Abstract
Background and Objectives
Helium plasma skin regeneration (PSR) is a novel skin rejuvenation technology with significant differences compared with nitrogen PSR technology but that may exert similar skin tissue effects. Study objectives included a comparison of acute and chronic skin tissue changes among the two plasmas in a porcine animal model.
Study Design/Materials and Methods
In this study, both helium and nitrogen gas plasmas were used to treat the dorsal skin of Yorkshire cross mini pigs with 20% (8.6 J/cm2) and 40% (17.8 J/cm2) power helium plasma single pass treatment (4 liter gas flow, continuous energy delivery, and linear non‐overlapping passes) compared with high energy nitrogen plasma double pass treatment (PSR3 @ 14.1 J/cm2: 4.0 J, 2.5 Hz pulse rate, overlapping horizontal, and vertical passes). Acute and chronic skin contraction, maximum acute depth of injury and chronic reparative healing depth were assessed along with representative histopathology in each treatment paradigm.
Results
High‐energy nitrogen plasma treatment exhibited greatest mean depth of acute tissue injury 4 hours post‐treatment whereas helium plasma treatment exhibited greater acute skin tissue contraction. Then, 20% and 40% power helium plasma treatment results were each very similar among animals as a percentage of nitrogen plasma treatment results for both depths of acute tissue injury and acute skin tissue contraction. Mean depths of reparative tissue healing were similar among treatment paradigms 30 days after treatment with significant intra‐ and inter‐animal variability observed within each treatment paradigm. Thirty‐day mean skin tissue contraction was greater for helium plasma treatment; however, the data varied significantly between animals in all paradigms. Histopathologic tissue evaluation after 30 days showed similar findings among the treatment paradigms with epidermal hyperplasia, flattening of rete ridges and with regenerative granulation tissue expanding the superficial and papillary dermis.
Conclusions
This study demonstrates modestly reduced depth of the thermal effect, greater skin tissue contraction and similarity of acute and chronic histopathological findings for helium plasma when compared with nitrogen plasma in a porcine animal model. © 2019 The Authors. Lasers in Surgery and Medicine published by Wiley Periodicals, Inc.
Keywords: nitrogen plasma, helium plasma, radiofrequency, skin regeneration, coagulation, impedance, energy density
INTRODUCTION
Nitrogen plasma skin regeneration (PSR) remains a viable option for skin rejuvenation. The unique heat signature and healing profile of nitrogen plasma results in a non‐ablative (at time of treatment), non‐chromophore‐dependent thermal wound with initial preservation of the upper layers of desiccated skin tissue that serve as a natural biological dressing during the skin's regenerative healing process 1, 2. The lack of an open wound, relatively rapid epidermal recovery (typically within 7 days after treatment), non‐fractionated (effectively full field) energy delivery and suitability for diverse skin types are among the more significant clinical merits of this skin rejuvenation treatment 3, 4.
Nitrogen PSR histological studies (porcine, human) showed significant neo‐collagenesis and a corresponding reduction in elastosis in the upper dermis 4, 5, 6. Clinical evaluation of nitrogen PSR treatment effectiveness demonstrated benefits in the appearance of treated skin with improvements in dyschromia, photodamage and skin texture including reduction of acne scarring and rhytidosis 1, 2, 4, 7, 8, 9. Complications resulting from nitrogen PSR treatment include focally delayed healing, post‐inflammatory hyperpigmentation, and rare hypertrophic scarring events 1, 4, 10. Despite treatment protocols that include darker skin types permanent hypopigmentation has not been reported.
Benefits of nitrogen PSR include relatively quick healing, low incidence of complications, suitability for intermediate skin types (e.g., Fitzpatrick III, IV), full‐field treatment of dyschromia and photodamage, diversity of protocols for various skin conditions, high patient acceptance and the potential for improvement of rhytidosis. One significant disadvantage, however, is that reduction of moderate to severe rhytidosis remains inferior to conventional full‐field, multi‐pass, ablative CO2 or erbium YAG laser skin resurfacing. A novel, previously Food and Drug Administration (FDA) cleared helium gas plasma soft tissue coagulation device (initially developed for surgical use) with potential for skin resurfacing applications was evaluated for similar treatment outcomes versus nitrogen PSR (the positive control) prior to initiating clinical studies—including initial characterization of helium gas plasma skin tissue effects and comparison to nitrogen PSR in a live porcine skin tissue treatment model.
METHODS
The animal studies were conducted at American Preclinical Services (Minneapolis, MN) using four juvenile porcine Yorkshire cross animals. The animal studies were conducted at Americal Preclinical Services using four juvenile porcine Yorkshire cross animals, with two animals survived four hours following treatment and two animals survived thirty days following treatment. Thirty‐six test locations were identified and marked along the dorsum of each animal using tattoo ink. The dorsum was chosen for this initial study due to the need to survive the animal for 30 days while minimizing the potential for additional non‐study‐related injury to the treatment sites.
The helium gas plasma soft tissue coagulation device (Bovie J‐Plasma; Bovie Medical Corporation, Clearwater, FL) was evaluated in a commercially available FDA‐approved format with energies selected based on the depth of thermal effects observed for non‐dermatologic tissues at much higher energy levels 11. Helium plasma energy delivery consisted of single, linear, non‐overlapping passes with continuous energy delivery and 4 L/min helium gas flow at 20% and 40% power levels correlating to energy densities below (8.6 J/cm2) and above (17.8 J/cm2) that used for nitrogen PSR treatment (14.1 J/cm2).
The nitrogen PSR device (Energist Medical Group formerly Rhytec, Swansea, United Kingdom) was evaluated with the highest energy (4.0 J, 2.5 Hz pulse rate and energy density 14.1 J/cm2—see Table 1) double pass (PSR3) treatment protocol available to maximize depth of skin tissue effects and to enable this device to serve as the positive control for these studies. Of note, the technical specifications for the more recently FDA‐approved device (NeoGen PSR System, 2013) are identical to those of the original FDA‐approved device (Portrait PSR System). While highest energy, double pass nitrogen PSR treatment is certainly not appropriate for all skin types or conditions in the clinical setting, these parameters are appropriate for patients with lighter skin types and deep rhytids in the cheek and peri‐oral areas.
Table 1.
Comparison of Thermal and Physical Properties of Helium and Nitrogen Gas Plasmas Used in this Study
| Helium plasma | Nitrogen plasma | |
|---|---|---|
| Molar heat volume (J/mol·K) | 12.4 | 19.9 |
| Plasma generation | Direct discharge | Local discharge |
| Top‐down thermal conduction | Yes | Yes |
| In‐depth joule tissue heating | Yes | No |
| Plasma beam diameter at target | 3 mm | 6 mm |
| Energy delivery | Continuous (or pulsed) | Pulsed (1.0–2.5 Hz) |
| Study treatment energy (Watts or J/s) | 20% power = 3.2 | 4 Joules @ 2.5 Hz = 10 |
| 40% power = 6.6 | ||
| Energy density (J/cm2) | 20% power = 8.6 | 14.1 |
| 40% power = 17.8 |
The study treatment energies for the helium plasma soft tissue coagulation device were determined with calorimetry performed with continuous energy delivery. Because energy delivery with the helium plasma device is dynamic (treatment tip continuously moving over the tissue) an average tip velocity of 1 cm/s was used to determine tissue area treated in 1 second (3 mm spot width × 10 mm length) with resulting oval surface area of 0.37 cm2 used in the helium plasma energy density calculations.
All measurements, necropsies, tissue preparation, and histological staining (Hemoatoxylin and Eosin, Masson's trichrome) were performed by American Preclinical Services Pathology Department. The study pathologist (co‐author AS) was blinded to the treatment identification information for each site during histopathologic slide review. This was a GLP (Good Laboratory Practice) study performed in compliance with the study protocol and amendments and Pre‐Clinical Pathology Consulting Service standard operating procedures.
Dermal tissue injury was evaluated for each treatment site with a depth of injury measured from the dermal‐epidermal junction (basement membrane) to the deepest focus of dermal injury. This focus was measured in the approximate center of the treatment site where possible with a depth of the skin tissue injury measurements captured using a validated ocular reticle in the Olympus BX45 microscope with each site measured in millimeters. The treatment site squares had been previously outlined with blue tattoo ink. The linear dimensions of the upper and lower horizontal sides and the right and left vertical sides were measured in millimeters with the horizontal and vertical measurements averaged for each square and then multiplied to obtain average surface area dimensions for each square. Raw depth data and treatment site dimensions obtained in each animal consisted of eight treatment sites for the helium plasma groups and the high‐energy nitrogen plasma group with the exception of only four treatment sites for high energy nitrogen plasma in animal 18P0439. These data were analyzed and converted into a graphical format using Kaleidagraph 4.0 (Synergy Software, Reading, Pennsylvania).
RESULTS
Acute Histopathology and Injury Depth Data
In the acute animals, nitrogen PSR treatment (double pass or PSR3, 4.0 J) and helium gas plasma treatment at both 20% and 40% power all resulted in acute thermal injury histopathologically evidenced by necrotic epidermis adherent to basement membrane and deeper dermal soft tissue coagulative (necrotic) injury extending into the superficial reticular dermis (Fig. 1, note retention of scarlet stain in tissue heated above its denaturation temperature as well as the relatively sharp demarcation between the scarlet‐stained tissue above and the airline blue‐stained tissue below with the latter representative of non‐denatured tissue deeper in the dermis 12). Depth of acute tissue injury varied among animals where high energy nitrogen plasma treatments exhibited greatest depth and where 20% and 40% power helium plasma treatments were each very similar among animals as a percentage of nitrogen plasma treatment depth (Fig. 2). Acute tissue injury depth for 20% and 40% helium plasma treatment in each of two animals was 54% and 57% and 66% and 71%, respectively, that of nitrogen plasma. Measured acute injury depths below the basement membrane (all sites, both animals) averaged 404 μm for nitrogen plasma treatment and 222 and 281 μm for 20% and 40% helium plasma, respectively.
Figure 1.
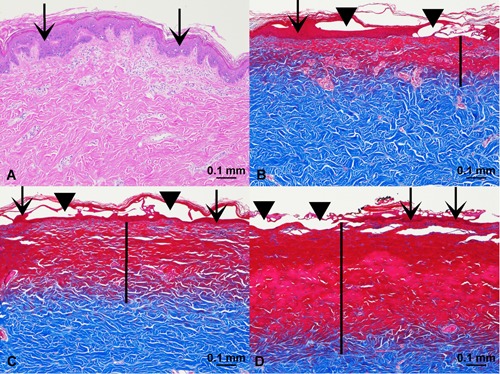
Acute tissue injury. (A) Control, hematoxylin and eosin (H&E) stain, ×10 magnification. (B) Helium plasma 20% power (single pass), Masson's trichrome stain, ×10 magnification. (C) Helium plasma 40% power (single pass), Masson's trichrome stain, ×10 magnification. (D) Nitrogen plasma PSR3 (4.0 J), Masson's trichrome stain, ×10 magnification. Scale bars = 0.1 mm. Vertical black bars designate depth of dermal injury, extending into the papillary and reticular dermis. Scarlet stained tissue in 1B, C, D indicates denatured tissue characterized by coagulative necrosis.
Figure 2.
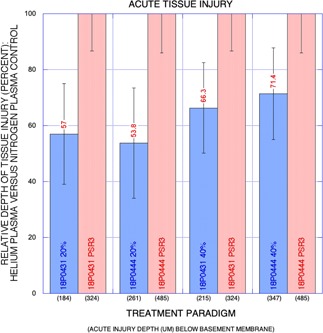
Graph: Relative depth of acute tissue injury (percent) for helium plasma versus nitrogen plasma control (y‐axis) with treatment paradigm on x‐axis and mean depth of tissue injury in micrometers below basement membrane also shown. Note the greater depth of effect for the nitrogen plasma treatment paradigm and non‐overlap of helium and nitrogen plasma standard deviation error bars. While the mean depth of tissue injury was greater in one animal (18P0444), the relative depth of injury as a percentage of nitrogen plasma injury depth was remarkably similar among the helium plasma treatment paradigms in the two animals.
Thirty‐Day Histopathology and Reparative Healing Depth Data
In the 30‐day‐survived animals, nitrogen PSR treatment (double pass or PSR3, 4.0 J) and helium gas plasma treatment at both 20% and 40% power all exhibited epidermal hyperplasia, loss of rete ridges and regenerative granulation tissue expanding the superficial and papillary dermis (Fig. 3). Significant intra‐ and inter‐animal variability was observed within each treatment paradigm and mean depths of reparative tissue healing were similar among treatment paradigms at 30 days after treatment (Fig. 4). Measured chronic reparative healing depths below the basement membrane (all sites, both animals) averaged 360 μm for nitrogen plasma treatment and 283 and 272 μm for 20% and 40% helium plasma, respectively.
Figure 3.
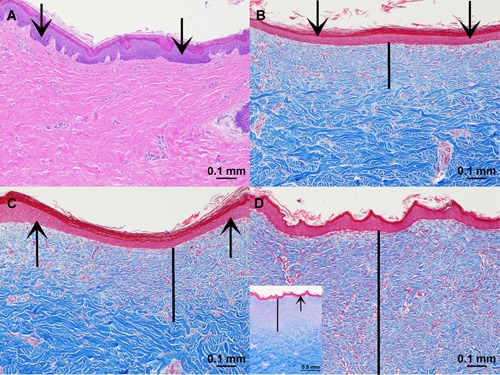
Thirty‐day reparative healing depth. (A) Control, hematoxylin and eosin (H&E) stain, ×10 magnification. (B) Helium plasma 20% power (single pass), Masson's trichrome stain, ×10 magnification. (C) Helium plasma 40% power (single pass), Masson's trichrome stain, ×10 magnification. (D) Nitrogen plasma PSR3 (4.0 J), Masson's trichrome stain, ×10 magnification with smaller inset lower left at ×4 magnification for orientation. Scale bars (except inset in D) = 0.1 mm. Vertical black bars designate the depth of reparative tissue healing extending into the dermis.
Figure 4.
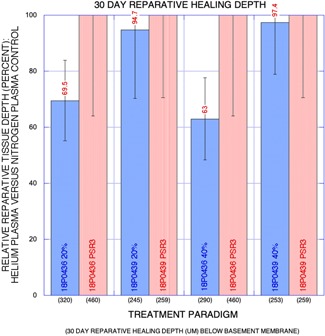
Graph: Relative 30‐day reparative healing depth (percent) for helium plasma versus nitrogen plasma control (y‐axis) with a treatment paradigm on x‐axis and mean reparative healing depth in micrometers below basement membrane are also shown. Although the greater depth of effect was observed for the nitrogen plasma treatment paradigm, overlap of nitrogen and helium plasma standard deviation error bars was observed in both paradigms in each animal. Depth of reparative healing tissue was greater in one animal (18P0436) for both helium and nitrogen plasma treatment paradigms.
Acute and 30‐Day Skin Contraction Data
Representative gross treatment site photographs for acute pre‐ and post‐treatment and chronic pre‐treatment and pre‐necropsy at 30 days are shown in Figures 5 and 6. Acute skin tissue contraction was observed in all treatment paradigms (Fig. 7) and averaged −5.4% among the animals for nitrogen plasma treatment. Acute skin tissue contraction was much greater for helium plasma treatments at both 20% and 40% power with low inter‐animal variability (4–16%) as a percentage of nitrogen plasma treatment (193–231% greater contraction at 20% power and 258–269% greater contraction at 40% power, Fig. 7). The treatment site area reductions were also observed in all treatment paradigms at thirty days following treatment. Significant inter‐animal variability was observed for all treatment paradigms at 30 days following treatment with greatest skin tissue contraction observed for helium plasma treatment (Fig. 8). A potentially important factor impacting these results is that the survived animals gained 10% body mass (and a corresponding increase in girth that resulted in an increase in treatment site areas for the untreated control sites) during the 30‐day post‐treatment interval—the increase in area of the controls was accounted for in the calculations as a further decrease in area that had been overcome by the treatment.
Figure 5.
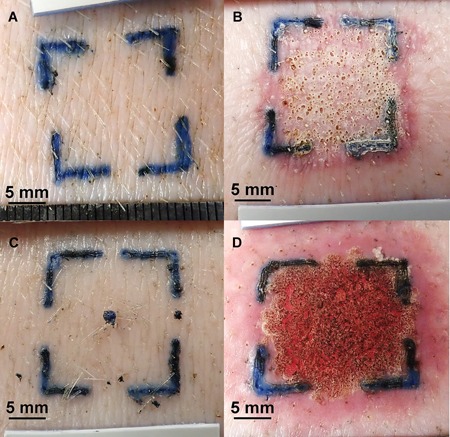
Gross photographs (animal 18P0444): (A) Forty percent of helium plasma pre‐treatment. (B) Forty percent of helium plasma 4 hours post‐treatment. (C) PSR3 pre‐treatment. (D) PSR3 4 hours post‐treatment. Scale bars = 5 mm. Note the similarity of superficial coagulation (necrotic epidermis) in (B) and (D) and the greater erythematous reaction of surrounding tissue in (D) along with grossly visible tissue contraction in (B) versus (A) and (D) versus (C).
Figure 6.
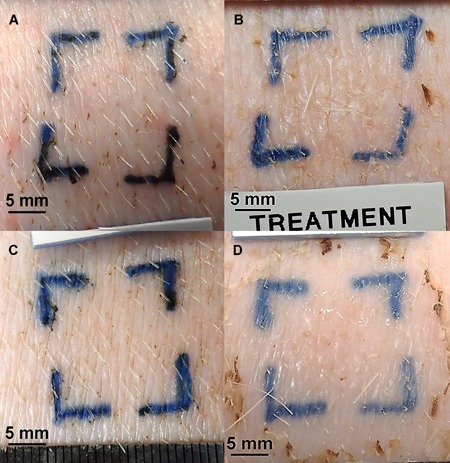
Gross photographs (animal 18P0436): (A) Forty percent of helium plasma pre‐treatment. (B) Forty percent of helium plasma 30 days post‐treatment. (C) PSR3 pre‐treatment. (D) PSR3 30 days post‐treatment. Scale bars = 5 mm. Note the similarity of healing in (B) and (D) and ambiguity of visible tissue contraction in (B) versus (A) and (D) versus (C) (see text).
Figure 7.
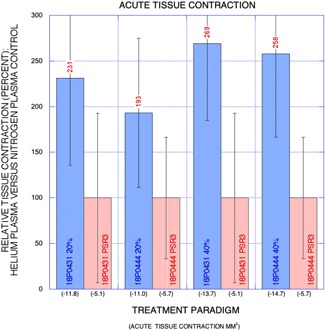
Graph: Relative net acute change in treatment site area (percent) for helium plasma versus nitrogen plasma control (y‐axis) with treatment paradigm on x‐axis and mean change in treatment site area in millimeters squared are also shown. Greatest percent reduction in treatment site area was observed for the 40% helium plasma treatment paradigm with similar findings among the two animals.
Figure 8.
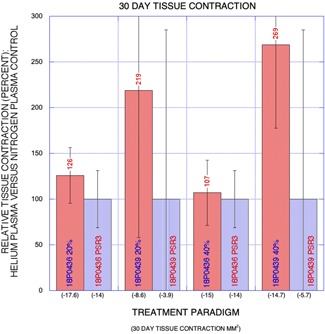
Graph: Relative net 30‐day change in treatment site area (percent) for helium plasma versus nitrogen plasma control (y‐axis) with treatment paradigm on the x‐axis and mean change in treatment site area in millimeters squared are also shown. Greatest tissue contraction was observed for both helium plasma treatment paradigms and the relative area decrease was much higher in one animal (18P0439).
DISCUSSION
The purpose of this study was to evaluate the suitability of helium gas plasma for skin resurfacing procedures in a pre‐clinical model and to compare the technology to the predicate nitrogen PSR technology that is currently FDA cleared for that indication. Thermal injury depth measurements provided data related to the safety of the helium plasma device for the application. Soft tissue contraction measurements provided further insight as to the potential efficacy of the helium plasma device for wrinkle reduction through the contraction of soft tissue. Representative histology from the two plasmas provided a detailed comparison of the skin tissue effects at both time points.
To better understand and analyze the results of the study, it is important to outline differences in the mechanism of plasma beam generation and in the exerted skin tissue effects between the helium and nitrogen plasma devices. Nitrogen PSR treatment delivers heat to the tissue through the expulsion of energy generated by ionizing nitrogen gas through the use of microwave energy. Helium PSR treatment delivers heat to the tissue in a similar fashion but also via electrical current traveling from one electrode to another through an ionized helium gas bridge interface.
While both nitrogen and helium gases are stable, inert, ideal gases helium gas is monatomic with three degrees of freedom for subatomic thermal energy transitions whereas nitrogen gas is diatomic with five degrees of freedom for such energy transitions [note two additional degrees of freedom (total of seven) related to vibrational energy are also found at very high temperatures far exceeding those encountered in clinical applications] 13. The additional two degrees of freedom give nitrogen gas greater ability to store heat energy and comparison on an equi‐molar basis (where the same number of atoms of helium or molecules of nitrogen are arriving at the surface per unit time and transferring thermal energy) results in a higher molar volume heat capacity for nitrogen than for helium (Table 1: 19.9 vs. 12.4 J/mol·K, respectively).
This greater heat capacity for nitrogen gas is ideally suited for delivery of heat to the tissue in single brief pulses. The nitrogen beam applicator uses a “local discharge” process with the ionization process confined to the handpiece where microwaves heat a tungsten wire (susceptor) that then heats a flow of nitrogen gas as well as partially ionizing the nitrogen gas. Once the ionized nitrogen gas leaves the applicator nozzle the excited electrons move to lower energy states and emit the characteristic yellow optical emission (Lewis Rayleigh afterglow) as photons of a specifically visible wavelength escape and the plasma converts back to the stable gas state. Heat distribution within the nitrogen plasma pulse is Gaussian in nature and tissue heating occurs with top‐down thermal transfer from the flowing hot nitrogen gas as much of the nitrogen gas may no longer be ionized at the time of thermal energy transfer.
In contrast, with its lower heat capacity and unique electrical properties helium gas is more suited for a very different energy delivery paradigm wherein helium plasma is created through a “direct discharge” process where a continuous electrical discharge path exists from the tip of the handpiece (positive electrode or cathode) to the target tissue (negative ground or anode). While a small percentage of the helium gas is ionized in the non‐heated state (cold atmospheric plasma) addition of radiofrequency (RF) energy only incrementally increases ionization of the helium gas flow (e.g., <0.1%) but does so continuously along the beam path down to the target tissue. RF energy is delivered to the handpiece by the generator and used to energize the positive electrode. When helium gas is passed over the energized electrode, helium plasma is generated, which enables heat to be applied to tissue in two distinct modes.
First, top‐down thermal transfer from the flowing hot helium gas occurs with heat generated by the actual production of the plasma beam itself through the ionization and rapid neutralization of the helium atoms. The neutralization of the helium atoms gives rise to the characteristic violet optical emission (Lewis Raleigh afterglow). Second, as plasmas are very good electrical conductors, a portion of the RF energy used to energize the electrode and generate the plasma passes from the electrode to the tissue. Electrical displacement current is formed in the tissue region immediately surrounding the helium plasma beam contact point, both across the tissue surface and in‐depth. In one‐half cycle, current flows into this region, accumulating charge that is withdrawn in the next half‐cycle. The flow of current through the resistance of the tissue creates Joule (resistive) heating.
The direct discharge process and the supply gas utilized by the investigative device may account for the differences in skin tissue effects noted in this study when compared with the nitrogen PSR system. The conductive helium plasma beam produced by the direct discharge process can be thought of as a flexible wire or electrode bridge that “connects” to the tissue that represents the path of least resistance to the flow of the RF energy. The tissue that represents the path of least resistance is typically either the tissue that is in closest proximity to the tip of the device or the tissue that has the lowest impedance (is the easiest to pass energy through). As tissue is treated, it coagulates and desiccates, and the impedance of the tissue increases. As this occurs, the path of least resistance is constantly changing, and subsequently, the permissive, seemingly chaotic energy (plasma beam) branches out laterally from treated, higher impedance tissue to untreated, lower impedance tissue. This results in non‐Gaussian energy deposition with impedance‐dependent lateral thermal spread and a decrease in the depth of thermal effect for the helium plasma device. As helium gas has a simple molecular structure consisting of only two electrons it is easily ionized by low current RF energy. Since the current of the RF energy is so low, it is dispersed before it is able to penetrate deep into the tissue. This allows for effective soft tissue heating and contraction with a relatively shallow depth of the thermal effect. This self‐limiting nature of helium plasma's thermal depth of tissue effect has been demonstrated in several other tissue types and with much higher energy settings than used in this study 11.
Variables that may potentially impact skin tissue effects of both plasma devices include handpiece velocity, treatment tip configuration and distance from the tissue surface as well as gas flow rates, energy pulsing schemes, energy “beam” configuration and number of passes. The treatment tip to target tissue distance may be of more importance for the nitrogen plasma treatment as an increase or decrease from the recommended offset distance will have a similar direct effect on the depth of tissue injury. For the helium plasma device increasing the distance from the target tissue beyond a minimum electrical coupling distance (e.g., 5 mm) will cause the helium plasma RF bridge to sever resulting in no tissue effect. Moving the helium plasma electrode closer to the target tissue will not significantly increase the depth of effect because of the more stable nature of the energized gas flow, continuously changing skin tissue impedance that effectively expands the beam peripherally (away from areas with higher impedance and towards areas with lower impedance) and device characteristics that may limit energy density‐independent of handpiece velocity. The electrocoupling characteristics and distance may be somewhat advantageous as holding the treatment tip closer to the skin surface will not significantly increase energy density and holding the treatment tip beyond the electrocoupling distance will sever the radiofrequency bridge resulting in no tissue effect—versus inverse relationship between treatment tip distance from skin surface and resulting tissue effects with the predicate device (increased/reduced tissue effect with decreasing/increasing distance between treatment tip and the skin). Additional specific variables that may affect helium plasma skin tissue effects include extrinsic factors that may affect the skin tissue impedance (e.g., injected local or tumescent anesthesia; e.g., topical anesthetic creams, gels, ointments) and device electrical configuration (e.g., reduced current limiting Joule tissue heating).
Available energy densities for the helium and nitrogen plasma systems overlap with maximum energy density for nitrogen PSR (14.1 J/cm2) corresponding to a power level of approximately 32% for helium plasma (Fig. 9). Although the molar heat volume capacity of nitrogen gas is higher than for helium gas, the helium plasma system is capable of much higher energy densities due to its RF generator design (available power range from 2 W up to 40 W), smaller plasma beam diameter at the target (spot size), continuous pulse train energy delivery (vs. maximum single‐pulse rate of 2.5 Hz for nitrogen PSR) and its additional Joule tissue heating (enabled by maintenance of the helium gas plasma bridge via continuous re‐ionization of helium along the beam path) beyond the common top‐down or surface tissue heating of both systems (Table 1).
Figure 9.
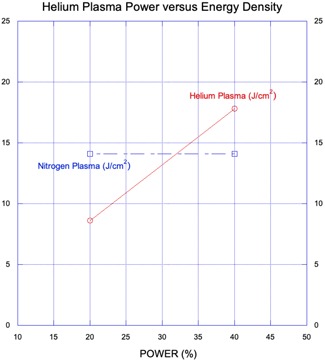
Graph: Helium plasma power (percent, x‐axis) versus helium plasma energy density (J/cm2, y1‐axis) with an overlay of nitrogen plasma energy density (J/cm2, y2‐axis). The energy density for helium plasma at 20% and 40% power is approximately 40% less and 20% higher, respectively, than that of nitrogen plasma. Maximum energy density for the nitrogen plasma system as tested is approximately equivalent to that of the helium plasma system at 32% power.
Despite these perceived advantages, the depth of RF Joule tissue heating during treatment with the helium gas plasma soft tissue coagulation device is again subject to skin tissue impedance and device characteristics (voltage, duration of current flow). Whereas unregulated RF energy and increased skin tissue impedance may paradoxically result in a greater extent of skin tissue injury 14 the helium plasma device's low current and continuous (five thousand times per second) impedance monitoring are designed to prevent unintended electrical current flow deep into the target tissue. Even so, it is established that full field and even fractional energy‐based skin resurfacing treatments at high energy densities may result in delayed healing and scarring, especially in more permissive treatment areas (e.g., neck) where the skin is thinner and/or less vascular 15.
Previous study of nitrogen PSR tissue effects in a Yucatan minipig model showed quick epidermal recovery with preservation of rete ridge formation at lower fluences and more extensive epidermal injury with irreversible thermal damage extending into the superficial dermis at higher fluences 3; the overall depth of irreversible tissue injury was more superficial than that observed in this study. We believe that the differences in histopathology observed in this study may be related to the enhanced depths of tissue effects and the still relatively early tissue sampling at a recovery timepoint of just 30 days. A longer post‐treatment recovery period before tissue sampling may help resolve this question. The use of a different pig breed (Yorkshire cross) in the current study may have affected the depth of tissue injury findings since prior evaluation of skin thickness in these two breeds demonstrated that the epidermis is approximately one‐third thinner in the Yorkshire breed 16. Differences in nitrogen plasma treatment tip position relative to the skin surface may have also contributed to the relative increased depth of tissue injury noted in this study. Inadvertently holding the nitrogen plasma treatment tip closer to or farther away from the skin surface directly impacts the effective treatment energy observed at the skin surface, increasing or lowering energy density, respectively. It is possible that the nitrogen plasma treatment tip position was held relatively closer to the skin in this study. Of note, similar movement of the helium plasma treatment tip does not significantly impact the effective treatment energy observed at the skin surface unless increased treatment tip to skin surface distance becomes sufficient to disrupt the RF bridge interface.
CONCLUSIONS
The differences between the helium and nitrogen plasma devices evaluated in this study result in differences in depth of thermal effect and in skin tissue contraction. The lower depths of thermal effect and larger percentages of skin tissue contraction for the helium plasma device compared to the FDA cleared nitrogen plasma device point to its potential suitability for use in skin resurfacing procedures. Despite significant differences in plasma generation and characteristics and in plasma—skin tissue interaction, high energy double‐pass nitrogen plasma and helium gas plasma treatment (20% and 40% power) of porcine skin (continuous energy delivery, single‐pass) exhibit similar acute and chronic histopathological changes. The greater skin tissue contraction exhibited by the helium plasma system at the lower energy density evaluated (40% lower than nitrogen PSR) may be explained by its unique bimodal energy delivery and its more complete full‐field energy delivery to the tissue. These effects may be compounded at the higher energy density evaluated (20% higher than nitrogen PSR).
The helium plasma device has been available commercially in an FDA‐approved format for general indications of ablation, coagulation, and cutting of soft tissue even as these pre‐clinical studies have been conducted. During this time the device has been in use (off label) by many practitioners for facial skin rejuvenation, both in previous generator design and in the updated generator design used in this study. While anecdotal clinical evidence shows much promise for this new technology for the treatment of facial rhytidosis, detailed study of helium plasma skin tissue effects and a formal clinical study evaluating the efficacy and safety are ongoing.
References
REFERENCES
- 1. Foster KW, Moy RL, Fincher EF. Advances in plasma skin regeneration. J Cosmet Dermatol 2008;7(3):169–179. [DOI] [PubMed] [Google Scholar]
- 2. Holcomb JD. Nitrogen plasma skin regeneration and aesthetic facial surgery: Multicenter evaluation of concurrent treatment. Arch Facial Plast Surg 2009;11(3):184–193. [DOI] [PubMed] [Google Scholar]
- 3. Fitzpatrick R, Bernstein E, Iyer S, Brown D, Andrews P, Penny K. A histopathologic evaluation of the plasma skin regeneration system (PSR) versus a standard carbon dioxide resurfacing laser in an animal model. Lasers Surg Med 2008;40(2):93–99. [DOI] [PubMed] [Google Scholar]
- 4. Kilmer S, Semchyshyn N, Shah G, Fitzpatrick R. A pilot study on the use of a plasma skin regeneration device (Portrait® PSR3) in full facial rejuvenation procedures. Lasers Med Sci 2007;22(2):101–109. [DOI] [PubMed] [Google Scholar]
- 5. Elsaie ML, Kammer JN. Evaluation of plasma skin regeneration technology for cutaneous remodeling. J Cosmet Dermatol 2008;7(4):309–311. [DOI] [PubMed] [Google Scholar]
- 6. Bogle MA, Arndt KA, Dover JS. Evaluation of plasma skin regeneration technology in low‐energy full‐facial rejuvenation. Arch Dermatol 2007;143(2):168–174. [DOI] [PubMed] [Google Scholar]
- 7. Potter MJ, Harrison R, Ramsden A, Bryan B, Andrews P, Gault D. Facial acne and fine lines: Transforming patient outcomes with plasma skin regeneration. Ann Plast Surg 2007;58(6):608–613. [DOI] [PubMed] [Google Scholar]
- 8. Bentkover SH. Plasma skin resurfacing: Personal experience and long‐term results. Facial Plast Surg Clin North Am 2012;20(2):145–162. [DOI] [PubMed] [Google Scholar]
- 9. Theppornpitak N, Udompataikul M, Chalermchai T, Ophaswongse S, Limtanyakul P. Nitrogen plasma skin regeneration for the treatment of mild‐to‐moderate periorbital wrinkles: A prospective, randomized, controlled evaluator‐blinded trial. J Cosmet Dermatol 2019;18(1):163–168. [DOI] [PubMed] [Google Scholar]
- 10. Kono T, Groff WF, Sakurai H, Yamaki T, Soejima K, Nozaki M. Treatment of traumatic scars using plasma skin regeneration (PSR) system. Lasers Surg. Med. 2009;41(2):128–130. [DOI] [PubMed] [Google Scholar]
- 11. Pedroso JD, Gutierrez MM, Volker KW, Howard DL. Thermal effect of J‐Plasma® in a porcine tissue model: Implications for minimally invasive surgery. Surg Technol Int 2017;30:19–24. [PubMed] [Google Scholar]
- 12. Flint MH, Lyons MF. The effect of heating and denaturation on the staining of collagen by the Masson trichome procedure. Histochem J 1975;7(6):547–555. [Google Scholar]
- 13. Current JD, editor. Physics Related to Anesthesia (Chapter 3, Vaporization [Heat Capacity]. 2nd edition Mainz, Germany: PediaPress GmbH, p 209. [Google Scholar]
- 14. Lee RC. Tissue injury from exposure to power frequency electrical fields In: Lin J, editor. Advances in Electromagnetic Fields in Living Systems. Germany: Springer; 1994. pp 81–127. [Google Scholar]
- 15. Avram MM, Tope WD, Yu T, Szachowicz E, Neslon JS. Hypertrophic scarring of the neck following ablative fractional carbon dioxide laser resurfacing. Lasers Surg Med 2009;41(3):185–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Eggleston TA, Roach WP, Mitchell MA, Smith K, Oler D, Johnson TE. Comparison of two porcine (Sus scrofa domestica) skin models for in vivo near‐infrared laser exposure. Comparative Med 2000;50(4):391–397. [PubMed] [Google Scholar]


