Abstract
In combination with bulky substituents at the core, fourfold benzannulation at the cata‐positions stabilizes a nonacene sufficiently to allow its isolation and characterization by 1H NMR and X‐ray analysis. The four benzo units blueshift the absorption spectrum in comparison to a solely linear nonacene, but significantly increase the stability in the solid state.
Keywords: acenes, benzannulation, Cava reaction, nonacene, X-ray diffraction
Attachment of four benzo units electronically stabilizes a nonacene core without heteroatom substitution at/on the aromatic backbone. The four benzo units blueshift the absorption spectrum in comparison to a linear nonacene and significantly increase the stability in the solid state.
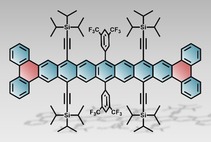
The higher acenes have been a magnificent challenge, accepted since Clar's first synthesis of pentacene.1 Unsubstituted, they are both insoluble and vulnerable towards ambient conditions. Anthony et al.2 have introduced bulky silylethynyl substituents to the larger acenes, and, depending on size and steric demand, even heptacenes can be stabilized (Figure 1).3 However, the stabilization of octacenes and nonacenes remains challenging. Apart from surface‐4 or matrix‐based5 approaches, to the best of our knowledge only two approaches yielded nonacene‐type structures. Miller et al. employed thioether substituents,6 while Anthony et al. combined steric repulsion with fluorination7 to achieve the stabilization of nonacene derivative Non. Other methods of stabilization allow a significant number of linearly annulated benzene rings but with starkly diminished acene character.8 We recently prepared stable tetrabenzoheptacenes (and azaheptacenes) such as B4Hep, exhibiting hexacene‐like absorption maxima, the blueshift being a consequence of the diminished conjugation of the quadruply annulated acene core.9 Here we extend this approach to a reasonably stable nonacene derivative, B4Non, by employing a modification of Anthony's route.
Figure 1.
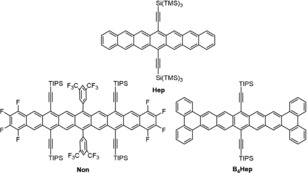
Heptacene Hep, nonacene Non and tetrabenzoheptacene B4Hep.
Bis(bromomethyl)phenanthrene 1 and an excess of p‐benzoquinone furnished 2 (Scheme 1); a second Cava reaction with 3 results in 4 (crude yield 87 %), fourfold ethynylation of which gave intermediate 5 (40 %) using a large excess of lithium acetylide (≈100 equiv). Reductive aromatization with SnCl2 furnishes tetrabenzononacene B4Non. The concentration was adjusted to precipitate B4Non during synthesis. B4Non, unlike other higher acenes,7 is surprisingly stable in the solid state. Its proton NMR spectrum shows sharp resonances (see the Supporting Information), in contrast to the broad signals observed for other nonacenes7—the well‐resolved signals being due to the stabilization of its closed‐shell ground state.
Scheme 1.
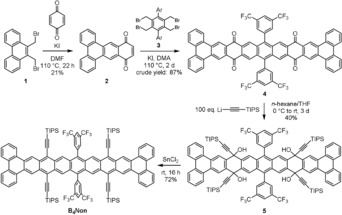
Synthesis of tetrabenzononacene B4Non.
Figure 2.
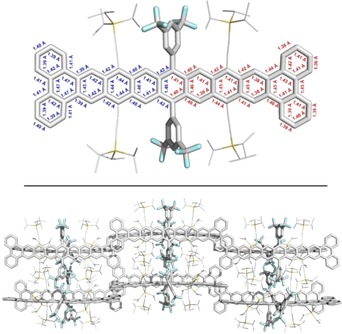
X‐ray structure (top) and packing motif (bottom) of B4Non including average bond lengths determined by crystal analysis (red) vs. calculated bond lengths (blue; DFT, B3LYP/6‐311+G*). For the sake of clarity, all protons were omitted and TIPS‐ethynyl substituents were reduced in size.
Single crystals were grown by subsequently layering n‐hexane and MeOH on a THF solution of B4Non under nitrogen; solvent molecules are included in the crystal packing but are heavily disordered and cannot be resolved. B4Non crystallizes with two independent molecules per unit cell with a minor π–π interaction of two phenanthrenylenes. These molecules form 1D‐stacks and are oriented perpendicular to each other, effectively preventing dimerization but also pronounced π–π interactions in the solid state. The independent molecules’ average bond lengths of the innermost ring of the formal triphenylene units are elongated (1.44–1.47 Å) compared to that of the other aromatic C−C backbone bonds (1.38–1.46 Å).9 Calculated and experimentally determined bond lengths of B4Non are in agreement with each other and the expected values.
Non‐fluorescent B4Non exhibits structured, finger‐like absorption bands with a lowest energy absorption maximum at 958 nm (Figure 3, Table 1), 576 cm−1 blue‐shifted in comparison to that of Anthony's nonacene Non and red‐shifted by 2859 cm−1 compared to the p‐band of B4Hep. The first reduction potential of B4Non occurs at −1.19 V (cyclovoltammetry, reversible, Table 1, SI), more negative than that of Non (−0.51 eV) due to the absence of electron‐withdrawing fluorine substituents.7, 9 The first oxidation potential is at 0.98 V (irreversible).
Figure 3.
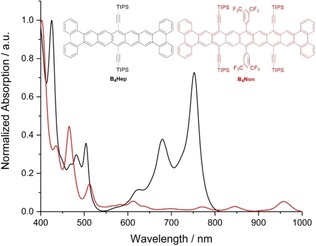
UV/vis absorption spectra of B4Hep (black) and B4Non (red) in n‐hexane (c≈10−6 m) at room temperature.
Table 1.
Photophysical and calculated properties.
|
Acene |
λ max,abs [nm][a] |
E 1/2 red1 [V][b] |
EA CV [eV][c] |
E LUMO,DFT [eV][d] |
|---|---|---|---|---|
|
B4Non |
958 |
−1.19 |
−3.61 |
−3.79 |
|
Non 7 |
1014 |
−0.51 |
−4.29 |
−4.27 |
|
B4Hep 9 |
752 |
−1.34 |
−3.46 |
−3.26 |
|
Hep 3 |
835 |
−0.83* |
−3.97 |
−3.38 |
[a] Lowest energy absorption maxima. [b] First reduction potentials measured by cyclic voltammetry (CV) in CH2Cl2 using Bu4NPF6 as electrolyte and Fc/Fc+ as internal standard (−4.80 eV) at 0.2 V s−1/ * vs. SCE.11 [c] Electron affinities estimated from first reduction potentials. [d] DFT‐calculated LUMOs using TURBOMOLE B3LYP/ def2 TZVP//Gaussian 09, B3LYP/6‐311++G**. TMS substituents were used instead of TIPS to simplify calculations.12
Similar to most higher acenes,3a, 7, 10 there is a dramatic difference between solid state and solution persistability: B4Non is stable for more than 6 weeks under nitrogen in the solid state, although its half‐life in n‐hexane solution is only 30 min under ambient conditions (see SI) and 7 h under nitrogen atmosphere (Figure 4). In air probably the endo‐peroxide forms (see SI),7 while the mode of decomposition under nitrogen is less clear.
Figure 4.
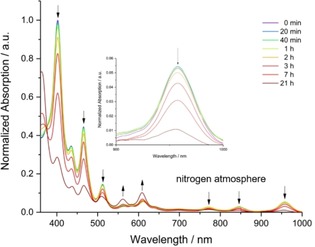
Change in UV/vis absorption intensity of B4Non under an atmosphere of nitrogen in n‐hexane at room temperature.
Analysis of the decomposition products via recycling gel permeation chromatography (see SI) suggests the formation of dimeric and oligomeric species, both common degradation products for higher acenes.3a, 10 Compared to Non, B4Non is less stable under air and nitrogen in solution (Figure 4), as Non's electron‐withdrawing halogen substituents retard endo‐peroxide formation. In contrast, Non is persistent for 2 d at 10 °C in the solid state,7 while B4Non is stable for weeks.
Figure 5 displays the calculated NICS values for B4Non′. The system shows NICS(1) values in accord with expectations, with the formal inner triphenylene ring being the least aromatic one—“empty” in the Clar formalism—and the other rings displaying high aromaticity. This is further illustrated in FMO calculations (see SI), in which the central triphenylene rings show small coefficients in the outer rings compared to their nonacene congeners.
Figure 5.
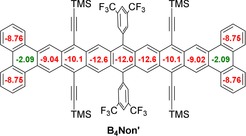
NICS(1) values of B4Non′ DFT‐calculated at the B3LYP/6–311+G* level; TMS substituents were used to simplify calculations.12
In conclusion, we have prepared a novel, reasonably stable tetrabenzononacene, B4Non, in which the stabilization is due to the attachment of four benzo units at the cata‐position of the acene unit. The material displays sharp NMR resonances and a slightly blue‐shifted absorption in comparison to Anthony's nonacene Non, yet it is only one of the very few isolated and structurally characterized nonacenes. B4Non is highly stable in the solid state. Benzo‐wings therefore should allow stabilization of other, hitherto only moderately persistent, reactive aromatics.
Experimental Section
CCDC https://www.ccdc.cam.ac.uk/services/structures?id=doi:10.1002/anie.201909614 (B4Non) contains the supplementary crystallographic data for this publication. These data can be obtained free of charge from The http://www.ccdc.cam.ac.uk/. The synthetic details for precursors and remaining materials can be found in the SI.
Synthesis of 6: (Tri‐iso‐propylsilyl)acetylene (1.10 g, 1.36 mL, 6.02 mmol, 100 equiv.) was dissolved in n‐hexane (20 mL) and cooled to 0 °C. n‐BuLi (2.5 m in n‐hexane, 2.29 mL, 5.72 mmol, 95.0 equiv.) was added dropwise and the mixture was stirred for 1 h at room temperature. 4 (70.0 mg, 60.2 μmol, 1.00 equiv.) was added and the suspension was stirred for 3 d at room temperature. Sat. NHCl4 solution and DCM were added, the layers were separated and the aqueous layer was extracted with DCM. The combined organic layers were dried over MgSO4, filtered and the solvent was removed under reduced pressure. The crude product was subjected to column chromatography (SiO2, PE/DCM 80:20 to 25:75) to yield 6 as a yellow powder (46.0 mg, 24.3 μmol, 40 %). Mp: >350 °C. 1H NMR (600 MHz, CD2Cl2) δ=9.41 (s, 4 H), 8.80 (dd, J=7.2, 1.8 Hz, 4 H), 8.70 (m, 4 H), 8.47 (s, 4 H), 8.29 (d, J=2.0 Hz, 2 H), 8.18 (d, J=1.6 Hz, 4 H), 7.71 (qd, J=7.2, 1.6 Hz, 8 H), 3.43 (s, 4 H), 1.08 (m, 42 H), 0.97 ppm (m, 84 H). 13C{1H} NMR (151 MHz, CD2Cl2) δ=140.7, 137.7, 136.8, 135.8, 132.5, 132.3, 130.5, 130.4, 130.3, 129.5, 128.1, 127.6, 124.6, 124.6, 124.1, 123.6, 122.8, 122.0, 109.4, 108.9, 104.6, 90.3, 85.7, 69.4, 18.5, 18.5, 18.5, 11.4, 11.3 ppm. IR (neat): ν (cm−1)=2942, 2863, 1464, 1335, 1274, 1141, 1042, 888, 750, 670. HRMS (MALDI+, DCTB): m/z calcd for C114H116F12O4Si4: [M+H]+ 1891.7988, found: 1891.7974, correct isotope distribution.
Synthesis of B4Non: 6 (20.0 mg, 10.6 μmol, 1.00 equiv.) was dissolved in MeCN/THF (1:1, 2 mL) in a glove box. Anhydrous SnCl2 (40.1 mg, 211 μmol, 20.0 equiv.) was added and the mixture was stirred at room temperature overnight. The resulting precipitate was filtered, washed with MeCN and dried to give almost pure B4Non (crude yield 72 %). The material can further be purified by washing it thoroughly with DCM to yield pure B4Non (total yield: 3.0 mg, 1.64 μmol, 16 %). Suitable specimen for single crystal analysis were grown by subsequently layering n‐hexane and MeOH on a THF solution under nitrogen. Mp: >350 °C. 1H NMR (600 MHz, [D8]THF) δ=10.83 (s, 2 H), 9.72 (s, 4 H), 8.95 (s, 4 H), 8.76 (d, J=8.0 Hz, 4 H), 8.61 (d, J=8.0 Hz, 4 H), 8.49 (m, 4 H), 7.64 (m, 8 H), 1.26 ppm (m, 84 H). The compound was not soluble enough for 13C{1H} NMR analysis. IR (neat): ν (cm−1)=2939, 2855, 1453, 1369, 1213, 1110, 1019, 882, 753, 506. HRMS (MALDI+, DCTB): m/z calcd for C114H105F12Si4: [M+H]+ 1823.7879, found: 1823.7891, correct isotope distribution.
Conflict of interest
The authors declare no conflict of interest.
Supporting information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supplementary
Acknowledgements
M.M. thanks the Fonds der Chemischen Industrie for a PhD scholarship. We thank the DFG (SFB 1249) for generous support, Prof. M. Mastalerz for access to the cyclic voltammetry/ recycling gel permeation chromatography system, and L. Ahrens for support with cyclic voltammetry measurements.
M. Müller, S. Maier, O. Tverskoy, F. Rominger, J. Freudenberg, U. H. F. Bunz, Angew. Chem. Int. Ed. 2020, 59, 1966.
References
- 1. Clar E., Polycyclic Hydrocarbons, Vol. 1, 2nd Ed., Springer, Berlin, 1964. [Google Scholar]
- 2.
- 2a. Anthony J. E., Chem. Rev. 2006, 106, 5028–5048; [DOI] [PubMed] [Google Scholar]
- 2b. Anthony J. E., Angew. Chem. Int. Ed. 2008, 47, 452–483; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2008, 120, 460–492; [Google Scholar]
- 2c. Purushothaman B., Parkin S. R., Anthony J. E., Org. Lett. 2010, 12, 2060–2063; [DOI] [PubMed] [Google Scholar]
- 2d. Purushothaman B., Parkin S. R., Kendrick M. J., David D., Ward J. W., Yu L., Stingelin N., Jurchescu O. D., Ostroverkhova O., Anthony J. E., Chem. Commun. 2012, 48, 8261–8263. [DOI] [PubMed] [Google Scholar]
- 3.
- 3a. Payne M. M., Parkin S. R., Anthony J. E., J. Am. Chem. Soc. 2005, 127, 8028–8029; [DOI] [PubMed] [Google Scholar]
- 3b. Chun D., Cheng Y., Wudl F., Angew. Chem. Int. Ed. 2008, 47, 8380–8385; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2008, 120, 8508–8513; [Google Scholar]
- 3c. Qu H., Chi C., Org. Lett. 2010, 12, 3360–3363. [DOI] [PubMed] [Google Scholar]
- 4.
- 4a. Zuzak R., Dorel R., Kolmer M., Szymonski M., Godlewski S., Echavarren A. M., Angew. Chem. Int. Ed. 2018, 57, 10500–10505; [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew. Chem. 2018, 130, 10660–10665; [Google Scholar]
- 4b. Krüger J., García F., Eisenhut F., Skidin D., Alonso J. M., Guitián E., Pérez D., Cuniberti G., Moresco F., Peña D., Angew. Chem. Int. Ed. 2017, 56, 11945–11948; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2017, 129, 12107–12110; [Google Scholar]
- 4c. Zugermeier M., Gruber M., Schmid M., Klein B. P., Ruppenthal L., Müller P., Einholz R., Hieringer W., Berndt R., Bettinger H. F., Nanoscale 2017, 9, 12461–12469; [DOI] [PubMed] [Google Scholar]
- 4d. Colazzo L., Mohammed M. S. G., Dorel R., Nita P., García Fernández C., Abufager P., Lorente N., Echavarren A. M., de Oteyza D. G., Chem. Commun. 2018, 54, 10260–10263; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4e. Clevenger R. G., Kumar B., Menuey E. M., Lee G.-H., Patterson D., Kilway K. V., Chem. Eur. J. 2018, 24, 243–250; [DOI] [PubMed] [Google Scholar]
- 4f. Clevenger R. G., Kumar B., Menuey E. M., Kilway K. V., Chem. Eur. J. 2018, 24, 3113–3116. [DOI] [PubMed] [Google Scholar]
- 5.
- 5a. Tönshoff C., Bettinger H. F., Angew. Chem. Int. Ed. 2010, 49, 4125–4128; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2010, 122, 4219–4222; [Google Scholar]
- 5b. Shen B., Tatchen J., Sanchez-Garcia E., Bettinger H. F., Angew. Chem. Int. Ed. 2018, 57, 10506–10509; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2018, 130, 10666–10669; [Google Scholar]
- 5c. Mondal R., Shah B. K., Neckers D. C., J. Am. Chem. Soc. 2006, 128, 9612–9613; [DOI] [PubMed] [Google Scholar]
- 5d. Mondal R., Tönshoff C., Khon D., Neckers D. C., Bettinger H. F., J. Am. Chem. Soc. 2009, 131, 14281–14289. [DOI] [PubMed] [Google Scholar]
- 6. Kaur I., Jazdzyk M., Stein N. N., Prusevich P., Miller G. P., J. Am. Chem. Soc. 2010, 132, 1261–1263. [DOI] [PubMed] [Google Scholar]
- 7. Purushothaman B., Bruzek M., Parkin S. R., Miller A.-F., Anthony J. E., Angew. Chem. Int. Ed. 2011, 50, 7013–7017; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2011, 123, 7151–7155. [Google Scholar]
- 8.
- 8a. Zhang G., Rominger F., Zschieschang U., Klauk H., Mastalerz M., Chem. Eur. J. 2016, 22, 14840–14845; [DOI] [PubMed] [Google Scholar]
- 8b. Wang Z., Gu P., Liu G., Yao H., Wu Y., Li Y., Rakesh G., Zhu J., Fu H., Zhang Q., Chem. Commun. 2017, 53, 7772–7775; [DOI] [PubMed] [Google Scholar]
- 8c. Kohl B., Rominger F., Mastalerz M., Angew. Chem. Int. Ed. 2015, 54, 6051–6056; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2015, 127, 6149–6154; [Google Scholar]
- 8d. Cortizo-Lacalle D., Mora-Fuentes J. P., Strutyński K., Saeki A., Melle-Franco M., Mateo-Alonso A., Angew. Chem. Int. Ed. 2018, 57, 703–708; [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew. Chem. 2018, 130, 711–716; [Google Scholar]
- 8e. Gu P.-Y., Wang Z., Liu G., Yao H., Wang Z., Li Y., Zhu J., Li S., Zhang Q., Chem. Mater. 2017, 29, 4172–4175. [Google Scholar]
- 9.
- 9a. Müller M., Rüdiger E. C., Koser S., Tverskoy O., Rominger F., Hinkel F., Freudenberg J., Bunz U. H. F., Chem. Eur. J. 2018, 24, 8087–8091; [DOI] [PubMed] [Google Scholar]
- 9b. Müller M., Reiss H., Tverskoy O., Rominger F., Freudenberg J., Bunz U. H. F., Chem. Eur. J. 2018, 24, 12801–12805; [DOI] [PubMed] [Google Scholar]
- 9c. Müller M., Koser S., Tverskoy O., Rominger F., Freudenberg J., Bunz U. H. F., Chem. Eur. J. 2019, 25, 6082–6086. [DOI] [PubMed] [Google Scholar]
- 10.
- 10a. Zade S. S., Zamoshchik N., Reddy A. R., Fridman-Marueli G., Sheberla D., Bendikov M., J. Am. Chem. Soc. 2011, 133, 10803–10816; [DOI] [PubMed] [Google Scholar]
- 10b. Maliakal A., Raghavachari K., Katz H., Chandross E., Siegrist T., Chem. Mater. 2004, 16, 4980–4986. [Google Scholar]
- 11. Cardona C. M., Li W., Kaifer A. E., Stockdale D., Bazan G. C., Adv. Mater. 2011, 23, 2367–2371. [DOI] [PubMed] [Google Scholar]
- 12.Frisch et al., Gaussian 09, Wallingford, 2009 (see SI for details).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supplementary


