ABSTRACT
Preserved melanin pigments have been discovered in fossilised integumentary appendages of several amniote lineages (fishes, frogs, snakes, marine reptiles, non‐avialan dinosaurs, birds, and mammals) excavated from lagerstätten across the globe. Melanisation is a leading factor in organic integument preservation in these fossils. Melanin in extant vertebrates is typically stored in rod‐ to sphere‐shaped, lysosome‐derived, membrane‐bound vesicles called melanosomes. Black, dark brown, and grey colours are produced by eumelanin, and reddish‐brown colours are produced by phaeomelanin. Specific morphotypes and nanostructural arrangements of melanosomes and their relation to the keratin matrix in integumentary appendages create the so‐called 'structural colours'. Reconstruction of colour patterns in ancient animals has opened an exciting new avenue for studying their life, behaviour and ecology. Modern relationships between the shape, arrangement, and size of avian melanosomes, melanin chemistry, and feather colour have been applied to reconstruct the hues and colour patterns of isolated feathers and plumages of the dinosaurs Anchiornis, Sinosauropteryx, and Microraptor in seminal papers that initiated the field of palaeocolour reconstruction. Since then, further research has identified countershading camouflage patterns, and informed subsequent predictions on the ecology and behaviour of these extinct animals. However, palaeocolour reconstruction remains a nascent field, and current approaches have considerable potential for further refinement, standardisation, and expansion. This includes detailed study of non‐melanic pigments that might be preserved in fossilised integuments. A common issue among existing palaeocolour studies is the lack of contextualisation of different lines of evidence and the wide variety of techniques currently employed. To that end, this review focused on fossil amniotes: (i) produces an overarching framework that appropriately reconstructs palaeocolour by accounting for the chemical signatures of various pigments, morphology and local arrangement of pigment‐bearing vesicles, pigment concentration, macroscopic colour patterns, and taphonomy; (ii) provides background context for the evolution of colour‐producing mechanisms; and (iii) encourages future efforts in palaeocolour reconstructions particularly of less‐studied groups such as non‐dinosaur archosaurs and non‐archosaur amniotes.
Keywords: palaeocolour, melanin, melanosomes, exceptional preservation, amniotes, taphonomy
I. INTRODUCTION
Colours and their macroscopic patterns are critical to understanding the life and behaviour of an animal (Burley, Krantzberg & Radman, 1982; Butcher & Rohwer, 1989). Animals routinely employ colouration for aposematism, crypsis, sociosexual selection, and physiological purposes (Cuthill et al., 2017). The age, sex, and species of extant animals are often identified based on colour patterns (Peterson, 1999). Integumentary colour‐producing mechanisms contribute towards functions such as thermoregulation, resistance of the integument to mechanical abrasion, and protection from stressful environmental conditions such as ultraviolet (UV) radiation, humidity, pathogens, and long‐term climate change (Roulin, 2014). Given the diverse range of biological information provided by colouration it is important that descriptions of fossil taxa include palaeocolour reconstructions. For the purposes of this review, we distinguish between colour and colour patterns, with the former representing a general hue and the latter being a complex macroscale feature created by localised concentrations of pigments and structural mechanisms.
The colour palette of extant animals is fashioned from complex permutations and combinations of pigments (e.g. carotenoids, porphyrins, psittacofulvins and melanins) and structural components (e.g. keratin and collagen) (Fig. 1A). Naturally occurring pigment molecules (i.e. biochromes) produce colour by preferentially absorbing certain wavelengths of light while permitting others to be reflected (Hill & McGraw, 2006b) (Fig. 1B). It is these wavelengths of reflected light that impart the observable colour, whereas 'structural colours' are produced when light is scattered at the interfaces of layered nanoscale arrangements of reflective tissue constituents (e.g. arrays of different morphotypes of melanosomes in keratinous matrices of vertebrate integumentary structures, chitins in arthropods) that vary in refractive indices (Prum, 1999; Hill & McGraw, 2006b) (Fig. 1C, D). Additionally, colour changes through rapid spatial dispersal of pigment molecules, pigment‐containing vesicles, and reflective structures in ectothermic animals (e.g. crustaceans, cephalopods, fishes, amphibians, and non‐avialan/non‐dinosaurian reptiles) are controlled by neuroendocrine stimulation of cellular assemblies. These cellular assemblies termed chromatophores are categorised into classes according to the hues imparted (xanthophores: yellow; erythrophores: red; melanophores: black/brown; leucophores: white; cyanophores: blue; iridophores: reflective/iridescent) with the process causing these rapid colour changes termed metachrosis (Boyer & Swierk, 2017).
Figure 1.
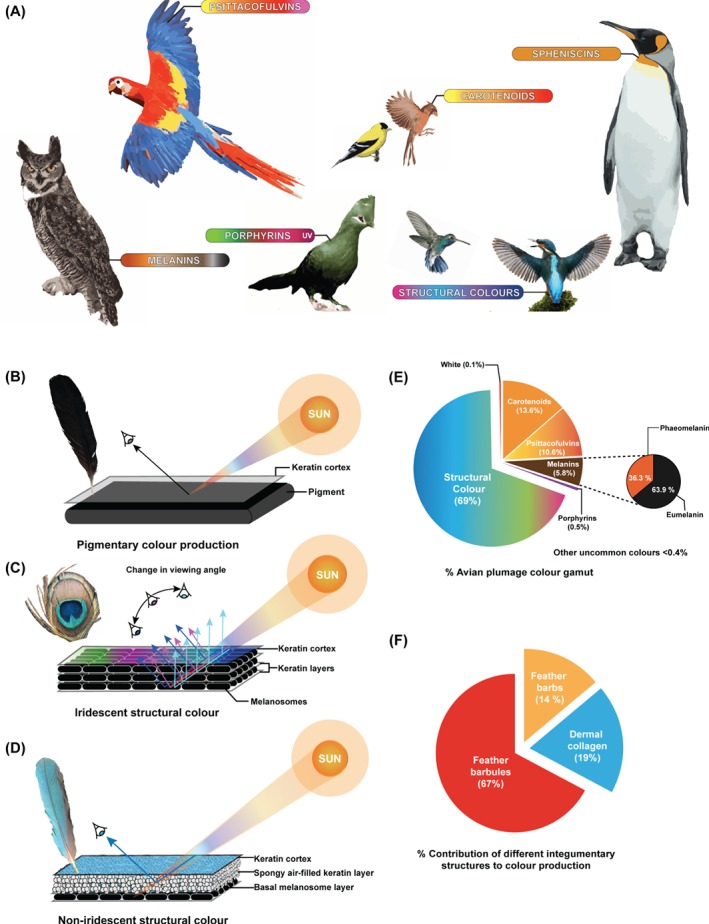
(A) Different colour‐production mechanisms in birds and the ranges of colours produced. (B–D) Optical mechanisms of colour production in bird feathers: pigmentary (B), iridescent structural (C), and non‐iridescent structural (D). (E) Approximate percentage contribution of different colour‐producing mechanisms to the avian plumage gamut based on data in Stoddard & Prum (2008, 2011). (F) Contribution of different integumentary structures to the production of colours based on data in Hill & McGraw (2006b). Artwork in A created using reference photographs from Wikipedia licenced under the Creative Commons attribution 4.0.
Melanin is of particular interest in fossilised organisms due to its resilience to diagenesis (i.e. thermal stability) (Glass et al., 2012, 2013; Colleary et al., 2015) since it is argued that long‐term thermal stability through diagenesis is the ultimate factor conferring organic fossilisation potential to biomolecules (Parry et al., 2018; Saitta, Kaye & Vinther, 2018d). The earliest known preserved fossil melanin dates to the late Carboniferous, ∼307 million years ago (Gabbott et al., 2016). The ability of melanin and melanin‐bearing membrane‐bound vesicles called melanosomes to be preserved within fossilised integumentary structures such as skin and feathers in non‐avialan dinosaurs, early birds, non‐dinosaurian reptiles and mammals has brought forth a unique opportunity to infer the actual colour patterns of these extinct animals (Vinther et al., 2008; Lindgren et al., 2014, 2015, 2018; Colleary et al., 2015; Vinther, 2015; Manning et al., 2019; Yang et al., 2019), enabling a range of novel hypotheses to be articulated (e.g. predator–prey interactions, aposematism, crypsis, sexual selection, behaviour, and habitat choice) (Vinther et al., 2008, 2016; Lindgren et al., 2010; Zhang et al., 2010; Li et al., 2012; Smithwick et al., 2017; Saitta et al., 2018c). Thus far, different studies have used different methods to infer palaeocolour and there is no overarching framework to maximise the repeatability and accuracy of reconstructions. Here, we review the different procedures currently available and propose a holistic protocol for palaeocolour reconstruction focussed on amniotes that accounts for taphonomic loss of information, pigment types and chemistry, morphology and arrangement of pigment‐bearing vesicles, products of pigment diagenesis, and preserved macroscopic colour patterns.
II. PIGMENTARY MECHANISMS OF COLOUR PRODUCTION IN AMNIOTES
Much work on fossil colour reconstruction has focused on filamentous integuments of non‐avialan dinosaurs, birds and closely allied species (Vinther et al., 2008; Lindgren et al., 2010; Li et al., 2012; Vinther, 2015). Extant birds, the only living dinosaurs, and mammals can act as a useful extant phylogenetic bracket (Witmer, 1995) for studying pigmentation and colours in fossilised filamentous integuments, a key adaptation in the evolution of bird‐line archosaurs parallel to mammalian hair (Fig. 2). The rationale for this choice is: (i) the relatively close morphological, developmental, and molecular similarities of their various epidermal integumentary outgrowths (e.g. scales, filaments, feathers and hairs) (Dhouailly, 2009; Dhouailly et al., 2019) and (ii) their shared pigmentary and structural colour‐producing mechanisms (Hofreiter & Schöneberg, 2010; Schartl et al., 2016).
Figure 2.
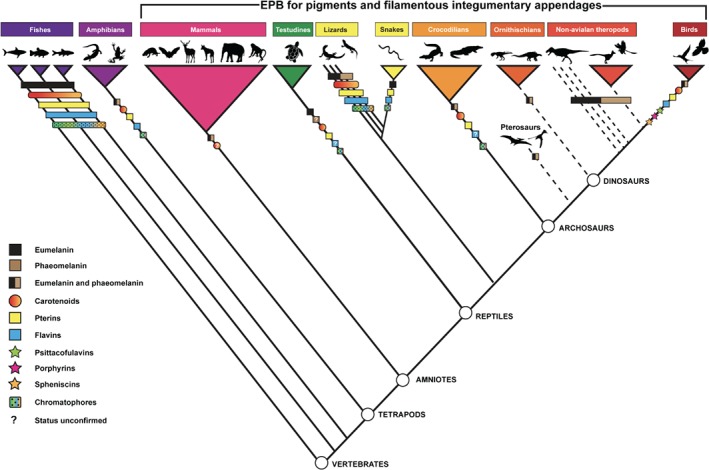
Simplified cladogram showing the distribution of different types of colour‐producing pigments in vertebrates: fishes (Courts, 1960; Johnson & Fuller, 2014; Sefc, Brown & Clotfelter, 2014; Kottler, Künstner & Schartl, 2015; Cal et al., 2017), amphibians (Obika & Negishi, 1972; Czeczuga, 1980; Thorsteinsdottir & Frost, 1986; Ichikawa, Ohtani & Miura, 1998; Thibaudeau & Altig, 2012), mammals (Ito & Wakamatsu, 2003; Galván et al., 2016b), testudines (Gopalakrishnakone, 1986; Roulin, Mafli & Wakamatsu, 2013; Steffen et al., 2015; Brejcha & Kleisner, 2016), lizards (Taylor & Hadley, 1970; Fitze et al., 2009; Cuervo, Belliure & Negro, 2016; Boyer & Swierk, 2017; Megía‐Palma, Jorge & Reguera, 2018), snakes (Blair & Graham, 1954; Kikuchi, Seymoure & Pfennig, 2014), crocodilians (Alibardi, 2011), extinct archosaurs (Li et al., 2014) and birds (Stoddard & Prum, 2011; Cuthill et al., 2017). Dotted lines indicate stem groups; bold lines indicate crown groups. Note that this cladogram shows the distribution of colour systems confirmed by prior published research but does not comment on ancestral states or the mode of evolution of colour‐producing mechanisms. Iridescence is not included on this cladogram because the mechanisms producing iridescence differ between birds and non‐avian vertebrates. Silhouettes for cyprinid fish (illustration by Ellen Edmonson, vectorised by Timothy J. Bartley), batrachid (illustration by Nobu Tamura, vectorised by T. Michael Keesey), rodent (vectorised by Michael B.H.), canid (illustration by Sam Fraser‐Smith, vectorised by T. Michael Keesey), elephant (T. Michael Keesey), lizard (illustration by Nobu Tamura, vectorised by T. Michael Keesey), Psittacosaurus (vectorised by Pete Bucholz), Tyrannosaurus rex (vectorised by Scott Hartman), Velociraptor (vectorised by Emily Willoughby), and troodontid dinosaur (vectorised by Emily Willoughby) were downloaded from http://www.phylopic.org. Remaining silhouettes, free of copyright, were also downloaded from http://www.phylopic.org. All silhouettes used are subject to CC Public Domain Dedication 1.0 licence.
The vast repertoire of colours and patterns in vertebrates are generated by 378 known genetic loci (Montoliu, Oetting & Bennett, 2011). Pigmentation in the pelage of several mammals including mice (Robbins et al., 1993), rabbits (Fontanesi et al., 2006), sheep (Våge et al., 1999), dogs (Newton et al., 2000), big cats (Eizirik et al., 2003), cows (Klungland et al., 1995), horses (Marklund et al., 1996) and humans (Ito & Wakamatsu, 2003) have been analysed thus far, and there is consensus that mammalian integuments are dominated by two variants of melanin pigments: eumelanin and phaeomelanin (Ito & Wakamatsu, 2003). Two proteins, melanocortin 1 receptor (MC1R) and agouti signalling protein (ASIP), play critical roles in vertebrates in determining which variant of melanin is expressed within hair and in regulating the spatial distribution of these pigments, resulting in complex patterning (Hofreiter & Schöneberg, 2010). Polymorphism in the colouration of mammalian pelage in the wild and in domesticated species also occurs due to mutations in the MC1R loci (Switonski, Mankowska & Salamon, 2013). Loss‐of‐function mutations usually lead to phaeomelanic, paler, reddish/yellowish colours (Ha et al., 2003; Rees, 2003), whereas gain‐of‐function mutations lead to eumelanic black/darker‐brown colours (Kijas et al., 1998; Våge et al., 1999). No other form of pigmentation in mammalian skin or hair is currently known, except the carotenoid pigmentation in the skin and hairs of bats (Bolívar‐Cimé, Clarke & Racey, 2012; Galván et al., 2016b) and the porphyrin‐mediated UV fluorescence in the pelage of New World flying squirrels (Glaucomys spp.) (Kohler et al., 2019). Blue structural colours in mammals have been studied in the rump and facial skin of mandrills (Mandrillus sphinx), the scrotum of the vervet monkey (Cercopithecus aethiops), mouse opossum (Marmosa mexicana) and woolly opossum (Caluromys derbianus) and are produced by light scattering in dermal collagen arrays (Prum & Torres, 2004).
In homeotherms, dead keratinous epidermal tissues undergo melanisation. By contrast, ectotherms like fishes, lissamphibians, and reptiles (e.g. turtles, tortoises, crocodilians, lizards and snakes) have pigmentation placed under neuronal and hormonal control within specialised chromatophore assemblies of the metabolically active dermis (Fujii, 1993; Holman, 2003; Mathger et al., 2003; Kerney, 2011). The integumentary ultrastructure, histology, distribution of pigments, and mechanisms producing colour patterns in crocodilians and testudines have only recently been investigated in detail (Alibardi, 2011; Rowe et al., 2013). In general, modern crocodilians have adopted dull skin colours rather than brighter hues, likely given their restricted ecological range (i.e. semi‐aquatic). Dull and pale cryptic patterns on their scaly skin (e.g. dark stripes, spots, splotches) have been shown to function in camouflage (Webb, Manolis & Whitehead, 1987). While the darker colours in crocodilians are generated by melanosome‐bearing chromatophores (melanophores), the diffuse lighter colours are generated using pterin‐ and carotenoid‐containing chromatophores (xanthophores) and guanine‐crystal‐bearing chromatophores (iridophores). Colours of the paler regions vary from grey or white (in Alligatoridae), through to pale brown (e.g. Crocodylus niloticus), and orange‐yellow (e.g. Crocodylus porosus). The only detailed histological study of pigment systems in crocodilians suggests that eumelanin is the sole type of melanin found in epidermal melanocytes and dermal melanophores (Alibardi, 2011). The localisation, concentration, and distribution of melanocytes in different layers of skin and scales control the intensity of the darker colours as well as macroscopic colour patterning (Alibardi, 2011). Rapid and reversible changes in colours of reptile skin are caused by the translocation of pigments under neuronal or hormonal control within the chromatophores in the epidermis and dermis, in response to changes in the environment (Taylor & Hadley, 1970; Sherbrooke & Frost, 1989; Kuriyama et al., 2006; Merchant et al., 2018). However, in crocodilians and testudines, the temporal onset of this colour change is much more gradual (i.e. days to weeks) compared to that of other reptiles, such as anole lizards (i.e. minutes to hours) (Rowe et al., 2013; Merchant et al., 2018; Staniewicz, Youngprapakorn & Jones, 2018).
Scaly integuments have been suggested to be the ancestral condition in non‐avialan dinosaurs since filamentous integuments are currently unknown in most ornithischians, all sauropodomorphs and some early theropod lineages such as ceratosaurids, abelisaurids, and allosauroids, with many of these groups instead preserving extensive, well‐developed scale impressions (Bonaparte, Novas & Coria, 1990; Coria & Currie, 2006; Coria & Chiappe, 2007). Feather‐like epidermal structures have been suggested to be the derived condition in the common ancestor of all coelurosaurian dinosaurs (Barrett, Evans & Campione, 2015). However, homoplastic loss of filaments in the scaly integuments of coelurosaurian tyrannosaurids has also been suggested (Bell et al., 2017), although others argue that this might be influenced by taphonomic bias against organic feather preservation (Saitta et al., 2018b) in North American tyrannosaurids. Among ornithischians, scales in Kulindadromeus have been suggested to be secondarily derived from feathers based on hypotheses relating to evolutionary development (Dhouailly, 2009; Godefroit et al., 2014; Dhouailly et al., 2019). Additionally, the presence of branched filamentous pycnofibres in pterosaurs, similar to primitive feathers in dinosaurs, also hints at the possibility that the ancestral state of all avemetatarsalians was filamentous (Yang et al., 2019). If this is the case, then the evolution of morphologically disparate integumentary filaments may show complex patterns of multiple independent filament losses across the archosaur phylogeny. A key factor that has the potential to bias these clade‐wide studies of integumentary structures is taphonomy, given that preservation of integumentary structures varies widely across different fossiliferous sites and deep time (Davis & Briggs, 1995; Wilson et al., 2016), particularly in Triassic localities in which we find the earliest representatives of dinosaurs and their close relatives (Clarke, 2013).
The integumentary structures of dinosaur and pterosaur fossils share close morphological and, presumably, partial functional homology with modern bird feathers (Xu, Zhou & Prum, 2001; Barrett et al., 2015; Mayr et al., 2016; Yang et al., 2019) and given the avian‐like physiology of dinosaurs (O'Connor & Claessens, 2005; Schachner, Lyson & Dodson, 2009; Schachner et al., 2011), the similarity of fossilised melanosomes in exceptionally preserved fossil dinosaur feathers to those of modern birds (Li et al., 2010, 2012; Eliason, Shawkey & Clarke, 2016), and the ability of melanosome morphology and melanin chemistry to elucidate palaeocolour in dinosaurs, a discussion of colour mechanisms in modern birds is warranted.
The myriad colours in bird feathers are fashioned by a complex interplay of pigments and structural mechanisms (Fig. 1E, F)(Shawkey, Morehouse & Vukusic, 2009). The major pigments contributing to colour production in the avian integument include carotenoids, melanins, porphyrins, and psittacofulvins (Hill & McGraw, 2006a, b ; Cuthill et al., 2017). Other pigments such as pterins and flavins play a relatively minor role in producing feather colours. Additionally, spheniscins are specialised pigments that are limited to penguins. Key research by Stoddard & Prum (2008) on avian visual sensitivities and later work (Stoddard & Prum, 2011) using 965 feather samples examined from 111 avian species led to the quantification of the approximate percentage of colours contributing to avian plumage colour gamut. While the largest and smallest proportions of the avian colour gamut are occupied by structural colours (∼69%) and non‐pigmented white (∼0.1%), respectively, simple pigmentary colour prevalence is as follows: carotenoids (∼13.6%), psittacofulvins (∼10.6%), melanins (∼5.8%), and porphyrins (∼0.5%) (Fig. 1E). Avian pigments are briefly discussed below.
(1). Carotenoids
Carotenoids are linear, conjugated 40‐C tetraterpenoid molecules divided into two major categories based on different functional groups (Matsuno, 1989). Non‐substituted and non‐polar carotenoids with only carbon and hydrogen atoms are referred to as carotenes (Fig. 3C) whereas substituted, polar carotenoids with oxygen‐containing functional groups are collectively designated as xanthophylls (Fig. 3F) (Lu & Li, 2008). These pigments comprise over 1100 distinct chemical entities (Yabuzaki, 2017). Carotenoids are synthesised de novo by various organisms (e.g. bacteria, plants, algae, and fungi) and acquire their yellow/orange to red colours from the 'chromophore centre'. Chromophore centres (not to be confused with dermal chromatophores) are molecules or functional groups containing alternating single/double/triple bonds (i.e. conjugation) directly responsible for absorption of light and imparting colour (McHale, 2017). Greater degrees of conjugation within the chromophore centre result in greater absorption of short wavelengths in the violet to green region of the visible spectrum (400–550 nm) (Hill & McGraw, 2006b). A key difference between carotenoids and other pigments is that de novo biosynthesis of carotenoids does not occur in animals due to absence of the enzyme phytoene synthase. Thus, carotenoid‐based colouration in animals is accomplished through dietary uptake (Brockmann & Völker, 1934; Matsuno, 1989; Goodwin, 1992; Sefc et al., 2014) or through symbiotic association (e.g. marine filter‐feeders) (Maoka, 2011). Although carotenoids are not synthesised by animals, the metabolic framework to process them after uptake does exist, allowing for conversion into non‐dietary forms widely prevalent in avian feathers, beaks, and skin. After ingestion and metabolic processing, these pigments enter the bloodstream through both lipid‐dependent and lipid‐independent mechanisms and are eventually deposited in keratinous dead tissue through passive diffusion (Parker, 1996). It has been shown that only yellow carotenoids are assimilated by animals and can be bio‐converted to make red ones (Goodwin, 1986). Blends of different carotenoid types can then create intermediate hues. Not all types of ingested carotenoids become incorporated into keratinised tissue (Hill & McGraw, 2006b). The process of carotenoid incorporation is governed by multiple selective factors such as diet (Rock et al., 1992; Williams, Boileau & Erdman, 1998), intestinal endoparasites (Ruff & Fuller, 1975), toxins (Osborne et al., 1982), diffusion thresholds (Parker, 1996), and unequal binding affinity for different lipoprotein types (Hill & McGraw, 2006b). Hence, the intensity of carotenoid‐based colours can provide honest signals of diet and health for sexual selection to act upon (Weaver et al., 2018).
Figure 3.
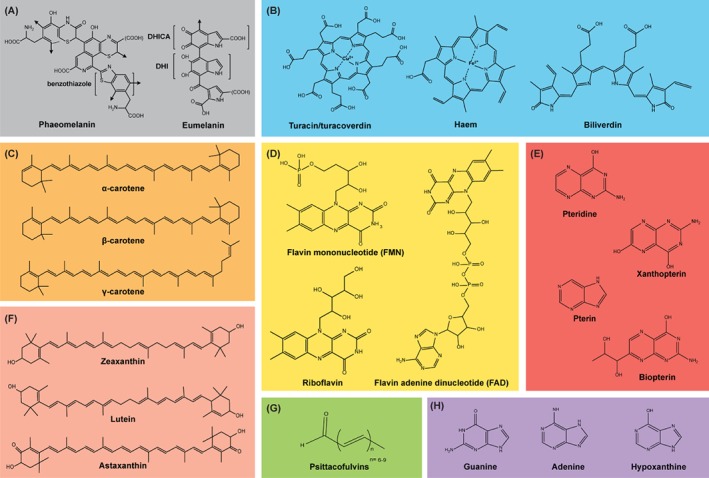
Molecular structures of some common examples of pigments in the animal kingdom: melanins (A), porphyrins (B), carotenes (C), flavins (D), pterins (E), xanthophylls (F), psittacofulvins (generalised molecular structure) (G) and purines (H).
(2). Psittacofulvins
Psittacofulvins are a class of highly colourful pigments restricted to the plumage of parrots (Psittaciformes). Initially mistaken for carotenoids, this unique class of lipid‐soluble, red‐yellow pigments was first named by Krukenberg (1882). Further research (Völker, 1936, 1937) highlighted that these pigments were independent of dietary uptake, unlike carotenoids. The detailed chemistry of psittacofulvins has only recently been revealed by high‐performance liquid chromatography (HPLC) coupled with ultraviolet–visible (UV/VIS) spectroscopy and mass spectrometry (MS) (Stradi, Pini & Celentano, 2001) (Fig. 3G). This new approach identified at least four different variants of psittacofulvins and suggested a linear polyenal (i.e. long‐chain‐conjugated aldehyde) structure for each, differing only in the number of C=C conjugations. Based on this structure they also postulated two putative metabolic pathways by which psittacofulvins could be synthesised: through a polyketide pathway derived from acetyl‐CoA or through enzymatic desaturation of fatty acids. Further work by McGraw & Nogare (2004) discovered a fifth psittacofulvin variant in parrots and demonstrated that these pigments are exclusively limited in distribution to feathers and do not impart colouration to any other tissue of the body. Mundy (2018) confirmed that red colours in parrot feathers are produced by long‐chain‐conjugated polyenals whereas yellows are produced by polyunsaturated 14‐, 16‐, or 18‐C fatty acids. Additionally, Mundy (2018) identified a point mutation in the gene MuPKS, a member of the polyketide synthase gene family implicated in fatty acid biosynthesis, which causes green budgerigars (a result of structural blue combined with yellow psittacofulvins) to develop blue colouration. Therefore, MuPKS is likely responsible for generating psittacofulvins. Polyketide synthases can cyclically add 2‐C moieties to produce yellow psittacofulvins, which can then generate red psittacofulvins with further downstream processing that mirrors the synthesis of carotenoid pigments (Mundy, 2018). The reflectance properties of psittacofulvins have been investigated by various researchers (Krukenberg, 1882; Hudon & Brush, 1992; McGraw & Nogare, 2005). Characteristic absorption peaks at wavelengths shorter than those of carotenoids (Völker, 1936) and small reflectance peaks in the UV region (Pearn, Bennett & Cuthill, 2001; McGraw & Nogare, 2005) have been identified. Parrots can also blend colours by combining psittacofulvins with structural mechanisms. As in the budgerigar example, structural blue with yellow psittacofulvins produces green (for detailed mechanisms, see Section III).
(3). Melanin
The term melanin, derived from the ancient Greek μϵλανoς (‘melanos’) meaning ‘dark’, was first coined by the Swedish chemist Berzelius (1840) to designate a dark‐brown pigment isolated from retinal membranes. Several classification schemes have been proposed for melanin pigments during the last 50 years (Nickerson, 1946; Nicolaus, Piattelli & Narni, 1959; Nicolaus, 1968; Riley, 1992, 1997; Pezzella et al., 1997; Ito & Wakamatsu, 1998; Wakamatsu & Ito, 2002; Borovansky & Riley, 2011; Prota, 2012; d'Ischia et al., 2013), but no clear‐cut, all‐encompassing definition has emerged that sufficiently explains all of the properties of this diverse group of pigments (Prota, 1988, 2012; d'Ischia et al., 2013). Melanin is frequently found in integumentary structures like mammalian hair (Goding, 2007) and avian feathers (Stoddard & Prum, 2011) as well as vertebrate skin (Morales‐Guerrero et al., 2017; Parolini et al., 2018; Pshennikova & Voronina, 2018). It can also be found localised in other vertebrate organs, for instance, the retina of the eye, cochlea of the inner ear, and in certain regions of the brain and liver (Borovansky & Riley, 2011; Rossi, McNamara, Webbet et al., 2019). At the chemical level, current consensus has broadly defined melanins as groups of highly heterogenous molecules derived from the oxidation of phenolic compounds and downstream polymerisation of the resulting intermediates (Solano, 2014). Melanin pigments produce colours ranging from black, grey or dark‐brown, to reddish‐brown by near‐uniformly increasing broadband reflectance of visible wavelengths in the 300–700 nm range as well as wavelengths of the UV spectrum invisible to the human eye (Hill & McGraw, 2006b). Variation in the hues imparted by melanin is due to differences in the chemical units that make up these polymeric molecules (Riley, 1997).
Animal melanins (Fig. 3A) consist of eumelanin and phaeomelanin. Eumelanin is formed by the oxidative polymerization of 5,6‐dihydroxyindole (DHI) and 5,6‐dihydroxyindole‐2‐carboxylic acid (DHICA) (Ito & Wakamatsu, 1998). Phaeomelanin is formed by the spontaneous combination of dopaquinone (an aromatic derivative of l‐dihydroxyphenylalanine, an intermediate of the tyrosine catabolic pathway) with the amino acid cysteine to generate cysteinyl‐dopa which undergoes further oxidation to become phaeomelanin (Greco et al., 2011). Eumelanin principally produces hues ranging from darker shades of brown to black, while phaeomelanin produces rufous/reddish‐brown colours. The presence of a higher number of carbonyl groups (C=O) in eumelanin causes strong absorption in the red region of the visible spectrum which makes it appear dark brown/black, whereas fewer carbonyl groups in the benzothiazole‐rich (sulphur‐containing) phaeomelanin produces paler brown to buff colours (Nickerson, 1946). Functionally, melanins can be further categorised in terms of their source (i.e. animal, plant, fungal, and synthetic) (Bell & Wheeler, 1986; Riley, 1997; Solano, 2014; Xiao et al., 2015), but alternative terminology (i.e. eumelanin, phaeomelanin, neuromelanin, sepiamelanin, allomelanin, and pyomelanin) also appears frequently in the literature (Pezzella et al., 1997; Schmaler‐Ripcke et al., 2009; Solano, 2014; Varga et al., 2016). Neuromelanin is thought to be made up of mixtures of eumelanin and phaeomelanin and is found in the catecholaminergic neurons of substantia nigra (SN) of the brain (Graham, 1979; Carstam et al., 1991; Zecca et al., 2001; Solano, 2014), whereas eumelanin in cephalopod ink has been characterised as sepiomelanin (Pezzella et al., 1997; Palumbo, 2003). Allomelanin and pyomelanin are plant, fungal, or bacterial in nature (Varga et al., 2016). The enormous heterogeneity shown by these polymeric molecules can be attributed to differences in phenolic and quinone intermediates produced during oxidation steps as well as the types of intermediates eventually ending up as monomers that undergo polymerisation into the final product. Thus, the use of ambiguous terminology has reduced 'melanin' to something of a wastebasket term for any black/dark‐brown pigment from any source, irrespective of chemical characterisation, although similarities exist.
Animal melanins are typically stored in microscopic, lipid membrane‐bound, sub‐cellular vesicles called melanosomes (Schraermeyer, 1996; Marks & Seabra, 2001). Melanosomes vary in morphology and distribution in a tissue‐ and taxon‐specific way (Hong et al., 2006; Borovansky & Riley, 2011; Eliason et al., 2016). In bird feathers and mammalian hair, eumelanin has been noted to be stored in rod‐shaped eumelanosomes, whereas phaeomelanin has been reported to be found in spherical‐shaped phaeomelanosomes (Vinther et al., 2008). In some tissues, such as the vertebrate retinal pigment epithelia, two distinct melanosome morphologies can be observed: ovoid and rods arranged basoapically (Kim & Choi, 1998). In amphibians, integumentary melanosome morphology is transitional between rod and spherical shapes, resembling a laterally condensed ellipsoid (Colleary et al., 2015). For many years, the binary categorisation of animal melanins into eumelanin and phaeomelanin prompted authors to classify melanin‐based colours discretely in birds, with eumelanin pigment localising exclusively to rod‐shaped 'eumelanosomes' and phaeomelanin to spherical 'phaeomelanosomes' (Trinkaus, 1948; Somes & Smyth, 1965). However, it is now thought that both pigments can co‐occur within melanised feathers (McGraw et al., 2004). The relative proportion of phaeomelanin and eumelanin is key to the final shade imparted to the feathers. For example, the total melanin concentration is roughly the same in the pale reddish‐buff feathers of red‐winged blackbirds (Agelaius phoeniceus) and the black feathers of zebra finches (Taeniopygia guttata), but due to differences in the relative proportions of the two pigments (83% phaeomelanin in the former and 92% eumelanin in the latter) they show markedly different colour shades (McGraw et al., 2004). This raises the question of co‐localisation of eumelanin and phaeomelanin within a single type of melanosomes in feathers, as suggested by the 'casing model' of neuromelanin whereby phaeomelanin granules form a core and eumelanin molecules aggregate onto the surface of that core (Ito, 2006). Additionally, the correlation between melanosome shape and colour does not hold in retinal pigment epithelium, within which eumelanin is stored in both shapes of melanosomes (Wang, Dillon & Gaillard, 2006). Therefore, the melanosome shape–colour correlation in feathers would be better explained by characterising rod‐shaped melanosomes as eumelanin‐dominant and spherical melanosomes as phaeomelanin‐dominant. Liu et al. (2014) tested the hypothesis that different colours of feathers correspond to relative proportions of eumelanin and phaeomelanin using laser desorption synchrotron post‐ionisation (synchrotron‐LDPI)‐MS. Peak probability contrast from MS data showed that black feather colours can be attributed to oxidised forms of DHICA/DHI units of eumelanin and are most clearly distinguishable from other colours. Brown is dominated by higher proportions of phaeomelanin composed of oxidised versions of benzothiazine, benzothiazole, and isoquinolines. Grey colour is derived from phaeomelanin with nominal amounts of eumelanin and isoquinoline derivatives. Galván & Wakamatsu (2016) largely corroborated the conclusions of Liu et al. (2014) using an expanded colour gamut from birds and mammals but suggested that carboxylated DHICA and benzothiazole unit concentrations could act as correlates of the intensity of blacks and reddish‐brown colours, respectively. Additionally, they also noted that colour phenotypes are not produced by DHICA or benzothiazoles in isolation but are composed of varying proportions of both. The chemical methods of Liu et al. (2014) and Galván & Wakamatsu (2016) could potentially be useful in reconstructing palaeocolour from preserved melanin signatures in fossils.
(4). Porphyrins
Porphyrins are a class of pigments characterised by square‐planar, macrocyclic, and nitrogen‐containing tetrapyrrole rings connected via methine (=CH−) bridges (Finar, 1956). These molecules, apart from being integumentary colourants, also play important physiological roles in non‐integumentary tissues ranging from the oxygen‐bearing haem co‐factor in the respiratory metalloprotein haemoglobin in animals to the key light‐absorbing component of chlorophyll in plants (Rimington, 1957). In birds, porphyrins (Fig. 3B) can be classified into three groups: (i) natural porphyrins (e.g. uroporphyrin, coproporphyrin, and protoporphyrin) limited to brown eggshells and rusty‐hued feathers in owls (Strigiformes), bustards (Otididae), and nightjars (Caprimulgidae), (ii) metalloporphyrins in blood (i.e. haem), (iii) red and green colour‐imparting porphyrins (i.e. turacin and turacoverdin) in turacos (Musophagiformes), and (iv) bilins in blue egg shells (e.g. biliverdin) (Derrien & Turchini, 1925; Völker, 1938; Ponka, 1999; Weidensaul et al., 2011; Galván et al., 2016a). Natural porphyrins are derived from succinyl CoA (an intermediate of the Krebs cycle), and the amino acid glycine (Needham, 2012). Metalloporphyrins are produced by enzymatic addition of metal ions such as iron, as in haem (Ponka, 1999), or copper, as in turacins (Rimington, 1939). Bilins are formed further downstream by oxidative degradation of haem in the liver but have also been found transported to more peripheral tissues like oviducts for deposition in egg shells (Poole, 1965, 1966). The spectral absorption of porphyrin, like other pigments, owes its origin to conjugated double‐bond chromophore centres within the molecules, but porphyrins are unique in their ability to fluoresce red under UV light (Derrien & Turchini, 1925). Relatively large, polymerised porphyrins in brown egg shells show broad‐spectral absorbance of wavelengths similar to melanins (Hill & McGraw, 2006b), but the aromatic double‐bonded structure that confers wide spectral absorbance also makes them highly photolabile (Moan, 1988; Arakane et al., 1996; Rotomskis, Bagdonas & Streckyte, 1996), causing them to fade over time. Additionally, the linking of these pigments to proteins and metal ions impacts not only the final colour produced but quenches the intense red fluorescence. Notably, although the fluorescent properties of porphyrin‐containing feathers are not conspicuous under normal illumination, transient salmon‐pink fluorescence in hidden, basal barbs of belly feathers has been observed in Otididae, which is proposed to function in short‐duration sexual displays (Galván et al., 2016a).
(5). Spheniscins
Spheniscins are a newly characterised category of endogenously synthesised, yellow‐orange pigments exclusively limited to penguins (Sphenisciformes) and are suggested to have evolved once in fossil stem penguins (Thomas et al., 2013). Chromatography and elemental composition (CHN) analysis highlighted their similarity to yellow and red pterin pigments (see Section II.6), although their solubility indicates crucial differences with other yellow‐red pigments (McGraw et al., 2007). Comparison of Raman and mid‐infrared spectra of spheniscins with other avian pigments reveals 17 unique spectral bands in spheniscins in the wave number (ν) range 300–1700 cm−1 with the five most intense bands at 1577 cm−1 (vs, very small), 1285 cm−1 (s, small), 683 cm−1 (m, medium), 1469 cm−1 (m, medium) and 1351 cm−1 (m, medium). Raman spectra also predict the putative chemical structure of sphensicins to contain aromatically bonded heterocyclic rings with the possibility of tautomeric rearrangement under low pH conditions. However, further work is required to clarify the chemical properties of spheniscins (Thomas et al., 2013).
(6). Other uncommon pigments
Pterins and related pigments (e.g. certain purines and flavins) are by‐products derived from catabolism of the purine nucleotides – adenosine triphosphate and/or guanosine triphosphate (Fig. 3H) (Hill & McGraw, 2006b; Nelson, Lehninger & Cox, 2008). Pterins (Fig. 3E) are nitrogenous heterocyclic compounds with two‐pyrimidine‐ring skeletons which differ in their linear or cyclic substituents and impart characteristic red‐orange‐yellow colours, although colourless variants have also been reported (Fox, 1976). The compounds are frequently encountered in insect wings and eyes (Pfleiderer, 1994). Among vertebrates, pterins have been found in the skin and eyes of some fishes (Grether, Hudon & Endler, 2001), amphibians (Thorsteinsdottir & Frost, 1986), and reptiles (Steffen & McGraw, 2007). In avian species, they have been reported only as ocular colourants and do not occur in integumentary structures (Oliphant & Hudon, 1993). While both purines and pterins absorb very short wavelengths of light (guanine, λmax = 246 and 273 nm; hypoxanthine, λmax = 249 nm), pterins additionally absorb light >300 nm (Needham, 2012).
Flavins (Fig. 3D) are heterocyclic compounds with the basic structure of 7,8‐dimethyl‐10‐alkylisoalloxazine (Rivlin, 2012; Edwards, 2014). The key dietary source of these pigments is vitamin B2 (riboflavin) and its derivatives, flavin mononucleotide (FMN) and flavin adenine dinucleotide (FAD) (Rivlin, 2012). Riboflavin has been described to impart yellow colouration to skin patches in fishes (Courts, 1960) and amphibians (Obika & Negishi, 1972). Riboflavin is sometimes co‐deposited with melanin to produce olive or dark‐green hues in snakes (Blair & Graham, 1954; Villela & Thein, 1967). Pure riboflavin in aqueous solution shows four distinct absorbance peaks (λmax) at 220, 266, 375, and 475 nm but is highly photolabile even under white‐light illumination. This photolability is a result of its fluorescence at longer wavelengths (∼530 nm), which causes the colour to bleach due to breakdown of the chromophore centre even under low energy/long wavelength illumination (Hill & McGraw, 2006b). Riboflavin has also been postulated to influence the colour of bird egg yolks (Gliszczyńska‐Świgło & Koziołowa, 2000), but additional studies are needed.
III. COMBINED STRUCTURAL AND PIGMENTARY MECHANISMS
Structural colour‐producing mechanisms greatly expand the range of vertebrate integumentary colours in isolation as well as in combination with pigmentary colours. In relation to the use of an extant phylogenetic bracket approach to uncovering possible structural colours in dinosaurs, there is ample evidence of structural colour mechanisms in modern birds (Fox, 1976; Prum, 1999; Vinther et al., 2010; McCoy et al., 2018) but little is known in crocodilian scales except for a minor role played by guanine‐crystal‐containing iridophores, which act as reflective platelets to produce extremely weak iridescence. This is due to the fact that reflective platelets occur at low spatial frequencies in the epidermis and dermis and are not arranged in highly ordered vertical arrays, a prerequisite for strong iridescence (Alibardi, 2011). Blue, white, and iridescent shimmering effects in bird feathers are produced by two types of light scattering (coherent and incoherent) caused by nanoscale architecture of the material making up the feathers. Incoherent scattering occurs when light is scattered in different directions by materials lacking an ordered assembly, with wavelengths of different scattered light waves out of phase with each other (Hulst & van de Hulst, 1981; Bohren & Huffman, 2008). Coherent scattering occurs when light waves are scattered by materials with a highly ordered internal structure, and emerge in phase and reinforce each other (Prum et al., 1999; Prum, Andersson & Torres, 2003; Prum & Torres, 2003b). The most common classes of colour‐producing structures in feathers are (i) non‐pigmented keratin with randomly oriented vacuoles, which produce a white colour through incoherent light scattering, (ii) feather barbules with nanostructural arrays of pigment and β‐keratin, which typically produce iridescence through coherent scattering with colour depending on the viewing angle, and (iii) the spongy medullary layer of the barb rami or main feather rachis, which produces non‐iridescent blues, violet, green, and UV colours (Hill & McGraw, 2006b). Recently, a fourth mechanism was discovered whereby 'super‐black' feathers in birds‐of‐paradise (Paradisaeidae) achieve their colour from highly flattened bottlebrush‐like barbules that are devoid of pigments and are extremely effective at trapping light through multiple instances of internal scattering, resulting in directionally mediated structural absorption (McCoy et al., 2018).
Iridescence‐producing melanosome–keratin arrays recognised in 14 out of 32 bird orders can be assembled into either flat laminar nanostructures, crystal‐like lattices with a square or hexagonal unit cell, or irregular to quasi‐ordered arrays (Hill & McGraw, 2006b). The morphology of the melanosomes is closely related to the type of ordering in these repeating arrays. Peafowls possess solid square unit cells of rod‐shaped melanosomes (Zi et al., 2003), whereas trogons possess closely packed hollow melanosomes with hexagonal unit cells (Durrer, 1986). In some species like the scrub euphonia (Euphonia affinis), hollow melanosomes are arranged into uneven quasi‐ordered structures (Hill & McGraw, 2006b). Rod‐shaped melanosomes (solid or hollow) can be organised into structures ranging from exclusively laminar to three‐dimensional lattices to produce iridescent structural colour. However, platelet‐like melanosomes found in hummingbirds can only be packed into laminar sheets to produce iridescence (Greenewalt, Brandt & Friel, 1960). Likewise, the structure of air spaces within these arrangements can range from single cavities to multiple small compartments interspersed between the melanosomes. Thus, laminar and crystal‐like lattices produce strong iridescence, whereas quasi‐ordered structures impart weak or non‐iridescent colours (Hill & McGraw, 2006b).
Non‐iridescent structural colours are usually created in two ways: through modification of the internal structure of feather barbules (Dyck, 1987) or by three‐dimensional quasi‐ordered arrays of box‐shaped medullary cells in feather barbs and rami, filled with a spongy matrix of β‐keratin and air (Dyck, 1971; Prum et al., 1999). In the first category, iridescence is offset through equivalent backscattering at all viewing angles by arranging keratin–melanosome layers along curvatures in specialised rounded ridges on the barbule surface. The architecture of the second category is like Swiss cheese, with larger volumes of air compared to keratin that permit coherent light scattering (Hill & McGraw, 2006a, b ). Melanosomes in the basal pigmentary layer of non‐iridescent feathers although ellipsoidal, are morphologically distinct from those producing black, brown and iridescent colours but overlap significantly with grey‐colour‐producing melanosomes (Babarović et al., 2019).
Structural colour can also occur in avian integumentary tissues other than feathers. Non‐iridescent structurally coloured skin, bill sheaths, caruncles and scales are present in at least 250 bird species. This was first investigated in 19 avian families from 11 orders by Auber (1974) under the assumption that all blue or green colours are structural as opposed to being pigmentary due to the rarity of blue or green pigments in avian integument (Fox, 1976). The anatomy and mechanism of colour production in structurally coloured skin, beaks, and scales was later explored in 31 species of birds (from 17 families and 10 orders ranging from passerines to palaeognaths) (Prum & Torres, 2003a). This study illustrated coherent scattering of light from arrays of parallel collagen fibres in the dermis and that variations in colour can be produced by altering the size and spacing of these fibres. The arrangement of these collagen fibres functionally resembles the rounded quasi‐ordered nanostructures in feather barbules and scatters light waves equivalently in all directions perpendicular to the collagen fibres.
Pigmentary and structural mechanisms imparting integumentary colours in animals have traditionally been studied as separate components. However, mounting evidence indicates that many colours are rendered through combined mechanisms and are difficult to produce by either mechanism in isolation (Dyck, 1971; Shawkey et al., 2009; Stoddard & Prum, 2011; Shawkey & D'Alba, 2017). The complex interplay of pigmentary systems and optical and/or material properties of the integumentary structures are discussed in the following sections.
(1). Pigments and ordered nanostructures
Colour production through the combination of pigments and ordered nanostructures is frequently observed in birds (Noh et al., 2010). Pigmentary green colours are comparatively rare except for turacoverdin, which is limited exclusively to Musophagidae. Since turacoverdin‐based green colours are dependent on large quantities of Cu2+ ions, a trace dietary mineral (Dyck, 1976), shades of green colours in most birds are produced by an interaction of structural and pigmentary mechanisms in one of three ways: (a) coherent scattering in the feather barbules at the interface of highly ordered melanosome–keratin arrays yielding iridescent green (Hill & McGraw, 2006b), (b) interaction between carotenoid pigments in the barbs and melanin‐bearing melanosome layers in the barbules yielding olive‐green (Dyck, 1976), and (c) quasi‐ordered spongy keratin layers containing carotenoid or psittacofulvin yielding non‐iridescent green (Dyck, 1976; Stavenga et al., 2011; Stoddard & Prum, 2011; D'Alba, Kieffer & Shawkey, 2012). Previous hypotheses suggested that blue‐colour‐producing spongy keratin nanostructures combine with yellow pigments to generate the green colours (Hill & McGraw, 2006b). However, new evidence suggests that the keratin layer is also predisposed to producing peak reflectance in the green spectral region by itself, but that the spectral reflectance curve is wide enough to include blue wavelengths (D'Alba et al., 2012; Shawkey & D'Alba, 2017). The pigment molecules absorb these blue wavelengths and significantly augment the saturation of the green colours (Shawkey & D'Alba, 2017). New research also posits that the production of non‐iridescent blue colours has a substantial contribution from the ordered melanosome arrays lying basal to the spongy keratin layer (Parnell et al., 2015). The basal melanosome layer plays a critical role of siphoning away the incoherently scattered white wavelengths (owing to the broad‐based spectral absorption of melanin) which would otherwise greatly diminish the blue hue to impart a more whitish colour (Shawkey & Hill, 2006; Zhang et al., 2015).
(2). Pigments and disordered nanostructures
Pigment molecules, by definition, produce colours by absorbing only certain wavelengths of light and reflecting all others. However, optical/material properties of sheathing biopolymers (e.g. feather keratin, arthropod chitin, or plant cellulose) also impact the wavelengths absorbed/reflected and influence the final colour imparted. Texture and thickness of the encasing material can regulate brightness – a thicker array of keratin with randomly oriented vacuoles will promote greater incoherent scattering, and when combined with deposited pigments (carotenoid/psittacofulvin), can produce a bright yellow/green colour. By contrast, thin arrays of disordered keratinous material combined with pigments produce duller colours. Whether pigments or structural biopolymers have the greatest effect in imparting the final colour is a matter of considerable debate (Shawkey et al., 2006; Jacot et al., 2010; Evans & Sheldon, 2011; Shawkey & D'Alba, 2017).
(3). Dermal chromatophore assemblies
Colour changes also occur through rapid spatial dispersal of pigment molecules, pigment‐containing vesicles, and reflective structures in ectothermic animals (e.g. crustaceans, cephalopods, fishes, amphibians and reptiles). These are controlled by neuroendocrine stimulation of cellular assemblies (Bagnara & Hadley, 1973). Diverse colours and dynamic colour changes result from the interaction between three‐dimensionally organised layers of dermal cells including various types of pigments and reflective nanostructures which are organised into 'dermal chromatophore units' (Bagnara, Taylor & Hadley, 1968). The chromatophore units are composed of three layers of cells. These layers have been recognised in ectothermic vertebrates such as fishes (Fujii, 1993), amphibians (Hadley & Bagnara, 1969; Ichikawa et al., 1998), and reptiles (Taylor & Hadley, 1970; Sherbrooke & Frost, 1989). The most superficial pigmented layer, lying immediately below the epidermis and basal lamella, consists of pterin‐ and/or carotenoid‐bearing xanthophores (yellow‐orange) and erythrophores (red). Lying below the xanthophores and erythrophores is a layer made up of nanoscale reflective platelets (guanine/purine crystals) embedded within iridophores and leucophores (Kuriyama et al., 2006; Boyer & Swierk, 2017). The shape, orientation, and three‐dimensional organisation of the platelets influence the production of structural colours ranging from white to violet via incoherent scattering, coherent scattering, thin‐film interference, and/or diffraction. The innermost layer, called the melanophore layer, contains melanin pigments and produces black/brown colours. The fine‐scale and rapid colour change (i.e. metachrosis), which can yield wide variation in macroscale integumentary patterns, is facilitated by the fine‐tuned co‐localisation and dynamic regulation of the ratios of different pigment‐bearing chromatophore types (Boyer & Swierk, 2017; Shawkey & D'Alba, 2017). Metachrosis is a key mechanism behind predator‐evasion behaviours like camouflage (Cacciali et al., 2018).
IV. DIAGENETIC TRANSFORMATIONS OF PIGMENTS AND STRUCTURAL COLOURS IN FOSSILS
The taphonomy of pigments other than melanin, carotenoids and porphyrin have not yet been studied in detail within fossils. The known diagenetic pathways of pigments and structural colour are discussed below.
(1). Melanins
Most of what is known about the chemistry of eu‐ and phaeomelanin is through alkaline peroxide degradation and hydrolysis (Ito et al., 2011). HPLC and MS have been used to characterise the resulting melanin degradation products from this treatment (Ito & Wakamatsu, 1998). These products have since been used as diagnostic markers for eumelanin and phaeomelanin in modern samples treated with alkaline peroxide degradation and hydrolysis. Pyrrole‐2,3,5‐tricarboxylic acid (PTCA), pyrrole‐2,3,4‐tricarboxylic acid (iso‐PTCA), pyrrole‐2,3,4,5‐tetracarboxylic acid (PTeCA), and pyrrole‐2,3‐dicarboxylic acid (PDCA) are markers for eumelanin. Thiazole‐4,5‐dicarboxylic acid (TDCA), 4‐amino‐3‐hydroxyphenylalanine (4‐AHP), and thiazole‐2,4,5‐tricarboxylic acid (TTCA) have been suggested as markers for phaeomelanin (Pezzella et al., 1997; Ito & Wakamatsu, 1998; Ward et al., 2008; Ito et al., 2011). These markers have also been recovered as products of pyrolytic gas chromatography of fossil samples (Glass et al., 2012). Glass et al. (2013) further suggested that diagenesis and prolonged thermal maturation cause eumelanin subunits to crosslink together, granting exceptional stability through deep time (Fig. 4A). Although the precise diagenetic pathway for phaeomelanin currently remains unknown, Glass et al. (2012) noted that the pyrolysis gas chromatography mass spectra for fossil tissues known to contain eumelanin sometimes additionally indicated the presence of sulphur‐containing molecular fragments (e.g. thiophenes, alkylthiophenes, etc.). These sulphur‐bearing molecular fragments were attributed to the incorporation of sulphur into eumelanin by early diagenetic processes occurring chiefly in euxinic marine palaeoenvironments, resulting in organic preservation through a vulcanisation‐like process of this biopolymer whose natural thermal stability already allows for organic fossilization (McNamara et al., 2016b). McNamara et al. (2016b) showed that pyrite‐rich freshwater palaeoenvironments (e.g. oil shales from Messel, Germany and Libros, Spain) can yield similar sulphur signals in mass spectra of fossil eumelanin. One example of this type of euxinic depositional environment is in the preservation of the nodosaur Borealopelta markmitchelli, which has been suggested to preserve fossil phaeomelanin (Brown et al., 2017). This raises an important question of whether the inference of fossil phaeomelanin chemical markers from euxinic environments could potentially be erroneous (i.e. derived from taphonomic incorporation of environmental sulphur rather than from endogenous phaeomelanin). Colleary et al. (2015), McNamara et al. (2016a) and Brown et al. (2017) however, agree that there is a low chance of conflation of eumelanin and phaeomelanin in fossils from mass spectra data. The potentially environmental thiophene and its derivatives differ chemically from the endogenous nitrogen‐containing markers in phaeomelanin (e.g. 4‐AHP for benzothiazine moieties). Furthermore, melanosome morphology, tissue type, and phylogenetic placement of the specimen could potentially provide independent lines of evidence to distinguish fossil phaeomelanin from fossil eumelanin.
Figure 4.
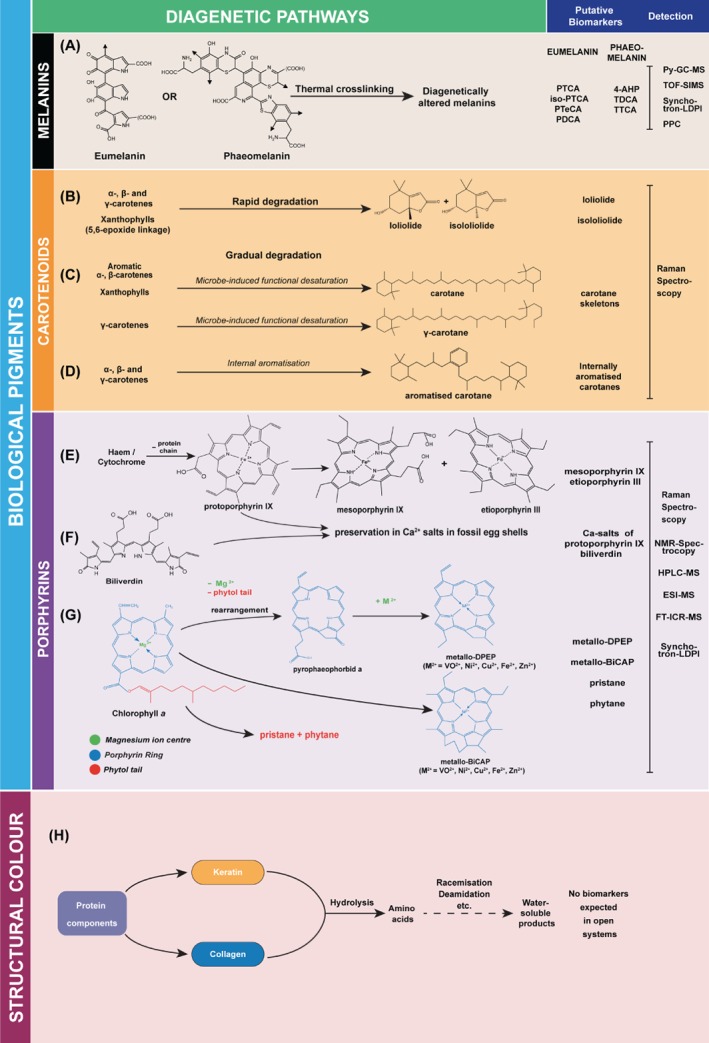
Diagenetic pathways for biological pigments and structural colour‐producing mechanisms along with their potential biomarkers. 4‐AHP, 4‐amino‐3‐hydroxyphenylalanine; ESI‐MS, electrospray ionisation mass spectrometry; FT‐ICR‐MS, Fourier‐transform ion cyclotron resonance mass spectrometry; HPLC‐MS, high performance liquid chromatography; iso‐PTCA, pyrrole‐2,3,4‐tricarboxylic acid; NMR, nuclear magnetic resonance; PDCA, pyrrole‐2,3‐dicarboxylic acid; PPC, peak‐probability contrast; PTCA, pyrrole‐2,3,5‐tricarboxylic acid; PTeCA, pyrrole‐2,3,4,5‐tetracarboxylic acid; Py‐GC‐MS, pyrolysis‐gas chromatography‐mass spectroscopy; synchotron‐LDPI, laser desorption‐ionisation; TDCA, thiazole‐4,5‐dicarboxylic acid; TOF‐SIMS, time of flight secondary‐ion mass spectroscopy; TTCA, thiazole‐2,4,5‐tricarboxylic acid.
Differentiating integumentary melanosomes from those of visceral organs in two‐dimensionally flattened fossils has been suggested to be complicated. Recent work posits that weak post‐burial hydrodynamic disturbances in quiet or stagnated lakebeds can cause visceral organ melanosomes to be redistributed to the integumentary surface (McNamara et al., 2018a). However, two‐dimensional flattening of carcasses has been suggested to have minimal effect on at least the lateral expansion or distortion of non‐biomineralised fossil tissues (Briggs & Williams, 1981), and preliminary experimental taphonomy has suggested this to hold true in the case of avian plumage/carcasses (Saitta, Clapham & Vinther, 2018a) as well as lizard carcasses and beetle exoskeletons (Saitta et al., 2018d). Furthermore, organic preservation of melanin‐containing organs such as the retina and liver can show discrete localisation patterns in fossils without evidence of lateral distortion (Sallan & Coates, 2014; Smithwick et al., 2017). The fact that three‐dimensional ultrastructural melanosome alignment in feathers (Vinther et al., 2010; Li et al., 2012; Vitek et al., 2013) and macroscopic colour patterns (Vinther et al., 2008; Smithwick et al., 2017) can be preserved unaltered in fossils suggests that taphonomic disturbance of melanosome distribution does not occur to any significant degree during carbonaceous fossilisation and compression. Therefore, it is unclear whether melanosome redistribution within a carcass would be a taphonomic factor of great concern. Regardless, sampling from regions of the fossil clearly attributable to integument or integumentary structures would be a simple solution.
(2). Carotenoids
Bacteria, halophilic archaea, photosynthetic eukaryotes, and a host of non‐photosynthetic organisms have been reported to produce carotenoid pigments (Britton, 1995). Fossil carotenoids have been detected in sediments as far back as the Precambrian using gas chromatography‐tandem mass spectrometry (GC–MS–MS) (Marshall & Marshall, 2010; French et al., 2015). Most carotenoids, however, do not survive long‐term diagenesis in forms that are readily linkable to their biological precursors and exact taxonomic origin. During early sedimentary diagenesis, different types of carotenoids vary in stability. Those containing 5,6‐epoxide linkages (i.e. three‐carbon ring with an oxygen atom) rapidly degrade in early diagenesis within anoxic and stagnant lakebed sediments and are converted to loliolide, isololiolide, and other derivatives in aqueous phase (Fig. 4B) (Repeta, 1989). However, there is evidence that some aromatic carotenoids (e.g. β‐carotenes, lutein, zeaxanthin) retain their chemical backbone structures in the form of saturated perhydro derivatives (e.g. carotanes, lycopane) (Fig. 4C). These carotenoids are degraded primarily though microbial oxidation in a much more gradual manner compared to those containing 5,6‐epoxide linkages, as noted from recent anoxic environments (Repeta, 1989). Such carotenoids can potentially become molecular fossils. β‐carotenes can also undergo internal aromatisation reactions to generate several novel products that have also been noted as potential fossil biomarkers (Fig. 4D) (Koopmans, De Leeuw & Damsté, 1997). Recently some advances have been made detecting the molecular remnants of perhydro derivatives using Raman spectroscopy (Marshall & Marshall, 2010). Raman spectroscopy combined with a near‐infrared laser is capable of detecting distinctive absorption bands for unaltered carotenoids in modern feathers placed behind an amber matrix but failed to do so in a fossil feather sample preserved as a carbonaceous compression as well as fossil feather amber inclusions (Thomas et al., 2014). A further test of the efficacy of this technique would be to repeat the analysis with a larger sample size of fossil feathers since the fossils examined could have lacked carotenoids in vivo.
It should be noted that carotenoids are generally acquired through microbial/plant‐based diet in birds and detection of carotenoids in fossils whose matrices contain large amounts of organic microbial/plant matter could potentially lead to erroneous colour prediction, a serious issue when organic melanin preservation often coincides with lagerstätten representing lake or lagoonal palaeoenvironments. Therefore, comparison of the fossil with the surrounding matrix is vital to exclude false positive carotenoid detections. Irrespective of the challenges of detecting fossil carotenoids that are demonstrably endogenous to a fossil rather than environmental, predicting their original hue (i.e. red, orange, or yellow) could be challenging due to changes in conjugation through diagenesis which alter the produced hue. Morphological interpretation of xanthophores (i.e. chromatophores containing carotenoid and/or pterins) has been suggested in a 11.2–8.7‐million‐year‐old fossil snake (McNamara et al., 2016a) but the inorganic, phosphatic preservation of these structures in this specimen is unusual given that such pigments might be expected to preserve organically, warranting further investigation. Experimental demonstration of xanthophore morphology preserved via authigenic mineralization as phosphate would provide strong evidence for such a claim.
(3). Porphyrins
Geoporphyrins (i.e. diagenetically altered porphyrins from various sources) are abundant in sediments, coal, and oil both across the globe and in deep time (Van Berkel, Quirke & Filby, 1989; Huseby & Ocampo, 1997; Junium, Freeman & Arthur, 2015). Two categories of about a hundred geoporphyrins have been described with the first sourced from the diagenesis of haems, cytochromes, and natural tetrapyrroles and the second from the diagenesis of the chlorophylls of phototrophs (Montforts & Glasenapp‐Breiling, 2002). The typical first step in haem/cytochrome diagenesis is the hydrolytic detachment of the porphyrin ring from the protein components followed by a series of reactions leading to the formation of a wide variety of porphyrin derivatives that are either free or complexed with metal ions, including mesoporphyrin IX and etioporphyrin III (Killops & Killops, 2013) (Fig. 4E).
Biological pigments produced in vivo by catabolism of haems, biliverdin, and protoporphyrin, all of which impart colouration to modern bird eggs, have also been detected in fossil eggs ranging from the Miocene‐Holocene subfossil blue‐green eggshells of the upland Moa (Megalapteryx didinus) and pale brown eggs of North Island Moa (Euryapteryx curtus) (Igic et al., 2010) to the blue‐green Late Cretaceous macrooolithiid eggshells attributed to the oviraptorid Heyuannia huangi (Wiemann et al., 2017). Wiemann, Yang & Norell (2018) examined a larger sample of fossilised non‐avialan dinosaur and early bird eggshells and suggested that the evolution of coloured eggshells with macroscale patterns (speckles and spots) can potentially be traced to a single origin at the base of paravians. While protoporphyrin is relatively unreactive due to resonance stabilisation of conjugated double bonds, biliverdin is much more labile due to its linear, oxidised nature (Gorchein, Lim & Cassey, 2009). It has been suggested that formation of calcium salts and entrapment in the eggshell matrix facilitates the preservation of biliverdin (Fig. 4F) and protoporphyrin in these fossils (Wiemann et al., 2017, 2018).
Although not produced in vertebrates, it is of interest to discuss chlorophyll diagenesis as it relates to challenges in studying fossil vertebrate porphyrins due to complications from environmental sources of geoporphyrins. In plants, chlorophyll a (C55H72MgN4O5) quickly undergoes post‐mortem chemical transformation in aquatic sediments and loses its green colour (Fig. 4G). Of the three functional groups of the porphyrin ring that characterise chlorophyll molecules and influence colour production, carboxylic acid and a centrally coordinated metal ion are lost, while the phytol chain can be preserved (albeit hydrolytically cleaved from the porphyrin ring). Post‐separation from the molecule, the phytol chain undergoes reactions of various types in the sediment and forms unsaturated carbon skeletons of the diastereomers phytane and pristane (Killops & Killops, 2013). The porphyrin ring can be preserved through one of two different diagenetic pathways (Fig. 4E–G): (i) a product of one early diagenetic pathway of chlorophyll a is pyrophaeophaeophorhbid a, which ultimately leads to metallodeoxyphylloerythrethioporphyrin (metallo‐DPEP) (Killops & Killops, 2013). The type of metal ion in metallo‐DPEP (e.g., VO2+, Ni2+, Cu2+, Fe2+, Zn2+) inserted into the coordination centre is dependent on the pH, redox potential (Eh), metal availability, and thermal maturity of the sediments (Junium et al., 2015). (ii) Alternatively, phaeophytin (i.e. chlorophyll lacking the Mg2+ ion) undergoes a ring cleavage followed by cyclisation to form bicycloalkanoporphyrines (BiCAPs) with or without metal ions (e.g. VO2+, Ni2+, Cu2+, Fe2+, Zn2+). A major challenge in studying geoporphyrins is the occurrence of highly altered fossil porphyrins in matured kerogen pools that do not always unambiguously indicate their original biological source (Killops & Killops, 2013), thus contamination from surrounding sediments could be difficult to rule out in some fossils suspected to contain integumentary porphyrins.
(4). Structural colour
While the structural colour‐producing mechanisms in feathers have been studied in detail in modern birds (Hill & McGraw, 2006b) and in a limited manner in the feathers of fossil paravians (Vinther et al., 2010; Li et al., 2012; Vitek et al., 2013; Hu et al., 2018), reports on such mechanisms in the skin of non‐avialan dinosaurs or early birds do not exist, leaving crucial gaps in our reconstruction of palaeocolour. This is due to the fact that proteins like keratin and dermal collagen are prone to denaturation, hydrolysis, deamidation, racemisation, thermal degradation and dissolution of certain constituent amino acids through diagenesis (Ortiz et al., 2018) and do not appear readily to fossilise organically (Briggs & Summons, 2014; Saitta et al., 2017; Smithwick et al., 2017) (Fig. 4H). Therefore, inferences regarding structural colouration derived from keratin frameworks and dermal collagen in the fossil record are likely to be difficult. Since keratin does not appear to fossilise organically (Armstrong et al., 1983; Saitta et al., 2017, 2018b), only the pigment components/layers of structural colour‐imparting tissue arrays will be available to identify structural colouration in fossils, as is the case for the few known examples of iridescence in fossils of originally keratinous structures (Vinther et al., 2010; Li et al., 2012; Vitek et al., 2013; Hu et al., 2018).
It has been suggested that keratin protein can preserve phosphatically (McNamara et al., 2018b). However, Saitta et al. (2018b) note that most fossil keratinous structures are easily explained via preservation of endogenous calcium phosphate without needing to invoke taphonomically induced phosphatization. For example, most phosphatically preserved keratinous structures include feather rachises and claw, beak, and osteoderm sheaths (Murphy, Trexler & Thompson, 2006; Christiansen & Tschopp, 2010) – structures known to be calcified in vivo in order to increase the hardness of the keratinous structure (Blakey, Earland & Stell, 1963; Blakey & Lockwood, 1968). Experimental decay experiments capable of inducing microbially mediated phosphatisation of keratin would provide strong support for the idea that keratin protein can be authigenically mineralised and would thereby provide the opportunity to investigate fossil structural colour that involves keratin nanostructural components. However, such support is currently lacking, and it appears that keratin protein loss during fossilisation is the most conservative explanation.
V. EXISTING PALAEOCOLOUR RESEARCH
Colour patterns and organic staining from fossil pigments across broad geographical and temporal ranges have long been recognised in the fossil record by the naked eye (Williams, 1930; Carpenter, 1970; Kříž & Lukeš, 1974; Pan et al., 2013). Pigments have been discovered in phylogenetically diverse fossils preserved as dark carbonaceous remnants, including fossil algae (Wolkenstein, Gross & Falk, 2010), leaves (Rieseberg & Soltis, 1987), cephalopods (Glass et al., 2012; Williams, 2017), trilobites (McRoberts et al., 2013), crinoids, eurypterids, graptolites (Vinther, 2015), insects (McNamara et al., 2013b), stem lampreys like Tullimonstrum (Clements et al., 2016; McCoy et al., 2016), fishes (Gabbott et al., 2016), frogs (Colleary et al., 2015), snakes (McNamara et al., 2016a), marine reptiles (Whitear, 1956; Lindgren et al., 2014), non‐avialan dinosaurs (Li et al., 2010, 2012; Field et al., 2013; Vinther et al., 2016; Peteya et al., 2017; Hu et al., 2018), birds (Vinther et al., 2008, 2010; Gren et al., 2017; Peteya et al., 2017) and mammals (Colleary et al., 2015; Manning et al. 2019). The earliest chemical study on palaeocolour noted the similarity between infrared (IR) spectra of melanin in fossilised and modern cephalopod ink (Beyermann & Hasenmaier, 1973). However, it was the application of electron microscopy to fossils that provided the catalyst for the development of palaeocolour reconstruction by allowing nanometre‐ to micron‐scale objects to be imaged for the first time. Electron micrographs of fossil feathers and hairs from the middle Eocene Messel lagerstätten (Wuttke, 1983) showed large fabrics of micron‐sized rod‐shaped and spherical to sub‐spherical structures, which were originally interpreted as preserved microbial biofilms associated with decay of the original tissue. This microbial interpretation was supported in later work (Davis & Briggs, 1995; Martill & Frey, 1995) until these microbodies were alternatively identified decades later as melanin‐bearing organelles called melanosomes (Vinther et al., 2008; Zhang et al., 2010).
Subsequent research linked colour patterns in isolated fossil feathers to melanosome localisation whereby melanosomes were present in the darker, carbonaceous regions of the fossil but were lacking in intervening non‐stained areas (Vinther et al., 2010; Vitek et al., 2013), paving the way for reconstructions of palaeocolour in non‐avialan dinosaur and early bird integument (Clarke et al., 2010; Li et al., 2010, 2012; Field et al., 2013; Vinther et al., 2016; Peteya et al., 2017; Hu et al., 2018). Despite mounting evidence from their structure, chemistry, and localisation patterns in favour of a melanosome identity for these fossil microbodies, many studies continued to argue in favour of a microbial identification (McNamara et al., 2009; Iniesto et al., 2013; Moyer et al., 2014; Schweitzer, Lindgren & Moyer, 2015), while others suggested the identities of these structures should be determined on a case‐by‐case basis (Lindgren et al., 2012, 2015).
Fossil colour reconstruction has progressed significantly since the early work on isolated feathers (Vinther et al., 2008, 2010; Zhang et al., 2010; Vitek et al., 2013) with palaeocolour reconstructions produced for iconic fossils. A comparative dataset of melanosome morphology (i.e. aspect ratio and size) in 167 modern bird species and use of their shape–colour relationships to predict colour in fossil samples through quadratic discriminant analysis (QDA) has been used on the paravians Anchiornis huxleyi and Microraptor gui (Li et al., 2010, 2012).
Melanosomes sampled from various regions of Anchiornis led to its reconstruction with reddish‐brown head feathers, a grey feathered body, and black‐tipped white feathers along the wings and tail (Li et al., 2010). Saitta et al. (2018c) provided additional details on its feathering and a refined reconstruction sporting fluffy, likely open‐vaned, bifurcated contour feathers. Microraptor, on the other hand was reconstructed to possess iridescent black feathers all over its body (Li et al., 2012). The initial dataset (Li et al., 2010, 2012) has been augmented by the addition of a further 129 modern taxa bearing iridescence‐producing melanosomes (Nordén et al., 2018). However, QDA has its own caveats. Since the distributions of the melanosome morphology variables (aspect ratio, long axis, short axis, etc.) are not Gaussian, parametric predictive modelling like QDA yields limited statistical accuracy (63–73%) and cannot support nominal multistate variables in combination (e.g. melanosome shape and hollow/flat/solid). Multinomial logistic regression (MLR) proposed by Nordén et al. (2018) is much more accurate (yielding a statistical modelling accuracy of 83%) and does not possess the shortcomings of QDA. However, they also acknowledge that the expanded dataset may not be entirely representative of the morphological diversities of melanosomes in 10000+ modern avian species. Improvements to statistical methodology have the potential to revolutionise palaeocolour reconstruction.
Despite robust statistical analysis, a whole‐body reconstruction might still require extensive destructive sampling of the fossil because colour can vary across the body of an organism and specimens are often too large to be viewed whole in an electron microscope. To reduce permanent damage to fossil specimens, palaeontologists are often limited in the extent of their sampling, which may be insufficient to ascribe patterns of melanin‐based hues across the entire body.
Ideally, methods to determine fossil pigmentation should be non‐destructive and quantitative. Through combined use of synchrotron rapid‐scanning X‐ray fluorescence (SRS‐XRF), X‐ray absorption near‐edge structure (XANES) and X‐ray absorption spectroscopy, the preservation of trace metal cations chelated by melanin within fossils has been studied (Wogelius et al., 2011; Manning et al., 2013, 2019). Cu2+/Zn2+ ions can be chelated to melanin during its synthesis and were suggested to be biomarkers mapping the colouration of fossils. However, there is no strong support for (i) trace metal chelation happening uniquely during the synthesis of eumelanin/phaeomelanin versus the synthesis of other biomolecules, and (ii) the ability of these techniques to distinguish ions incorporated during eumelanin synthesis from those taken up secondarily during post‐mortem taphonomic processes. Additionally, in cases where oxidative weathering has removed the carbonaceous fossil melanin from certain regions in the fossil, a lack of Cu2+/Zn2+ associated with the mouldic impressions of melanosomes in sediment could lead to the erroneous conclusion that these regions were unmelanised in vivo. Therefore, Cu2+/Zn2+ does not meet the definition of a biomarker for melanin in that it is not specific to melanin and is not expected to persist when the melanin it is chelated to is lost (Vinther, 2015). Indeed, many organic compounds other than melanin chelate copper ions, including other pigments (e.g. porphyrins) and humic acids from decomposed organic substances (Premović et al., 2000). A widespread presence of Cu2+ has also been found in a 48‐million‐year‐old Dawn Redwood (Metasequoia) leaf (Edwards et al., 2014), which would not have possessed melanin. In a more recent work on a 3‐million‐year‐old fossil mammal (Manning et al., 2019), if the Cu2+/Zn2+ ions were bound to phaeomelanin benzothiazole moieties and organosulphur residues from the diagenesis of keratin, the signal is likely to be of limited use on account of variability and significant peak overlap of the phaeomelanin signal with background remnants of keratin breakdown products, beyond the issue of potential taphonomic incorporation of Cu2+/Zn2+ ions.
Incomplete preservation remains a major hurdle for palaeocolour reconstruction. For example, the single supposedly matte black feather (Carney et al., 2012), whose ascription to Archaeopteryx has been questioned (Kaye et al., 2019b), is clearly not enough confidently to reconstruct the colour pattern of the entire animal. Among the various theropod specimens for which palaeocolour has been reconstructed, many have large patches of missing soft tissue preservation such as the tail feathers of the Anchiornis specimen studied by Li et al. (2010), the leg region of Caudipteryx (Zhang et al., 2010; Li et al., 2014), the neck region of Beipiaosaurus (Zhang et al., 2010), tail portions of Caihong (Hu et al., 2018), and parts of the abdominal region of Sinosauropteryx (Zhang et al., 2010; Smithwick et al., 2017). In some instances, such gaps in carbonaceous staining might not simply have represented white colouration due to absence of melanin considering that other pigments and/or structural mechanisms could have been present but either not preserved (e.g. protein‐based structural colour) or yet to be detected reliably in fossil feathers and scales (e.g. carotenoids or porphyrins) (Table 1). However, certain patterns of carbonaceous soft‐tissue preservation might be very consistent with a truly non‐pigmented, white reconstruction (e.g. countershading or complex repeating patterns such as spots or stripes). Furthermore, much as the osteological completeness of fossil specimens is often less than 100%, incomplete soft tissue preservation could be due to scavenging, physical perturbation, or microbial/autolytic decay of even relatively thermally stable biomolecules. Another consideration is that in non‐avialan dinosaurs with simple feathers (e.g. non‐pennaceous or barbule‐lacking) and presumably higher predation risk due to terrestrial lifestyles, colouration might have been more limited to drab or cryptic melanin‐based colours compared to the diversity of plumage colours seen in modern birds.
Table 1.
Current lines of evidence for palaeocolour reconstruction
| Observational evidence | Examples | Considerations |
|---|---|---|
| Macroscopic carbonaceous stains (Vinther et al., 2008; Zhang et al., 2010; Smithwick et al., 2017) | Colour patterns (e.g., stripes, mottling, bars, 'bandit masks', countershading). | Absence of stains may be due to: (i) early taphonomic processes (e.g. scavenging, decay, physical perturbation); (ii) non‐pigmented integument; (iii) structurally coloured integument that lacks fossilisation potential through diagenesis; (iv) non‐melanin‐based pigmentation; (v) late taphonomic processes (e.g. oxidative weathering of organics). |
| Melanosome morphology and organisation (Vinther et al., 2008, Li et al., 2010, 2012, Zhang et al., 2010) |
Aspect ratio, shape, and size from organic preservation or mouldic impression in sediment (e.g. oblong, oblate, platelet). Internal structure (e.g. solid/hollow). Arrangement of melanosomes relative to each other can reveal structural colouration (e.g. melanosome lattices). |
Melanosome organic structure can be lost through oxidative weathering or through aqueous conditions during thermal maturation/diagenesis.Thermal maturation/diagenesis results in some minor (<10%) shrinkage of melanosomes. Many structural colour arrays involve proteinaceous components that likely do not fossilise. |
| Organic chemistry (Colleary et al., 2015) | Chemical signatures consistent with fossil pigment – precise signature dependent on the pigment and analytical technique used (e.g. secondary ions, pyrolysates, infrared absorption spectra, etc.). | Some sulphur moieties can be derived from phaeomelanin or from taphonomic incorporation of sulphur into eumelanin. Chemical makeup of biomolecules can alter during diagenesis. |
Fossil feather morphologies are critical factors in relation to colour and must be considered in an evolutionary perspective. The organisation of barbs and barbules into the planar vanes of feathers might have permitted the evolution of structural iridescence through nanoscale alignment of reflective keratin–melanosome arrays (Koschowitz, Fischer & Sander, 2014). Coinciding with the evolution of pennaceous feathers in maniraptorans, a pleiotropic increase in melanosome morphological diversity linked to physiological factors such as elevated metabolic rates has been suggested to have greatly expanded the integumentary colour gamut (Li et al., 2014). Prior to this, melanosome morphological diversity was reported to be limited in filamentous integumentary structures of pterosaurs and non‐maniraptoran dinosaurs, which were then hypothesised to have plesiomorphically used spherical to sub‐spherical melanosomes to confer black, brown, or grey colours (Li et al., 2014). Li et al. (2014) also suggest that the lack of diversity in melanosome shapes of non‐maniraptoran archosaurs actually calls into question the attribution of brown colour to filamentous integuments by QDA (Li et al., 2010, 2012) simply on the basis of spherical/sub‐spherical melanosomes. This limited melanosome diversity might be viewed as analogous to the limited diversity seen in modern day ratites (Eliason et al., 2016), although characterising ratites as having low metabolic rates as per the metabolic hypothesis is likely inappropriate when viewed within the broader archosaur phylogeny, and because their flightlessness may have limited colour/melanosome diversity due to predation pressures. However, this metabolic hypothesis of low melanosome morphology (Eliason et al., 2016; Eliason & Clarke, 2018) is difficult to test in extinct animals, and it would be surprising if pterosaurs capable of powered flight had low metabolic rates. Additionally, non‐maniraptoran archosaurs, particularly those with a filamentous integument, are under‐represented compared to maniraptorans in palaeocolour studies, and so their limited melanosome diversity may be influenced by small sample size or terrestrial lifestyles with high predation risk. If melanosome diversity is instead increased to compensate for a lack of alternative colour‐producing mechanisms in dead filamentous tissue as compared to metabolically active skin, then the fact that melanosome diversity is high in more endothermic animals might represent correlation rather than causation since animals with filamentous integument tend towards elevated metabolisms, at least in extant species. Therefore, the shift of colour production from skin to filaments remains the main alternative hypothesis (Vinther, 2015) to the pleiotropic metabolic hypothesis (Li et al., 2014) for the evolutionary drivers of increased melanosome diversity.
Methods predicting palaeocolour using melanosome characteristics might need to account for diagenetic effects on melanosome morphology and chemistry. McNamara et al. (2013a) conducted thermal maturation experiments on feathers, however their study erroneously reported their methodology. Colleary et al. (2015) subjected feathers of different colours to a pressure of 250 bars and temperature regimes of 200°C and 250°C and yielded comparable and quantifiable results. With increasing maturation conditions, melanosomes shrank by no more than 10% in the experiments of Colleary et al. (2015) due to volatile loss and dehydration with potentially minimal effects on aspect ratio. McNamara et al. (2013a) reported as much as 20% shrinkage, but their experimental duration was only 1 h and not 24 h as originally reported (McNamara et al., 2017). Additionally, the chemical signature of the samples of Colleary et al. (2015) as determined by time of flight–secondary ion mass spectrometry (TOF‐SIMS) approached that of fossil melanin samples with increasing maturation conditions and remained distinct from non‐melanin controls. Aqueous maturation conditions, however, can completely obliterate melanosome morphology (possibly those with high phaeomelanin concentration in particular), even though the amorphous pigments might still remain, similar to that seen in some fossils (Colleary et al., 2015). Although fossil melanin is chemically altered from the original molecule (Glass et al., 2013; Colleary et al., 2015) and can also diagenetically incorporate sulphur (McNamara et al., 2016a), melanin is still considered to be relatively stable through diagenesis (Briggs & Summons, 2014), and is therefore capable of being fossilised due to its innate thermal stability. A novel method of sediment‐encased maturation of feathers and lizards (Saitta et al., 2018a) has supported the hypothesis that melanosomes remain exposed on the sediment while surrounding keratin protein is lost during diagenesis (Saitta et al., 2017, 2018d). The method promises a means to test the efficacy of palaeocolour reconstruction. Predictions about the original colour of the feather based on methods used to study fossil feathers could be validated against the known colour of the experimental feather prior to its maturation. It might also be used to determine what chemical signatures would be expected from non‐melanin pigments in fossils and if these pigments can leave behind any macro‐ or microscopic staining or textures. One weakness of the current method, however, is that due to a lack of concurrent compaction during thermal maturation, melanosome organisation is lost, hindering comparisons to fossil feathers with reported structural colouration based on preserved melanosome arrangement (Saitta et al., 2018d).
Palaeocolour reconstructions (see Table 2 for a list of fossil specimens for which colours have been reconstructed, and Fig. 5 for illustrations of key taxa) have been used to infer aspects of extinct organisms' ecology or behaviour, such as sexual displays and camouflage. Iridescent plumage in Microraptor (Li et al., 2012) and Caihong (Hu et al., 2018), rufous head feathers and spangled remiges and coverts of Anchiornis (Li et al., 2010), striped retrices in Caudipteryx (Qiang et al., 1998), melanised rectrices in Jeholornis (O'Connor et al., 2013), elongate ribbon‐like rectrices in Epidexipteryx (Zhang et al., 2008), and possibly sexually dimorphic streamer‐like retrices, spotted wings, and head feathers in Confuciusornis (Zhang et al., 2010; Li et al., 2018), among others, might be consistent with the presence of sexual selection and visual displays, at least among non‐avialan maniraptoran dinosaurs and early birds (Clarke, 2013).
Table 2.
Predictions of palaeocolour in fossil amniotes with gaps in current knowledge
| Specimens | Colour patterns predicted | Gaps in knowledge |
|---|---|---|
| theropod dinosaurs | ||
| Inkayacu (Clarke et al., 2010) | Secondary remiges and an isolated body contour feather were brown, whereas preserved covert and tertials were grey. | Remaining body colour unknown. No chemical data available. |
| Eocypselus (Ksepka et al., 2013) | Feathers on the top of the head were glossy black. | Melanosomes were also found in the wing feathers but have not been analysed or interpreted as any specific colour. No chemical data available. |
| Messelornis (Colleary et al., 2015) | A single feather was found to be iridescent. | Unclear exactly where the sample was taken but appears to have been a wing feather. No chemical data available. |
| Primotrogon (Nordén et al., 2018) | Black, iridescent, and grey colours detected. | Incomplete preservation. Differentiating between covert and body feathers is difficult on both specimens. No chemical data available. |
| Eocoracias (Babarović et al., 2019) | First instance of a fossil bird showing non‐iridescent structural blue colour. | Colour reconstructed using ancestral state reconstruction of non‐iridescent structural blue in Coraciidae. However, the morphology of melanosomes producing this colour overlaps significantly with grey colour producing ones. |
| Scaniacypselsus (Nordén et al., 2018) | Black and grey wings with grey body. | Reconstruction based on combined data from three different individuals. Incomplete preservation in all three fossils leaves gaps in reconstruction. In two sampling points, statistical predictions offer equal probability for black/iridescent and grey/brown colours. No chemical data available. |
| Changzuiornis (Huang et al., 2016) | Preserved wing and tail feathers were black. | Remaining body colour unknown. No chemical data available. |
| Unnamed enantiornithine CUGB P1202 (Peteya et al., 2017) | Feathers of nape, head, and body were iridescent. | Melanosomes were also found in a wing feather but had degraded too much to determine colour based on morphology. No chemical data available. |
| Confuciusornis (Li et al., 2018; Zheng, 2009) | Contour feathers were black all over the body with lighter wings. One specimen (CUGB P1401) had small spots on the wings, coverts, crest, and throat. Cryptic colouration in wing coverts, crest, and throat. | Sexual dimorphism in pair of long tail feathers unclear. No chemical data available. |
| Eoconfuciusornis (Zheng et al., 2017) | Feathers making up the wing coverts, nape, and tail were black. A dark spotted pattern is visible on the secondary remiges. Feathers on the hindlimb and top of the head were grey. Feathers on the throat were brown. | Colour not predicted beyond identifying melanosome morphology. No chemical data available. |
| Archaeopteryx (Carney et al., 2012) | The isolated holotype feather (possibly a covert wing feather) was black. | Remaining body colour unknown. Uncertain if feather belongs to Archaeopteryx (Kaye, Pittman, Mayr et al., 2019b). |
| Anchiornis (Li et al., 2010) | Body contour feathers were dark grey. Forelimb and hindlimb coverts and remiges were white with black tips. Feathers on the top of the head were reddish brown. Flecks of reddish brown were also present on the face. | Colouration of tail feathers unknown, as the specimen examined did not preserve a tail, but other specimens' tails show similar spotting as in the wings. No chemical data available. |
| Caihong (Hu et al., 2018) | Iridescent feathers as in hummingbirds. | Precise hue created by light scattering from the platelet‐like melanosomes cannot be reconstructed because it is determined by the spacing of the photonic nanostructures in vivo as well as the distribution of keratin. |
| Microraptor (Li et al., 2012) | Feathers sampled across the body were iridescent. Most conservative possibility is that they were glossy black. | |
| Sinornithosaurus (Zhang et al., 2010) | Rod and spherical melanosomes detected in different samples, interpreted as black and rufous feather colouration. | Feathers are from pieces of the counterpart with uncertain location. No chemical data available. |
| Caudipteryx (Zhang et al., 2010; Li et al., 2014) | Feathers sampled across the body were black. Tail feathers preserve a visible banding pattern. | Colour not predicted beyond identifying melanosome morphology. No chemical data available. |
| Yi (Xu et al., 2015) | Feathers near the skull contain both oblong (as do feathers near neck, tibia and ulna) and oblate (as do membranous tissue) melanosomes. | Colour not predicted beyond identifying melanosome morphology. No chemical data available. |
| Beipiaosaurus (Li et al., 2014) | Feathers sampled from the neck were brown. | Remaining body colour unknown. No chemical data available. |
| Sinosauropteryx (Smithwick et al., 2010) | Feathers sampled from the tail were reddish brown with intervening non‐melanised bands. More detailed examination of preserved feather distribution across several specimens suggests a counter shaded pattern on the body and a "bandit mask" on the face. | Reconstruction based on combined data from different individuals of different sizes. No chemical data analysed for a taxon that is suggested by some (Eliason, Shawkey & Clarke, 2016; Li et al., 2014) to lack the correlation between melanosome morphology and colour. |
| ornithischian dinosaurs | ||
| Psittacosaurus (Vinther et al., 2016) | Scales sampled across the body were melanised. The face, ankle, ischial region, cloacal region, and some large scales on the shoulder were particularly heavily pigmented. Countershading present. | Colour reconstruction purely based on melanosome morphology. No chemical data available. |
| Borealopelta (Brown et al., 2017) | No melanosomes with preserved structure were found, but chemical signatures suggest high concentrations of phaeomelanin based on benzothiazole detected from TOF‐SIMS interpreted to give a reddish‐brown colouration. Countershading present. | No melanosomes preserved, so the colour predictions are based purely on chemistry. |
| marine reptiles | ||
| Stenopterygius (Lindgren et al., 2018) | Abundant branched melanophores and melanosomes across the flank but conspicuously absent in belly ‐ evidence of dark dorsum and a light ventrum (i.e., countershading). | Skeleton and soft tissue preservation not complete. Parts of snout and regions beyond the pelvis missing. Incomplete model for melanophore preservation mechanism. |
| Platecarpus (Lindgren et al., 2010) | Melanosomes preserved in the eye. Macroscopic colour patterns in preserved scales. | Colour not reconstructed. |
| pterosaurs | ||
| Pterorhynchus (Czerkas & Ji, 2002) | Macroscopic colour patterns noted in the head crest. | Colour not reconstructed. |
| Tupandactylus (Pinheiro et al., 2012) | Swaths of melanosomes preserved in large head crest misinterpreted as fossilised microbial consortia. | Colour not reconstructed. |
| Anurognathid pterosaurs NJU‐57003, CAGS‐Z070 (Yang et al., 2019) | Most likely covered in pycnofibres with various degrees of branching. Melanosomes of diverse morphologies reported. | Brown and black colour in filaments. Colour patterns not reconstructed. |
| mammals | ||
| Palaeochiropteryx (Colleary et al., 2015) | Phaeomelanin signature identified using TOF‐SIMS. Spherical to oblate melanosomes observed in hair filaments. Brown pelage. | Entire body colour not reconstructed. |
| Hassianycteris (Colleary et al., 2015) | Phaeomelanin signature identified using TOF‐SIMS. Spherical to oblate melanosomes observed in hair filaments. Brown pelage. | Entire body colour not reconstructed. |
| Apodemus (Manning et al., 2019) | Phaeomelanin pigment remnants reportedly mapped using Cu2+/Zn2+ ions bound to organosulphur residues through a combination of SRS‐XRF and XAS. Brown pelage. | The use of chelating metal ions is not a reliable method of colour reconstruction. |
Figure 5.

Key fossil taxa used for palaeocolour reconstruction: (A) Caihong juji (Hu et al., 2018), (B) a pristine specimen of Anchiornis huxleyi showing macroscale colour patterns (image credit: Xiaoli Wang), (C) specimen of Anchiornis used for prediction of plumage colour (Li et al., 2010), (D) Eoconfuciusornis zhengi (O'Connor & Claessens, 2005; Pan et al., 2016), (E) Psittacosaurus (Vinther et al., 2016), (F) Eocypselus rowei (Ksepka et al., 2013), (G) Sinosauropteryx prima (Zhang et al., 2010; Smithwick et al., 2017), (H) Microraptor gui (Li et al., 2012), and (I) Borealopelta markmitchelli (Brown et al., 2017). Scale bars: A, 10 cm; B, 10 cm; C, 10 cm; D, 20 mm; E, 20 mm; F, 10 cm; G, 50 cm; H, 10 cm; I, 0.5 m.
Countershading is a form of camouflage in which an animal's body is lighter in the ventral regions and darker in the dorsal regions (Stevens & Merilaita, 2009; Penacchio et al., 2015). This pigmentation pattern counteracts the shadow the body casts when it is illuminated from above to reduce three‐dimensional appearance to a relatively two‐dimensional flattened appearance. This makes the animal more difficult to recognise against its background. Countershading is widespread among animals and is consistent with the ventrally non‐pigmented abdomen in Psittacosaurus (Vinther et al., 2016), Borealopelta (Brown et al., 2017), and Sinosauropteryx (Smithwick et al., 2017). Additionally, a bandit mask, common in modern diurnal birds and mammals possibly to reduce glare in open environments and mask the presence of eyes, has been reported in Sinosauropteryx (Smithwick et al., 2017). One type of analysis of countershading in fossils involves the creation of a uniformly grey three‐dimensional volume‐rendering model, considering soft tissues such as musculature, that is photographed in different natural and laboratory light conditions. The negative images of the illuminated model are then matched against the carbonaceous stains of macroscale pigmentation patterns in the fossil to determine which lighting conditions most closely estimate the countershading pattern of the animal. Animals in open, well‐illuminated habitats normally show sharp and more dorsally situated boundaries between dark and light colours, whereas smoother and ventrally situated boundaries are common among animals in diffusely lit habitats. Such analysis of countershading patterns enables ecological hypotheses for extinct taxa to be proposed: closed, shaded environments for Psittacosaurus (Vinther et al., 2016) versus open, illuminated environments for Sinosauropteryx (Smithwick et al., 2017). Predator–prey interactions have also been predicted by comparing the relationships between body size and extent of countershading in modern animals with those of fossils in order to predict predation pressure (Brown et al., 2017). Once predation pressure is reduced due to the evolution and development of large body size, countershading can be lost, allowing for comparisons of predation pressure on large‐bodied animals across time. However, it must be kept in mind that fully articulated fossils with well‐preserved pigment preservation capable of being reasonably modelled in three dimensions are the exception rather than the norm.
VI. DISCUSSION
Under the framework presented herein (Fig. 6), the fossil specimen is first identified and placed within a phylogenetic framework, followed by macroscopic examination of carbonaceous stains and visible colour patterns in the integument (e.g. stripes, spots, splotches, spangles, etc.) in both the part and counterpart, if present. Since, the macrostructure of feathers (pennaceous versus plumulaceous vane, branching patterns, shapes of barbs and barbules) can be critical to colour production, particularly structural colour, a range of imaging is recommended to visualise their preserved morphology as far as possible. For example, UV imaging can clarify integumentary details and sometimes reveal hidden information (Frey et al., 2003; Tischlinger, 2005; Chiappe & Göhlich, 2010; Hone et al., 2010; Kellner et al., 2010; Rauhut et al., 2012; Tischlinger & Arratia, 2013; Foth, Tischlinger & Rauhut, 2014). Laser stimulated fluorescence (LSF) is a next‐generation fluorescent imaging method that offers substantial benefits in these areas (Mayr et al., 2002; Kaye et al., 2015, 2019a, b ; Falk et al., 2016; Vinther et al., 2016; Wang et al., 2017; Yang et al., 2019). The utility of these fluorescent imaging methods appears to stem from the preservation of soft tissues like patagial membranes, skin, scales, or keratinous sheaths via endogenous phosphates (Murphy et al., 2006; Christiansen & Tschopp, 2010). Although fossil melanin does not fluoresce, fluorescing compounds in the sediment matrix can often backlight the fossil to reveal the morphology of organically preserved integument (Kaye et al., 2015; Wang et al., 2017). For feathers, unless the rachis/calamus is calcified, then it is likely that it will not fluoresce and will have to be visualised through backlighting. LSF can also help identify preservational gaps within the fossil, to provide an estimate for the loss of information due to partial decay and disarticulation (e.g. Anchiornis: BMNHC PH828 and Yi: STM 31‐2), as well as any artificial reconstructions added to the fossil (Mateus, Overbeeke & Rita, 2008; Kaye et al., 2015). While several types of pigment biomolecules (e.g. porphyrins) can fluoresce, their preservation potential is either uncertain or their biomarkers cannot be reliably linked to their original sources and distinguished from environmental sources.
Figure 6.
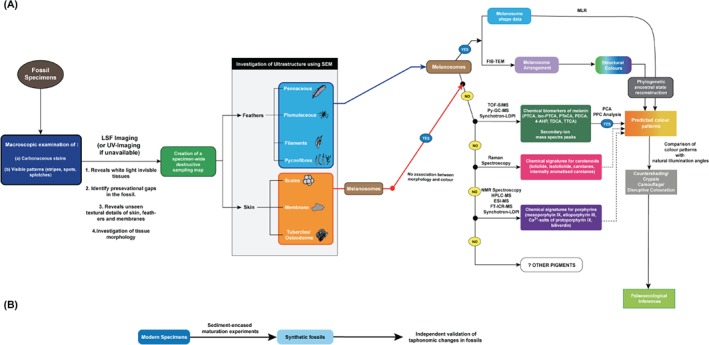
(A) Proposed holistic schematic framework for the reconstruction of fossil colour. Solid lines indicate confirmed steps; dashed lines indicate potentially useful, but yet untested, steps in palaeocolour reconstruction. (B) Sediment‐encased maturation can be used to experimentally validate taphonomic changes in fossils. ESI‐MS, electrospray ionisation mass spectrometry; FIB‐TEM, focused ion beam‐transmission electron microscopy; FT‐ICR‐MS, Fourier‐transform ion cyclotron resonance mass spectrometry; HPLC‐MS, high performance liquid chromatography; LSF, laser stimulated fluorescence; MLR, multinomial logistic regression; NMR, nuclear magnetic resonance; PCA, principal components analysis; PPC, peak probability contrast; Py‐GC‐MS, pyrolysis‐gas chromatography‐mass spectroscopy; synchotron‐LDPI, laser desorption‐ionisation; TDCA, thiazole‐4,5‐dicarboxylic acid; TOF‐SIMS, time of flight secondary‐ion mass spectroscopy; UV, ultra‐violet.
Once both white‐light visible and white‐light invisible tissues have been identified, the next step would ideally be to create a detailed map that encompasses all identified integumentary tissue types and macroscopic colour patterns for destructive sampling. While destructive sampling is carried out by flaking off small regions of organic stains, care must be taken to acquire the least material that is necessary. Following this, samples can be coated with gold/silver for ultrastructural analysis using scanning electron microscopy (SEM) to determine melanosome morphology and organisation. Additionally, if the fossil specimen is small enough to be placed into the SEM chamber, it can imaged uncoated under variable pressure conditions (VP‐SEM) (Vinther et al., 2008; Zhang et al., 2010). If melanosome morphology is preserved (i.e. not taphonomically altered into amorphous fossil melanin) and appears minimally undistorted by diagenesis as evidenced, for example, by organic melanosomes residing inside mouldic impressions with minimal shrinkage, then the melanosome shape data can be compared to that of modern species using QDA or MLR (Nordén et al., 2018). If melanosomes seem to be arranged in ordered nanostructural arrays, then sections perpendicular to barb/barbule long axes made using focused ion beam‐transmission electron microscopy (FIB‐TEM) can at least partially reveal the nature of the repeating units in three‐dimensional space (Hu et al., 2018).
Even if the melanosome morphology is obliterated or rendered unusable by diagenesis, melanin chemistry can still be exploited to identify the diagenetically altered melanin and ascertain the type of melanin pigment. Different variants of mass spectroscopy techniques such as synchotron‐LDPI (Liu et al., 2014), TOF‐SIMS (Colleary et al., 2015), or peroxidation followed by pyrolysis‐gas chromatography‐mass spectroscopy (Py‐GC‐MS) (Glass et al., 2012, 2013) can be used to detect chemical biomarkers of melanin. Melanin‐based colours can be estimated through comparison with modern bird feathers through statistical methods such as peak probability contrast (PPC) (Liu et al., 2014), which identifies the most important melanin peaks for identifying colour in a training database of modern birds and predicts the colour in fossil samples based on the presence/absence of those peaks. One point to note is that the morphology of melanosomes which produce non‐iridescent structural colours is not readily distinguishable from those of grey‐colour‐imparting ones and this has important bearings on palaeocolour reconstruction (Babarović et al., 2019). In these cases, simple statistical classifications or chemical analysis may not be sufficient to pin‐point exact colours in fossils. Phylogenetic ancestral state reconstruction has shown much promise in adequately distinguishing between grey and non‐iridescent‐colour‐producing melanosomes in fossil birds (Babarović et al., 2019).
Although pigments other than melanin have yet to be fully utilised in vertebrate palaeocolour reconstructions, we present some possible analytical techniques here. Carotenoid biomarkers (e.g. loliolide, isololiolide and carotanes) can be probed for by Raman spectroscopy. Porphyrin biomarkers (e.g. metallo‐DPEP and metallo‐BiCAP) can be detected with nuclear magnetic resonance (NMR) spectroscopy, HPLC‐MS, electrospray ionisation mass spectrometry (ESI‐MS), Fourier‐transform ion cyclotron resonance mass spectrometry (FT‐ICR‐MS), or synchotron‐LDPI (Mironov et al., 2017; Zheng et al., 2018). However, caution is urged in linking these biomarkers with carotenoids or porphyrins in carbonaceous fossils since they might be derived from exogenous, environmental sources.
A systematic understanding of the molecular changes that occur through pigment diagenesis will help to guide a search for chemical signatures in fossils, and so researchers attempting to reconstruct palaeocolour may wish to validate their conclusions under experimental frameworks. Experimental taphonomy, particularly thermal maturation, should help to identify chemical and morphological signatures of fossil melanin and other pigments and might lead to the possibility of using pigments other than melanin for palaeocolour reconstruction (Colleary et al., 2015; Saitta et al., 2017, 2018d).
VII. CONCLUSIONS
(1) There is currently no single formalised methodological framework that can be referenced for all cases of fossil colour reconstruction. Additionally, limitations on the preservation of biological tissues, sampling, and diagenesis should be accounted for. For example, information loss in fossilised feathers due to incomplete preservation of structural colour‐imparting keratin protein arrays or uncertainties in the preservation/identification of other fossil pigments should be borne in mind as combined pigmentary and structural mechanisms may have produced colour blends in extinct taxa that are undetectable in fossils.
(2) The correlation between melanosome morphology and the chemistry of the melanin pigment within, and therefore the hue produced, does not persist in tissues other than feathers and hair (i.e. non‐filamentous integument) (Vinther, 2015). Other workers have gone further by suggesting that the relationship does not persist in some non‐maniraptoran dinosaurs based on the hypothesis that melanosome morphology is dependent on pleiotropic gene relationships with other metabolic processes (Kellner et al., 2010; Li et al., 2014), but this hypothesis is currently untestable in extinct organisms.
(3) Given that colour patterns, such as camouflage and countershading, are now being used to inform ecological aspects such as habitat preference and predator–prey interactions (Vinther et al., 2016; Brown et al., 2017; Smithwick et al., 2017), it has become especially important to discuss thoroughly the nuances of palaeocolour reconstruction. Herein, we synthesised different methodologies to provide a thorough framework to recognise melanin and fossilisable structural colour‐based patterns in fossils, especially among amniotes.
(4) Only once colour patterns have been reconstructed can predictions be made regarding the visual ecology and behaviour of ancient animals, such as signalling, camouflage, habitat preference, or predator–prey interactions. Strong caution has been advised by Negro, Finlayson & Galván (2018) in inferring colour patterns and predicting life‐history traits in fossils. While some of their criticisms are quite valid, it should be kept in mind that there are numerous limitations commonly faced by palaeontologists, such as an incomplete fossil record, poor preservation, destructive sampling techniques, and the inability to conduct observational or experimental behavioural or ecological studies on extinct organisms, all of which will hinder palaeocolour reconstruction efforts. Although biases should be recognised and accounted for, these difficulties should not preclude attempts by researchers to conduct objective palaeobiological investigations to the best of their abilities, thus making the field of palaeocolour reconstruction an exciting and stimulating endeavour.
(5) This review provides a framework incorporating a comprehensive battery of tests informed by taphonomy that can serve to guide palaeocolour reconstruction, especially among amniotes.
VIII. ACKNOWLEDGEMENTS
We would like to thank the attendees of the International Pennaraptoran Dinosaur Symposium (2018) hosted at the University of Hong Kong for the helpful discussion and feedback following the talk presented by A.R. Their feedback greatly improved the quality of this article. A.R. is supported by the Hong Kong PhD Fellowship (HKPF PF16‐09281). This study was also supported by the Faculty of Science of the University of Hong Kong and the HKU MOOC course Dinosaur Ecosystems (both to M.P.).
Contributor Information
Arindam Roy, Email: arindam.roy@connect.hku.hk.
Michael Pittman, Email: mpittman@hku.hk.
IX. REFERENCES
- Alibardi, L. (2011). Histology, ultrastructure, and pigmentation in the horny scales of growing crocodilians. Acta Zoologica 92, 187–200. [Google Scholar]
- Arakane, K. , Ryu, A. , Hayashi, C. , Masunaga, T. , Shinmoto, K. , Mashiko, S. , Nagano, T. & Hirobe, M. (1996). Singlet oxygen (1Δg) generation from coproporphyrin in Propionibacterium acneson irradiation. Biochemical and Biophysical Research Communications 223, 578–582. [DOI] [PubMed] [Google Scholar]
- Armstrong, W. , Halstead, L. , Reed, F. & Wood, L. (1983). Fossil proteins in vertebrate calcified tissues. Philosophical Transactions of the Royal Society of London. B: Biological Sciences 301, 301–343. [Google Scholar]
- Auber, L. (1974). Formation of 'polyhedral'cell cavities in cloudy media of bird feathers. Proceedings of the Royal Society of Edinburgh, Section B: Biological Sciences 74, 27–41. [Google Scholar]
- Babarović, F. , Puttick, M. N. , Zaher, M. , Learmonth, E. , Gallimore, E. J. , Smithwick, F. M. , Mayr, G. & Vinther, J. (2019). Characterization of melanosomes involved in the production of non‐iridescent structural feather colours and their detection in the fossil record. Journal of the Royal Society Interface 16, 20180921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagnara, J. T. & Hadley, M. E. (1973). Chromatophores and Color Change. Prentice Hall, New Jersey. [Google Scholar]
- Bagnara, J. T. , Taylor, J. D. & Hadley, M. E. (1968). The dermal chromatophore unit. The Journal of Cell Biology 38, 67–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett, P. M. , Evans, D. C. & Campione, N. E. (2015). Evolution of dinosaur epidermal structures. Biology Letters 11, 20150229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell, A. A. & Wheeler, M. H. (1986). Biosynthesis and functions of fungal melanins. Annual Review of Phytopathology 24, 411–451. [Google Scholar]
- Bell, P. R. , Campione, N. E. , Persons, W. S. T. , Currie, P. J. , Larson, P. L. , Tanke, D. H. & Bakker, R. T. (2017). Tyrannosauroid integument reveals conflicting patterns of gigantism and feather evolution. Biology Letters 13, 20170092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berzelius, J. J. (1840). Lehrbuch der Chemie, Fourth Edition. Arnoldische Buchhandlung, Dresden und Leipzig. [Google Scholar]
- Beyermann, K. & Hasenmaier, D. (1973). Identifizierung 180 Millionen Jahre alten, wahrscheinlich unverändert erhaltenen Melanins. Fresenius' Zeitschrift für Analytische Chemie 266, 202–205. [Google Scholar]
- Blair, J. & Graham, J. (1954). The pigments of snake skins. 1. The isolation of riboflavin as a pigment of the skins of the green snakes Philothamnus semivariegatus and Dispholidus typus . Biochemical Journal 56, 286–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakey, P. & Lockwood, P. (1968). The environment of calcified components in keratins. Calcified Tissue Research 2, 361–369. [DOI] [PubMed] [Google Scholar]
- Blakey, P. , Earland, C. & Stell, J. (1963). Calcification of keratin. Nature 198, 481. [Google Scholar]
- Bohren, C. F. & Huffman, D. R. (2008). Absorption and Scattering of Light by Small Particles. Wiley‐VCH Verlag GmbH & Co. KGaA, Weinheim. [Google Scholar]
- Bolívar‐Cimé, B. , Clarke, F. M. & Racey, P. A. (2012). Transient yellow colouration of the bat Artibeus jamaicensis coincides with pollen consumption. Mammalian Biology‐Zeitschrift für Säugetierkunde 77, 221–223. [Google Scholar]
- Bonaparte, J. F. , Novas, F. E. & Coria, R. A. (1990). Carnotaurus sastrei Bonaparte, the Horned, Lightly Built Carnosaur from the Middle Cretaceous of Patagonia. Contributions in Science, Natural History Museum of Los Angeles County, California. [Google Scholar]
- Borovansky, J. & Riley, P. A. (2011). Melanins and Melanosomes: Biosynthesis, Structure, Physiological and Pathological Functions. Wiley‐VCH Verlag & Co. KGaA, Weinheim. [Google Scholar]
- Boyer, J. F. F. & Swierk, L. (2017). Rapid body color brightening is associated with exposure to a stressor in an Anolis lizard. Canadian Journal of Zoology 95, 213–219. [Google Scholar]
- Brejcha, J. & Kleisner, K. (2016). Turtles are not just walking stones: conspicuous coloration and sexual selection in freshwater turtles. Biosemiotics 9, 247–266. [Google Scholar]
- Briggs, D. E. & Summons, R. E. (2014). Ancient biomolecules: their origins, fossilization, and role in revealing the history of life. BioEssays 36, 482–490. [DOI] [PubMed] [Google Scholar]
- Briggs, D. E. G. & Williams, S. H. (1981). The restoration of flattened fossils. Lethaia 14, 157–164. [Google Scholar]
- Britton, G. J. C. (1995). Carotenoids today and challenges for the future. In Carotenoids, vol. 1A (Eds. George T. B., Pfander S., Liaaen‐Jensen H., Pfander H.), pp. 13–26, Birkhäuser Verlag. Basel, Boston, Berlin.
- Brockmann, H. & Völker, O. (1934). Der gelbe federfarbstoff des kanarienvogels [Serinus canaria canaria (L.)] und das vorkommen von carotinoiden bei vögeln. Hoppe‐Seyler´'s Zeitschrift für Physiologische . Chemie 224, 193–215. [Google Scholar]
- Brown, C. M. , Henderson, D. M. , Vinther, J. , Fletcher, I. , Sistiaga, A. , Herrera, J. & Summons, R. E. (2017). An exceptionally preserved three‐dimensional armored dinosaur reveals insights into coloration and Cretaceous predator‐prey dynamics. Current Biology 27, 2514–2521 e3. [DOI] [PubMed] [Google Scholar]
- Burley, N. , Krantzberg, G. & Radman, P. (1982). Influence of colour‐banding on the conspecific preferences of zebra finches. Animal Behaviour 30, 444–455. [Google Scholar]
- Butcher, G. S. & Rohwer, S. (1989). The evolution of conspicuous and distinctive coloration for communication in birds In Current Ornithology, vol. 6 (Ed. Power D. M.), pp. 51–108, Springer–Verlag, New York City. [Google Scholar]
- Cacciali, P. , Lotzkat, S. , Gamble, T. & Köhler, G. (2018). Cryptic diversity in the Neotropical gecko genus Phyllopezus Peters, 1878 (Reptilia: Squamata: Phyllodactylidae): a new species from Paraguay. International Journal of Zoology 2018, 3958327. [Google Scholar]
- Cal, L. , Suarez‐Bregua, P. , Cerdá‐Reverter, J. M. , Braasch, I. & Rotllant, J. (2017). Fish pigmentation and the melanocortin system. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology 211, 26–33. [DOI] [PubMed] [Google Scholar]
- Carney, R. M. , Vinther, J. , Shawkey, M. D. , D'Alba, L. & Ackermann, J. (2012). New evidence on the colour and nature of the isolated Archaeopteryx feather. Nature Communications 3, 637. [DOI] [PubMed] [Google Scholar]
- Carpenter, F. M. (1970). Fossil insects from New Mexico. Psyche 77, 400–412. [Google Scholar]
- Carstam, R. , Brinck, C. , Hindemith‐Augustsson, A. , Rorsman, H. & Rosengren, E. (1991). The neuromelanin of the human substantia nigra. Biochimica et Biophysica Acta (BBA) ‐ Molecular Basis of Disease 1097, 152–160. [DOI] [PubMed] [Google Scholar]
- Chiappe, L. M. & Göhlich, U. B. (2010). Anatomy of Juravenator starki (Theropoda: Coelurosauria) from the Late Jurassic of Germany. Neues Jahrbuch für Geologie und Paläontologie‐Abhandlungen 258, 257–296. [Google Scholar]
- Christiansen, N. A. & Tschopp, E. (2010). Exceptional stegosaur integument impressions from the Upper Jurassic Morrison Formation of Wyoming. Swiss Journal of Geosciences 103, 163–171. [Google Scholar]
- Clarke, J. (2013). Feathers before flight. Science 340, 690–692. [DOI] [PubMed] [Google Scholar]
- Clarke, J. A. , Ksepka, D. T. , Salas‐Gismondi, R. , Altamirano, A. J. , Shawkey, M. D. , D'Alba, L. , Vinther, J. , DeVries, T. J. & Baby, P. (2010). Fossil evidence for evolution of the shape and color of penguin feathers. Science 330, 954–957. [DOI] [PubMed] [Google Scholar]
- Clements, T. , Dolocan, A. , Martin, P. , Purnell, M. A. , Vinther, J. & Gabbott, S. E. (2016). The eyes of Tullimonstrum reveal a vertebrate affinity. Nature 532, 500–503. [DOI] [PubMed] [Google Scholar]
- Colleary, C. , Dolocan, A. , Gardner, J. , Singh, S. , Wuttke, M. , Rabenstein, R. , Habersetzer, J. , Schaal, S. , Feseha, M. , Clemens, M. , Jacobs, B. F. , Currano, E. D. , Jacobs, L. L. , Sylvestersen, R. L. , Gabbott, S. E. & Vinther, J. (2015). Chemical, experimental, and morphological evidence for diagenetically altered melanin in exceptionally preserved fossils. Proceedings of the National Academy of Sciences of the United States of America 112, 12592–12597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coria, R. A. & Chiappe, L. M. (2007). Embryonic skin from Late Cretaceous sauropods (Dinosauria) of Auca Mahuevo, Patagonia, Argentina. Journal of Paleontology 81, 1528–1532. [Google Scholar]
- Coria, R. A. & Currie, P. J. (2006). A new carcharodontosaurid (Dinosauria, Theropoda) from the Upper Cretaceous of Argentina. Geodiversitas 28, 71–118. [Google Scholar]
- Courts, A. (1960). Occurrence of riboflavin in sturgeon skin. Nature 185, 463–464. [Google Scholar]
- Cuervo, J. J. , Belliure, J. & Negro, J. J. (2016). Coloration reflects skin pterin concentration in a red‐tailed lizard. Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology 193, 17–24. [DOI] [PubMed] [Google Scholar]
- Cuthill, I. C. , Allen, W. L. , Arbuckle, K. , Caspers, B. , Chaplin, G. , Hauber, M. E. , Hill, G. E. , Jablonski, N. G. , Jiggins, C. D. & Kelber, A. (2017). The biology of color. Science 357, eaan0221. [DOI] [PubMed] [Google Scholar]
- Czeczuga, B. (1980). Investigations on carotenoids in Amphibia—II. Carotenoids occurring in various parts of the body of certain species. Comparative Biochemistry and Physiology Part B: Comparative Biochemistry 65, 623–630. [Google Scholar]
- Czerkas, S. A. & Ji, Q. (2002). A new rhamphorhynchoid with a headcrest and complex integumentary structures In Feathered Dinosaurs and the Origin of Flight, vol. 1 (ed. Czerkas S.), pp. 15–41. The Dinosaur Museum, Blanding. [Google Scholar]
- D'Alba, L. , Kieffer, L. & Shawkey, M. D. (2012). Relative contributions of pigments and biophotonic nanostructures to natural color production: a case study in budgerigar (Melopsittacus undulatus) feathers. Journal of Experimental Biology 215, 1272–1277. [DOI] [PubMed] [Google Scholar]
- d'Ischia, M. , Wakamatsu, K. , Napolitano, A. , Briganti, S. , Garcia‐Borron, J. C. , Kovacs, D. , Meredith, P. , Pezzella, A. , Picardo, M. & Sarna, T. (2013). Melanins and melanogenesis: methods, standards, protocols. Pigment Cell & Melanoma Research 26, 616–633. [DOI] [PubMed] [Google Scholar]
- Davis, P. G. & Briggs, D. E. (1995). Fossilization of feathers. Geology 23, 783–786. [Google Scholar]
- Derrien, E. & Turchini, J. (1925). Nouvelles observations de fluorescences rouges chez les animaux. Comptes Rendus des Séances de la Société de Biologie 92, 1030–1031. [Google Scholar]
- Dhouailly, D. (2009). A new scenario for the evolutionary origin of hair, feather, and avian scales. Journal of Anatomy 214, 587–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhouailly, D. , Godefroit, P. , Martin, T. , Nonchev, S. , Caraguel, F. & Oftedal, O. (2019). Getting to the root of scales, feather and hair: as deep as odontodes? Experimental Dermatology 28, 503–508. [DOI] [PubMed] [Google Scholar]
- Durrer, H. (1986). Colouration In Biology of the Integument (Eds. Bereiter‐Hahn J., Matoltsy A. G., Richards K. S.), pp. 239–247. Springer, Berlin, Heidelberg. [Google Scholar]
- Dyck, J. (1971). Structure and spectral reflectance of green and blue feathers of the rose‐faced lovebird (Agapornis roseicollis). Biologiske Skrifter 18, 1–67, Munksgaard. [Google Scholar]
- Dyck, J. (1976). Structural colours. Proceedings of the International Ornithological Congress 16, 426–437. [Google Scholar]
- Dyck, J. (1987). Structure and Light Reflection of Green Feathers of Fruit Doves (Ptilinopus spp.) and an Imperial Pigeon (Ducula concinna). Biologiske Skrifter 30, 5–43, Munksgaard, Copenhagen, Denmark. [Google Scholar]
- Edwards, A. M. (2014). Structure and general properties of flavins In Flavins and Flavoproteins‐ Methods and Protocols, (Eds Weber S., Schleicher E.), pp. 3–13. Springer, New York. [DOI] [PubMed] [Google Scholar]
- Edwards, N. , Manning, P. , Bergmann, U. , Larson, P. , van Dongen, B. , Sellers, W. , Webb, S. , Sokaras, D. , Alonso‐Mori, R. & Ignatyev, K. (2014). Leaf metallome preserved over 50 million years. Metallomics 6, 774–782. [DOI] [PubMed] [Google Scholar]
- Eizirik, E. , Yuhki, N. , Johnson, W. E. , Menotti‐Raymond, M. , Hannah, S. S. & O'Brien, S. J. (2003). Molecular genetics and evolution of melanism in the cat family. Current Biology 13, 448–453. [DOI] [PubMed] [Google Scholar]
- Eliason, C. M. & Clarke, J. A. (2018). Metabolic physiology explains macroevolutionary trends in the melanic colour system across amniotes. Proceedings of the Royal Society B 285, 20182014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliason, C. M. , Shawkey, M. D. & Clarke, J. A. (2016). Evolutionary shifts in the melanin‐based color system of birds. Evolution 70, 445–455. [DOI] [PubMed] [Google Scholar]
- Evans, S. R. & Sheldon, B. C. (2011). Quantitative genetics of a carotenoid‐based color: heritability and persistent natal environmental effects in the great tit. The American Naturalist 179, 79–94. [DOI] [PubMed] [Google Scholar]
- Falk, A. R. , Kaye, T. G. , Zhou, Z. & Burnham, D. A. (2016). Laser fluorescence illuminates the soft tissue and life habits of the Early Cretaceous bird Confuciusornis. PLoS One 11, e0167284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field, D. J. , D'Alba, L. , Vinther, J. , Webb, S. M. , Gearty, W. & Shawkey, M. D. (2013). Melanin concentration gradients in modern and fossil feathers. PLoS One 8, e59451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finar, I. L. (1956). Organic Chemistry, Volume 2: Stereochemistry and the Chemistry of Natural Products. Journal of Chemical Education Longmans, Edinburgh. [Google Scholar]
- Fitze, P. S. , Cote, J. , San‐Jose, L. M. , Meylan, S. , Isaksson, C. , Andersson, S. , Rossi, J.‐M. & Clobert, J. (2009). Carotenoid‐based colours reflect the stress response in the common lizard. PLoS One 4, e5111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontanesi, L. , Tazzoli, M. , Beretti, F. & Russo, V. (2006). Mutations in the melanocortin 1 receptor (MC1R) gene are associated with coat colours in the domestic rabbit (Oryctolagus cuniculus). Animal Genetics 37, 489–493. [DOI] [PubMed] [Google Scholar]
- Foth, C. , Tischlinger, H. & Rauhut, O. W. (2014). New specimen of Archaeopteryx provides insights into the evolution of pennaceous feathers. Nature 511, 79–82. [DOI] [PubMed] [Google Scholar]
- Fox, D. L. (1976). Animal Biochromes and Structural Colours: Physical, Chemical, Distributional & Physiological Features of Coloured Bodies in the Animal World. University of California Press, Oakland. [Google Scholar]
- French, K. , Rocher, D. , Zumberge, J. & Summons, R. G. (2015). Assessing the distribution of sedimentary C40 carotenoids through time. Geobiology 13, 139–151. [DOI] [PubMed] [Google Scholar]
- Frey, E. , Tischlinger, H. , Buchy, M. C. & Martill, D. M. (2003). New specimens of Pterosauria (Reptilia) with soft parts with implications for pterosaurian anatomy and locomotion. Geological Society, London, Special Publications 217, 233–266. [Google Scholar]
- Fujii, R. (1993). Cytophysiology of fish chromatophores In International Review of Cytology, vol. 143 (Eds Jean K. W., Friedlander M., Jarvik J.), pp. 191–255. Elsevier, Amsterdam. [Google Scholar]
- Gabbott, S. E. , Donoghue, P. C. , Sansom, R. S. , Vinther, J. , Dolocan, A. & Purnell, M. A. (2016). Pigmented anatomy in Carboniferous cyclostomes and the evolution of the vertebrate eye. Proceedings of the Royal Society of London B: Biological Sciences 283, 20161151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galván, I. & Wakamatsu, K. (2016). Color measurement of the animal integument predicts the content of specific melanin forms. RSC Advances 6, 79135–79142. [Google Scholar]
- Galván, I. , Camarero, P. R. , Mateo, R. & Negro, J. J. (2016a). Porphyrins produce uniquely ephemeral animal colouration: a possible signal of virginity. Scientific Reports 6, 39210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galván, I. , Garrido‐Fernández, J. , Ríos, J. , Pérez‐Gálvez, A. , Rodríguez‐Herrera, B. & Negro, J. J. (2016b). Tropical bat as mammalian model for skin carotenoid metabolism. Proceedings of the National Academy of Sciences of the United States of America 113, 10932–10937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass, K. , Ito, S. , Wilby, P. R. , Sota, T. , Nakamura, A. , Bowers, C. R. , Vinther, J. , Dutta, S. , Summons, R. , Briggs, D. E. , Wakamatsu, K. & Simon, J. D. (2012). Direct chemical evidence for eumelanin pigment from the Jurassic period. Proceedings of the National Academy of Sciences of the United States of America 109, 10218–10223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass, K. , Ito, S. , Wilby, P. R. , Sota, T. , Nakamura, A. , Bowers, C. R. , Miller, K. E. , Dutta, S. , Summons, R. E. , Briggs, D. E. G. , Wakamatsu, K. & Simon, J. D. (2013). Impact of diagenesis and maturation on the survival of eumelanin in the fossil record. Organic Geochemistry 64, 29–37. [Google Scholar]
- Gliszczyńska‐Świgło, A. & Koziołowa, A. (2000). Chromatographic determination of riboflavin and its derivatives in food. Journal of Chromatography A 881, 285–297. [DOI] [PubMed] [Google Scholar]
- Godefroit, P. , Sinitsa, S. M. , Dhouailly, D. , Bolotsky, Y. L. , Sizov, A. V. , McNamara, M. E. , Benton, M. J. & Spagna, P. (2014). Dinosaur evolution. A Jurassic ornithischian dinosaur from Siberia with both feathers and scales. Science 345, 451–455. [DOI] [PubMed] [Google Scholar]
- Goding, C. R. (2007). Melanocytes: the new black. International Journal of Biochemistry and Cell Biology 39, 275–279. [DOI] [PubMed] [Google Scholar]
- Goodwin, T. (1986). Metabolism, nutrition, and function of carotenoids. Annual Review of Nutrition 6, 273–297. [DOI] [PubMed] [Google Scholar]
- Goodwin, T. (1992). Distribution of carotenoids In Methods in Enzymology Vol. 213 (Ed. Packer L.), pp. 167–172. Elsevier, Amsterdam. [Google Scholar]
- Gopalakrishnakone, P. (1986). The structure of the pigment cells in the turtle Trionyx sinensis . Archivum Histologicum Japonicum 49, 421–435. [DOI] [PubMed] [Google Scholar]
- Gorchein, A. , Lim, C. & Cassey, P. (2009). Extraction and analysis of colourful eggshell pigments using HPLC and HPLC/electrospray ionization tandem mass spectrometry. Biomedical Chromatography 23, 602–606. [DOI] [PubMed] [Google Scholar]
- Graham, D. G. (1979). On the origin and significance of neuromelanin. Archives of Pathology & Laboratory Medicine 103, 359–362. [PubMed] [Google Scholar]
- Greco, G. , Panzella, L. , Verotta, L. , d'Ischia, M. & Napolitano, A. (2011). Uncovering the structure of human red hair pheomelanin: benzothiazolylthiazinodihydroisoquinolines as key building blocks. Journal of Natural Products 74, 675–682. [DOI] [PubMed] [Google Scholar]
- Greenewalt, C. H. , Brandt, W. & Friel, D. D. (1960). Iridescent colors of hummingbird feathers. Journal of the Optical Society of America 50, 1005–1013. [Google Scholar]
- Gren, J. A. , Sjovall, P. , Eriksson, M. E. , Sylvestersen, R. L. , Marone, F. , Clauss, K. G. V. S. , Taylor, G. J. , Carlson, S. , Uvdal, P. & Lindgren, J. (2017). Molecular and microstructural inventory of an isolated fossil bird feather from the Eocene Fur Formation of Denmark. Palaeontology 60, 73–90. [Google Scholar]
- Grether, G. F. , Hudon, J. & Endler, J. A. (2001). Carotenoid scarcity, synthetic pteridine pigments and the evolution of sexual coloration in guppies (Poecilia reticulata). Proceedings of the Royal Society of London B: Biological Sciences 268, 1245–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha, T. , Naysmith, L. , Waterston, K. , Oh, C. , Weller, R. & Rees, J. (2003). Defining the quantitative contribution of the melanocortin 1 receptor (MC1R) to variation in pigmentary phenotype. Annals of the New York Academy of Sciences 994, 339–347. [DOI] [PubMed] [Google Scholar]
- Hadley, M. E. & Bagnara, J. T. (1969). Integrated nature of chromatophore responses in the in vitro frog skin bioassay. Journal of Endocrinology 84, 69–82. [DOI] [PubMed] [Google Scholar]
- Hill, G. & McGraw, K. (2006a). Bird Coloration. Function and Evolution, Edition (Volume 2). Harvard University Press, Cambridge. [Google Scholar]
- Hill, G. E. & McGraw, K. J. (2006b). Bird Coloration: Mechanisms and Measurements. Harvard University Press, Cambridge. [Google Scholar]
- Hofreiter, M. & Schöneberg, T. (2010). The genetic and evolutionary basis of colour variation in vertebrates. Cellular and Molecular Life Sciences 67, 2591–2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holman, J. A. (2003). Fossil Frogs and Toads of North America. Indiana University Press, Bloomington. [Google Scholar]
- Hone, D. W. , Tischlinger, H. , Xu, X. & Zhang, F. (2010). The extent of the preserved feathers on the four‐winged dinosaur Microraptor gui under ultraviolet light. PLoS One 5, e9223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong, L. , Garguilo, J. , Anzaldi, L. , Edwards, G. S. , Nemanich, R. J. & Simon, J. D. (2006). Age‐dependent photoionization thresholds of melanosomes and lipofuscin isolated from human retinal pigment epithelium cells. Photochemistry and Photobiology 82, 1475–1481. [DOI] [PubMed] [Google Scholar]
- Hu, D. Y. , Clarke, J. A. , Eliason, C. M. , Qiu, R. , Li, Q. G. , Shawkey, M. D. , Zhao, C. L. , D'Alba, L. , Jiang, J. K. & Xu, X. (2018). A bony‐crested Jurassic dinosaur with evidence of iridescent plumage highlights complexity in early paravian evolution. Nature Communications 9, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, J. , Wang, X. , Hu, Y. , Liu, J. , Peteya, J. A. & Clarke, J. A. (2016). A new ornithurine from the Early Cretaceous of China sheds light on the evolution of early ecological and cranial diversity in birds. PeerJ 4, e1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudon, J. & Brush, A. H. (1992). Identification of carotenoid pigments in birds In Methods in Enzymology Vol. 213 (Ed. Packer L.), pp. 312–321. Elsevier, Amsterdam. [Google Scholar]
- Hulst, H. C. & van de Hulst, H. C. (1981). Light Scattering by Small Particles. Dover Publications Inc., New York. [Google Scholar]
- Huseby, B. & Ocampo, R. (1997). Evidence for porphyrins bound, via ester bonds, to the Messel oil shale kerogen by selective chemical degradation experiments. Geochimica et Cosmochimica Acta 61, 3951–3955. [Google Scholar]
- Ichikawa, Y. , Ohtani, H. & Miura, I. (1998). The erythrophore in the larval and adult dorsal skin of the brown frog, Rana ornativentris: its differentiation, migration, and pigmentary organelle formation. Pigment Cell Research 11, 345–354. [DOI] [PubMed] [Google Scholar]
- Igic, B. , Greenwood, D. R. , Palmer, D. J. , Cassey, P. , Gill, B. J. , Grim, T. , Brennan, P. L. , Bassett, S. M. , Battley, P. F. & Hauber, M. E. (2010). Detecting pigments from colourful eggshells of extinct birds. Chemoecology 20, 43–48. [Google Scholar]
- Iniesto, M. , Lopez‐Archilla, A. I. , Fregenal‐Martínez, M. , Buscalioni, A. D. & Guerrero, M. C. (2013). Involvement of microbial mats in delayed decay: an experimental essay on fish preservation. Palaios 28, 56–66. [Google Scholar]
- Ito, S. (2006). Encapsulation of a reactive core in neuromelanin. Proceedings of the National Academy of Sciences of the United States of America 103, 14647–14648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito, S. & Wakamatsu, K. (1998). Chemical degradation of melanins: application to identification of dopamine‐melanin. Pigment Cell Research 11, 120–126. [DOI] [PubMed] [Google Scholar]
- Ito, S. & Wakamatsu, K. (2003). Quantitative analysis of eumelanin and pheomelanin in humans, mice, and other animals: a comparative review. Pigment Cell Research 16, 523–531. [DOI] [PubMed] [Google Scholar]
- Ito, S. , Nakanishi, Y. , Valenzuela, R. K. , Brilliant, M. H. , Kolbe, L. & Wakamatsu, K. (2011). Usefulness of alkaline hydrogen peroxide oxidation to analyze eumelanin and pheomelanin in various tissue samples: application to chemical analysis of human hair melanins. Pigment Cell & Melanoma Research 24, 605–613. [DOI] [PubMed] [Google Scholar]
- Jacot, A. , Romero‐Diaz, C. , Tschirren, B. , Richner, H. & Fitze, P. S. (2010). Dissecting carotenoid from structural components of carotenoid‐based coloration: a field experiment with great tits (Parus major). The American Naturalist 176, 55–62. [DOI] [PubMed] [Google Scholar]
- Johnson, A. M. & Fuller, R. C. (2014). The meaning of melanin, carotenoid, and pterin pigments in the bluefin killifish, Lucania goodei . Behavioral Ecology 26, 158–167. [Google Scholar]
- Junium, C. K. , Freeman, K. H. & Arthur, M. A. (2015). Controls on the stratigraphic distribution and nitrogen isotopic composition of zinc, vanadyl and free base porphyrins through Oceanic Anoxic Event 2 at Demerara Rise. Organic Geochemistry 80, 60–71. [Google Scholar]
- Kaye, T. G. , Falk, A. R. , Pittman, M. , Sereno, P. C. , Martin, L. D. , Burnham, D. A. , Gong, E. , Xu, X. & Wang, Y. (2015). Laser‐stimulated fluorescence in paleontology. PLoS One 10, e0125923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaye, T. G. , Pittman, M. , Marugan‐Lobon, J. , Martin‐Abad, H. , Sanz, J. L. & Buscalioni, A. D. (2019a). Fully fledged enantiornithine hatchling revealed by Laser‐Stimulated Fluorescence supports precocial nesting behavior. Scientific Reports 9, 5006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaye, T. G. , Pittman, M. , Mayr, G. , Schwarz, D. & Xu, X. (2019b). Detection of lost calamus challenges identity of isolated Archaeopteryx feather. Scientific Reports 9, 1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellner, A. W. , Wang, X. , Tischlinger, H. , de Almeida Campos, D. , Hone, D. W. & Meng, X. (2010). The soft tissue of Jeholopterus (Pterosauria, Anurognathidae, Batrachognathinae) and the structure of the pterosaur wing membrane. Proceedings of the Royal Society of London B: Biological Sciences 277, 321–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerney, R. (2011). Embryonic staging table for a direct‐developing salamander, Plethodon cinereus (Plethodontidae). The Anatomical Record: Advances in Integrative Anatomy and Evolutionary Biology 294, 1796–1808. [DOI] [PubMed] [Google Scholar]
- Kijas, J. , Wales, R. , Törnsten, A. , Chardon, P. , Moller, M. & Andersson, L. (1998). Melanocortin receptor 1 (MC1R) mutations and coat color in pigs. Genetics 150, 1177–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi, D. W. , Seymoure, B. M. & Pfennig, D. W. (2014). Mimicry's palette: widespread use of conserved pigments in the aposematic signals of snakes. Evolution and Development 16, 61–67. [DOI] [PubMed] [Google Scholar]
- Killops, S. D. & Killops, V. J. (2013). Introduction to Organic Geochemistry. Blackwell Publishing Ltd., Oxford. [Google Scholar]
- Kim, I. T. & Choi, J. B. (1998). Melanosomes of retinal pigment epithelium‐distribution, shape, and acid phosphatase activity. Korean Journal of Ophthalmology 12, 85–91. [DOI] [PubMed] [Google Scholar]
- Klungland, H. , Vage, D. , Gomez‐Raya, L. , Adalsteinsson, S. & Lien, S. (1995). The role of melanocyte‐stimulating hormone (MSH) receptor in bovine coat color determination. Mammalian Genome 6, 636–639. [DOI] [PubMed] [Google Scholar]
- Kohler, A. M. , Olson, E. R. , Martin, J. G. & Anich, P. S. (2019). Ultraviolet fluorescence discovered in New World flying squirrels (Glaucomys). Journal of Mammalogy 100, 21–30. [Google Scholar]
- Koopmans, M. P. , De Leeuw, J. W. & Damsté, J. S. S. (1997). Novel cyclised and aromatised diagenetic products of β‐carotene in the Green River Shale. Organic Geochemistry 26, 451–466. [Google Scholar]
- Koschowitz, M. C. , Fischer, C. & Sander, M. (2014). Evolution. Beyond the rainbow. Science 346, 416–418. [DOI] [PubMed] [Google Scholar]
- Kottler, V. A. , Künstner, A. & Schartl, M. (2015). Pheomelanin in fish? Pigment Cell & Melanoma Research 28, 355–356. [DOI] [PubMed] [Google Scholar]
- Kříž, J. & Lukeš, P. (1974). Color patterns on Silurian Platyceras and Devonian Merista from the Barrandian area, Bohemia, Czechoslovakia. Journal of Paleontology, 48, 41–48. [Google Scholar]
- Krukenberg, C. F. W. (1882). Die federfarbstoffe der psittaciden. Vergleichend‐physiologische Studien Reihe 2, 29–36. [Google Scholar]
- Ksepka, D. T. , Clarke, J. A. , Nesbitt, S. J. , Kulp, F. B. & Grande, L. (2013). Fossil evidence of wing shape in a stem relative of swifts and hummingbirds (Aves, Pan‐Apodiformes). Proceedings of the Royal Society of London B: Biological Sciences 280, 20130580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuriyama, T. , Miyaji, K. , Sugimoto, M. & Hasegawa, M. (2006). Ultrastructure of the dermal chromatophores in a lizard (Scincidae: Plestiodon latiscutatus) with conspicuous body and tail coloration. Zoological Science 23, 793–799. [DOI] [PubMed] [Google Scholar]
- Li, Q. , Gao, K. Q. , Vinther, J. , Shawkey, M. D. , Clarke, J. A. , D'Alba, L. , Meng, Q. , Briggs, D. E. & Prum, R. O. (2010). Plumage color patterns of an extinct dinosaur. Science 327, 1369–1372. [DOI] [PubMed] [Google Scholar]
- Li, Q. , Gao, K. Q. , Meng, Q. , Clarke, J. A. , Shawkey, M. D. , D'Alba, L. , Pei, R. , Ellison, M. , Norell, M. A. & Vinther, J. (2012). Reconstruction of Microraptor and the evolution of iridescent plumage. Science 335, 1215–1219. [DOI] [PubMed] [Google Scholar]
- Li, Q. G. , Clarke, J. A. , Gao, K. Q. , Zhou, C. F. , Meng, Q. J. , Li, D. L. , D'Alba, L. & Shawkey, M. D. (2014). Melanosome evolution indicates a key physiological shift within feathered dinosaurs. Nature 507, 350–353. [DOI] [PubMed] [Google Scholar]
- Li, Q. , Clarke, J. A. , Gao, K. Q. , Peteya, J. A. & Shawkey, M. D. (2018). Elaborate plumage patterning in a Cretaceous bird. PeerJ 6, e5831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindgren, J. , Caldwell, M. W. , Konishi, T. & Chiappe, L. M. (2010). Convergent evolution in aquatic tetrapods: insights from an exceptional fossil mosasaur. PLoS One 5, e11998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindgren, J. , Uvdal, P. , Sjovall, P. , Nilsson, D. E. , Engdahl, A. , Schultz, B. P. & Thiel, V. (2012). Molecular preservation of the pigment melanin in fossil melanosomes. Nature Communications 3, 824. [DOI] [PubMed] [Google Scholar]
- Lindgren, J. , Sjovall, P. , Carney, R. M. , Uvdal, P. , Gren, J. A. , Dyke, G. , Schultz, B. P. , Shawkey, M. D. , Barnes, K. R. & Polcyn, M. J. (2014). Skin pigmentation provides evidence of convergent melanism in extinct marine reptiles. Nature 506, 484–488. [DOI] [PubMed] [Google Scholar]
- Lindgren, J. , Moyer, A. , Schweitzer, M. H. , Sjovall, P. , Uvdal, P. , Nilsson, D. E. , Heimdal, J. , Engdahl, A. , Gren, J. A. , Schultz, B. P. & Kear, B. P. (2015). Interpreting melanin‐based coloration through deep time: a critical review. Proceedings of the Royal Society of London B: Biological Sciences 282, 20150614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindgren, J. , Sjövall, P. , Thiel, V. , Zheng, W. , Ito, S. , Wakamatsu, K. , Hauff, R. , Kear, B. P. , Engdahl, A. & Alwmark, C. (2018). Soft‐tissue evidence for homeothermy and crypsis in a Jurassic ichthyosaur. Nature 564, 359–365. [DOI] [PubMed] [Google Scholar]
- Liu, S. Y. , Shawkey, M. D. , Parkinson, D. , Troy, T. P. & Ahmed, M. (2014). Elucidation of the chemical composition of avian melanin. RSC Advances 4, 40396–40399. [Google Scholar]
- Lu, S. & Li, L. (2008). Carotenoid metabolism: biosynthesis, regulation, and beyond. Journal of Integrative Plant Biology 50, 778–785. [DOI] [PubMed] [Google Scholar]
- Manning, P. L. , Edwards, N. P. , Wogelius, R. A. , Bergmann, U. , Barden, H. E. , Larson, P. L. , Schwarz‐Wings, D. , Egerton, V. M. , Sokaras, D. & Mori, R. A. (2013). Synchrotron‐based chemical imaging reveals plumage patterns in a 150 million year old early bird. Journal of Analytical Atomic Spectrometry 28, 1024–1030. [Google Scholar]
- Manning, P. L. , Edwards, N. P. , Bergmann, U. , Anné, J. , Sellers, W. I. , van Veelen, A. , Sokaras, D. , Egerton, V. M. , Alonso‐Mori, R. & Ignatyev, K. (2019). Pheomelanin pigment remnants mapped in fossils of an extinct mammal. Nature Communications 10, 2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maoka, T. (2011). Carotenoids in marine animals. Marine Drugs 9, 278–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marklund, L. , Moller, M. J. , Sandberg, K. & Andersson, L. (1996). A missense mutation in the gene for melanocyte‐stimulating hormone receptor (MCIR) is associated with the chestnut coat color in horses. Mammalian Genome 7, 895–899. [DOI] [PubMed] [Google Scholar]
- Marks, M. S. & Seabra, M. C. (2001). The melanosome: membrane dynamics in black and white. Nature Reviews Molecular Cell Biology 2, 738–748. [DOI] [PubMed] [Google Scholar]
- Marshall, C. P. & Marshall, A. O. (2010). The potential of Raman spectroscopy for the analysis of diagenetically transformed carotenoids. Philosophical Transactions of the Royal Society of London A: Mathematical, Physical and Engineering Sciences 368, 3137–3144. [DOI] [PubMed] [Google Scholar]
- Martill, D. & Frey, E. (1995). Colour patteming preserved in Lower Cretaceous birds and insects: The Crato Formation of NE Brazil. Neues Jahrbuch fur Geologie und Palaontologie‐Monatshefte, 2, 118–128. [Google Scholar]
- Mateus, O. , Overbeeke, M. & Rita, F. (2008). Dinosaur frauds, hoaxes and “Frankensteins”: how to distinguish fake and genuine vertebrate fossils. Journal of Paleontological Techniques 2, 1–5. [Google Scholar]
- Mathger, L. M. , Land, M. F. , Siebeck, U. E. & Marshall, N. J. (2003). Rapid colour changes in multilayer reflecting stripes in the paradise whiptail, Pentapodus paradiseus . Journal of Experimental Biology 206, 3607–3613. [DOI] [PubMed] [Google Scholar]
- Matsuno, T. (1989). Animal carotenoids In Carotenoids, (Eds. Krinsky N. I., Mathews‐Roth M. M., Taylor R. F.) pp. 59–74. Springer, Boston. [Google Scholar]
- Mayr, G. , Peters, S. D. , Plodowski, G. & Vogel, O. (2002). Bristle‐like integumentary structures at the tail of the horned dinosaur Psittacosaurus . Naturwissenschaften 89, 361–365. [DOI] [PubMed] [Google Scholar]
- Mayr, G. , Pittman, M. , Saitta, E. , Kaye, T. G. & Vinther, J. (2016). Structure and homology of Psittacosaurus tail bristles. Palaeontology 59, 793–802. [Google Scholar]
- McCoy, V. E. , Saupe, E. E. , Lamsdell, J. C. , Tarhan, L. G. , McMahon, S. , Lidgard, S. , Mayer, P. , Whalen, C. D. , Soriano, C. & Finney, L. (2016). The 'Tully monster'is a vertebrate. Nature 532, 496–499. [DOI] [PubMed] [Google Scholar]
- McCoy, D. E. , Feo, T. , Harvey, T. A. & Prum, R. O. (2018). Structural absorption by barbule microstructures of super black bird of paradise feathers. Nature Communications 9, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGraw, K. & Nogare, M. (2004). Carotenoid pigments and the selectivity of psittacofulvin‐based coloration systems in parrots. Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology 138, 229–233. [DOI] [PubMed] [Google Scholar]
- McGraw, K. J. & Nogare, M. C. (2005). Distribution of unique red feather pigments in parrots. Biology Letters 1, 38–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGraw, K. J. , Wakamatsu, K. , Ito, S. , Nolan, P. M. , Jouventin, P. , Dobson, F. S. , Austic, R. E. , Safran, R. J. , Siefferman, L. M. & Hill, G. E. (2004). You can't judge a pigment by its color: carotenoid and melanin content of yellow and brown feathers in swallows, bluebirds, penguins, and domestic chickens. The Condor 106, 390–395. [Google Scholar]
- McGraw, K. J. , Toomey, M. B. , Nolan, P. M. , Morehouse, N. I. , Massaro, M. & Jouventin, P. (2007). A description of unique fluorescent yellow pigments in penguin feathers. Pigment Cell Research 20, 301–304. [DOI] [PubMed] [Google Scholar]
- McHale, J. L. (2017). Molecular Spectroscopy. CRC Press, Boca Raton. [Google Scholar]
- McNamara, M. E. , Orr, P. J. , Kearns, S. L. , Alcalá, L. , Anadón, P. & Penalver Molla, E. (2009). Soft‐tissue preservation in Miocene frogs from Libros, Spain: insights into the genesis of decay microenvironments. Palaios 24, 104–117. [Google Scholar]
- McNamara, M. E. , Briggs, D. E. , Orr, P. J. , Field, D. J. & Wang, Z. (2013a). Experimental maturation of feathers: implications for reconstructions of fossil feather colour. Biology Letters 9, 20130184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara, M. E. , Briggs, D. E. , Orr, P. J. , Gupta, N. S. , Locatelli, E. R. , Qiu, L. , Yang, H. , Wang, Z. , Noh, H. & Cao, H. (2013b). The fossil record of insect color illuminated by maturation experiments. Geology 41, 487–490. [Google Scholar]
- McNamara, M. E. , Orr, P. J. , Kearns, S. L. , Alcala, L. , Anadon, P. & Penalver, E. (2016a). Reconstructing carotenoid‐based and structural coloration in fossil skin. Current Biology 26, 1075–1082. [DOI] [PubMed] [Google Scholar]
- McNamara, M. E. , van Dongen, B. E. , Lockyer, N. P. , Bull, I. D. & Orr, P. J. (2016b). Fossilization of melanosomes via sulfurization. Palaeontology 59, 337–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara, M. E. , Briggs, D. E. , Orr, P. J. , Field, D. J. & Wang, Z. (2017). Correction to 'Experimental maturation of feathers: implications for reconstructions of fossil feather colour'. Biology Letters 13, 20170128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara, M. E. , Kaye, J. S. , Benton, M. J. , Orr, P. J. , Rossi, V. , Ito, S. & Wakamatsu, K. (2018a). Non‐integumentary melanosomes can bias reconstructions of the colours of fossil vertebrates. Nature Communications 9, 2878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara, M. E. , Zhang, F. , Kearns, S. L. , Orr, P. J. , Toulouse, A. , Foley, T. , Hone, D. W. , Rogers, C. S. , Benton, M. J. & Johnson, D. (2018b). Fossilized skin reveals coevolution with feathers and metabolism in feathered dinosaurs and early birds. Nature Communications 9, 2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRoberts, C. A. , Hegna, T. A. , Burke, J. J. , Stice, M. L. , Mize, S. K. & Martin, M. J. (2013). Original spotted patterns on Middle Devonian phacopid trilobites from western and central New York. Geology 41, 607–610. [Google Scholar]
- Megía‐Palma, R. , Jorge, A. & Reguera, S. (2018). Raman spectroscopy reveals the presence of both eumelanin and pheomelanin in the skin of lacertids. Journal of Herpetology 52, 67–73. [Google Scholar]
- Merchant, M. , Hale, A. , Brueggen, J. , Harbsmeier, C. & Adams, C. (2018). Crocodiles alter skin color in response to environmental color conditions. Scientific Reports 8, 6174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mironov, N. A. , Abilova, G. R. , Sinyashin, K. O. , Gryaznov, P. I. , Borisova, Y. Y. , Milordov, D. V. , Tazeeva, E. G. , Yakubova, S. G. , Borisov, D. N. & Yakubov, M. R. (2017). Chromatographic isolation of petroleum vanadyl porphyrins using sulfocationites as sorbents. Energy & Fuels 32, 161–168. [Google Scholar]
- Moan, J. (1988). A change in the quantum yield of photoinactivation of cells observed during photodynamic treatment. Lasers in Medical Science 3, 93–97. [Google Scholar]
- Montforts, F. P. & Glasenapp‐Breiling, M. (2002). Naturally occurring cyclic tetrapyrroles In Progress in the Chemistry of Organic Natural Products/Fortschritte der Chemie organischer Naturstoffe (Eds. Glasenapp‐Breiling M., Jagtap P. G., Kingston D. G. I., Montforts F. P., Samala L., Yuan H.) pp. 1–51. Springer, Vienna. [DOI] [PubMed] [Google Scholar]
- Montoliu, L. , Oetting, W. S. & Bennett, D. C. (2011). Color Genes. European Society for Pigment Cell Research; http://www.espcr.org/micemut [Google Scholar]
- Morales‐Guerrero, B. , Barragan‐Vargas, C. , Silva‐Rosales, G. R. , Ortega‐Ortiz, C. D. , Gendron, D. , Martinez‐Levasseur, L. M. & Acevedo‐Whitehouse, K. (2017). Melanin granules melanophages and a fully‐melanized epidermis are common traits of odontocete and mysticete cetaceans. Veterinary Dermatology 28, 213–e50. [DOI] [PubMed] [Google Scholar]
- Moyer, A. E. , Zheng, W. , Johnson, E. A. , Lamanna, M. C. , Li, D. Q. , Lacovara, K. J. & Schweitzer, M. H. (2014). Melanosomes or microbes: testing an alternative hypothesis for the origin of microbodies in fossil feathers. Scientific Reports 4, 4233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundy, N. I. (2018). Colouration genetics: pretty polymorphic parrots. Current Biology 28, R113–R114. [DOI] [PubMed] [Google Scholar]
- Murphy, N. L. , Trexler, D. & Thompson, M. (2006). “Leonardo”, a mummified Brachylophosaurus from the Judith River Formation In Horns and Beaks: Ceratopsian and Ornithopod Dinosaurs (Ed. Carpenter K.) pp. 117–133. Indiana University Press, Bloomington. [Google Scholar]
- Needham, A. E. (2012). The Significance of Zoochromes. Springer Science & Business Media, Berlin, Heidelberg. [Google Scholar]
- Negro, J. J. , Finlayson, C. & Galván, I. (2018). Melanins in fossil animals: is it possible to infer life history traits from the coloration of extinct species? International Journal of Molecular Sciences 19, 230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson, D. L. , Lehninger, A. L. & Cox, M. M. (2008). Lehninger Principles of Biochemistry. Macmillan, London. [Google Scholar]
- Newton, J. , Wilkie, A. L. , He, L. , Jordan, S. A. , Metallinos, D. L. , Holmes, N. G. , Jackson, I. J. & Barsh, G. S. (2000). Melanocortin 1 receptor variation in the domestic dog. Mammalian Genome 11, 24–30. [DOI] [PubMed] [Google Scholar]
- Nickerson, M. (1946). Relation between black and red melanin pigments in feathers. Physiological Zoology 19, 66–77. [DOI] [PubMed] [Google Scholar]
- Nicolaus, R. A. (1968). Melanins. Hermann, Paris. [Google Scholar]
- Nicolaus, R. , Piattelli, M. & Narni, G. (1959). The structure of sepiomelanin. Tetrahedron Letters 1, 14–17. [Google Scholar]
- Noh, H. , Liew, S. F. , Saranathan, V. , Mochrie, S. G. J. , Prum, R. O. , Dufresne, E. R. & Cao, H. (2010). How noniridescent colors are generated by quasi‐ordered structures of bird feathers. Advanced Materials 22, 2871–2880. [DOI] [PubMed] [Google Scholar]
- Nordén, K. K. , Faber, J. , Babarović, F. , Stubbs, T. L. , Selly, T. , Schiffbauer, J. D. , Peharec Štefanić, P. , Mayr, G. , Smithwick, F. M. & Vinther, J. (2018). Melanosome diversity and convergence in the evolution of iridescent avian feathers—implications for paleocolor reconstruction. Evolution 73, 15–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor, P. M. & Claessens, L. P. (2005). Basic avian pulmonary design and flow‐through ventilation in non‐avian theropod dinosaurs. Nature 436, 253–256. [DOI] [PubMed] [Google Scholar]
- O'Connor, J. , Wang, X. , Sullivan, C. , Zheng, X. , Tubaro, P. , Zhang, X. & Zhou, Z. (2013). Unique caudal plumage of Jeholornis and complex tail evolution in early birds. Proceedings of the National Academy of Sciences of the United States of America 110, 17404–17408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obika, M. & Negishi, S. (1972). Melanosome of toad: a storehouse of riboflavin. Experimental Cell Research 70, 293–300. [DOI] [PubMed] [Google Scholar]
- Oliphant, L. W. & Hudon, J. (1993). Pteridines as reflecting pigments and components of reflecting organelles in vertebrates. Pigment Cell Research 6, 205–208. [DOI] [PubMed] [Google Scholar]
- Ortiz, J. E. , Sánchez‐Palencia, Y. , Gutiérrez‐Zugasti, I. , Torres, T. & González‐Morales, M. (2018). Protein diagenesis in archaeological gastropod shells and the suitability of this material for amino acid racemisation dating: Phorcus lineatus (da Costa, 1778). Quaternary Geochronology 46, 16–27. [Google Scholar]
- Osborne, D. , Huff, W. , Hamilton, P. & Burmeister, H. (1982). Comparison of ochratoxin, aflatoxin, and T‐2 toxin for their effects on selected parameters related to digestion and evidence for specific metabolism of carotenoids in chickens. Poultry Science 61, 1646–1652. [DOI] [PubMed] [Google Scholar]
- Palumbo, A. (2003). Melanogenesis in the ink gland of Sepia officinalis . Pigment Cell Research 16, 517–522. [DOI] [PubMed] [Google Scholar]
- Pan, Y. , Sha, J. , Zhou, Z. & Fürsich, F. T. (2013). The Jehol Biota: definition and distribution of exceptionally preserved relicts of a continental Early Cretaceous ecosystem. Cretaceous Research 44, 30–38. [Google Scholar]
- Pan, Y. , Zheng, W. , Moyer, A. E. , O'Connor, J. K. , Wang, M. , Zheng, X. , Wang, X. , Schroeter, E. R. , Zhou, Z. & Schweitzer, M. H. (2016). Molecular evidence of keratin and melanosomes in feathers of the Early Cretaceous bird Eoconfuciusornis . Proceedings of the National Academy of Sciences of the United States of America 113, E7900–E7907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker, R. S. (1996). Absorption, metabolism, and transport of carotenoids. The FASEB Journal 10, 542–551. [PubMed] [Google Scholar]
- Parnell, A. J. , Washington, A. L. , Mykhaylyk, O. O. , Hill, C. J. , Bianco, A. , Burg, S. L. , Dennison, A. J. , Snape, M. , Cadby, A. J. & Smith, A. (2015). Spatially modulated structural colour in bird feathers. Scientific Reports 5, 18317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parolini, M. , Iacobuzio, R. , Bassano, B. , Pennati, R. & Saino, N. (2018). Melanin‐based skin coloration predicts antioxidant capacity in the brown trout (Salmo trutta). Physiological and Biochemical Zoology 91, 1026–1035. [DOI] [PubMed] [Google Scholar]
- Parry, L. A. , Smithwick, F. , Nordén, K. K. , Saitta, E. T. , Lozano‐Fernandez, J. , Tanner, A. R. , Caron, J. B. , Edgecombe, G. D. , Briggs, D. E. & Vinther, J. (2018). Soft‐bodied fossils are not simply rotten carcasses–toward a holistic understanding of exceptional fossil preservation: exceptional fossil preservation is complex and involves the interplay of numerous biological and geological processes. BioEssays 40, 1700167. [DOI] [PubMed] [Google Scholar]
- Pearn, S. M. , Bennett, A. T. & Cuthill, I. C. (2001). Ultraviolet vision, fluorescence and mate choice in a parrot, the budgerigar Melopsittacus undulatus . Proceedings of the Royal Society of London B: Biological Sciences 268, 2273–2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penacchio, O. , Lovell, P. G. , Cuthill, I. C. , Ruxton, G. D. & Harris, J. M. (2015). Three‐dimensional camouflage: exploiting photons to conceal form. The American Naturalist 186, 553–563. [DOI] [PubMed] [Google Scholar]
- Peterson, R. T. (1999). A Field Guide to the Birds: Eastern and Central North America. Houghton Mifflin Harcourt, Boston, New York. [Google Scholar]
- Peteya, J. A. , Clarke, J. A. , Li, Q. G. , Gao, K. Q. & Shawkey, M. D. (2017). The plumage and colouration of an enantiornithine bird from the Early Cretaceous of China. Palaeontology 60, 55–71. [Google Scholar]
- Pezzella, A. , d'Ischia, M. , Napolitano, A. , Palumbo, A. & Prota, G. (1997). An integrated approach to the structure of Sepia melanin. Evidence for a high proportion of degraded 5, 6‐dihydroxyindole‐2‐carboxylic acid units in the pigment backbone. Tetrahedron 53, 8281–8286. [Google Scholar]
- Pfleiderer, W. (1994). Natural pteridine pigments‐pigments found in butterflies wings and insect eyes. Chimia 48, 488–489. [Google Scholar]
- Pinheiro, F. L. , Horn, B. L. , Schultz, C. L. , de Andrade, J. A. & Sucerquia, P. A. (2012). Fossilized bacteria in a Cretaceous pterosaur headcrest. Lethaia 45, 495–499. [Google Scholar]
- Ponka, P. (1999). Cell biology of heme. The American Journal of the Medical Sciences 318, 241–256. [DOI] [PubMed] [Google Scholar]
- Poole, H. (1965). Spectrophotometric identification of eggshell pigments and timing of superficial pigment deposition in the Japanese quail. Proceedings of the Society for Experimental Biology and Medicine 119, 547–551. [Google Scholar]
- Poole, H. (1966). Relative oöporphyrin content and porphyrin forming capacity of wild‐type and white‐egg Japanese quail uterine tissue. Proceedings of the Society for Experimental Biology and Medicine 122, 596–598. [DOI] [PubMed] [Google Scholar]
- Premović, P. I. , Nikolić, N. D. , Tonsa, I. R. , Pavlović, M. S. , Premović, M. P. & Dulanović, D. T. (2000). Copper and copper (II) porphyrins of the Cretaceous–Tertiary boundary at Stevns Klint (Denmark). Earth and Planetary Science Letters 177, 105–118. [Google Scholar]
- Prota, G. (1988). Progress in the chemistry of melanins and related metabolites. Medicinal Research Review 8, 525–556. [DOI] [PubMed] [Google Scholar]
- Prota, G. (2012). Melanins and Melanogenesis. Academic Press, Cambridge. [Google Scholar]
- Prum, R. O. (1999). The anatomy and physics of avian structural colours. Proceedings of the International Ornithological Congress 22, 1633–1653. [Google Scholar]
- Prum, R. O. & Torres, R. (2003a). Structural colouration of avian skin: convergent evolution of coherently scattering dermal collagen arrays. Journal of Experimental Biology 206, 2409–2429. [DOI] [PubMed] [Google Scholar]
- Prum, R. O. & Torres, R. H. (2003b). A Fourier tool for the analysis of coherent light scattering by bio‐optical nanostructures. Integrative and Comparative Biology 43, 591–602. [DOI] [PubMed] [Google Scholar]
- Prum, R. O. & Torres, R. H. (2004). Structural colouration of mammalian skin: convergent evolution of coherently scattering dermal collagen arrays. Journal of Experimental Biology 207, 2157–2172. [DOI] [PubMed] [Google Scholar]
- Prum, R. O. , Torres, R. , Williamson, S. & Dyck, J. (1999). Two‐dimensional Fourier analysis of the spongy medullary keratin of structurally coloured feather barbs. Proceedings of the Royal Society of London B: Biological Sciences 266, 13–22. [Google Scholar]
- Prum, R. O. , Andersson, S. & Torres, R. H. (2003). Coherent scattering of ultraviolet light by avian feather barbs. Auk 120, 163–170. [Google Scholar]
- Pshennikova, E. S. & Voronina, A. S. (2018). Melanophores inside frogs. International Letters of Natural Sciences 71, 1–9. [Google Scholar]
- Qiang, J. , Currie, P. J. , Norell, M. A. & Shu‐An, J. (1998). Two feathered dinosaurs from northeastern China. Nature 393, 753–761. [Google Scholar]
- Rauhut, O. W. M. , Foth, C. , Tischlinger, H. & Norell, M. A. (2012). Exceptionally preserved juvenile megalosauroid theropod dinosaur with filamentous integument from the late Jurassic of Germany. Proceedings of the National Academy of Sciences of the United States of America 109, 11746–11751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees, J. L. (2003). Genetics of hair and skin color. Annual Review of Genetics 37, 67–90. [DOI] [PubMed] [Google Scholar]
- Repeta, D. J. (1989). Carotenoid diagenesis in recent marine sediments: II. Degradation of fucoxanthin to loliolide. Geochimica et Cosmochimica Acta 53, 699–707. [Google Scholar]
- Rieseberg, L. H. & Soltis, D. E. (1987). Flavonoids of fossil Miocene Platanus and its extant relatives. Biochemical Systematics and Ecology 15, 109–112. [Google Scholar]
- Riley, P. A. (1992). Materia‐melanica ‐ further dark thoughts. Pigment Cell Research 5, 101–106. [DOI] [PubMed] [Google Scholar]
- Riley, P. (1997). Melanin. International Journal of Biochemistry and Cell Biology 29, 1235–1239. [DOI] [PubMed] [Google Scholar]
- Rimington, C. (1939). A reinvestigation of turacin, the copper porphyrin pigment of certain birds belonging to the Musophagidae. Proceedings of the Royal Society of London B: Biological Sciences 127, 106–120. [Google Scholar]
- Rimington, C. (1957). Haem pigments and porphyrins. Annual Review of Biochemistry 26, 561–586. [DOI] [PubMed] [Google Scholar]
- Rivlin, R. (2012). Riboflavin. Springer Science & Business Media, Berlin. [Google Scholar]
- Robbins, L. S. , Nadeau, J. H. , Johnson, K. R. , Kelly, M. A. , Roselli‐Rehfuss, L. , Baack, E. , Mountjoy, K. G. & Cone, R. D. (1993). Pigmentation phenotypes of variant extension locus alleles result from point mutations that alter MSH receptor function. Cell 72, 827–834. [DOI] [PubMed] [Google Scholar]
- Rock, C. L. , Swendseid, M. E. , Jacob, R. A. & McKee, R. W. (1992). Plasma carotenoid levels in human subjects fed a low carotenoid diet. The Journal of Nutrition 122, 96–100. [DOI] [PubMed] [Google Scholar]
- Rotomskis, R. , Bagdonas, S. & Streckyte, G. (1996). Spectroscopic studies of photobleaching and photoproduct formation of porphyrins used in tumour therapy. Journal of Photochemistry and Photobiology B: Biology 33, 61–67. [DOI] [PubMed] [Google Scholar]
- Rossi, V. , McNamara, M. E. , Webb, S. M. , Ito, S. , & Wakamatsu, K. (2019). Tissue‐specific geometry and chemistry of modern and fossilized melanosomes reveal internal anatomy of extinct vertebrates. Proceedings of the National Academy of Sciences, 201820285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roulin, A. (2014). Melanin‐based colour polymorphism responding to climate change. Global Change Biology 20, 3344–3350. [DOI] [PubMed] [Google Scholar]
- Roulin, A. , Mafli, A. & Wakamatsu, K. (2013). Reptiles produce pheomelanin: evidence in the eastern Hermann's tortoise (Eurotestudo boettgeri). Journal of Herpetology 47, 258–261. [Google Scholar]
- Rowe, J. W. , Clark, D. L. , Shaw, D. M. & Wittle, L. W. (2013). Histological basis of substrate color‐induced melanization and reversal of melanization in Painted Turtles (Chrysemys picta marginata). Chelonian Conservation and Biology 12, 246–251. [Google Scholar]
- Ruff, M. D. & Fuller, H. L. (1975). Some mechanisms of reduction of carotenoid levels in chickens infected with Eimeria acervulina or E. tenella . The Journal of Nutrition 105, 1447–1456. [DOI] [PubMed] [Google Scholar]
- Saitta, E. T. , Rogers, C. , Brooker, R. A. , Abbott, G. D. , Kumar, S. , O'Reilly, S. S. , Donohoe, P. , Dutta, S. , Summons, R. E. & Vinther, J. (2017). Low fossilization potential of keratin protein revealed by experimental taphonomy. Palaeontology 60, 547–556. [Google Scholar]
- Saitta, E. T. , Clapham, C. & Vinther, J. (2018a). Experimental subaqueous burial of a bird carcass and compaction of plumage. Paläontologische Zeitschrift, 92(4), 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitta, E. T. , Fletcher, I. , Martin, P. , Pittman, M. , Kaye, T. G. , True, L. D. , Norell, M. A. , Abbott, G. D. , Summons, R. E. , Penkman, K. & Vinther, J. (2018b). Preservation of feather fibers from the Late Cretaceous dinosaur Shuvuuia deserti raises concern about immunohistochemical analyses on fossils. Organic Geochemistry 125, 142–151. [Google Scholar]
- Saitta, E. T. , Gelernter, R. , Vinther, J. & Benson, R. (2018c). Additional information on the primitive contour and wing feathering of paravian dinosaurs. Palaeontology 61, 273–288. [Google Scholar]
- Saitta, E. T. , Kaye, T. G. & Vinther, J. (2018d). Sediment‐encased maturation: a novel method for simulating diagenesis in organic fossil preservation. Palaeontology 62, 135–150. [Google Scholar]
- Sallan, L. C. & Coates, M. I. (2014). The long‐rostrumed elasmobranch Bandringa zangerl, 1969, and taphonomy within a carboniferous shark nursery. Journal of Vertebrate Paleontology 34, 22–33. [Google Scholar]
- Schachner, E. R. , Lyson, T. R. & Dodson, P. (2009). Evolution of the respiratory system in nonavian theropods: evidence from rib and vertebral morphology. The Anatomical Record: Advances in Integrative Anatomy and Evolutionary Biology 292, 1501–1513. [DOI] [PubMed] [Google Scholar]
- Schachner, E. R. , Farmer, C. , McDonald, A. T. & Dodson, P. (2011). Evolution of the dinosauriform respiratory apparatus: new evidence from the postcranial axial skeleton. The Anatomical Record: Advances in Integrative Anatomy and Evolutionary Biology 294, 1532–1547. [DOI] [PubMed] [Google Scholar]
- Schartl, M. , Larue, L. , Goda, M. , Bosenberg, M. W. , Hashimoto, H. & Kelsh, R. N. (2016). What is a vertebrate pigment cell? Pigment Cell & Melanoma Research 29, 8–14. [DOI] [PubMed] [Google Scholar]
- Schmaler‐Ripcke, J. , Sugareva, V. , Gebhardt, P. , Winkler, R. , Kniemeyer, O. , Heinekamp, T. & Brakhage, A. A. (2009). Production of pyomelanin, a second type of melanin, via the tyrosine degradation pathway in Aspergillus fumigatus . Applied and Environmental Microbiology 75, 493–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schraermeyer, U. (1996). The intracellular origin of the melanosome in pigment cells: a review of ultrastructural data. Histology and Histopathology 11, 445–462. [PubMed] [Google Scholar]
- Schweitzer, M. H. , Lindgren, J. & Moyer, A. E. (2015). Melanosomes and ancient coloration re‐examined: a response to Vinther 2015 (DOI 10.1002/bies.201500018). BioEssays 37, 1174–1183. [DOI] [PubMed] [Google Scholar]
- Sefc, K. M. , Brown, A. C. & Clotfelter, E. D. (2014). Carotenoid‐based coloration in cichlid fishes. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology 173, 42–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shawkey, M. D. & D'Alba, L. (2017). Interactions between colour‐producing mechanisms and their effects on the integumentary colour palette. Philosophical Transactions of the Royal Society B: Biological Sciences 372, 20160536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shawkey, M. D. & Hill, G. E. (2006). Significance of a basal melanin layer to production of non‐iridescent structural plumage color: evidence from an amelanotic Steller's jay (Cyanocitta stelleri). Journal of Experimental Biology 209, 1245–1250. [DOI] [PubMed] [Google Scholar]
- Shawkey, M. D. , Hill, G. E. , McGraw, K. J. , Hood, W. R. & Huggins, K. (2006). An experimental test of the contributions and condition dependence of microstructure and carotenoids in yellow plumage coloration. Proceedings of the Royal Society of London B: Biological Sciences 273, 2985–2991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shawkey, M. D. , Morehouse, N. I. & Vukusic, P. (2009). A protean palette: colour materials and mixing in birds and butterflies. Journal of the Royal Society Interface 6, S221–S231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherbrooke, W. C. & Frost, S. K. V. (1989). Integumental chromatophores of a color‐change, thermoregulating lizard, Phrynosoma modestum (Iguanidae, Reptilia). American Museum Novitates 2943, 1–14. [Google Scholar]
- Smithwick, F. M. , Nicholls, R. , Cuthill, I. C. & Vinther, J. (2017). Countershading and stripes in the theropod dinosaur Sinosauropteryx reveal heterogeneous habitats in the Early Cretaceous Jehol biota. Current Biology 27, 3337–3343. [DOI] [PubMed] [Google Scholar]
- Solano, F. (2014). Melanins: skin pigments and much more—types, structural models, biological functions, and formation routes. New Journal of Science 2014, 1–28. [Google Scholar]
- Somes, Jr R. G. & Smyth Jr, J. R. (1965). Feather phaeomelanin intensity in Buff Orpington, New Hampshire and Rhode Island Red breeds of fowl: 2. Inheritance studies from whole feather extracts. Poultry Science 44, 47–52. [DOI] [PubMed] [Google Scholar]
- Staniewicz, A. , Youngprapakorn, U. & Jones, G. (2018). First report of physiological color change in a crocodilian. Copeia 106, 264–267. [Google Scholar]
- Stavenga, D. G. , Tinbergen, J. , Leertouwer, H. L. & Wilts, B. D. (2011). Kingfisher feathers–colouration by pigments, spongy nanostructures and thin films. Journal of Experimental Biology 214, 3960–3967. [DOI] [PubMed] [Google Scholar]
- Steffen, J. E. & McGraw, K. J. (2007). Contributions of pterin and carotenoid pigments to dewlap coloration in two anole species. Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology 146, 42–46. [DOI] [PubMed] [Google Scholar]
- Steffen, J. E. , Learn, K. M. , Drumheller, J. S. , Boback, S. M. & McGraw, K. J. (2015). Carotenoid composition of colorful body stripes and patches in the painted turtle (Chrysemys picta) and red‐eared slider (Trachemys scripta). Chelonian Conservation and Biology 14, 56–63. [Google Scholar]
- Stevens, M. & Merilaita, S. (2009). Animal camouflage: current issues and new perspectives. Philosophical Transactions of the Royal Society B: Biological Sciences 364, 423–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoddard, M. C. & Prum, R. O. (2008). Evolution of avian plumage color in a tetrahedral color space: a phylogenetic analysis of new world buntings. The American Naturalist 171, 755–776. [DOI] [PubMed] [Google Scholar]
- Stoddard, M. C. & Prum, R. O. (2011). How colorful are birds? Evolution of the avian plumage color gamut. Behavioral Ecology 22, 1042–1052. [Google Scholar]
- Stradi, R. , Pini, E. & Celentano, G. (2001). The chemical structure of the pigments in Ara macao plumage. Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology 130, 57–63. [DOI] [PubMed] [Google Scholar]
- Switonski, M. , Mankowska, M. & Salamon, S. (2013). Family of melanocortin receptor (MCR) genes in mammals‐mutations, polymorphisms and phenotypic effects. Journal of Applied Genetics 54, 461–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor, J. D. & Hadley, M. E. (1970). Chromatophores and color change in the lizard, Anolis carolinensis . Zeitschrift für Zellforschung und Mikroskopische Anatomie 104, 282–294. [DOI] [PubMed] [Google Scholar]
- Thibaudeau, G. & Altig, R. (2012). Coloration of anuran tadpoles (Amphibia): development, dynamics, function, and hypotheses. ISRN Zoology. 1–16, 10.5402/2012/725203. [DOI] [Google Scholar]
- Thomas, D. B. , McGoverin, C. M. , McGraw, K. J. , James, H. F. & Madden, O. (2013). Vibrational spectroscopic analyses of unique yellow feather. Journal of the Royal Society Interface 10, 20121065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas, D. B. , Nascimbene, P. C. , Dove, C. J. , Grimaldi, D. A. & James, H. F. (2014). Seeking carotenoid pigments in amber‐preserved fossil feathers. Scientific Reports 4, 5226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorsteinsdottir, S. & Frost, S. K. (1986). Pigment cell differentiation: the relationship between pterin content, allopurinol treatment, and the melanoid gene in axolotls. Cell Differentiation 19, 161–172. [DOI] [PubMed] [Google Scholar]
- Tischlinger, H. (2005). Ultraviolet light investigations of fossils from the Upper Jurassic Plattenkalks of Southern Frankonia. Zitteliana B 26, 26. [Google Scholar]
- Tischlinger, H. & Arratia, G. (2013). Ultraviolet light as a tool for investigating Mesozoic fishes, with a focus on the ichthyofauna of the Solnhofen archipelago. Mesozoic Fishes 5, 549–560. [Google Scholar]
- Trinkaus, J. P. (1948). Factors concerned in the response of melanoblasts to estrogen in the Brown Leghorn fowl. Journal of Experimental Zoology 109, 135–169. [DOI] [PubMed] [Google Scholar]
- Våge, D. I. , Klungland, H. , Lu, D. & Cone, R. D. (1999). Molecular and pharmacological characterization of dominant black coat color in sheep. Mammalian Genome 10, 39–43. [DOI] [PubMed] [Google Scholar]
- Van Berkel, G. J. , Quirke, J. M. E. & Filby, R. H. (1989). The Henryville Bed of the New Albany shale—I. Preliminary characterization of the nickel and vanadyl porphyrins in the bitumen. Organic Geochemistry 14, 119–128. [Google Scholar]
- Varga, M. , Berkesi, O. , Darula, Z. , May, N. V. & Palágyi, A. (2016). Structural characterization of allomelanin from black oat. Phytochemistry 130, 313–320. [DOI] [PubMed] [Google Scholar]
- Villela, G. & Thein, M. (1967). Riboflavin in the blood serum, the skin and the venom of some snakes of Burma. Cellular and Molecular Life Sciences 23, 722. [DOI] [PubMed] [Google Scholar]
- Vinther, J. (2015). A guide to the field of palaeo colour: melanin and other pigments can fossilise: reconstructing colour patterns from ancient organisms can give new insights to ecology and behaviour. BioEssays 37, 643–656. [DOI] [PubMed] [Google Scholar]
- Vinther, J. , Briggs, D. E. , Prum, R. O. & Saranathan, V. (2008). The colour of fossil feathers. Biology Letters 4, 522–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinther, J. , Briggs, D. E. , Clarke, J. , Mayr, G. & Prum, R. O. (2010). Structural coloration in a fossil feather. Biology Letters 6, 128–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinther, J. , Nicholls, R. , Lautenschlager, S. , Pittman, M. , Kaye, T. G. , Rayfield, E. , Mayr, G. & Cuthill, I. C. (2016). 3D camouflage in an ornithischian dinosaur. Current Biology 26, 2456–2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitek, N. S. , Vinther, J. , Schiffbauer, J. D. , Briggs, D. E. & Prum, R. O. (2013). Exceptional three‐dimensional preservation and coloration of an originally iridescent fossil feather from the Middle Eocene Messel Oil Shale. Paläontologische Zeitschrift 87, 493–503. [Google Scholar]
- Völker, O. (1936). Über den gelben federfarbstoff des Wellensittichs (Melopsittacus undulatus (Shaw)). Journal für Ornithologie 84, 618–630. [Google Scholar]
- Völker, O. (1937). Über fluoreszierende, gelbe federpigmente bei papageien, eine neue klasse von federfarbstoffen. Journal für Ornithologie 85, 136–146. [Google Scholar]
- Völker, O. (1938). Porphyrin in vogelfedern. Journal für Ornithologie 86, 436–456. [Google Scholar]
- Wakamatsu, K. & Ito, S. (2002). Advanced chemical methods in melanin determination. Pigment Cell Research 15, 174–183. [DOI] [PubMed] [Google Scholar]
- Wang, Z. , Dillon, J. & Gaillard, E. (2006). Antioxidant properties of melanin in retinal pigment epithelial cells. Photochemistry and Photobiology 82, 474–479. [DOI] [PubMed] [Google Scholar]
- Wang, X. , Pittman, M. , Zheng, X. , Kaye, T. G. , Falk, A. R. , Hartman, S. A. & Xu, X. (2017). Basal paravian functional anatomy illuminated by high‐detail body outline. Nature Communications 8, 14576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward, W. C. , Lamb, E. C. , Gooden, D. , Chen, X. , Burinsky, D. J. & Simon, J. D. (2008). Quantification of naturally occurring pyrrole acids in melanosomes. Photochemistry and Photobiology 84, 700–705. [DOI] [PubMed] [Google Scholar]
- Weaver, R. J. , Santos, E. S. , Tucker, A. M. , Wilson, A. E. & Hill, G. E. (2018). Carotenoid metabolism strengthens the link between feather coloration and individual quality. Nature Communications 9, 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb, G. , Manolis, S. C. & Whitehead, P. J. (1987). Wildlife Management: Crocodiles and Alligators. Surrey Beatty & Sons, Chipping Norton. [Google Scholar]
- Weidensaul, C. S. , Colvin, B. A. , Brinker, D. F. & Huy, J. S. (2011). Use of ultraviolet light as an aid in age classification of owls. The Wilson Journal of Ornithology 123, 373–377. [Google Scholar]
- Whitear, M. (1956). XCVII.—On the colour of an ichthyosaur. Journal of Natural History 9, 742–744. [Google Scholar]
- Wiemann, J. , Yang, T.‐R. , Sander, P. N. , Schneider, M. , Engeser, M. , Kath‐Schorr, S. , Müller, C. E. & Sander, P. M. (2017). Dinosaur origin of egg color: oviraptors laid blue‐green eggs. PeerJ 5, e3706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiemann, J. , Yang, T. R. & Norell, M. A. (2018). Dinosaur egg colour had a single evolutionary origin. Nature 563, 555–558. [DOI] [PubMed] [Google Scholar]
- Williams, J. S. (1930). A color pattern on a new Mississippian trilobite. American Journal of Science 5, 61–64. [Google Scholar]
- Williams, S. T. (2017). Molluscan shell colour. Biological Reviews 92, 1039–1058. [DOI] [PubMed] [Google Scholar]
- Williams, A. W. , Boileau, T. W. & Erdman, J. W. Jr. (1998). Factors influencing the uptake and absorption of carotenoids. Proceedings of the Society for Experimental Biology and Medicine 218, 106–108. [DOI] [PubMed] [Google Scholar]
- Wilson, P. , Parry, L. A. , Vinther, J. & Edgecombe, G. D. (2016). Unveiling biases in soft‐tissue phosphatization: extensive preservation of musculature in the Cretaceous (Cenomanian) polychaete Rollinschaeta myoplena (Annelida: Amphinomidae). Palaeontology 59, 463–479. [Google Scholar]
- Witmer, L. M. (1995). The extant phylogenetic bracket and the importance of reconstructing soft tissues in fossils In Functional Morphology in Vertebrate Paleontology Vol.1 (Ed. Thomason J. J.), pp. 19–33, Cambridge University Press, Cambridge. [Google Scholar]
- Wogelius, R. , Manning, P. , Barden, H. , Edwards, N. , Webb, S. , Sellers, W. , Taylor, K. , Larson, P. , Dodson, P. & You, H. (2011). Trace metals as biomarkers for eumelanin pigment in the fossil record. Science 333, 1622–1626. [DOI] [PubMed] [Google Scholar]
- Wolkenstein, K. , Gross, J. H. & Falk, H. (2010). Boron‐containing organic pigments from a Jurassic red alga. Proceedings of the National Academy of Sciences of the United States of America 107, 19374–19378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wuttke, M. (1983). Weichteil‐erhaltung'durch lithifizierte mikroorganismen bei mittel‐eozänen vertebraten aus den ölschiefern der 'grube messel'bei darmstadt. Senckenbergiana Lethaea 64, 509–527. [Google Scholar]
- Xiao, M. , Li, Y. , Allen, M. C. , Deheyn, D. D. , Yue, X. , Zhao, J. , Gianneschi, N. C. , Shawkey, M. D. & Dhinojwala, A. (2015). Bio‐inspired structural colors produced via self‐assembly of synthetic melanin nanoparticles. ACS Nano 9, 5454–5460. [DOI] [PubMed] [Google Scholar]
- Xu, X. , Zhou, Z.‐h. & Prum, R. O. (2001). Branched integumental structures in Sinornithosaurus and the origin of feathers. Nature 410, 200–204. [DOI] [PubMed] [Google Scholar]
- Xu, X. , Zheng, X. , Sullivan, C. , Wang, X. , Xing, L. , Wang, Y. , Zhang, X. , O'Connor, J. K. , Zhang, F. & Pan, Y. (2015). A bizarre Jurassic maniraptoran theropod with preserved evidence of membranous wings. Nature 521, 70–73. [DOI] [PubMed] [Google Scholar]
- Yabuzaki, J. (2017). Carotenoids Database: structures, chemical fingerprints and distribution among organisms. Database 2017, bax004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, Z. , Jiang, B. , McNamara, M. E. , Kearns, S. L. , Pittman, M. , Kaye, T. G. , Orr, P. J. , Xu, X. & Benton, M. (2019). Pterosaur integumentary structures with complex feather‐like branching. Nature Ecology and Evolution 3, 24–30. [DOI] [PubMed] [Google Scholar]
- Zecca, L. , Tampellini, D. , Gerlach, M. , Riederer, P. , Fariello, R. & Sulzer, D. (2001). Substantia nigra neuromelanin: structure, synthesis, and molecular behaviour. Molecular Pathology 54, 414–418. [PMC free article] [PubMed] [Google Scholar]
- Zhang, F. , Zhou, Z. , Xu, X. , Wang, X. & Sullivan, C. (2008). A bizarre Jurassic maniraptoran from China with elongate ribbon‐like feathers. Nature 455, 1105–1108. [DOI] [PubMed] [Google Scholar]
- Zhang, F. , Kearns, S. L. , Orr, P. J. , Benton, M. J. , Zhou, Z. , Johnson, D. , Xu, X. & Wang, X. (2010). Fossilized melanosomes and the colour of Cretaceous dinosaurs and birds. Nature 463, 1075–1078. [DOI] [PubMed] [Google Scholar]
- Zhang, Y. , Dong, B. , Chen, A. , Liu, X. , Shi, L. & Zi, J (2015). Using cuttlefish ink as an additive to produce non‐iridescent structural colors of high color visibility. Advanced Materials 27, 4719–4724. [DOI] [PubMed] [Google Scholar]
- Zheng, X. (2009). The Origin of Birds. Shandong Science Technology Press, Jinan. [Google Scholar]
- Zheng, X. T. , O'Connor, J. K. , Wang, X. L. , Pan, Y. H. , Wang, Y. , Wang, M. & Zhou, Z. H. (2017). Exceptional preservation of soft tissue in a new specimen of Eoconfuciusornis and its biological implications. National Science Review 4, 441–452. [Google Scholar]
- Zheng, F. , Hsu, C. S. , Zhang, Y. , Sun, Y. , Wu, Y. , Lu, H. , Sun, X. , Shi, Q. J. (2018). Simultaneous detection of vanadyl, nickel, iron, and gallium porphyrins in marine shales from the Eagle Ford Formation, South Texas. Energy & Fuels 32, 10382–10390. [Google Scholar]
- Zi, J. , Yu, X. , Li, Y. , Hu, X. , Xu, C. , Wang, X. , Liu, X. & Fu, R. (2003). Coloration strategies in peacock feathers. Proceedings of the National Academy of Sciences of the United States of America 100, 12576–12578. [DOI] [PMC free article] [PubMed] [Google Scholar]


