Abstract
Background
Different interventions are offered to children with cerebral palsy (CP) to improve the activity domain of the international classification of functioning (ICF). In therapy settings, the focus is mostly on motor capacity, but the ultimate goal is to improve motor performance. We therefore examined if changes in motor capacity outcomes are accompanied by changes in objectively measured motor performance after a 3‐month intensive treatment period in ambulatory children with CP.
Methods
A secondary analysis on prospective clinical trial data was performed using multivariate linear regression. Sixty‐five children (37 boys and 28 girls) with spastic CP, mean age 7 years and 3 months, Gross Motor Function Classification System (GMFCS) levels I–III were involved in a distinct 3‐month intensive treatment period. Motor capacity (Gross Motor Function Measure [GMFM], functional muscle strength [FMS], and walking speed [WS]) and motor performance (using three Actigraph‐GT3X+‐derived outcome measures) were measured at baseline, 12 and 24 weeks.
Results
No significant associations were found for any of the change scores (∆12) between motor capacity and motor performance after a 12‐week intensive treatment period. After 24 weeks, ∆24FMS (p = .042) and ∆24WS (p = .036) were significantly associated with changes in motor performance outcome measure percentage of time spent sedentary (∆24%sedentary). In this model, 16% of variance of ∆24%sedentary was explained by changes in motor capacity (p = .030).
Conclusions
Changes in motor capacity are mostly not accompanied by changes in objectively measured motor performance after an intensive treatment period for ambulatory children with CP. These findings should be taken into account during goal setting and are important to manage expectations of both short‐ and longer term effects of treatment programmes.
Keywords: Actigraph, cerebral palsy, motor capacity, motor performance, physical behaviour
Key messages.
Changes in motor capacity outcomes are mostly not accompanied by changes in actual motor performance after one distinct intensive treatment period.
The lack of a clear longitudinal capacity–performance relationship should be taken into account during goal setting and expectation management in treatment of individual patients.
Future treatment programmes should focus more specifically on motor performance next to motor capacity.
Other factors than just motor capacity (such as social and environmental opportunities) should be taken into account to achieve an improvement in motor performance.
1. INTRODUCTION
Cerebral palsy (CP) is a disorder of development of movement and posture with a wide variety of consequences in different domains of functioning such as physical behaviour, sensation, perception, communication, cognition, and behaviour (Rosenbaum et al., 2007). Children with CP are not only heterogeneous in their level of functioning in these domains but also in aetiology, presentation, comorbidities, and response to treatment (Shevell, 2018).
Different interventions are offered to improve the level of functioning in children with CP (Novak et al., 2013; Ryan, Cassidy, Noorduyn, & O'Connell, 2017). The effectiveness of these interventions for childhood CP can be assessed on different levels of the World Health Organization's international classification of functioning (ICF) (World Health Organization, 2001). Although all ICF levels are important in ambulatory children with CP in their primary school age, the activity level, with its classifiers capacity (what children can do in a standardized environment) and performance (what children actually do in daily life) (World Health Organization, 2001), are of special interest (Holsbeeke, Ketelaar, Schoemaker, & Gorter, 2009; Novak et al., 2013; Ryan et al., 2017). Optimizing performance is, however, the ultimate goal for children with CP in order to keep up at school, come along with their friends, and participate in other daily activities.
From the perspective of motor functioning, several outcome measures can be used to assess capacity and performance. Of the presently available outcome measures for motor capacity in CP, the valid Gross Motor Function Measure (GMFM) can be considered as the “gold” standard (Harvey, Robin, Morris, Graham, & Baker, 2008). Motor performance can be assessed with self‐reported methods (such as questionnaires) or objective techniques (such as activity monitors). Accelerometry‐based activity monitors have shown good reliability and validity for measuring physical behaviour in children with CP and are therefore the preferred method to assess motor performance (Mitchell, Ziviani, & Boyd, 2015; O'Neil et al., 2016).
Most children with CP get lifelong therapies in which becoming less sedentary and more active (i.e. improving motor performance) is a common treatment goal, in particular for the ambulatory subgroup in their primary school age. However, during a treatment period, the focus is mostly on motor capacity, for which the GMFM is routinely used, and not on motor performance. The reason for this might be that most of the time activity monitor measurements are not feasible or that experience is lacking to perform such measurements. Another reason might be that in daily practice, it is often reasoned that improvements in actual motor performance can be achieved and assessed indirectly by improvements in motor capacity.
From a clinical viewpoint, it is especially important to check this assumption and to determine if an intervention‐related change in motor capacity is actually accompanied by a change towards a more optimal motor performance. So far, only few studies studied this “capacity–performance relationship” longitudinally (Ho, Chang, Granlund, & Hwang, 2017; Smits et al., 2014; van Eck, Dallmeijer, Voorman, & Becher, 2009; Wright et al., 2008). Significant relationships were found in three studies, but these relationships showed to be highly variable (Ho et al., 2017; Smits et al., 2014; van Eck et al., 2009). Only the study of Wright et al. (2008) was in the context of one delineated intensive treatment period and showed mainly nonsignificant change score relationships. It is important to note that in all studies, motor performance was not assessed objectively, but with self‐reported outcome measures.
To our knowledge, no study so far has longitudinally described the capacity–performance relationship in the context of one distinct intensive treatment period in children with CP, using an objective performance outcome measure that represents what the children actually do in daily life. To better guide treatment for individuals with CP, we examined if changes in motor capacity outcomes are accompanied by a change in objectively measured motor performance immediately after a 3‐month intensive treatment period in ambulatory children with CP, and 3 months thereafter.
2. METHODS
2.1. Design and participants
A secondary analysis on prospective clinical trial data was performed, using the dataset from the Dutch SPACE BOP study (“SPAstic cerebral palsy; Cost‐Effectiveness of BOtulinum toxin and Physiotherapy”, inclusion period: October 2009 to September 2013) (Schasfoort, Dallmeijer, et al., 2018; Schasfoort, Pangalila, et al., 2018). The primary aim of this study was to compare the effectiveness of botulinum toxin combined with comprehensive rehabilitation with comprehensive rehabilitation alone. The SPACE BOP study was a pragmatically designed, single‐blind, multicentre trial. The study was not fully randomized: Randomized participants and those who had objections to randomization (because they strongly preferred one of the interventions) were both enrolled in the study. Initially, all parents of eligible children were invited to participate on a randomized basis. Randomization was stratified by Gross Motor Function Classification System (GMFCS) level. If parents declined randomization, they could participate in the treatment group of their preference. Physiotherapists, outcome assessors, and data analysts were blinded for both randomized and not‐randomized participants. Randomized participants were blinded until after baseline measurements. Power calculations showed that a sample size of 60 children was sufficient (Schasfoort, Dallmeijer, et al., 2018). The power of this secondary analysis was considered sufficient considering the general rule of 10 to 15 participants per variable.
In total, 65 children 4–12 years of age with a diagnosis of spastic CP with primarily lower extremity involvement (unilateral or bilateral), GMFCS levels I–III, and an indication for botulinum toxin treatment were included in the study (40% participated with randomization). Botulinum toxin treatment <6 months or CP‐related surgery <12 months at enrolment, being cognitively unable to understand instructions, presence of contractures or severe comorbidity were reasons for exclusion. Children were recruited from two university hospitals and five rehabilitation centres in the Netherlands. The study was approved by the ethics committee of Erasmus MC and written informed consent was obtained from parents/primary caregivers.
2.2. Procedures
For the present analysis, we used the data of the 65 included children as one group. All children followed a period of 12 weeks of intensive functional physiotherapy (ideally three 45‐ to 60‐minute sessions each week) with the purpose to improve motor capacity and motor performance. A subgroup of children received botulinum toxin 1 week prior to the start of the physiotherapy period. If indicated by the spasticity management team, children got 2–4 weeks of serial casting (1 week after physiotherapy had started) and/or new or realignment of ankle foot orthoses (AFOs), as soon as possible. All children (independent of whether they received botulinum toxin or not) could be considered for these additional treatments. The motor capacity and performance outcome measures were measured at baseline, 12 weeks (primary endpoint and end of the intensive treatment period) and 24 weeks.
2.3. Outcome measures
Motor capacity was assessed using three outcome measures: gross motor function (GMFM), functional muscle strength (FMS), and walking speed (WS). We used the Gross Motor Function Measure 66 Item Set version (GMFM66‐IS) to measure gross motor function. Russell et al. (2010) have shown that this short version of the GMFM‐66 is a valid and reliable method for children with CP. A difference of 0.8 on the overall GMFM‐66 score is considered a minimum clinically important difference (Oeffinger et al., 2008). FMS was measured by the 30‐seconds sit‐to‐stand test (STS); this involves the maximum number of sit‐to‐stands in 30 seconds. This test has shown good reliability and moderate validity for children with CP (Chrysagis, Skordilis, & Koutsouki, 2014). WS (in m/s), at a comfortable pace with shoes and, if applicable, AFOs, was measured during a two‐dimensional (2D) instrumented gait analysis on a 10 meter walkway. WS was calculated using the sagittal spatiotemporal information of the lateral malleolus from the 2D space calibrated middle 3 meter of the walkway.
Motor performance, comprising both physical activity and sedentary behaviour (Bussmann & van den Berg‐Emons, 2013), was measured objectively by the Actigraph activity monitor (Actigraph‐GT3X+). This device has been widely validated for measuring daily activity in children with CP (Mitchell et al., 2015; O'Neil et al., 2016). All children were asked to wear the Actigraph for 7 days. Children had to wear the device for at least 480 minutes (8 hours) during daytime; the data for days with shorter weartime were excluded from the analysis. When swimming, bathing/showering, or sleeping the Actigraph was taken off. ActiLife software (version 6.6.2) was used to analyse the Actigraph signals. Based on the three axes, a vector magnitude was calculated. A period of zero counts for 15 minutes or longer was considered as a nonwear period. Average intensity of physical activity counts per minute (CPM) for “all day” and “after school” (defined as the time after 2 p.m. on weekdays and full weekend days) and average percentage of the day spent sedentary (%sedentary) were calculated. Evenson cut‐off points (based on the vertical axis) were used to define %sedentary (0–25 counts per 15 seconds) (Evenson, Catellier, Gill, Ondrak, & McMurray, 2008). We calculated the CPM for both the all day and after school period because we assumed that children have less opportunities for actual performance of physical activities at school compared with their time after school.
2.4. Statistical analysis
Descriptive statistics were used to provide an overview of patient characteristics and to calculate mean (SD) scores. From here, change scores were calculated (12 weeks—baseline [∆12] and 24 weeks—baseline [∆24]) for all outcome measures. It is important to look at change scores at both 12 and 24 weeks because a change in motor performance generally takes time. Paired T tests were used to assess if motor capacity and motor performance outcome measures changed significantly after 12 and 24 weeks and to determine differences between ∆CPM all day and ∆CPM after school.
Subsequently, blockwise multivariate linear regression analyses were performed to investigate if changes in motor capacity were associated with changes in motor performance, and to determine the relative contribution of changes in motor capacity outcome measures and child characteristics to (variance of) changes in motor performance outcome measures. Motor performance change scores after 12 and 24 weeks (∆CPM all day, ∆CPM after school, and ∆%sedentary) were used as dependent variables in the models (enter method). We performed separate analyses for the data after 12 and 24 weeks using identical blocks. In the first block, motor capacity change scores (∆GMFM, ∆FMS, and ∆WS), respectively after 12 and 24 weeks, were entered as independent variables. GMFCS level and age were added to the model in the second block. Finally, we created scatterplots to see if the longitudinal capacity–performance relationship differed for GMFCS levels. P values below .05 were considered statistically significant. SPSS 22.0 and 25.0 were used for all statistical analyses.
3. RESULTS
A total of 65 children were enrolled in the SPACE BOP study. Baseline characteristics are outlined in Table 1. The study population had a mean age of 7 years and 3 months, and more boys than girls were included. Children were equally distributed across GMFCS Levels I, II, and III, and most children had the bilateral type of CP. Everyone received intensive physiotherapy for a period of 12 weeks, and 41 children were treated with botulinum toxin prior to comprehensive rehabilitation. Additional interventions during this period were casting (32 children) or new/realigned AFOs (21 children), of whom 15 children had both casting and AFOs.
Table 1.
Patient characteristics at baseline, n = 65
| Patient characteristics | n (%) |
|---|---|
| Mean age in years and months (SD) | 7 years and 3 months (2 years and 4 months) |
| Gender | |
| Boy | 37 (57%) |
| Girl | 28 (43%) |
| GMFCS | |
| Level I | 19 (29%) |
| Level II | 23 (35%) |
| Level III | 23 (35%) |
| CP type | |
| Unilateral | 14 (22%) |
| Bilateral | 51 (78%) |
| Treatment with botulinum toxin | 41 (63%) |
| Casting period (unilateral or bilateral) | 32 (49%) |
| New/realignment AFOs | 21 (32%) |
Abbreviations: AFOs, ankle foot orthoses; CP, cerebral palsy; GMFCS, Gross Motor Function Classification System.
Outcomes at baseline and change scores after 12 (∆12) and 24 weeks (∆24) from baseline are outlined in Table 2. Overall, statistically significant changes were found for motor capacity outcome measures after 12 and 24 weeks, in contrast to the motor performance outcome measures. There were no significant differences in improvement of CPM after school compared with CPM all day (35 vs. 18 CPM after 12 weeks, P = .23, and 56 vs. 30 CPM after 24 weeks, P = .10).
Table 2.
Baseline scores and change scores after 12 and 24 weeks for motor capacity and performance outcome measures
| Baseline | Change score after 12 weeks (∆12) | Change score after 24 weeks (∆24) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean (SD) | Range | N | Mean (SD) | (%) | Range | N | P | Mean (SD) | (%) | Range | N | P | |
| Weartime in days | 6.1 (1.2) | 5.5 (1.4)a | 5.4 (1.6)b | ||||||||||
| Motor capacity | |||||||||||||
| GMFM (score) | 68.5 (12.7) | 43.0–92.1 | 65 | 1.0 (2.5) | 1.5 | −6.4–7.4 | 65 | .002 * | 2.0 (3.3) | 2.9 | −9.8–9.1 | 65 | <.001 * |
| FMS (number STS) | 12.8 (3.7) | 5.0–23.0 | 65 | 1.1 (2.9) | 8.9 | −6–10 | 65 | .003 * | 1.3 (2.9) | 10 | −7–9 | 65 | <.001 * |
| Walking speed with shoes/AFOs (m/s) | 0.90 (0.27) | 0.39–1.60 | 62 | 0.01 (0.24) | 0.7 | −0.61–0.55 | 60 | .847 | 0.11 (0.25) | 12 | −0.55–0.78 | 60 | .001 * |
| Motor performance | |||||||||||||
| CPM all day (counts) | 819 (321) | 310–1701 | 65 | 18 (200) | 2.2 | −565–464 | 61 | .477 | 30 (173) | 3.7 | −469–429 | 60 | .185 |
| CPM after school (counts) | 850 (353) | 212–1757 | 65 | 35 (262) | 4.1 | −583–718 | 61 | .307 | 56 (236) | 6.6 | −486–937 | 60 | .07 |
| % Sedentary (%) | 72.1 (8.6) | 40.8–87.3 | 65 | −0.7 (5.3) | −1.0 | −15.1–14.8 | 61 | .295 | −0.4 (4.6) | −0.6 | −9.8–9.1 | 60 | .476 |
Abbreviations: AFOs, ankle foot orthoses; CPM, counts per minute; FMS, functional muscle strength; GMFM, Gross Motor Function Measure; STS, sit‐to‐stand test. The bold entries are used to highlight the statistical significant results
Mean weartime in days at 12 weeks (SD).
Mean weartime in days at 24 weeks (SD).
P < .05.
Results from blockwise multivariate linear regression analyses, with motor performance change scores after 12 and 24 weeks as dependent variables, are presented in Tables 3 and 4, respectively. After 12 weeks, changes in motor capacity outcomes were not significantly associated with changes in motor performance outcomes (Block 1), and the child characteristics (age and GMFCS level) also did not contribute significantly to changes in motor performance outcomes (Block 2). The total explained percentage of variance of both blocks was low (ranged from 1% to 6%) and not statistically significant. After 24 weeks, ∆24FMS and ∆24WS showed significant negative associations with ∆24%sedentary in Blocks 1 and 2 (P = .036 to P = .042): Improvements in FMS and WS were associated with a decrease in %sedentary. Only for the dependent variable ∆24%sedentary the model in Block 1 was statistically significant, with a total explained percentage of variance of 16% (P = .030). The capacity–performance relationship for GMFM with CPM all day after 12 weeks did not differ for differently GMFCS levels (Figure 1). This was also the case for all other relationships between changes in motor capacity and motor performance after 12 weeks (not presented as figure).
Table 3.
Blockwise multiple regression analyses for changes in three performance outcome measures with capacity change scores and patient characteristics (age, GMFCS) as independent variables (0–12 weeks)
| Multiple regression with 0–12 weeks change scores for performance outcomes as dependent | ∆12CPM all day (n = 56) | ∆12CPM after school (n = 56) | ∆12%Sedentary (n = 56) | |||
|---|---|---|---|---|---|---|
| Β | P | β | P | Β | P | |
| Block 1: capacity change scores 0–12 weeks | ||||||
| ∆12GMFM (score) | −3.4 | .766 | 5.3 | 0.726 | −0.03 | .907 |
| ∆12FMS (number STS) | 6.0 | .525 | 14.0 | 0.259 | −0.4 | .126 |
| ∆12Walking speed with shoes/AFOs (m/s) | −40.1 | .736 | −75.2 | 0.627 | 2.1 | .497 |
| R 2 total | 0.01 | .902 | 0.04 | 0.573 | 0.06 | .362 |
| Block 2: capacity change scores 0–12 weeks, adjusted for age and GMFCS level | ||||||
| ∆12GMFM (score) | −3.1 | .801 | 8.4 | 0.593 | −0.1 | .877 |
| ∆12FMS (number STS) | 6.1 | .528 | 14.7 | 0.241 | −0.4 | .131 |
| ∆12Walking speed with shoes/AFOs (m/s) | −42.0 | .731 | −92.1 | 0.559 | 2.2 | .475 |
| Age (in years) | 1.2 | .922 | 9.9 | 0.544 | −0.01 | .967 |
| GMFCS levels (I–III) | −5.0 | .895 | −43.2 | 0.372 | 0.4 | .698 |
| R 2 change | 0.00 | .989 | 0.02 | 0.620 | 0.003 | .925 |
| R 2 total | 0.01 | .989 | 0.06 | 0.708 | 0.06 | .654 |
Abbreviations: AFOs, ankle foot orthoses; CPM, counts per minute; FMS, functional muscle strength; GMFM, Gross Motor Function Measure; STS, sit‐to‐stand test.
P < .05.
Table 4.
Blockwise multiple regression analyses for changes in three performance outcome measures with capacity change scores and patient characteristics (age, GMFCS) as independent variables (0–24 weeks)
| Multiple regression with 0–24 weeks change scores for performance outcomes as dependent | ∆24CPM all day (n = 55) | ∆24CPM after school (n = 55) | ∆24%Sedentary (n = 55) | |||
|---|---|---|---|---|---|---|
| Β | P | β | P | β | P | |
| Block 1: capacity change scores 0–24 weeks | ||||||
| ∆24GMFM (score) | 0.1 | .993 | 4.5 | .668 | −0.01 | .972 |
| ∆24FMS (number STS) | 8.8 | .314 | 14.6 | .220 | −0.5 | .042 * |
| ∆24Walking speed with shoes/AFOs (m/s) | 156.1 | .102 | 208.4 | .107 | −5.0 | .036 * |
| R 2 total | 0.07 | .285 | 0.09 | .168 | 0.16 | .030 * |
| Block 2: capacity change scores 0–24 weeks, adjusted for age and GMFCS level | ||||||
| ∆24GMFM (score) | 0.3 | .973 | 3.2 | .763 | 0.01 | .959 |
| ∆24FMS (number STS) | 8.4 | .363 | 17.4 | .161 | −0.5 | .036 * |
| ∆24Walking speed with shoes/AFOs (m/s) | 157.3 | .123 | 218.4 | .111 | −5.3 | .039 * |
| Age (in years) | −1.3 | .907 | 11.0 | .457 | −0.2 | .536 |
| GMFCS levels (I–III) | 8.0 | .805 | −32.7 | .455 | 0.3 | .722 |
| R 2 change | 0.00 | .967 | 0.02 | .637 | 0.01 | .801 |
| R 2 total | 0.07 | .581 | 0.11 | .314 | 0.17 | .101 |
Abbreviations: AFOs, ankle foot orthoses; CPM, counts per minute; GMFM, Gross Motor Function Measure; FMS, functional muscle strength; STS, sit‐to‐stand test.
P < .05.
Figure 1.
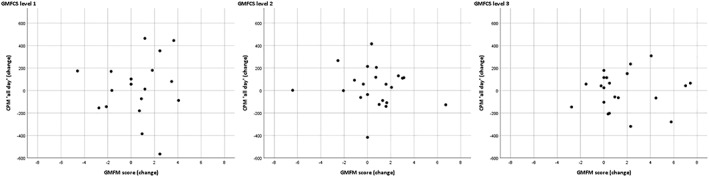
Illustrative scatterplots for the associations between GMFM and CPM all day change scores between baseline and 12 weeks for GMFCS levels I–III. CPM, counts per minute; GMFM, Gross Motor Function Measure
4. DISCUSSION
We found that changes in motor capacity were mostly not accompanied by changes in motor performance in ambulatory children with CP within the context of one distinct intensive treatment period. The few associations that were found, might indicate that the relationship between changes in motor capacity and motor performance depends on measurement interval and type of motor capacity and performance outcome measure. In the end, only one model was statistically significant with a total explained percentage of variance of 16%, suggesting that multiple other factors play a role in the capacity–performance relationship.
Our findings are generally in line with a previous study (Wright et al., 2008), although they mainly looked at relationships between changes in outcomes covering different domains of the ICF (body functions and structures, activities, and participation). In contrast, we specifically focused on the relationship between changes in motor capacity and motor performance within the ICF activity domain. Different to our results, other colleagues found stronger evidence of significant associations between changes in motor capacity and motor performance (Ho et al., 2017; Smits et al., 2014; van Eck et al., 2009; Wright et al., 2008). The populations in these studies differed somewhat from our population: They all included children with, predominantly spastic, CP from all GMFCS levels (Ho et al., 2017; Smits et al., 2014; van Eck et al., 2009) (vs. children with spastic CP, GMFCS levels I–III in our study), and two studies had a study population that was slightly older (Ho et al., 2017; van Eck et al., 2009). The most important differences, however, are follow‐up period (1–3 years vs. 3–6 months in our study), the fact that we looked at results of one distinct treatment period (vs. “natural” development of motor capacity and motor performance), and the use of self‐reported vs. objective outcome measures for motor performance in our study. Maybe, stronger associations would have been found for change scores when our study had longer follow‐up time, especially considering that achieving changes in motor performance may take time. Other studies mainly looked at associations between changes in motor capacity and motor performance in children's (natural) development over time, but we do not know the intensity of their therapies and to what degree these children were involved in interventions. Another important difference is that we know that self‐reported outcome measures differ from objective outcome measures and are more susceptible to recall and social desirability bias (Adamo et al., 2009; Prince et al., 2008). We therefore believe that measuring motor performance objectively with activity monitors (for a few days) gives a more valid representation of intervention‐related changes in what children actually do during their daily life.
The finding that motor capacity changes (∆24FMS and ∆24WS) were only significantly associated with changes in the motor performance outcome measure ∆24%sedentary, and not with ∆24CPM all day and ∆24CPM after school, confirms that sedentary behaviour indeed is a different and independent construct of motor performance (Bussmann & van den Berg‐Emons, 2013). Also, our results seem to support the assumption that changing motor performance generally takes time.
Only few significant associations between changes in motor capacity and motor performance were found. Regarding the interpretation of findings from our analyses, it is important to realize that results depend on the effects and effect sizes of the intervention that children received. In our case, motor performance outcomes showed small nonsignificant changes after a period of intensive treatment. This finding is not strange, because maintaining the level of functioning is often considered an important treatment goal in chronic rehabilitation populations such as CP. Moreover, longitudinal developmental trajectories of physical activity for children with CP have shown a decrease over time in the amount and intensity of physical activity in a group similar to the presently studied ambulatory age group (Bjornson et al., 2019). This should be taken into account together with the large variation in motor performance in general (Bjornson et al., 2019) and the heterogeneity that is inherent to CP (Damiano, 2014; Shevell, 2018).
The lack of a clear association between changes in motor capacity and changes in (especially the physical activity part of) motor performance after an intensive treatment period raises the question if we should change the way of treatment or add other components to treatment focusing more on performance and individual treatment goals. This is supported by Novak et al. (2013) who showed that interventions work on no more than one level of the ICF. Bjornson, Zhou, Stevenson, and Christakis (2013) already suggested interventions focusing on motor performance instead of motor capacity. And more recently, Reedman, Boyd, and Sakzewski (2017) found a modest (but clinically insufficient) effect of therapy and behaviour change interventions on motor performance (as measured by activity monitors) in a systematic review. The limited environmental and social possibilities of children with CP may also play a role, and maybe we should focus more on creating these possibilities. We think that one of the most important factors is the (school)programme children have to follow during the day. Despite the fact that our data showed no statistically significant difference between change scores of CPM all day and CPM after school, we still consider the finding that children are more active in the after school period of important clinical relevance. Our assumption is also supported by previous studies in healthy children showing that children spent less time sedentary and more time active in the after school period (Arundell, Hinkley, Veitch, & Salmon, 2015; Verloigne et al., 2017). Therefore, maybe we should incorporate more activities in the (school)programme of children with CP and break up long periods of sedentary time during the day.
Some limitations should be considered. First, general limitations known to ambulatory monitoring techniques, such as compliance in wearing time, inability to measure activities involving water, and seasonal influences, should be taken into account. Because motor performance varies during the day and between days, we cannot fully guarantee that individual Actigraph measurements were gathered on representative days. However, this factor is minimalized by a mean wearing time of 6 days (of at least 8 hours a day). Second, we like to address the possible ceiling effect of the GMFM. This may have played a role but is a general issue known to the population of ambulatory children with CP. Despite this, the GMFM remains the gold standard to measure motor capacity, and we did find statistically significant and clinically relevant improvements in GMFM scores (Harvey et al., 2008). Finally, our data were limited to measurements after 12 and 24 weeks in a relative small group of 65 ambulatory children with CP. It would have been interesting to study the capacity–performance relationship in a larger group, after a longer follow‐up period, and also take personal and environmental barriers and facilitators into account (Bloemen et al., 2015).
In conclusion, we found that changes in motor capacity are mostly not accompanied by changes in objectively measured motor performance. Type of outcome measures, measurement interval, and multiple other factors seem to be of influence. Clinicians and parents should be aware of this so these findings can be taken into account during goal setting and management of expectations of the effects of (intensive) treatment programmes. Because of the lack of a clear longitudinal capacity–performance relationship, it is important to evaluate our current treatment programmes that are mainly focused on improving motor capacity and less on improving motor performance. Incorporating interventions more specifically focusing on improving motor performance into current treatment programmes (based on individual patient goals and needs), creating social and environmental opportunities to optimize the use of motor capacity in daily life and evaluation of normal day to day programmes of children with CP probably is a good starting point in reaching long‐term improvement in both motor capacity and motor performance.
ACKNOWLEDGEMENTS
The work described here is a secondary analysis of data from the SPACE BOP study: “SPAstic cerebral palsy; Cost‐Effectiveness of Botulinum toxin and Physiotherapy”. The authors would like to thank the Netherlands Organisation for Health Research and Development (ZonMW Grant: 170995003) and Rijndam Rehabilitation for their funding. We also would like to thank the SPACE BOP study group members Professor Dr Henk Stam, Professor Dr Jules Becher, Professor Dr Ewout Steyerberg, Dr Herwin Horemans, Dr Annet Dallmeijer, Dr Suzanne Polinder, Dr Eline Bolster, Irma Viola, Karlijn van Beek, and Johannes Verheijden. Also, we would like to acknowledge Dr Majanka Heijenbrok‐Kal, Mirjam van Pelt‐Zoutendijk, Mariëtte Koster, Monique Arts, and Nicky Kerkhof for their contribution.
Halma E, Bussmann JBJ, van den Berg‐Emons HJG, et al. Relationship between changes in motor capacity and objectively measured motor performance in ambulatory children with spastic cerebral palsy. Child Care Health Dev. 2020;46:66–73. 10.1111/cch.12719
REFERENCES
- Adamo, K. B. , Prince, S. A. , Tricco, A. C. , Connor‐Gorber, S. , & Tremblay, M. (2009). A comparison of indirect versus direct measures for assessing physical activity in the pediatric population: A systematic review. International Journal of Pediatric Obesity, 4(1), 2–27. 10.1080/17477160802315010 [DOI] [PubMed] [Google Scholar]
- Arundell, L. , Hinkley, T. , Veitch, J. , & Salmon, J. (2015). Contribution of the after‐school period to children's daily participation in physical activity and sedentary behaviours. PLoS ONE, 10(10), e0140132 10.1371/journal.pone.0140132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjornson, K. , Fiss, A. , Avery, L. , Wentz, E. , Kerfeld, C. , Cicirello, N. , & Hanna, S. E. (2019). Longitudinal trajectories of physical activity and walking performance by Gross Motor Function Classification System level for children with cerebral palsy. Disability and Rehabilitation, 1–9. 10.1080/09638288.2018.1534995 [DOI] [PubMed] [Google Scholar]
- Bjornson, K. F. , Zhou, C. , Stevenson, R. , & Christakis, D. A. (2013). Capacity to participation in cerebral palsy: Evidence of an indirect path via performance. Archives of Physical Medicine and Rehabilitation, 94(12), 2365–2372. 10.1016/j.apmr.2013.06.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloemen, M. A. , Backx, F. J. , Takken, T. , Wittink, H. , Benner, J. , Mollema, J. , & de Groot, J. F. (2015). Factors associated with physical activity in children and adolescents with a physical disability: A systematic review. Developmental Medicine and Child Neurology, 57(2), 137–148. 10.1111/dmcn.12624 [DOI] [PubMed] [Google Scholar]
- Bussmann, J. B. , & van den Berg‐Emons, R. J. (2013). To total amount of activity … and beyond: Perspectives on measuring physical behavior. Frontiers in Psychology, 4, 463 10.3389/fpsyg.2013.00463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrysagis, N. , Skordilis, E. K. , & Koutsouki, D. (2014). Validity and clinical utility of functional assessments in children with cerebral palsy. Archives of Physical Medicine and Rehabilitation, 95(2), 369–374. 10.1016/j.apmr.2013.10.025 [DOI] [PubMed] [Google Scholar]
- Damiano, D. L. (2014). Meaningfulness of mean group results for determining the optimal motor rehabilitation program for an individual child with cerebral palsy. Developmental Medicine and Child Neurology, 56(12), 1141–1146. 10.1111/dmcn.12505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evenson, K. R. , Catellier, D. J. , Gill, K. , Ondrak, K. S. , & McMurray, R. G. (2008). Calibration of two objective measures of physical activity for children. Journal of Sports Sciences, 26(14), 1557–1565. 10.1080/02640410802334196 [DOI] [PubMed] [Google Scholar]
- Harvey, A. , Robin, J. , Morris, M. E. , Graham, H. K. , & Baker, R. (2008). A systematic review of measures of activity limitation for children with cerebral palsy. Developmental Medicine and Child Neurology, 50(3), 190–198. 10.1111/j.1469-8749.2008.02027.x [DOI] [PubMed] [Google Scholar]
- Ho, P. C. , Chang, C. H. , Granlund, M. , & Hwang, A. W. (2017). The relationships between capacity and performance in youths with cerebral palsy differ for GMFCS levels. Pediatric Physical Therapy, 29(1), 23–29. 10.1097/PEP.0000000000000332 [DOI] [PubMed] [Google Scholar]
- Holsbeeke, L. , Ketelaar, M. , Schoemaker, M. M. , & Gorter, J. W. (2009). Capacity, capability, and performance: Different constructs or three of a kind? Archives of Physical Medicine and Rehabilitation, 90(5), 849–855. 10.1016/j.apmr.2008.11.015 [DOI] [PubMed] [Google Scholar]
- Mitchell, L. E. , Ziviani, J. , & Boyd, R. N. (2015). Variability in measuring physical activity in children with cerebral palsy. Medicine and Science in Sports and Exercise, 47(1), 194–200. 10.1249/MSS.0000000000000374 [DOI] [PubMed] [Google Scholar]
- Novak, I. , McIntyre, S. , Morgan, C. , Campbell, L. , Dark, L. , Morton, N. , … Goldsmith, S. (2013). A systematic review of interventions for children with cerebral palsy: State of the evidence. Developmental Medicine and Child Neurology, 55(10), 885–910. 10.1111/dmcn.12246 [DOI] [PubMed] [Google Scholar]
- Oeffinger, D. , Bagley, A. , Rogers, S. , Gorton, G. , Kryscio, R. , Abel, M. , … Tylkowski, C. (2008). Outcome tools used for ambulatory children with cerebral palsy: Responsiveness and minimum clinically important differences. Developmental Medicine and Child Neurology, 50(12), 918–925. 10.1111/j.1469-8749.2008.03150.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neil, M. E. , Fragala‐Pinkham, M. , Lennon, N. , George, A. , Forman, J. , & Trost, S. G. (2016). Reliability and validity of objective measures of physical activity in youth with cerebral palsy who are ambulatory. Physical Therapy, 96(1), 37–45. 10.2522/ptj.20140201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince, S. A. , Adamo, K. B. , Hamel, M. E. , Hardt, J. , Connor Gorber, S. , & Tremblay, M. (2008). A comparison of direct versus self‐report measures for assessing physical activity in adults: A systematic review. International Journal of Behavioral Nutrition and Physical Activity, 5, 56 10.1186/1479-5868-5-56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reedman, S. , Boyd, R. N. , & Sakzewski, L. (2017). The efficacy of interventions to increase physical activity participation of children with cerebral palsy: A systematic review and meta‐analysis. Developmental Medicine and Child Neurology, 59(10), 1011–1018. 10.1111/dmcn.13413 [DOI] [PubMed] [Google Scholar]
- Rosenbaum, P. , Paneth, N. , Leviton, A. , Goldstein, M. , Bax, M. , Damiano, D. , … Jacobsson, B. (2007). A report: The definition and classification of cerebral palsy April 2006. Developmental Medicine and Child Neurology. Supplement, 109, 8–14. [PubMed] [Google Scholar]
- Russell, D. J. , Avery, L. M. , Walter, S. D. , Hanna, S. E. , Bartlett, D. J. , Rosenbaum, P. L. , … Gorter, J. W. (2010). Development and validation of item sets to improve efficiency of administration of the 66‐item Gross Motor Function Measure in children with cerebral palsy. Developmental Medicine and Child Neurology, 52(2), e48–e54. 10.1111/j.1469-8749.2009.03481.x [DOI] [PubMed] [Google Scholar]
- Ryan, J. M. , Cassidy, E. E. , Noorduyn, S. G. , & O'Connell, N. E. (2017). Exercise interventions for cerebral palsy. Cochrane Database of Systematic Reviews, 6, CD011660 10.1002/14651858.CD011660.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schasfoort, F. , Dallmeijer, A. , Pangalila, R. , Catsman, C. , Stam, H. , Becher, J. , … Bussmann, J. (2018). Value of botulinum toxin injections preceding a comprehensive rehabilitation period for children with spastic cerebral palsy: A cost‐effectiveness study. Journal of Rehabilitation Medicine, 50(1), 22–29. 10.2340/16501977-2267 [DOI] [PubMed] [Google Scholar]
- Schasfoort, F. , Pangalila, R. , Sneekes, E. M. , Catsman, C. , Becher, J. , Horemans, H. , … Bussmann, J. B. J. (2018). Intramuscular botulinum toxin prior to comprehensive rehabilitation has no added value for improving motor impairments, gait kinematics and goal attainment in walking children with spastic cerebral palsy. Journal of Rehabilitation Medicine, 50(8), 732–742. 10.2340/16501977-2369 [DOI] [PubMed] [Google Scholar]
- Shevell, M. (2018). Cerebral palsy to cerebral palsy spectrum disorder: Time for a name change? Neurology. 10.1212/WNL.0000000000006747 [DOI] [PubMed] [Google Scholar]
- Smits, D. W. , Gorter, J. W. , van Schie, P. E. , Dallmeijer, A. J. , Ketelaar, M. , & PERRIN+ Study Group (2014). How do changes in motor capacity, motor capability, and motor performance relate in children and adolescents with cerebral palsy? Archives of Physical Medicine and Rehabilitation, 95(8), 1577–1584. 10.1016/j.apmr.2014.04.013 [DOI] [PubMed] [Google Scholar]
- van Eck, M. , Dallmeijer, A. J. , Voorman, J. M. , & Becher, J. G. (2009). Longitudinal study of motor performance and its relation to motor capacity in children with cerebral palsy. Developmental Medicine and Child Neurology, 51(4), 303–310. 10.1111/j.1469-8749.2008.03263.x [DOI] [PubMed] [Google Scholar]
- Verloigne, M. , Ridgers, N. D. , Chinapaw, M. , Altenburg, T. M. , Bere, E. , Van Lippevelde, W. , … De Bourdeaudhuij, I. (2017). Patterns of objectively measured sedentary time in 10‐ to 12‐year‐old Belgian children: An observational study within the ENERGY‐project. BMC Pediatrics, 17(1), 147 10.1186/s12887-017-0894-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . (2001). International classification of functioning, disability, and health: ICF: Geneva.
- Wright, F. V. , Rosenbaum, P. L. , Goldsmith, C. H. , Law, M. , & Fehlings, D. L. (2008). How do changes in body functions and structures, activity, and participation relate in children with cerebral palsy? Developmental Medicine and Child Neurology, 50(4), 283–289. 10.1111/j.1469-8749.2008.02037.x [DOI] [PubMed] [Google Scholar]


