Abstract
Objectives
Investigation of novel vertical radiation shield (VRS) in reducing operator radiation exposure.
Background
Radiation exposure to the operator remains an occupational health hazard in the cardiac catheterization laboratory (CCL).
Methods
A mannequin simulating an operator was placed near a computational phantom, simulating a patient. Measurement of dose equivalent and Air Kerma located the angle with the highest radiation, followed by a common magnification (8 in.) and comparison of horizontal radiation absorbing pads (HRAP) with or without VRS with two different: CCL, phantoms, and dosimeters. Physician exposure was subsequently measured prospectively with or without VRS during clinical procedures.
Results
Dose equivalent and Air Kerma to the mannequin was highest at left anterior oblique (LAO)‐caudal angle (p < .005). Eight‐inch magnification increased mGray by 86.5% and μSv/min by 12.2% compared to 10‐in. (p < .005). Moving 40 cm from the access site lowered μSv/min by 30% (p < .005). With LAO‐caudal angle and 8‐in. magnification, VRS reduced μSv/min by 59%, (p < .005) in one CCL and μSv by 100% (p = .016) in second CCL in addition to HRAP. Prospective study of 177 procedures with HRAP, found VRS lowered μSv by 41.9% (μSv: 15.2 ± 13.4 vs. 26.2 ± 31.4, p = .001) with no difference in mGray. The difference was significant after multivariate adjustment for specified variables (p < .001).
Conclusions
Operator radiation exposure is significantly reduced utilizing a novel VRS, HRAP, and distance from the X‐ray tube, and consideration of lower magnification and avoiding LAO‐caudal angles to lower radiation for both operator and patient.
Keywords: Air Kerma, distance, dose equivalent, horizontal radiation absorbing pad, magnification
1. INTRODUCTION
Radiation exposure in the cardiac catheterization laboratory (CCL) is known to have both stochastic and deterministic effects on the patient and the operator.1, 2, 3, 4, 5 Fluoroscopy‐guided transcatheter interventions have become more complex over the past decade with chronic total occlusion techniques, high‐risk coronary interventions, mechanical support devices, and structural interventions in a population with rising body mass index (BMI) that may increase radiation exposure.6, 7, 8 Simple methods and devices to reduce radiation include avoidance of left anterior oblique (LAO) or steep caudal or cranial angles, lower fluoroscopy frame rates, several shields, and greater distance from the X‐ray tube.9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23 A variety of more expensive, although very effective systems have been developed in reducing operator radiation exposure, including the use of a suspended radiation protection system and vascular robotics.24, 25, 26, 27 The primary aim of this study was to compare operator radiation exposure with or without a novel vertical radiation shield (VRS) using first a mannequin and a human computational phantom, followed by evaluation during clinical procedures in the CCL.
2. METHODS
2.1. Computational human phantom models
2.1.1. Phantom Model A
Prior to comparing various shields, a computational human phantom (United States Department of Energy) was placed on a CCL table (Toshiba Infinix, Irving, CA) to simulate a patient and to locate the position with the highest impact on scatter radiation. Various angles (measured in degrees), magnifications, and distances were evaluated in triplicate. Both Air Kinetic Energy Released per unit Mass (Kerma) measured in mGray per hour (mGy/hr) provided by the CCL. X‐ray radiation exposure or dose equivalent to the wrist of the mannequin(operator) was measured at 10 mm below the skin in microSieverts per minute (μSv/min) provided by a dosimeter (Fluke RaySafe 2, Glenwood, IL) after fluoroscopy for a period of time until the exposure was stable (Figure 1a). Source to image distance (SID) was measured between 107 and 116 cm and interventional reference point was 15 cm from the isocenter to the X‐ray source for measurement of Air Kerma. Measurement of radiation was collected with the following angles: LAO‐Caudal (Caud); LAO‐Cranial (Cran); right anterior oblique (RAO)‐Cran, and RAO‐Caud. After defining the angle with the highest radiation exposure, this angle was used in comparison of magnification zoom fields set at 6, 8, and 10 in. Using this same angle and an 8 in. field (as this magnification is used by most operators in this institution), radiation exposure was compared with the mannequin positioned at the access site, then moved 40 and 120 cm caudal from the access site. This was followed by comparison with use of HRAP (Radpad Yellow, Worldwide Innovations & Technologies, Kansas City, MO) alone or in conjunction with a VRS (Steradian, Radux Devices, MapleGrove, MN).
Figure 1.
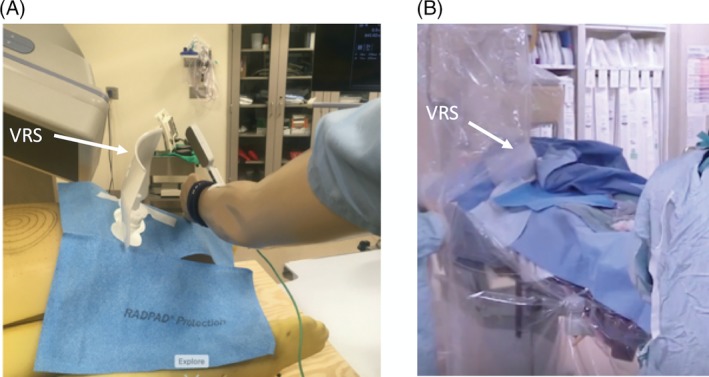
(a) Position of mannequin, when testing both horizontal radiation absorbing pad (HRAP) and vertical radiation shield (VRS; white arrow) with human computational phantom. (b) Example of VRS placement (white arrow) with both the ceiling‐mounted shield and HRAP with left radial access during a clinical procedure [Color figure can be viewed at https://wileyonlinelibrary.com]
2.1.2. Phantom Model B
The above methods were repeated with a different computational human phantom (Alderson RANDO, Imaging Solutions, Cypress, TX), CCL (Phillips, Allura system FD 10 and FD 20, Andover, MA) with a HRAP (Microtek, Ecolab, St. Paul, MN), and dose equivalent measured at the wrist also 10 mm below the skin with RAD‐60R dosimeter (RADOS, Turku, Finland) on the same mannequin. SID was measured between 103 and 119 cm with and interventional reference point was 15 cm from the isocenter to the X‐ray source. Both Air Kerma (mGy) and dose equivalent in milli Roentgen equivalent man (converted to μSv) was measured in triplicate after 15 seconds of cine.
2.2. Clinical procedures
Operator radiation exposure was measured in a prospective manner in the setting of two CCLs (Phillips, Allura system, Andover, MA): CCL A installed in 2008 and CCL B installed in 2016 with clarity software,19 among four interventional cardiologists with internal review board approval. Operator radiation exposure was measured and recorded after each case using dosimeter RAD‐60R (RADOS, Turku, Finland) placed on the left wrist of an interventional cardiologist under their sterile gown. All cases had at least two HRAP (Radpad Yellow, Worldwide Innovations & Technologies, Kansas City, MO), a ceiling‐mounted upper body radiation protection shield with a patient contour cutout, and a lower lead shield attached to the side of the CCL Table. A series of cases were done with and without the VRS, placed between the access site and the detector in the area not covered by the ceiling‐mounted shield (Figure 1b). The VRS was adhered to the drape and flexed at the base to conform to body habitus and location.
2.3. Statistical analyses
Use of t‐test with two samples assuming equal variances on analysis of radiation using the human phantom and mannequin for data acquired in both CCL with both human phantoms. For the data acquired from the clinical cases, use of descriptive statistics, t‐test with two samples assuming unequal variances compared the variables in those with and without use of VRS. Inferential statistics with MANCOVA to determine whether there were significant differences in μSv to the wrist between the two groups after controlling for covariates of interest including BMI, magnification (8 or 10 in.), percutaneous intervention, additional large injection such as left ventriculography/subclavian or femoral injection, access site (radial, femoral, or both radial/femoral). MANCOVA is a combination of a MANOVA preceded by a regression analysis. A level of significance of .05 was used in the MANCOVA. The data of the skewness statistics (1.94 and 2.71) were not greater than three and kurtosis statistics (5.44 and 8.73) were not in the range of 10–20 for non‐normality. Thus, the data of both μSv and mGray exhibited a normal distribution. Significance level was set at two‐sided alpha of .05. Statistical analyses were performed using Microsoft Exel (Version 16.16.10) and IBM SPSS programs.
3. RESULTS
3.1. Modifiable procedural factors associated with radiation exposure
Comparison in phantom Model A of μSv/min and mGy/hr in phantom Model A at magnification of 8 in. demonstrated that LAO/Caud (30/28°) angle resulted in two to four times more radiation compared to the LAO/Cran (38/20°), RAO/Cran (05/41°), and RAO/Caud (33/25°), p < .005 (Figure 2a). Comparison of radiation in phantom Model A at LAO/Caud (30/28°) with SID of 115 cm demonstrated both μSv/min and mGy/hr significantly decreased with 10 in. compared to both 6 and 8 in. magnification (Figure 2b). With the detector at LAO/Caud angle, 8 in. magnification (standard magnification for most operators), distance from the source lowered μSv/min by 30% at 40 cm and 47% at 120 cm caudal from the access site with no change in mGy/hr (Figure 2c).
Figure 2.
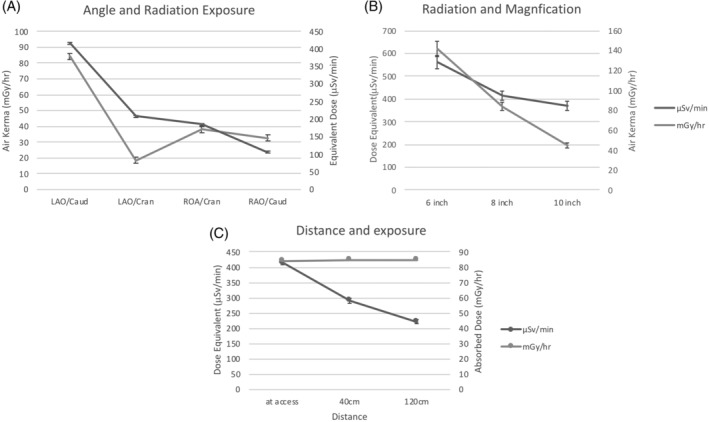
(a) Comparison of radiation exposure to the mannequin and human computational phantom with four angles of the detector. Left anterior oblique (LAO); Right anterior oblique (RAO); caudal (Caud); cranial (Cran). LAO/Caud with significantly more radiation measured by μSv/min and mGy/hr, p < .005. (b) Increase in magnification significantly increases exposure measured with μSv/min and mGy/hr (p < .005). (c) Significant decline in μSv/min (p < .001) with no change in mGy/hr produced by the X‐ray tube (p = .732) when mannequin positioned 40 and 120 cm from femoral access site of phantom
3.2. HRAP and VRS impact on radiation exposure
In Phantom Model A with the detector at LAO/Caud angle, on 8 in. magnification, shielding with HRAP (Radpad) and VRS compared to neither shield resulted in a 57% reduction in μSv/min (p < .005); the combination of both HRAP and VRS resulted in 80% (p < .005) reduction in μSv/min, and VRS lowered μSv/min by 59% (p < .005) when added to HRAP, all with no significant change in mGy/hr (Figure 3a). In Phantom Model B with the detector at LAO/Caud angle, on 8 in. magnification, shielding with HRAP (Microtek) and VRS compared to HRAP alone resulted in 100% reduction in μSv, p < .016, with no significant change in mGy, respectively (Figure 3b).
Figure 3.
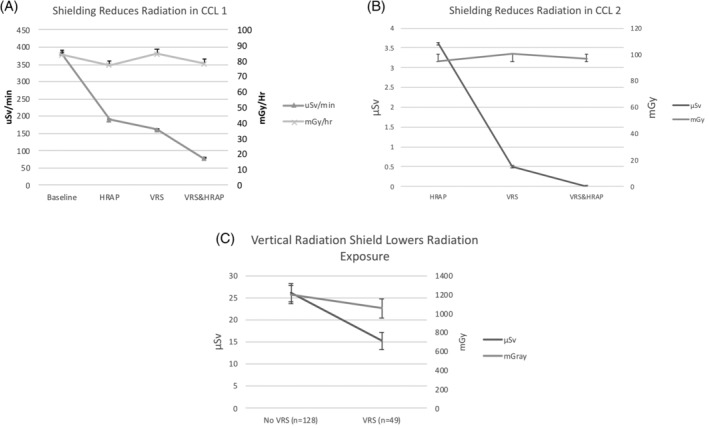
Radiation exposure using horizontal and vertical shields. HRAP = horizontal radiation absorbing pad; VRS = vertical radiation shield (Steradian). (a) CCL 1 (Toshiba) with significant decline in μSv/min with use of both HRAP (RadPad) and VRS (Steradian) compared to neither HRAP or VRS shields (baseline); and combination of both VRS and HRAP with further improvement in radiation protection (p < .005), with no change in mGy/hr from the X‐ray tube (p = .226). (b) CCL 2 (Phillips) with significant drop in μSv with use of VRS compared to HRAP (Microtek; p = .019) and further reduction with combination of both HRAP and VRS (p = .016), with no change in in mGy generated from the X‐ray tube (p = .353, p = .797). (c) Significant shielding in clinical cases from radiation shown as decline in μSv with the VRS and HRAP to HRAP alone (p = .001) with no significant change in mGy produced from the X‐ray tube (p = .297)
3.3. Prospective clinical procedure radiation evaluation
A total of 184 procedures were performed as part of the current study. Seven procedures were excluded from the analysis as no coronary angiography was performed (three were atrial septal defect closures, one was a balloon aortic valvuloplasty, one was an intra‐balloon pump insertion and two were right heart catheterization alone). Table 1 demonstrates the patient and procedural characteristics of clinical procedures with VRS (n = 49) versus those without (n = 128). Clinical procedures with VRS had significantly higher magnification and more men, but lower fluoroscopy time compared with the group that did not use VRS. Mean comparison found a 41.9% lower μSv in the group with VRS compared to those without VRS (Figure 3c), with no change in mGy. μSv remained lower with the use of VRS versus without VRS even after adjustment of covariates of interest (F[1, 170] = 8.61, p < .001, partial η 2 = 0.05).
Table 1.
Demographics and procedure information
| No VRS (n = 128) | VRS (n = 49) | p value | |
|---|---|---|---|
| Age (years) | 67.6 ± 11.3 | 68.7 ± 12.7 | .606 |
| Sex (%male) | 61.7 | 81.6 | .005 |
| BMI | 32 ± 8.14 | 32.8 ± 9.7 | .627 |
| Room 2 (%) | 84.4 | 79.6 | .474 |
| Sheath Ext (%) | 42.2 | 34.7 | .360 |
| Radial access (%) | 83.6 | 81.6 | .763 |
| Femoral access (%) | 26.6 | 16.3 | .205 |
| Radial/femoral (%) | 9.4 | 2 | .149 |
| Case w/8″ mag (%) | 2 | 47 | <.005 |
| RHC/case | 0.08 ± 0.35 | 0.04 ± 0.20 | .374 |
| HRAP (#) | 2.0 ± 0.15 | 2.0 ± 0.14 | .902 |
| CABG (graft/case) | 0.21 ± 0.7 | 0.59 ± 1.2 | .037 |
| FFRorIVUS/case | 0.15 ± 0.2 | 0.35 ± 0.78 | .111 |
| Large injection/case | 0.23 ± 0.44 | 0.29 ± 0.5 | .469 |
| PCI/case | 0.69 ± 0.85 | 0.53 ± 0.87 | .282 |
| Contrast (mL) | 122 ± 75 | 120 ± 72 | .857 |
| Dose Eq (μSv) | 26.2 ± 31.4 | 15.2 ± 13.4 | .001 |
| Air Kerma (mGy) | 1,199 ± 998 | 1,054 ± 743 | .297 |
| Frame count | 17.1 ± 12 | 17 ± 13 | .915 |
| Flourotime (min) | 13.9 ± 16.7 | 10 ± 8.8 | .044 |
Note: VRS = vertical radiation shield; BMI = body mass index; CCL A = Phillips Allura built 2008 and remaining % used CCL B (Phillips Allura built 2016); Sheath Ext = sheath extension (StandTall); 8″ Mag = magnification is set 8 in.; RHC/case = right heart catheterization performed per case; HRAP = horizontal radiation absorbing pad; CABG (graft/case) = coronary artery bypass grafts per case; FFR or IVUS = fractional flow reserve or intravascular ultrasound; Large Injection/case = left ventriculogram, aortic, iliac, femoral or subclavian angiogram performed per case; PCI = percutaneous coronary intervention; Dose Eq = dose equivalent.
4. DISCUSSION
Occupational hazards in the catheterization laboratory impact operators and staff.1, 2, 3, 4, 5 Maneuvers are well described to lower radiation, including avoidance of LAO, steep cranial or caudal views.9, 10, 11 In a controlled setting using a human computational phantom simulating a patient, and a mannequin in the place of an operator we discovered the LAO‐Caudal angle has the highest operator and patient radiation exposure. Raising magnification from 10 to 8 in. field size will almost double radiation exposure. Moving only16 in. (40 cm) caudal from the access site can lower operator exposure by approximately one third. In both the controlled CCL above and a prospective study with physicians in clinical cases, we discovered the addition of a novel VRS (Steradian) placed between the operator and detector, significantly lowered by almost half the operator radiation exposure. The reduction in operator exposure was significant after controlling for variables including magnification, BMI, percutaneous coronary intervention, additional large injection, or access site location.
5. STUDY LIMITATIONS
The VRS was not evaluated at other angles, magnification, or distance, clinical data collected were not blinded or randomized with a sham control arm. Radiation exposure to the head was not obtained given the dosimeter used would not safely attach to the operator. Fluoroscopy time was lower in the group with VRS, but no difference was found in frame count or Air Kerma (mGy).
6. CONCLUSIONS
In summary, operator radiation exposure is significantly reduced utilizing the novel VRS with horizontal radiation absorbing pads and distance from the X‐ray tube, and consideration of lower magnification and avoiding LAO‐caudal angles to lower radiation for both operator and patient.
ACKNOWLEDGMENTS
The authors would like to acknowledge Robert Wilson, MD, Betsy Wilson, Elizabeth Bisinov MD, Bridget Ales.
Panetta CJ, Galbraith EM, Yanavitski M, et al. Reduced radiation exposure in the cardiac catheterization laboratory with a novel vertical radiation shield. Catheter Cardiovasc Interv. 2020;95:7–12. 10.1002/ccd.28629
Funding information Radux Devices
EDITORIAL COMMENT: Expert Article Analysis for: https://doi.org/10.1002/ccd.28681
REFERENCES
- 1. Chambers CE, Fetterly KA, Holzer R, et al. Radiation safety program for the cardiac catheterization laboratory. Cathet Cardiovasc Interv. 2011;2011:546‐556. [DOI] [PubMed] [Google Scholar]
- 2. Andreassi MG, Piccaluga E, Gargani L, et al. Subclinical carotid atherosclerosis and early vascular aging from long‐term low‐dose ionizing radiation exposure. J Am Coll Cardiol Intv. 2015;8:616‐627. [DOI] [PubMed] [Google Scholar]
- 3. Andreassi MG, Piccaluga E, Guagliumi G, et al. Occupational health risks in cardiac catheterization laboratory workers. Circ Cardiovasc Interv. 2016;9:e003273. [DOI] [PubMed] [Google Scholar]
- 4. Best JMP, Skelding KA, Mehran R, et al. SCAI consensus document on occupational radiation exposure to the pregnant cardiologist and technical personnel. EuroIntervention. 2011;6(7):866‐874. [DOI] [PubMed] [Google Scholar]
- 5. Klein LW, Miller DL, Balter S, et al. Occupational health hazards of interventional cardiologists in the current decade: results of the 2014 SCAI membership survey. Cathet Cardiovasc Interv. 2015;86(5):913‐924. [DOI] [PubMed] [Google Scholar]
- 6. NCD Risk Factor Collaboration (NCD‐RisC) . Trends in adult body mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population‐based measurement studies with 19.2 million participants. Lancet. 2016;387:1377‐1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sauren LD, van Garsse L, van Ommen V, Kemerink GJ. Occupational radiation dose during transcatheter aortic valve im‐ plantation. Catheter Cardiovasc Interv. 2011;78:770‐776. [DOI] [PubMed] [Google Scholar]
- 8. Madder RD, VanOosterhout S, Mulder A, et al. Patient body mass index and physician radiation dose during coronary angiography. Circ Cardiovasc Interv. 2019;12:e006823. [DOI] [PubMed] [Google Scholar]
- 9. Pitney MR, Allan RM, Giles RW, et al. Modifying fluoroscopic views reduces operator radiation exposure during coronary angioplasty. J Am Coll Cardiol. 1994;24:1560‐1563. [DOI] [PubMed] [Google Scholar]
- 10. Kuon E, Dahm JB, Empen K, Robinson DM, Reuter G, Wucherer M. Identification of less‐irradiating tube angulations in invasive cardiology. J Am Coll Cardiol. 2004;44:1420‐1428. [DOI] [PubMed] [Google Scholar]
- 11. Agarwal S, Parashar A, Bajaj NS, et al. Relationship of beam angulation and radiation exposure in the cardiac catheterization laboratory. J Am Coll Cardiol Intv. 2014;7:558‐566. [DOI] [PubMed] [Google Scholar]
- 12. Abdelaal E, Plourde G, MacHaalany J, et al. Effectiveness of low rate fluoroscopy at reducing operator and patient radiation dose during transradial coronary angiography and inventions. J Am Coll Cardiol Intv. 2014;7(5):567‐747. [DOI] [PubMed] [Google Scholar]
- 13. Hansen JW, Foy A, Schmidt T, Ghahramani M, Chambers CE. Fluoroscopy pulse rate reduction during diagnostic and therapeutic imaging in the cardiac catheterization laboratory: an evaluation of radiation dose, procedure complications and outcomes. Cathet Cardiovasc Interv. 2017;89:665‐670. [DOI] [PubMed] [Google Scholar]
- 14. de Buck S, la Gerche A, Ector J, et al. Asymmetric collimation can significantly reduce patient radiation dose during pulmonary vein isolation. Europace. 2012;14:437‐444. [DOI] [PubMed] [Google Scholar]
- 15. Wassef AWA, Hiebert B, Ravandi A, et al. Radiation dose reduction in the cardiac catheterization laboratory utilizing a novel protocol. J Am Coll Cardiol Intv. 2014;7(5):550‐557. [DOI] [PubMed] [Google Scholar]
- 16. den Boer A, de Feyter PJ, Humel WA, et al. Reduction of radiation exposure while maintaining high‐quality fluoroscopic images during interventional cardiology using novel X‐ray tube technology with extra beam filtering. Circulation. 1994;89:2710‐2714. [DOI] [PubMed] [Google Scholar]
- 17. Hamer OW, Sirlin CB, Strotzer M, et al. Chest radiography with a flat‐panel detector: image quality with dose reduction after copper filtration. Radiology. 2005;237:691‐700. [DOI] [PubMed] [Google Scholar]
- 18. Lloyd P, Lowe D, Harty DS, Eyes B. The secondary radiation grid; its effect on fluoroscopic dose‐area product during barium enema examinations. Br J Radiol. 1998;71:303‐306. [DOI] [PubMed] [Google Scholar]
- 19. Eloot L, Thierens H, Taeymans Y, et al. Novel X‐ray imaging technology enables significant patient dose reduction in interventional cardiology while maintaining diagnostic image quality. Catheter Cardiovasc Interv. 2015. Nov;86(5):E205‐E212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Subramanian S, Waller BR, Winders N, et al. Clinical Evaluation of a radio‐protective cream for the hands of the pediatric interventional cardiologist. Cathet Cardiovasc Interv. 2017;89:709‐716. [DOI] [PubMed] [Google Scholar]
- 21. Fetterly K, Schueler B, Grams M, Sturchio G, Bell M, Gulati R. Head and neck radiation dose and radiation safety for interventional physicians. J Am Coll Cardiol Intv. 2017;10:520‐528. [DOI] [PubMed] [Google Scholar]
- 22. Wieneke V, Delewi R, Sjauw KD, et al. Efficacy of the RADPAD protection drape in reducing operators' radiation exposure in the catheterization laboratory a sham‐controlled randomized trial. Circ Cardiovasc Interv. 2017;10:e006058. [DOI] [PubMed] [Google Scholar]
- 23. Politi L, Biondi‐Zoccai G, Nocetti L, et al. Reduction of scatter radiation during transradial percutaneous coronary angiography: a randomized trial using a lead‐free radiation shield. CCI. 2012;79:97‐102. [DOI] [PubMed] [Google Scholar]
- 24. Wilson R, Gainor J, Valeti U, et al. A new device to markedly reduce cardiac Cath lab radiation levels. J Am Coll Cardiol. 2018;72(13):103. [Google Scholar]
- 25. Fattal P, Goldstein JA. A novel complete radiation protection system eliminates physician radiation exposure and leaded aprons. Cathet Cardiovasc Interv. 2013;81:11‐16. [DOI] [PubMed] [Google Scholar]
- 26. Savage C, Seale FM, Shaw CJ, et al. Evaluation of a suspended personal radiation protection system vs. conventional apron shields in clinical interventional procedures. Open J Radiol. 2013;3:143‐151. [Google Scholar]
- 27. Granada JF, Delgado JA, Uribe MP, et al. First‐in‐human evaluation of a novel robotic‐assisted coronary angioplasty system. J Am Coll Cardiol Intv. 2011;4(4):460. [DOI] [PubMed] [Google Scholar]


