Abstract
Background
Treatment for both facial and truncal acne has not sufficiently been studied.
Objectives
To evaluate the long‐term safety and efficacy of trifarotene in both facial and truncal acne.
Methods
In a multicentre, open‐label, 52‐week study, patients with moderate facial and truncal acne received trifarotene 50 μg/g cream (trifarotene). Assessments included local tolerability, safety, investigator and physician's global assessments (IGA, PGA) and quality of life (QOL). A validated QOL questionnaire was completed by the patient at Baseline, Week 12, 26 and 52/ET.
Results
Of 453 patients enrolled, 342 (75.5%) completed the study. Trifarotene‐related treatment‐emergent adverse events (TEAEs) were reported in 12.6% of patients, and none was serious. Most related TEAEs were cutaneous and occurred during the first 3 months. Signs and symptoms of local tolerability were mostly mild or moderate and severe signs, and symptoms were reported for 2.2% to 7.1% of patients for the face and 2.5% to 5.4% for the trunk. Local irritation increased during the first week of treatment on the face and up to Weeks 2 to 4 on the trunk with both decreasing thereafter.
At Week 12, IGA and PGA success rates were 26.6% and 38.6%, respectively. Success rates increased to 65.1% and 66.9%, respectively at Week 52. Overall success (both IGA and PGA success in the same patient) was 57.9% at Week 52.
At Week 52 visit, 92/171 (53.8%) patients who had completed their assessments had scores from 0 to 1 (i.e. no effect of acne on their QOL) vs. 47/208 (22.6%) patients at Baseline visit.
Conclusion
In this 52‐week study, trifarotene was safe, well tolerated and effective in moderate facial and truncal acne.
Introduction
Acne is estimated to affect 9.4% of the global population, making it the eighth most prevalent disease worldwide.1 Although typically a disease of adolescence, it also affects a significant proportion of adults, including up to 22% of adult women.2, 3, 4, 5 It may persist for years with permanent consequences including scarring, low self‐esteem and embarrassment due to the visible nature of these marks.6 Acne on the face is the most common, and often the most visible form of the disease and clinical studies has evaluated the efficacy of acne therapy on the face. However, back and chest acne have been estimated to occur in 61% and 45% of acne patients, respectively.7 Until now, acne on the back has received only little attention in clinical studies.
Facial and truncal acne are considered pathophysiologically and clinically similar, with both locations presenting comparable types of lesions, although it may be possible to treat acne in both locations with similar therapeutic approaches.8, 9 Even though many topical and systemic treatments have been used in the past to treat acne, none has been specifically developed and clinically studied for the treatment of both facial and truncal acne.
Currently, topical retinoids are the mainstay of acne treatment, with adapalene, tretinoin and tazarotene available in various concentrations, formulations and combinations as well as orally administered isotretinoin. Retinoids have a central role in acne therapy because they inhibit the development of microcomedones, have anti‐inflammatory and immunomodulatory properties and reduce acne lesions.10 Thus, they are considered a foundation of acne treatment. Retinoids can be combined with other topical therapies such as benzoyl peroxide or topical antibiotics, as well as systemic treatments including antibiotics and or oral contraceptives in more severe cases.11, 12
Trifarotene cream 50 μg/g cream (hereafter trifarotene, developed by Nestlé Skin Health – Galderma) has shown in acne strong anti‐comedogenic, antipigmenting activity and anti‐inflammatory properties in vivo.13 This represents a new generation of retinoids, with a RAR‐γ selectivity that is highly expressed in the skin. It has a good in vivo metabolic stability in cultured keratinocytes and is rapidly metabolized in human liver microsomes, predicting an improved safety profile due to low systemic levels. In the Rhino mouse model, trifarotene showed a dose‐dependent comedolytic activity that is fully efficacious at a 10‐fold lower concentration than that for tazarotene 0.1% cream to achieve similar results.13 No accumulation is expected with long‐term use.13, 14
The primary objective of this study was to assess the long‐term safety of trifarotene for up to 52 weeks of treatment in patients with both moderate facial acne and truncal acne. Secondary objectives were the efficacy of trifarotene for up to 52 weeks and patient‐reported quality of life (QoL).
Patient selection and methods
This was a long‐term, multicentre, open‐label, non‐comparative, phase 3, safety and efficacy study in patients with moderate acne on the face and trunk. The study was conducted between February 2015 and February 2017.
The study complied with all local legal requirements for the conduct of a clinical study and was conducted in accordance with good clinical practices and the Principles of the Declaration of Helsinki. Prior to the inclusion of any patient, the study received local ethics committee or local independent review board approval. Patients provided written informed consent before participation. The study was registered in the US ClinicalTrials.gov register (Identifier n°: NCT02189629) and the European clinical trials register (EudraCT n°: 2014‐001755‐23).
In total, 453 patients were to be enrolled at 32 sites in Europe (18 sites) and the USA (14 sites). The study included patients 9 years of age or older at enrolment, with an Investigator Global Assessment (IGA, for face) score of 3 (moderate) on a 5‐point scale (from 0 = clear to 4 = severe), ≥20 inflammatory lesions and ≥25 non‐inflammatory lesions on the face, a physician's global assessment (PGA, for trunk) score of 3 (moderate) on a 5‐point scale (from 0 = clear to 4 = severe), and ≥20 inflammatory lesions and ≥20 non‐inflammatory lesions on the trunk (chest, shoulders, upper back, mid and lower back, if applicable). All investigators and study personnel were harmonized to the study‐specific measurements.
After the first application of trifarotene at study site, patients were instructed to apply for the first 12 weeks trifarotene at home once daily in the evening after washing and drying the skin. Patients were encouraged to use a non‐comedogenic and hypoallergenic moisturizer of their choice and were instructed not to apply moisturizer one hour before or one hour after study drug application. The investigator could reduce trifarotene application frequency, if tolerability issues arose. If, during the study, IGA and PGA met treatment success criteria, the protocol instructed: (i) If a patient's IGA = 0 and PGA = 0, treatment was discontinued; however, the patient had to continue to attend the scheduled study visits. (ii) If a patient's IGA = 0 and a PGA ≥ 1, treatment was discontinued on the face only. These patients were asked to continue to apply their treatment on the truncal region. (iii) If a patient's IGA was ≥1 and PGA = 0, treatment was discontinued on the truncal region, and the patient was instructed to continue to apply the treatment on the face.
Local tolerability signs and symptoms including erythema, scaling, dryness and stinging/burning were evaluated on a 4‐point scale [0 (none) to 3 (severe) at Baseline and at Weeks 12, 20, 26, 38 and 52/ET]. Assessments were conducted separately for the face and trunk. Treatment‐emergent adverse events (TEAE) were assessed through the study period. A TEAE was defined as an adverse event that occurred, or increased in severity, on or after the date of the first application of trifarotene. TEAEs were summarized per quarter (Q), based on the study day: 1 to 89 (Q1), 90 to 179 (Q2), 180 to 269 (Q3) and ≥270 (Q4).
Routine laboratory parameters (biochemistry, haematology and urinalysis) and pregnancy tests, when appropriate were monitored at defined time points during the study.
Efficacy parameters were assessed at Baseline and at Weeks 12, 20, 26, 38 and 52/ET. IGA was used to assess the face, and PGA was used to assess the reachable areas of the upper truncal region. For children aged 9–11 years who did not meet truncal acne inclusion criteria at the Screening and Baseline visits, PGA was performed at all planned scheduled visits. If, during the study, acne appeared on the trunk, treatment with trifarotene and tolerability assessments were begun at the investigator's discretion; these data were evaluated in the safety analysis.
Overall treatment success was defined at a particular study visit if a patient had an IGA or PGA score of ‘0’ (clear) or ‘1’ (almost clear) and at least a 2‐grade improvement from Baseline, depending of the anatomical area assessed.
Patients evaluated their facial acne improvement compared with their baseline disease status at Weeks 12, 26 and 52/ET, based on a 6‐point scale (from 0 = complete improvement to 5 = worse).
The dermatology life quality index (DLQI) and the children‐DLQI (C‐DLQI), for patients aged up to 16 years at Baseline, were completed by each patient at Baseline and Weeks 12, 26 and 52/ET.15, 16 The DLQI/C‐DLQI measured the dermatology‐related limitations of functional ability on patients’ lives. The questionnaires were conducted prior to any acne assessment by the investigator, to avoid influencing the patient's answers. The higher the score (ranging from 0 to 30), the more the QOL was impaired.
For statistical analysis purposes, ‘Baseline’ evaluations were defined as the last measurements prior to the first application of trifarotene. No inferential statistical analysis was planned. All data were summarized descriptively using observed data. No imputation methods were used to impute the missing values.
For summary statistics, categorical variables were summarized by frequency and percentage for each response category. Continuous variables were summarized using means and standard deviations (mean ± SD). All local tolerability and efficacy endpoints, as well as DLQI/C‐DLQI, were summarized by visit.
SAS version 9.4 or above was used for all statistical analyses.
Results
Study population
A total of 453 (99.6%) patients were enrolled and received trifarotene, and 348 (76.5%) completed the study. The most common reason for discontinuation was patient's request 53 (11.6%). A total of 376 (83%) patients completed at least 180 days, and 342 (75.5%) patients completed 360 days. Details about patient disposition are provided in Fig. 1. The mean patient age was 18.3 ± 6.6 years, ranging from 9 to 54 years. Demographic and baseline characteristics for the PGA safety population were similar to the IGA safety population.
Figure 1.
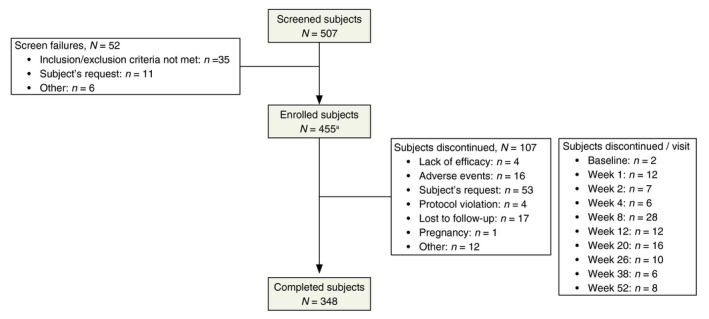
Patient disposition. Screened patients were those who had signed the ICF. Enrolled patients were those who did not fail screening and were given trifarotene at Baseline visit. aOf the 455 patients enrolled, 453 were treated (i.e. two patients did not apply study drug and returned the unused study drug).
Detailed demographic and baseline characteristics are provided in Table 1.
Table 1.
Demographic and baseline characteristics
| Safety population (SAF, N = 453) | Safety PGA population (SAFT, N = 444) | |
|---|---|---|
| Age (years) | ||
| Mean (SD) | 18.3 ± 6.6 | 18.4 ± 6.5 |
| Age group, n (%) | ||
| <18 years | 286 (63.1) | 277 (62.4) |
| ≥18 years | 167 (36.9) | 167 (37.6) |
| 9–11 years | 18 (4.0) | 9 (2.0) |
| Gender, n (%) | ||
| Male | 227 (50.1) | 227 (51.1) |
| Female | 226 (49.9) | 217 (48.9) |
| Race, n (%) | ||
| White | 432 (95.4) | 424 (95.5) |
| Black or African American | 12 (2.6) | 11 (2.5) |
| Asian | 3 (0.7) | 3 (0.7) |
| American Indian or Alaska Native | 1 (0.2) | 1 (0.2) |
| Native Hawaiian or Other Pacific Islander | 3 (0.7) | 3 (0.7) |
| Multiple | 2 (0.4) | 2 (0.5) |
| Other | 0 | 0 |
| Ethnicity, n (%) | ||
| Hispanic or Latino | 47 (10.4) | 44 (9.9) |
| Not Hispanic or Latino | 406 (89.6) | 400 (90.1) |
| Skin phototype, n (%) | ||
| Type I | 13 (2.9) | 13 (2.9) |
| Type II | 188 (41.5) | 182 (41.0) |
| Type III | 184 (40.6) | 183 (41.2) |
| Type IV | 53 (11.7) | 52 (11.7) |
| Type V | 7 (1.5) | 7 (1.6) |
| Type VI | 2 (0.4) | 1 (0.2) |
| Missing | 6 (1.3) | 6 (1.4) |
| Baseline inflammatory facial lesion count | ||
| n | 453 | 444 |
| Mean (SD) | 15.0 | 15.2 |
| Baseline non‐inflammatory facial lesion count | ||
| n | 453 | 444 |
| Mean (SD) | 58.2 ± 36.7 | 58.5 ± 37.0 |
| Baseline inflammatory truncal lesion count | ||
| n | 446 | 444 |
| Missing | 7 | 0 |
| Mean (SD) | 43.4 ± 28.6 | 43.5 ± 28.5 |
| Baseline non‐inflammatory truncal lesion count | ||
| Missing | 7 | 0 |
| Mean (SD) | 56.1 ± 39.5 | 56.3 ± 39.4 |
Safety Population (SAF): All patients that were randomized and applied the study drug at least once. It includes all patients with facial acne/trunk acne and facial acne/without truncal acne.
Safety PGA Population (SAFT): All patients that were randomized and applied the study drug at least once on the trunk. It includes all patients with facial acne/truncal acne.
Local tolerability and safety
At Baseline, ˃80% of the patients did not exhibit any local tolerability signs/symptoms on the face and trunk. Table 2 provides percentages of patients reporting local tolerability signs/symptoms by severity observed with trifarotene on the face and trunk.
Table 2.
Local tolerability parameters worsened from Baseline (worst post‐baseline) on the face and trunk (SAFT Population)
| Face ( N = 453) | |
|---|---|
| Erythema | |
| n | 449 |
| Mild, n (%) | 210 (46.8) |
| Moderate, n (%) | 111 (24.7) |
| Severe, n (%) | 10 (2.2) |
| Scaling | |
| n | 449 |
| Mild, n (%) | 210 (46.8) |
| Moderate, n (%) | 131 (29.2) |
| Severe, n (%) | 10 (2.2) |
| Dryness | |
| n | 449 |
| Mild, n (%) | 195 (43.4) |
| Moderate, n (%) | 140 (31.2) |
| Severe, n (%) | 26 (5.8) |
| Stinging/Burning | |
| n | 449 |
| Mild, n (%) | 169 (37.6) |
| Moderate, n (%) | 95 (21.2) |
| Severe, n (%) | 32 (7.1) |
| Trunk ( N = 446) | |
|---|---|
| Erythema | |
| n | 442 |
| Mild, n (%) | 131 (29.6) |
| Moderate, n (%) | 76 (17.2) |
| Severe, n (%) | 24 (5.4) |
| Scaling | |
| n | 442 |
| Mild, n (%) | 137 (31.0) |
| Moderate, n (%) | 48 (10.9) |
| Severe, n (%) | 11 (2.5) |
| Dryness | |
| n | 442 |
| Mild, n (%) | 152 (34.4) |
| Moderate, n (%) | 63 (14.3) |
| Severe, n (%) | 12 (2.7) |
| Stinging/Burning | |
| n | 442 |
| Mild, n (%) | 111 (25.1) |
| Moderate, n (%) | 42 (9.5) |
| Severe, n (%) | 21 (4.8) |
Safety PGA Population (SAFT): All patients that were randomized and applied the study drug at least once on the trunk. It includes all patients with facial acne/truncal acne.
The mean tolerability score, including worst score for each parameter during the study period, is presented in Fig. 2 for the face and in Fig. 3 for the trunk.
Figure 2.
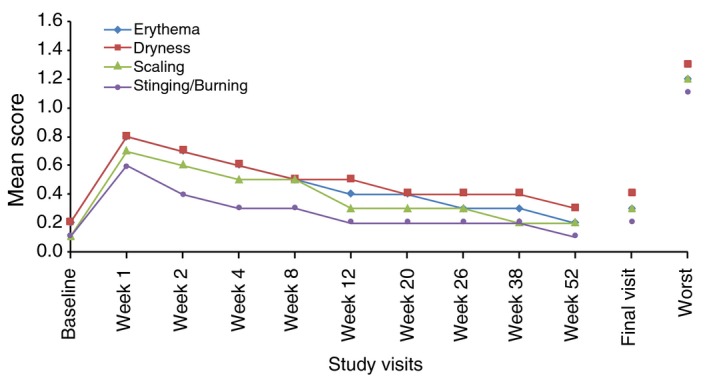
Local tolerability by visit: mean scores of signs and symptoms on the face (SAF population). Safety Population (SAF): All patients that were randomized and applied the study drug at least once. It includes all patients with facial acne/trunk acne and facial acne/without truncal acne. Local tolerability parameters including erythema, scaling, dryness and stinging/burning were evaluated at each visit on a 4‐point scale (0 = none, 1 = mild, 2 = moderate and 3 = severe) at Baseline and at Weeks 12, 20, 26, 38 and 52/ET.
Figure 3.
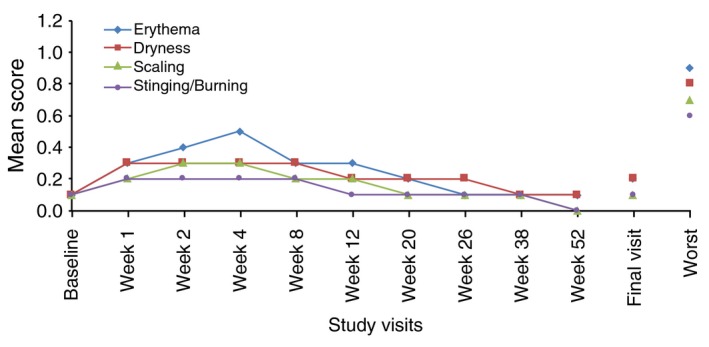
Local tolerability by visit: mean scores of signs and symptoms on the trunk (SAFT population). Safety PGA Population (SAFT): All patients that were randomized and applied the study drug at least once on the trunk. It includes all patients with facial acne/truncal acne. Local tolerability parameters including erythema, scaling, dryness and stinging/burning were evaluated at each visit on a 4‐point scale (0 = none, 1 = mild, 2 = moderate and 3 = severe) at Baseline and at Weeks 12, 20, 26, 38 and 52/ET.
Overall, 468 TEAEs were reported in 218/453 (48.1%) patients during the whole study period, of which 57 (12.6%) patients had cutaneous AES related to trifarotene. Thirteen (2.9%) patients had AES related to trifarotene leading to discontinuation. The majority of all TEAEs that occurred during the first 3 months of the study. Serious TEAEs occurred in 10 (2.2%) of the patients; none were trifarotene‐related.
The most common cutaneous trifarotene‐related TEAEs were pruritus [21 (4.6%) patients], irritation [19 (4.2%) patients] and sunburn [8 (1.8%) patients]; these were mainly observed on treated areas and were of mild severity. Severe trifarotene‐related TEAEs occurred in 3 (0.7%) different patients: application site irritation, pruritus and erythema.
A total of 16 (3.5%) patients discontinued the study due to TEAEs, among which 13 (2.9%) discontinued due to related TEAEs. The latter were considered AESIs – 10 events were skin irritation and three events were worsening of acne. All occurred on the face and trunk during the first 3 months were related and non‐serious. All patients discontinued from the study and followed up until complete resolution or stabilization.
Treatment‐emergent adverse events were also analysed among the subset of patients who extended the application of trifarotene to their middle and/or lower back and applied the cream at least once during the study period in that area. The safety profile in these patients (n = 231) was similar to that of the overall safety population.
No clinically meaningful change in routine laboratory parameter values was observed during the study.
Efficacy
Both IGA and PGA success rates continuously improved over time, IGA success rates increased from 26.6% at Week 12 to 65.1% at Week 52; PGA success rates increased from 38.6% at Week 12 to 66.9% at Week 52. IGA and PGA success rates are given in Fig. 4. Overall success (both IGA and PGA success in the same patient) was 57.9% at Week 52 (Fig. 5).
Figure 4.
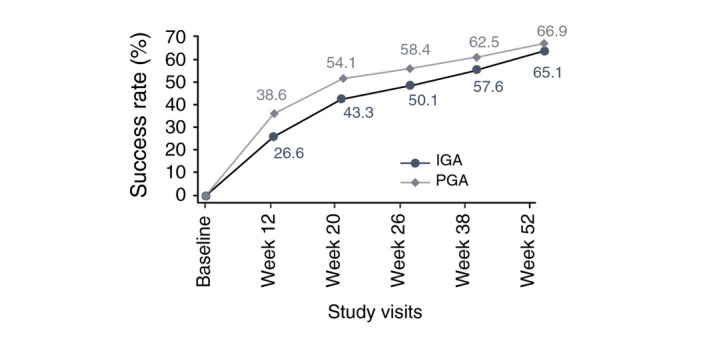
Evolution of the percent investigator's global assessment and physician global assessment success rates at baseline and weeks 12, 20, 26, 38 and 52 (SAFT population). Investigator's Global Assessment (IGA) and Physician Global Assessment (PGA) were scored on a 5‐point scale (from 0 = clear to 4 = severe).
Figure 5.
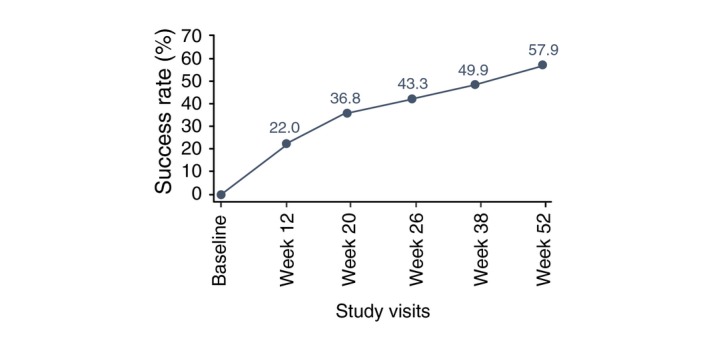
Evolution of the overall percent investigator's global assessment and physician global assessment success in the same patient over 52 weeks (SAFT population). Overall treatment success was defined at a particular study visit if the same patient had an IGA/PGA score of “clear” or “almost clear” and at least a 2‐grade improvement from Baseline at any study visit.
The percentage of patients who reported a marked or complete improvement of facial acne was 41.4% at Week 12, 54.8% at Week 26 and 66.6% at Week 52.
Figure 6 provides visual support of improvement of severity of acne on the face and trunk at different time points during the study.
Figure 6.
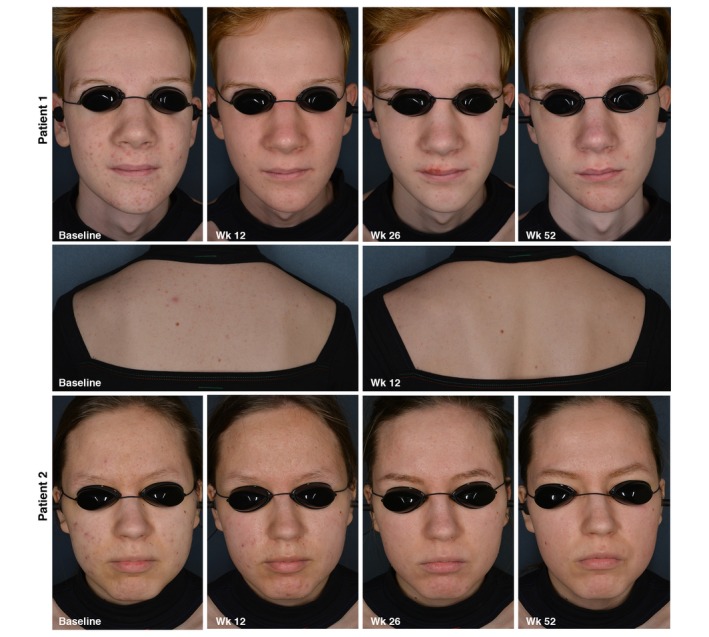
Acne severity of two patients at different time points. Patient n°1: Face, Acne severity on the face decreased from an IGA score of 3 to an IGA score of 1 at 12 weeks; Trunk, Acne severity on the trunk decreased from a PGA score of 3 to a PGA score of 2 at Week 12. Patient n°2: Face, Acne severity on the face decreased from an IGA score of 3 to an IGA score of 1 after 52 weeks.
Quality of life
At Week 52 visit, 92/171 (53.8%) patients who had completed their assessments had scores from 0 to 1 (i.e. no effect of acne on their QOL) vs. 47/208 (22.6%) patients at Baseline visit (Table 3).
Table 3.
Impact of acne on the patient's quality of life using the Dermatology Life Quality Index and the Children Dermatology Life Quality Index at Baseline and Week 52 (SAF Population)
| Baseline | Week 52 | |
|---|---|---|
| DLQI | ( N = 208) | ( N = 171) |
| No effect, n (%) | 47 (22.6) | 92 (53.8) |
| Small effect, n (%) | 85 (40.9) | 61 (35.7) |
| Moderate effect, n (%) | 43 (20.7) | 14 (8.2) |
| Very large effect, n (%) | 33 (15.9) | 4 (2.3) |
| Extremely large effect, n (%) | 0 | 0 |
| C‐DLQI | ( N = 246) | ( N = 177) |
| No effect, n (%) | 76 (30.9) | 96 (54.2) |
| Small effect, n (%) | 115 (46.7) | 71 (40.1) |
| Moderate effect, n (%) | 49 (19.9) | 10 (5.6) |
| Very large effect, n (%) | 6 (2.4) | 0 |
| Extremely large effect, n (%) | 0 | 0 |
Safety Population (SAF): All patients that were randomized and applied the study drug at least once. It includes all patients with facial acne/trunk acne and facial acne/without truncal acne.
DLQI: Patients 17 years of age and older at Baseline visit; C‐DLQI: Patients aged ≤16 years at Baseline visit.
The meaning of the DLQI total scores was interpreted as follows: no effect (0–1), small effect (2–6), moderate effect (7–12), very large effect (13–18) or extremely large effect (19–30).
Discussion and conclusions
This 52‐week long‐term study assessed for the first time the safety and efficacy of the first‐in‐class selective RAR‐γ agonist trifarotene. Moreover, this study is the first to systematically evaluate a treatment for both moderate facial acne and truncal acne in a prospective long‐term tolerability, safety and efficacy study.
A total of 348 (76.5%) patients completed the study; a high completion rate for a 52‐week study, especially when considering the age of the population (more than 63% of the patients were younger than 18 years of age). Relatively few patients aged between 9 and 11 years were enrolled. This was due the low prevalence with truncal acne found in this group.
During this study, 103 trifarotene‐related TEAEs were reported in 57 (12.6%) of the patients. Only 16 (3.5%) of these TEAEs led to study discontinuation, with a majority (2.9%) occurring during the first quarter after treatment initiation. The most common cutaneous TEAEs related to trifarotene were application site pruritus [in 21 (4.6%) of the patients], application site irritation [in 19 (4.2%) of the patients] and sunburn [in 8 (1.8%) of the patients]; all were observed on treated areas. As observed with other retinoids, the majority of these local adverse events occurred during the first weeks of treatment, subsiding thereafter; all were mild or moderate in intensity.17, 18, 19 Local tolerability on the trunk was better than on the face. A possible explanation could be that the epidermis of the back is thicker than of the face. Facial and truncal skin are exposed to different environmental challenges. Facial skin is more frequently exposed to environmental sources of irritation and damage, and often remains unprotected against external triggers such as UV light, temperature and pollution, while the skin on the trunk is more frequently exposed to heat, moisture and occlusion.9 The concomitant use of moisturizer facilitated management of ‘retinoid dermatitis’ as reported recently.20 To limit retinoid‐related side‐effects, short contact therapy (SCT) was proposed for topical treatments with retinoids.21 To address this issue, SCT study was performed during the development programme of trifarotene. Results from this pilot study showed a better tolerability but reduced efficacy of trifarotene (unpublished results).
Overall success (IGA and PGA success in the same subject) increased continuously with a greater acne improvement on the trunk than on the face. At 52 weeks of treatment, 57.9% of the patients experienced overall success, a significant therapeutic benefit. The continued improvement, reaching high rates of overall success (face and trunk clear or almost clear in the same subject) over 52 weeks, is important for healthcare providers to motivate and encourage patients to follow long‐term topical therapy with the expectation of further benefits.
The QOL of adults and children substantially improved over the study period and was positively impacted by the improved acne severity.
Patients had a high treatment compliance of 95.3% for both face and trunk. The treatment regimen and manageable safety profile might have played a role in the observed treatment compliance, an observation reported by previous investigations.22
The open‐label and non‐comparative design of this study has certain inherent limitations and biases. Nevertheless, the design of this study was appropriate for the intended objectives, with evaluation of local tolerance and safety of trifarotene in patients with acne on both the face and trunk being the primary aim. The results obtained during the study confirmed the primary study objectives demonstrating that trifarotene is safe and well tolerated. One yet unpublished phase 2 12‐week study in patients with moderate to severe acne evaluated three increasing therapeutic concentrations of trifarotene cream (25, 50 and 100 μg/g) vs. vehicle and tazarotene 0.1% gel. The dose strength of trifarotene 50 μg/g was shown to be more effective than the lower concentration (trifarotene 25 μg/g) and with regard to the higher concentration (trifarotene 100 μg/g) in 2‐point reduction of Investigator's Global Assessment (IGA) scores and per cent decrease in inflammatory lesion count but less effective in reduction of total lesion count than trifarotene 100 μg/g cream. Although both doses of 50 and 100 μg/g of trifarotene provided a positive benefit/risk ratio in the treatment of acne vulgaris when compared to vehicle, the superior safety and local tolerability profile of trifarotene 50 μg/g cream over trifarotene 100 μg/g and tazarotene 0.1% gel argued in favour of carrying this dose forward in phase 3. Two vehicle well‐controlled, identical phase 3 studies in the target population further substantiated the clinical benefit of trifarotene 50 μg/g cream of both facial and truncal acne.23
In conclusion, in this 52‐week study, once‐daily application of trifarotene 50 μg/g cream was safe, well tolerated and effective in patients with moderate facial and truncal acne, providing continuous improvement over time to patients. The present results also support the acceptable safety profile of trifarotene. Moreover, this is the first comprehensive long‐term study of truncal acne using a validated methodology. Thus, this study design could serve as a model for future investigations of this largely ignored clinical condition.
Acknowledgments
The authors would like to thank the Trifarotene LTS study teams in Sophia Antipolis, France and Fort Worth – Texas, USA; all investigators and research staff in Europe and the United States who participated in this clinical trial and acknowledge the writing support of Karl Patrick Göritz, SMWS – Scientific and Medical Writing Services, France.
Conflict of interest
Ulrike Blume‐Peytavi has received honoraria lectures from Nestlé Skin Health – Galderma and has received fees for the conduct of clinical studies. Joseph Fowler received grant support as principal investigator of this study. Thomas Dirschka received lecture fees from Almirall, Biofrontera, Nestlé Skin Health Care – Galderma R&D, Leo, Meda, Riemser, Janssen and unrestricted grants from Meda and Nestlé Skin Health Care – Galderma R&D; moreover, he is a member of advisory boards for Almirall, Biofrontera, Leo, Meda, Novartis, Riemser and Janssen. Zoe Draelos, Fran Cook‐Bolden, Lajos Kemény and Lawrence Eichenfield have served as investigators and consultants for Nestlé Skin Health Care – Galderma. Phoebe Rich has received consulting fees from Abbvie, Allergan, Anacor, Novartis, Polichem, Valeant and Meiji. Emil Tanghetti has served as a consultant and investigator for clinical research for Hologic, Nestlé Skin Health Care – Galderma R&D, Allergan and Ortho Dermatologics. Michael Graeber, Faiz Ahmad and Alessandra Alió Saenz are employees of Galderma Research and Development LLC, Fort Worth, TX, USA. Karl Patrick Göritz has no financial interest to disclose.
Funding sources
The study was funded by Nestlé Skin Health Care – Galderma R&D.
References
- 1. Tan JK, Bhate K. A global perspective on the epidemiology of acne. Br J Dermatol 2015; 172(Suppl 1): 3–12. [DOI] [PubMed] [Google Scholar]
- 2. Goulden V, Stables GI, Cunliffe WJ. Prevalence of facial acne in adults. J Am Acad Dermatol 1999; 41: 577–580. [PubMed] [Google Scholar]
- 3. Poli F, Dreno B, Verschoore M. An epidemiological study of acne in female adults: results of a survey conducted in France. J Eur Acad Dermatol Venereol 2001; 15: 541–545. [DOI] [PubMed] [Google Scholar]
- 4. Perkins AC, Maglione J, Hillebrand GG, Miyamoto K, Kimball AB. Acne vulgaris in women: prevalence across the life span. J Women's Health 2002 2012; 21: 223–230. [DOI] [PubMed] [Google Scholar]
- 5. Tanghetti EA, Kawata AK, Daniels SR, Yeomans K, Burk CT, Callender VD. Understanding the burden of adult female acne. J Clin Aesthet Dermatol 2014; 7: 22–30. [PMC free article] [PubMed] [Google Scholar]
- 6. Zaenglein AL, Pathy AL, Schlosser BJ et al Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol 2016; 74: 945–973. e33 [DOI] [PubMed] [Google Scholar]
- 7. Tan JK, Tang J, Fung K et al Prevalence and severity of facial and truncal acne in a referral cohort. J Drugs Dermatol 2008; 7: 551–556. [PubMed] [Google Scholar]
- 8. Del Rosso JQ. Management of truncal acne vulgaris: current perspectives on treatment. Cutis 2006; 77: 285–289. [PubMed] [Google Scholar]
- 9. Mandy S. Chest and back acne: a retrospective review. Adv Ther 1995; 12: 321–322. [Google Scholar]
- 10. Leyden J, Stein‐Gold L, Weiss J. Why topical retinoids are mainstay of therapy for acne. Dermatol Ther (Heidelb) 2017; 7: 293–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nast A, Dreno B, Bettoli V et al European evidence‐based (S3) guideline for the treatment of acne – update 2016 – short version. J Eur Acad Dermatol Venereol 2016; 30: 1261–1268. [DOI] [PubMed] [Google Scholar]
- 12. Thiboutot DM, Dreno B, Abanmi A et al Practical management of acne for clinicians: an international consensus from the Global Alliance to Improve Outcomes in Acne. J Am Acad Dermatol 2018; 78: S1–S23.e1 [DOI] [PubMed] [Google Scholar]
- 13. Aubert J, Piwnica D, Bertino B et al Non‐clinical and human pharmacology of the potent and selective topical RARγ agonist trifarotene. Br J Dermatol 2018; 179: 442–456. [DOI] [PubMed] [Google Scholar]
- 14. Thoreau E, Arlabosse J, Boui‐Peter C et al Structure‐based design of Trifarotene (CD5789), a potent and selective RARγ agonist for the treatment of acne. Bioorg Med Chem Lett 2018; 28: 1736–1741. [DOI] [PubMed] [Google Scholar]
- 15. Morgan M, McCreedy R, Simpson J, Hay RJ. Dermatology quality of life scales–a measure of the impact of skin diseases. Br J Dermatol 1997; 136: 202–206. [PubMed] [Google Scholar]
- 16. Lewis‐Jones MS, Finlay AY. The Children's Dermatology Life Quality Index (CDLQI): initial validation and practical use. Br J Dermatol 1995; 132: 942–949. [DOI] [PubMed] [Google Scholar]
- 17. Fluhr JW, Vienne MP, Lauze C, Dupuy P, Gehring W, Gloor M. Tolerance profile of retinol, retinaldehyde and retinoic acid under maximized and long‐term clinical conditions. Dermatology 1999; 199(Suppl 1): 57–60. [DOI] [PubMed] [Google Scholar]
- 18. Weiss JS, Thiboutot DM, Hwa J, Liu Y, Graeber M. Long‐term safety and efficacy study of adapalene 0.3% gel. J Drugs Dermatol 2008; 7(6 Suppl): s24–s28. [PubMed] [Google Scholar]
- 19. Culp L, Moradi Tuchayi S, Alinia H, Feldman SR. Tolerability of topical retinoids: are there clinically meaningful differences among topical retinoids? J CutMed Surg 2015; 19: 530–538. [DOI] [PubMed] [Google Scholar]
- 20. Balak DMW. Topical trifarotene: a new retinoid. Br J Dermatol 2018; 179: 231–232. [DOI] [PubMed] [Google Scholar]
- 21. Veraldi S, Barbareschi M, Benardon S, Schianchi R. Short contact therapy of acne with tretinoin. J Dermatolog Treat 2013; 24: 374–376. [DOI] [PubMed] [Google Scholar]
- 22. Koo J. How do you foster medication adherence for better acne vulgaris management? Skinmed 2003; 2: 229–233. [DOI] [PubMed] [Google Scholar]
- 23. Tan J, Thiboutot D, Popp G et al Randomized phase 3 evaluation of trifarotene 50 μg/g cream treatment of moderate facial and truncal ACNE. J Am Acad Dermatol 2019; 80: 1691–1699. [DOI] [PubMed] [Google Scholar]


