOI] [PubMed] [Google Scholar]
- 190. Mukherjee A, Patel B, Koga H, Cuervo AM, Jenny A. Selective endosomal microautophagy is starvation‐inducible in drosophila. Autophagy. 2016;12(11):1984‐1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Murrow L, Malhotra R, Debnath J. ATG12‐ATG3 interacts with Alix to promote basal autophagic flux and late endosome function. Nat Cell Biol. 2015;17(3):300‐310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Kowal J, Arras G, Colombo M, et al. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc Natl Acad Sci U S A. 2016;113(8):E968‐E977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Simons M, Raposo G. Exosomes–vesicular carriers for intercellular communication. Curr Opin Cell Biol. 2009;21(4):575‐581. [DOI] [PubMed] [Google Scholar]
- 194. Colombo M, Moita C, van Niel G, et al. Analysis of ESCRT functions in exosome biogenesis, composition and secretion highlights the heterogeneity of extracellular vesicles. J Cell Sci. 2013;126(24):5553‐5565. [DOI] [PubMed] [Google Scholar]
- 195. Baietti MF, Zhang Z, Mortier E, et al. Syndecan‐syntenin‐ALIX regulates the biogenesis of exosomes. Nat Cell Biol. 2012;14(7):677‐685. [DOI] [PubMed] [Google Scholar]
- 196. Ghossoub R, Lembo F, Rubio A, et al. Syntenin‐ALIX exosome biogenesis and budding into multivesicular bodies are controlled by ARF6 and PLD2. Nat Commun. 2014;5:3477. [DOI] [PubMed] [Google Scholar]
- 197. Trajkovic K, Hsu C, Chiantia S, et al. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science (New York, NY). 2008;319(5867):1244‐1247. [DOI] [PubMed] [Google Scholar]
- 198. Denzer K, van Eijk M, Kleijmeer MJ, Jakobson E, de Groot C, Geuze HJ. Follicular dendritic cells carry MHC class II‐expressing microvesicles at their surface. J Immunol. 2000;165(3):1259‐1265. [DOI] [PubMed] [Google Scholar]
- 199. Thery C, Ostrowski M, Segura E. Membrane vesicles as conveyors of immune responses. Nat Rev Immunol. 2009;9(8):581‐593. [DOI] [PubMed] [Google Scholar]
- 200. Kalra H, Simpson RJ, Ji H, et al. Vesiclepedia: a compendium for extracellular vesicles with continuous community annotation. PLoS Biol. 2012;10(12):e1001450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Katzmann DJ, Odorizzi G, Emr SD. Receptor downregulation and multivesicular‐body sorting. Nat Rev Mol Cell Biol. 2002;3(12):893‐905. [DOI] [PubMed] [Google Scholar]
- 202. Mageswaran SK, Dixon MG, Curtiss M, Keener JP, Babst M. Binding to any ESCRT can mediate ubiquitin‐independent cargo sorting. Traffic. 2014;15(2):212‐229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Gillooly DJ, Morrow IC, Lindsay M, et al. Localization of phosphatidylinositol 3‐phosphate in yeast and mammalian cells. EMBO J. 2000;19(17):4577‐4588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Babst M. MVB vesicle formation: ESCRT‐dependent, ESCRT‐independent and everything in between. Curr Opin Cell Biol. 2011;23(4):452‐457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Miranda AM, Lasiecka ZM, Xu Y, et al. Neuronal lysosomal dysfunction releases exosomes harboring APP C‐terminal fragments and unique lipid signatures. Nat Commun. 2018;9(1):291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Lozach PY, Huotari J, Helenius A. Late‐penetrating viruses. Curr Opin Virol. 2011;1(1):35‐43. [DOI] [PubMed] [Google Scholar]
- 207. Abrami L, Lindsay M, Parton RG, Leppla SH, van der Goot FG. Membrane insertion of anthrax protective antigen and cytoplasmic delivery of lethal factor occur at different stages of the endocytic pathway. J Cell Biol. 2004;166(5):645‐651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Jae LT, Raaben M, Herbert AS, et al. Virus entry. Lassa virus entry requires a trigger‐induced receptor switch. Science (New York, NY). 2014;344(6191):1506‐1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Shtanko O, Nikitina RA, Altuntas CZ, Chepurnov AA, Davey RA. Crimean‐Congo hemorrhagic fever virus entry into host cells occurs through the multivesicular body and requires ESCRT regulators. PLoS Pathog. 2014;10(9):e1004390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Grassel L, Fast LA, Scheffer KD, et al. The CD63‐Syntenin‐1 complex controls post‐Endocytic trafficking of oncogenic human papillomaviruses. Sci Rep. 2016;6:32337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Sakurai Y, Kolokoltsov AA, Chen CC, et al. Ebola virus. Two‐pore channels control Ebola virus host cell entry and are drug targets for disease treatment. Science (New York, NY). 2015;347(6225):995‐998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Carette JE, Raaben M, Wong AC, et al. Ebola virus entry requires the cholesterol transporter Niemann‐pick C1. Nature. 2011;477(7364):340‐343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Cote M, Misasi J, Ren T, et al. Small molecule inhibitors reveal Niemann‐pick C1 is essential for Ebola virus infection. Nature. 2011;477(7364):344‐348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Khor R, McElroy LJ, Whittaker GR. The ubiquitin‐vacuolar protein sorting system is selectively required during entry of influenza virus into host cells. Traffic. 2003;4(12):857‐868. [DOI] [PubMed] [Google Scholar]
- 215. Huotari J, Meyer‐Schaller N, Hubner M, et al. Cullin‐3 regulates late endosome maturation. Proc Natl Acad Sci U S A. 2012;109(3):823‐828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Gschweitl M, Ulbricht A, Barnes CA, et al. A SPOPL/Cullin‐3 ubiquitin ligase complex regulates endocytic trafficking by targeting EPS15 at endosomes. elife. 2016;5:e13841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Nour AM, Li Y, Wolenski J, Modis Y. Viral membrane fusion and nucleocapsid delivery into the cytoplasm are distinct events in some flaviviruses. PLoS Pathog. 2013;9(9):e1003585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Gruenberg J, van der Goot FG. Mechanisms of pathogen entry through the endosomal compartments. Nat Rev Mol Cell Biol. 2006;7(7):495‐504. [DOI] [PubMed] [Google Scholar]
- 219. Zaitseva E, Yang ST, Melikov K, Pourmal S, Chernomordik LV. Dengue virus ensures its fusion in late endosomes using compartment‐specific lipids. PLoS Pathog. 2010;6(10):e1001131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Roth SL, Whittaker GR. Promotion of vesicular stomatitis virus fusion by the endosome‐specific phospholipid bis(monoacylglycero)phosphate (BMP). FEBS Lett. 2011;585(6):865‐869. [DOI] [PubMed] [Google Scholar]
- 221. Patel A, Mohl BP, Roy P. Entry of bluetongue virus capsid requires the late endosome‐specific lipid Lysobisphosphatidic acid. J Biol Chem. 2016;291(23):12408‐12419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Radulovic M, Stenmark H. ESCRTs in membrane sealing. Biochem Soc Trans. 2018;46(4):773‐778. [DOI] [PubMed] [Google Scholar]
- 223. Mittal E, Skowyra ML, Uwase G, et al. Mycobacterium tuberculosis type VII secretion system effectors differentially impact the ESCRT endomembrane damage response. MBio. 2018;9(6):e01765‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Nolte‐'t Hoen E, Cremer T, Gallo RC, Margolis LB. Extracellular vesicles and viruses: are they close relatives? Proc Natl Acad Sci U S A. 2016;113(33):9155‐9161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Ramakrishnaiah V, Thumann C, Fofana I, et al. Exosome‐mediated transmission of hepatitis C virus between human hepatoma Huh7.5 cells. Proc Natl Acad Sci U S A. 2013;110(32):13109‐13113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Tamai K, Shiina M, Tanaka N, et al. Regulation of hepatitis C virus secretion by the Hrs‐dependent exosomal pathway. Virology. 2012;422(2):377‐385. [DOI] [PubMed] [Google Scholar]
- 227. Dreux M, Garaigorta U, Boyd B, et al. Short‐range exosomal transfer of viral RNA from infected cells to plasmacytoid dendritic cells triggers innate immunity. Cell Host Microbe. 2012;12(4):558‐570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Bukong TN, Momen‐Heravi F, Kodys K, Bala S, Szabo G. Exosomes from hepatitis C infected patients transmit HCV infection and contain replication competent viral RNA in complex with Ago2‐miR122‐HSP90. PLoS Pathog. 2014;10(10):e1004424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Chahar HS, Bao X, Casola A. Exosomes and their role in the life cycle and pathogenesis of RNA viruses. Viruses. 2015;7(6):3204‐3225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Urbanelli L, Buratta S, Tancini B, et al. The role of extracellular vesicles in viral infection and transmission. Vaccines (Basel). 2019;7(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Feng Z, Hensley L, McKnight KL, et al. A pathogenic picornavirus acquires an envelope by hijacking cellular membranes. Nature. 2013;496(7445):367‐371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. Rivera‐Serrano EE, Gonzalez‐Lopez O, Das A, Lemon SM. Cellular entry and uncoating of naked and quasi‐enveloped human hepatoviruses. elife. 2019;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233. Miao Y, Li G, Zhang X, Xu H, Abraham SN. A TRP Channel senses lysosome neutralization by pathogens to trigger their expulsion. Cell. 2015;161(6):1306‐1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234. Scott CC, Gruenberg J. Ion flux and the function of endosomes and lysosomes: pH is just the start: the flux of ions across endosomal membranes influences endosome function not only through regulation of the luminal pH. BioEssays. 2011;33(2):103‐110. [DOI] [PubMed] [Google Scholar]
- 235. Li P, Gu M, Xu H. Lysosomal ion channels as decoders of cellular signals. Trends Biochem Sci. 2019;44(2):110‐124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236. Sterea AM, Almasi S, El Hiani Y. The hidden potential of lysosomal ion channels: a new era of oncogenes. Cell Calcium. 2018;72:91‐103. [DOI] [PubMed] [Google Scholar]
- 237. Grimm C, Butz E, Chen CC, Wahl‐Schott C, Biel M. From mucolipidosis type IV to Ebola: TRPML and two‐pore channels at the crossroads of endo‐lysosomal trafficking and disease. Cell Calcium. 2017;67:148‐155. [DOI] [PubMed] [Google Scholar]
- 238. Jentsch TJ, Pusch M. CLC chloride channels and transporters: structure, function, physiology, and disease. Physiol Rev. 2018;98(3):1493‐1590. [DOI] [PubMed] [Google Scholar]
- 239. Xu H, Ren D. Lysosomal physiology. Annu Rev Physiol. 2015;77:57‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240. Forgac M. Vacuolar ATPases: rotary proton pumps in physiology and pathophysiology. Nat Rev Mol Cell Biol. 2007;8(11):917‐929. [DOI] [PubMed] [Google Scholar]
- 241. Mindell JA. Lysosomal acidification mechanisms. Annu Rev Physiol. 2012;74:69‐86. [DOI] [PubMed] [Google Scholar]
- 242. Smith AE, Helenius A. How viruses enter animal cells. Science (New York, NY). 2004;304(5668):237‐242. [DOI] [PubMed] [Google Scholar]
- 243. Zhong XZ, Yang Y, Sun X, Dong XP. Methods for monitoring Ca(2+) and ion channels in the lysosome. Cell Calcium. 2017;64:20‐28. [DOI] [PubMed] [Google Scholar]
- 244. Patel S. Function and dysfunction of two‐pore channels. Sci Signal. 2015;8(384):re7. [DOI] [PubMed] [Google Scholar]
- 245. Wang X, Zhang X, Dong XP, et al. TPC proteins are phosphoinositide‐ activated sodium‐selective ion channels in endosomes and lysosomes. Cell. 2012;151(2):372‐383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246. Medina DL, Di Paola S, Peluso I, et al. Lysosomal calcium signalling regulates autophagy through calcineurin and TFEB. Nat Cell Biol. 2015;17(3):288‐299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247. Chen CC, Keller M, Hess M, et al. A small molecule restores function to TRPML1 mutant isoforms responsible for mucolipidosis type IV. Nat Commun. 2014;5:4681. [DOI] [PubMed] [Google Scholar]
- 248. Tian X, Gala U, Zhang Y, et al. A voltage‐gated calcium channel regulates lysosomal fusion with endosomes and autophagosomes and is required for neuronal homeostasis. PLoS Biol. 2015;13(3):e1002103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249. Lloyd‐Evans E, Morgan AJ, He X, et al. Niemann‐pick disease type C1 is a sphingosine storage disease that causes deregulation of lysosomal calcium. Nat Med. 2008;14(11):1247‐1255. [DOI] [PubMed] [Google Scholar]
- 250. Shen D, Wang X, Li X, et al. Lipid storage disorders block lysosomal trafficking by inhibiting a TRP channel and lysosomal calcium release. Nat Commun. 2012;3:731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251. Gomez NM, Lu W, Lim JC, et al. Robust lysosomal calcium signaling through channel TRPML1 is impaired by lysosomal lipid accumulation. FASEB J. 2018;32(2):782‐794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252. Corbett EF, Michalak M. Calcium, a signaling molecule in the endoplasmic reticulum? Trends Biochem Sci. 2000;25(7):307‐311. [DOI] [PubMed] [Google Scholar]
- 253. Christensen KA, Myers JT, Swanson JA. pH‐dependent regulation of lysosomal calcium in macrophages. J Cell Sci. 2002;115(Pt 3):599‐607. [DOI] [PubMed] [Google Scholar]
- 254. Meldolesi J, Pozzan T. The endoplasmic reticulum Ca2+ store: a view from the lumen. Trends Biochem Sci. 1998;23(1):10‐14. [DOI] [PubMed] [Google Scholar]
- 255. Coe H, Michalak M. Calcium binding chaperones of the endoplasmic reticulum. Gen Physiol Biophys. 2009;28:F96‐F103. [PubMed] [Google Scholar]
- 256. Morgan AJ, Platt FM, Lloyd‐Evans E, Galione A. Molecular mechanisms of endolysosomal Ca2+ signalling in health and disease. Biochem J. 2011;439(3):349‐374. [DOI] [PubMed] [Google Scholar]
- 257. Segawa K, Nagata S. An apoptotic 'Eat Me' signal: Phosphatidylserine exposure. Trends Cell Biol. 2015;25(11):639‐650. [DOI] [PubMed] [Google Scholar]
- 258. Akutsu H, Seelig J. Interaction of metal ions with phosphatidylcholine bilayer membranes. Biochemistry. 1981;20(26):7366‐7373. [DOI] [PubMed] [Google Scholar]
- 259. Huster D, Arnold K, Gawrisch K. Strength of Ca(2+) binding to retinal lipid membranes: consequences for lipid organization. Biophys J. 2000;78(6):3011‐3018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260. Melcrova A, Pokorna S, Pullanchery S, et al. The complex nature of calcium cation interactions with phospholipid bilayers. Sci Rep. 2016;6:38035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261. Melcr J, Martinez‐Seara H, Nencini R, Kolafa J, Jungwirth P, Ollila OHS. Accurate binding of sodium and calcium to a POPC bilayer by effective inclusion of electronic polarization. J Phys Chem B. 2018;122(16):4546‐4557. [DOI] [PubMed] [Google Scholar]
- 262. Papahadjopoulos D, Nir S, Duzgunes N. Molecular mechanisms of calcium‐induced membrane fusion. J Bioenerg Biomembr. 1990;22(2):157‐179. [DOI] [PubMed] [Google Scholar]
- 263. Razi M, Futter CE. Distinct roles for Tsg101 and Hrs in multivesicular body formation and inward vesiculation. Mol Biol Cell. 2006;17(8):3469‐3483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264. Pons V, Luyet P‐P, Morel E, et al. Hrs and SNX3 functions in sorting and membrane invagination within multivesicular bodies. PLoS Biol. 2008;6(9):e214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265. Wemmer M, Azmi I, West M, Davies B, Katzmann D, Odorizzi G. Bro1 binding to Snf7 regulates ESCRT‐III membrane scission activity in yeast. J Cell Biol. 2011;192(2):295‐306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266. Falguières T, Luyet PP, Bissig C, Scott CC, Velluz M‐C, Gruenberg J. In vitro budding of intralumenal vesicles into late endosomes is regulated by Alix and Tsg101. Mol Biol Cell. 2008;19:4942‐4955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267. Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome‐mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9(6):654‐659. [DOI] [PubMed] [Google Scholar]
- 268. Yu B, Kim HW, Gong M, et al. Exosomes secreted from GATA‐4 overexpressing mesenchymal stem cells serve as a reservoir of anti‐apoptotic microRNAs for cardioprotection. Int J Cardiol. 2015;182:349‐360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269. Bethune J, Artus‐Revel CG, Filipowicz W. Kinetic analysis reveals successive steps leading to miRNA‐mediated silencing in mammalian cells. EMBO Rep. 2012;13(8):716‐723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270. Griffiths G. Back R, marsh M. a quantitative analysis of the endocytic pathway in baby hamster kidney cells. J Cell Biol. 1989;109(6 Pt 1):2703‐2720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271. Chakraborty K, Leung K, Krishnan Y. High lumenal chloride in the lysosome is critical for lysosome function. elife. 2017;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272. Park SH, Hyun JY, Shin I. A lysosomal chloride ion‐selective fluorescent probe for biological applications. Chem Sci. 2019;10(1):56‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273. Kilpatrick BS, Eden ER, Schapira AH, Futter CE, Patel S. Direct mobilisation of lysosomal Ca2+ triggers complex Ca2+ signals. J Cell Sci. 2013;126(1):60‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274. Wang W, Zhang X, Gao Q, et al. A voltage‐dependent K(+) channel in the lysosome is required for refilling lysosomal Ca(2+) stores. J Cell Biol. 2017;216(6):1715‐1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275. Melchionda M, Pittman JK, Mayor R, Patel S. Ca2+/H+ exchange by acidic organelles regulates cell migration in vivo. J Cell Biol. 2016;212(7):803‐813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276. Garrity AG, Wang W, Collier CM, Levey SA, Gao Q, Xu H. The endoplasmic reticulum, not the pH gradient, drives calcium refilling of lysosomes. elife. 2016;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Movie S1 The Movie shows the tomographic reconstruction of a late endosome/endolysosome in Cos cells, which illustrates the complexity of the intralumenal membrane system, including multilamellar regions with a Russian Doll‐like organization. In this tomogram, ILVs are clearly visible (and definitely bilayered), as well as other intralumenal membranes, including presumably remnants of organelles delivered by autophagy. Cells were fast frozen, and then processed for rapid freeze substitution. Images represent a 300 nm section of a typical endosome. [Courtesy of Robert G. Parton, Brisbane, Australia]


