Abstract
Background
Sixty percent of patients with stage IV melanoma may develop brain metastases, which result in significantly increased morbidity and a poor overall prognosis. Phase 3 studies of melanoma usually exclude patients with untreated brain metastases; therefore, clinical data for intracranial responses to treatments are limited.
Methods
A multicenter, retrospective case series investigation of consecutive BRAF‐mutant patients with melanoma brain metastases (MBMs) treated with a combination of BRAF inhibitor encorafenib and MEK inhibitor binimetinib was conducted to evaluate the antitumor response. Assessments included the intracranial, extracranial, and global objective response rates (according to the modified Response Evaluation Criteria in Solid Tumors, version 1.1); the clinical benefit rate; the time to response; the duration of response; and safety.
Results
A total of 24 patients with stage IV BRAF‐mutant MBMs treated with encorafenib plus binimetinib in 3 centers in the United States were included. Patients had received a median of 2.5 prior lines of treatment, and 88% had prior treatment with BRAF/MEK inhibitors. The intracranial objective response rate was 33%, and the clinical benefit rate was 63%. The median time to a response was 6 weeks, and the median duration of response was 22 weeks. Among the 21 patients with MBMs and prior BRAF/MEK inhibitor treatment, the intracranial objective response rate was 24%, and the clinical benefit rate was 57%. Similar outcomes were observed for extracranial and global responses. The safety profile for encorafenib plus binimetinib was similar to that observed in patients with melanoma without brain metastases.
Conclusions
Combination therapy with encorafenib plus binimetinib elicited intracranial activity in patients with BRAF‐mutant MBMs, including patients previously treated with BRAF/MEK inhibitors. Further prospective studies are warranted and ongoing.
Keywords: antitumor, binimetinib, BRAF inhibitor, encorafenib, MEK inhibitor, melanoma, melanoma‐associated brain metastasis
Short abstract
All clinical trials to date with encorafenib and binimetinib (US Food and Drug Administration–approved in June 2018 for BRAF‐mutated metastatic melanoma) have excluded untreated melanoma brain metastases. This case series provides the first clinical evidence of intracranial activity of the combination of encorafenib plus binimetinib in patients with BRAF‐mutant melanoma with active brain metastases. Intracranial clinical activity is observed for the first time in patients previously treated with BRAF/MEK inhibitors, a population that has not been previously investigated.
Introduction
Metastatic melanoma has a high risk of spreading to the central nervous system.1, 2 Among all cancers, melanoma is the third most common cause of brain metastases,3 and it has an observed incidence ranging from 43% to 75% in clinical and autopsy series, respectively.2, 4 Data predating the advent of effective systemic therapy for metastatic melanoma showed an estimated 3‐month survival rate of 43% for patients with melanoma brain metastases (MBMs). Patients with brain metastases have a poor prognosis and account for up to 54% of deaths in patients with melanoma.5, 6, 7
Surgery, whole brain radiation therapy, and, more recently, stereotactic radiosurgery have been the accepted standard‐of‐care management of MBMs. Although surgery and radiation therapy continue to have a role in the management of symptomatic and large MBMs, recent prospective and retrospective clinical trials evaluating targeted therapies and checkpoint inhibitors have demonstrated significant clinical activity in patients with melanoma and active brain metastases.8, 9, 10, 11, 12, 13, 14 BRAF mutations, the most frequent genetic alterations, are observed in 50% of melanomas and may predispose patients to developing brain metastases.15, 16, 17 BRAF mutations drive constitutive MAPK pathway activation, which leads to increased tumor cell survival and proliferation. Treatment with BRAF inhibitors, in combination with MEK inhibitors, can elicit profound and early clinical responses in patients with BRAF‐mutated melanoma.18 A combination of BRAF and MEK inhibitor therapy has been shown to improve survival in comparison with BRAF inhibitor monotherapy. The combinations of dabrafenib plus trametinib, vemurafenib plus cobimetinib, and, most recently, encorafenib plus binimetinib are approved by the US Food and Drug Administration for advanced BRAF‐mutant melanoma.18, 19, 20, 21, 22, 23
The combination of dabrafenib and trametinib showed a high intracranial response rate (58%) in asymptomatic patients with untreated MBMs.14 Vemurafenib demonstrated an intracranial response rate of 18% in patients with untreated brain metastases in a prospective phase 2 trial.22 The combination of encorafenib plus binimetinib has demonstrated clinical activity and tolerability in the phase 3 COLUMBUS (Combined LGX818 [encorafenib] Used with MEK162 [binimetinib] in BRAF mutant Unresectable Skin cancer) study in patients with BRAF V600–mutated melanoma.18, 23 However, the combination has not been formally studied in trials including patients with active brain metastases. In this analysis, we report the results of a retrospective case series evaluating the antitumor activity of encorafenib plus binimetinib in patients with BRAF‐mutant melanoma and active brain metastases, including those who were treatment‐naive and those with prior treatment with BRAF/MEK combinations.
Materials and Methods
The objective of this retrospective study was to assess the intracranial antitumor activity of encorafenib plus binimetinib in patients with BRAF‐mutant melanoma harboring brain metastases. Consecutive patients were included if they had stage IV melanoma with brain metastases confirmed by imaging studies, had a confirmed tumor BRAF mutation, and had been treated with encorafenib plus binimetinib at 1 of the 3 participating study centers (Mount Sinai Comprehensive Cancer Center, Miami Beach, Florida; Levine Cancer Institute, Atrium Health, Charlotte, North Carolina; and The University of Texas MD Anderson Cancer Center, Houston, Texas). Patients were excluded if they did not have measurable intracranial disease by magnetic resonance imaging (MRI) or if pre/post brain MRI imaging was not performed.
Retrospective data collection included patients’ demographic characteristics, clinical history of melanoma and brain metastases (including the clinical features at diagnosis, course of the disease, treatment received, and outcomes), and encorafenib‐binimetinib treatment exposure. Assessments included tumor responses to treatment (intracranial, extracranial, and global responses) evaluated with the modified Response Evaluation Criteria in Solid Tumors, version 1.1 (mRECIST1.1); the time to response; and the duration of response. For the purposes of this study, intracranial refers to intra‐axial lesions (ie, not intracranial lesions that were extra‐axial). Safety data were collected from a chart review and included adverse events, laboratory abnormalities, and intolerance to encorafenib‐binimetinib therapy. Lactate dehydrogenase (LDH) levels were recorded at the start of treatment with encorafenib plus binimetinib and at the time of response or progression.
For the response analysis, extracranial lesions (a minimum of 10 mm in diameter for measurable nonnodal lesions) were assessed according to the Response Evaluation Criteria in Solid Tumors, version 1.1. For the assessment of brain lesions, the Response Evaluation Criteria in Solid Tumors were modified to allow up to 5 intracranial target lesions, as described previously.8, 12, 24 Imaging criteria were enhancing lesions on MRI brain axial T1 with contrast. Intracranial lesions were measured only with gadolinium‐enhanced MRI and were considered measurable if the longest diameter was at least 5 mm. Global responses were assessed with the mRECIST1.1 criteria for brain lesions and systemic disease to encompass all index lesions in the brain and systemic compartments. Results for all assessments and baseline data were summarized descriptively. The objective response rate was defined as the percentage of complete and partial responses as evaluated with mRECIST1.1; the clinical benefit rate was defined as the percentage of patients who had a complete response, partial response, or stable disease for 4 months or longer (the 4‐month threshold corresponds to the scanning frequency). All case reports were reviewed in accordance with Helsinki principles and were approved by the institutional review boards at the individual institutions.
Results
Study Patients
Information on patient disposition can be found in Figure 1. A total of 29 patients were screened, and 24 patients met the inclusion criteria for this analysis as of the data cutoff date of February 28, 2019. Of the 24 patients, 2 were treated at the Mount Sinai Comprehensive Cancer Center, 7 were treated at the Levine Cancer Institute, and 15 were treated at The University of Texas MD Anderson Cancer Center. The reasons for screened patients to be excluded were a lack of measurable disease (3 patients) and a lack of scans (2 patients). Patients were initiated on the full doses of encorafenib (450 mg once daily) and binimetinib (45 mg twice daily) with the exception of 4 patients who required dose reductions of one or both agents.
Figure 1.

Patient disposition.
A summary of the patient demographics and clinical characteristics is shown in Table 1. For the 24 patients included in this study, the mean age was 52.8 years, and a majority were male (58%). Most patients had an Eastern Cooperative Oncology Group performance status of 0 or 1 (20 patients [83%]). The median time from the melanoma diagnosis (ie, the time from the primary melanoma diagnosis to the start of encorafenib‐binimetinib treatment) was 505 days, and the median time since the diagnosis of metastatic brain lesions was 59.5 days. At the baseline, a majority of the patients (54%) had 1 to 10 metastatic brain lesions, although 8 patients (33%) had more than 20 lesions (3 patients [13%] had 0‐3 metastatic brain lesions, 10 patients [42%] had 4‐10 lesions, and 11 patients [46%] had more than 10 lesions). Lesion sizes ranged from 0.5 to 3.5 cm with a median size of 1.0 cm. Seven patients had LDH levels higher than 250 U/L at the initiation of encorafenib plus binimetinib. A total of 21 patients (88%) had previously received brain‐directed treatment, with the most common treatments being stereotactic radiosurgery (SRS) and surgery. All patients received prior systemic treatment, with the median number of prior lines being 2.5. The most common prior systemic treatment regimens were dabrafenib plus trametinib (88%) and PD‐1–targeted monotherapy (46%). For the patients included in the analysis, only 3 (13%) were BRAF/MEK treatment–naive, 10 (42%) progressed on prior BRAF/MEK therapy, and 11 (46%) discontinued prior BRAF/MEK therapy because of intolerance. Of the 21 patients who had prior BRAF/MEK therapy, 6 had an intervening therapy before the initiation of encorafenib plus binimetinib (the median interval from prior BRAF/MEK therapy was 2 months). Eleven patients (46%) received steroids during encorafenib‐binimetinib treatment.
Table 1.
Baseline Clinical and Demographic Characteristics (n = 24)
| Characteristic | Value |
|---|---|
| Sex, No. (%) | |
| Female | 10 (42) |
| Male | 14 (58) |
| Age, y | |
| Mean (SD) | 52.8 (13.4) |
| Median | 52.5 |
| Range | 25‐72 |
| ECOG performance status, No. (%) | |
| 0 | 10 (42) |
| 1 | 10 (42) |
| 2 | 3 (13) |
| 3 | 1 (4) |
| Time since melanoma diagnosis, da | |
| Mean (SD) | 508.3 (337.8) |
| Median | 505 |
| Time since diagnosis of metastatic brain lesions, d | |
| Mean (SD) | 88.5 (60.2) |
| Median | 59.5 |
| No. of metastatic brain lesions, No. (%) | |
| 0‐3 | 3 (13) |
| 4‐10 | 10 (42) |
| >10 | 11 (46) |
| LDH, U/L | |
| Mean (SD) | 258 (175) |
| Median | 190 |
| Range | 82‐774 |
| Previous brain‐directed treatment, No. (%) | |
| Whole brain radiation | 14 (58) |
| Stereotactic radiation | 4 (17) |
| Gamma knife | 9 (38) |
| Surgery | 8 (33) |
| None | 3 (13) |
| Prior systemic treatment, No. (%) | 24 (100) |
| No. of prior lines, mean (SD) | 2.8 (1.6) |
| No. of prior lines, median | 2.5 |
| Prior BRAF/MEK inhibitor therapy, No. (%) | 21 (88) |
| Discontinued because of poor tolerability | 11 |
| Discontinued because of progression | 10 |
| Previous systemic treatment (most common), No. (%) | |
| Dabrafenib + trametinib | 21 (88) |
| Vemurafenib + cobimetinib | 7 (29) |
| PD‐1–targeted monotherapy | 11 (46) |
| Ipilimumab + nivolumab | 9 (38) |
| Chemotherapy | 5 (21) |
Abbreviations: ECOG, Eastern Cooperative Oncology Group; LDH, lactate dehydrogenase.
Time from the primary melanoma diagnosis to the start of the encorafenib‐binimetinib treatment.
Tumor Response
A summary of assessments for intracranial, extracranial, and global responses is shown in Table 2. According to mRECIST1.1 criteria, the intracranial objective response was 33%: 3 patients (13%) achieved a complete response, and 5 (21%) achieved a partial response. An additional 11 patients (46%) had stable disease as their best response, and the intracranial clinical benefit rate was 63%. The best percentage decrease in the intracranial tumor burden for each patient is depicted in Figure 2. Similar results were observed for extracranial responses, although there were more patients with stable disease, and no complete extracranial responses were observed. For the global response, the objective response rate was 42%, and the clinical benefit rate was 63%. Seven of the 8 patients who had an intracranial response had at least stable disease for extracranial tumors; 2 patients had both intracranial and extracranial responses.
Table 2.
Response to Treatment
| BRAF/MEK Inhibitor–Naive (n = 3) | Prior BRAF/MEK Inhibitor | Total (n = 24) | ||
|---|---|---|---|---|
| Poor Tolerability (n = 11) | Progressed (n = 10) | |||
| Intracranial response | ||||
| Best overall response, No. (%) | ||||
| CR | 2 (67) | 1 (9) | — | 3 (13) |
| PR | 1 (33) | 1 (9) | 3 (30) | 5 (21) |
| SD | — | 6 (55) | 5 (50) | 11 (46) |
| PD | — | 2 (18) | 2 (20) | 4 (17) |
| Could not be evaluated | — | 1 (9) | — | 1 (4) |
| Objective response, No. (%) | 3 (100) | 2 (18) | 3 (30) | 8 (33) |
| CR + PR + SD, No. (%) | 3 (100) | 8 (73) | 8 (80) | 19 (79) |
| Clinical benefit, No. (%) | 3 (100) | 6 (55) | 6 (60) | 15 (63) |
| Extracranial | ||||
| Best overall response, No. (%) | ||||
| CR | — | — | — | — |
| PR | 1 (33) | 3 (27) | 1 (10) | 5 (21) |
| SD | 2 (67) | 5 (45) | 7 (70) | 14 (58) |
| PD | — | 2 (18) | 1 (10) | 3 (13) |
| Could not be evaluated | — | 1 (10) | 1 (10) | 2 (8) |
| Objective response, No. (%) | 1 (33) | 3 (27) | 1 (10) | 5 (21) |
| CR + PR + SD, No. (%) | 3 (100) | 8 (73) | 8 (80) | 19 (79) |
| Clinical benefit, No. (%) | 3 (100) | 5 (45) | 4 (40) | 12 (50) |
| Global response | ||||
| Best overall response, No. (%) | ||||
| CR | — | 1 (9) | — | 1 (4) |
| PR | 3 (100) | 3 (27) | 3 (30) | 9 (38) |
| SD | — | 4 (36) | 6 (60) | 10 (42) |
| PD | — | 3 (27) | 1 (10) | 4 (17) |
| Could not be evaluated | — | — | — | — |
| Objective response, No. (%) | 3 (100) | 4 (36) | 3 (30) | 10 (42) |
| CR + PR + SD, No. (%) | 3 (100) | 8 (73) | 9 (90) | 20 (83) |
| Clinical benefit, No. (%) | 3 (100) | 6 (55) | 6 (60) | 15 (63) |
Abbreviations: CR, complete response; PD, progressive disease; PR, partial response; SD, stable disease.
An objective response is defined as CR + PR; a clinical benefit is defined as CR + PR + SD for 4 months or longer.
Figure 2.
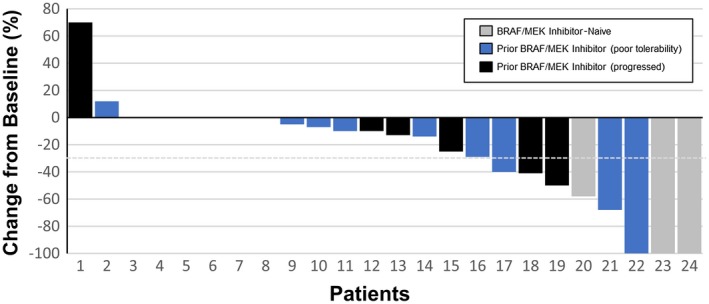
Waterfall plot for the best percentage intracranial response for each patient.
The median time to an intracranial response was 6 weeks (which coincided with the first imaging assessment), and the median duration of response was 22 weeks (Fig. 3). A total of 5 patients had an ongoing response, and 13 patients were still on treatment at the time of data collection. An intracranial response was observed in the 3 BRAF/MEK inhibitor treatment–naive patients (Table 2), with 2 responses ongoing (>4 months); the third patient was switched to immunotherapy after 3 months. Of the 21 patients who had previously received BRAF/MEK inhibitor therapy and discontinued treatment for either poor tolerability or disease progression, the objective response rate was 24% (patients who discontinued for either poor tolerability [18%] or progression [30%]), and the clinical benefit rate was 57% (patients who discontinued for either poor tolerability [55%] or progression [60%]). The 6 patients with an intervening therapy after the prior BRAF/MEK inhibitor therapy and before the receipt of encorafenib plus binimetinib had a partial response (n = 3) and stable disease (n = 3) as the best intracranial response. The results for patients with no intervening therapy after their prior BRAF/MEK inhibitor included a complete response (n = 1), a partial response (n = 1), stable disease (n = 8), and progressive disease (n = 3) as the best intracranial response (1 patient was not evaluable); this indicated that using a different BRAF/MEK inhibitor combination right after a previous BRAF/MEK inhibitor regimen may be a viable option for some patients. For example, a patient who progressed on dabrafenib and trametinib achieved a partial response with encorafenib plus binimetinib, with a 41% reduction in intracranial lesions (response ongoing). Another patient received encorafenib plus binimetinib after not tolerating dabrafenib plus trametinib and achieved a complete response (response ongoing).
Figure 3.
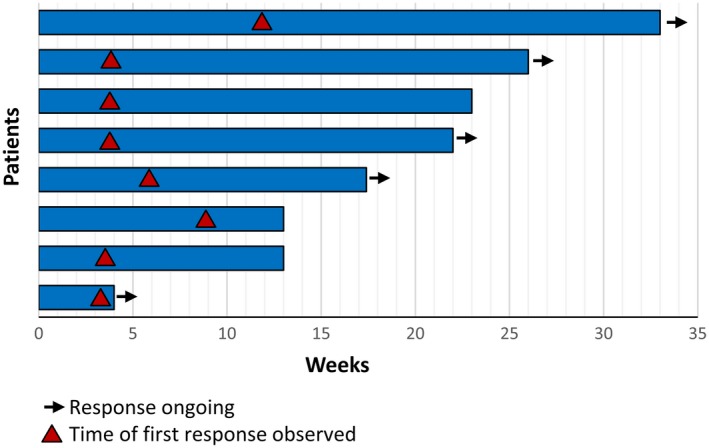
Duration of intracranial response.
The median LDH level before the initiation of treatment with encorafenib plus binimetinib was 190 U/L (range, 82‐774 U/L), whereas the median LDH level was 184 U/L (range, 69‐409 U/L) at response. One of the 4 patients with progressive disease as the best response had elevated LDH at the baseline. The 7 patients who had LDH above 250 U/L at the baseline had an intracranial objective response rate (29%) similar to that of those patients who had an LDH level below 250 U/L at the baseline (35%).
Safety
Adverse events were observed in 16 patients (67%; Table 3). The most common adverse events were fatigue (17%) and myalgia (13%). Adverse events were grade 1 or 2 in severity with the exception of 2 patients who experienced grade 3 myalgia. One patient had a fever, and 2 patients experienced a retinal detachment. One patient presented with intraretinal and subretinal fluid after 1 dose of encorafenib plus binimetinib, and this was diagnosed as drug‐induced serous retinal detachment. Binimetinib was discontinued, and the event resolved 1 week later. The patient resumed binimetinib after 11 days and continued without recurrence. The second patient presented with blurred vision 2 months after initiating encorafenib and binimetinib. He was diagnosed to have bilateral acute iritis, posterior vitreous detachment in both eyes, and allergic conjunctivitis on the right side. He was treated with topical steroid drops with a resolution of symptoms and continued his encorafenib at 450 mg once daily and binimetinib at 45 mg twice daily without a break. Laboratory abnormalities were observed in 4 patients (2 patients with elevated liver function tests (LFTs), 1 patient with elevated creatinine, and a case of severe pancytopenia). A total of 4 patients (17%) dose‐reduced one or both agents during the study because of adverse events (myalgia [n = 2], neuropathy, and nausea/vomiting/fatigue). Of these 4 patients, 2 were still on treatment, 1 progressed, and 1 discontinued because of an adverse event (neuropathy). One patient who reduced binimetinib (nausea/vomiting/fatigue) re‐escalated the dose when the events resolved.
Table 3.
Adverse Event Summary
| Adverse Events | No. (%) |
|---|---|
| Patients experiencing adverse event | 16 (67) |
| Adverse events in ≥5% of patients | |
| Fatigue | 4 (17) |
| Myalgia | 3 (13) |
| Retinal detachment | 2 (8) |
| Rheumatoid arthritis | 2 (8) |
| Nausea | 2 (8) |
Discussion
The development of brain metastases in melanoma is associated with a poor prognosis, and identifying therapies with intracranial activity remains an area of critical unmet need. In this retrospective case series, BRAF/MEK inhibition with the combination of encorafenib plus binimetinib demonstrated intracranial antitumor activity in BRAF‐mutant patients with MBMs. The intracranial objective response rate in this heavily pretreated population was 33%, and the intracranial clinical benefit rate was 63% with a median duration of response of 22 weeks (6 patients with an ongoing response). Extracranial responses were generally concordant with intracranial responses. These results are particularly noteworthy because the majority of the patients had previously progressed on BRAF/MEK inhibitors or had discontinued treatment on account of poor tolerance; most of these patients (15 of 21 [71%]) did not have an intervening therapy after the prior BRAF/MEK inhibitor treatment. Although this was consistent with previous observations of successful BRAF/MEK inhibition rechallenge, the combination of encorafenib plus binimetinib showed no new safety concerns and a tolerable toxicity profile.25, 26, 27, 28, 29 The potential for physicians to offer patients an alternative course of BRAF/MEK inhibitor therapy would expand the therapeutic armamentarium for improving outcomes while limiting toxicity and preserving quality of life for patients with MBMs.
The safety data observed in this analysis are consistent with the known profile of encorafenib plus binimetinib in patients with BRAF‐mutant metastatic melanoma who do not have brain metastases. Notably, although there are limited data on the ability of BRAF/MEK inhibitor combinations to cross the blood‐brain barrier, intracranial antitumor activity reported in this study suggests that the regimen is indeed able to penetrate the central nervous system to reach these metastases.30 Also, it should be noted that encorafenib has a much longer dissociation constant from its target and may achieve more sustainable MAPK inhibition once it crosses the blood‐brain barrier, which may underlie its clinical activity, although it remains to be determined whether this will result in a longer time to progression. Ongoing preclinical research is further characterizing the ability of encorafenib and other BRAF inhibitors to cross the blood‐brain barrier.31
Even though the majority of the patients in this analysis had been previously treated with BRAF/MEK inhibitors, these data are largely consistent with the available literature for BRAF/MEK inhibitor treatment of brain metastases in patients with melanoma, none of which has included patients with prior BRAF/MEK inhibitor treatment. Intracranial response rates varying between 20% and 40% have been observed for dabrafenib and vemurafenib in exploratory studies.8, 9, 10, 11, 14 In addition, a recent prospective study evaluating the combination of dabrafenib plus trametinib in a cohort of patients with MBMs who had BRAF V600E mutations and no prior central nervous system–directed therapy or prior BRAF/MEK inhibitor reported a median progression‐free survival of 5.6 months and a median overall survival of 10.8 months.14 These results were also consistent with a recent retrospective case series in 65 patients with melanoma and active brain metastases treated with a combination of BRAF/MEK inhibitors, in which a median progression‐free survival of 5.3 months and a median overall survival of 9.5 months were observed.11 Notably for dabrafenib‐trametinib data, an intracranial progression‐free survival of 5.6 months14 is significantly shorter than the median progression‐free survival of 10.1 months reported in phase 3 studies,8 indicating a shorter duration of response and disease control for targeted therapy in the brain. This is also highlighted by the fact that the majority of progression events in COMBI‐MB (Study to Evaluate Treatment of Dabrafenib Plus Trametinib in Subjects With BRAF Mutation‐Positive Melanoma That Has Metastasized to the Brain) were in the brain.
There are several limitations to this report. First, this case series does not include sufficient median follow‐up time to allow for an analysis of progression‐free survival or overall survival. Also, interpretation of the study results is limited because the study was retrospective, nonrandomized, and uncontrolled. However, our population may have been more representative of real‐world patients because of the generally more restrictive inclusion criteria in most clinical trials. Because this is a retrospective analysis, the collection of data is more challenging in comparison with a formal prospective clinical trial and may suffer from unclear reporting or underreporting of adverse events. Finally, this evaluation is based on observations at only 3 sites with 24 patients, and more definitive conclusions would require a prospective evaluation of a much larger sample size. Because of the promising results observed, further research is warranted. Notably, a phase 2, open‐label, randomized, multicenter trial of encorafenib plus binimetinib evaluating a standard‐dose regimen (encorafenib at 450 mg once daily and binimetinib at 45 mg twice daily) and a high‐dose regimen (encorafenib at 300 mg twice daily and binimetinib at 45 mg twice daily) in patients with BRAF V600–mutant MBMs is underway (NCT03911869).
In summary, this retrospective case series showed intracranial activity for the combination of encorafenib plus binimetinib in patients with BRAF‐mutant MBMs. Responses were observed in both BRAF/MEK inhibitor–naive and pretreated patients. Because of these promising exploratory results and the limited options available for the treatment of brain metastases in BRAF‐mutant metastatic melanoma, further prospective studies are warranted to confirm the efficacy and sequencing of BRAF/MEK inhibitor regimens in this population.
Funding Support
Editorial support, funded by Array Biopharma, Inc and under the direction of the authors, was provided by JD Cox, PhD and Mayville Medical Communications in accordance with Good Publication Practice 3 guidelines. The University of Texas MD Anderson Cancer Center is supported by the National Institutes of Health (grant P30 CA016672).
Conflict of Interest Disclosures
Jose Lutzky reports belonging to speakers bureaus for Array, Regeneron, and Novartis and to advisory boards for Novartis, Regeneron, Array, and Bristol‐Myers Squibb. Michael A. Davies reports being compensated for membership in advisory boards for Array, GSK, Roche/Genentech, Novartis, Bristol‐Myers Squibb, Sanofi‐Aventis, Vaccinex, and Syndax; being an uncompensated consultant for Nanostring; receiving grants from Bristol‐Myers Squibb; and being the principal investigator for research grants to his institution from AstraZeneca, Roche/Genentech, GSK, Myriad, Oncothyreon, and Sanofi‐Aventis. Jessica Michaud Davis reports belonging to a speakers bureau for Exelixis and to an advisory board for Array. Isabella C. Glitza reports receiving research support from Bristol‐Myers Squibb and Merck, consultant fees from Array and Bristol‐Myers Squibb, and speaker fees from Array. Rodabe N. Amaria reports research funding from Merck, Bristol‐Myers Squibb, Genentech, Array, and Iovance. Sapna P. Patel reports belonging to advisory boards for Cardinal Health, Castle Biosciences, and Incyte and to data and safety monitoring boards for Immunocore and Reata; receiving clinical trial support from Bristol‐Myers Squibb, Deciphera, Ideaya, Novartis, Provectus, and Reata; and receiving nonpromotional speaker fees from Merck. Asim Amin reports belonging to advisory boards for Exelixis, Dynavax Technologies, Novartis, Bristol‐Myers Squibb, Genzyme Corporation, Regeneron, and Merck and to speakers bureaus for Novartis, Bristol‐Myers Squibb, Genzyme Corporation, and Regeneron and performing clinical trial research for Bristol‐Myers Squibb, Dynavax Technologies Corporation, and Merck & Co. Hussein Tawbi reports consulting for Merck, Novartis, Genentech, Bristol‐Myers Squibb, and Array and institutional research funding from Merck, Celgene, Bristol‐Myers Squibb, Genentech, and GSK. The other authors made no disclosures.
Author Contributions
Kourtney Holbrook: Study design, data collection, and manuscript development. Jose Lutzky: Study design, data collection, and manuscript development. Michael A. Davies: Study design, data collection, and manuscript development. Jessica Michaud Davis: Study design, data collection, and manuscript development. Isabella C. Glitza: Study design, data collection, and manuscript development. Rodabe N. Amaria: Study design, data collection, and manuscript development. Adi Diab: Study design, data collection, and manuscript development. Sapna P. Patel: Study design, data collection, and manuscript development. Asim Amin: Study design, data collection, and manuscript development. Hussein Tawbi: Study design, data collection, and manuscript development.
The first 2 authors contributed equally to this article as first authors.
The last 2 authors contributed equally to this article as final authors.
We thank the individuals and their families for allowing their data to be included in this analysis.
References
- 1. Cohen JV, Tawbi H, Margolin KA, et al. Melanoma central nervous system metastases: current approaches, challenges, and opportunities. Pigment Cell Melanoma Res. 2016;29:627‐642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sampson JH, Carter JH Jr, Friedman AH, Seigler HF. Demographics, prognosis, and therapy in 702 patients with brain metastases from malignant melanoma. J Neurosurg. 1998;88:11‐20. [DOI] [PubMed] [Google Scholar]
- 3. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5‐29. [DOI] [PubMed] [Google Scholar]
- 4. Long GV, Margolin KA. Multidisciplinary approach to brain metastasis from melanoma: the emerging role of systemic therapies. Am Soc Clin Oncol Educ Book. 2013;33:393‐398. [DOI] [PubMed] [Google Scholar]
- 5. Budman DR, Camacho E, Wittes RE. The current causes of death in patients with malignant melanoma. Eur J Cancer. 1978;14:327‐330. [DOI] [PubMed] [Google Scholar]
- 6. Davies MA, Liu P, McIntyre S, et al. Prognostic factors for survival in melanoma patients with brain metastases. Cancer. 2011;117:1687‐1696. [DOI] [PubMed] [Google Scholar]
- 7. Ray S, Dacosta‐Byfield S, Ganguli A, Bonthapally V, Teitelbaum A. Comparative analysis of survival, treatment, cost and resource use among patients newly diagnosed with brain metastasis by initial primary cancer. J Neurooncol. 2013;114:117‐125. [DOI] [PubMed] [Google Scholar]
- 8. Long GV, Trefzer U, Davies MA, et al. Dabrafenib in patients with Val600Glu or Val600Lys BRAF–mutant melanoma metastatic to the brain (BREAK‐MB): a multicentre, open‐label, phase 2 trial. Lancet Oncol. 2012;13:1087‐1095. [DOI] [PubMed] [Google Scholar]
- 9. Falchook GS, Long GV, Kurzrock R, et al. Dabrafenib in patients with melanoma, untreated brain metastases, and other solid tumours: a phase 1 dose‐escalation trial. Lancet. 2012;379:1893‐1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dummer R, Goldinger SM, Turtschi CP, et al. Vemurafenib in patients with BRAF(V600) mutation–positive melanoma with symptomatic brain metastases: final results of an open‐label pilot study. Eur J Cancer. 2014;50:611‐621. [DOI] [PubMed] [Google Scholar]
- 11. Drago JZ, Lawrence D, Livingstone E, et al. Clinical experience with combination BRAF/MEK inhibitors for melanoma with brain metastases: a real‐life multicenter study. Melanoma Res. 2019;29:65‐69. [DOI] [PubMed] [Google Scholar]
- 12. Tawbi HA, Forsyth PA, Algazi A, et al. Combined nivolumab and ipilimumab in melanoma metastatic to the brain. N Engl J Med. 2018;379:722‐730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Goyal S, Silk AW, Tian S, et al. The clinical management of multiple melanoma brain metastases: a systematic review. JAMA Oncol. 2015;1:668‐676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Davies MA, Saiag P, Robert C, et al. Dabrafenib plus trametinib in patients with BRAFV600‐mutant melanoma brain metastases (COMBI‐MB): a multicentre, multicohort, open‐label, phase 2 trial. Lancet Oncol. 2017;18:863‐873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jakob JA, Bassett RL Jr, Ng CS, et al. NRAS mutation status is an independent prognostic factor in metastatic melanoma. Cancer. 2012;118:4014‐4023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cicenas J, Tamosaitis L, Kvederaviciute K, et al. KRAS, NRAS and BRAF mutations in colorectal cancer and melanoma. Med Oncol. 2017;34:26. [DOI] [PubMed] [Google Scholar]
- 17. Center for Drug Evaluation and Research . Approved drugs–trametinib and dabrafenib. Accessed March 4, 2019. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-dabrafenib-plus-trametinib-adjuvant-treatment-melanoma-braf-v600e-or-v600k-mutations
- 18. Dummer R, Ascierto PA, Gogas HJ, et al. Encorafenib plus binimetinib versus vemurafenib or encorafenib in participants with BRAF‐mutant melanoma (COLUMBUS): a multicentre, open‐label, randomised phase 3 trial. Lancet Oncol. 2018;19:603‐615. [DOI] [PubMed] [Google Scholar]
- 19. Webster RM, Mentzer SE. The malignant melanoma landscape. Nat Rev Drug Discov. 2014;13:491‐492. [DOI] [PubMed] [Google Scholar]
- 20. Long GV, Stroyakovskiy D, Gogas H, et al. Dabrafenib and trametinib versus dabrafenib and placebo for Val600 BRAF–mutant melanoma: a multicentre, double‐blind, phase 3 randomised controlled trial. Lancet. 2015;386:444‐451. [DOI] [PubMed] [Google Scholar]
- 21. Robert C, Karaszewska B, Schachter J, et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. N Engl J Med. 2015;372:30‐39. [DOI] [PubMed] [Google Scholar]
- 22. McArthur GA, Maio M, Arance A, et al. Vemurafenib in metastatic melanoma patients with brain metastases: an open‐label, single‐arm, phase 2 multicentre study. Ann Oncol. 2017;28:634‐641. [DOI] [PubMed] [Google Scholar]
- 23. Dummer R, Ascierto PA, Gogas HJ, et al. Overall survival in patients with BRAF‐mutant melanoma receiving encorafenib plus binimetinib versus vemurafenib or encorafenib (COLUMBUS): a multicentre, open‐label, randomised, phase 3 trial. Lancet Oncol. 2018;19:1315‐1327. [DOI] [PubMed] [Google Scholar]
- 24. Tawbi HA, Boutros C, Kok D, Robert C, McArthur G. New era in the management of melanoma brain metastases. Am Soc Clin Oncol Educ Book. 2018;38:741‐750. [DOI] [PubMed] [Google Scholar]
- 25. Seghers AC, Wilgenhof S, Lebbe C, Neyns B. Successful rechallenge in two patients with BRAF‐V600–mutant melanoma who experienced previous progression during treatment with a selective BRAF inhibitor. Melanoma Res. 2012;22:466‐472. [DOI] [PubMed] [Google Scholar]
- 26. Roux J, Pages C, Malouf D, et al. BRAF inhibitor rechallenge in patients with advanced BRAF V600–mutant melanoma. Melanoma Res. 2015;25:559‐563. [DOI] [PubMed] [Google Scholar]
- 27. Desvignes C, Abi Rached H, Templier C, et al. BRAF inhibitor discontinuation and rechallenge in advanced melanoma patients with a complete initial treatment response. Melanoma Res. 2017;27:281‐287. [DOI] [PubMed] [Google Scholar]
- 28. Valpione S, Carlino MS, Mangana J, et al. Rechallenge with BRAF‐directed treatment in metastatic melanoma: a multi‐institutional retrospective study. Eur J Cancer. 2018;91:116‐124. [DOI] [PubMed] [Google Scholar]
- 29. Viñal D, Martinez D, Espinosa E. Efficacy of rechallenge with BRAF inhibition therapy in patients with advanced BRAFV600 mutant melanoma. Clin Transl Oncol. 2019;21:1061‐1066. [DOI] [PubMed] [Google Scholar]
- 30. Eroglu Z, Holmen SL, Chen Q, et al. Melanoma central nervous system metastases: an update to approaches, challenges, and opportunities. Pigment Cell Melanoma Res. 2019;32:458‐469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wang YI, Abaci HE, Shuler ML. Microfluidic blood‐brain barrier model provides in vivo‐like barrier properties for drug permeability screening. Biotechnol Bioeng. 2017;114:184‐194. [DOI] [PMC free article] [PubMed] [Google Scholar]


