Abstract
Transfaunation is supposed to stimulate normal rumen function and has been used as an ancillary treatment for indigestion. Although it is widely recommended, there are little research data on the efficacy and the necessary volume.
The objective of the prospective clinical trial was the evaluation of the therapeutic efficacy of two different transfaunation volumes which can be obtained under practical conditions.
Forty‐five cattle suffering from indigestion were included in the study. A scoring system for the classification of rumen fluid was used. Scores were given in accordance with the importance of the parameter as an indication of microbial dysfunction. Animals with disturbed rumen fluid composition and activity were randomly assigned into 3 groups. Group 1 received 1 L of rumen fluid, group 2 received 5 L of rumen fluid and group 3 (control group) received 5 L of body temperature water. Rumen fluid analysis was repeated on days 1 and 4 after transfaunation. The feed intake of the animals was recorded.
After the transfaunation of 1 L and 5 L, the rumen fluid score improved significantly from day 0 to days 1 and 4. Rumen fluid samples in the control group showed no significant improvement from day 0 to day 1.
No significant differences were observed between the two treatment groups. But significant differences between the improvement of group 1 and the control group on days 1 and 4 and significant differences between group 2 and the control group on day 1 were detected. Small volumes of rumen fluid are easily obtainable by stomach tubes fitted with suction pumps. In summary, the transfaunation of as little as 1 L of rumen fluid caused significant improvement in the activity of rumen flora in cows suffering from indigestion.
Keywords: cattle, indigestion, rumen fluid, transfaunation
Abbreviations
- LDA
left abomasal displacement
- RDA
right abomasal displacement
1. INTRODUCTION
Transfaunation is the process of transferring rumen fluid containing microbes and nutrients from healthy animals into animals with impaired rumen digestion. This technique is believed to stimulate rumen function and has been used as an ancillary treatment for ketosis, anorexia and several causes of indigestion such as rumen acidosis. The method has been recommended in numerous text books and practical guides to bovine medicine as mentioned in the review by Depeters and George (2014). Although widely recommended, the authors are only aware of one research study evaluating the effect of 10 L rumen fluid in cows after surgical correction of LDA (Rager, George, House, & Depeters, 2004). In this study, transfaunated cows showed a greater milk yield, increased dry matter intake and lesser frequency of ketonuria after surgery than control animals which were not transfaunated. Leo‐Penu, Fitzpatrick, Zerby, and Parker ( also applied 10 kg of rume fluid in Bos indicus bulls after transportation. However, the large volume of 10 L rumen fluid is restricted to veterinary hospitals or larger cattle operations, which have access to rumen cannulated cattle or fresh rumen fluid obtained after slaughter. Smaller volumes, however, can be easily obtained by stomach tube from healthy cows on farm (e.g. 2 L, Steiner et al., 2015), but it seems that no studies on the effect of administering these smaller volumes to adult animals have been performed to date. There are a considerable number of recommendations in various text books and journal articles for administering volumes between 1 and 5 L rumen fluid to animals suffering from primary and secondary indigestion, enteritis, ketosis and lameness. None of these recommendations have justified the volume used or evaluated the efficacy of the procedure (Anderson, 2008; Dennnison et al., 2002; DePeters & Georges, 2014; Diernhofer, 1952; Kafka, 1951; Laflin & Gnad, 2008; Mieth, 1958; Pounden & Hibbs, 1949; Quin, 1949; Rings & Rings, 1993; Stöber, 1958; Van Metre, Callan, Holt, & Garry, 2005).
The main objective of this study was to evaluate the therapeutic efficacy of a single transfaunation using smaller volumes (1 and 5 L) of active rumen fluid to cows suffering from indigestion. It was hypothesized that the transfaunation of rumen fluid in volumes of 1 or 5 litres would have a greater effect on rumen fluid characteristics and feed intake when compared to cattle administered 5 L of water.
2. MATERIAL AND METHODS
A convenience sample of adult cattle (n = 45) was selected from cases (n = 94) which were referred to the University Clinic for Ruminants of the Vetmeduni Vienna (Austria). The primary criterion was that all animals showed decreased feed intake. The primary diseases of these animals were diagnosed, recorded and treated. After evaluation of the case history and clinical examination, rumen fluid sampling was performed by stomach tube (Selekt Rumen Fluid Collector, Nimrod Veterinary Products). Immediately after sampling, a rumen fluid analysis was performed that included the evaluation of odour, colour, consistency, pH value, number and viability of protozoa, sedimentation time and methylene blue reduction time. Odour, colour and consistency of the samples were assessed organoleptically (Constable, Hinchcliff, Done, & Grünberg, 2017). The pH values were measured with a portable pH meter (CG 818, Schott Austria GmbH.) (Steiner et al., 2015). The number of protozoa and the percentage of living protozoa were evaluated microscopically using a Fuchs‐Rosenthal counting chamber (Dehority, 1984). The durations of the sedimentation and flotation were measured with the methylene blue reduction test (Dirksen & Smith, 1987).
A scoring system assessing rumen function has been developed based on literature and clinical experience (Dirksen & Smith, 1987; Rings & Rings, 1993; Steiner et al., 2015). Rumen fluid parameters indicative of microbial dysfunction were allocated points based on an objective assessment as displayed in Table 1. The score was calculated by summing the points. Additional to the decreased feed intake, the second inclusion criterion was decreased rumen function; cattle with rumen fluid scores of more than 8 points were included in the study.
Table 1.
Scoring system for calculation of the overall rumen fluid score
| Parameter | Possible results | Score |
|---|---|---|
| Odour |
Aromatic Stale Sour; putrid |
0 .5 1 |
| Colour |
Olive green Non‐olive green |
0 1 |
| Consistency |
(Slightly) viscous Highly viscous/ Watery/foamy |
0 1 1 |
| Ph value |
6.2 – 7.0 <6.2;>7.0 <5.8;>7.4 <5.5;>7.7 |
0 2 4 6 |
| Number of protozoa per ml |
≥ 160,000 159,999 – 120,000 <120,000 – 80,000 <80,000 – 40,000 <40,000 – 800 <800 |
0 2 4 6 8 10 |
| Viable protozoa (%) |
100 – 91 90 – 86 85 – 76 75 – 66 65 – 56 55 – 46 <46 |
0 1 2 3 4 5 6 |
| Methylene blue reduction time (min) |
≤3 >3 >6 >10 >15 |
0 1 2 4 6 |
| Sedimentation and flotation time (min) |
4 – 8 <4;>8 |
0 1 |
Based on history, clinical examination and rumen function score, a convenience sample size of 45 adult animals from the total of 94 cases was included in the study. The mean age was 4.59 ± 2.06 years; 69.6% of the animals were Fleckvieh (Simmental), 15.2% were Holstein Friesian, 10.9% Red Friesian and 4.3% Brown Swiss, which is the typical breed distribution for the region. Different underlying diseases which had caused the decreased appetite and changes in rumen fluid were diagnosed. Six animals suffered from primary indigestion (5 inactivity of rumen microbial flora and 1 frothy bloat), a further 39 animals suffered from secondary indigestion caused by LDA (13), RDA/AV (10), mastitis (5), traumatic reticuloperitonitis (5), intestinal obstruction (5) and neurological disease (1). Additionally, ketosis (6), omasal paresis (3), metritis (6) and lameness (5) were diagnosed as concomitant diseases. Table 2 represents the distribution of the diseases in the study groups.
Table 2.
Diseases of study animals and distribution in the study groups
| Number of study animals | ||||
|---|---|---|---|---|
| Total | Group 1 | Group 2 | Group 3 | |
| Primary indigestion | ||||
| Inactivity of rumen microbial flora | 5 | 3 | 1 | 1 |
| Frothy bloat | 1 | 0 | 1 | 0 |
| Secondary indigestion | ||||
| Lda | 13 | 3 | 2 | 8 |
| Rda | 10 | 4 | 5 | 1 |
| Mastitis | 5 | 2 | 2 | 1 |
| Reticuloperitonitis traumatica | 5 | 2 | 1 | 2 |
| Bowel obstruction | 5 | 1 | 2 | 2 |
| Neurological disease | 1 | 0 | 1 | 0 |
| Total number of cows | 45 | 15 | 15 | 15 |
| Concomitant diseases | ||||
| Ketosis | 6 | 4 | 1 | 1 |
| Metritis | 6 | 3 | 3 | 0 |
| Lameness | 5 | 3 | 2 | 0 |
| Omasal paresis | 3 | 1 | 2 | 0 |
In 76.1% of the animals, surgery had to be performed, in 50.4% because of abomasal displacement, the remainder because of intestinal obstruction or traumatic reticuloperitonitis. Animals received antibiotics parenterally, non‐steroideal anti‐inflammatory drugs and intravenous fluid therapy, but no oral treatment other than the study therapy. For the study, the cattle were randomly assigned (by lot) into 3 groups, in the order of their admission to the veterinary hospital. On day 0 all 15 animals in group 1 received 1 L of rumen fluid, 15 animals in group 2 received 5 L of rumen fluid and 15 animals in group 3, the control group, received 5 L of warm water (38°C).
Rumen fluid for treatment was obtained from two rumen‐fistulated cows. Cows were non‐lactating, fed hay ad libitum and received commercial concentrate for maintenance requirements. Small rumen fluid samples were taken, analysed and scored soon before study treatment was performed, scores of 0 – 2 were accepted for transfaunation. The transfaunate was collected by a stomach tube fitted with a suction pump (Selekt Rumen Fluid Collector, Nimrod Veterinary Products) directly via the rumen cannula, in equal volumes and mixed thoroughly before transfaunation. The rumen fluid was immediately transfaunated into the study animals of group 1 and 2 using a drenching system (Flux Long Rumen Line, profs products). The same system was used to introduce the water to the animals of the control group.
Rumen fluid sampling and analysis of the study animals were repeated on days 1 and 4 after the initial administration of rumen fluid or water. Rumen fluid samples were scored again using described procedure. The personnel who examined the rumen fluid were blinded to treatment.
Appetite was scored as 0 for inappetence, 1 for moderately reduced feed intake (less than 1/3 of the ration), 2 for mildly reduced feed intake (between 1/3 and 2/3 of the ration) and 3 for good appetite (more than 2/3 of the ration). The ration was calculated for maintenance and 20 L milk yield, and consisted of hay, grass silage and pelleted concentrate (4 kg/d). Scoring was assessed by visual appraisal. Since the animals were held in the veterinary hospital, the feed intake could be observed continuously over 8 hr (next feeding). The person who performed the clinical examination and assessed feed intake was also blinded to treatment.
A reduction in the total points scoring the rumen fluid and an increase in feed intake were defined as positive effects of the transfaunation.
The study was authorized by institutional and governmental animal protection boards according to the Austrian national legislation (GZ 68.205/127‐II/10b/2008).
A statistical software (IBM SPSS Statistics version 19) was used for analyses and figures. All rumen fluid data were measured on ordinal scales or transferred into scores and used as ordinal data (Table 1). Data are presented as median (1st/3rd quartiles), non‐parametric tests were performed. Friedman test was used for statistical analyses comparing scored parameters of odour, colour, consistency, pH, number of protozoa, vital protozoa methylene blue reduction time, sedimentation and flotation time and the overall score between the days 0,1 4 within the treatment groups. Since Friedman test does not provide a post hoc test, the Wilcoxon signed‐rank test was used as post hoc test when required. Differences of the scored parameters as described between the three study groups at days 0, 1 or 4 were determined using the Mann‐Whitney U test. Differences were considered statistically significant if p ≤ .05.
3. RESULTS
The follow‐up examinations of rumen fluid were possible on day 1 in all animals. On day 4, only 35 animals could be examined, 10 animals died or had to be euthanized (3 in group 1, 4 in group 2 and 3 in group 3) because of deterioration of their clinical condition caused by the primary disease. On day 0, the mean scores (1st/3rd quartile) of the rumen fluids of the animals in the three groups were 12 (10/14.2) in group 1, 11.5 (10/15) in group 2 and 11.5 (10/12) in group 3. There were no significant differences between the three groups.
After the administration of 1 L rumen fluid (group 1), the score improved to 7.0 (2.5/8.5) on day 1 and to 2.0 (0.9/4.2) on day 4. The score significantly decreased from day 0 to day 1 (p = .001) and from day 1 to day 4 (p = .002).
After the transfaunation of 5 L rumen fluid (group 2), the score also improved to 6.8 (4.5/10.4) on day 1 and to 5.0 (2.75/9.5) on day 4. There was a significant improvement from day 0 to day 1 (p = .003) and from day 0 to day 4 (p = .022), but no significant difference between day 1 and day 4. The control group, in which the cows received 5 L water, the rumen fluid showed only a slight, non‐significant improvement to 9.0 (7.2/11.5) on day 1. A significant improvement was observed from day 0 and day 1 to day 4 (p = .010; p = .010) on which the rumen fluid score was 6.8 (5.4/9.1). Detailed results of the three groups and significant differences are presented in Table 3.
Table 3.
Median (1st/3rd quartiles) for scored of rumen fluid parameters from cattle treated with 1 litre (group 1), 5 litres (group 2) of rumen fluid or 5 litres of water (group 3)
| Parameter | 1 litre rumen fluid (group 1) | 5 litres rumen fluid (group 2) | 5 litres water (group 3) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| day | day 0 | day 1 | day 4 | day 0 | day 1 | day 4 | day 0 | day 1 | day 4 | |
| odour |
0.5 (0.5/0.5) *;§ |
0 (0/0) †;§ |
0 (0/0.2) †;§ |
0.5 (0.5/1) *;§ | 0.25 (0/0.5) *;§ |
0.5 (0/0.5) *;§ |
.5 (0/.5) *;§ |
.5 (.5/.5) *;¶ |
0 (0/.5) *;§ |
|
| colour |
0 (0/0) *;§ |
0 (0/0) *;§ |
0 (0/0) *;§ |
0 (0/0) *;§ |
0 (0/0) *;§ |
0 (0/0) *;§ |
0 (0/0) *;§ |
0 (0/0) *;§ |
0 (0/0) *;§ |
|
| consistency |
0 (0/0) *;§ |
0 (0/0) *;§ |
0 (0/0) *;§ |
0 (0/0) *;§ |
0 (0/0) *;§ |
0 (0/0) *;§ |
0 (0/0) *;§ |
0 (0/0) *;§ |
0 (0/0) *;§ |
|
| pH value |
0 (0/2) *;§ |
0 (0/1) *;§ |
0 (0/2) *;§ |
2 (0/2 *;§ |
0 (0/2) *;§ |
0 (0/3) *;§ |
2 (0/2) *;§ |
0 (0/0) †;§ |
0 (0/.5) †;§ |
|
| number of protozoa |
4 (2/4) *;§ |
0 (0/2) †;§ |
0 (0/0) ‡;§ |
4 (2/6) *;§ |
1 (0/2) †;§ |
0 (0/0) †;§ |
2 (2/4) *;§ |
2 (2/4) *,†;¶ |
2 (0/2.5) †;§ |
|
| vital protozoa |
5 (4/5.5) *;§ |
2 (1/3.5) †;§ |
1 (0/2) ‡;§ |
5 (3.8/6) *;§ |
2.5 (2.0/4.25) †;§ |
2 (1.5/4) †;§,¶ |
4 (3/4.5) *;§ |
4 (3/6) *;¶ |
3 (2/4) *;¶ |
|
| methylene blue reduction time |
1 (1/4) *;§ |
1 (0/1) †;§ |
0 (0/0) †;§ |
1 (1/2) *;§ |
0 (0/2) †;§ |
0 (0/.5) †;§ |
2 (1/4) *;§ |
1 (1/2) *,†;§ |
1 (0/1.25) †;§ |
|
| sedimentation and flotation time |
1 (0/1) *;§ |
0 (0/1) *,†;§ |
0 (0/0) †;§ |
1 (1/1 *;§ |
1 (0/1) †;§ |
1 (.5/1) *,†;§ |
1 (.5/1.0) *,†;§ |
1 (1/1) *;§ |
.5 (0/1) †;§ |
|
| overall score |
12 (10/14.2) *;§ |
7 (2.5/8.5) †;§ |
2 (.9/4.2) †;§ |
11.5 (10/15) *;§ |
6.8 (4.5/10.4) †;§ |
5 (2.75/9.5) †;§,¶ |
11.5 (10/12) *;§ |
9.0 (7.2/11.5) *;¶ |
6.8 (5.4/9.1) †;¶ |
|
,†,‡ different indices represent significant differences between treatment days within a treatment group;
,¶ different symbols indicate significant differences between treatment groups within treatment days
The various parameters of rumen fluid examination in the three groups over time are illustrated in Figures 1, 2, 3, 4, 5, 6. No significant differences were observed between the two treatment groups (group 1 and 2). But significant differences between group 1 and the control group on days 1 (p = .000) and 4 (p = .000) and significant differences between group 2 and the control group on day 1 (p = .004) were detected. The most obvious improvements in group 1 were observed for the parameter of odour from day 0 to day 1 (p = .003) and from day 0 to day 4 (p = .020), number of protozoa from day 0 to day 1 (p = .004) and from day 1 to day 4 (p = .023) and from day 0 to day 4 (0.004), percentage of vital/motile protozoa from day 0 to day 1 (p = .001), from day 1 to day 4 (p = .007) and from day 0 to day 4 (p = .007) and methylene blue reduction time from day 0 to day 1 (p = 0,005) and from day 1 to day 4 (p = 0,003). The other parameters did not differ significantly. In group 2, significant improvements were observed for the parameters of number of protozoa from day 0 to day 1 (p = .003) and from day 0 to day 4 (p = .024), the number of vital protozoa from day 0 to day 1 (p = .001) and from day 0 to day 4 (p = .040), the time for methylene blue reduction from day 0 to day 1 (p = .046) and times for sedimentation and flotation from day 0 to day 1 (p = .014). The other parameters did not differ. In the control group, significant improvements were observed in the categories in number of protozoa from day 0 to day 4 (p = .024), sedimentation and flotation times from day 1 to day 4 (p = .025) and methylene blue reduction time from day 0 to day 4 (p = .017). Only the percentage of animals with normal pH values increased significantly from day 0 to day 1 (p = .007) and from day 1 to day 4 (p = .035) after drenching with water.
Figure 1.
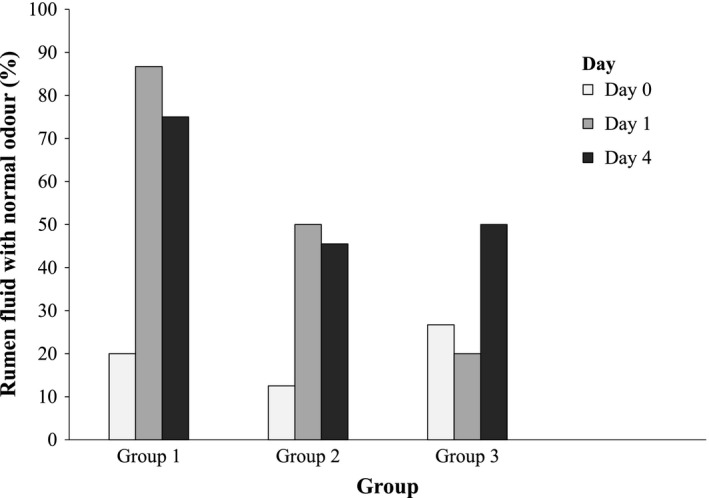
Percentage of rumen fluids (raw data) with normal odour in the three study groups on days 0, 1 and 4 (results of statistical analyses provided in Table 3, please note that for statistical analysis, the scored data have been used)
Figure 2.
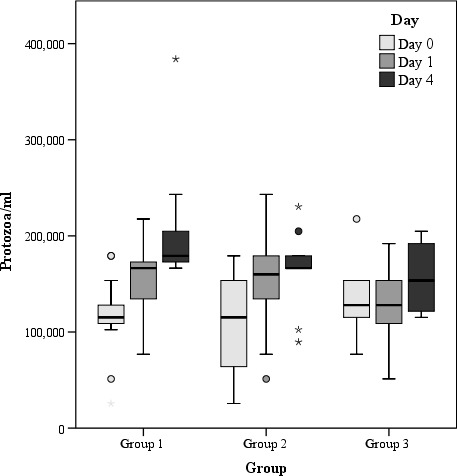
Number of protozoa/ml in rumen fluids (raw data) of the three study groups on days 0, 1 and 4 (results of statistical analyses provided in Table 3, please note that for statistical analysis, the scored data have been used)
Figure 3.
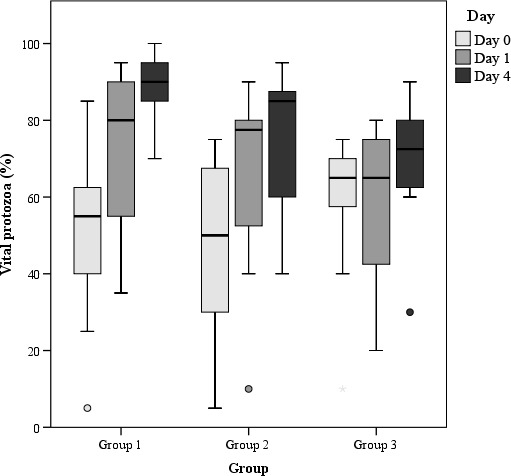
Percentage of vital protozoa in rumen fluids (raw data) of the three study groups on days 0, 1 and 4 (results of statistical analyses provided in Table 3, please note that for statistical analysis, the scored data have been used)
Figure 4.
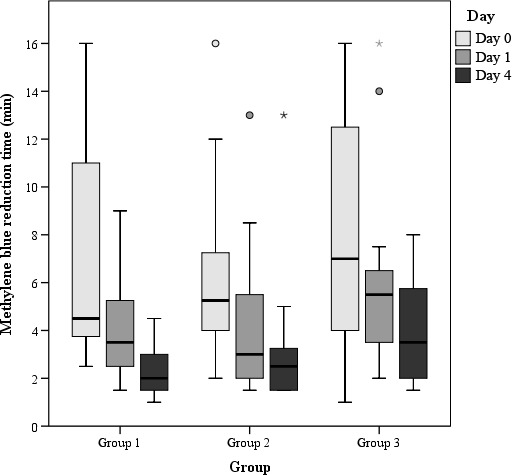
Time for methylene blue reduction in rumen fluids of the three study groups on days 0, 1 and 4 (results of statistical analyses provided in Table 3, please note that for statistical analysis, the scored data have been used)
Figure 5.
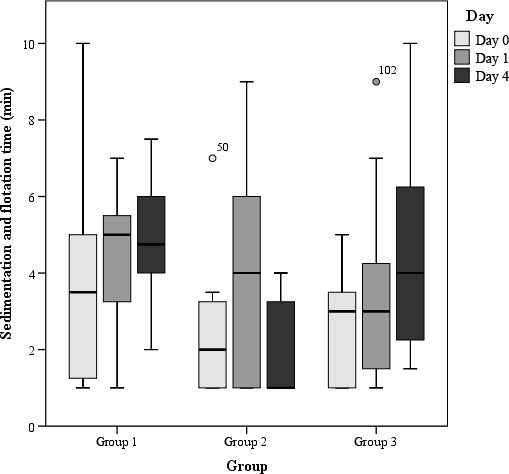
Time for sedimentation and flotation in rumen fluids of the three study groups on days 0, 1 and 4 (results of statistical analyses provided in Table 3, please note that for statistical analysis, the scored data have been used)
Figure 6.
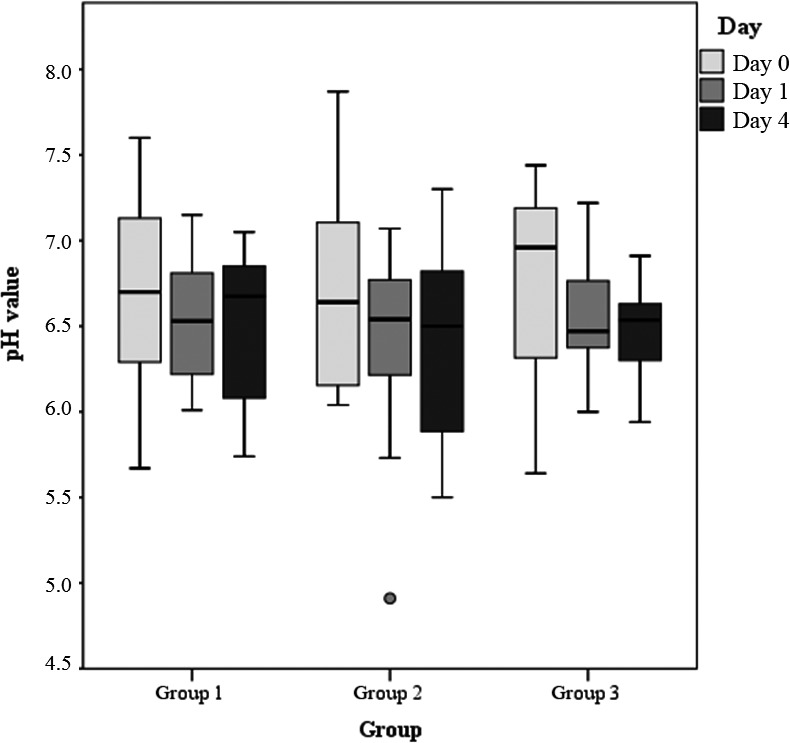
pH values of rumen fluids of the three study groups on days 0, 1 and 4 (results of statistical analyses provided in Table 3, please note that for statistical analysis, the scored data have been used)
The administration of rumen fluid also had a positive effect on the feed intake. Animals that received 1 L rumen fluid showed significantly greater feed intakes on days 1 (p = .003) and day 4 (p = .006) compared with day 0. Group 2 showed only a significant improvement from day 0 to day 1 (p = .037), and the control group showed a significant improvement only from days 0 to day 4 (p = .026). Detailed results of feed intake are illustrated in Figure 7.
Figure 7.
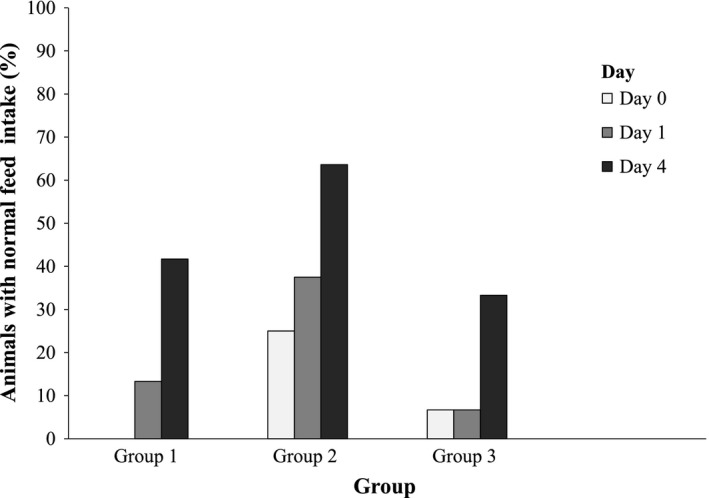
Percentage of animals with full feed intakes on days 0, 1 and 4 (results of statistical analyses provided in Table 3, please note that for statistical analysis, the scored data have been used)
4. DISCUSSION
Rumen transfaunation has a long history, being considered a valuable treatment for poor thrift calves and for cows suffering from indigestion from some 60 years ago (Kafka, 1951; Pounden & Hibbs, 1949; Quin, 1949). Since then the recommended volumes for adult animals have ranged from 1 to 10 L (Pounden & Hibbs, 1949; Quin, 1949; Kafka, 1951; Diernhofer, 1952; Mieth, 1985; Stöber, 1958; Rings & Rings, 1993; Dennnison et al., 2002; Rager et al., 2004; Van Metre et al., 2005; Anderson, 2008; Laflin & Gnad, 2008). However, it seems that these volumes were all derived from clinical experience and that there have been no controlled, experimental studies to support the recommendations. We believe that Rager and others (2004) were the first to attempt to conduct a study on the effect of rumen fluid on health and productivity in adult cows. They treated cows after surgical correction of LDA with rumen fluid and examined dry matter intake, milk yield and some metabolic parameters. They used the previously recommended maximum of 10 L rumen fluid and found positive effects. As mentioned previously, greater volumes of rumen fluid are difficult, if not impossible, to obtain using stomach tubes in the field. However, as we did not compare transfaunation of 10 L of rumen fluid in the present study, we are not able to draw any further conclusions.
The animals in the present study suffered from different primary diseases. Some were admitted to the clinic because of primary indigestion, but most animals were submitted because of other gastrointestinal diseases, mastitis or lameness causing secondary indigestions. The primary diseases had to be treated first. Therefore, 76.1% of the study animals underwent surgery, often a lifesaving procedure. Animals received no additional oral treatment, neither to treat the indigestion nor to support energy metabolism. The lack of homogeneity of the patients is a typical limitation of a field trial; however, the initial differences (day 0) between the cows of the groups were not significant (Table 3).
It is reasonable to assume that improvement of the decreased biochemical activity of rumen fluid at day 0 can be considered mainly as a result of transfaunation; however, an additional effect of infusion fluid in the rumen can be expected. This assumption is also corroborated by the fact that in both treatment groups, the main improvements were observed in the parameters of number of protozoa, percentage of vital protozoa, methylene blue reduction time and sedimentation and flotation times. These parameters are influenced by the introduction of living microorganisms by transfaunation and are considered to be useful and objective indicators of biochemical activity. The increase in the number and the percentage of viable protozoa also allows conclusions about bacterial and biochemical activity in the rumen, which is more difficult to examine under field conditions (Holtenius, Björck, & Hoflund, 1959).
The overall rumen fluid scores decreased significantly after transfaunation from day 0 to day 1. On day 4, significant improvements were also seen in the control group, most likely because of the progression of recovery from the primary disease. Although antibiotics were given systemically (not orally) to cows which underwent surgery, the medication may have influenced rumen microbiota and rumen fermentation (Fiore, Morgante, Muraro, Boso, & Gianesella, 2016). Since the proportion of cows which received antibiotics was not different between the groups, this would have been a systemic effect over all groups.
Because the animals originated from different farms and had different primary diseases, the values of milk yield and laboratory parameters such as haematology and blood biochemistry were very variable, so no analyses of these parameters were performed. Aside from the improvement of the quality of rumen fluid, feed intake also significantly increased after transfaunation as described by Rager et al., (2004) and Leo‐Penu et al., (). It is reasonable to believe that an increased feed intake may contribute to reduction of negative energy balance. It seems likely that repeated administration of rumen fluid could even increase the positive effects but this would need to be confirmed.
The main result of this study is that smaller transfaunation volumes, even as little as 1 L, had positive effects on relatively inactive rumen fluid and surprisingly no significant differences in the reduction of scores were observed between the two treatments groups (1 L and 5 L). It could be speculated that unrestricted exponential growth of rumen bacteria may be one reason for this. It seems possible that in an inactive rumen fluid prior to transfaunation, there is relatively little competition from existing fauna, so the transfaunation introduces fauna directly into the logarithmic phase of growth. However, to our knowledge there are currently no data proving this hypothesis. Overall, these results are of practical significance. One litre rumen fluid is easily and within 2 min obtainable using stomach tubes fitted with suction pumps, without considerable defence reactions of donor cows (Steiner et al., 2015). No rumen cannulated cows are necessary. Furthermore, the sampling of small volumes such as 1 L is very unlikely to cause any negative effects on the health of the donor. In our clinical practice, we never recorded any adverse reaction from the rumen fluid donors. Healthy donors from the same farm, in the same state of lactation and fed the same ration as the patient should be selected. Using animals from the same farm reduces the risk for transmission of infectious agents, which should be taken into biosecurity consideration.
In conclusion, transfaunation of small volumes of rumen fluid proved to be very efficient at improving rumen function and feed intake. It should be used more widely as a valuable practical supportive treatment accelerating recovery after surgical correction or medical treatment, and as a simple therapy for indigestion. It is inexpensive, fast and does not result in withdrawal periods.
ACKNOWLEDGEMENT
The authors would like to thank Prof. D. Logue (Glasgow University) and Prof D. Arney (Estonian University of Life Science) for their support in preparing the manuscript.
Steiner S, Linhart N, Neidl A, Baumgartner W, Tichy A, Wittek T. Evaluation of the therapeutic efficacy of rumen transfaunation. J Anim Physiol Anim Nutr. 2020;104:56–63. 10.1111/jpn.13232
REFERENCES
- Anderson, D. E. (2008). Surgical Disease of the small intestine. Veterinary Clinics of North America, 24, 383–401. 10.1016/j.cvfa.2008.02.004 [DOI] [PubMed] [Google Scholar]
- Constable, P. D. , Hinchcliff, K. W. , Done, S. H. , & Grünberg, W. (2017). Examination of rumen fluid In Constable P. D., Hinchcliff K. W., Done S. H., & Grünberg W. (Eds.), Veterinary Medicine. A textbook of the diseases of cattle, horses, sheep, pigs, and goats, 11th ed. (pp. 448–449). Elsevier, St. Louis. [Google Scholar]
- Dehority, B. A. (1984). Evaluation of subsampling and fixation procedures used for counting rumen protozoa. Applied and Environmental Microbiology, 48, 182–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennison, A. C. , VanMetre, D. C. , Callan, R. J. , Dinsmore, P. , Mason, G. L. , & Ellis, R. P. (2002). Hemorrhagic bowel syndrome in dairy cattle: 22 cases (1997–2000). Journal of the American Veterinary Medical Association, 221, 686–689. 10.2460/javma.2002.221.686 [DOI] [PubMed] [Google Scholar]
- DePeters, E. J. , & George, L. W. (2014). Rumen transfaunation. Immunology Letters, 162, 69–76. 10.1016/j.cvfa.2008.02.004 [DOI] [PubMed] [Google Scholar]
- Diernhofer, K. (1952). Verabreichung von panseninhalt bei verdauungsstörungen der wiederkäuer. Veterinary Medicine Austria, 40, 398–402. [Google Scholar]
- Dirksen, G. , & Smith, M. C. (1987). Acquisition and analysis of bovine rumen fluid. Bovine Practitioner, 22, 108–116. [Google Scholar]
- Fiore, E. , Morgante, M. , Muraro, M. , Boso, M. , & Gianesella, M. (2016). Methaphylactic effect of tulathromycin treatment on rumen fluid parameters in feedlot beef cattle. Canadian Journal of Veterinary Research, 80, 60–65. [PMC free article] [PubMed] [Google Scholar]
- Holtenius, P. , Björck, G. , & Hoflund, S. (1959). Die untersuchung von pansensaftproben. Deutsche Tierarztliche Wochenschrift, 66, 554–558. [Google Scholar]
- Kafka, H. (1951). Die übertragung von panseninhalt vom gesunden zum kranken rind. Veterinary Medicine Austria, 38, 576–577. [Google Scholar]
- Laflin, S. L. , & Gnad, D. P. (2008). Rumen cannulation: Procedure and use of a cannulated bovine. Veterinary Clinics of North America, 24, 335–340. 10.1016/j.cvfa.2008.02.007 [DOI] [PubMed] [Google Scholar]
- Leo‐Penu, C. L. O. , Fitzpatrick, L. A. , Zerby, H. N. , & Parker, A. J. . Treating bos indicus bulls with rumen transfaunation after 24 hours of transportation does not replete muscle glycogen. Animal Production Science, 56, 1738–1744. 10.1071/AN14632. [DOI] [Google Scholar]
- Mieth, K. (1958). Zur behandlung der vormagenstörungen der rinder durch pansensaftübertragung. Berlin Münchner Tierärztliche Wochenschrift, 71, 1–3. [Google Scholar]
- Pounden, W. D. , & Hibbs, J. W. (1949). Rumen inoculation in young calves. Journal of the American Veterinary Medical Association, 114, 33–35. [PubMed] [Google Scholar]
- Quin, A. H. (1949). Some advancements in veterinary therapy. Journal of the American Veterinary Medical Association, 115, 343–346. [Google Scholar]
- Rager, K. D. , George, L. W. , House, J. K. , & Depeters, E. J. (2004). Evaluation of rumen transfaunation after surgical correction of left‐sided displacement of the abomasum in cows. Journal of the American Veterinary Medical Association, 225, 915–920. 10.2460/javma.2004.225.915 [DOI] [PubMed] [Google Scholar]
- Rings, M. , & Rings, M. B. (1993). Rumen fluid analysis. Agri‐practice, 14, 26–29. [Google Scholar]
- Steiner, S. , Neidl, A. , Linhart, N. , Tichy, A. , Gasteiner, J. , Gallob, K. , … Wittek, T. (2015). Randomised prospective study compares efficacy of five different stomach tubes for rumen fluid sampling in dairy cows. Veterinary Record, 176, 50–55. 10.1136/vr.102399 [DOI] [PubMed] [Google Scholar]
- Stöber, M. , & Tiefenbach, B. (1958). Pansensaftgewinnung und vormagenentleerung zu therapeutischen zwecken – prüfung der brauchbarkeit von drei instrumenten. Deutsche Tierärztliche Wochenschrift, 65, 11–16. [Google Scholar]
- Van Metre, D. C. , Callan, R. J. , Holt, T. N. , & Garry, F. B. (2005). Abdominal emergencies in cattle. Veterinary Clinics of North America, 21, 655–696. 10.1016/j.cvfa.2005.06.003 [DOI] [PubMed] [Google Scholar]


