Abstract
Objective
The WHISPER randomized controlled trial (RCT) evaluates safety and clinical effectiveness of subperception spinal cord stimulation (SCS) at ≤1.2 kHz in subjects previously implanted with an SCS system for treatment of chronic, neuropathic pain.
Methods
WHISPER is a prospective, multicenter RCT with a crossover design sponsored by Boston Scientific, Marlborough, MA (http://clinicaltrials.gov: NCT02314000). Eligible subjects were randomized (N = 140) to receive subperception or supraperception for three months and then crossed over to receive the alternative. Upon completion of crossover period, subjects who preferred subperception were followed up to one year. Overall pain, quality‐of‐life, and other outcomes were collected in the study. The primary endpoint was the overall pain responder rate (≥50% improvement from baseline) with no increase in medications. Secondary endpoints consisted of pain scores, physical disability, quality of life, and treatment preference.
Results
The study met its primary endpoint and demonstrated noninferiority between supraperception and subperception in a prespecified cohort of 70 randomized subjects (Interim Analysis). Thirty‐nine percent of subjects with subperception settings and 29% with supraperception settings had a greater than or equal to 50% reduction in their overall pain scores with no increase in average daily medication at three‐months post‐activation as compared with baseline. Further assessment of all participating study subjects (N = 140) revealed similar results. Subjects were previously implanted 3.8 ± 2 years and had a disability score (Oswestry Disability Index) of 70.2 ± 11.4 at study start. Of the randomized subjects that completed the End of Period 2 Visit, 93 (66%) preferred subperception SCS and their mean overall pain reduced from 7.3 ± 1.1 (N = 89) at baseline to 4.0 ± 2.1 (N = 80) at 12‐months post‐activation. Post hoc analysis also demonstrated that multiple options provide superior outcomes, as supported by a 74% increase in the responder rate when subjects could choose their most effective option (47%) compared with supraperception alone (27%).
Discussion
Subperception SCS at ≤1.2 kHz is safe and effective in subjects with extreme physical disability and previously implanted for chronic pain. Further, by providing study participants with different waveform options, increased pain relief was achieved.
Keywords: Chronic pain, randomized controlled trial, SCS, spinal cord stimulation, subperception
INTRODUCTION
Spinal cord stimulation (SCS) is an effective treatment for chronic intractable pain associated with a variety of conditions. Historically, SCS has been administered with the understanding that pain relief required stimulation‐induced paresthesia to overlap the painful area. Recent studies have demonstrated that pain relief may be obtained without generating paresthesia 1, 2, 3, 4. Results from a recently published double‐blind, randomized controlled trial (RCT) provided level 1 evidence that equivalent pain relief using stimulation frequencies from 1 to 10 kHz with appropriate neural dosing was achieved in a cohort of 20 subjects 1. Furthermore, the recharge burden with 1 kHz was significantly less than with higher frequencies. North et al. demonstrated in a randomized crossover‐controlled trial of 22 subjects, that 1 kHz subperception stimulation provided significant improvement in pain and disability compared with conventional paresthesia‐based settings 4. These subjects had undergone a successful trial and initial implant but later presented with inadequate pain relief. Subperception SCS with high‐frequency stimulation (10 kHz) has also been reported to be clinically effective 2, 3. However, higher frequency stimulation has potential drawbacks including greater charging burden for patients that might possibly lead to noncompliance. Additionally, not all patients respond to 10 kHz stimulation 2, 3, 5. Based on pain diary VAS scores in the SENZA study, approximately 1/3 of subjects with low back pain were nonresponders at 12 months 6. Thus, there is a need for subperception SCS devices that are less burdensome to patients while still clinically effective.
Subperception SCS at 1 kHz has recently been demonstrated to be an effective treatment option for de novo subjects (i.e. new to SCS for treatment of chronic pain) 1. The question remains though if patients who have been previously implanted and are using paresthesia‐based stimulation, would indeed benefit from subperception SCS as an alternative treatment option for management of their chronic pain. Thus, we describe here a prospective, multicenter, RCT with a crossover design to evaluate the safety and effectiveness of subperception SCS at frequencies ≤1.2 kHz in a cohort of previously implanted subjects with chronic low back and/or leg pain. We sought additionally to assess if providing multiple treatment options facilitates successful SCS outcomes in this cohort of long‐term implanted subjects with extreme disability.
MATERIALS AND METHODS
Study Design
WHISPER is a prospective, multicenter randomized, controlled crossover trial designed to demonstrate the safety and effectiveness of subperception SCS at frequencies up to 1.2 kHz. Following consent, subjects' eligibility to participate in the study was determined based on predefined criteria (see the Subject Selection section). During the baseline period (seven days), all subjects' SCS systems were turned off. At the end of the baseline period, subjects reported their overall pain scores and physical disability with their SCS devices turned “off.” This served to determine study eligibility and provide baseline data for the purpose of analysis. All subjects who passed screening were randomized in a 1:1 ratio to receive either paresthesia‐based SCS (supraperception) followed by subperception SCS (or vice versa) for 90 days each (noted as periods 1 and 2, as shown in Fig. 1). During each period, based on treatment assignment, all study participants' devices were programmed to achieve pain relief. A washout period of 3–7 days between both the periods mitigated against carryover effect of previous settings. At the end of each period, data related to pain outcomes (verbal rating scale, VRS), quality of life (global impression of change as assessed by subjects, PGIC), and disability (Oswestry Disability Index, ODI), and percent pain relief (PPR) was collected 7. At End of Period 2, only those subjects who chose subperception continued to participate in the study up to 12 months post‐randomization.
Figure 1.
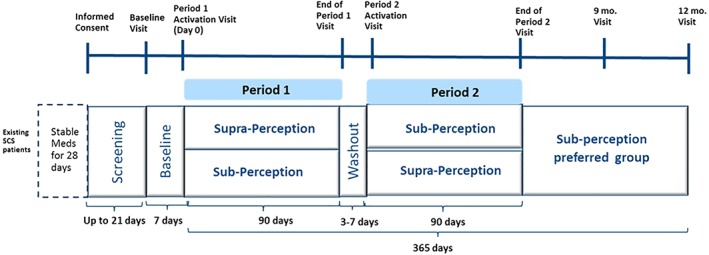
Study schematic. [Color figure can be viewed at http://wileyonlinelibrary.com]
Subjects' pain medications were held constant up to the End of Period 2. However, certain rescue medications were allowed per study protocol during baseline and Washout when stimulation was turned off.
The study was conducted in compliance with the U.S. Code of Federal Regulations and Declaration of Helsinki. The study protocol and informed consent forms were approved by study sites' Institutional Review Board prior to study start. Study monitoring was completed by Sponsor personnel and verified by source documentation maintained at site to ensure accuracy and correctness of data.
The study is registered on http://clinicaltrials.gov with NCT02314000. The study was funded by Boston Scientific Corporation.
Subject Selection
Subjects offered participation in the study were previously implanted on‐label with an SCS System (Precision or Precision Spectra System, Boston Scientific, Valencia, CA, USA) capable of multiple independent current control, for at least six months and with an interest in SCS‐induced pain relief without paresthesia were offered participation in the study. Subjects were to be at least 22 years of age, with chronic pain of trunk and/or limbs and on stable pain medications for at least 28 days prior to consent. Key inclusion criteria included: 1) overall pain of at least 6 (on a 0–10 scale) during baseline period; 2) at least a 30% reduction in overall pain intensity with the use of their SCS System with/without medications as reported by PPR at time of consent; 3) average total daily morphine equivalent of ≤300 mg prior to consent. Subjects with any significant cognitive impairment or pain‐related diagnosis that may confound study outcomes and/or terminal illness with anticipated survival <12 months were excluded. Only subjects who were willing to provide signed informed consent and able to comply with all protocol‐required procedures and assessments/evaluations were included in the study.
Data Collection and Analysis
Outcome data were collected at baseline and at the end of each period. For those subjects who chose subperception settings at End of Period 2, additional data were collected at 9‐ and 12‐month visits. Pain scores (0–10) were collected based on a seven‐day recall (VRS). Additionally, assessments such as global impression of change as assessed by clinicians and PGIC, ODI, SF‐36, and PPR were also collected over the course of the study. Subject demographics including age, gender, diagnosis for receiving the implant, duration of SCS implant, and so on was also collected. Adverse events (AEs) were collected.
The primary endpoint of the study was based on the proportion of subjects with 50% or greater reduction from baseline in average daily overall pain intensity at 90 days postactivation with no increase in baseline average daily medications intake used to treat pain.
A minimum sample size of 130 randomized subjects was required to show the responder rate in the treatment group (subperception) was noninferior (with a margin 20%) to the control group (supraperception), assuming there is no difference in the responder rate in two groups and a 90% power. With a 10% attrition rate, the planned sample size was 146 randomized subjects. The study included an interim analysis at 70 randomized subjects for effectiveness and futility. Secondary endpoints included outcomes related to low back pain and overall pain numerical rating scale, PPR, and treatment satisfaction. Post hoc analyses of best response and the subgroup of supra perception subjects were performed.
The analyses of efficacy and safety endpoints were based on the Intent‐to‐Treat (ITT) and Per‐Protocol sets. These analyses and their methods were predefined in the statistical analysis plan prior to performing any of the analysis. Continuous variables were summarized using descriptive statistics, which include number of nonmissing observations, mean, median, standard deviation, minimum, maximum, or 95% confidence interval. For categorical variables, descriptive statistics include frequencies and percentages of categories. All the preplanned statistical analysis was provided by the sponsor.
For safety analyses, rates of occurrence of all device and procedure related nonserious AEs and all serious AEs through the end of the study were reported descriptively.
RESULTS
During January 2015 to March 2017, the study enrolled 229 subjects at 22 participating U.S. centers. Of the 229 enrolled subjects, 140 were randomized to either receive supraperception followed by subperception or vice versa as shown in Figure 2, to form ITT group. Fifty‐nine subjects did not meet eligibility criteria and eight withdrew once enrolled, and hence were not randomized. In addition, data collected from 22 subjects were not included as they were enrolled under an earlier version of the study. An interim analysis based on 70 prespecified subjects was completed. We also report the study outcomes for all participating subjects in the study (N = 140 randomized subjects).
Figure 2.
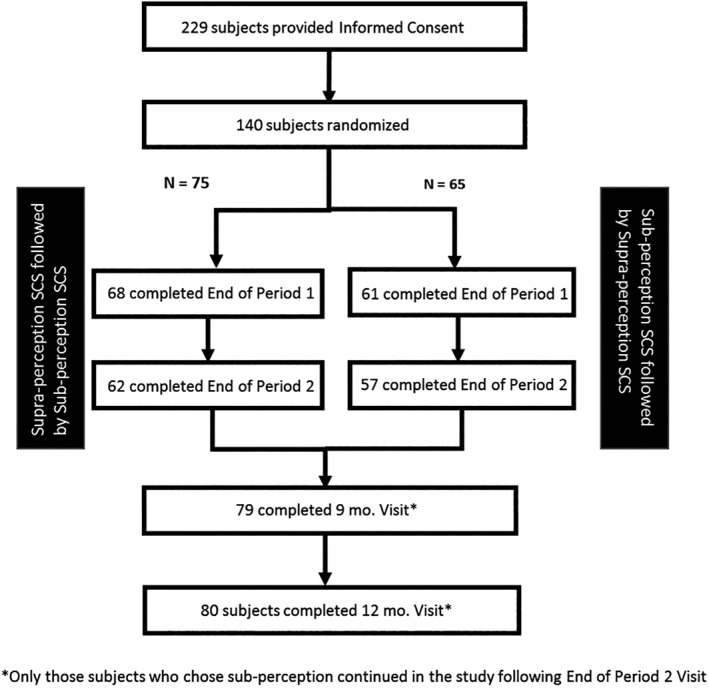
Subject disposition in the study. The table summarizes the disposition of all subjects in the study.
Subject demographics and baseline characteristics for all subjects are summarized in Table 1. Subjects were aged 33–87 years (mean 59.8 years, N = 140) with a majority under the age of 60 (52%). In addition, subjects had low back/leg pain for a mean duration of 17 ± 12.7 years and were previously implanted with an SCS System for a mean duration of 3.8 ± 2 years at study start. Ninety‐seven percent of subjects reported receiving their system for low back pain within a range of diagnoses as shown in Table 1 (not mutually exclusive). At baseline, subjects had a mean overall pain score (VRS) of 7.3 ± 1.2 and exhibited severe physical disability as reflected by a mean ODI score of 70.2 ± 11.4 (defined as “crippling” with SCS device turned off). These baseline characteristics were similarly noted in the prespecified cohort of 70 randomized subjects for interim analysis.
Table 1.
Study demographics/clinical characteristics.
| Study cohort (N = 140) | |
|---|---|
| Duration of implant prior to study enrollment (years)—Mean (SD) N | 3.8 (2.0) 114 |
| Age (years)—Mean (SD) N | 59.8 (11.3) 140 |
| Gender—Male (N%) | 41% (57/140) |
| Overall Pain (VRS)—Mean (SD) N | 7.3 (1.3) 131 |
| Disability (Oswestry Disability Index [ODI])—Mean (SD) N | 70.2 (11.4) 140 |
| Diagnosis for SCS implant (some subjects have multiple diagnoses) | |
| Failed back surgery syndrome (FBSS) | 46% (64/140) |
| Complex regional pain syndrome (CRPS) | 17% (24/140) |
| Radiculopathy | 46% (65/140) |
| Other | 45% (63/70) |
The study successfully met its primary endpoint in a prespecified analysis cohort of 70 randomized subjects as part of interim analysis and demonstrated noninferiority between subperception and supraperception settings (p < 0.001). Thirty‐nine percent (27 of 70 subjects) of subjects with subperception settings and 29% (20 of 70 subjects) with supraperception settings had a greater than or equal to 50% reduction in their overall pain scores with no increase in average daily medication at three‐months postactivation as compared with baseline. The study also achieved all secondary endpoints. This trend was similar when the analysis was repeated with all the subjects in the study (N = 140).
Post hoc analysis (N = 140), as summarized in the Methods section, demonstrated that if subjects could choose the most effective therapy option (i.e., subperception or supraperception), the overall responder rate increased by 74% as compared with supraperception alone in 140 randomized subjects (Fig. 3). This was tested for superiority and the data demonstrated that providing multiple waveform options elicits superior outcomes more than one option only (either supraperception or subperception SCS only). A subgroup analysis based on subjects' response to supraperception (paresthesia responders) during the randomized phase showed that 35 of 70 subjects had more than 50% improvement in pain relief. In this subcohort, if subjects could choose the stimulation settings that provided the most effective relief, a significant reduction (Δ = 4.5‐point improvement, p < 0.0001) in overall pain was determined (Fig. 4).
Figure 3.

Responder rate when subjects could choose the most effective therapy option (N = 140)—post hoc analysis based on pain scores collected during the crossover phase. A 74% increase in responder rate was noted when subjects could choose the most effective option compared with use of supraperception only. [Color figure can be viewed at http://wileyonlinelibrary.com]
Figure 4.
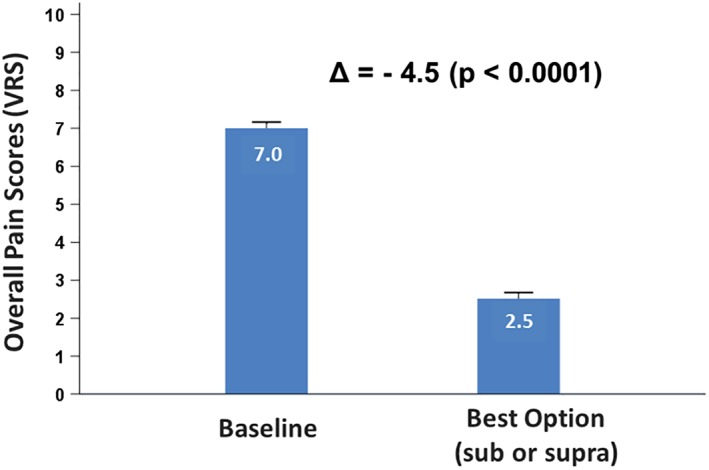
Outcomes for paresthesia responders (N = 35) only. [Color figure can be viewed at http://wileyonlinelibrary.com]
Of the 140 randomized subjects that completed the End of Period 2 Visit, 93 (66%) preferred subperception SCS and were followed out to 12 months postactivation as required by study protocol. A significant improvement in their overall pain scores was noted at three months and was sustained up to 12 months postactivation as shown in Figure 5. Mean overall pain reduced from 7.3 ± 1.1 (N = 89) at baseline to 4.4 ± 1.9 (N = 93) at three months and 4.0 ± 2.1 (N = 80) at 12 months postactivation. A similar trend was noted in low back and leg pain scores. At 12‐month postactivation, mean leg pain reduced from 6.2 ± 2.2 (N = 89) at baseline to 3.2 ± 2.1 (N = 80) and mean low back pain reduced from 7.1 ± 1.3 (N = 89) at baseline to 4.0 ± 2.1 (N = 80). PPR at 12‐month postactivation for mean overall, low back, and leg pain was determined to be 65.3, 65.4, and 66.9, respectively (Tables 2 and 3). Subjects also noted a significant improvement (12.6‐point improvement, p < 0.001) in their disability at 12‐month postactivation as assessed by ODI scores with subperception settings. Subjects' satisfaction measured using PGIC revealed that 87.6% of subjects (70 of 80) reported being “better” or “great deal better." Finally, safety was also monitored during the trial and no unanticipated events were reported.
Figure 5.
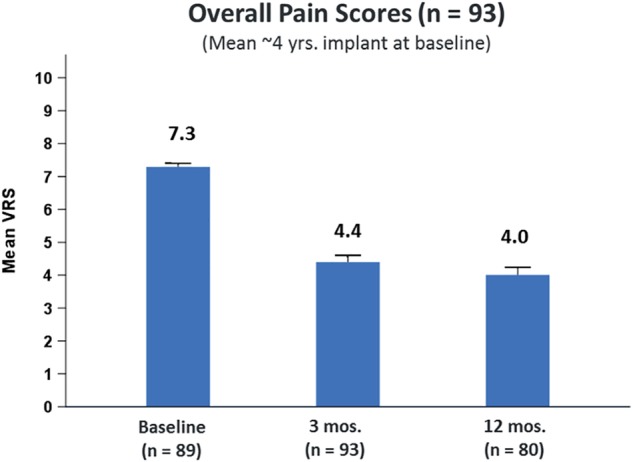
Subperception preferred group—mean VRS up to 12 months. (n = 93). [Color figure can be viewed at http://wileyonlinelibrary.com]
Table 2.
Pain Scores in All Randomized Subjects (N = 140) Based on Their Treatment Assignment.
| Outcome | Baseline mean (SD) N | Subperception SCS mean (SD) N | Supraperception SCS Mean (SD) N |
|---|---|---|---|
| Mean overall pain (VRS) | 7.3 (1.1) 131 | 4.7 (1.9) 123 | 5.1 (1.9) 124 |
| Mean low back pain (VRS) | 6.9 (1.6) 131 | 4.2 (1.9) 123 | 4.8 (1.8) 124 |
| Mean leg pain (VRS) | 6.1 (2.3) 131 | 3.9 (2.2) 123 | 4.3 (2.2) 124 |
| Mean overall pain (PPR) | NA | 57.4 (24.0) 119 | 54.9 (25.6) 124 |
| Mean low back pain (PPR) | NA | 56.1 (24.1) 118 | 51.9 (27.7) 122 |
| Mean leg pain (PPR) | NA | 55.0 (26.4) 112 | 52.7 (28.5) 119 |
Table 3.
Pain and Disability Scores in Subjects Who Preferred Subperception at 3 and 12 Months Follow‐Up (N = 93).
| Outcome | Baseline mean (SD) N | Three‐month follow‐up mean (SD) N | 12‐month follow‐up mean (SD) N |
|---|---|---|---|
| Mean overall pain (VRS) | 7.3 (1.1) 89 | 4.4 (2.0) 93 | 4.0 (2.1) 80 |
| Mean low back pain (VRS) | 7.1 (1.3) 89 | 4.1 (1.9) 93 | 4.0 (2.1) 80 |
| Mean leg pain (VRS) | 6.1 (2.2) 89 | 3.5 (2.2) 93 | 3.2 (2.1) 80 |
| Mean overall pain (PPR) | NA | NA | 65.3 (25.1) 79 |
| Mean low back pain (PPR) | NA | NA | 65.4 (25.3) 78 |
| Mean leg pain (PPR) | NA | NA | 66.9 (25.2) 74 |
| Disability (ODI) | 71.2 (10.6) 93 | 60.2 (14.2) 93 | 58.5 (15.1) 80 |
| [“crippling”] | [severe] | [severe] |
DISCUSSION
The WHISPER RCT is the first, large RCT to provide outcomes of previously implanted SCS subjects using subperception at ≤1.2 kHz. The population in this study is particularly difficult to treat given the ODI assessment corresponding to a classification of severely disabled or “crippled” at study entry and included both good and poor paresthesia responders who had experienced chronic pain for several years. Most other RCTs, as summarized in Table 4, typically enroll subjects who are naïve to SCS (de novo) and have several other limiting factors including disability, workers compensation, and so on. This trial is unique relative to other previously published RCTs assessing SCS for chronic pain in that it enrolled subjects who were already implanted and had fewer restrictions around study eligibility, thus making it a practical RCT, and thereby better representing outcomes that may be achieved in the real‐world versus previous RCTs implemented with an extensive list of required inclusion/exclusion criteria. As such, the WHISPER trial investigated a rarely studied cohort of subjects who were implanted long term and represent one of the most challenging patient populations using SCS for chronic pain. Moreover, the design of the study required subjects to be on stable pain medications during the randomized phase and thus trial outcomes were less likely to be confounded by interaction with opioids, a known factor that interacts with SCS outcomes and one of the noted limitations of other published RCTs 2, 8.
Table 4.
Comparison of WHISPER RCT vs. Other Published Studies.
| Study aspects | WHISPER | Senza* , † | PROCO‡ | Sunburst§ |
|---|---|---|---|---|
| RCT | Yes | Yes | Yes | Yes |
| Subjects considered for study | Previously implanted with Precision or Spectra SCS systems | De novo | De novo | De novo |
| Mean duration of implant at time of consent (year) | 3.8 | 0 | 0 | 0 |
| Mean duration of chronic pain (low back/leg pain) (year) | 17 | 13 | 10.9 | 12.8 |
| Mean disability as assessed by Oswestry Disability Index (ODI) scores | 70.2 | 54 | 58.9 | 49.1 |
| (Crippling) | (Severe) | (Severe) | (Severe) |
The study successfully met its prespecified primary and secondary endpoints based on an interim analysis of 70 randomized subjects. The study outcomes in this interim analysis cohort were similar to those reported in the entire study population (N = 140 randomized subjects). Post hoc analysis also showed that if subjects could choose their most effective treatment, multiple options provided superior outcomes than supraperception settings alone. In a subcohort of 35 subjects who were responders to supraperception (greater than or equal to 50% improvement in overall pain) during the randomized phase, a statistically significant (p < 0.0001) improvement was noted when they could choose their most effective therapy.
At the end of the randomized phase, the majority of subjects (66%) chose subperception as their preferred choice for SCS. If given the choice, 62% (66 of 107 subjects who completed the questionnaire) preferred to keep both options of subperception and supraperception for the management of their pain. Improvement in overall pain with subperception SCS was sustained at one‐year follow‐up.
Published results from a recent RCT provided level 1 evidence that significant pain relief is achieved using 1 kHz SCS in de novo subjects 1. The outcomes of this study are consistent with the data from the PROCO study in that positive outcomes with 1 kHz SCS can also be achieved in previously implanted subjects as well. This also is reflective of preclinical data obtained in a chronic rat model where the inhibitory effects of different frequencies on mechanical hypersensitivity were investigated. In that study, 1 kHz stimulation showed significantly greater response rates than low rate SCS (50 Hz) with no difference between 1 and 10 kHz 9. It is possible that a different mechanism of action, such as recruitment of dorsal horn fibers, is activated at frequencies of 1–10 kHz compared with low rate SCS.
In a recently published retrospective chart review, Van Buyten et al. reported a 19% explant rate due to inadequate pain in a cohort of 955 SCS patients at five years after implant 10. It could be reasoned that at least some, if not many, participating WHISPER study subjects would have been probable candidates for explant in the real‐world clinical setting due to their severe disability and the extended length of time of previous SCS implantation prior to study participation. However, on the basis of the post hoc analysis in this study, sustained overall pain relief is realistically attainable in previously implanted subjects with severe, preexisting physical disability. Further, when given the choice, many study subjects preferred to keep both supra‐ and subperception SCS settings for selective use in managing their chronic pain. Comparable results have been documented by other reports in the literature showing patient preference for multiple available options 11, 12. Collectively, the data from these previously reported studies and our results in this study therefore would suggest that the accessibility itself to more than one selectable option (vs. having one stimulation setting only for available use) is the key feature that can facilitate long‐term pain relief and the continued benefit from SCS, and thereby in turn possibly preclude the need for device explantation.
IMMPACT guidelines for chronic pain trials recommend the use of a global rating of improvement and satisfaction so subjects may incorporate not only pain relief but also overall improvements in physical/emotional functioning, convenience, and so on into one single rating 13. This is likely more reliable than reporting of pain scores alone, which is subjective and dependent on a variety of factors. As such, PGIC, a 7‐point scale, as recommended by IMMPACT was administered in the study. Notably, of de novo subjects who used 10 kHz for 12 months (SENZA), only 52.8% of subjects reported being better or a great deal better 6. While both approaches (≤ 1.2 kHz and 10 kHz) are paresthesia‐free therapies, subjects in the WHISPER trial reported higher PGIC scores. At this time, one can only speculate if this result is due to the use of subperception SCS at up to 1.2 kHz but use of lower stimulation frequencies has been shown to foster a lower charging burden as demonstrated by Thomson et al. 1. In addition, it is also possible that these subjects reported better satisfaction because of their access to multiple neurostimulative choices instead of only one option alone for use in treating their chronic pain.
Limitations
The study has a few limitations in that subjects and/or clinicians assessing the pain outcomes were not blinded. Given the nature of the comparators, i.e., supraperception vs. subperception, it would not be possible to blind each group. One other limitation is the lack of historical data related to pain in study participants prior to SCS device implantation. While an attempt to address this was made by using a seven‐day baseline period where stimulation was turned off, it may not have necessarily captured the overall pain scores prior to intervention—one drawback of using previously implanted subjects. The study recruited previously implanted subjects and one may speculate that they intended to achieve better outcomes thereby adding a bias; however, the study required that all subjects have at least a 30% improvement (current) with their SCS system. Finally, the study only collected long‐term results (post hoc) for those subjects who preferred sub perception up to 12 months, thereby lacking long‐term data for other subjects who preferred supraperception to make a direct comparison.
CONCLUSIONS
The WHISPER RCT provides clinical evidence for the effectiveness and safety of subperception SCS at up to 1.2 kHz in study participants who have been implanted long term with a neurostimulation device for treatment of chronic, intractable pain. Furthermore, the noninferiority of subperception versus supraperception stimulation settings when using SCS for chronic pain was effectively demonstrated. Notably, these results are consistent with other RCTs that evaluated subperception SCS at or near ~1 kHz, which showed positive outcomes in de novo subjects and improved outcomes in previously implanted SCS patients who were paresthesia failures 1, 4. Significant improvement in pain relief was sustained out to 12 months when using subperception SCS. Additionally, access to more than one selectable treatment approach (sub‐ or supraperception) provided better outcomes when subjects could choose their most effective therapy. Thus, the outcomes of the WHISPER RCT suggest that providing multiple options to patients has the potential to extend the duration of effective treatment when using SCS for chronic pain and may in turn reduce the need for device explantation.
Authorship Statement
Drs. North, Loudermilk, Lee, Sachdeva, Kaiafas, Washabaugh, Sheth, Scowcroft, Mekhail, Lampert, Yearwood, Shaw, Atallah, McLeod, Han, Yu, Sedrak, Lucas, Trobridge, Hegarty, and Miller carried out the study including collecting patient data. Mrs. Jain and Dr. North contributed to study design and helped prepare the manuscript. Statistical support in analyzing the data was carried out by Dr. Chen with additional input from Mrs. Jain. All authors critically reviewed and approved the submitted manuscript.
COMMENTS
The time from when randomized controlled trials are conceived and finally published is long but welcome even though our field continues to move forward at a pace. The purpose of this study is to show the safety and effectiveness of up to 1.2kHz frequency in SCS patients so allowing this mode of sub‐perception SCS to be marketed by the sponsor. However there are many useful features that arise from this work.
Firstly it confirms the effectiveness of sub‐perception programming even at lower kHz frequencies since we already know that outcome is not affected by kHz frequency alone.
Secondly it shows the effectiveness of SCS in patients with long‐term pain who have been treated with SCS for more than 4 years but also how optimization of programming with paresthesia based or sub‐perception can improve clinical outcomes.
Thirdly it shows that patients may respond optimally to different modes of SCS programming.
It is an awkward study to understand. The population selected is patients who were “interested in sub‐perception programming”. Some due to relatively poor effect of their existing SCS pain relief, but others who had good pain relief but wanted to participate in such a trial. The study has no blinding, although not dissimilar to all other commercially sponsored RCT to date.
Having said all that, the study objectives are met. The clinical scientist in me wants more scientific rigor for all future RCT in our field.
Simon Thomson, MBBS
Basildon, United Kingdom
***
This is a quality RCT validating the efficacy of the Whisper settings for subparesthesia spinal cord stimulation. There is good evidence of a subset of patients who respond to this therapy (35%). Thus conversely 65% do not. Adding in the option of tonic stimulation raises the responder rate to 47%. Thus conversely 53% do not respond to just these two waveforms. What does this tell us? To me it says that human nervous systems in chronic pain work in different (and mysterious) ways and the key to success will lie in a suite of waveforms available to the patient in order to find the one that meshes best with the patients neural transmission. How many will be needed? That is an unknown and depends very much on the relative efficacy of each one but a best guess might be six unique waveforms where each has a partial population of responders.
I believe we will see a move away from “one waveform to rule them all” in existing IPGs to a convergence of options within a pulse generator. That process has started already and will be the story of the 2020s.
Marc Russo, MBBS
Sydney, Australia
Comments not included in the Early View version of this paper.
Acknowledgements
The authors would like to thank Daniel Halperin, PhD, for writing and editorial assistance during the preparation of this manuscript. The data, analytic methods, and study materials for this clinical trial will be made available to other researchers in accordance with the Boston Scientific Data Sharing Policy (https://www.bostonscientific.com/).
For more information on author guidelines, an explanation of our peer review process, and conflict of interest informed consent policies, please go to https://www.wiley.com/WileyCDA/Section/id-301854.html
Conflict of Interest: Drs. James Scowcroft and Joseph Atallah have received consulting fees from Boston Scientific Corporation. Dr. Cong Yu has received personal and consulting fees from Boston Scientific, Medtronic, and St. Jude Medical (Abbott). Dr. Nagy Mekhail has received consulting fees from Boston Scientific and Abbott. Drs. James North and Benjamin Lampert have received consulting fees and travel sponsorship from Boston Scientific Corporation. Dr. Erik Shaw has received speaking fees from Jazz Pharmaceuticals. Dr. Andrew Trobridge has received consulting fees from Flowonix, Nuvectra and Boston Scientific Corporation. Dr. Thomas Yearwood has received speaking fees from Boston Scientific and Abbott and is CMO for Meagan Medical and a consultant for Neuronano. Mrs. Roshini Jain and Dr. Lilly Chen are salaried employees of Boston Scientific. All other listed investigators not listed in the COI statement have no relevant COI to report.
Source(s) of financial support: This study was sponsored by Boston Scientific Corporation.
[The copyright line for this article was changed on 22 October 2019 after original publication.]
REFERENCES
- 1. Thomson SJ, Tavakkolizadeh M, Love‐Jones S et al. Effects of rate on analgesia in kilohertz frequency spinal cord stimulation: results of the PROCO randomized controlled trial. Neuromodulation 2018;21:67–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kapural L, Yu C, Doust MW et al. Novel 10‐kHz high‐frequency therapy (HF10 therapy) is superior to traditional low‐frequency spinal cord stimulation for the treatment of chronic back and leg pain: the SENZA‐RCT randomized controlled trial. Anesthesiology 2015;123:851–860. [DOI] [PubMed] [Google Scholar]
- 3. Russo M, Verrills P, Mitchell B, Salmon J, Barnard A, Santarelli D. High frequency spinal cord stimulation at 10 kHz for the treatment of chronic pain: 6‐month Australian clinical experience. Pain Phys 2016;19:267–280. [PubMed] [Google Scholar]
- 4. North JM, Hong KSJ, Cho PY. Clinical outcomes of 1 kHz subperception spinal cord stimulation in implanted patients with failed paresthesia‐based stimulation: results of a prospective randomized controlled trial. Neuromodulation 2016;19:731–737. [DOI] [PubMed] [Google Scholar]
- 5. Kapural L, Yu C, Doust MW et al. Comparison of 10‐kHz high‐frequency and traditional low‐frequency spinal cord stimulation for the treatment of chronic back and leg pain: 24‐month results from a multicenter, randomized, controlled pivotal trial. Neurosurgery 2016;79:667–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. FDA Summary of Safety and Effectiveness Data , Senza Spinal Cord Stimulation System, PMA P130022, page 44–45, Table 20.
- 7. Tunis SR, Stryer DB, Clancy CM. Practical clinical trials: increasing the value of clinical research for decision making in clinical and health policy. JAMA 2003;290:1624–1632. [DOI] [PubMed] [Google Scholar]
- 8. Deer T, Slavin KV, Amirdelfan K et al. Success using neuromodulation with BURST (SUNBURST) study: results from a prospective, randomized controlled trial using a novel Burst waveform. Neuromodulation 2018;21:56–66. [DOI] [PubMed] [Google Scholar]
- 9. Shechter R, Yang F, Xu Q et al. Conventional and kilohertz‐frequency spinal cord stimulation produces intensity‐ and frequency‐dependent inhibition of mechanical hypersensitivity in a rat model of neuropathic pain. Anesthesiology 2013;119:422–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Van Buyten JP, Wille F, Smet I et al. Therapy‐related explants after spinal cord stimulation: results of an international retrospective chart review study. Neuromodulation 2017;20:642–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Berg AP, Mekel‐Bobrov N, Goldberg E, Huynh D, Jain R. Utilization of multiple spinal cord stimulation (SCS) waveforms in chronic pain patients. Expert Rev Med Devices 2017;14:663–668. [DOI] [PubMed] [Google Scholar]
- 12. Kriek N, Groeneweg JG, Stronks DL, de Ridder D, Huygen FJ. Preferred frequencies and waveforms for spinal cord stimulation in patients with complex regional pain syndrome: a multicentre, double‐blind, randomized and placebo‐controlled crossover trial. Eur J Pain 2017;21:507–519. [DOI] [PubMed] [Google Scholar]
- 13. Dworkin RH, Turk DC, Farrar JT et al. Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain 2005;113:9–19. [DOI] [PubMed] [Google Scholar]


