Abstract
Aim
Our aim was to update the recommendations for the diagnosis, treatment and follow‐up of the first febrile urinary tract infection in young children, which were endorsed in 2012 by the Italian Society of Pediatric Nephrology.
Methods
The Italian recommendations were revised on the basis of a review of the literature published from 2012 to October 2018. We also carried out an ad hoc evaluation of the risk factors to identify children with high‐grade vesicoureteral reflux or renal scarring, which were published in the previous recommendations. When evidence was not available, the working group held extensive discussions, during various meetings and through email exchanges.
Results
Four major modifications have been introduced. The method for collecting urine for culture and its interpretation has been re‐evaluated. We have reformulated the algorithm that guides clinical decisions to proceed with voiding cystourethrography. The suggested antibiotics have been revised, and we have recommended further restrictions of the use of antibiotic prophylaxis.
Conclusion
These updated recommendations have now been endorsed by the Italian Society of Pediatric Nephrology and the Italian Society for Pediatric Infectivology. They can also be used to compare other recommendations that are available, as a worldwide consensus in this area is still lacking.
Keywords: antibiotic treatment, children, febrile urinary tract infection, prophylaxis, vesicoureteral reflux

Abbreviations
- BC
bladder catheterization
- CVU
clean voided urine
- LE
leucocyte esterase
- RBUS
renal and bladder ultrasound
- SPA
suprapubic aspiration
- UC
urine culture
- UTI
urinary tract infection
- VCUG
voiding cystourethrography
- VUR
vesicoureteral reflux
Key notes.
We updated the 2012 Italian recommendations for the first febrile urinary tract infection in young children and introduced four major modifications.
The method for collecting urine for culture and its interpretation has been re‐evaluated, and we have reformulated the algorithm that guides clinical decisions to proceed with voiding cystourethrography.
The suggested antibiotics have been revised and we have recommended further restrictions of the use of antibiotic prophylaxis.
1. INTRODUCTION
In this paper, we present the updated recommendations for the diagnosis, treatment and follow‐up of the first febrile urinary tract infection (UTI) in young children, endorsed by the Italian Society of Pediatric Nephrology and the Italian Society for Pediatric Infectivology. Our previous document 1 has been revised 6 years after its publication, on the basis of recently published literature and the results of an ad hoc evaluation of the risk factors previously proposed to guide clinicians in the identification of children with high‐grade vesicoureteral reflux (VUR).2 As regards risk factors, only the presence of a pathogen other than Escherichia coli significantly predicted high‐grade reflux both in the univariate (odds ratio 2.52, 95% Confidence Interval 1.32‐4.81, P < .005) and multivariate analyses (odds ratio 2.74, 95% CI: 1.39‐5.41, P = .003). The other three most frequent risk factors, abnormal renal and bladder ultrasound (RBUS), abnormal prenatal ultrasound, male younger than 6 months at UTI occurrence, were neither significantly nor independently associated with the presence of high‐grade reflux.2
As in the previous version, these recommendations apply to infants and young children, 2 months to 3 years of age, with a first febrile UTI, based on a temperature of at least 38°C. We excluded infants younger than 2 months of age, because of their specific features and specific treatment needs and children older than 3 years of age because of the lower risk of nephro‐urologic abnormalities and different clinical presentation. Children with immunodeficiency, a previous workup for congenital malformation of the kidney or urinary tract, or those requiring admission to an intensive care unit were also excluded. The updated recommendations follow the same structure as previously, considering 4 major topics: diagnosis, treatment, imaging and antibiotic prophylaxis and grading the evidence on the basis of the SORT criteria: strong (grade A), moderate (grade B) or weak (grade C) in support of a particular intervention.3
The recommendations are intended for use by all physicians dealing with febrile infants and children inside and outside the hospital and by specialists in paediatric and adult nephrology and urology.
2. DIAGNOSIS
2.1. When to suspect a UTI
A diagnosis of UTI should be considered in children presenting with fever of 38°C or higher,4 with no apparent source (grade A).5 In children aged 2 to 3 months, fever may be absent and clinical manifestations may include lethargy, irritability and vomiting.6 The absence of fever in the first 3 months of life does not correlate with a less severe condition,6 and the risk of complications, such as sepsis and meningitis, at this age is greater.7 In older children, frequency, dysuria and changes in continence habits may be early symptoms, while abdominal pain and loin tenderness can be associated with fever. The presence of malodorous urine is neither specific nor sensitive enough to help in the diagnosis of febrile UTI (grade B).8 Poor growth has also been reported as a possible sign of UTI, but in our opinion poor growth is mainly related to recurrent infections or to associated conditions such as chronic kidney disease.
2.2. What to do when a UTI is suspected
Urine should be collected and analysed by dipstick or microscopy to identify children in whom UTI is very likely,9 and, if urinalysis is abnormal, by urine culture (UC) to obtain a definitive diagnosis5 (grade A). The presence of leucocyturia and bacteriuria in a fresh urine specimen and/or positivity for leucocyte esterase (LE) at dipstick suggests a diagnosis of UTI in symptomatic children. Urine culture can confirm the diagnosis based on the growth of a single bacterial strain.5, 10 The interpretation of urine dipstick which is positive for nitrites and negative for leucocytes is not simple, as infection stimulates an inflammatory response in the host, represented in UTI by the presence of urinary leucocytes. Therefore, the positivity of urine culture in the absence of leucocyturia has to be evaluated with caution, as it could represent bacteriuria and not UTI. If fever persists we recommend to repeat dipstick to check for the appearance of leucocyturia.
2.3. How to collect urine
Collecting urine in small children represents a hard practical task. Four methods of urine collection are utilised, with no agreement in the literature: urinary bag, clean voided urine (CVU), transurethral bladder catheterisation (BC) and suprapubic aspiration (SPA). Each method has to be performed following standardised procedures.11, 12, 13, 14, 15, 16, 17, 18 As regards dipstick and urinalysis implementation, any method for urine collection is feasible (grade A).5, 11, 12, 19, 20, 21, 22
How to collect urine for UC has been extensively analysed by the NICE Working Group4 and by Whiting et al23 and is summarised in Table 1. Suprapubic aspiration and transurethral BC are the least likely to yield a contaminated growth (grade A). These methods are cumbersome to implement in primary care as a routine procedure, but feasible in hospital settings.5, 11, 19 In particular, SPA should be recommended in some circumstances, such as severe phimosis, vulvar synechia, infections or malformations of the external genitalia.20 The use of CVU as an alternative to invasive methods is controversial 20, 21; it is recommended by the Australian and English guidelines,4, 11, 23 while the American Academy of Pediatrics does not consider it a valid method for UC.5 When CVU is used in infants, a simple, quick and effective method to stimulate micturition has been reported, though contamination has not been evaluated.14, 15, 16, 17, 18 The use of a bag to collect a UC sample is only recommended by our previous guidelines1 and the 2018 NICE guidelines 4; in the recent literature, most authors recommend its use only in order to perform dipstick analysis.5, 11, 12, 19, 20, 21, 22
Table 1.
Urine culture collection methods
| Method | Recommendation | References |
|---|---|---|
| Bag | Not recommended | 5, 11, 19, 21 |
| CVU |
Recommended in primary care Second choice in hospital settings (consider micturition stimulating methods in infants <6 mo, <10 kg) |
11, 14, 15, 16, 17, 18, 19, 21, 23 |
| Transurethral BC | First choice in hospital settings and mandatory in critically ill children | 5, 11, 19, 21, 26 |
| SPA | Gold standard, but not feasible as a routine procedure in primary care | 5, 11, 12, 19, 20, 21 |
Abbreviations: BC, bladder catheterisation; CVU, clean voided urine; SPA, suprapubic aspiration.
On the basis of these data from the literature and following extensive discussions within our working group, we recommend obtaining urine for culture according to the child's clinical condition. In a febrile child in poor general clinical condition or in a severely ill appearing child, urine must be collected by transurethral BC or SPA4 (grade A). In a febrile child in good clinical condition, a ‘two‐step’ approach is feasible5, 22; urine can be collected by CVU or bag for dipstick.5, 11, 12, 19, 21, 22, 25 If dipstick shows the presence of LE with or without nitrites, urine for UC should be collected by CVU or transurethral BC4, 11, 19, 21, 25, 26 (grade A). If dipstick does not show the presence of LE and nitrites, we do not recommend UC but a clinical follow‐up and a new dipstick if fever persists after 24‐48 hours (grade B).
2.4. What is the clinical significance of urine dipstick, microscopy and culture?
The sensitivity and specificity of dipstick and microscopy have been well summarised in a metanalysis 9 and are reported in Table 2.
Table 2.
Sensitivity and specificity of urinary dipstick (leucocyte esterase and nitrite) and microscopy (white blood cells and bacteria) for diagnosis of urinary tract infection (adapted with permission from Williams GJ)9
| Test | Sensitivity % ( range) | Specificity % ( range) |
|---|---|---|
| Leucocyte esterase | 79 (73‐84) | 87 (80‐92) |
| Nitrite | 49 (41‐57) | 98 (96‐99) |
| Leucocyte esterase or nitrite positive | 88 (82‐91) | 79 (69‐87) |
| Both leucocyte esterase and nitrite positive | 45 (30‐61) | 98 (96‐99) |
| Microscopy: white blood cells | 74 (67‐80) | 86 (82‐90) |
| Microscopy: unstained bacteria | 88 (75‐94) | 92 (83‐96) |
| Microscopy: Gram stain | 91 (80‐96) | 96 (92‐98) |
Results of the LE test are comparable to those of white blood cells by microscopy. Microscopy with Gram staining for bacterial differentiation is the rapid test with the highest sensitivity and specificity; however, this test is almost never performed in routine settings in Italy. While in our previous recommendations the use of dipstick in children <2 years of age was discouraged due to its unreliability,1, 27 more recent data in the literature agree on the use of the dipstick test also in children <2years.4, 28, 29, 30, 31 A practical approach, based on the results of LE and nitrite dipstick analysis, is suggested in Table 3.
Table 3.
Interpretation and suggested practical approach following the result of nitrite and leucocyte esterase urine dipstick
|
Nitrite positive Leucocyte esterase positive |
UTI very likely | Perform urine culture and start antibiotics empirically |
|
Nitrite negative Leucocyte esterase positive |
UTI likely | Perform urine culture and start antibiotics empirically |
|
Nitrite negative Leucocyte esterase negative |
UTI quite unlikely | Search for alternative diagnosis |
Abbreviation: UTI, urinary tract infection.
When urine microscopy is employed, it should be performed on a fresh specimen by an expert operator (grade B). Urine culture is required to confirm the diagnosis (grade A): it is considered positive if the culture demonstrates the growth of a single organism with a colony count threshold which depends on the method used for collection, as indicated in Table 4 (grade C). However, it is difficult to establish a precise cut‐off for interpreting the results of UC, because of the heterogeneity and variability of the available literature, as well summarised by some authors.10, 21 Of course, each result has to be evaluated against anamnestic, clinical and laboratory data (fever, leucocyturia, bacteremia).10 We give some recommendations on cut‐offs, keeping in mind that the UC result must be evaluated in the context of the clinical situation.
Table 4.
Cut‐off for a significant colony count in urine culture according to urine collection method
| Method |
Cut‐off values indicated in the literature (Reference number) |
Our Recommendation (Grade C)* |
|---|---|---|
| SPA |
>50.000 CFU/mL (or less if fever and leucocyturia)5 |
>10.000 CFU/mL |
| Transurethral BC | >50.000 CFU/mL5, 10 Or >10.000 CFU/mL11, 19 if fever and leucocyturia 5, 10 | >10.000 CFU/mL |
| CVU | >100.000 CFU/mL4, 11, 19, 20, 21 | >50.000 CFU/mL |
| Bag** | >100.000 CFU/mL20, 21 | >100.000 CFU/mL |
Abbreviations: BC, Bladder catheterisation; CVU, clean voided urine; SPA, suprapubic aspiration.
It refers to children with fever ≥38°C and leucocyturia.
Not recommended.
In our settings, where we mainly use CVU samples, we believe that both urinary leucocytes and a significant colony count in UC (Table 4) are needed for the diagnosis of UTI (grade B).
2.5. Are blood tests necessary when a UTI is suspected?
Routine blood tests are not necessary to identify the site of infection. If the child is hospitalised, a complete blood count, C‐reactive protein, procalcitonin and renal function tests are indicated (grade B), and always recommended in infants <3 months.4, 11, 32
2.6. When should a child be hospitalised?
We recommend to hospitalise any critically ill child (sepsis, dehydration and vomiting) (grade A), when serious concern of noncompliance is present (grade B) and when fever persists after 3 days of appropriate antibiotic treatment, as shown by sensitivity testing (grade A).33, 34
3. TREATMENT
In a febrile child with suggestive clinical signs and/or positive urine dipstick or microscopy, antibiotic treatment has to be initiated soon after a urine specimen for UC has been obtained. Prompt antibiotic treatment is necessary to eradicate the infection, to prevent bacteremia (in particular, during the first months of life) and to improve clinical condition (grade A).20, 35, 36 As regards the risk of UTI‐related renal scarring, it is now an established fact the time to initiation of antibiotic treatment makes no difference in the frequency and severity of scarring, as long as it is initiated within 3‐4 days from the onset of fever.35, 37, 38, 39, 40 Many studies have demonstrated that starting treatment either orally or parenterally is of equal effectiveness, and the clinician should base their choice of the route of administration on practical considerations5, 33, 34, 41, 42, 43, 44, 45, 46, 47, 48, 49: if the UTI is complicated, that is when the child appears septic or severely dehydrated or is vomiting, or if concerns regarding compliance are present, treatment should be started parenterally and continued with an oral antibiotic as soon as the clinical conditions of the child allow it (grade A); if the UTI is not complicated, that is when the febrile child is in good clinical condition and able to retain oral fluids and medications and compliance is expected, treatment should be administered via the oral route (grade A) 5, 33, 34, 35, 46, 47, 48, 49 (Figure 1). The results of oral versus parenteral route do not differ in terms of duration of fever, recurrence of UTI or incidence of UTI‐related renal scarring.5, 11, 33, 34, 42, 43
Figure 1.
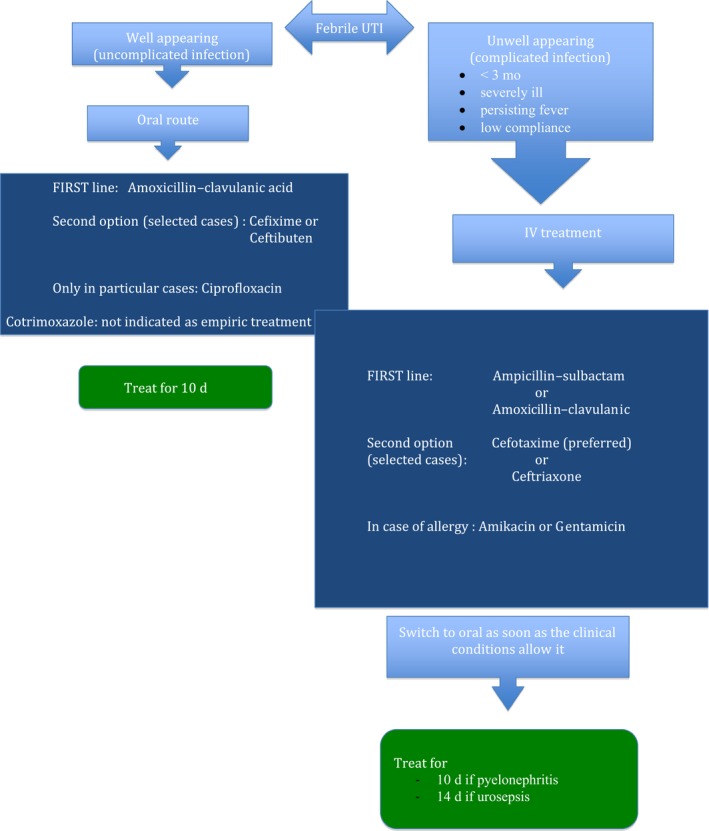
Treatment of urinary tract infection
Clinicians should also base their choice of the antibiotic on local antimicrobial sensitivity patterns (if available) and adjust it according to sensitivity testing of the isolated uropathogen (grade A).5, 11, 33, 34, 49 Escherichia coli remains the predominant uropathogen isolated in acute community‐acquired uncomplicated infections (80%), followed by Klebsiella, Enterobacter, Proteus species and Enterococci. Many of the characteristics of these pathogens are changing, particularly due to antimicrobial resistance.50, 51, 52, 53, 54, 55, 56 According to our national pattern of resistance,57, 58, 59, 60, 61, 62 we recommend amoxicillin‐clavulanic acid as the first‐line oral antibiotic and ampicillin‐sulbactam or amoxicillin‐clavulanic acid if the intravenous route is indicated (grade B). The increasing resistance of Escherichia coli to third‐generation cephalosporins (about 30% in Italy) is mainly due to the widespread and not always appropriate use of this class of antibiotics.62, 63, 64 Therefore, we suggest considering cephalosporins (cefixime or ceftibuten for the oral route and cefotaxime or ceftriaxone for iv administration) in children with severe infections.5, 11, 33, 34, 45, 46, 47, 48, 49, 62, 65, 66 In effect cephalosporins have a superior efficacy and rapidity of action, making the possible onset of resistance a less important issue (grade C). Because ceftriaxone is known to cause cholestasis,67 it should be used with caution in infants with jaundice or those younger than 3 months; cefotaxime should be preferred, also due to pharmacokinetic/pharmacodynamic considerations and especially because of its renal excretion (grade C).
If UC results show resistance to the prescribed antibiotic but the patient's condition is improving, treatment should be continued without change (grade C).68, 69 In children who are allergic to beta‐lactams, an aminoglycoside, such as amikacin or gentamicin, is the best choice (grade A), bearing in mind that Pseudomonas Aeruginosa quickly develops antibiotic resistance when aminoglycosides are used as a monotherapy.70, 71
Because of the high rate of resistance, the empirical use of trimethoprim must be avoided; it should be used only on the basis of antibiogram sensitivity.72
The use of ciprofloxacin in paediatric patients is controversial. The use of quinolones should be limited to patients whose clinical condition is severe or who are unresponsive to other antibiotics, only on the basis of sensitivity patterns, as indicated in recent recommendations.73, 74 The worrying increase in resistance due to the widespread use of quinolones in adults should also be taken into consideration.75
Agents that are excreted in the urine but do not achieve therapeutic blood concentrations, such as nitrofurantoin, should not be used to treat febrile UTIs, because parenchymal and serum antimicrobial concentrations may be insufficient to treat pyelonephritis or urosepsis (grade A).47
The suggested dosages of the aforementioned antibiotics are outlined in Table 5.
Table 5.
Suggested dosage for antibiotic treatment of febrile urinary tract infections
| Treatment | Dose |
|---|---|
| Intravenous | |
| Penicillins | |
|
Ampicillin‐Sulbactam Amoxicillin‐clavulanic acid |
100 mg/kg/d of ampicillin in 3‐4 doses 100 mg/kg/d of amoxicillin in 3‐4 doses |
| Cephalosporins | |
|
Cefotaxime Ceftriaxone |
150‐200 mg/kg/d in 3‐4 doses* 75‐100 mg/kg/d in 1 dose* |
| Aminoglycosides | |
|
Amikacin Gentamicin |
15 mg/kg/d in 1 dose** 6‐7.5 mg/kg/d in 1 dose** |
| Oral route | |
| Amoxicillin‐clavulanic acid | 50‐90 mg/kg/d of amoxicillin in 3 doses |
| Cephalosporins | |
| Cefixime | 8 mg/kg twice/d 1st d, once daily thereafter |
| Ceftibuten | 9 mg/kg twice/d 1st d, once daily thereafter |
| Ciprofloxacin | 20‐40 mg/kg/d in 2 doses |
| Trimethoprim‐sulfamethoxazole | 8‐12 mg/kg/d of trimethoprim in 2 doses*** |
Note: Dosages, in accordance with those cited in References (1, 35) and with the Sanford Guide to Antimicrobial Therapy, may vary from those used in some Institutions or trials. Always compare with current product monographs.
The highest dose in children with urosepsis.
Serum levels must be monitored and dosage adjusted accordingly.
To be used only on the basis of antibiogram sensitivity, because of the high resistance rate.
There is no consensus in the literature on the optimal duration of antimicrobial therapy5, 11, 33, 34, 45, 46, 47, 48, 49, 76, 77, 78; we suggest a 10‐day course for pyelonephritis. For urosepsis, we recommend a 14‐day course to be started parenterally; however, parenteral therapy can be limited to 3 days in most cases (grade B).
There is insufficient evidence and no recommendations on the use of methylprednisolone in the management of acute pyelonephritis, with one small study showing a significant reduction in scarring in the treatment arm that warrants a larger series.48, 79
4. IMAGING
4.1. When and how should ultrasound be performed?
We recommend performing RBUS in all children, 2‐4 weeks after the first febrile UTI, in order to detect renal and urinary tract anomalies (grade B). We do not recommend a RBUS during the febrile UTI, unless it is complicated, atypical or severe (presence of any of the following: septic state, fever persisting after 3 days of appropriate antibiotic treatment, elevated plasma creatinine, oliguria)(grade B).49
The RBUS report should always describe the characteristics of the kidneys, and in particular renal length, echogenicity and thickness of the parenchyma. Other important characteristics are the features of the calices, the antero‐posterior diameter of the renal pelvis at the exit from the renal parenchyma, the maximum diameter of the ureter, the wall thickness of the bladder and, if possible, pre‐ and post‐void bladder volume. We also recommend that the presence or absence of renal pelvic uroepithelial thickening is reported.
It is, however, important to point out that a great deal of evidence exists on the low predictive value of renal ultrasound as regards the presence of VUR. This examination is frequently normal in children with low‐grade, and even in some with high‐grade, VUR, and while mild and transient renal pelvic or ureteral distension is common, it is often not associated with VUR. On the other hand, abnormal RBUS findings represent a risk factor for UTI‐related renal scarring and are present in up to 86% of patients with high‐grade VUR.80 Among the abnormal findings, of particular relevance are as follows: mono‐ or bilateral renal hypoplasia, major pelvi‐calyceal dilatation, ureteral dilatation and uroepithelial thickening of the renal pelvis.81
4.2. When and how should imaging to detect VUR be performed?
We recommend imaging in order to detect VUR after the first febrile UTI when RBUS reveals mono‐ or bilateral renal hypoplasia, anomalies of parenchymal echogenicity, ureteral dilatation, uroepithelial thickening of the renal pelvis and pelvi‐calyceal dilatation, particularly if associated with uroepithelial thickening, bladder abnormalities (grade B). An isolated dilatation of the renal pelvis generally does not represent an indication for further imaging (grade B). In addition, imaging for the detection of VUR should be performed when the UTI is caused by a pathogen other than Escherichia coli (grade A)2, 82 and in children with recurrent febrile UTIs (Figure 2).
Figure 2.
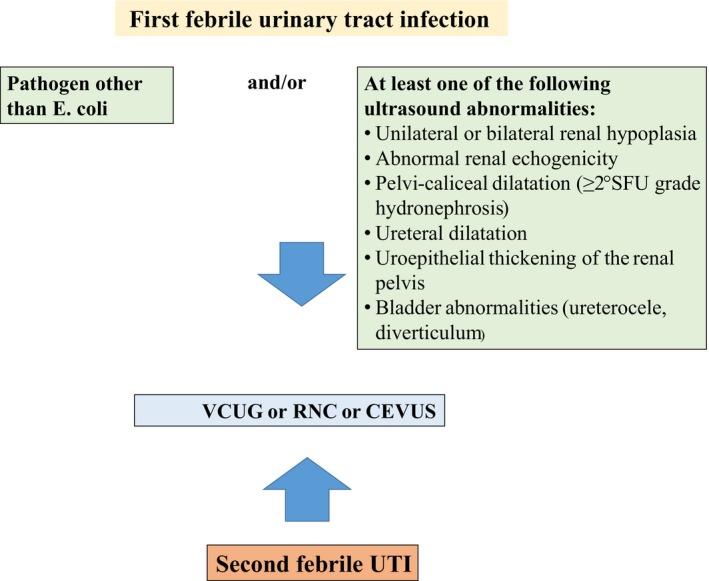
When should imaging to detect vesicoureteral reflux be performed? Legends: SFU, Society for foetal Urology; VCUG, fluoroscopic contrast voiding cystourethrography; RNC, direct radionuclide cystography; CEVUS, contrast enhanced voiding ultrasonography
4.2.1. Imaging options
Currently, there are four imaging modalities available for the detection of VUR.
Fluoroscopic contrast voiding cystourethrography (VCUG) is the standard method for the diagnosis of VUR, the assessment of the degree of reflux and the anatomy of the male urethra (grade A). A standardised protocol for VCUG performance has recently been issued: in boys, lidocaine gel is instilled in the urethra before catheterisation; a small age‐appropriate (3.5‐8 French) non‐balloon catheter is inserted by means of a sterile procedure; the bladder should be filled until voiding occurs and if VUR is not identified on the first void, a second filling with the same catheter should be performed to increase the chance for detection of VUR.83
Vesico‐ureteric reflux is detected with equal or superior sensitivity by direct radionuclide cystography, which delivers much less radiation than VCUG, but is less readily available and does not provide anatomic details of the male urethra; it could represent the first choice in females (grade B).
Contrast enhanced voiding ultrasonography is a sensitive modality used to detect VUR84, 85; in addition, a second‐generation contrast agent and a transperineal approach enables a precise evaluation of the bladder and male urethra.86 It is less commonly performed because it is time consuming, expensive and not available on a large scale.
The last option is indirect radioisotopic cystography, which can be obtained during the last phases of a MAG 3 scintigraphy; however, it has a low sensitivity and specificity.
4.3. Is antibiotic treatment necessary at the time of catheter insertion for imaging?
Even though it is widely prescribed in clinical practice, prescription of antibiotic therapy is debated: some guidelines recommend its use,11 but recent data show that the risk of UTI after VCUG is very low.87 We suggest administering antibiotic treatment at full dosage for three days in infants, especially within the first 12 months of life, or when major urinary tract abnormalities are present at RBUS (grade C).
4.4. Scintigraphy
Scintigraphy is not routinely recommended after the first UTI. The implementation of a renal cortical scintigraphy (with DMSA) is recommended in all children with VUR grades IV and V, which have been recognised as major risk factors for permanent renal damage (grade B).88, 89 In order to evaluate the presence of UTI‐related renal scarring, a renal scan has to be performed at least 6 months after the febrile UTI, the time required to avoid misinterpretation of transient changes related to the acute infection.
5. WHAT TO DO AFTER THE FIRST FEBRILE UTI?
Most febrile UTIs in children are uneventful infections, occurring in otherwise normal children who have an excellent prognosis. A relatively small number of children (6%‐10%) will develop recurrences, generally during the following year.46 Recurrence risk factors are high‐grade reflux, age below 1 year in males, female sex and bladder bowel dysfunction.
Generally speaking, it is important to instruct parents to recognise UTI symptoms and to prevent modifiable risk factors for recurrent UTIs and in particular constipation and bladder bowel dysfunction.90, 91 We believe that also a low fluid intake has to be taken into consideration (grade C).
Circumcision is a conceivable option in selected cases of males with high‐grade VUR and with recurrent febrile UTIs despite other efforts to prevent infections.
As regards antibiotic prophylaxis, it has been used for decades in children with VUR, with the assumption that renal damage and its progression would be prevented if recurrent UTIs were avoided. Currently, its effectiveness is under debate. A number of recent randomized controlled trials have shown no or a minimal effect of antibiotic prophylaxis in reducing the recurrence of UTIs.92, 93, 94, 95, 96 Various meta‐analyses have been published 97, 98, 99; among those, the one published by De Bessa et al99appears of particular interest, as the authors separated dilating (grades III‐IV‐V) and non‐dilating (grades I‐II) VUR as far as breakthrough infections are concerned. Analysing the first published studies, the authors found that antibiotic prophylaxis would be beneficial only in children with high‐grade VUR. With the addition of the data from the RIVUR study,100 these results changed, supporting antibiotic prophylaxis in all children with VUR. It has to be underlined that the RIVUR trial evaluated 607 children (92% female) with an age range of 2‐71 months, 126 were toilet trained, 71 of them had bladder bowel dysfunction, and 92% had grade I to III reflux. We believe that the treatment showed statistical, but not clinical significance: 22 patient‐years of antibiotics were required to prevent one febrile UTI. Therefore, the analysis of the data regarding recurrent infections does not stand in favour of the use of antibiotic prophylaxis, at least in children with low‐grade reflux.
An additional concern is the propensity of antibiotics to induce bacterial resistance. A recent meta‐analysis by Selekman et al101 showed that prophylaxis increases the risk of multidrug resistance (children receiving prophylaxis had 6.4 times the odds), with important implications in the risk‐benefit assessment of prophylaxis.
Concurrently, it has become clear that prophylaxis does not reduce the appearance and progression of permanent renal damage, as shown by multiple recent meta‐analyses.97, 98, 99, 102 Furthermore, the treatment group from the RIVUR trial received together over 600 years of prophylaxis, without a demonstrable effect on scar formation.
In conclusion, antibiotic prophylaxis is not routinely recommended in infants and children after the first febrile UTI (grade A). It may be considered in infants and children after treatment of the acute episode until VCUG is performed (grade C), with reflux grades IV and V (grade C), and with recurrent febrile UTIs, defined as >3 febrile UTIs within 12 months (grade C).
These recommendations are in line with the main international guidelines.4, 5, 11
As a first choice prophylactic agent, we suggest amoxicillin‐clavulanic acid, while ceftibuten or nitrofurantoin should be regarded as secondary options, keeping in mind that nitrofurantoin may cause gastrointestinal intolerance and is inactive against most strains of Proteus.103 There is insufficient evidence to recommend a specific dose; however, traditionally, the dose used for prophylaxis has been one‐quarter to one‐third of the treatment dose, given once per day. There are no data on the efficacy of the practice of alternating prophylactic antibiotics.
Similarly, the optimal duration of prophylaxis has not been established. According to the longer susceptibility to UTI in girls than in boys, we suggest 12‐24 months in girls and 6‐12 months in boys (grade C).
5.1. Other interventions for preventing UTI
Several interventions, other than antibiotic prophylaxis, have been used for the prevention of recurrent UTIs, but evidence for their effectiveness in infants and children is lacking.104
The efficacy of cranberry juice remains questionable. In a study on children aged 1‐6 years, with recurrent UTIs, but no or minor urologic malformations, the intervention (cranberry for 6 months) did not significantly reduce the number of children who experienced a recurrence of UTI, but it was effective in reducing the actual number of recurrences and related antimicrobial use.105
Few studies are available on probiotics and, at present, no significant benefit has been demonstrated for UTI prevention.106
6. HEALTH BENEFITS, POTENTIAL RISKS AND LIMITATIONS OF OUR RECOMMENDATIONS
Our recommendations are useful in helping the practicing clinician to determine the diagnostic and therapeutic approach to a child with a febrile UTI. Furthermore, the clinical use of the recommendations will lead to a reduction in the number of performed VCUG, and therefore to a reduction of radiation and financial costs. On the other hand, reducing the number of VCUG could produce the risk of missing a small number of high‐grade VUR after the first febrile UTI; anyhow, a second febrile UTI represents in our recommendation an indication to further imaging. Another health benefit may be represented by a more restricted use of antibiotic prophylaxis, also addressing the problem of growing antibiotic resistance; of course, the risk of increasing UTI recurrences has to be kept in mind.
7. FUTURE RESEARCH
The authors of these recommendations have found some gaps in the knowledge on UTI in infants and children, needing further studies. In particular, we suggest the need to study the number of colony count needed to make a diagnosis of UTI and the scientific rationale to recommend different numbers of colony counts for different urine collection modalities. Other points are the meaning of nitrites in the absence of leucocyturia, the role of steroids in preventing the appearance of scarring, the role of a high fluid intake in preventing recurrent infections and the need of antibiotic prophylaxis in children with high‐grade reflux.
Further research should also establish if a shorter duration of antibiotic treatment is warranted. Most importantly, needed to establish the potential morbidity of UTIs in the long‐term,107 are prospective studies following a first febrile UTI in children with normal kidneys as well as in children with prenatally diagnosed hypodysplasia.
1. UTI recommendations at a glance
Diagnosis. The diagnosis of UTI should be considered in children presenting with fever (38°C or higher) and no apparent source. Urine should be collected and analysed by microscopy or dipstick to identify children in whom UTI is very likely (leucocyturia with or without nitrites) and by urine culture to make a definitive diagnosis.
Urine culture. To collect urine, a two‐step approach is feasible in febrile children in good clinical condition. Urine can be collected by clean voided method or bag for dipstick. If the dipstick shows the presence of leucocytes with or without nitrites, a sample for urine culture should be collected by clean voided urine or transurethral bladder catheterisation. If the dipstick is normal, there is no need to perform a urine culture and a new dipstick is recommended after 24‐48 h, if the fever persists. In a severely ill febrile child urine must be collected by transurethral bladder catheterisation or suprapubic aspiration.
Treatment. In a febrile child with positive urine dipstick or microscopy, antibiotic treatment must be initiated soon after urine culture is obtained. Suggested antibiotics are as follows: amoxicillin‐clavulanic acid as first line if the oral route is possible in children who appear well and ampicillin‐sulbactam or amoxicillin‐clavulanic acid if the intravenous route is indicated in severely ill children.
Imaging. A renal and bladder ultrasound is suggested in all children, 2‐4 wk after the febrile UTI, while voiding cystourethrography is indicated when ultrasound reveals major anomalies of the kidney and/or urinary tract and/or when the UTI is caused by a pathogen other than Escherichia coli.
Antibiotic prophylaxis. This is not routinely recommended after the first febrile UTI. It may be considered in children with reflux grade IV and V, or with recurrent febrile UTIs, defined as more than three febrile UTIs within 12 mo.
CONFLICTS OF INTEREST
The authors have no conflicts of interest to declare.
FUNDING INFORMATION
This study did not receive any specific funding.
ACKNOWLEDGEMENTS
Alexandra Teff provided linguistic advice. The non‐profit association ‘Il Sogno di Stefano (Stefano's Dream)' provided financial support for the meetings held in preparation of these recommendations.
Ammenti A, Alberici I, Brugnara M, et al; on behalf of the Italian Society of Pediatric Nephrology . Updated Italian recommendations for the diagnosis, treatment and follow‐up of the first febrile urinary tract infection in young children. Acta Paediatr. 2020;109:236–247. 10.1111/apa.14988
Funding information
This study did not receive any specific funding.
REFERENCES
- 1. Ammenti A, Cataldi L, Chimenz R, et al. Febrile urinary tract infections in young children: recommendations for the diagnosis, treatment and follow‐up. Acta Pædiatr. 2012;101:451‐457. [DOI] [PubMed] [Google Scholar]
- 2. Alberici I, La Manna A, Pennesi M, et al. First urinary tract infections in children: the role of the risk factors proposed by the Italian recommendations. Acta Pædiatr. 2019;108:544‐550. [DOI] [PubMed] [Google Scholar]
- 3. Ebell MH, Siwek J, Weiss BD, et al. Strength of recommendation taxonomy (SORT): a patient‐centered approach to grading evidence in the medical literature. Am Fam Physician. 2004;69:549‐557. [PubMed] [Google Scholar]
- 4. National Institute for Health and Clinical Excellence (NICE) . Clinical Guideline. Urinary tract infection under 16s: diagnosis and management. Published August 2007. Last updated: October 2018. https://www.nice.org.uk/guidance/cg54 Accessed January 8, 2019
- 5. AAP Subcommittee on Urinary Tract Infection . Reaffirmation of AAP clinical practice guideline: the diagnosis and management of the initial urinary tract infection in febrile infants and young children 2–24 months of age. Pediatrics. 2016;138:e20163026. [DOI] [PubMed] [Google Scholar]
- 6. Hernandez‐Bou S, Trenchs V, Alarcon M, Luaces C. Afebrile very young infants with urinary tract infection and the risk for bacteremia. Pediatr Infect Dis J. 2014;33:244‐247. [DOI] [PubMed] [Google Scholar]
- 7. Bachur RG, Harper MB. Predictive model for serious bacterial infections among infants younger than 3 months of age. Pediatrics. 2001;108:311‐316. [DOI] [PubMed] [Google Scholar]
- 8. Gauthier M, Gouin S, Phan V, Gravel J. Association of malodorous urine with urinary tract infection in children aged 1 to 36 months. Pediatrics. 2012;129:885‐890. [DOI] [PubMed] [Google Scholar]
- 9. Williams GJ, Macaskill P, Chan SF, Turner R, Hodson E, Graig JC. Absolute and relative accuracy of rapid urine tests for urinary tract infection in children: a meta‐analysis. Lancet Infect Dis. 2010;10:240‐250. [DOI] [PubMed] [Google Scholar]
- 10. Roberts KB, Wald ER. The diagnosis of UTI: colony count criteria revisited. Pediatrics. 2018;141:e20173239. [DOI] [PubMed] [Google Scholar]
- 11. McTaggart S, Danchin M, Ditchfield M, et al. KHA‐CARI guideline: diagnosis and treatment of urinary tract infection in children. Nephrology. 2015;20:55‐60. [DOI] [PubMed] [Google Scholar]
- 12. Bajaj L, Bothner J. Urine collection techniques in infants and children with suspected urinary tract infection. Uptodate 2018 (literature review current through: Feb 2019. Topic last updated: dec 04, 2018. http://www.uptodate.com/store).
- 13. Baron EJ, Miller JM, Weinstein MP, et al. executive summary: a guide to utilization of the microbiology laboratory for diagnosis of infectious diseases: 2013 recommendations by the infectious diseases society of America (IDSA) and the American society for microbiology (ASM)(a). Clin Infect Dis. 2013;57:485‐488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kaufman J, Fitzpatrick P, Tosif S, et al. The QuickWee trial: protocol for a randomised controlled trial of gentle suprapubic cutaneous stimulation to hasten non‐invasive urine collection from infants. BMJ Open. 2016;6:e011357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kaufman J, Tosif S, Fitzpatrick P, et al. Quick‐Wee: a novel non‐ invasive urine collection method. Emerg Med J. 2017;34:63‐64. [DOI] [PubMed] [Google Scholar]
- 16. Tran A, Fortier C, Giovannini‐Chami L, et al. Evaluation of the bladder stimulation technique to collect midstream urine in infants in a pediatric emergency department. PLoS ONE. 2016;11:e0152598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Herreros ML, Tagarro A, Garcia‐Pose A, Sanchez A, Cañete A, Gili P. Accuracy of a new clean‐catch technique for diagnosis of urinary tract infection in infants younger than 90 days of age. Pediatr Child Health. 2015;20:e30‐e32. [PMC free article] [PubMed] [Google Scholar]
- 18. Labrosse M, Levy A, Autmizguine J, Grave J. Evaluation of a new strategy for clean catch urine in infants. Pediatrics. 2016;138:e2 0160573. [DOI] [PubMed] [Google Scholar]
- 19. Robinson JL, Finlay JC, Lang ME, Bortolussi R, Canadian Pediatric Society, Infectious Disease and Immunization Committee, Community Paediatrics Committee . Urinary tract infections in Infants and children: diagnosis and management. Paediatr Child Health. 2014;19(6):315‐319. Reaffirmed: Jan 30 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Simões e Silva AC, Oliveira EA. Update on the approach of urinary tract infection in childhood. J Pediatr (Rio J). 2015;91:S2‐S10. [DOI] [PubMed] [Google Scholar]
- 21. LaRocco MT, Franek J, Leibach EK, et al. Effectiveness of preanalytic practices on contamination and diagnostic accuracy of urinary cultures: a laboratory medicine best practices systematic review and meta‐analysis. Clin Microbiol Rev. 2016;29:105‐147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lavelle JM, Blackstone MM, Funari MK, et al. Two‐step process for ED UTI screening in febrile young children: reducing catheterization rates. Pediatrics. 2016;138(1):e20153023. [DOI] [PubMed] [Google Scholar]
- 23. Whiting P, Westwood M, Bojke L, et al. Clinical effectiveness and cost‐effectiveness of tests for the diagnosis and investigation of urinary tract infection in children: a systematic review and economic model. Health Technol Assess. 2006;10:1‐154. [DOI] [PubMed] [Google Scholar]
- 24. Shaikh N, Morone NE, Lopez J, et al. Does this child have a urinary tract infection? JAMA. 2007;298:2895‐2904. [DOI] [PubMed] [Google Scholar]
- 25. Al‐Orifi F, McGillivray D, Tange S, Kramer MS. Urine culture from bag in young children: are the risks too high? J Pediatr. 2000;137:221‐226. [DOI] [PubMed] [Google Scholar]
- 26. Etoubleau C, Reveret M, Brouet D, et al. Moving from bag to catheter for urine collection in non‐toilet‐trained children suspected of having urinary tract infection: a paired comparison of urine cultures. J Pediatr. 2009;154:803‐806. [DOI] [PubMed] [Google Scholar]
- 27. Mori R, Yonemoto N, Fitzgerald A, Tullus K, Verrier‐Jones K, Lakhanpaul M. Diagnostic performance of urine dipstick testing in children with suspected UTI: a systematic review of relationship with age and comparison with microscopy. Acta Pædiatr. 2010;99:581‐584. [DOI] [PubMed] [Google Scholar]
- 28. Glissmeyer EW, Korgenski EK, Wilkes J, et al. Dipstick screening for urinary tract infection in febrile infants. Pediatrics. 2014;133:e1121‐e1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kanegaye JT, Jacob JM, Malikli D. Automated urinalysis and urine dipstick in the emergency evaluation of young febrile children. Pediatrics. 2014;134:523‐529. [DOI] [PubMed] [Google Scholar]
- 30. Schroeder AR, Chang PW, Shen MW, Biondi EA, Greenhow TL. Diagnostic accuracy of the urinalysis for urinary tract infection in infants <3 months of age. Pediatrics. 2015;135:965‐971. [DOI] [PubMed] [Google Scholar]
- 31. Velasco R, Benito H, Mozun R, et al. Using a urine dipstick to identify a positive urine culture in young febrile infants is as effective as in older patients. Acta Paediatr. 2015;104:e39‐e44. [DOI] [PubMed] [Google Scholar]
- 32. Shaikh N, Borrel JL, Evrol J, Procalcitonin L. C‐reactive protein and erythrocyte sedimentation rate for the diagnosis of acute pyelonephritis in children. Cochrane Database Syst Rev. 2015;1:CD009185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. National Institute of Health and Clinical Excellence . Urinary tract infection in children: diagnosis, treatment and long‐term management. London, UK: NICE Clinical Guideline; 2007. [Google Scholar]
- 34. Morello W, La Scola C, Alberici I, Montini G. Acute pyelonephritis in children. Pediatr Nephrol. 2016;3:1253‐1265. [DOI] [PubMed] [Google Scholar]
- 35. Montini G, Tullus K, Hewitt I. Febrile urinary tract infections in children. N Engl J Med. 2011;365:239‐250. [DOI] [PubMed] [Google Scholar]
- 36. Doganis D, Sinaniotis K. Early antibiotic treatment of pyelonephritis in children is still mandatory. Pediatrics. 2009;123:e173‐e174. [DOI] [PubMed] [Google Scholar]
- 37. Karavanaki KA, Soldatou A, Koufadaki AM, Tsentidis C, Haliotis FA, Stefanidis CJ. Delayed treatment of the first febrile urinary tract infection in early childhood increased the risk of renal scarring. Acta Paediatr. 2017;106:149‐154. [DOI] [PubMed] [Google Scholar]
- 38. Hewitt IK, Zucchetta P, Rigon L, et al. Early treatment of acute pyelonephritis in children fails to reduce renal scarring: data from the Italian renal infection study trials. Pediatrics. 2008;122:486‐490. [DOI] [PubMed] [Google Scholar]
- 39. Coulthard MG, Verber I, Jani JC, et al. Can prompt treatment of childhood UTI prevent kidney scarring? Pediatr Nephrol. 2009;24:2059‐2063. [DOI] [PubMed] [Google Scholar]
- 40. Oh MM, Kim JW, Park MG, Kim JJ, Yoo KH, Moon DG. The impact of therapeutic delay time on acute scintigraphic lesion and ultimate scar formation in children with first febrile UTI. Eur J Pediatr. 2012;171:565‐570. [DOI] [PubMed] [Google Scholar]
- 41. Iacobelli S, Bonsante F, Guignard JP. Infections urinaires en pediatrie. Arch Pediatr. 2009;16:1073‐1079. [DOI] [PubMed] [Google Scholar]
- 42. Montini G, Toffolo A, Zucchetta P, et al. Antibiotic treatment for pyelonephritis in children: multicentre randomised controlled non‐inferiority trial. BMJ. 2007;335:386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bocquet N, Sergent Alaoui A, Jais J‐P, et al. Randomized trial of oral versus sequential IV/oral antibiotic for acute pyelonephritis in children. Pediatrics. 2012;129:e269‐e275. [DOI] [PubMed] [Google Scholar]
- 44. Arshad M, Seed PC. Urinary tract infections in the infant. Clin Perinatol. 2015;42(17–28):17–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Stein R, Dogan HS, Hoebeke P, et al. Urinary tract infections in children: EAU/ESPU guidelines. Eur Urol. 2015;67:546‐558. [DOI] [PubMed] [Google Scholar]
- 46. American Academy of Pediatrics . Subcommittee on urinary tract infection, steering committee on quality improvement and management. urinary tract infection: clinical practice guideline for the diagnosis and management of the initial UTI in febrile infants and children 2 to 24 months. Pediatrics. 2011;128:595‐610. [DOI] [PubMed] [Google Scholar]
- 47. Masson P, Matheson S, Webster AC, Craig JC. Meta‐analyses in prevention and treatment of urinary tract infections. Infect Dis Clin North Am. 2009;23:355‐385. [DOI] [PubMed] [Google Scholar]
- 48. Schmidt B, Copp HL. Work‐up of pediatric urinary tract infection. Urol Clin North Am. 2015;42:519‐526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Traisman ES. Clinical management of urinary tract infections. Pediatr Ann. 2016;45: e108–e111. Review. [DOI] [PubMed] [Google Scholar]
- 50. Mårild S, Jodal U. Incidence rate of first‐time symptomatic urinary tract infection in children under 6 years of age. Acta Paediatr. 1998;87:549‐552. [DOI] [PubMed] [Google Scholar]
- 51. O'Brien K, Stanton N, Edwards A, Hood K, Butler CC. Prevalence of urinary tract infection (UTI) in sequential acutely unwell children presenting in primary care: exploratory study. Scand J Prim Health Care. 2011;29:19‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kanellopoulos TA, Salakos C, Spiliopoulou I, Ellina A, Nikolakopoulou NM, Papanastasiou DA. First urinary tract infection in neonates, infants and young children: a comparative study. Pediatr Nephrol. 2006;21:1131‐1137. [DOI] [PubMed] [Google Scholar]
- 53. Zorc JJ, Kiddoo DA, Shaw KN. Diagnosis and management of pediatric urinary tract infections. Clin Microbiol Rev. 2005;18:417‐422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ismaili K, Lolin K, Damry N, Alexander M, Lepage P, Hall M. Febrile urinary tract infections in 0‐to 3‐month‐old infants: a prospective follow‐up study. J Pediatr. 2011;158:91‐94. [DOI] [PubMed] [Google Scholar]
- 55. Saadeh SA, Mattoo TK. Managing urinary tract infections. Pediatr Nephrol. 2011;26:1967‐1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Tamma PD, Sklansky DJ, Palazzi DL, Swami SK, Milstone AM. Antibiotic susceptibility of common pediatric uropathogens in the United States. Clin Infect Dis. 2014;59:750‐752. [DOI] [PubMed] [Google Scholar]
- 57. Mazzone C, Laneve M, Resta F. Indagine epidemiologica locale delle infezioni delle vie urinarie in età pediatrica: eziologia e profilo di sensibilità agli antibiotici. RIMeL/IJLaM. 2008;4:280‐284. [Google Scholar]
- 58. Giardino S, Bandettini R, Perotti M, et al. Gram‐negative urinary tract infections and increasing isolation of ESBL‐producing or ceftazidime‐resistant strains in children: results from a single‐centre survey. Infez Med. 2013;21:29‐33. [PubMed] [Google Scholar]
- 59. Calitri C, Scolfaro C, Colombo S, et al. Extended‐spectrum beta lactamase‐producing enterobacteriaceae among the pediatric population: who is at risk and why? Results from a single‐centre prospective study. Infez Med. 2016;24:318‐325. [PubMed] [Google Scholar]
- 60. Calzi A, Grignolo S, Caviglia I, et al. Resistance to oral antibiotics in 4569 Gram‐negative rods isolated from urinary tract infection in children. Eur J Pediatr. 2016;175:1219‐1225. [DOI] [PubMed] [Google Scholar]
- 61. Caracciolo A, Bettinelli A, Bonato C, et al. Antimicrobial resistance among Escherichia coli that cause childhood community‐acquired urinary tract infections in Northern Italy. Ital J Pediatr. 2011;37:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. De Luca M, Donà D, Montagnani C, et al. Antibiotic prescriptions and prophylaxis in Italian children. Is it time to change? Data from the ARPEC project. PLoS ONE. 2016;11:e0154662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Malloy AM, Campos JM. Extended‐spectrum beta‐lactamases: a brief clinical update. Pediatr Infect Dis. 2011;30:1092‐1093. [DOI] [PubMed] [Google Scholar]
- 64. Nicolini G, Sperotto F, Esposito S. Combating the rise of antibiotic resistance in children. Minerva Pediatr. 2014;66:31‐39. [PubMed] [Google Scholar]
- 65. Jackson EC. Urinary tract infections in children: knowledge updates and a salute to the future. Pediatr Rev. 2015;36:153‐164. [DOI] [PubMed] [Google Scholar]
- 66. Mårild S, Jodal U, Sandberg T. Ceftibuten versus trimethoprim‐sulfamethoxazole for oral treatment of febrile urinary tract infection in children. Pediatr Nephrol. 2009;24:521‐526. [DOI] [PubMed] [Google Scholar]
- 67. Dinleyici EC, Bor O, Kebapci M, Aydogdu SD. Ceftriaxone‐associated cholelithiasis:30 min drip infusion versus bolus injection. Pediatr Int. 2010;52:890. [DOI] [PubMed] [Google Scholar]
- 68. Abel zur Wiesch P, Clarelli F, Cohen T. Using chemical reaction kinetics to predict optimal antibiotic treatment strategies. PLoS Comput Biol. 2017;13:e1005321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Opatowski L, Mandel J, Varon E, Boëlle PY, Temime L, Guillemot L. Antibiotic dose impact on resistance selection in the community: a mathematical model of β‐lactams and streptococcus pneumoniae dynamics. Antimicrob Agents Chemother. 2010;54:2330‐2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Han SB, Lee SC, Lee SY, Jeong DC, Kang JH. Aminoglycoside therapy for childhood urinary tract infection due to extended‐spectrum β‐lactamase‐producing Escherichia coli or Klebsiella pneumoniae . BMC Infect Dis. 2015;15:414 10.1186/s12879-015-1153-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Poey N, Madhi F, Biscardi S, Béchet S, Cohen R. aminoglycosides monotherapy as first‐line treatment for febrile urinary tract infection in children. Pediatr Infect Dis J. 2017;36:1104‐1107. [DOI] [PubMed] [Google Scholar]
- 72. Duffy MA, Hernandez‐Santiago V, Orange G, Davey PG, Guthrie B. Trimethoprim prescription and subsequent resistance in childhood urinary infection: multilevel modelling analysis. Br J Gen Pract. 2013;63:e238‐e243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Koyle MA, Barqawi A, Wild J, Passamaneck M, Furness PD 3rd. Pediatric urinary tract infections: the role of fluoroquinolones. Pediatr Infect Dis J. 2003;22:1133‐1137. [DOI] [PubMed] [Google Scholar]
- 74. European Medicines Agency . Disabling and potentially permanent side effects lead to suspension or restrictions of quinolone and fluoroquinolone antibiotics. EMA/795349/2018.
- 75. Gökçe İ, Çiçek N, Güven S, et al. Changes in bacterial resistance patterns of pediatric urinary tract infections and rationale for empirical antibiotic therapy. Balkan Med J. 2017;34:432‐435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Benador D, Neuhaus TJ, Papazyan JP, et al. Randomised controlled trial of three day versus 10 day intravenous antibiotics in acute pyelonephritis: effect on renal scarring. Arch Dis Child. 2001;84:241‐246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Beetz R, Bachmann H, Gatermann S, et al. Urinary tract infections in infants and children–a consensus on diagnostic, therapy and prophylaxis [in German]. Urologe A. 2007;46(112):114‐118, 120–3. [DOI] [PubMed] [Google Scholar]
- 78. Brady PW, Conway PH, Goudie A. Length of intravenous antibiotic therapy and treatment failure in infants with urinary tract infections. Pediatrics. 2010;126:196‐203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Huang YY, Chen MJ, Chiu NT, Chou HH, Lin KY, Chiou YY. Adjunctive oral methylprednisolone in pediatric acute pyelonephritis alleviates renal scarring. Pediatrics. 2011;128:e496‐e504. [DOI] [PubMed] [Google Scholar]
- 80. Lee HY, Soh BH, Hong CH, Kim MJ, Han SW. The efficacy of ultrasound and dimercaptosuccinic acid scan in predicting vesicoureteral reflux in children below the age of 2 years with their first febrile urinary tract infection. Pediatr Nephrol. 2009;24:2009‐2013. [DOI] [PubMed] [Google Scholar]
- 81. Gordon ZN, McLeod DJ, Becknell B, Bates DG, Alpert SA. Uroepithelial thickening on sonography improves detection of vesicoureteral reflux in children with first febrile urinary tract infection. J Urol. 2015;194:1074‐1079. [DOI] [PubMed] [Google Scholar]
- 82. Pauchard JY, Chehade H, Kies CZ, Girardin E, Cachat F, Gehri M. Avoidance of voiding cystourethrography in infants younger than 3 months with Escherichia coli urinary tract infection and normal renal ultrasound. Arch Dis Child. 2017;102:804‐808. [DOI] [PubMed] [Google Scholar]
- 83. Frimberger D, Mercado‐Deane MG, AAP Section on Urology; AAP Section on Radiology . Establishing a standard protocol for the voiding cystourethrography. Pediatrics. 2016;138:e20162590. [DOI] [PubMed] [Google Scholar]
- 84. Darge K. Voiding urosonography with US contrast agents for the diagnosis of vesicoureteric reflux in children. II. Comparison with radiological examinations. Pediatr Radiol. 2008;38:54‐63. [DOI] [PubMed] [Google Scholar]
- 85. Mane N, Sharma A, Patil A, Gadekar C, Andankar M, Pathak H. Comparison of contrast‐enhanced voiding urosonography with voiding cystourethrography in pediatric vesicoureteral reflux. Turk J Urol. 2018;44:261‐267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Papadopoulou F, Ntoulia A, Siomou E, Darge K. Contrast‐enhanced voiding urosonography with intravesical administration of a second‐generation ultrasound contrast agent for diagnosis of vesicoureteral reflux: prospective evaluation of contrast safety in 1,010 children. Pediatr Radiol. 2014;44:719‐728. [DOI] [PubMed] [Google Scholar]
- 87. Johnson EK, Malhotra NR, Shannon R, et al. Urinary tract infection after voiding cystourethrogram. J Pediatr Urol. 2017;13:384. [DOI] [PubMed] [Google Scholar]
- 88. Shaikh N, Ewing AL, Bhatnagar S, Hoberman A. Risk of renal scarring in children with a first urinary tract infection: a systematic review. Pediatrics. 2010;126:1084‐1091. [DOI] [PubMed] [Google Scholar]
- 89. Shaikh N, Craig JC, Rovers MM, et al. Identification of children and adolescents at risk for renal scarring after a first urinary tract infection: a meta‐analysis with individual patient data. JAMA Pediatr. 2014;168:893‐900. [DOI] [PubMed] [Google Scholar]
- 90. Keren R, Shaikh N, Pohl H, et al. Risk factors for recurrent urinary tract infection and renal scarring. Pediatrics. 2015;136:e13‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Shaikh N, Hoberman A, Keren R, et al. Recurrent urinary tract infection in children with bladder and bowel dysfunction. Pediatrics. 2016;137:e20152982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Garin EH, Olavarria F, Garcia Nieto V, Valenciano B, Campos A, Young L. Clinical significance of primary vesicoureteral reflux and urinary antibiotic prophylaxis after acute pyelonephritis: a multicenter, randomized, controlled study. Pediatrics. 2006;117:626‐632. [DOI] [PubMed] [Google Scholar]
- 93. Roussey‐Kesler G, Gadjos V, Idres N, et al. Antibiotic prophylaxis for the prevention of recurrent urinary tract infection in children with low‐grade vesicoureteral reflux: results from a prospective randomized study. J Urol. 2008;179:674‐679. [DOI] [PubMed] [Google Scholar]
- 94. Montini G, Rigon L, Zucchetta P, et al. Prophylaxis after first febrile urinary tract infection in children? A multicenter, randomized, controlled, noninferiority trial. Pediatrics. 2008;122:1064‐1071. [DOI] [PubMed] [Google Scholar]
- 95. Pennesi M, Travan L, Peratoner L, et al. East Italy prophylaxis in VUR study group. Is antibiotic prophylaxis in children with vesicoureteral reflux effective in preventing pyelonephritis and renal scars? A randomized, controlled trial. Pediatrics. 2008;121:e1489‐e1494. [DOI] [PubMed] [Google Scholar]
- 96. Craig JC, Simpson JM, Williams GJ, et al. Prevention of recurrent urinary tract infection in children with vesicoureteric reflux and normal renal tracts (PRIVENT) investigators. N Engl J Med. 2009;361:1748‐1759. [DOI] [PubMed] [Google Scholar]
- 97. Dai B, Liu Y, Jia J, Mei C. Long‐term antibiotics for the prevention of recurrent urinary tract infection in children: a systematic review and meta‐analysis. Arch Dis Child. 2010;95:499‐508. [DOI] [PubMed] [Google Scholar]
- 98. Wang H‐H, Gbadegesin RA, Foreman JW, et al. Efficacy of antibiotic prophylaxis in children with vesicoureteral reflux: systematic review and meta‐analysis. J Urol. 2015;193:963‐969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. de Bessa J, de Carvalho Mrad FC, Mendes EF, et al. Antibiotic prophylaxis for prevention of febrile urinary tract infections in children with vesicoureteral reflux: a meta‐analysis of randomized, controlled trials comparing dilated to nondilated vesicoureteral reflux. J Urol. 2015;193(5 Suppl):1772‐1777. [DOI] [PubMed] [Google Scholar]
- 100. The RIVUR Trial Investigators . Antimicrobial prophylaxis for children with vesicoureteral reflux. N Engl J Med. 2014;370:2367‐2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Selekman RE, Shapiro DJ, Boscardin J, et al. Uropathogen resistance and antibiotic prophylaxis: a meta‐analysis. Pediatrics. 2018;142:e20180119 10.1542/peds2018-0119 Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Hewitt IK, Pennesi M, Morello W, Ronfani L, Montini G. Antibiotic prophylaxis for urinary tract infection‐related renal scarring: a systematic review. Pediatrics. 2017;139:e20163145. [DOI] [PubMed] [Google Scholar]
- 103. Huttner A, Verhaegh EM, Harbarth S, Muller AE, Theuretzbacher U, Mouton JW. Nitrofurantoin revisited: a systematic review and meta‐analysis of controlled trials. J Antimicrob Chemother. 2015;70:2456‐2464. [DOI] [PubMed] [Google Scholar]
- 104. Tewary K, Narchi H. Recurrent urinary tract infections in children: preventive interventions other than prophylactic antibiotics. World J Methodol. 2015;5:13‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Salo J, Uhari M, Helminen M, et al. Cranberry juice for the prevention of recurrences of urinary tract infections in children: a randomized placebo‐controlled trial. Clin Infect Dis. 2012;54:340‐346. [DOI] [PubMed] [Google Scholar]
- 106. Schwenger EM, Tejani AM, Loewen PS. Probiotics for preventing urinary tract infections in children and adults. Cochrane Database Syst Rev. 2015;23(12), Art.No.:CD008772. 10.1002/14651858.CD008772.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Toffolo A, Ammenti A, Montini G. Long‐term clinical consequences of urinary tract infections during childhood: a review. Acta Paediatr. 2012;101(10):1018‐1031. [DOI] [PubMed] [Google Scholar]


