Abstract
Background
The extent of breast cancer outcome disparity can be measured by comparing Surveillance, Epidemiology, and End Results (SEER) breast cancer‐specific survival (BCSS) by region and with institutional cohort (IC) rates.
Methods
Patients who were diagnosed with a first primary, de novo, stage IV breast cancer at ages 25 to 84 years from 1990 to 2011 were studied. The change in 5‐year BCSS over time from 1990 to 2011 was compared using the SEER 9 registries (SEER 9) without the Seattle‐Puget Sound (S‐PS) region (n = 12,121), the S‐PS region alone (n = 1931), and the S‐PS region IC (n = 261). The IC BCSS endpoint was breast cancer death confirmed from chart and/or death certificate and cause‐specific survival for SEER registries. BCSS was estimated using the Kaplan‐Meier method. Hazard ratios (HzR) were calculated using Cox proportional‐hazards models.
Results
For SEER 9 without the S‐PS region, 5‐year BCSS improved 7% (from 19% to 26%) over time, it improved 14% for the S‐PS region (21% to 35%), and it improved 27% for the S‐PS IC (29% to 56%). In the IC Cox proportional‐hazards model, recent diagnosis year, chemotherapy, surgery, and age <70 years were associated with better survival. For SEER 9, additional significant factors were white race and positive hormone receptor status and S‐PS region was associated with better survival (HzR, 0.87; 95% CI, 0.84‐0.90). In an adjusted model, hazard of BC death decreased in the most recent time period (2005‐2011) by 28% in SEER 9 without S‐PS, 43% in the S‐PS region and 45% in the IC (HzR, 0.72 [95% CI, 0.67‐0.76], 0.57 [95% CI, 0.49‐0.66], and 0.55 [95% CI, 0.39‐0.78], respectively).
Conclusions
Over 2 decades, the survival of patients with metastatic breast cancer improved nationally, but with regional survival disparity and differential improvement. To achieve equitable outcomes, access and treatment approaches will need to be identified and adopted.
Keywords: differential survival, disease-specific survival (DSS), metastatic breast cancer, regional disparity
Short abstract
The observation of a greater improvement in survival with metastatic breast cancer by region indicates progress in treatment and a possible statistical cure, in that patients may be able to live long enough with disease to die of other causes. The direct identification of specific factors related to differential survival rates, such as access to care and molecular subtype‐appropriate treatment, is warranted.
Introduction
Variation in breast cancer recurrence and survival may be influenced by age, race, access to care, insurance coverage, socioeconomic status, geographic area of residence (urban/rural or metropolitan/nonmetropolitan), and timely diagnosis and treatment.1, 2, 3, 4 From national statistics, factors contributing to state variations in cancer incidence rates include risk factor prevalence, access to and utilization of early detection services, and completeness of reporting.5 Despite survival improvements across poverty levels for all stages of disease, relative survival remains lower among women residing in poor areas compared with affluent women.6 Some evidence links guideline compliance to improved and optimal outcomes, but a lack of ability to compare guideline adherence in national databases inhibits the ability to evaluate widespread adherence or efficacy.7, 8
We previously observed significant improvement in 5‐year disease‐specific survival of patients with de novo stage IV metastatic breast cancer (MBC) over time from 1990 to 2010 without a concurrent improvement in the survival of patients with recurrent MBC from our study of an institutional cohort of breast cancer registry patients.9 The 5‐year breast cancer‐specific survival (BCSS) rates in our institutional cohort of patients with stage IV breast cancer were significantly higher than the rates previously reported for stage IV breast cancer from Surveillance, Epidemiology, and End Results (SEER) registry data.10
Regional disparity in breast cancer outcomes can be measured by comparing BCSS rates from SEER across geographic regions and with the rates from a SEER‐embedded institutional cohort. We compared SEER aggregate data to the regional subset from the Seattle‐Puget Sound (S‐PS) area registry and to an institutional cohort (IC) located in the S‐PS registry area whose cases are included in the S‐PS Cancer Surveillance System (SEER 9 without S‐PS, n = 12,121; S‐PS, n = 1931; and Seattle IC, n = 261). Our objectives were to compare survival rates to evaluate regional disparity in de novo MBC survival, to compare survival rate improvement over time by region and institution, and to assess the impact of temporal advances in systemic therapies on trends in de novo stage IV MBC survival rates. In particular, our focus was on regional survival differences and the potential for survival rate improvement over time as patients with metastatic disease have a poor prognosis and are often treated with palliative rather than with stabilizing or curative intent.
Materials and Methods
The analysis included patients aged 25 to 84 years with first primary breast cancer who were diagnosed with de novo stage IV breast cancer from 1990 to 2011 in the SEER 9 registries and an institutional cohort (IC) located in the SEER 9 S‐PS region (vital status through 2016). We calculated 5 ‐year breast cancer‐specific survival (BCSS) for 3 time periods (1990‐1998, 1999‐2004, and 2005‐2011), during which adjuvant chemotherapy treatments changed significantly and was available for the IC patients (Table 1).11 For the IC, the BCSS endpoint was breast cancer death confirmed from chart and/or death certificate. For SEER, SEER*Stat‐documented cause‐specific survival was used.12 The SEER S‐PS region was used separately for comparison with SEER 9 without S‐PS and the IC. Five‐year BCSS and 95% CIs and Cox proportional hazard models were calculated using SPSS 25.0 (IBM Corporation) for the institutional cohort and STATA (StataCorp LLC) for SEER 9.13, 14 BCSS was estimated as the net measure representing survival from death caused by the primary diagnosed breast cancer in the absence of other causes of death. Patients who died of causes other than those specified were considered to be censored.15
Table 1.
Change in Systemic Therapy From 1990 to 2011: Stage IV Breast Cancer, IC Patients only, n = 261
| Systemic Therapy | No. of Patients (%) | P | ||
|---|---|---|---|---|
| 1990‐1998 | 1999‐2004 | 2005‐2011 | ||
| Initial chemotherapy, n = 175 | 51 (64) | 40 (66) | 84 (70) | .629 |
| Taxane therapy, n = 99 | 11 (21) | 24 (60) | 64 (76) | <.001 |
| Anthracycline therapy, n = 114 | 43 (83) | 28 (70) | 43 (51) | .001 |
| Trastuzumab therapy: HER‐2–positive patients, n = 45 | 0 (0) | 8 (68) | 25 (100) | <.001 |
| Neoadjuvant therapy, n = 64 | 18 (23) | 7 (12) | 39 (33) | .007 |
| Hormone therapy: HR‐positive patients, n = 193 | 48 (86) | 41 (89) | 83 (91) | .583 |
Abbreviation: HR, hormone receptor.
Cox proportional hazards modelling was used to estimate adjusted hazard ratios (HzR) with corresponding 95% CIs, with death from disease as the endpoint. The IC was used to build an a priori model informed by a chi‐square analysis and tested by stepwise entry into the model with a subsequent forced‐entry model to include all variables of interest in the SEER 9 population. The proportional hazards assumption was evaluated graphically using the log(‐log[survival]) versus log of survival time. We found no evidence suggesting violation of the proportionality assumption. All P values were 2‐sided using a .05 level of significance.
Data from the SEER 9 population‐based cancer registries (Connecticut, Detroit, Atlanta, San Francisco‐Oakland, Hawaii, Iowa, New Mexico, Seattle‐Puget Sound, and Utah) were included in our analysis.16 The SEER program is funded by the National Institutes of Health and the National Cancer Institute and represents cancer incidence data for approximately 28% of the US population.
The institutional cohort (IC) breast cancer registry database, which was created in 1990, contains detailed information on diagnosis, pathology, staging, surgery, chemotherapy, radiation therapy, tumor markers, and vital status at follow‐up, including cause‐specific death. Incident breast cancer cases are entered at the time of diagnosis in a Health Insurance Portability and Accountability Act of 1996 (HIPAA)‐compliant and Institutional Review Board (IRB)‐approved research registry. This project was HIPAA compliant and IRB approved. Patient vital and disease status, including date, site and type of recurrence, and date and cause of death, is collected prospectively through annual updates by a certified cancer registrar. Follow‐up is obtained from: 1) electronic chart review; 2) an IRB‐approved, physician‐directed follow‐up letter; 3) an institutional cancer registry; and 4) the SEER S‐PS registry.17
Results
The SEER 9 without S‐PS population and the SEER S‐PS region population were both older than the IC patients (mean age, 61 vs 55 years). More IC and S‐PS patients identified as white race (IC, 81%; S‐PS, 89%) than SEER 9 without S‐PS patients (75%) (Table 2). Of all invasive breast cancers in the populations, 5% of those in SEER 9 without S‐PS, 4% of those in the S‐PS region, and 3% of those in the IC were de novo stage IV. Patients in the S‐PS region and in the IC were more often hormone receptor (HR)‐positive (66% and 74%, respectively, vs 56% in SEER 9 without S‐PS). Stage IV surgical treatment was received by ≥50% of patients in all 3 groups (SEER 9 without S‐PS, 58%; S‐PS, 56%; IC, 50%). Patients in the IC were treated less often with surgery (50%) and radiation (46%) and more often with chemotherapy (67%) than the SEER population (range, 51%‐53%). Patients in the IC more often resided in a metropolitan area with a population >1 million (86%) compared with patients in SEER 9 without S‐PS (61%) and patients in the S‐PS region (58%) (Table 2).
Table 2.
Baseline Demographic and Clinical Characteristics of Patients With Stage IV Breast Cancer by Location and Data Source
| Characteristic | No. of Patients (%) | P | ||
|---|---|---|---|---|
| SEER 9 Without S‐PS, n = 12,121 | SEER S‐PS, n = 1931 | Institutional Cohort, n = 271 | ||
| Age: Mean [range], y | 61 [25‐84] | 61 [25‐84] | 55 [28‐84] | <.001 |
| Race | ||||
| White | 9121 (75) | 1723 (89) | 211 (81) | <.001 |
| Black | 2019 (17) | 79 (4) | 16 (6) | |
| Other | 898 (7) | 129 (7) | 34 (13) | |
| Diagnosis year | ||||
| 1990‐1998 | 4423 (36) | 658 (34) | 80 (31) | <.001 |
| 1999‐2004 | 3255 (27) | 516 (27) | 61 (23) | |
| 2005‐2011 | 4443 (37) | 757 (39) | 120 (46) | |
| Proportion of invasive BC: de novo Stage IV | 12,121 (5) | 1931 (4) | 261 (3) | <.001 |
| Follow‐up: Median [range], y | 1.67 [0.08‐25] | 2.08 [0.08‐25] | 5.24 [0.05‐22] | <.001 |
| Hormone receptor status | ||||
| Positive | 6798 (75) | 1275 (77) | 193 (77) | .013 |
| Negative | 2321 (25) | 372 (23) | 57 (23) | |
| Unknown: No. (% of total) | 3002 (25) | 284 (15) | 11 (4) | |
| Surgery | ||||
| Yes | 6768 (56) | 1129 (58) | 130 (50) | .029 |
| No | 5236 (43) | 783 (41) | 131 (50) | |
| Unknown | 117 (1) | 19 (1) | 0 (0) | |
| Radiation | ||||
| Yes | 7771 (64) | 1135 (60) | 121 (46) | .002 |
| No/unknown | 4350 (36) | 762 (40) | 140 (54) | |
| Chemotherapy | ||||
| Yes | 6199 (51) | 1033 (53) | 175 (67) | .055 |
| No/unknown | 5922 (49) | 898 (47) | 86 (33) | |
| Location of residence | ||||
| Nonmetro urban | 1615 (14) | 187 (10) | 8 (3) | <.001 |
| Nonmetro rural | 161 (1) | 15 (1) | 3 (1) | |
| Metro population <1 million | 2818 (14) | 603 (31) | 26 (10) | |
| Metro population ≥1 million | 7284 (61) | 1126 (58) | 224 (86) | |
Abbreviations: BC, breast cancer; Metro, metropolitan; Nonmetro, nonmetropolitan; SEER 9, Surveillance, Epidemiology, and End Results 9 registries; S‐PS, Seattle‐Puget Sound.
Data on the type of chemotherapy treatments used from 1990 to 2011 were available for the stage IV IC cohort. Chemotherapy treatment increased from 64% to 70% for patients who had stage IV disease, with taxane treatment increasing (from 21% to 76%) and anthracycline treatment decreasing (from 83% to 51%) (Table 1). The receipt of hormone therapy in HR‐positive patients increased from 86% to 91% over time. Trastuzumab treatment became available in 1999, and treatment increased over time to 100% of patients with HER‐2–positive, de novo, stage IV breast cancer in the most recent time period. Twelve percent of chemotherapy regimens received by patients in the IC who had de novo stage IV disease were considered nonstandard.
Over time, among patients in SEER 9 without S‐PS who had stage IV breast cancer, 5‐year BCSS improved 7%, from 19% to 26% (1990‐1998: 19%; 95% CI 18%, 21%; 1999‐2004: 23%; 95% CI 21%, 24%; 2005‐2011: 26%; 95% CI 24%, 27%; log‐rank test 53.51, P < .001); and, among patients in the SEER S‐PS region who had stage IV breast cancer, BCSS improved 14%, from 21% to 35% (1990‐1998: 21%; 95% CI 18%, 24%; 1999‐2004: 27%; 95% CI 23%, 31%; 2005‐2011: 35%; 95% CI 32%, 39%; log‐rank test 27.48, P < .001) (Fig. 1). Among patients in the IC who had stage IV breast cancer, 5‐year BCSS improved 27% over the same period, from 29% to 56% (1990‐1998: 29%; 95% CI 18%, 37%; 1999‐2004: 47% 95% CI, 34%, 59%; 2005‐2010: 56%; 95% CI 45%, 65%; log‐rank test 10.97; P = .004) (Fig. 2, Table 3).
Figure 1.
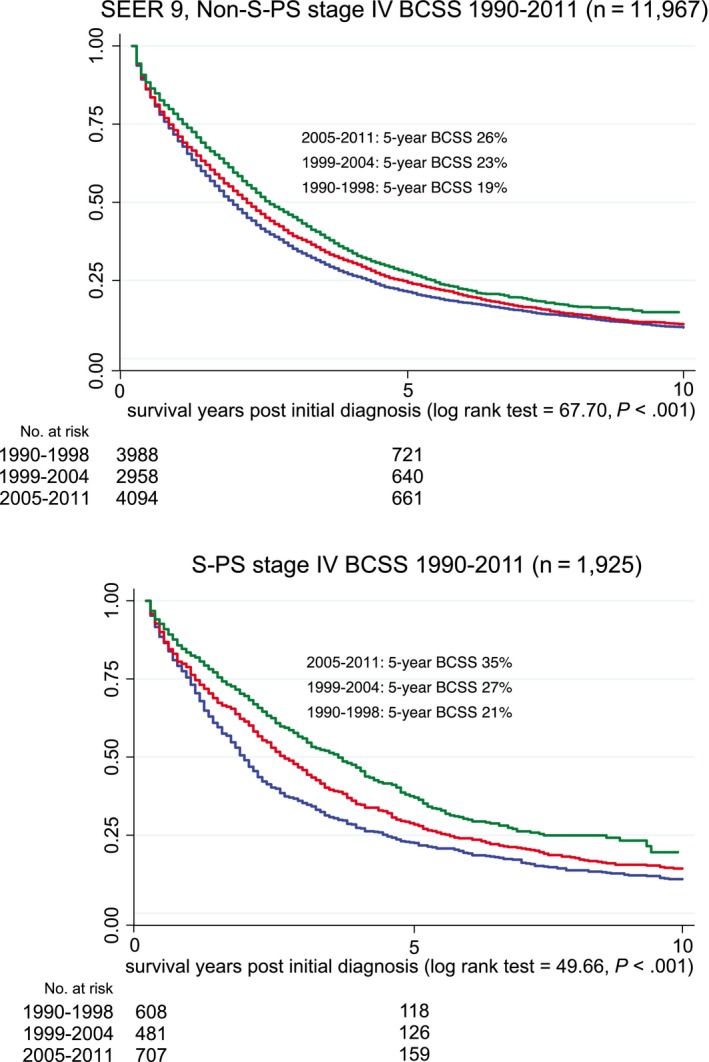
Stage IV breast cancer‐specific survival (BCSS) is illustrated for the Surveillance, Epidemiology, and End Results (SEER) 9 registries without the Seattle‐Puget Sound (S‐PS) region and for the S‐PS region alone from 1990 to 2011.
Figure 2.
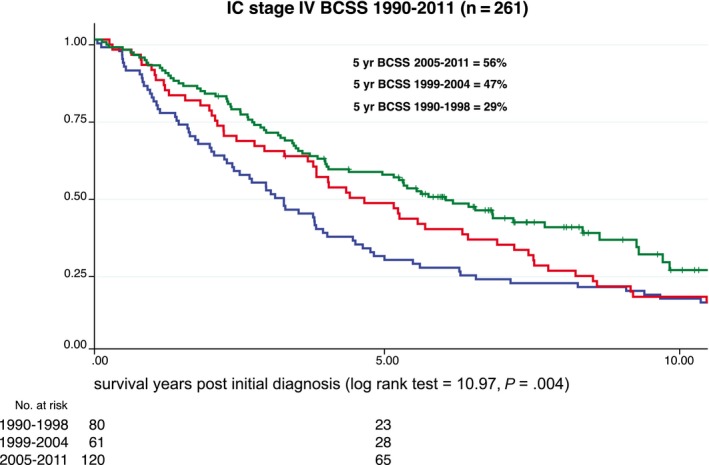
Stage IV breast cancer‐specific survival (BCSS) is illustrated for the institutional cohort (IC) from 1990 to 2011.
Table 3.
Changes in 5‐Year Breast Cancer‐Specific Survival and Overall Survival in Patients Aged 25 to 84 Years With Stage IV Breast Cancer From 1990 to 2011 by Surveillance, Epidemiology, and End Results Region and in the Institutional Cohort
| Population/Cohort | 5‐Year Survival Rates (95% CI) | P | ||
|---|---|---|---|---|
| 1990‐1998 | 1999‐2004 | 2005‐2011 | ||
| BCSS | ||||
| SEER 9 without SEER S‐PS | 19 (18‐21) | 23 (21‐24) | 26 (24‐27) | <.001 |
| SEER S‐PS | 21 (18‐24) | 27 (23‐31) | 35 (32‐39) | <.001 |
| institutional cohort | 29 (18‐37) | 47 (34‐59) | 56 (45‐65) | .004 |
| OS | ||||
| SEER 9 without SEER S‐PS | 16 (15‐18) | 20 (19‐21) | 23 (22‐24) | <.001 |
| SEER S‐PS | 18 (15‐21) | 25 (21‐28) | 32 (28‐35) | <.001 |
| institutional cohort | 29 (18‐37) | 47 (34‐59) | 56 (45‐65) | .004 |
Abbreviations: BCSS, breast cancer‐specific survival; OS, overall survival; SEER 9, Surveillance, Epidemiology, and End Results 9 registries; S‐PS, Seattle‐Puget Sound.
In the first period (1990‐1998), the 5‐year BCSS rate ranged from 19% to 29%, with overlapping 95% CIs for both populations and for the IC (Table 3). In the second period, 95% CIs in the 2 SEER populations overlapped, but the IC did not (5‐year BCSS: 23%, 27%, and 47%, respectively). In the most recent period (2005‐2011), the 5‐year BCSS rate for SEER 9 without S‐PS was 26%, for S‐PS it was 35%, and for the IC it was 56% without 95% CI overlap, indicating a significant difference between all 3 (P = .017) (Table 3), The breast cancer‐specific (BCSS) and overall survival (OS) rates were equivalent for patients in the IC. For the SEER cohort, the change over time was the same for OS and BCSS, and the 95% CIs overlapped, indicating no statistical difference between OS and BCSS (Table 3).
Among patients from the IC who had stage IV disease (n = 261), we ran a Cox proportional hazards forward conditional entry model with breast cancer‐specific death as the outcome. Significant variables by order of entry were: 1) surgery (yes vs no: HzR 0.51; 95% CI 0.37, 0.71), 2) more recent diagnosis year (1990‐1998 [reference]; 1999‐2004: HzR 0.63; 95% CI 0.42, 0.94; 2005‐2011: HzR 0.55; 95% CI 0.39, 0.78), 3) age (<70 vs ≥70 years: HzR 0.62; 95% CI 0.41, 0.92), and 4) initial chemotherapy (yes vs no: HzR 0.71; 95% CI 0.52, 0.98). Radiation therapy, HR status, and race (white/nonwhite) were not significant in the model. HER‐2 status was run on the subset of patients after 1998 who had HER‐2 test results, and the variable was not significant in the model (Table 4).
Table 4.
Cox Proportional Hazards Model of Breast Cancer‐Specific Death in an Institutional Cohort of Patients With Stage IV Breast Cancer, 1990 to 2011 (n = 261)
| By Order of Entry into the Model: | HzR (95% CI) | P |
|---|---|---|
| Surgery | ||
| No | Reference | <.001 |
| Yes | 0.51 (0.37, 0.71) | |
| Diagnosis year | ||
| 1990‐1998 | Reference | |
| 1999‐2004 | 0.63 (0.42, 0.94) | .001 |
| 2005‐2011 | 0.55 (0.39, 0.78) | |
| Age, y | ||
| ≥70 | Reference | .018 |
| <70 | 0.62 (0.41, 0.92) | |
| Chemotherapy | ||
| No | Reference | .036 |
| Yes | 0.71 (0.52, 0.98) | |
| Radiation therapy | ||
| No | Reference | NS |
| Yes | 0.75 (0.52, 1.07) | |
| Hormone receptor status | ||
| Negative | Reference | NS |
| Positive | 0.76 (0.51, 1.13) | |
| Race | ||
| Nonwhite | Reference | NS |
| White | 0.95 (0.64, 1.40) |
Abbreviations: HzR, hazard ratio; NS, nonsignificant.
In the SEER 9 (n = 14,052), SEER 9 without S‐PS region (n = 12,121), and SEER S‐PS region (n = 1931) cohorts, we ran separate Cox proportional hazards models for breast cancer‐specific death in each population. We used the model that was developed and tested in the IC study group but added race (white/black), HR status, and region (SEER 9 without S‐PS and the SEER S‐PS region) to the SEER 9 analysis (Table 5). Reduced hazard was associated with surgery, diagnosis year interval, and age <70 versus ≥70 years and was very similar, with overlapping 95% CIs, indicating no difference in HzR values between the SEER 9 groups. Initial chemotherapy was significant in SEER 9 and SEER 9 without S‐PS, but not in the S‐PS region alone. Radiation therapy was not significant in any of the SEER groups. HR status was significant in all 3 SEER groups, with the HzR ranging from 0.43 to 0.47 and overlapping 95% CIs. White race had a survival advantage in SEER 9 and SEER 9 without S‐PS, but not in the S‐PS region. In the adjusted model, the SEER S‐PS region had a significant, independent 13% reduced hazard of mortality (HzR, 0.87; 95% CI, 0.79‐0.88; reference: SEER 9 without S‐PS).
Table 5.
Cox Proportional Hazards Model of Breast Cancer‐Specific Death in Patients With Stage IV Breast Cancer From the Surveillance, Epidemiology, and End Results 9 Registries, 1990 to 2011
| Variable | HzR | 95% CI | P |
|---|---|---|---|
| SEER 9 registries, n = 14,052 | |||
| Surgery | |||
| No | Reference | ||
| Yes | 0.58 | 0.55, 0.61 | <.001 |
| Diagnosis year | |||
| 1990‐1998 | Reference | ||
| 1999‐2004 | 0.83 | 0.78, 0.89 | <.001 |
| 2005‐2011 | 0.69 | 0.65, 0.73 | <.001 |
| Age, y | |||
| ≥70 | Reference | ||
| <70 | 0.76 | 0.72, 0.81 | <.001 |
| Chemotherapy | |||
| No | Reference | ||
| Yes | 0.92 | 0.87, 0.96 | .001 |
| Radiation therapy | |||
| No | Reference | ||
| Yes | 1.02 | 0.97, 1.07 | .479 |
| Hormone receptor status | |||
| Negative | Reference | ||
| Positive | 0.47 | 0.44, 0.49 | <.001 |
| Race | |||
| Nonwhite | Reference | ||
| White | 0.83 | 0.79, 0.88 | <.001 |
| SEER 9 without the S‐PS region | |||
| S‐PS region | 0.87 | 0.84, 0.90 | <.001 |
| SEER S‐PS region, n = 1931 | |||
| Surgery | |||
| No | Reference | ||
| Yes | 0.57 | 0.50, 0.66 | <.001 |
| Diagnosis year | |||
| 1990‐1998 | Reference | ||
| 1999‐2004 | 0.74 | 0.63, 0.87 | <.001 |
| 2005‐2011 | 0.57 | 0.49, 0.66 | <.001 |
| Age, y | |||
| ≥70 | Reference | ||
| <70 | 0.72 | 0.62, 0.84 | <.001 |
| Chemotherapy | |||
| No | Reference | ||
| Yes | 0.91 | 0.78, 1.05 | .199 |
| Radiation therapy | |||
| No | Reference | ||
| Yes | 1.05 | 0.92, 1.19 | .505 |
| Hormone receptor status | |||
| Negative | Reference | ||
| Positive | 0.43 | 0.37, 0.51 | <.001 |
| Race | |||
| Nonwhite | Reference | ||
| White | 0.86 | 0.71, 1.05 | .135 |
| SEER 9 without the S‐PS region, n = 12,121 | |||
| Surgery | |||
| No | Reference | ||
| Yes | 0.58 | 0.55, 0.61 | <.001 |
| Diagnosis year | |||
| 1990‐1998 | Reference | ||
| 1999‐2004 | 0.85 | 0.80, 0.91 | <.001 |
| 2005‐2011 | 0.72 | 0.67, 0.76 | <.001 |
| Age, y | |||
| ≥70 | Reference | ||
| <70 | 0.77 | 0.73, 0.82 | <.001 |
| Chemotherapy | |||
| No | Reference | ||
| Yes | 0.91 | 0.86, 0.97 | .002 |
| Radiation therapy | |||
| No | Reference | ||
| Yes | 1.02 | 0.97, 1.07 | .525 |
| Hormone receptor status | |||
| Negative | Reference | ||
| Positive | 0.47 | 0.44, 0.50 | <.001 |
| Race | |||
| Nonwhite | Reference | ||
| White | 0.84 | 0.79, 0.89 | <.001 |
Abbreviations: HzR, hazard ratio; SEER 9, Surveillance, Epidemiology, and End Results 9 registries; S‐PS, Seattle‐Puget Sound.
In SEER 9, the survival of patients with stage IV disease improved significantly over time from 1990 to 2011, with the most improvement in the SEER S‐PS region and less improvement in SEER 9 without S‐PS (Table 5). In the adjusted model, compared with 1990 to 1998, SEER S‐PS patients in 2005 to 2011 had a 43% reduced hazard of BC mortality (HzR, 0.57; 95% CI, 0.49‐0.66), and a 28% reduction among SEER 9 without S‐PS patients (HzR, 0.72; 95% CI, 0.67‐0.76).
Discussion
Five‐year, stage IV, de novo metastatic BCSS improved significantly over time from 1990 to 2011 by 7% for SEER 9 without S‐PS (from 20% to 27%), by 14% for the SEER S‐PS region (from 21% to 35%) and by 20% in the IC located in the SEER S‐PS region (from 29% to 56%). The relatively large, de novo, 5‐year metastatic BCSS improvement observed over time, which was differential by region, is unexpected for metastatic disease, suggesting that a more aggressive approach, depending on the extent of disease, patient characteristics, and tumor characteristics, may extend survival.
The SEER S‐PS region population and the IC included a higher percentage of white patients compared with SEER 9 without S‐PS, and the patients more often were HR‐positive, which may be interrelated because of the differential racial composition of the region's population.18, 19, 20 Patients with stage IV disease in the SEER 9 without S‐PS and S‐PS region populations received more surgery and radiation but less initial chemotherapy than the IC patients. Adjusting for these factors and age, race, and HR status in a Cox proportional hazards model in the SEER 9 population, the S‐PS region was independently associated with improved 5‐year MBC survival.
In the IC Cox model, HR status and race were not associated with improved survival, but diagnosis time period coincident with temporal advances in systemic therapies was associated with an improvement. Improved survival over the same time periods was consistent in the SEER 9 population but to a lesser degree. The CIs for the SEER S‐PS region and for SEER 9 without S‐PS did not overlap, indicating a significant difference in survival improvement over time, with better survival in the SEER S‐PS population. The IC had the highest chemotherapy treatment rate, the largest survival improvement over time, and the best 5‐year survival in the most recent time period (56%). The finding of a differential IC survival improvement over time, confirming the results in the SEER S‐PS region, indicates more aggressive chemotherapy for patients with stage IV breast cancer may be a factor in this stage IV survival divergence.
In a meta‐analysis conducted by Caswell‐Jin et al, among 8 studies of de novo MBC, the median survival increased significantly from 20 to 31 months between 1990 and 2010 coincident with significant advances in adjuvant treatment.21 However, the study mixed TNM stage IV and “distant” SEER summary score, which is nonequivalent to TNM stage IV MBC.22 In a SEER study by Dawood et al (1988‐2003), a modest improvement in stage IV breast cancer survival was observed.23 A Netherlands study found a relative median survival improvement from 1995 to 2008 related to age and extent of treatment.24 In a Czech Republic study of survival trends from 2000 to 2008, no change was seen in stage IV survival.25 Using SEER data, Di Meglio et al found a significant, modest improvement in de novo stage IV MBC survival over time for patients with ductal, but not lobular, stage IV disease (1990‐2011).26 Although the study by Jemal et al was not representative solely of patients with TNM stage IV MBC because of admixture with patients who had stage III disease, those authors reported substantial 5‐year survival improvement in SEER 9 “distant” breast cancer, from 19% (1975‐1977) to 34% (2006‐2012); and the Cancer Statistics, 2019 report indicated a 27% 5‐year relative survival rate for “distant” breast cancer (SEER 18 registries, 2008‐2014).27, 28
Younger patients with stage IV breast cancer have better survival than their older counterparts.29, 30 Race is a significant factor in MBC survival, with historic survival variation by black, white, Asian, and other racial categories. Recently, DeSantis et al and others have identified closing disparity in several US states between black and white survival rates.31, 32, 33
Over time from 1992 to 2006, patients who had HR‐positive, stage IV breast cancer had significantly better and improving survival, nearly twice that of those with HR‐negative breast cancer (2006: 40% vs 18%).34 In a more recent study that added HER‐2 status, HR‐positive/HER‐2–positive stage IV breast cancer had the best 4‐year survival at 47%, followed HR‐positive/HER‐2–negative, HR‐negative/HER‐2–positive, and HR‐negative/HER‐2–negative, with very poor comparative survival at approximately 12%.35 These findings indicate that, with current advances in systemic therapy, the extent to which patients with HR‐positive and/or HER‐2–positive stage IV breast cancer receive molecular subtype‐appropriate therapy, survival can be dramatically improved.36 In a SEER analysis, stage IV breast cancer surgery was associated with increased survival, although stage IV breast cancer surgery declined in the United States over time (1988‐2011).37
The IC data are from a registry database with dedicated medical record abstraction and follow‐up for vital status, including cause of death. The SEER registry data endpoints are estimated using SEER*Stat cause‐specific survival. The validity and accuracy of SEER registry data have been evaluated and graded for accuracy, with some data elements assessed as more accurate (age and stage) than others (such as treatment).38 HER‐2 status was not available from SEER data during study years 1990 through 2009 so was not included in the models. The degree to which HER‐2–positive patients receive appropriate treatment could play a factor in the differential survival observed, but HER‐2 testing and treatment have been widely available and well documented since 1999/2001. Because neither HER‐2 status nor treatment was available, we cannot say whether they were a factor. Site of metastatic disease was not available for our analysis as the SEER registry did not record them until 2010.
The inability to directly compare treatment factors constituting optimal care between the SEER 9 population and the IC limited the direct identification of specific factors related to differential survival rates. We can postulate that the IC patients who were treated in the S‐PS region urban area and followed in a carefully curated database may have received optimal care.
Primary, de novo, metastatic BCSS has improved over time toward prolonged disease control as treatments have advanced, especially in patients with favorable tumor characteristics, younger age (<70 years), and low‐volume metastatic disease. Although only 3% to 5% of invasive breast cancers are currently diagnosed as stage IV disease, as fewer patients with early breast cancer suffer distant recurrences because of improved adjuvant treatment, those with de novo stage IV disease represent nearly one‐half of the estimated 155,000 patients with MBC living in the United States today.9, 39, 40 Our current results indicate that the stage IV population that is living longer may be benefiting from many of the same therapies used to treat early breast cancer, especially for patients who are able to handle adjuvant chemotherapy treatment and are HR‐positive.
However, the lag in survival improvement across different population‐based, geographic regions suggests that some groups and regions may benefit unequally from treatment advances as well as timely diagnosis.41, 42, 43, 44 Phelan et al hypothesize that such disparities persist because individuals with higher socioeconomic status and more resources also gain more immediate access to new medical treatments and technologies.45 In a study of geographic distribution and survival among clinical trial participants, rural and urban patients with cancer who had uniform access to clinical trials had similar outcomes.46 As precision medicine and targeted therapy oncology practice continue to progress, the potential for worsening disparities for MBC treatment and outcomes among under‐resourced populations may grow if uniform access to care is not provided. It is not clear whether MBC survival improvement is achievable or aspirational in the current health care environment.
It appears from these results that we may be at a crossroads for MBC treatment and survival.47 Access to appropriate, timely, and up‐to‐date diagnosis, care, treatment, and surveillance could turn this fatal disease into a chronic and treatable phenomenon, depending on patient factors, molecular subtype, and insurance capacity to pay for treatment. The potential for an improvement in MBC survival indicates progress in treatment and a possible statistical cure, in that patients may be able to live long enough with disease to die of other causes. Strategies to educate the broader population and improve access to early diagnosis and screening, new drugs and drug combinations, and clinical trials will be critical if we are to reduce the disparities seen here and allow all to benefit from the significant advances that continue to be made.
Funding Support
This work was supported by the Kaplan Cancer Research Fund, the Metastatic Breast Cancer Alliance, and the Surveillance, Epidemiology, and End Results (SEER) Cancer Surveillance System (CSS) program of the National Cancer Institute (contract HHSN261201300012I; National Cancer Institute control number N01 PC‐2013‐00012) for additional case ascertainment.
Conflict of Interest Disclosures
The authors made no disclosures.
Author Contributions
All authors made substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work; drafting the work or revising it critically for important intellectual content; had final approval of the version to be published; and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
We acknowledge and sincerely thank Dr. Marc Hurlbert for his invaluable assistance.
References
- 1. Henley SJ, Anderson RN, Thomas CC, Massetti GM, Peaker B, Richardson LC. Invasive cancer incidence, 2004‐2013, and deaths, 2006‐2015, in nonmetropolitan and metropolitan counties—United States. MMWR Surveill Summ. 2017;66:1‐13. doi: 10.15585/mmwr.ss6614a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Moss JL, Liu B, Feuer EJ. Urban/rural differences in breast and cervical cancer incidence: the mediating roles of socioeconomic status and provider density. Womens Health Issues. 2017;27:683‐691. doi: 10.1016/j.whi.2017.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Singh GK, Williams SD, Siahpush M, Mulhollen A. Socioeconomic, rural‐urban, and racial inequalities in US cancer mortality: part I—all cancers and lung cancer and part II—colorectal, prostate, breast, and cervical cancers. J Cancer Epidemiol. 2011;2011:107497. doi: 10.1155/2011/107497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brawley OW. Health disparities in breast cancer. Obstet Gynecol Clin North Am. 2013;40:513‐523. doi: 10.1016/j.ogc.2013.06.001 [DOI] [PubMed] [Google Scholar]
- 5. Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71‐96. doi: 10.3322/CA.2007.0010 [DOI] [PubMed] [Google Scholar]
- 6. DeSantis C, Siegel R, Bandi P, Jemal A. Breast cancer statistics, 2011. CA Cancer J Clin. 2011;61:409‐418. doi: 10.3322/casc.20134 [DOI] [PubMed] [Google Scholar]
- 7. Schwentner L, Wockel A, Konig J, et al. Adherence to treatment guidelines and survival in triple‐negative breast cancer: a retrospective multi‐center cohort study with 9,156 patients. BMC Cancer. 2013;13:487. doi: 10.1186/1471-2407-13-487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Vaddepally RK, Hejab A, Haythem Y. Institutional adherence to National Comprehensive Cancer Network (NCCN) guidelines in neoadjuvant treatment of breast cancer and its correlation to outcomes [abstract]. J Clin Oncol. 2018;36(30 suppl):47. doi: 10.1200/JCO.2018.36.30_suppl.47 [DOI] [Google Scholar]
- 9. Malmgren JA, Mayer M, Atwood MK, Kaplan HG. Differential presentation and survival of de novo and recurrent metastatic breast cancer over time: 1990‐2010. Breast Cancer Res Treat. 2018;167:579‐590. doi: 10.1007/s10549-017-4529-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gloeckler Ries LA, Reichman ME, Reidel Lewis D, Hankey BF, Edwards BK. Cancer survival and incidence from the Surveillance, Epidemiology, and End Results (SEER) program. Oncologist. 2003;8:541‐552. [DOI] [PubMed] [Google Scholar]
- 11. Narod SA, Giannakeas V, Sopik V. Time to death in breast cancer patients as an indicator of treatment response. Breast Cancer Res Treat. 2018;172:659‐669. doi: 10.1007/s10549-018-4935-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Surveillance Research Program, National Cancer Institute . SEER*Stat software (seer.cancer.gov/seerstat) version 8.3.5. National Cancer Institute; 2018. [Google Scholar]
- 13. IBM Corporation . IBM SPSS Statistics for Windows, Version 25.0. IBM Corporation; 2017. [Google Scholar]
- 14. StataCorp LLC . Stata Statistical Software: Release 15. StataCorp LLC; 2017. [Google Scholar]
- 15. Marubini E, Valsecchi MG. Analyzing Survival Data From Clinical Trials and Observational Studies. John Wiley & Sons Inc; 1995. [Google Scholar]
- 16. Surveillance, Epidemiology, and End Results (SEER) Program . SEER*Stat Database: Incidence‐SEER 9 Registries Research Data, November 2017 Submission (1973‐2015) <Katrina/Rita Population Adjustment>‐Linked To County Attributes‐Total U.S., 1969‐2016 Counties. National Cancer Institute, Division of Cancer Control and Population Sciences, Surveillance Research Program; 2018. [Google Scholar]
- 17. Cancer Surveillance System of the Fred Hutchinson Cancer Research Center . Contract No. N01‐CN‐67009. Surveillance, Epidemiology and End Results (SEER) Program of the National Cancer Institute; 2004. Accessed September 18, 2019. https://www.fredhutch.org/en/research/divisions/public-health-sciencesdivision/research/epidemiology/cancer-surveillance-system.html [Google Scholar]
- 18. Gapstur SM, Dupuis J, Gann P, Collila S, Winchester DP. Hormone receptor status of breast tumors in black, Hispanic, and non‐Hispanic white women. An analysis of 13,329 cases. Cancer. 1996;77:1465‐1471. doi: [DOI] [PubMed] [Google Scholar]
- 19. Warner ET, Tamimi RM, Hughes ME, et al. Racial and ethnic differences in breast cancer survival: mediating effect of tumor characteristics and sociodemographic and treatment factors. J Clin Oncol. 2015;33:2254‐2261. doi: 10.1200/JCO.2014.57.1349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ren JX, Gong Y, Ling H, Hu X, Shao ZM. Racial/ethnic differences in the outcomes of patients with metastatic breast cancer: contributions of demographic, socioeconomic, tumor and metastatic characteristics. Breast Ca Res Treat. 2019;173:225‐237. doi: 10.1007/s10549-018-4956-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Caswell‐Jin JL, Plevritis SK, Tian L, et al. Change in survival in metastatic breast cancer with treatment advances: meta‐analysis and systematic review. JNCI Cancer Spectr. 2018;2:pky062. doi: 10.1093/jncics/pky062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Walters S, Maringe C, Butler J, Brierley JD, Rachet B, Coleman MP. Comparability of stage data in cancer registries in six countries: lessons from the International Cancer Benchmarking Partnership. Int J Cancer. 2013;132:676‐685. doi: 10.1002/ijc.27651 [DOI] [PubMed] [Google Scholar]
- 23. Dawood S, Broglio K, Gonzalez‐Angulo AM, Buzdar AU, Hortobagyi GN, Giordano SH. Trends in survival over the past two decades among white and black patients with newly diagnosed stage IV breast cancer. J Clin Oncol. 2008;26:4891‐4898. doi: 10.1200/JCO.2007.14.1168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ruiterkamp J, Ernst MF, de Munch L, et al. Improved survival of patients with primary distant metastatic breast cancer in the period of 1995‐2008. A nationwide population‐based study in the Netherlands. Breast Cancer Res Treat. 2011;128:495‐503. doi: 10.1007/s10549-011-1349-x [DOI] [PubMed] [Google Scholar]
- 25. Pavlik T, Majek O, Buchler T, et al. Trends in stage‐specific population‐based survival of cancer patients in the Czech Republic in the period 2000‐2008. Cancer Epidemiol. 2014;38:28‐34. doi: 10.1016/j.canep.2013.11.002 [DOI] [PubMed] [Google Scholar]
- 26. Di Meglio A, Freedman RA, Lin NU, et al. Time trends in incidence rates and survival of newly diagnosed stage IV breast cancer by tumor histology: a population‐based analysis. Breast Cancer Res Treat. 2016;157:587‐596. doi: 10.1007/s10549-016-3845-5 [DOI] [PubMed] [Google Scholar]
- 27. Jemal A, Ward EW, Johnson CJ, et al. Annual Report to the Nation on the status of cancer, 1975‐2014, featuring survival. J Natl Cancer Inst. 2017;109:djx030. doi: 10.1093/jnci/djx030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7‐34. doi: 10.3322/caac.21551 [DOI] [PubMed] [Google Scholar]
- 29. Eng LG, Dawood S, Sopik V, et al. Ten‐year survival in women with primary stage IV breast cancer. Breast Cancer Res Treat. 2016;160:145‐152. doi: 10.1007/s10549-016-3974-x [DOI] [PubMed] [Google Scholar]
- 30. Chen MT, Sun HF, Zhao Y, et al. Comparison of patterns and prognosis among distant metastatic breast cancer patients by age groups: a SEER population‐based analysis. Sci Rep. 2017;7:9254. doi: 10.1038/s41598-017-10166-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. DeSantis CE, Fedewa SA, Goding Sauer A, Kramer JL, Smith RA, Jemal A. Breast cancer statistics, 2015: convergence of incidence rates between black and white women. CA Cancer J Clin. 2016;66:31‐42. doi: 10.3322/caac.21320 [DOI] [PubMed] [Google Scholar]
- 32. DeSantis CE, Ma J, Goding Sauer A, Newman LA, Jemal A. Breast cancer statistics, 2017, racial disparity in mortality by state. CA Cancer J Clin. 2017;67:439‐448. doi: 10.3322/caac.21412 [DOI] [PubMed] [Google Scholar]
- 33. Hunt BR, Hurlbert MS. Black:white disparities in breast cancer mortality in the 50 largest cities in the United States. Cancer Epidemiol. 2016;45:169‐173. doi: 10.1016/j.canep.2016.07.018 [DOI] [PubMed] [Google Scholar]
- 34. Chen L, Linden HM, Anderson BO, Li C. Trends in 5‐year survival rates among breast cancer patients by hormone receptor status and stage. Breast Cancer Res Treat. 2014;147:609‐616. doi: 10.1007/s10549-014-3112-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Howlader N, Cronin KA, Kurian AW, Andridge R. Differences in breast cancer survival by molecular subtypes in the United States. Cancer Epidemiol Biomarkers Prev. 2018;27:619‐626. doi: 10.1158/1055-9965.EPI-17-0627 [DOI] [PubMed] [Google Scholar]
- 36. Waks AG, Winer EP. Breast cancer treatment, a review. JAMA. 2019;321:288‐300. doi: 10.1001/jama.2018.19323 [DOI] [PubMed] [Google Scholar]
- 37. Thomas A, Khan SA, Chrischilles EA, Schroeder MC. Initial surgery and survival in stage IV breast cancer in the United States, 1988‐2011. JAMA Surg. 2016;151:424‐431. doi: 10.1001/jamasurg.2015.4539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Noone AM, Lund JL, Mariotto A, et al. Comparison of SEER treatment data with Medicare claims. Med Care. 2016;54:e55‐e64. doi: 10.1097/MLR.0000000000000073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Malmgren J, Hurlbert M, Atwood M, Kaplan HG. Examination of a paradox: recurrent metastatic breast cancer incidence decline without improved distant disease survival: 1990‐2011. Breast Cancer Res Treat. 2019;174:505‐514. doi: 10.1007/s10549-018-05090-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mariotto AB, Etzioni R, Hurlbert M, Penberthy L, Mayer M. Estimation of the number of women living with metastatic breast cancer in the United States. Cancer Epidemiol Biomarkers Prev. 2017;26:809‐815. doi: 10.1158/1055-9965.EPI-16-0889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zhang Y, Franzini L, Chan W, Xu H, Du XL. Effects of health insurance on tumor stage, treatment, and survival in large cohorts of patients with breast and colorectal cancer. J Health Care Poor Underserved. 2015;26:1336‐1358. doi: 10.1353/hpu.2015.0119 [DOI] [PubMed] [Google Scholar]
- 42. Iqbal J, Ginsburg O, Rochon PA, Sun P, Narod SA. Differences in breast cancer stage at diagnosis and cancer‐specific survival by race and ethnicity in the United States. JAMA. 2015;313:165‐173. doi: 10.1001/jama.2014.17322 [DOI] [PubMed] [Google Scholar]
- 43. Agarwal S, Ying J, Boucher KM, Agarwal JP. The association between socioeconomic factors and breast cancer‐specific survival varies by race. PLoS One. 2017;12:e0187018. doi: 10.1371/journal.pone.0187018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Nguyen‐Pham S, Leung J, McLaughlin D. Disparities in breast cancer stage at diagnosis in urban and rural adult women: a systematic review and meta‐analysis. Ann Epidemiol. 2014;24:228‐235. doi: 10.1016/j.annepidem.2013.12.002 [DOI] [PubMed] [Google Scholar]
- 45. Phelan JC, Link BG, Tehranifar P. Social conditions as fundamental causes of health inequalities theory, evidence, and policy implications. J Health Soc Behav. 2010;51(1 suppl):S28‐S40. [DOI] [PubMed] [Google Scholar]
- 46. Unger JM, Moseley A, Symington B, Chavez‐Macgregor M, Ramsey SD, Hershman DL. Geographic distribution and survival outcomes for rural patients with cancer treated in clinical trials. JAMA Netw Open. 2018;1:e181235. doi: 10.1001/jamanetworkopen.2018.1235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sledge GW Jr. Curing metastatic breast cancer. J Oncol Pract. 2015;12:6‐11. doi: 10.1200/jop.2015.008953 [DOI] [PubMed] [Google Scholar]


