Abstract
Background
“Traditional” spinal cord stimulation (SCS) trials with percutaneous electrodes externalized to a pulse generator (PG) are typically limited in duration due to risk of infection. Newer miniaturized wireless SCS technology eliminates the percutaneous extension (as well as PGs implanted for chronic use), thus facilitating a single‐stage implantation after which the device can remain indefinitely.
Objective
To evaluate fully implanted wireless SCS devices during a 30‐day screening trial in subjects with chronic low back pain and leg pain and a history of lumbosacral spine surgery.
Methods
In a randomized controlled trial of single‐stage wireless SCS using a wireless percutaneous system, 99 subjects received either 10 kHz high frequency stimulation (HFS) or lower frequency stimulation (LFS) below 1500 Hz (Bolash R, Creamer M, Rauck R, et al. Wireless high frequency spinal cord stimulation (10 kHz) compared to multi‐waveform low frequency spinal cord stimulation in the management of chronic pain in failed back surgery syndrome subjects: preliminary results of a multicenter, prospective, randomized controlled study. Pain Med 2019, https://doi.org/10.1093/pm/pnz019). In this report, we assess the 30‐day trial success rate (≥50% pain relief from baseline) and complications.
Results
The overall trial success rate was 88% (87/99): 92% (46/50) for HFS and 84% (41/49) for LFS (NS). The trial success rate in the 64 subjects with predominant low back pain was 92% (59/64) vs. 80% (28/35) in those with leg pain ≥ low back pain (NS). During the screening trial, one infection occurred (1%) and one subject withdrew and was explanted (1%). Electrode migrations were seen on routine follow‐up x‐rays in 10 cases (10%).
Conclusion
Using wireless SCS devices that allow for an extended trial period and evaluation of various waveforms, we observed a high rate trial success rate with both HFS and LFS waveforms, with minimal incidence of infection. Long‐term follow‐up will address the cost‐effectiveness and morbidity associated with this technology, which facilitates single‐stage treatment.
Keywords: Back and leg pain, failed back surgery syndrome, screening trial, spinal cord stimulation, wireless stimulation
INTRODUCTION
Spinal cord stimulation (SCS) occupies an important position among the growing number of modalities used to treat chronic pain and, in some cases, can reduce or eliminate the need for opioids. SCS is a reversible augmentative treatment that acts on the intact (though sometimes damaged) nervous system. Since the 1970s, SCS has been trialed using a temporarily percutaneous electrode connected to an external pulse generator (PG) to mimic long‐term treatment and identify subjects who are likely to have a successful response 1. The percutaneous external connector between the temporary electrode and the external PG limits the duration of such SCS screening trials due to potential contamination that would increase the risk of infection 2, 3, 4. In addition, temporary percutaneous electrodes have been reported to cause inflammatory and fibrous tissue formation, which can interfere with second stage placement of percutaneous or paddle electrodes for chronic use 5.
Newer wireless technology has eliminated the need for a percutaneous electrical connection, allowing the trial phase to be extended as long as necessary, testing as many waveforms and settings as needed to achieve and confirm success or failure of treatment 6. A successful implant may then remain in place permanently, eliminating the need for its removal and for implantation of new components (generator, extension cables, and new electrodes when required) that expose patients to additional discomfort and the risk of additional cost to payers 7, 8, 9.
Here we report clinical outcomes and complications associated with the wireless implanted system in the 30‐day trial phase of a randomized controlled trial (RCT) comparing 10 kHz high frequency stimulation (HFS) with lower frequency stimulation (LFS), of 1500 Hz or less.
METHODS
Patient Selection Criteria
Subjects all suffered from chronic back or back and leg pain (including predominant low back pain) following lumbosacral spine surgery, with an average back pain score of at least 5 on a 0 to 10 visual analog scale (VAS) recorded on a pain diary for 14 days after baseline assessment. As detailed in Appendix, the inclusion and exclusion criteria were those generally used in SCS studies in patients with persistent or recurrent pain following lumbosacral spine surgery.
Implant Procedure
Wireless SCS systems were implanted in a single stage: Under local anesthesia and minimal, if any, sedation, two 8‐contact permanent stimulators (Freedom‐8A SCS System, Stimwave®, Pompano Beach, FL) were placed percutaneously—One with the distal tip at the cephalad margin of T8, and the other with the distal tip at the cephalad margin of T9, as shown in Fig. 1. A receiver was advanced down the lumen of each stimulator and position was confirmed by fluoroscopy. A small, external, rechargeable transmitter was used for paresthesia mapping to guide positioning on or near the physiologic midline. Each device with mated receiver was then secured to the supraspinous ligament using nonabsorbable sutures and, at practitioner discretion, any of several standard commercially available anchors. No practitioner chose to use adhesive with any anchor 10. A tunneling tool was then used to pass each device with mated receiver distally to a separate, small incision, where it was knotted, looped, and secured in a subcutaneous pocket. The incisions were then closed. Fluoroscopy was used to confirm the location of marker bands on the receiver to provide guidance for external antenna placement.
Figure 1.
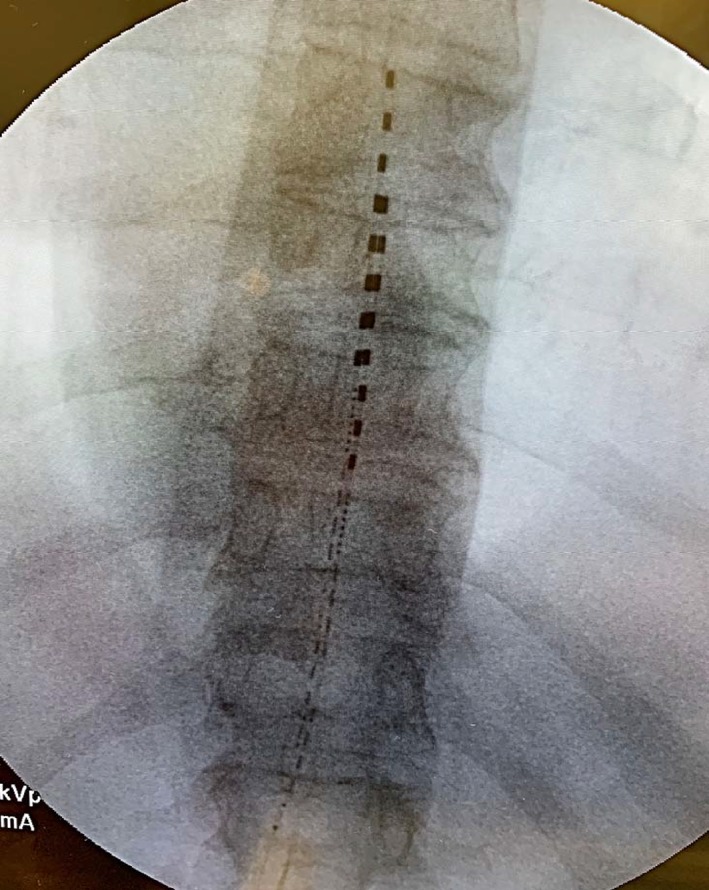
Anterior–posterior image showing placement of two 8‐contact dorsal epidural electrode arrays with tips at the superior endplates of T8 and T9 vertebral levels, per protocol for all subjects in the study. [Color figure can be viewed at http://wileyonlinelibrary.com]
Randomization and Programming
Subjects were randomized (1:1) to receive either HFS, 10 kHz at a pulse width of 30 μsec, or LFS, 50 to 1500 Hz with pulse widths between 30 and 1000 μsec. The devices were activated and programmed during the implant procedures, per randomized treatment assignments transmitted in numbered sealed opaque envelopes. Active electrode selections could be reprogrammed during the trial period in response to feedback from the subjects. HFS pulse parameters were fixed, and the active contact positions were varied during the trial based on reported pain relief. LFS parameters included 1) conventional “tonic” stimulation at 60 Hz with a pulse width of 300 μsec, 2) “high density” tonic stimulation at 1000 to 1500 Hz, and 3) burst stimulation consisting of between 4 and 10 pulses at a frequency of 500 to 1000 Hz, repeated 40 to 60 times per second. Patients assessed all three LFS waveforms on resulting pain relief.
Analysis
We defined trial success as at least 50% reduction in VAS score from baseline at 30 days; this was the primary outcome. We performed intention‐to‐treat analyses using Fisher's exact test statistics to evaluate the null hypothesis that the categorical variables were independent.
RESULTS
Table 1 presents demographics, baseline characteristics, and the trial responder rates. Of the 99 subjects randomized, 50 were male. The average age was 59.2 years, and the mean duration of pain was 10.5 years. All subjects had a history of lumbosacral spine surgery and most (64 of 99) reported predominant low back pain (based upon VAS ratings of low back and of leg pain at study entry).
Table 1.
Baseline Demographics, Characteristics, and 1‐Month Response Rates.
| HFS (N = 50) | LFS (N = 49) | Total (N = 99) | Success, N (%) | |
| Age mean years (SD) | 59.40 (12) | 59.00 (11) | 59.18 (12) | |
| Sex (male/female), N (%) | 23 (46)/27 (54) | 27 (55)/22 (45) | 50 (50.5)/49 (49.5) | |
| Height mean inches (SD) | 66.40 (4) | 66.60 (3) | 66.50 (4) | |
| Weight mean pounds (SD) | 202.00 (45) | 180.24 (44) | 185.50 (45) | |
| Pain duration mean years (SD) | 10.23 (9) | 10.88 (8) | 10.52 (9) | |
| Primary back pain, N (%) | 38 (60) | 34 (69) | 64 (65) | 59/64 (92) |
| Primary leg pain, N (%) | 18 (36) | 13 (27) | 31 (31) | 28/35 (80) |
| Equal back and leg pain, N (%) | 2 (4) | 2 (4) | 4 (4) | |
| Success, N (%) | 46/50 (92) | 41/49 (84) | 87/99 (88) |
SD = standard deviation; N = number.
Ninety‐eight of the 99 subjects completed the 30‐day trial (one subject in the LFS group withdrew after 1 week and was counted as a failure). The overall trial success rate was 88% (87/99), with an 84% success rate (41/49) for LFS and 92% success rate (46/50) for HFS (NS, p = 0.234, Fisher's exact test).
Of the 64 subjects with predominant low back pain, 92% (59/64) were successes vs. 80% (28/35) of those with leg pain exceeding or equaling low back pain. This difference was not statistically significant (Fisher's exact test, p = 0.107).
Table 2 presents the adverse events observed in the 99 subjects during the trial phase: one infection (1%), which was recorded as a “serious adverse event” (SAE) and led to removal of the device followed by reimplantation after treatment and 10 migrations. Most migrations occurred at the beginning of the trial period. Five LFS subjects experienced “unintended stimulation,” i.e., undesired paresthesia in an extraneous location (e.g., in the flank) or at a higher intensity than desired.
Table 2.
Treatment‐Related Adverse Events.
| HFS (N = 50), N (%) | LFS (N = 49), N (%) | Whole group (N = 99), N (%) | ||
| SAEs total | Subjects | 0 | 1 (2) | 1 (1) |
| AEs total | Subjects | 6 (12) | 19 (39) | 25 (25) |
| Type of AE | Migration | 3 (6) | 7 (14) | 10 (10) |
| Unintended stimulation | 0 | 5 (10) | 5 (5) | |
| Incisional pain | 0 | 3 (6) | 3 (3) | |
| Loss of stimulation | 0 | 2 (4) | 2 (2) | |
| Infection SAE | 0 | 1 (2) | 1 (1) | |
| Other | 3 (6) | 2 (4) | 5 (10) |
SAE = serious adverse event; AE = adverse event; HFS = high frequency stimulation; LFS = lower frequency stimulation; N = number.
DISCUSSION
We have no RCT evidence 1) to what extent SCS screening trials predict long‐term outcome; 2) if they do, what trial duration is optimal; and 3) whether their added cost (by comparison with single stage implantation) is justified. Percutaneous temporary electrode placement was introduced in the 1970's to accommodate an SCS screening trial 1, 11. This method was soon adopted for electrode implantation for chronic use, avoiding the need for a laminectomy for the second stage of treatment 12. Demonstration of pain relief with a temporary implant before implantation of a more costly and potentially more risky permanent system was required by Medicare in 1979 as a prerequisite for reimbursement. Some payers (e.g., the Belgian health care system, which requires a 30‐day trial) require longer screening with a temporary electrode connected to an external PG as a condition for reimbursement 13, 14.
Although SCS typically has been a two‐stage procedure, some clinicians have met the above requirements with “on table trials,” proceeding to PG implantation in a single stage and have reported overall results comparable to those achieved with more prolonged trials 15, 16. Indeed, the most‐cited, largest, and longest‐term studies have reported overall long‐term success rates on the order of 50% of those receiving SCS implants for chronic use after successful two‐stage screening trials 3, 17, indicating that trials have limited sensitivity. Moreover, Oakley reported in 2008 that permanent implants had worthwhile long‐term results in a small series of subjects whose trials did not reach the threshold for “success” 2, indicating that trial failure does not preclude long term SCS success. Thus, trials have limited specificity as well.
The percutaneous exit of a lead or catheter limits screening trial duration, as the external segment is by definition contaminated. If left in place for a “sufficient” amount of time, which cannot be predicted, it is reasonable to assume that the attached implant will eventually become contaminated and cause infection 14. Thus, even if the screening trial with externalized percutaneous components is abbreviated to minimize the risk of infection, this risk remains. Furthermore, if an infection does not become apparent until after a costly PG has been implanted, the entire implanted system generally requires removal, interrupting treatment until the infection has been successfully treated and a new system can be implanted. Unfortunately, some subjects never regain successful pain relief after removal of a system due to a complication.
Contemporary practice, thus, limits screening trial duration to the time required to achieve success, commonly defined as at least 50% relief of pain compared with baseline. If SCS screening trials were routinely extended to the 30 days used in this study, we would expect that some responses would reveal themselves as false positives and others as false negatives. Thus, we believe that short SCS screening trials increase the likelihood that an implant intended for chronic use will ultimately fail to provide pain relief and require removal or, conversely, will increase the possibility of a false negative result, leading to failure to treat a subject who would have been a treatment success. Both scenarios increase risks and generate additional health care costs.
If SCS were a drug, the typical screening trial duration would be weeks, even months, to allow for dose titration, management of side effects, and assessment of response. Similarly, novel waveforms developed in recent years, some of which have demonstrated superior results, should be included in SCS screening trial protocols. Because no single waveform has been shown to be uniformly effective in all patients, and a waveform that is inferior overall in a study population might nevertheless be the most effective choice for select patients, patients considering SCS should ideally be exposed to the multitude of programming parameters. A screening trial abbreviated in an attempt to reduce the risk of infection does not offer this possibility. Indeed, in clinical practice, the duration of an ideal screening trial should be as long as necessary to try as many waveforms as an individual subject requires. Obviously, this would be highly variable, and only wireless technology makes a sufficient trial duration feasible.
Limitations
Although the present study showed relatively high trial success rates for the wireless SCS system during 30‐day trials by comparison with prior SCS literature, we had no control group using alternative technology or shorter trials, and thus can draw no definitive conclusions. We hypothesize but have not proved that the results of longer “trials” will approach and thus better predict long‐term results. In fact, single‐stage wireless implants blur the distinction between the “trial” period and therapy with the “permanent” system, as the same implant is used for both.
This study protocol allowed the participants in the LFS arm to switch between various LFS waveforms, but the participants could not switch between LFS and HFS waveforms during the 30‐day period. High success rates were observed in both treatment arms, but crossover might have yielded additional successes during the trial period. In 2015, Smith et al. 18 reported that subjects who failed to obtain successful pain relief with LFS achieved success with HFS; conversely, in a crossover trial comparing LFS and higher frequency burst waveforms, a subset of subjects preferred LFS, even though the new waveform was found to be superior overall in that population 19. Thus, bidirectional crossover could have improved our screening trial yield.
The primary outcome of the RCT upon which this report is based 20 was a comparison of benefits and risks of different waveforms, not of different trial strategies. An RCT to address different trial strategies would require randomizing subjects to different methods of trialing. Such a study is presently underway 21. Given our observations in the present study, an RCT comparing screening trials using wireless implants with trials using externalized components might be deemed unnecessary and, thus, no longer be permitted by an institutional review board or ethics committee.
This protocol was limited to subjects with pain and a history of lumbosacral spine surgery; therefore, the level of electrode placement was limited to the thoracic spine. To the extent that high successes have been reported for SCS trials for other pain conditions, including those that require electrode placement at the cervical spine level, the present discussion might be relevant, but direct evidence is lacking.
The rate of migration observed in this study was comparable to that reported in many SCS series, but lower rates are achievable. A new anchoring system specifically for this wireless SCS system was introduced shortly after the conclusion of study enrollment and is expected to mitigate the problem.
CONCLUSIONS
Using wireless single‐stage SCS implants to allow for an extended screening trial period with various waveforms, we observed a high rate of trial success with LFS as well as HFS. We also observed a low incidence of infection (as expected because wireless implants eliminate percutaneous trial leads and extensions). Longer‐term follow‐up will allow assessment of the rate at which the subjects in this study have achieved lasting success while avoiding the morbidity and expense of a second device and a second procedure, thereby improving cost‐effectiveness.
Authorship Statement
Drs. Bolash, Creamer, Rauck, Vahedifar, Calodney, Fox, and Özaktay treated patients and collected the data at the study sites. Dr. Panchal is chairman of the DSMB for this study. Drs. North and Slavin and Mr. Vanquathem prepared the manuscript. All authors reviewed the manuscript critically and approved the final version.
COMMENTS
This is a spin off manuscript concerning the outcomes of trial stimulation using various modes of spinal cord stimulation using a new wireless SCS system. The authors report a high success rate, 88%, greater than 50% pain reduction at 30 days when comparing to baseline pain ratings. The long‐term outcome cohort will be published but unfortunately elsewhere. More interesting is how “the trial period” metamorphoses into a long‐term therapy. The manuscript includes a discussion concerning whether a short‐term trial period can predict long term outcome. This reviewer believes that clinical patient selection and education about chronic pain management is key to achieve good long‐term outcomes, not whether a trial period is effective or not.
To be honest, in healthcare systems like USA, you may be stuck with slavishly doing trials and implants because your reimbursement system rewards this methodology rather than careful multidisciplinary assessment teams. This is why a technological solution such as wireless SCS may help to break the impasse in the USA. Thankfully in my healthcare system we are able to develop the processes of SCS delivery more freely than rely on such technological change.
Simon Thomson, MBBS
Basildon, United Kingdom
***
The study reports a novel and interesting approach to trialing SCS made possible by the introduction of new technology. While it is tempting for the reader to theorize that a longer duration of SCS screening trial would increase the chances of long term SCS success, this is far from established fact.
It is equally possible that other factors related to CNS adaptation, long term failure of SCS targeting, or simply psychosocial factors constitute the major drivers of long term SCS failure and as such a longer duration trial may have little impact on the long term outcomes. As a matter of fact, the Belgian law requirement of a 4‐week trial has not been demonstrably associated with a lower rate of long term failures (1).
Sam Eldabe, MB ChB
Middlesbrough, United Kingdom
Reference
1. Van Buyten, J. P., et al. (2017). “Therapy‐Related Explants After Spinal Cord Stimulation: Results of an International Retrospective Chart Review Study.” Neuromodulation 20(7): 642–649.
***
This randomized multicenter study of 99 patients compares the effect of 10 kHz high frequency (HF) stimulation with the effect of lower frequency stimulation (LF, below 1.500 Hz) during a 30‐day screening period. The stimulation was accomplished with a fully implantable wireless SCS device in a single stage procedure with on‐table paresthesia testing.
The authors report a high trial success rate with both HF and LF waveforms (92% vs 85%, NS). In this study 9 out of 10 patients achieve at least 50% pain relief at 30 days. Interestingly, the group with predominant low back pain performed slightly better than the group with leg > low back pain (92% vs 80%, NS).
This study raises the question of what exactly is defined by the SCS trial. Is it the on‐table trial with paresthesia testing or the 30 days screening period? Additionally, how long does a SCS trial have to last?
And what factors contribute to the very good success rate? Is it the expertise of the physicians in selecting the right patients? Is it the good operation technique? Is it the exact electrode placement dependent on the paresthesia mapping in the awake Patient? Is it the screening period of 30 days? Or, is it the wireless stimulation device by itself? At least the category of wave form (HF vs LF) does not play a decisive role, since both groups have almost the same success rate. However, it would be of interest to analyze the utilized frequency and wave form in the group of patients treated with lower frequency stimulation (LF).
In arguing that the functionality of stimulation devices is based on the same technical principles, the achieved pain relief of this wireless device study can be transferred to fully implantable systems with primary cell or rechargeable pulse generators. In comparison to IPG devices, the infection rate of 1% is lower, while the rate of lead migration (10%) is almost the same.
As the authors noted, the second phase of the randomized controlled trial will provide results on the long‐term outcome.
Therefore, I am looking forward to seeing the results of the second study.
Dr. med. Matthias Winkelmüller
Hannover, Germany
Comments not included in the Early View version of this paper.
APPENDIX 1.
PATIENT SELECTION CRITERIA
≥18 years of age at informed consent.
History of lumbosacral spine surgery.
Chronic back or back and leg pain with an average back pain score >5 on a 0 to 10 scale recorded on a pain diary for 14 days after baseline assessment.
Pain refractory to conventional medical management with stable medication for at least 12 months prior to enrollment.
Able to comply with the study requirements, including completion of a pain diary and operating the programmer and recharging equipment.
Neuropsychosocially appropriate candidate as assessed by a clinical psychologist.
No postherpetic neuralgia or chronic condition requiring regular opioids.
Good surgical candidate with no health condition that contradicts SCS (e.g., anatomic abnormality that could jeopardize device placement, mechanical instability revealed on imaging within the past 6 months, a need for MRI or diathermy, uncontrolled diabetes, abnormal bleeding or coagulopathy, unresolved malignancy in the past 6 months, active systemic infection, immunocompromised, substance abuse).
No legal concern that could confound study results.
Not enrolled in another study.
No implanted device or previous SCS experience.
For women, not pregnant, using adequate birth control, or past child‐bearing age.
Life expectancy >1 year.
For more information on author guidelines, an explanation of our peer review process, and conflict of interest informed consent policies, please go to https://www.wiley.com/WileyCDA/Section/id-301854.html
Source(s) of financial support: All study sites received funding from Stimwave (Stimwave LLC, 1310 Park Central Blvd S, Pompano Beach, FL, USA).
Conflict of Interest: Mr. Vanquathem is an employee of Stimwave. Drs. Calodney and Panchal are consultants for Stimwave, and Dr. Panchal is a Stimwave shareholder. Dr. North's former employers (Johns Hopkins and Sinai Hospital) received research support from Boston Scientific, Medtronic, and St. Jude. Dr. North serves as an unpaid officer of the nonprofit Neuromodulation Foundation, Inc. to which (like his former employers Johns Hopkins University and Sinai Hospital) grants and support have been provided by Abbott, Boston Scientific Corp., Medtronic, Inc., Nevro Corp., Nuvectra, and Stimwave, Inc. He receives royalties from Abbott and consulting fees and royalties from Nuvectra. His wife holds shares in Stimwave, Inc. Dr. Bolash is a consultant for Medtronic, Nuvectra, Jazz Pharmaceuticals, and Pfizer. His institution receives research funding from Stimwave, Abbott, Nuvectra, Pfizer, and Mesoblast. Dr. Slavin serves as an advisor/consultant for Abbott (formerly St. Jude Medical), Biotronik, Boston Scientific, Insightec, Medtronic, Neuramodix, Nevro, Nuvectra, and Stimwave. He also conducts research for Abbott (formerly St. Jude Medical), Autonomic Technologies, Boston Scientific, Medtronic, and Neuros and has received honoraria or royalty from Karger and Wiley. The remaining authors have no conflicts of interest to report.
REFERENCES
- 1. Erickson DL. Percutaneous trial of stimulation for patient selection for implantable stimulating devices. J Neurosurg 1975;43:440–444. [DOI] [PubMed] [Google Scholar]
- 2. Oakley JC, Krames ES, Stamatos J, Foster AM. Successful long‐term outcomes of spinal cord stimulation despite limited pain relief during temporary trialing. Neuromodulation 2008;11:66–73. [DOI] [PubMed] [Google Scholar]
- 3. Kumar K, Hunter G, Demeria D. Spinal cord stimulation in treatment of chronic benign pain: challenges in treatment planning and present status, a 22‐year experience. Neurosurgery 2006;58:481–496. [DOI] [PubMed] [Google Scholar]
- 4. North RB. SCS trial duration. Neuromodulation 2003;6:4–5. [DOI] [PubMed] [Google Scholar]
- 5. Kin K, Agari T, Yasuhara T et al. The factors affecting the difficulty of percutaneous cylindrical electrode placement for spinal cord stimulation. World Neurosurg 2018;113:e391–e398. [DOI] [PubMed] [Google Scholar]
- 6. Perryman LT, Speck B, Garcia CM, Rashbaum R. Injectable spinal cord stimulator system: pilot study. Tech Reg Anesth Pain Manag 2012;16:102–105. [Google Scholar]
- 7. Pope JE, Deer TR, Falowski S et al. Multicenter retrospective study of neurostimulation with exit of therapy by explant. Neuromodulation 2017;20:543–552. [DOI] [PubMed] [Google Scholar]
- 8. Dietvorst S, Decramer T, Lemmens R, Morlion B, Nuttin B, Theys T. Pocket pain and neuromodulation: negligible or neglected? Neuromodulation 2017;20:600–605. [DOI] [PubMed] [Google Scholar]
- 9. Deer TR, Stewart CD. Complications of spinal cord stimulation: identification, treatment, and prevention. Pain Med 2008;9:S93–S101. [Google Scholar]
- 10. North RB, Recinos VR, Attenello FJ, Shipley J, Long DM. Prevention of percutaneous spinal cord stimulation electrode migration: a 15‐year experience. Neuromodulation 2014;17:670–677. [DOI] [PubMed] [Google Scholar]
- 11. Hosobuchi Y, Adams JE, Weinstein PR. Preliminary percutaneous dorsal column stimulation prior to permanent implantation. Technical note. J Neurosurg 1972;37:242–245. [DOI] [PubMed] [Google Scholar]
- 12. North RB, Fischell TA, Long DM. Chronic dorsal column stimulation via percutaneously inserted epidural electrodes. Preliminary results in 31 patients. Appl Neurophysiol 1977. ‐1978;40:184–191. [DOI] [PubMed] [Google Scholar]
- 13. Camberlin C, San Miguel L, Smit Y, Post P, Gerkens S, De Laet C. Neuromodulation for the management of chronic pain: implanted spinal cord stimulators and intrathecal analgesic delivery pumps Health Technology Assessment (HTA). KCE Report 189C. D/2012/10.273.76. Brussels: Belgian Health Care Knowledge Centre (KCE), 2012.
- 14. Logé D, De Coster O, Washburn S. Technological innovation in spinal cord stimulation: use of a newly developed delivery device for introduction of spinal cord stimulation leads. Neuromodulation 2012;15:392–401. [DOI] [PubMed] [Google Scholar]
- 15. Feler CA, Kaufman S. Spinal cord stimulation: one stage? Acta Neurochir 1992;117:91. [Google Scholar]
- 16. Weinand ME, Madhusudan H, Davis B, Melgar M. Acute vs. prolonged screening for spinal cord stimulation in chronic pain. Neuromodulation 2003;6:15–19. [DOI] [PubMed] [Google Scholar]
- 17. North RB, Kidd DH, Zahurak M, James CS, Long DM. Spinal cord stimulation for chronic, intractable pain: experience over two decades. Neurosurgery 1993;32:384–394. [DOI] [PubMed] [Google Scholar]
- 18. Smith H, Youn Y, Pilitsis JG. Successful use of high‐frequency spinal cord stimulation following traditional treatment failure. Stereotact Funct Neurosurg 2015;93:190–193. [DOI] [PubMed] [Google Scholar]
- 19. Deer T, Slavin KV, Amirdelfan K et al. Success using neuromodulation with burst (SUNBURST) study: results from a prospective randomized controlled trial using a novel burst waveform. Neuromodulation 2018;21:56–66. [DOI] [PubMed] [Google Scholar]
- 20. Bolash R, Creamer M, Rauck R et al. Wireless high frequency spinal cord stimulation (10 kHz) compared to multi‐waveform low frequency spinal cord stimulation in the management of chronic pain in failed back surgery syndrome subjects: preliminary results of a multicenter, prospective, randomized controlled study. Pain Med 2019; e‐pub ahead of print. 10.1093/pm/pnz019. [DOI] [PubMed] [Google Scholar]
- 21. Eldabe S, Gulve A, Thomson S et al. Does a screening trial for spinal cord stimulation in patients with chronic pain of neuropathic origin have clinical utility and cost‐effectiveness? (TRIAL‐STIM Study): study protocol for a randomised controlled trial. Trials 2018;19:633. [DOI] [PMC free article] [PubMed] [Google Scholar]


