Abstract
Patients with melanoma brain metastases (MBM) still have a very poor prognosis. Several treatment modalities have been investigated in an attempt to improve the management of MBM. This review aimed to evaluate the impact of current treatments for MBM on patient‐ and tumor‐related outcomes, and to provide treatment recommendations for this patient population. A literature search in the databases PubMed, Embase, Web of Science and Cochrane was conducted up to January 8, 2019. Original articles published since 2010 describing patient‐ and tumor‐related outcomes of adult MBM patients treated with clearly defined systemic therapy were included. Information on basic trial demographics, treatment under investigation and outcomes (overall and progression‐free survival, local and distant control and toxicity) were extracted. We identified 96 eligible articles, comprising 95 studies. A large variety of treatment options for MBM were investigated, either used alone or as combined modality therapy. Combined modality therapy was investigated in 71% of the studies and resulted in increased survival and better distant/local control than monotherapy, especially with targeted therapy or immunotherapy. However, neurotoxic side‐effects also occurred more frequently. Timing appeared to be an important determinant, with the best results when radiotherapy was given before or during systemic therapy. Improved tumor control and prolonged survival can be achieved by combining radiotherapy with immunotherapy or targeted therapy. However, more randomized controlled trials or prospective studies are warranted to generate proper evidence that can be used to change the standard of care for patients with MBM.
Keywords: brain metastases, melanoma, treatment, outcome
Abbreviations
- ACT
adoptive cell therapy
- anti‐PD1
anti‐programmed cell death protein 1
- BRAFi
B‐Raf inhibitor
- DC
distant control
- IPI
ipilimumab
- LC
local control
- MBM
melanoma brain metastases
- MEKi
MEK inhibitor
- OS
overall survival
- PFS
progression‐free survival
- RT
radiotherapy
- SRS
stereotactic radiosurgery
- SRT
stereotactic radiotherapy
- TMZ
temozolomide
- WBRT
whole brain radiation therapy
Introduction
Melanoma is the most aggressive subtype of skin cancer, comprising <5% of all cases. Nevertheless, morbidity is relatively high with approximately 50 000 deaths annually worldwide, especially due to the occurrence of metastases.1 After lung and breast cancer, melanoma is the third most common type of cancer likely to metastasize to the brain. An estimated 10–40% of melanoma patients will develop brain metastases.2 Prognosis of patients with melanoma brain metastases (MBM) is poor, with an expected overall survival (OS) of only 4 months,3, 4, 5 depending on factors like mutation status.6
Conventional therapy for MBM consists of whole brain radiation therapy (WBRT) for multiple metastases and stereotactic radiosurgery (SRS) or radiotherapy (SRT) for limited numbers of metastases. Despite these treatments, outcomes remain poor and the disease burden high. New therapies that could improve patient outcomes are therefore warranted.
The role of conventional chemotherapy and radiation is limited and even comparable to supportive care only in terms of progression‐free survival (PFS).7 Since the last decade, several new systemic drugs have been introduced, such as immunotherapy with checkpoint inhibitors like anti‐cytotoxic T‐lymphocyte‐associated protein 4 (ipilimumab [IPI]),8, 9 anti‐programmed cell death protein 1 (anti‐PD1) (nivolumab and pembrolizumab)10, 11 or a combination,12 and targeted therapy (BRAF, MEK inhibitors [BRAFi, MEKi]).13, 14, 15, 16 These therapies can be combined with RT. Currently, the precise impact of available treatment modalities for MBM on tumor‐ and patient‐related outcomes is unknown, as well as the impact of the timing of therapy (i.e., treatment can be given as neoadjuvant, adjuvant or concurrent with other treatment modalities).
This systematic review aimed to describe the impact of current treatment modalities on tumor‐ and patient‐related outcomes of patients with MBM. Given the lack of up to date guidelines on how to treat MBM patients, particularly with the introduction of new therapies, we provide recommendations for the treatment of MBM.
Methods
Search strategy
A literature search in the databases PubMed, Embase, Web of Science and Cochrane Library was conducted up to January 8, 2019, using a combination of search terms and synonyms for “melanoma,” brain metastases” and “systemic therapy” (Supplemental S1 for the PubMed search strategy).
All identified abstracts were screened independently by two reviewers (M.P.v.O. and L.D.), and full‐texts of potentially relevant articles were evaluated according to predefined in‐ and exclusion criteria (Supplemental S2). Reference lists of relevant articles were screened for additional eligible articles. Disagreements were resolved in consensus. Preferred Reporting Items for Systematic Reviews and Meta‐Analyses guidelines were followed.17
Data extraction
For each eligible article, information on study design, population characteristics, previously received treatment for MBM, treatment under investigation and outcomes (OS and PFS, local and distant control [LC and DC] and/or toxicity) were extracted. The results are summarized per treatment modality.
Statistics
Weighted medians or percentages for different outcomes were calculated based on the number of patients included in each study.
Results
Search results
The search strategy resulted in 1,172 unique abstracts. Of these, 148 abstracts were selected for full‐text screening of which 96, comprising 95 studies, were classified eligible according to our predefined criteria. See Figure 1 for an overview of the selection process.
Figure 1.
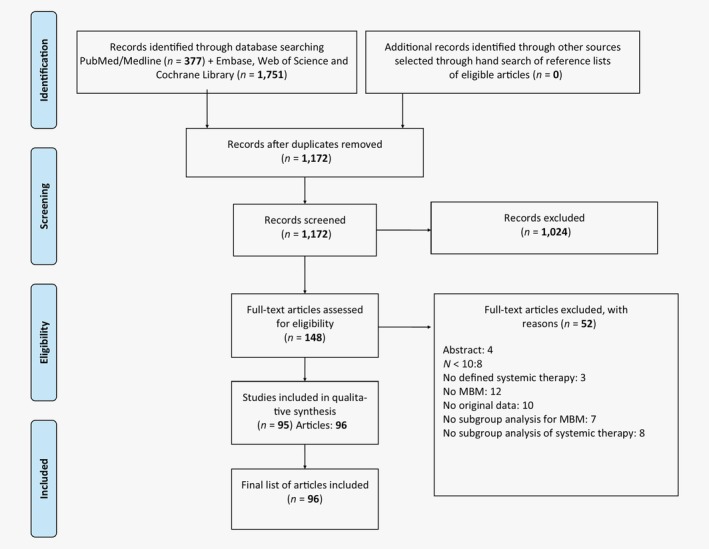
Schematic breakdown of literature search results. Abbreviation: MBM, melanoma brain metastases.
Study characteristics
Most included studies (79/95, 83%) had a retrospective study design, and the majority (71/95, 75%) was published in 2015 or later. The median number of MBM patients in the studies was 72 (range 10–3,219). Most commonly described treatments were chemotherapy (16/95, 17%), targeted therapy (45/95, 47%) and immunotherapy (60/95, 63%), either or not combined. Of the studies that included targeted therapy or checkpoint inhibitors, vemurafenib (16/45, 36%) and IPI (37/60, 62%) were mostly involved. Radiotherapy (SRS and/or WBRT) as (part of) treatment was investigated in 57/95 (60%) studies. Combined treatment was applied in 63/95 (66%) studies.
The most commonly described outcome was OS. Other outcomes that were reported were control rate (44/95, 46%)—including LC (=no increase in volume of the treated lesions) and DC (=freedom from development of new active disease apart from the treated lesions), PFS (36/95, 38%) and disease‐ and/or drug‐related toxicity (47/95, 49%). See Supplemental S3 for a description of the study characteristics and outcomes of each study.
Outcomes
Overall survival
Median OS varies considerably between different treatment modalities, whether given as monotherapy or combined with other modalities, and is significantly shortened in symptomatic patients18 and those with higher number of lesions.19, 20 The OS improved significantly in recent years, particularly with the introduction of targeted and immunotherapy (Figs. 2 a and 2 b).21, 22 For some studies, results on OS could not be reported as no subgroup analyses were presented.18, 19, 20, 23, 24, 25, 26
Figure 2.
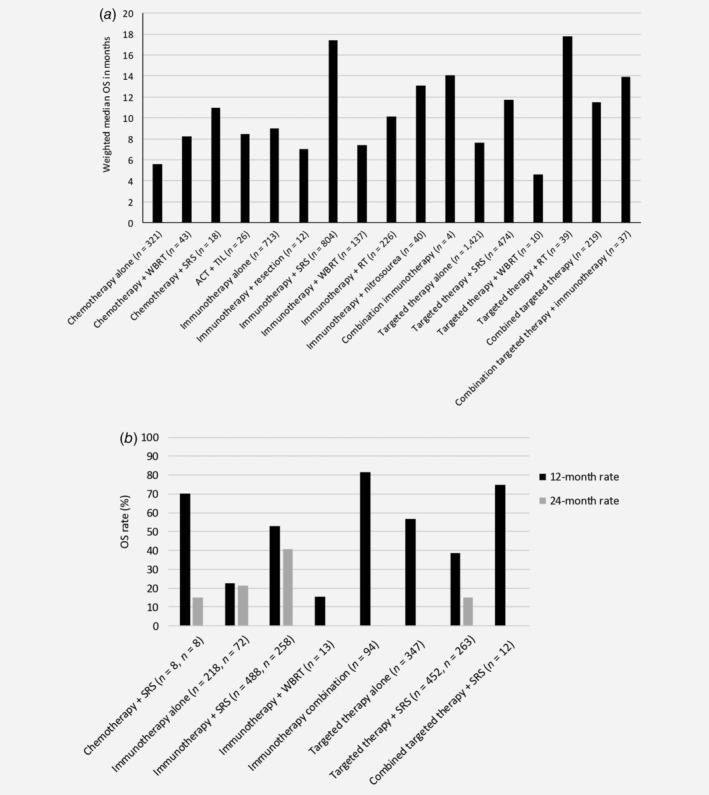
Weighted median overall survival (OS) in months (a), and 12‐ and 24‐month OS rates (b), separately for the different treatment strategies.
The weighted median OS for monotherapy chemotherapy was 5.6 months,27, 28, 29, 30, 31, 32 while the addition of WBRT or SRS resulted in prolonged survival, 8.233, 34 and 1135 months, respectively, and a 24‐month OS rate of 15%. Similarly, median OS of 8.5 months was found for combined adoptive cell therapy (ACT) with chemotherapy followed by the infusion of autologous tumor‐infiltrating lymphocytes.36
Weighted median OS with immunotherapy alone was similar to treatment with chemoradiation, that is, 9.0 months,30, 37, 38, 39, 40, 41, 42, 43, 44, 45 with weighted 12‐ and 24‐month OS rates of 22.5%44, 45 and 21.3%.45 The addition of surgery was not effective in terms of OS: median OS of only 7 months.46 Combining IPI with nitrosourea, or another immunotherapy (anti‐CLA‐4), did improve median OS to 13.147, 48 and 14.138 months, but the combination of immunotherapy with SRS is most effective, with a weighted median OS of 17.4 months.35, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65 Weighted 12‐ and 24‐month survival rates were 52.8%49, 51, 52, 57, 58, 63, 66, 67, 68, 69, 70, 71 and 40.7%56, 57, 63, 67, 68 with combined immunotherapy and SRS, and 12‐month OS rate was 81.5% for combined IPI and nivolumab.72 However, the combination with WBRT resulted in median OS of only 7.454, 62, 73, 74 and with combined SRS/WBRT in a median OS of 10.175, 76 months, with a 12‐month OS rate of 15.4%73 for combined WBRT+IPI. Timing of RT impacts OS, with higher median OS when RT was given before or during IPI compared to RT given after IPI.51, 56, 59, 62, 75 Also, the type of immunotherapy given has impact: SRS combined with an anti‐PD1 drug resulted in better survival outcomes than combined with an anti‐CTLA‐4 drug.38, 49, 50, 58, 67
Survival outcomes for targeted therapy are comparable to those of immunotherapy, with a weighted median OS of 7.6 months,30, 38, 77, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87 and a 12‐month OS rate of 56.8%.83, 87, 88 Results on the impact of the presence of specific mutations on the effectiveness of targeted therapy are conflicting.53, 84 Combining targeted therapy with SRS resulted in similar outcomes as the combination of dabrafenib with trametinib: weighted median OS of 11.735, 50, 89, 90, 91, 92, 93 versus 11.581, 94, 95 months, and 12‐month OS rates of 38.7%67, 89, 91, 92, 93, 96, 97 versus 48.4%,94 respectively. The 24‐month OS rate for targeted therapy with SRS was 15.2%.91, 93 Again, median OS was worse in patients also treated with WBRT, 4.6 months.98 With respect to timing, SRS before BRAFi resulted in significantly prolonged survival compared to SRS after BRAFi or concurrently to SRS.91 Lastly, combining targeted therapy (BRAFi/MEKi) with immunotherapy resulted in a weighted median OS of 13.9 months,30, 38 and the combination with SRS in a 12‐month OS rate of 75%.67
Progression‐free survival
Treatment with temozolomide (TMZ) chemotherapy alone resulted in PFS of 1.9 months,28 which improved to 4.7 months if combined with WBRT.33 The 6‐ and 12‐month PFS rates were 20% and 5% for combined chemotherapy with SRS, respectively (Fig. 3).67
Figure 3.
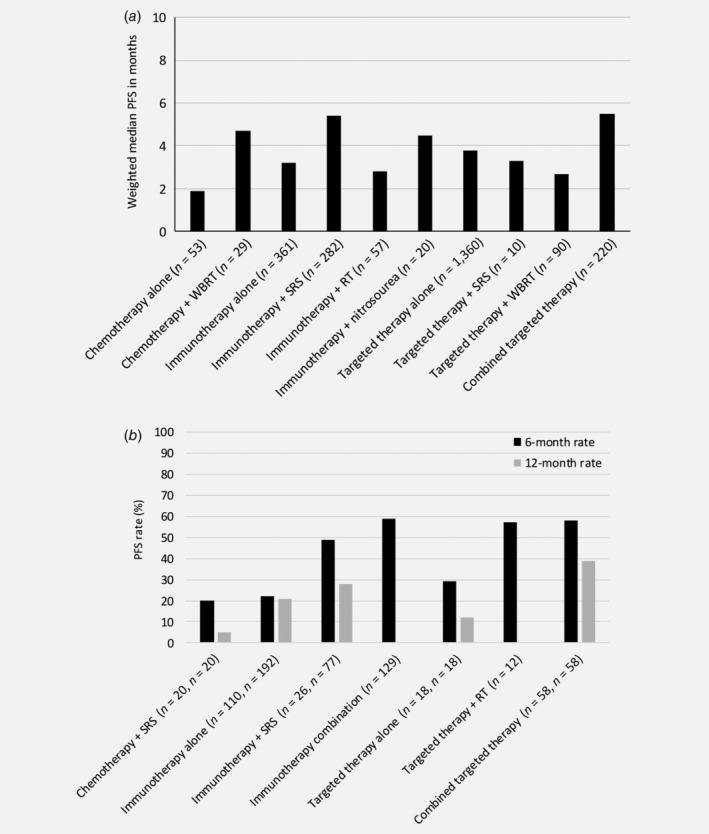
Weighted median progression‐free survival (PFS) in months (a), and 6‐ and 12‐month PFS rates (b), separately for the different treatment strategies.
Treatment with immunotherapy monotherapy resulted in weighted median PFS of 3.1 months,39, 41, 43, 44, 45, 99 and weighted 6‐month and 12‐month PFS rates of 22%41, 44, 67 and 21%,44, 67 respectively. Patients who received previous treatment for their MBM had better median PFS (5.0 vs. 1.2 months) compared to previously untreated patients.99 Combined treatment slightly improved PFS results. Immunotherapy with SRS or RT resulted in a weighted median PFS of 5.455, 57, 58, 59, 61, 100 and 2.875 months, respectively. The median immune‐related PFS for combined immunotherapy with nitrosourea (i.e., IPI + fotemustine) was 4.5 months.47, 74 Results regarding the optimal timing of combined immunotherapy and RT differed between studies, but the weighted median PFS was 9.2 months if SRS was given before or during immunotherapy versus 4.2 months when SRS was given nonconcurrently.43, 57, 59, 75 Finally, combining immunotherapies (i.e., IPI and nivolumab) resulted in high 6‐month intra‐ and extracranial PFS rates (64.2% and 75.9%, respectively).72
The weighted median PFS for patients treated with targeted monotherapy was 3.8 months,77, 78, 79, 80, 81, 82, 83, 84, 85, 87, 101 which is similar to treatment with immunotherapy alone, but could be increased to 5.5 months by combining BRAFi+MEKi.81, 94, 95 Patients with a specific BRAF mutation (Val600Lys) who received previous local treatment and were treated with dabrafenib had a similar median PFS as chemotherapy alone (1.9 months84), but was higher with a Val600Glu mutation and treatment with dabrafenib plus trametinib (7.2 months94). Initiating targeted therapy (mitogen‐activated protein kinase inhibitor) after the occurrence of MBM is more effective in preventing progression of metastases when compared to targeted treatment that was already initiated prior to the occurrence of MBM, 7.1 versus 2.1 months, respectively.87 Previous treatment for MBM did not change PFS in patients treated with targeted therapy.85 Combination of targeted therapy with WBRT or SRT resulted in weighted median PFS of 3.398 and 2.792 months, respectively, or 6‐month freedom‐from‐new‐MBM rate of 57%.102 The 6‐ and 12‐month PFS rates for patients treated with SRS plus targeted therapy (BRAFi) was 29% and 12%, respectively, which increased to 58% and 39% when BRAFi was combined with another targeted therapy (i.e., MEKi) as addition to SRS.67 In BRAF‐mutated patients, combined SRS + BRAFi resulted in a median PFS of 3.9 versus 1.7 months in those without a mutation (p = 0.02).92
Control rate
Mean 6‐ and 12‐month LC rates were similar when RT combined with either immunotherapy (79%49, 70, 103 and 85.4%,49, 66, 69, 71 respectively) or targeted therapy (86.3%89, 102 and 82.4%,66, 89, 93, 96, 97 respectively) (Fig. 4 a). MBM response rate was 22% with pembrolizumab only.104 However, when immunotherapy (pembrolizumab or IPI) was combined with SRS, LC rates were higher, ranging between 68% and 94.8%.55, 58, 63, 105 Of note, tumor control with combined therapy was lower in hemorrhagic versus nonhemorrhagic metastases (43% v. 83%, respectively68). Although not significant, local failure was lower when SRS was given concurrently with IPI versus noncurrent administration, 10% versus 19%.52 The overall response rate for vemurafenib monotherapy, defined as combined intra‐ and extracranial response, was reached in 10/24 (42%) patients in an open‐label Phase I trial.79 Targeted therapy with or without SRS resulted in a control rate of 92.5% in another study.92 Mean intracranial control rate was 37.8% and 55.8% for treatment with immunotherapy40, 41, 43 or targeted therapy81, 88, 106 alone, which increased to 52.5% when nivolumab and IPI were combined41, 72 and to 56.8% when dabrafenib and trametinib were combined.81, 94 Combined SRS with immunotherapy resulted in intracranial disease response after a median of 5.4 months,51 and was higher when IPI was administered before RT instead of after (40% v. 16.7%), although this was not statistically significant.62 If SRS is given after IPI, significantly prolonged intracranial control rates are reached when this is done within 5.5 months: 8.4 versus 3.6 months.107
Figure 4.
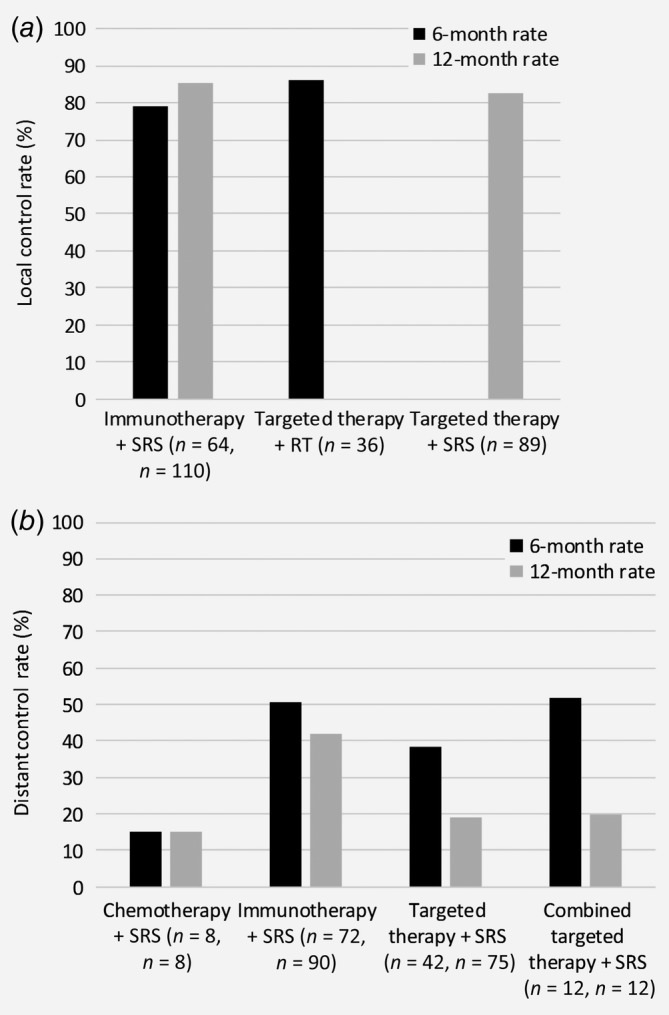
Weighted local (a) and distant (b) control rates, separately for the different treatment strategies.
Only one small study evaluated the DC rates (Fig. 4 b) in MBM patients for chemotherapy combined with SRS, showing 6‐ and 12‐month rates of 15% and 5%, respectively.67 The overall DC rate was 34.2% with immunotherapy alone,42 and intracranial response rates ranged between 18.8% and 24%.41 DC rates increased when immunotherapy was combined with RT, with mean 6‐ and 12‐month DC rates of 50.7%49, 67 and 42%,49, 66, 67 respectively. One small study even found a 24‐month DC rate of 95.4% after combined treatment of SRS with immunotherapy.57 Similarly, intracranial response rate was 48.6% when IPI and nivolumab were combined.41 DC rates were lower for the combination of targeted therapy with RT, that is, mean 6‐ and 12‐month DC rates of 38.6%67, 89 and 18.9%.66, 67, 89, 96 Nevertheless, combining BRAFi + MEKi and SRS resulted in higher 6‐ and 12‐month DC rates, 52% and 20%, respectively,67 or intracranial response rates between 44% and 65% after a median of 8.5 months follow‐up.94 The 12‐month distant failure rate could be significantly reduced for BRAF‐mutated patients treated with BRAFi, from 95% to 68%.26 Control rate can also be expressed in the number of new MBM while under treatment. One study found a significantly higher percentage of patients with new MBM under BRAFi treatment compared to the control group without BRAFi (60% vs. 15%, respectively); however, mean size of new lesions was smaller.108 The occurrence of new MBM appears to depend on the timing of treatment with vemurafenib: BRAF‐mutated patients without MBM who were treated with vemurafenib had a significantly decreased chance of developing MBM (incidence ratio = 0.51) when compared to patients who did not receive upfront vemurafenib.109
Toxicity
Treatment with chemotherapy obviously resulted in significantly more toxicity when compared to best supportive care alone,31 with moderate to severe toxicity in up to 30% of patients.28, 31 Grade 4 toxicities were uncommon (<2%).28 Grade 3 toxicity ranged from 3.4% for fatigue, neutropenia and lymphedema to 13.8% for thrombocytopenia when TMZ was combined with WBRT.33 Hemorrhage occurred in 5.9% of patients treated with combined chemotherapy and ACT.36
Mild to moderate toxicity was common with immunotherapy alone,45, 110 but Grade 3/4 toxicity was relatively low (weighted mean percentage of 8.4%).41, 44, 104 Although two studies showed that the combination of immunotherapy, IPI55, 111 or anti‐PD1,55 with RT resulted in significantly more brain toxicity than RT alone, the overall risk of Grade 3/4 toxicities was similar to that of immunotherapy alone, i.e. weighted mean of 8.1%.51, 64, 68, 69, 73, 74, 75, 112, 113 However, combining immunotherapy with nitrosourea resulted in a treatment‐related Grade 3/4 toxicity of 55%.47 Similarly, combining immunotherapies resulted in significant Grade 3/4 toxicity: weighted mean of 54.7%.41, 72 Radiation necrosis was observed in 30.4% of patients treated with pembrolizumab in one small study39 and in an average of 13.8% of patients receiving immunotherapy combined with RT,55, 58, 59, 61, 71, 100 and 11.6% of lesions.63, 65 Similarly, intracranial hemorrhage was observed in 23.3% of patients receiving combined treatment55, 70, 71 and intratumoral hemorrhage in all patients in one small study.73
Patients treated with targeted therapy alone experience Grade 3/4 toxicity more often than with immunotherapy alone, that is, an average of 37.2% of patients,78, 82, 84, 85 with skin lesions/rash being most common.83 The combination of targeted therapies (dabrafenib and trametinib) resulted in increased Grade 3/4 toxicity (48%).94 Combining vandetanib with WBRT resulted in Grade 3/4 toxicity rate of 50%, which was similar to WBRT with placebo.98 The average hemorrhage rate was similar when treated with targeted therapy alone or when combined with RT (11.2%84, 96 vs. 12.7%91, 92, 93, 97). Radiation necrosis occurred in an average of 16% of patients.97, 102
Discussion
In an attempt to improve the survival of MBM patients, several new systemic therapies have been introduced in the last decade, including targeted therapy and immunotherapy. This review showed that these new treatment modalities have been administered as monotherapy, but also in combination with conventional treatment modalities such as chemotherapy and RT. Not only the type of treatment was found to have an impact on treatment outcomes, also the timing of drug administration appears important. It should be noted that survival is not only determined by the presence and treatment of MBM, but also by the status of extracranial disease. A major limitation is that most studies included in this review are retrospective studies and that studies vary considerably in terms of the included patient population (e.g., performance status and extent of intra‐ and extracranial disease) and type of treatment used for these patients (e.g., previously SRT was only used for patients with a limited number of MBM and nowadays SRT is also used for patients with >10 MBM with limited total metastatic volume), hampering the conclusions that can be drawn. Nevertheless, we will provide recommendations for the treatment of MBM patients based on the available literature. These recommendations can be updated if better studies are published. It is important that the impact of treatment on all outcomes is considered. Although tumor‐related outcomes such as LC rate may be important to evaluate treatment effectiveness, this outcome may be less important for a patient. For example, the tumor may respond well, but if a patient is experiencing considerable treatment toxicity, this treatment may be less meaningful for that patient.
Treatment with immuno‐ and targeted therapy are preferred over treatment with chemotherapy alone as they improve both OS and PFS, particularly in combination with RT. A drawback is that significantly more radiation necrosis and Grade 3/4 toxicities were observed for combined treatment. However, it is difficult to discriminate between disease‐ and drug‐related toxicities. It is important to consider the timing of administrating the different treatment modalities. RT delivered before or during immune‐ or targeted therapy seems to result in longest OS51, 56, 59, 62, 75, 91 and PFS.43, 57, 59, 75
Although the results of the included studies are variable and, in some cases, contradictory, the general consensus seems to be that combined treatment of RT with immune or targeted therapy resulted in the highest control rates. Particularly the combination with BRAFi and MEKi seems valuable, which was also correlated with higher OS and PFS.67 Despite the fact that the safety of combining RT and targeted therapy has been established in several studies,92, 114 concerns have been raised with respect to possible increased toxicity, particularly with BRAF‐inhibitors.115 Further studies addressing the toxicity of this combined treatment are therefore warranted, including assessment of the impact on radiation necrosis, cognition and HRQoL.116 The combination of RT and immunotherapy, however, has not only proven to be efficacious, but also safe in terms of neurotoxicity.61, 103, 113, 117, 118, 119 Another issue is that targeted treatment with BRAFi and MEKi may result in resistance after long‐term use, suggesting that the best long‐term responses can be achieved by using immunotherapy after rapid tumor reduction with BRAFi/MEKi. The highest level of evidence for treatment of asymptomatic and untreated MBM patients with immunotherapy is provided by Long et al. and Tawbi et al., two relatively large (randomized) Phase II trials, showing that the combination of IPI and nivolumab resulted in relatively high intracranial control rates.41, 72 Recently, a randomized Phase II trial comparing IPI and nivolumab with concurrent intracranial SRT versus IPI and nivolumab alone in patients with asymptomatic, untreated MBM has opened for recruitment.120 Results of this trial will contribute to further improvement of treatment recommendations for this patient population.
To conclude, MBM patients seem to benefit most from treatment with targeted and immunotherapy, preferably combined with RT to create a synergistic effect, although toxicity may be increased with this strategy. Nevertheless, based on the available data, it is difficult to recommend one specific treatment for MBM patients. The exact treatment should therefore be based on the characteristics of individual patients (e.g., genetic profile and other tumor‐ and patient‐related characteristics), as well as their treatment preference. In order to achieve further improvements in the treatment of patients with MBM, it is essential to study the novel immunotherapies and targeted therapies, whether or not combined with RT (particularly SRS), in more (randomized controlled) trials to create more evidence‐based guidance. Finally, future research may emerge new targets for treatment which can also contribute to more patient‐specific treatments that can subsequently improve outcomes.
Supporting information
Appendix S1: Supporting information
Appendix S2: Supporting information
Appendix S3: Supporting information
Conflict of interest: E.H.W.K. reports research funding from BMS and advisory board fees from Roche, Novartis, Bristol‐Meyers Squibb, Pierre Fabre and Merck, outside the submitted work. All other authors declare that there is no conflict of interest.
References
- 1. Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 2010;127:2893–917. [DOI] [PubMed] [Google Scholar]
- 2. Bafaloukos D, Gogas H. The treatment of brain metastases in melanoma patients. Cancer Treat Rev 2004;30:515–20. [DOI] [PubMed] [Google Scholar]
- 3. Fife KM, Colman MH, Stevens GN, et al. Determinants of outcome in melanoma patients with cerebral metastases. J Clin Oncol 2004;22:1293–300. [DOI] [PubMed] [Google Scholar]
- 4. Raizer JJ, Hwu WJ, Panageas KS, et al. Brain and leptomeningeal metastases from cutaneous melanoma: survival outcomes based on clinical features. Neuro Oncologia 2008;10:199–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sampson JH, Carter JH Jr, Friedman AH, et al. Demographics, prognosis, and therapy in 702 patients with brain metastases from malignant melanoma. J Neurosurg 1998;88:11–20. [DOI] [PubMed] [Google Scholar]
- 6. Li Y, Umbach DM, Li L. Putative genomic characteristics of BRAF V600K versus V600E cutaneous melanoma. Melanoma Res 2017;27:527–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Goyal S, Silk AW, Tian S, et al. Clinical management of multiple melanoma brain metastases: a systematic review. JAMA Oncol 2015;1:668–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hodi FS, O'Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010;363:711–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Robert C, Thomas L, Bondarenko I, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med 2011;364:2517–26. [DOI] [PubMed] [Google Scholar]
- 10. Robert C, Schachter J, Long GV, et al. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med 2015;372:2521–32. [DOI] [PubMed] [Google Scholar]
- 11. Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med 2015;372:320–30. [DOI] [PubMed] [Google Scholar]
- 12. Wolchok JD, Chiarion‐Sileni V, Gonzalez R, et al. Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med 2017;377:1345–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med 2011;364:2507–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Larkin J, Ascierto PA, Dreno B, et al. Combined vemurafenib and cobimetinib in BRAF‐mutated melanoma. N Engl J Med 2014;371:1867–76. [DOI] [PubMed] [Google Scholar]
- 15. Long GV, Flaherty KT, Stroyakovskiy D, et al. Dabrafenib plus trametinib versus dabrafenib monotherapy in patients with metastatic BRAF V600E/K‐mutant melanoma: long‐term survival and safety analysis of a phase 3 study. Ann Oncol 2017;28:1631–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dummer R, Ascierto PA, Gogas HJ, et al. Overall survival in patients with BRAF‐mutant melanoma receiving encorafenib plus binimetinib versus vemurafenib or encorafenib (COLUMBUS): a multicentre, open‐label, randomised phase 3 trial. Lancet Oncol 2018;19:1315–27. [DOI] [PubMed] [Google Scholar]
- 17. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. J Clin Epidemiol 2009;62:1006–12. [DOI] [PubMed] [Google Scholar]
- 18. Tio M, Wang X, Carlino MS, et al. Survival and prognostic factors for patients with melanoma brain metastases in the era of modern systemic therapy. Pigment Cell Melanoma Res 2018;31:509–15. [DOI] [PubMed] [Google Scholar]
- 19. Bordia R, Zhong H, Lee J, et al. Melanoma brain metastases: correlation of imaging features with genomic markers and patient survival. J Neurooncol 2017;131:341–8. [DOI] [PubMed] [Google Scholar]
- 20. Vosoughi E, Lee JM, Miller JR, et al. Survival and clinical outcomes of patients with melanoma brain metastasis in the era of checkpoint inhibitors and targeted therapies. BMC Cancer 2018;18:490–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sloot S, Chen YA, Zhao XH, et al. Improved survival of patients with melanoma brain metastases in the era of targeted BRAF and immune checkpoint therapies. Cancer 2018;124:297–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Iorgulescu JB, Harary M, Zogg CK, et al. Improved risk‐adjusted survival for melanoma brain metastases in the era of checkpoint blockade immunotherapies: results from a national cohort. Cancer Immunol Res 2018;6:1039–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fang P, Boehling NS, Koay EJ, et al. Melanoma brain metastases harboring BRAF (V600K) or NRAS mutations are associated with an increased local failure rate following conventional therapy. J Neurooncol 2018;137:67–75. [DOI] [PubMed] [Google Scholar]
- 24. Vecchio S, Spagnolo F, Merlo DF, et al. The treatment of melanoma brain metastases before the advent of targeted therapies: associations between therapeutic choice, clinical symptoms and outcome with survival. Melanoma Res 2014;24:61–7. [DOI] [PubMed] [Google Scholar]
- 25. Wilkins A, Furness A, Corbett RW, et al. The melanoma‐specific graded prognostic assessment does not adequately discriminate prognosis in a modern population with brain metastases from malignant melanoma. Br J Cancer 2015;113:1275–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kotecha R, Miller JA, Venur VA, et al. Melanoma brain metastasis: the impact of stereotactic radiosurgery, BRAF mutational status, and targeted and/or immune‐based therapies on treatment outcome. J Neurosurg 2018;129:50–9. [DOI] [PubMed] [Google Scholar]
- 27. Staudt M, Lasithiotakis K, Leiter U, et al. Determinants of survival in patients with brain metastases from cutaneous melanoma. Br J Cancer 2010;102:1213–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Siena S, Crino L, Danova M, et al. Dose‐dense temozolomide regimen for the treatment of brain metastases from melanoma, breast cancer, or lung cancer not amenable to surgery or radiosurgery: a multicenter phase II study. Ann Oncol 2010;21:655–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Davies MA, Liu P, McIntyre S, et al. Prognostic factors for survival in melanoma patients with brain metastases. Cancer 2011;117:1687–96. [DOI] [PubMed] [Google Scholar]
- 30. Mangana J, Cheng PF, Kaufmann C, et al. Multicenter, real‐life experience with checkpoint inhibitors and targeted therapy agents in advanced melanoma patients in Switzerland. Melanoma Res 2017;27:358–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hofmann MA, Hauschild A, Mohr P, et al. Prospective evaluation of supportive care with or without CVD chemotherapy as a second‐line treatment in advanced melanoma by patient's choice: a multicentre dermatologic cooperative oncology group trial. Melanoma Res 2011;21:516–23. [DOI] [PubMed] [Google Scholar]
- 32. Ostheimer C, Bormann C, Fiedler E, et al. Malignant melanoma brain metastases: treatment results and prognostic factors—a single‐center retrospective study. Int J Oncol 2015;46:2439–48. [DOI] [PubMed] [Google Scholar]
- 33. Devito N, Yu M, Chen R, et al. Retrospective study of patients with brain metastases from melanoma receiving concurrent whole‐brain radiation and temozolomide. Anticancer Res 2011;31:4537–43. [PubMed] [Google Scholar]
- 34. Szyszka‐Charewicz B. The effectiveness of brain metastases radiotherapy in patients with melanoma. Nowotwory 2016;66:367–74. [Google Scholar]
- 35. Amaral T, Tampouri I, Eigentler T, et al. Immunotherapy plus surgery/radiosurgery is associated with favorable survival in patients with melanoma brain metastasis. Immunotherapy 2019;11:297–309. [DOI] [PubMed] [Google Scholar]
- 36. Hong JJ, Rosenberg SA, Dudley ME, et al. Successful treatment of melanoma brain metastases with adoptive cell therapy. Clin Cancer Res 2010;16:4892–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Du Four S, Janssen Y, Michotte A, et al. Focal radiation necrosis of the brain in patients with melanoma brain metastases treated with pembrolizumab. Cancer Med 2018;7:4870–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gaudy‐Marqueste C, Dussouil AS, Carron R, et al. Survival of melanoma patients treated with targeted therapy and immunotherapy after systematic upfront control of brain metastases by radiosurgery. Eur J Cancer 2017;84:44–54. [DOI] [PubMed] [Google Scholar]
- 39. Kluger HM, Chiang V, Mahajan A, et al. Long‐term survival of patients with melanoma with active brain metastases treated with pembrolizumab on a phase II trial. J Clin Oncol 2019;37:52–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ladwa R, Atkinson V. The changing paradigm of management in melanoma brain metastases. Asia Pac J Clin Oncol 2018;14:453–8. [DOI] [PubMed] [Google Scholar]
- 41. Long GV, Atkinson V, Lo S, et al. Combination nivolumab and ipilimumab or nivolumab alone in melanoma brain metastases: a multicentre randomised phase 2 study. Lancet Oncol 2018;19:672–81. [DOI] [PubMed] [Google Scholar]
- 42. Milsch L, Gesierich A, Kreft S, et al. Patterns of disease control and survival in patients with melanoma brain metastases undergoing immune‐checkpoint blockade. Eur J Cancer 2018;99:58–65. [DOI] [PubMed] [Google Scholar]
- 43. Parakh S, Park JJ, Mendis S, et al. Efficacy of anti‐PD‐1 therapy in patients with melanoma brain metastases. Br J Cancer 2017;116:1558–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Queirolo P, Spagnolo F, Ascierto PA, et al. Efficacy and safety of ipilimumab in patients with advanced melanoma and brain metastases. J Neurooncol 2014;118:109–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Margolin K, Ernstoff MS, Hamid O, et al. Ipilimumab in patients with melanoma and brain metastases: an open‐label phase 2 trial. Lancet Oncol 2012;13:459–65. [DOI] [PubMed] [Google Scholar]
- 46. Jones PS, Cahill DP, Brastianos PK, et al. Ipilimumab and craniotomy in patients with melanoma and brain metastases: a case series. Neurosurg Focus 2015;38:E5. [DOI] [PubMed] [Google Scholar]
- 47. Di Giacomo AM, Ascierto PA, Pilla L, et al. Ipilimumab and fotemustine in patients with advanced melanoma (NIBIT‐M1): an open‐label, single‐arm phase 2 trial. Lancet Oncol 2012;13:879–86. [DOI] [PubMed] [Google Scholar]
- 48. Di Giacomo AM, Ascierto PA, Queirolo P, et al. Three‐year follow‐up of advanced melanoma patients who received ipilimumab plus fotemustine in the Italian network for tumor biotherapy (NIBIT)‐M1 phase II study. Ann Oncol 2015;26:798–803. [DOI] [PubMed] [Google Scholar]
- 49. Ahmed KA, Stallworth DG, Kim Y, et al. Clinical outcomes of melanoma brain metastases treated with stereotactic radiation and anti‐PD‐1 therapy. Ann Oncol 2016;27:434–41. [DOI] [PubMed] [Google Scholar]
- 50. Choong ES, Lo S, Drummond M, et al. Survival of patients with melanoma brain metastasis treated with stereotactic radiosurgery and active systemic drug therapies. Eur J Cancer 2017;75:169–78. [DOI] [PubMed] [Google Scholar]
- 51. Cohen‐Inbar O, Shih HH, Xu Z, et al. The effect of timing of stereotactic radiosurgery treatment of melanoma brain metastases treated with ipilimumab. J Neurosurg 2017;127:1007–14. [DOI] [PubMed] [Google Scholar]
- 52. Diao K, Bian SX, Routman DM, et al. Combination ipilimumab and radiosurgery for brain metastases: tumor, edema, and adverse radiation effects. J Neurosurg 2018;129:1397–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Fang P, Jiang W, Allen P, et al. Radiation necrosis with stereotactic radiosurgery combined with CTLA‐4 blockade and PD‐1 inhibition for treatment of intracranial disease in metastatic melanoma. J Neurooncol 2017;133:595–602. [DOI] [PubMed] [Google Scholar]
- 54. Gabani P, Fischer‐Valuck BW, Johanns TM, et al. Stereotactic radiosurgery and immunotherapy in melanoma brain metastases: patterns of care and treatment outcomes. Radiother Oncol 2018;128:266–73. [DOI] [PubMed] [Google Scholar]
- 55. Kaidar‐Person O, Zagar TM, Deal A, et al. The incidence of radiation necrosis following stereotactic radiotherapy for melanoma brain metastases: the potential impact of immunotherapy. Anticancer Drugs 2017;28:669–75. [DOI] [PubMed] [Google Scholar]
- 56. Knisely JP, Yu JB, Flanigan J, et al. Radiosurgery for melanoma brain metastases in the ipilimumab era and the possibility of longer survival. J Neurosurg 2012;117:227–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Murphy B, Walker J, Bassale S, et al. Concurrent radiosurgery and immune checkpoint inhibition: improving regional intracranial control for patients with metastatic melanoma. Am J Clin Oncol 2019;42:253–7. [DOI] [PubMed] [Google Scholar]
- 58. Nardin C, Mateus C, Texier M, et al. Tolerance and outcomes of stereotactic radiosurgery combined with anti‐programmed cell death‐1 (pembrolizumab) for melanoma brain metastases. Melanoma Res 2018;28:111–9. [DOI] [PubMed] [Google Scholar]
- 59. Olson AC, Thomas S, Qin R, et al. Outcomes and toxicity of stereotactic radiosurgery for melanoma brain metastases in patients receiving ipilimumab. Melanoma Manag 2016;3:177–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Qian JM, Yu JB, Kluger HM, et al. Timing and type of immune checkpoint therapy affect the early radiographic response of melanoma brain metastases to stereotactic radiosurgery. Cancer 2016;122:3051–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Rahman R, Cortes A, Niemierko A, et al. The impact of timing of immunotherapy with cranial irradiation in melanoma patients with brain metastases: intracranial progression, survival and toxicity. J Neurooncol 2018;138:299–306. [DOI] [PubMed] [Google Scholar]
- 62. Silk AW, Bassetti MF, West BT, et al. Ipilimumab and radiation therapy for melanoma brain metastases. Cancer Med 2013;2:899–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Skrepnik T, Sundararajan S, Cui H, et al. Improved time to disease progression in the brain in patients with melanoma brain metastases treated with concurrent delivery of radiosurgery and ipilimumab. Oncoimmunology 2017;6:e1283461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Tazi K, Hathaway A, Chiuzan C, et al. Survival of melanoma patients with brain metastases treated with ipilimumab and stereotactic radiosurgery. Cancer Med 2015;4:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Yusuf MB, Amsbaugh MJ, Burton E, et al. Peri‐SRS administration of immune checkpoint therapy for melanoma metastatic to the brain: investigating efficacy and the effects of relative treatment timing on lesion response. World Neurosurg 2017;100:632–40. [DOI] [PubMed] [Google Scholar]
- 66. Acharya S, Mahmood M, Mullen D, et al. Distant intracranial failure in melanoma brain metastases treated with stereotactic radiosurgery in the era of immunotherapy and targeted agents. Adv Radiat Oncol 2017;2:572–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Ahmed KA, Abuodeh YA, Echevarria MI, et al. Clinical outcomes of melanoma brain metastases treated with stereotactic radiosurgery and anti‐PD‐1 therapy, anti‐CTLA‐4 therapy, BRAF/MEK inhibitors, BRAF inhibitor, or conventional chemotherapy. Ann Oncol 2016;27:2288–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Bauer‐Nilsen K, Trifiletti DM, Chatrath A, et al. Stereotactic radiosurgery for brain metastases from malignant melanoma and the impact of hemorrhagic metastases. J Neurooncol 2018;140:83–8. [DOI] [PubMed] [Google Scholar]
- 69. Kiess AP, Wolchok JD, Barker CA, et al. Stereotactic radiosurgery for melanoma brain metastases in patients receiving ipilimumab: safety profile and efficacy of combined treatment. Int J Radiat Oncol Biol Phys 2015;92:368–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Mathew M, Tam M, Ott PA, et al. Ipilimumab in melanoma with limited brain metastases treated with stereotactic radiosurgery. Melanoma Res 2013;23:191–5. [DOI] [PubMed] [Google Scholar]
- 71. Patel KR, Shoukat S, Oliver DE, et al. Ipilimumab and stereotactic radiosurgery versus stereotactic radiosurgery alone for newly diagnosed melanoma brain metastases. Am J Clin Oncol 2017;40:444–50. [DOI] [PubMed] [Google Scholar]
- 72. Tawbi HA, Forsyth PA, Algazi A, et al. Combined nivolumab and ipilimumab in melanoma metastatic to the brain. N Engl J Med 2018;379:722–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Gerber NK, Young RJ, Barker CA, et al. Ipilimumab and whole brain radiation therapy for melanoma brain metastases. J Neurooncol 2015;121:159–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Williams NL, Wuthrick EJ, Kim H, et al. Phase 1 study of ipilimumab combined with whole brain radiation therapy or radiosurgery for melanoma patients with brain metastases. Int J Radiat Oncol Biol Phys 2017;99:22–30. [DOI] [PubMed] [Google Scholar]
- 75. Schmidberger H, Rapp M, Ebersberger A, et al. Long‐term survival of patients after ipilimumab and hypofractionated brain radiotherapy for brain metastases of malignant melanoma: sequence matters. Strahlenther Onkol 2018;194:1144–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Stokes WA, Binder DC, Jones BL, et al. Impact of immunotherapy among patients with melanoma brain metastases managed with radiotherapy. J Neuroimmunol 2017;313:118–22. [DOI] [PubMed] [Google Scholar]
- 77. Azer MW, Menzies AM, Haydu LE, et al. Patterns of response and progression in patients with BRAF‐mutant melanoma metastatic to the brain who were treated with dabrafenib. Cancer 2014;120:530–6. [DOI] [PubMed] [Google Scholar]
- 78. Blank CU, Larkin J, Arance AM, et al. Open‐label, multicentre safety study of vemurafenib in 3219 patients with BRAFV600 mutation‐positive metastatic melanoma: 2‐year follow‐up data and long‐term responders' analysis. Eur J Cancer 2017;79:176–84. [DOI] [PubMed] [Google Scholar]
- 79. Dummer R, Goldinger SM, Turtschi CP, et al. Vemurafenib in patients with BRAF(V600) mutation‐positive melanoma with symptomatic brain metastases: final results of an open‐label pilot study. Eur J Cancer 2014;50:611–21. [DOI] [PubMed] [Google Scholar]
- 80. Dzienis MR, Atkinson VG. Response rate to vemurafenib in patients with B‐RAF‐positive melanoma brain metastases: a retrospective review. Melanoma Res 2014;24:349–53. [DOI] [PubMed] [Google Scholar]
- 81. Foppen MHG, Boogerd W, Blank CU, et al. Clinical and radiological response of BRAF inhibition and MEK inhibition in patients with brain metastases from BRAF‐mutated melanoma. Melanoma Res 2018;28:126–33. [DOI] [PubMed] [Google Scholar]
- 82. Gorka E, Fabo D, Gezsi A, et al. Dabrafenib therapy in 30 patients with melanoma metastatic to the brain: a single‐Centre controlled retrospective study in Hungary. Pathol Oncol Res 2017;24:401–6. [DOI] [PubMed] [Google Scholar]
- 83. Harding JJ, Catalanotti F, Munhoz RR, et al. Retrospective evaluation of vemurafenib as treatment for BRAF‐mutant melanoma brain metastases. Oncologist 2015;20:789–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Long GV, Trefzer U, Davies MA, et al. Dabrafenib in patients with Val600Glu or Val600Lys BRAF‐mutant melanoma metastatic to the brain (BREAK‐MB): a multicentre, open‐label, phase 2 trial. Lancet Oncol 2012;13:1087–95. [DOI] [PubMed] [Google Scholar]
- 85. McArthur GA, Maio M, Arance A, et al. Vemurafenib in metastatic melanoma patients with brain metastases: an open‐label, single‐arm, phase 2, multicentre study. Ann Oncol 2017;28:634–41. [DOI] [PubMed] [Google Scholar]
- 86. Seifert H, Hirata E, Gore M, et al. Extrinsic factors can mediate resistance to BRAF inhibition in central nervous system melanoma metastases. Pigment Cell Melanoma Res 2016;29:92–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Wattson DA, Sullivan RJ, Niemierko A, et al. Survival patterns following brain metastases for patients with melanoma in the MAP‐kinase inhibitor era. J Neurooncol 2015;123:75–84. [DOI] [PubMed] [Google Scholar]
- 88. Gibney GT, Gauthier G, Ayas C, et al. Treatment patterns and outcomes in BRAF V600E‐mutant melanoma patients with brain metastases receiving vemurafenib in the real‐world setting. Cancer Med 2015;4:1205–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Ahmed KA, Freilich JM, Sloot S, et al. LINAC‐based stereotactic radiosurgery to the brain with concurrent vemurafenib for melanoma metastases. J Neurooncol 2015;122:121–6. [DOI] [PubMed] [Google Scholar]
- 90. Gaudy‐Marqueste C, Carron R, Delsanti C, et al. On demand gamma‐knife strategy can be safely combined with BRAF inhibitors for the treatment of melanoma brain metastases. Ann Oncol 2014;25:2086–91. [DOI] [PubMed] [Google Scholar]
- 91. Mastorakos P, Xu Z, Yu J, et al. BRAF V600 mutation and BRAF kinase inhibitors in conjunction with stereotactic radiosurgery for intracranial melanoma metastases: a multicenter retrospective study. Neurosurgery 2019;84:868–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Wolf A, Zia S, Verma R, et al. Impact on overall survival of the combination of BRAF inhibitors and stereotactic radiosurgery in patients with melanoma brain metastases. J Neurooncol 2016;127:607–15. [DOI] [PubMed] [Google Scholar]
- 93. Xu Z, Lee CC, Ramesh A, et al. BRAF V600E mutation and BRAF kinase inhibitors in conjunction with stereotactic radiosurgery for intracranial melanoma metastases. J Neurosurg 2017;126:726–34. [DOI] [PubMed] [Google Scholar]
- 94. Davies MA, Saiag P, Robert C, et al. Dabrafenib plus trametinib in patients with BRAF(V600)‐mutant melanoma brain metastases (COMBI‐MB): a multicentre, multicohort, open‐label, phase 2 trial. Lancet Oncol 2017;18:863–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Drago JZ, Lawrence D, Livingstone E, et al. Clinical experience with combination BRAF/MEK inhibitors for melanoma with brain metastases: a real‐life multicenter study. Melanoma Res 2019;29:65–9. [DOI] [PubMed] [Google Scholar]
- 96. Ly D, Bagshaw HP, Anker CJ, et al. Local control after stereotactic radiosurgery for brain metastases in patients with melanoma with and without BRAF mutation and treatment. J Neurosurg 2015;123:395–401. [DOI] [PubMed] [Google Scholar]
- 97. Patel KR, Chowdhary M, Switchenko JM, et al. BRAF inhibitor and stereotactic radiosurgery is associated with an increased risk of radiation necrosis. Melanoma Res 2016;26:387–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Gupta A, Roberts C, Tysoe F, et al. RADVAN: a randomised phase 2 trial of WBRT plus vandetanib for melanoma brain metastases ‐ results and lessons learnt. Br J Cancer 2016;115:1193–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Dagogo‐Jack I, Lanfranchi M, Gainor JF, et al. A retrospective analysis of the efficacy of pembrolizumab in melanoma patients with brain metastasis. J Immunother 2017;40:108–13. [DOI] [PubMed] [Google Scholar]
- 100. Robin TP, Breeze RE, Smith DE, et al. Immune checkpoint inhibitors and radiosurgery for newly diagnosed melanoma brain metastases. J Neurooncol 2018;140:55–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Falchook GS, Long GV, Kurzrock R, et al. Dabrafenib in patients with melanoma, untreated brain metastases, and other solid tumours: a phase 1 dose‐escalation trial. Lancet 2012;379:1893–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Narayana A, Mathew M, Tam M, et al. Vemurafenib and radiation therapy in melanoma brain metastases. J Neurooncol 2013;113:411–6. [DOI] [PubMed] [Google Scholar]
- 103. Trommer‐Nestler M, Marnitz S, Kocher M, et al. Robotic stereotactic radiosurgery in melanoma patients with brain metastases under simultaneous anti‐PD‐1 treatment. Int J Mol Sci 2018;19:2653–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Goldberg SB, Gettinger SN, Mahajan A, et al. Pembrolizumab for patients with melanoma or non‐small‐cell lung cancer and untreated brain metastases: early analysis of a non‐randomised, open‐label, phase 2 trial. Lancet Oncol 2016;17:976–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Lonser RR, Song DK, Klapper J, et al. Surgical management of melanoma brain metastases in patients treated with immunotherapy. J Neurosurg 2011;115:30–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Menzies AM, Wilmott JS, Drummond M, et al. Clinicopathologic features associated with efficacy and long‐term survival in metastatic melanoma patients treated with BRAF or combined BRAF and MEK inhibitors Cancer 2015;121:3826–35. [DOI] [PubMed] [Google Scholar]
- 107. An Y, Jiang W, Kim BYS, et al. Stereotactic radiosurgery of early melanoma brain metastases after initiation of anti‐CTLA‐4 treatment is associated with improved intracranial control. Radiother Oncol 2017;125:80–8. [DOI] [PubMed] [Google Scholar]
- 108. Haueis SA, Kranzlin P, Mangana J, et al. Does the distribution pattern of brain metastases during BRAF inhibitor therapy reflect phenotype switching? Melanoma Res 2017;27:231–7. [DOI] [PubMed] [Google Scholar]
- 109. Gummadi T, Zhang BY, Valpione S, et al. Impact of BRAF mutation and BRAF inhibition on melanoma brain metastases. Melanoma Res 2015;25:75–9. [DOI] [PubMed] [Google Scholar]
- 110. Konstantinou MP, Dutriaux C, Gaudy‐Marqueste C, et al. Ipilimumab in melanoma patients with brain metastasis: a retrospective multicentre evaluation of thirty‐eight patients. Acta Derm Venereol 2014;94:45–9. [DOI] [PubMed] [Google Scholar]
- 111. Martin AM, Cagney DN, Catalano PJ, et al. Immunotherapy and symptomatic radiation necrosis in patients with brain metastases treated with stereotactic radiation. JAMA Oncol 2018;4:1123–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Anderson ES, Postow MA, Wolchok JD, et al. Melanoma brain metastases treated with stereotactic radiosurgery and concurrent pembrolizumab display marked regression; efficacy and safety of combined treatment. J Immunother Cancer 2017;5:76–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Diao K, Bian SX, Routman DM, et al. Stereotactic radiosurgery and ipilimumab for patients with melanoma brain metastases: clinical outcomes and toxicity. J Neurooncol 2018;139:421–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Patel BG, Ahmed KA, Johnstone PA, et al. Initial experience with combined BRAF and MEK inhibition with stereotactic radiosurgery for BRAF mutant melanoma brain metastases. Melanoma Res 2016;26:382–6. [DOI] [PubMed] [Google Scholar]
- 115. Kroeze SG, Fritz C, Hoyer M, et al. Toxicity of concurrent stereotactic radiotherapy and targeted therapy or immunotherapy: a systematic review. Cancer Treat Rev 2017;53:25–37. [DOI] [PubMed] [Google Scholar]
- 116. Tallet AV, Dhermain F, Le Rhun E, et al. Combined irradiation and targeted therapy or immune checkpoint blockade in brain metastases: toxicities and efficacy. Ann Oncol 2017;28:2962–76. [DOI] [PubMed] [Google Scholar]
- 117. Arscott WT, Zhu S, Plastaras JP, et al. Acute neurologic toxicity of palliative radiotherapy for brain metastases in patients receiving immune checkpoint blockade. Neurooncol Pract 2019;6:297–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Stera S, Balermpas P, Blanck O, et al. Stereotactic radiosurgery combined with immune checkpoint inhibitors or kinase inhibitors for patients with multiple brain metastases of malignant melanoma. Melanoma Res 2019;29:187–95. [DOI] [PubMed] [Google Scholar]
- 119. Chen L, Douglass J, Kleinberg L, et al. Concurrent immune checkpoint inhibitors and stereotactic radiosurgery for brain metastases in non‐small cell lung cancer, melanoma, and renal cell carcinoma. Int J Radiat Oncol Biol Phys 2018;100:916–25. [DOI] [PubMed] [Google Scholar]
- 120. Long, GV , Gonzalez, M . (2019). Anti‐PD 1 brain collaboration + radiotherapy extension (ABC‐X study) [online]. Available at: https://clinicaltrials.gov/ct2/show/NCT03340129 [Accessed 22 Aug. 2019].
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Supporting information
Appendix S2: Supporting information
Appendix S3: Supporting information


