Abstract
Background
Experimental and clinical studies have shown that tonic spinal cord stimulation (SCS) releases gamma‐aminobutyric acid (GABA) in the spinal dorsal horn. Recently, it was suggested that burst SCS does not act via spinal GABAergic mechanisms. Therefore, we studied spinal GABA release during burst and tonic SCS, both anatomically and pharmacologically, in a well‐established chronic neuropathic pain model.
Methods
Animals underwent partial sciatic nerve ligation (PSNL). Quantitative immunohistochemical (IHC) analysis of intracellular GABA levels in the lumbar L4 to L6 dorsal spinal cord was performed after 60 minutes of burst, tonic, or sham SCS in rats that had undergone PSNL (n = 16). In a second pharmacological experiment, the effects of intrathecal administration of the GABAA antagonist bicuculline (5 μg) and the GABAB antagonist phaclofen (5 μg) were assessed. Paw withdrawal thresholds to von Frey filaments of rats that had undergone PSNL (n = 20) were tested during 60 minutes of burst and tonic SCS 30 minutes after intrathecal administration of the drugs.
Results
Quantitative IHC analysis of GABA immunoreactivity in spinal dorsal horn sections of animals that had received burst SCS (n = 5) showed significantly lower intracellular GABA levels when compared to sham SCS sections (n = 4; P = 0.0201) and tonic SCS sections (n = 7; P = 0.0077). Intrathecal application of the GABAA antagonist bicuculline (5 μg; n = 10) or the GABAB antagonist phaclofen (5 μg; n = 10) resulted in ablation of the analgesic effect for both burst SCS and tonic SCS.
Conclusions
In conclusion, our anatomical and pharmacological data demonstrate that, in this well‐established chronic neuropathic animal model, the analgesic effects of both burst SCS and tonic SCS are mediated via spinal GABAergic mechanisms.
Keywords: gamma‐aminobutyric acid, burst spinal cord stimulation, GABAA/B receptor antagonist, peripheral nerve injury, mechanical hypersensitivity
Introduction
Tonic spinal cord stimulation (SCS) is a last‐resort treatment method for patients that suffer from intractable chronic neuropathic pain.1, 2, 3, 4, 5, 6 The standard tonic SCS protocol consists of continuous, tonic, electrical stimulation applied to the dorsal columns of the spinal cord with a frequency within the range of 40 to 80 Hz and a pulse width between 200 and 500 μS.2, 7, 8 The concept of SCS emerged as a direct application of the Gate Control Theory of Melzack and Wall in 1965.7, 9 It was postulated that antidromic stimulation of the non‐nociceptive Aβ fibers could close the “spinal gate,” located in the dorsal horn of the spinal cord. Closing of the “gate” is facilitated by inhibitory interneurons located in the superficial laminae of the dorsal horn and it is believed that the neurotransmitter gamma‐aminobutyric acid (GABA) plays a pivotal role in this process.8, 10, 11 During the development of neuropathic pain, Janssen and colleagues observed, in a partial sciatic nerve ligation (PSNL) model, increased intracellular levels of GABA in the dorsal horn.12 Later, it was demonstrated that tonic SCS decreased intracellular GABA immunoreactivity (IR) in the dorsal horn of these rats that had undergone PSNL.13 At the same time, extensive experimental microdialysis‐work has demonstrated that tonic SCS increases extracellular GABA levels in the dorsal horn of allodynic rats that had undergone PSNL.14, 15, 16 Thus, GABAergic interplay seems to be an important aspect of the analgesic mechanisms underlying tonic SCS in the PSNL model of chronic neuropathic pain. The role of GABA in segmental SCS mechanisms was further elucidated by the administration of pharmacological agents that specifically modulate GABA release in the dorsal horn during tonic SCS in neuropathic rats that had undergone PSNL. Local perfusion with a GABAB receptor antagonist in the dorsal horn transiently abolished the SCS‐induced effect in neuropathic rats,16 and rats not receiving adequate pain relief with tonic SCS (nonresponders) were turned into responders by administration of the GABAB receptor agonist baclofen.17 The aforementioned preclinical findings were successfully translated to the clinic, where patients with neuropathic pain who had experienced a deficient tonic SCS effect had improved pain relief following the intrathecal administration of baclofen,18 further confirming the theory that local spinal GABAergic mechanisms are pivotal for the effects of tonic SCS. While GABA plays a role in the underlying mechanism of tonic SCS, the neurotransmitters involved in other stimulation paradigms, such as burst SCS, have not been clearly identified. Because patients show different responses to different stimulation paradigms, the underlying mechanisms may be different.19 The burst waveform consists of closely spaced pulses delivered in a packet or burst, directly followed by a quiescent period or interburst interval.20 Burst SCS has proven to be effective in patients with failed back surgery syndrome and radiculopathy, and clinical trials have demonstrated its ability to help patients to reduce their analgesic intake.21, 22, 23 In addition, burst SCS reduces neuropathic pain without generating paresthesia in the affected limb or area.24, 25, 26 Yet, from a mechanistic point of view, the burst waveform is still in its infancy. Results of source‐localized electroencephalographic studies and patient questionnaires suggest that burst SCS preferentially activates the medial pain pathway, and hence modulates emotional‐affective pain aspects.25, 27 On a segmental level, an experimental electrophysiological study has aimed to elucidate the involvement of GABA in the spinal mechanism underlying burst SCS.28 It was found that the presence of a GABAB receptor antagonist blocked attenuation of dorsal horn neuronal firing during tonic SCS but not burst SCS. Furthermore, blood serum GABA measurements showed that systemic GABA levels were not increased following burst SCS. From this, the researchers concluded that burst SCS might not act via spinal GABAergic mechanisms.29 However, it should be mentioned that the aforementioned study was performed not only in an uncommon rat model for chronic neuropathic pain, the painful cervical nerve root compression model, but these experiments were also terminal; thus, no behavioral testing during the conscious state of the animals was performed.29 This makes interpretation of these data in the context of understanding the role of spinal GABA in a chronic neuropathic pain model difficult, as most experimental data on pain relief and the spinal GABAergic mechanism underlying tonic SCS have been documented and studied in the well‐described and validated PSNL model or a similar nerve injury model for peripheral mononeuropathy.13, 14, 15, 16, 17, 30 Therefore, in order to further understand the spinal mechanism underlying burst SCS we aimed to study, both anatomically and pharmacologically, the role of GABA in behavior and pain‐relieving mechanisms underlying burst and tonic SCS, in a well‐established chronic neuropathic PSNL model.
Methods
Ethics Statement
The experiments were performed in accordance with the European Directive for the Protection of Vertebrate Animals Used for Experimental and Other Scientific Purposes (86/609/EU). The protocol was approved by the Animal Research Committee of the Maastricht University Medical Centre (Project License number 2017‐022).
Animals
All experiments were performed using male Sprague Dawley rats (n = 36), which were young adults (5 weeks of age) at the start of the experiment (150 to 200 g). Sixteen animals were used in the first experiment, in which we assessed the quantitative immunohistochemical (IHC) analysis of GABA IR in spinal dorsal horn sections of animals after SCS (n = 16). Twenty animals were used in the second experiment, in which we intrathecally administered GABAA/B antagonists before SCS in order to assess the effects of these pharmacological agents on the behavioral pain‐relieving effects of SCS (n = 20). Animals were housed in groups of 2, in polycarbonate cages in a climate‐controlled vivarium maintained under controlled temperature (21 ± 1°C), relative humidity (55 ± 15%), and artificial lighting (12:12 reversed light/dark cycle) with distilled water and rodent food available ad libitum. The vivarium was equipped with a central radio system, continuously producing background music at 45 decibels, in order to desensitize the animals for experimenter‐related noise. All procedures were conducted between 09:00 and 16:00 hours. Experiments were conducted during the dark, active phase of the rodent circadian rhythm.
Partial Sciatic Nerve Ligation
A unilateral ligation of the left sciatic nerve was performed as described by Seltzer et al.,30 and previously applied in our laboratory.29, 31, 32, 33 In short, animals were anesthetized with 3% to 5% isoflurane (Abbott Laboratories Ltd., Kent, U.K.) and air enriched with 100% oxygen at a constant flow rate of 250 mL/min. By use of an automatic heating pad, body temperature was maintained at 37.5°C. The nervus ischiadicus from the left hind paw was exposed by blunt dissection and carefully freed from surrounding connective tissue. Subsequently, the nerve was partially ligated (by approximately one third) using 8/0 non‐absorbable silk sutures. The wound was then closed with 4/0 silk sutures. Development of mechanical hypersensitivity (mechanical allodynia) was monitored with the use of von Frey assays for 14 consecutive days. At day 14, the presence of mechanical hypersensitivity was confirmed if the log (50% paw withdrawal threshold [PWT]) was decreased by 0.2 units compared to baseline (day 0).34
Assessment of Mechanical Hypersensitivity (von Frey Assay)
Mechanical hypersensitivity was assessed by use of von Frey filaments. Von Frey assessment was always conducted in the same room, isolated from external sounds and equipped with artificial red light sources (temperature: 21 ± 1°C; relative humidity: 55 ± 15%). Prior to testing, animals were placed in the behavioral set‐up for 15 minutes in order to acclimate to the new surroundings. The assessment room was equipped with a mobile radio, continuously producing background music at 45 decibels. PWTs to von Frey filaments were assessed using the up‐down method.35 Von Frey filaments of linearly incrementing stiffness (bending forces 0.6, 1.2, 2.0, 3.6, 5.5, 111 8.5, 15.1 and 28.84 g) were applied to the plantar surface of the hind paw of the rats for 5 consecutive seconds. A negative response (the hind paw was not withdrawn) was followed by the subsequent filament with greater bending force. In contrast, a positive response (the hind paw was withdrawn) was followed by application of the previous filament with a lower bending force. After completion of a sequence of 6 consecutive responses, the 50% PWT was calculated.35 The predetermined cut‐off value was set at 28.84 g. For statistical analysis, the 50% PWTs were logarithmically transformed to yield a linear scale.
Tissue Preparation
For immunohistochemistry experiments, 16 animals in experiment 1 were divided into 3 groups: sham SCS (n = 4), tonic SCS (n = 7), and burst SCS (n = 5). Animals were killed 60 minutes after the start of (sham) SCS. Tissue perfusion was performed transcardially with a mixture of 4% paraformaldehyde and 15% picric acid in 0.2 m phosphate‐buffered saline (PBS; pH 7.6) after anesthesia with pentobarbital (100 mg/kg body weight). Then, lumbar spinal cord regions L4 to L6 were removed by a laminectomy, post‐fixated overnight at 4°C, and cryoprotected for 24 hours in 10% sucrose. This was followed by at least 72 hours’ incubation in 25% sucrose (in 0.1 m PBS; pH 7.6) at 4°C. Subsequently, tissues were frozen in solid carbon dioxide. Thirty‐micrometer thick transverse cryosections were mounted on gelatine‐coated glass slides and stored at −20°C until staining procedures were performed.
Immunohistochemical Detection of GABA
The immunohistochemistry protocol was performed as described by Janssen et al.13 In short, staining procedures were performed at room temperature unless stated otherwise. First, glass slides were air dried for 2 hours and subsequently washed in Tris‐buffered saline (TBS, 0.1 m, pH 7.6) including 0.3% Triton X‐100 (TBS‐T). Blocking was performed with 2% normal donkey serum (Sigma‐Aldrich, Zwijndrecht, The Netherlands). The serum was diluted in TBS‐T and applied for a 1‐hour incubation period. Then, the sections were incubated with a polyclonal rabbit anti‐GABA antibody (1:5,000 diluted in TBS‐T; Sigma‐Aldrich, A2052) for a time period of 48 hours. After 48 hours, the excess primary antibody was removed by use of TBS, after which sections were incubated with the secondary alexa fluor 488 donkey anti‐rabbit IgG antibody (1:100 diluted in TBS‐T; Invitrogen, Breda, The Netherlands, A21206) for 2 hours. Subsequently, sections were rinsed with TBS and coverslipped with TBS/glycerol (20%/80%).
Quantification of Immunostaining
Quantitative IHC analysis of spinal dorsal horn GABA staining was performed as described by Janssen et al.12, 13 Photomicrographs were taken of both ipsilateral and contralateral lumbar L4 to L6 spinal cord immunostained sections using a Provis AX70 fluorescent microscope (Olympus, Hamburg, Germany). The microscope was connected to a digital black and white video camera (U‐CMAD‐2; Olympus), equipped with CellP software. Rexed laminae 1 to 3 of the dorsal horn were determined as regions of interest for the GABA IR analysis.36 Grayscale values were calculated for these laminae. Analysis of gray scale spinal cord pictures was performed by a blinded observer using the AnalySIS software program CellP (Soft Imaging Systems, Münster, Germany).
Implantation of Spinal Cord Stimulation Device
Implantation of the SCS device was performed according to the standard protocol used in our institution.29, 32, 33, 37, 38 In short, a small laminectomy was made at T13, after which the spinal cord was exposed by use of a surgical rotary tool. A custom‐made cylindrical 4‐contact lead (0.72 mm diameter; Boston Scientific Neuromodulation, Valencia, CA, U.S.A.) was inserted into the epidural space in the caudal direction. Electrode configuration was set at alternating cathode and anode settings (rostral to caudal: + ‐ + ‐). Then, the electrode was secured to a spinous process with tissue adhesive (Histoacryl; B Braun Medical BV, Oss, The Netherlands) to prevent electrode migration. The electrode wires were tunneled subcutaneously to the neck of the animal, and the stimulator connectors were attached with 4/0 silk sutures. Animals were given 2 days for recovery prior to the initiation of SCS experiments.
Intrathecal Implantation
After successful implantation of the SCS device, 20 animals in experiment 2 underwent a small laminectomy at T12, after which the spinal cord was exposed by use of a surgical rotary tool. The membrane was carefully opened, after which a polyethylene catheter from Instech Laboratories (Plymouth Meeting, PA, U.S.A.; 27 gauge, length 1.5 cm) was inserted and tunneled intrathecally to the level of the L3 to L5 spinal segments as described by Truin et al.31 The catheter was secured to a spinous process with tissue adhesive (Histoacryl; B Braun Medical BV) to prevent migration. Subsequently, the catheter was slowly flushed with 10 μL of saline and the wound was closed with 4/0 silk sutures. Animals with signs of paralysis directly after surgery were excluded. Correct placement of the catheter was confirmed when lidocaine 2% injection (10 mg/mL) resulted in paralysis or dragging of the hind limbs.31
Spinal Cord Stimulation
For stimulation of the dorsal columns, an A‐M Systems stimulator (MultiStim: Programmable 8‐Channel Stimulator, model 3800, 220 V/50 Hz) fitted with a stimulus isolator (model 3820 for A‐M Systems MultiStim) was used. The stimulator was set to deliver constant current biphasic stimulation for both the tonic and burst SCS modes. The tonic SCS motor threshold (MT) was determined at the following settings: pulse width of 200 μS administered at a frequency of 2 Hz. Burst SCS MT was determined at the following settings: pulse width of 1,000 μS, 5 pulses (449 Hz intraburst frequency) administered at an interburst frequency of 2 Hz. The amplitude was gradually increased until symmetrical contractions of the hind limbs were perceived by hand and/or visually observed. Then, either a tonic SCS paradigm at 66% of MT (frequency 50 Hz, pulse width 200 μS),20 or a biphasic burst SCS paradigm at 50% MT (interburst frequency 40 Hz, pulse width 1,000 μS, 5 spikes at 449 Hz intraburst frequency) was applied for 60 minutes.24, 33 The SCS parameters were based on previously determined optimal settings for pain relief with burst SCS (50% MT) and tonic SCS (66% MT).29, 33 In all experiments, the PWTs to von Frey filaments were assessed before the start of SCS treatment (baseline), at 15, 30, 45, and 60 minutes after stimulation was turned on. The investigator was blinded to the stimulation condition throughout the whole experiment.
Intrathecal Administration of GABAA/B Receptor Antagonists (Experiment 2)
The concentration of the GABAA/B receptor antagonists bicuculline (5 μg; ≥97%; Sigma‐Aldrich) and phaclofen (5 μg; ≥97%; Sigma‐Aldrich) was chosen based on literature demonstrating a dose‐response curve for both antagonists.39 Because the peak dorsal horn drug concentrations occurred 30 minutes after intrathecal administration,39 antagonists were applied to the spinal cord 30 minutes before SCS and von Frey testing protocols were initiated. Intrathecal administration of the GABAA/B receptor antagonists (5 μg) was followed by the administration of 20 μL saline.
Vehicle administration consisted of 10 μL saline followed by the administration of 20 μL saline.
Data Analysis
Paw withdrawal threshold's to von Frey filaments are presented as mean ± standard error of the mean (SEM). In line with previous Von Frey studies,32, 33, 40 von Frey data were logarithmically transformed to obtain a linear scale and to account for Weber's Law.41 For statistical analysis of differences in the withdrawal thresholds over time within the groups of experiments 1 and 2, the nonparametric Friedman test was used, followed by Dunn's post hoc test. For the analysis of differences in the withdrawal thresholds between groups (ipsilateral and contralateral withdrawal thresholds), the nonparametric Mann–Whitney U‐test was used. Before statistical analysis of GABA IR gray values in experiment 1, a Shapiro‐Wilk normality test was performed. Then, the Kruskal‐Wallis test was performed in order to assess statistical differences over group means. Dunn's Multiple Comparison Test followed this in order to assess significant differences between the group means. All statistical analyses were performed with α = 0.05 using IBM SPSS statistics version 23 (IBM Corp., Armonk, NY, U.S.A.).
Results
Development of Mechanical Hypersensitivity (von Frey) and SCS
In experiment 1, after PSNL surgery, ipsilateral hindpaw PWTs were significantly lower than contralateral hindpaw PWTs (P = 0.019); all 16 animals qualified as hypersensitive due to increased response to mechanical stimulation by von Frey filaments (contralateral average PWTs: 11.2 ± 0.6 g (day post lesion [DPL] 7) vs. 1.1 ± 0.5 g [DPL 7]; P = 0.0049) (see Methods) (Figure 1A). Burst SCS (n = 5) significantly increased PWTs compared to baseline PWTs at 15 (P = 0.046), 30 (P = 0.025), 45 (P = 0.016), and 60 minutes of SCS (P = 0.019) (Figure 2A). For tonic SCS (n = 7), PWTs significantly differed from baseline PWTs at 15 (P = 0.027), 30 (P = 0.026), 45 (P = 0.033), and 60 minutes of SCS (P = 0.031) (Figure 2A). Sham SCS (n = 4) did not increase PWTs at any time points (see Figure 2A).
Figure 1.
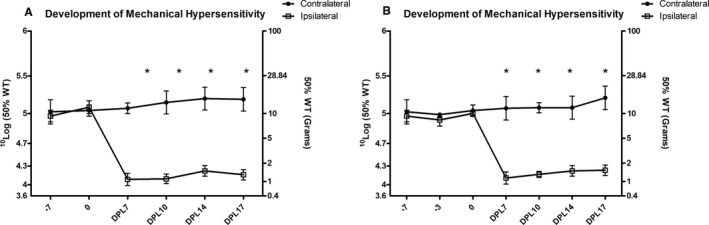
A, Development of mechanical hypersensitivity for experiment 1 (n = 16). B, Development of mechanical hypersensitivity for experiment 2 (n = 20). *P < 0.05 for ipsilateral vs. contralateral paw withdrawal thresholds. DPL, days post‐ligation; WT, withdrawal threshold.
Figure 2.
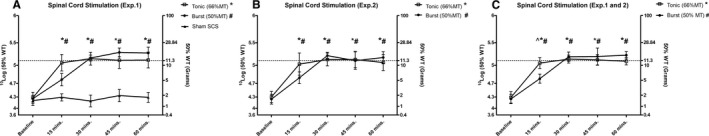
A, The effects of tonic spinal cord stimulation (SCS) (n = 7), burst SCS (n = 5), and sham SCS (n = 4) on pain withdrawal thresholds (PWTs) based on sensitivity to von Frey filaments in experiment 1. B, The effects of tonic SCS (n = 10) and burst SCS (n = 10) on PWTs in experiment 2. C, The effect of tonic SCS (n = 17) and burst SCS (n = 15) on PWT pooled data from experiments 1 and 2. The dotted line represents the average PWT baseline prior to sciatic nerve ligation (^ P < 0.05 for burst SCS vs. tonic SCS; # P < 0.05 for burst SCS vs. baseline; *P < 0.05 for tonic SCS vs. baseline). MT, motor threshold; WT, withdrawal threshold.
In experiment 2, after PSNL surgery, ipsilateral hindpaw PWTs were significantly lower than contralateral hindpaw PWTs (P = 0.0081) at DPL 7; all 20 animals qualified as hypersensitive (ipsilateral average PWTs: 10.9 ± 1.1 g [pre‐lesion] vs. 1.3 ± 0.7 g [post‐lesion]; P = 0.0064) (see Methods) (Figure 1B). For burst SCS (n = 10), PWTs significantly differed from baseline PWTs at 15 (P = 0.041), 30 (P = 0.027), 45 (P = 0.028), and 60 minutes of SCS (P = 0.022) (Figure 2B). For tonic SCS (n = 10), PWTs significantly differed from baseline PWTs at 15 (P = 0.029), 30 (P = 0.028), 45 (P = 0.031), and 60 minutes of SCS (P = 0.035) (see Figure 2B). No significant differences were reported between development of mechanical hypersensitivity in experiments 1 and 2. In addition, no significant differences were reported between SCS time points in experiments 1 and 2. When the SCS data of experiments 1 and 2 were pooled, burst SCS (n = 15) and tonic SCS (n = 17) significantly increased PWTs compared to baseline PWTs at 15 (P = 0.042 and P = 0.028, respectively), 30 (P = 0.027 and P = 0.026, respectlively), 45 (P = 0.021 and P = 0.032, respectively), and 60 minutes of stimulation (P = 0.025 and P = 0.033, respectively) (Figure 2C). Burst SCS PWTs and tonic SCS PWTs significantly differed at 15 minutes of SCS (P = 0.038) (see Figure 2C).
Spinal Dorsal Horn GABA Immunoreactivity
In experiment 1, anti‐GABA IHC revealed an intense IR predominantly in laminae 1 to 3 of the lumbar spinal dorsal horn with clear identification of GABA IR cell bodies (Figure 3B). Gray scale values were calculated for these laminae. The Shapiro‐Wilk normality test was not passed (P = 0.0395). Accordingly, a Kruskal‐Wallis test was performed, which showed a significant difference between mean gray values for the sham SCS, tonic SCS, and burst SCS groups (P = 0.0085, Kruskal‐Wallis statistic = 9.524). Statistical testing of GABA staining intensity in the spinal dorsal horn and gray values revealed that the tonic SCS group, although it showed a strong tendency, did not differ significantly from the sham SCS group (P = 0.1609). The burst SCS group differed significantly from the sham SCS group (P = 0.0201) and from the tonic SCS group (P = 0.0077).
Figure 3.
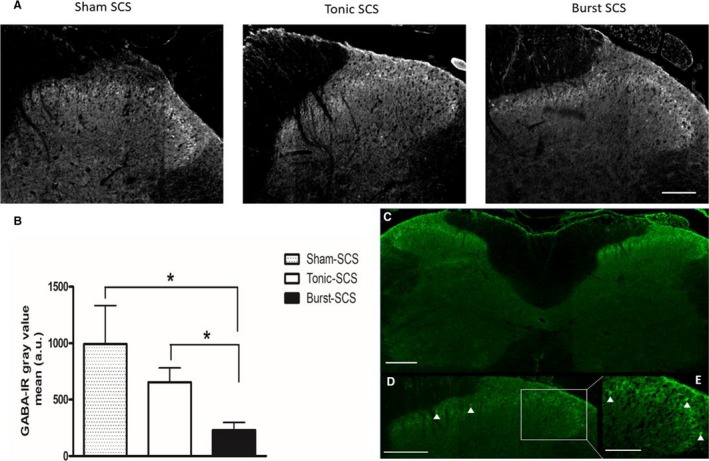
A, Spinal cord stimulation (SCS)‐induced alterations in intracellular GABA levels in the L4–L6 spinal dorsal horn. Representative gray scale photomicrographs of the upper laminae of the ipsilateral dorsal horn of a sham SCS animal, tonic SCS animal, and burst SCS animal. Scale bar = 100 μm. B, Quantified GABA‐IR in laminae 1 to 3 of the L4–L6 spinal dorsal horn of sham SCS animals (n = 4), tonic SCS animals (n = 7), and burst SCS animals (n = 5). *P < 0.05. C, Representative overview of the immunostaining of the left and right dorsal horn of the spinal cord. Scale bar = 100 μm. D, Arrowheads point out activated GABA‐IR neuronal profiles. Scale bar = 100 μm. E, Arrowheads point out activated GABA‐IR neuronal profiles. Scale bar = 50 μm. MT, motor threshold; WT, withdrawal threshold.
Intrathecal GABA Antagonist Administration
In experiment 2 (Figure 4), thresholds for mechanical hypersensitivity were not affected after 15 minutes of tonic SCS with intrathecal administration of the GABAA antagonist bicuculline, as PWTs were significantly increased as compared to baseline (P = 0.044) (Figure 5A). Meanwhile, at 30 minutes (P = 0.38), 45 minutes (P = 0.65), and 60 minutes (P = 0.32) of tonic SCS, thresholds for mechanical hypersensitivity were affected, since PWTs did not significantly differ from baseline PWTs. Administration of the GABAA antagonist bicuculline affected thresholds for mechanical hypersensitivity at all time points during burst SCS. PWTs did not significantly differ from baseline PWTs at 15 minutes (P = 0.74), 30 minutes (P = 0.49), 45 minutes (P = 0.52), and 60 minutes of burst SCS (P = 0.67) (see Figure 5A).
Figure 4.
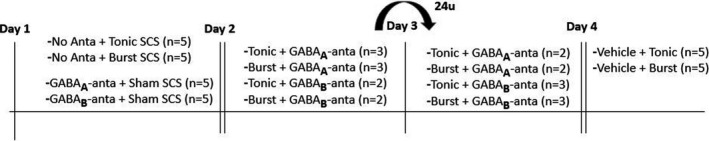
Timeline of experiment 2: pharmacological intrathecal experiments. SCS, spinal cord stimulation.
Figure 5.
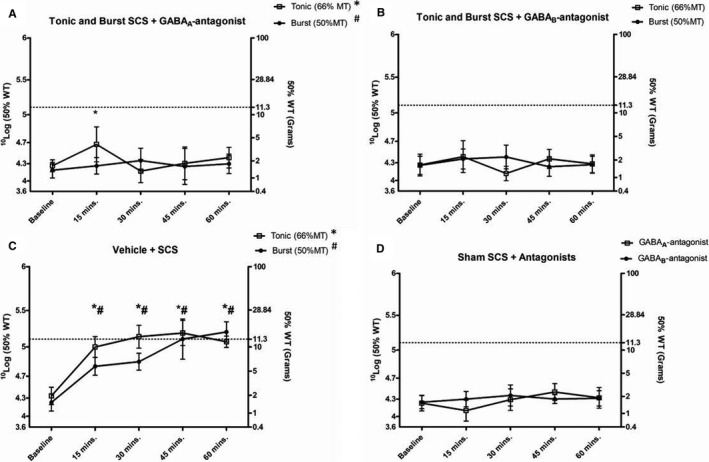
A, The effects of tonic spinal cord stimulation (SCS) (n = 10) and burst SCS (n = 10) on paw withdrawal thresholds (PWTs) based on sensitivity to von Frey filaments 30 minutes after GABAA antagonist bicuculline administration. PWTs were assessed at baseline and at 15, 30, 45, and 60 minutes of SCS. B, The effects of tonic SCS (n = 10) and burst SCS (n = 10) on PWTs 30 minutes after GABAB antagonist phaclofen administration. PWTs were assessed at baseline and at 15, 30, 45, and 60 minutes of SCS. C, The effects of tonic SCS (n = 5) and burst SCS (n = 5) on PWTs 30 minutes after vehicle administration. PWTs were assessed at baseline and at 15, 30, 45, and 60 minutes of SCS. D, The effects of sham SCS and GABA antagonist bicuculline (n = 10) or phaclofen (n = 10) administration on PWTs 30 minutes after antagonist administration. The dotted line represents the average PWT baseline prior to sciatic nerve ligation. # P < 0.05 for burst SCS vs. baseline; *P < 0.05 for tonic SCS vs. baseline.
After intrathecal administration of the GABAB antagonist phaclofen, thresholds for mechanical hypersensitivity did not differ from baseline for both tonic SCS and burst SCS (Figure 5B). PWTs at 15 minutes (P = 0.35 and P = 0.38, respectively), 30 minutes (P = 0.56 and P = 0.41, respectively), 45 minutes (P = 0.43 and P = 0.50, respectively), and 60 minutes of SCS (P = 064 and P = 0.59, respectively) did not significantly differ from baseline PWTs (see Figure 5B).
In a control experiment, administration of vehicle (saline minus antagonist) was administered. Thresholds for mechanical hypersensitivity were not affected, since PWTs significantly increased over time for both tonic SCS (n = 10) and burst SCS (n = 10) at 15 minutes (P = 0.01 and P = 0.01, respectively), 30 minutes (P = 0.02 and P = 0.01, respectively), 45 minutes (P = 0.01 and P = 0.03, respectively), and 60 minutes (P = 0.01 and P = 0.01, respectively) as compared to baseline (Figure 5C).
Administration of the GABAA antagonist bicuculline (n = 5) and the GABAB antagonist phaclofen (n = 5) 30 minutes prior to sham SCS did not increase PWTs as compared to baseline PWTs (Figure 5D).
Administration of the GABAA antagonist bicuculline (n = 5) and the GABAB antagonist phaclofen (n = 5) 30 minutes prior to Von Frey testing decreased PWTs of the contralateral paw (unaffected by the sciatic nerve ligation) at 15 minutes (P = 0.01 and P = 0.01, respectively), 30 minutes (P = 0.02 and P = 0.01, respectively), 45 minutes (P = 0.01 and P = 0.03, respectively), and 60 minutes (P = 0.01 and P = 0.01, respectively) of Von Frey testing as compared to PWTs 30 minutes before baseline (Figure 6).
Figure 6.
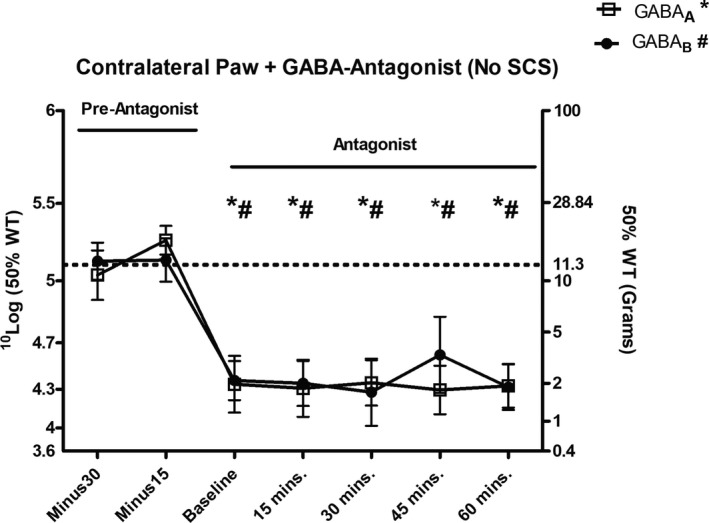
Paw withdrawal thresholds (PWTs) based on sensitivity to von Frey filaments of the contralateral paw 30 and 15 minutes before bicuculline (n = 10) or phaclofen administration (n = 10), and at baseline and 15, 30, 45, and 60 minutes after antagonist administration. The dotted line represents the average PWT baseline prior to sciatic nerve ligation (*P < 0.05 for bicuculline vs. −30 minutes; # P < 0.05 for phaclofen vs. −30 minutes). SCS, spinal cord stimulation; WT, withdrawal threshold.
Discussion
IHC analysis of dorsal horn sections showed a decrease in intracellular GABA levels immediately after burst SCS. Intracellular GABA levels were significantly decreased compared to sham SCS animals and tonic SCS animals. In a second experiment, administration of a GABAA and GABAB receptor antagonists abolished the analgesic effect of both tonic SCS and burst SCS. Based on these findings, we conclude that the analgesic effect of burst SCS is mediated via a spinal GABAergic mechanism. GABAergic interneurons are known to be important players in the pain gate system that is located in the dorsal horn of the spinal cord,39, 42, 43 which in its turn is a fundamental component of the working mechanisms underlying tonic SCS.8, 9, 10, 11, 13, 14, 15, 16, 17, 44 Both experimental and clinical studies have documented the importance of segmental GABA release by turning SCS nonresponders into responders via intrathecal administration of the GABAB agonist baclofen (in subeffective doses).16, 17, 18 The majority of experimental studies on the effects of tonic SCS were performed in sciatic nerve injury models, including the sciatic nerve ligation model (PSNL) and the chronic constriction injury model.13, 16, 17, 29, 31, 32, 33, 37, 38, 45 Therefore, in order to adequately compare and correlate our findings to previous literature, we deliberately chose to perform our experiments in the partial sciatic nerve ligation model. Initially, we found that intracellular GABA levels in the rat dorsal horn were significantly decreased following 60 minutes of burst SCS, hence indicating that burst SCS does induce the release of GABA in the dorsal horn. Our notion was further confirmed by the results of our second experiment, where we demonstrated that the GABAA antagonist phaclofen and GABAB antagonist bicuculline both abolished the pain‐relieving behavioral effect of burst and tonic SCS. Our findings on the effects and mechanisms of tonic SCS are in line with earlier work performed at our laboratory, where it was demonstrated that tonic SCS decreased intracellular GABA IR in the dorsal horn of rats that had undergone PSNL.13 Perhaps even more important is that, in line with our study, experimental pharmacological work has previously shown that local perfusion with a GABAB receptor antagonist in the dorsal horn of neuropathic rats transiently abolished the tonic SCS‐induced effect.16 Meanwhile, preclinical studies conducted in a sciatic nerve injury model demonstrated that intrathecal administration of the GABAB agonist baclofen and the GABAA agonist muscimol, administered intrathecally, resulted in a marked and long‐lasting increase of withdrawal thresholds in rats not achieving adequate pain relief with tonic SCS (nonresponders), although muscimol produced a less prominent threshold increase as compared to baclofen.46 This and our data show that tonic SCS operates by potentiating the spinal GABAergic systems in general, and that the tonic SCS effect depends on both GABAA receptor and GABAB receptor signaling, although the SCS effect seems to be more linked to the GABAB receptor system.45 In our study, administration of a GABAA and GABAB receptor antagonist prevented the pain‐relieving effect of burst SCS. Our conclusion that the pain‐relieving effect of burst SCS is mediated via spinal GABAergic mechanisms may conflict with findings reported by Crosby and colleagues.28 Based on electrophysiological analysis of neuronal firing after burst SCS, these investigators reported that the presence of a GABAB receptor antagonist did not block the attenuation of dorsal horn neuronal firing, when SCS was applied at 90% of the MT.28 Based on these findings the authors conclude that burst SCS does not act via a spinal GABAergic mechanism, whereas tonic SCS does.29 However, because the electrophysiological experiments were terminal, no behavioral testing was performed during the conscious state of the animals. Yet, behavioral analysis and thus assessment of the pain‐relieving effect is known to be the most important indicator of a treatment or compound's translational value.47 Furthermore, even though the experiments provide novel insights into the working mechanisms underlying tonic and burst SCS, the fact that the experiments were performed in a painful cervical nerve root compression rat model makes it difficult to compare the findings to the majority of experimental SCS literature, since most experimental data on pain relief and spinal GABAergic mechanisms underlying tonic and burst SCS have been documented and studied in peripheral nerve injury models for mononeuropathy.13, 14, 15, 16, 17, 30, 32, 33 It is interesting that in the study by Crosby and colleagues,29, 33 both burst and tonic SCS was applied at 90% MT, an intensity known to induce unwanted side effects in the animals. Our study and analysis was based on previously determined optimal settings for pain relief with burst SCS (50% MT) and tonic SCS (66% MT).29, 33 Moreover, according to the strength‐duration curve, with SCS administered at 90%, MT is above the perception threshold, while burst SCS in the clinical setting is usually applied below the perception threshold (paresthesia free).24, 25, 46, 48, 49 Experimental evidence and clinical observations indicate that burst SCS has a delayed wash‐in effect as compared to tonic SCS.32 An experimental study by Meuwissen et al.32 demonstrated that after 15 minutes, burst SCS does induce pain relief, but at a suboptimal level as compared to the plateau phase, which is reached after 30 minutes (when burst and tonic SCS reach similar levels of pain relief). It has been proposed that burst SCS is capable of modulating both the medial and the lateral pain pathways.25, 27 As stated by De Ridder, “the exact mechanism of the selective routing is unknown but could be related to diameter differences at a bifurcation, in which only the burst waveform is powerful enough to overcome the higher transmission resistance of the smaller C‐fibers that make up the medial pain pathway.”33 Meanwhile, evidence shows that tonic SCS, via descending pathways, exerts an inhibitory GABAergic effect in the spinal cord.50, 51, 52, 53, 54, 55 Therefore, it could be hypothesized that 5 minutes of burst SCS, as applied in the Crosby et al. study,28 was not sufficient to set in motion the delayed supraspinal mechanisms that lead to optimal pain relief as observed after the effects of burst SCS have plateaued (30+ minutes).32 Additionally, after only 5 minutes of burst SCS, Crosby and colleagues might have observed a GABA‐independent segmental mechanism of burst SCS, unaffected by the GABAB antagonist, while the supraspinal loop did not yet set in motion the descending pathways that exert an inhibitory effect via GABAergic mechanisms in the spinal cord. It should be mentioned that in our study we applied biphasic active recharge burst SCS, while Crosby and colleagues28 applied passive recharge burst SCS. With monophasic SCS, the pulse is given in only one direction from one electrode to the other. With a biphasic pulse, direction of the pulse is reversed by changing the polarity of the electrodes, allowing the system to actively recharge after each pulse. There are indications that biphasic stimulation is more safe56; however, whether or not there are physiological or clinical differences between the biphasic and monophasic burst waveforms is unknown.57, 58 To date, no data from clinical or preclinical studies have been performed that have directly compared passive recharge burst SCS and active recharge burst SCS; therefore, no conclusive statements regarding the efficacy of these burst waveforms can be made. However, future research that aims to elucidate the working mechanisms of, and (possible) differences between, these waveforms is desired. Interestingly, our results demonstrate that blocking of the GABAA receptor does not prevent a significant increase in PWTs after 15 minutes of tonic SCS, while the effect was successfully suppressed during the remaining minutes of stimulation. This suggests that tonic SCS possesses the ability to,initially, activate GABA‐independent pain‐relieving mechanisms. It is believed that the supraspinal mechanisms of tonic SCS arise from activation of the fast‐conducting lateral pain pathway. Once activated, supraspinal areas activated by the lateral pain pathway may modulate via descending pathways the pain signals coming in on a segmental level, through, for example, the release of serotonin and norepinephrine at the spinal dorsal horns.8, 11, 59
Conclusion
In conclusion, our data, based on experiments in a validated commonly used experimental chronic neuropathic pain model, demonstrate that spinal GABA plays a role in the working mechanisms of burst SCS in the attenuation of pain. Further analysis of the GABAergic mechanisms underlying burst SCS at different time points could shed more light on the exact mechanisms at play.
Conflicts of Interest
Jianwen Wendy Gu and Tianhe C. Zhang are employees of Boson Scientific Inc., and Elbert A.J. Joosten is a consultant for Boston Scientific Inc. Koen Meuwissen and Luuk de Vries have no conflicts of interest to declare.
Funding
This work was supported by a sponsored research contract (ISRNO060009) from Boston Scientific Inc., granted to Prof. Dr. E.A.J. Joosten.
Author Contributions
K.M. performed the experiments, analyzed the data, and wrote the manuscript. L.d.V. processed post‐mortem tissue and analyzed gray value data. J.W.G., T.C.Z., and E.J. edited the manuscript and contributed to the design of the experiment.
References
- 1. Kumar K, Rizvi S, Bnurs SB. Spinal cord stimulation is effective in management of complex regional pain syndrome I: fact or fiction. Neurosurgery. 2011;69:566–578; discussion 578–580. [DOI] [PubMed] [Google Scholar]
- 2. Kumar K, Abbas M, Rizvi S. The use of spinal cord stimulation in pain management. Pain Manag. 2012;2:125–134. [DOI] [PubMed] [Google Scholar]
- 3. Health Quality O. Spinal cord stimulation for neuropathic pain: an evidence‐based analysis. Ont Health Technol Assess Ser. 2005;5:1–78. [PMC free article] [PubMed] [Google Scholar]
- 4. Taylor RS, Van Buyten JP, Buchser E. Spinal cord stimulation for complex regional pain syndrome: a systematic review of the clinical and cost‐effectiveness literature and assessment of prognostic factors. Eur J Pain. 2006;10:91–101. [DOI] [PubMed] [Google Scholar]
- 5. Reig E, Abejon D. Spinal cord stimulation: a 20‐year retrospective analysis in 260 patients. Neuromodulation. 2009;12:232–239. [DOI] [PubMed] [Google Scholar]
- 6. Hou S, Kemp K, Grabois M. A systematic evaluation of burst spinal cord stimulation for chronic back and limb pain. Neuromodulation. 2016;19:398–405. [DOI] [PubMed] [Google Scholar]
- 7. Linderoth B, Foreman RD. Conventional and novel spinal stimulation algorithms: hypothetical mechanisms of action and comments on outcomes. Neuromodulation. 2017;20:525–533. [DOI] [PubMed] [Google Scholar]
- 8. Sdrulla AD, Guan Y, Raja SN. Spinal cord stimulation: clinical efficacy and potential mechanisms. Pain Pract. 2018;18:1048–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Melzack R, Wall PD. Pain mechanisms: a new theory. Science. 1965;150:971–979. [DOI] [PubMed] [Google Scholar]
- 10. Guan Y. Spinal cord stimulation: neurophysiological and neurochemical mechanisms of action. Curr Pain Headache Rep. 2012;16:217–225. [DOI] [PubMed] [Google Scholar]
- 11. Vallejo R, Bradley K, Kapural L. Spinal cord stimulation in chronic pain: mode of action. Spine (Phila Pa 1976). 2017;42(suppl 14):S53–S60. [DOI] [PubMed] [Google Scholar]
- 12. Janssen SP, Truin M, Van Kleef M, Joosten EA. Differential GABAergic disinhibition during the development of painful peripheral neuropathy. Neuroscience. 2011;184:183–194. [DOI] [PubMed] [Google Scholar]
- 13. Janssen SP, Gerard S, Raijmakers ME, Truin M, Van Kleef M, Joosten EA. Decreased intracellular GABA levels contribute to spinal cord stimulation‐induced analgesia in rats suffering from painful peripheral neuropathy: the role of KCC2 and GABA(A) receptor‐mediated inhibition. Neurochem Int. 2012;60:21–30. [DOI] [PubMed] [Google Scholar]
- 14. Linderoth B, Stiller CO, Gunasekera L, O'Connor WT, Ungerstedt U, Brodin E. Gamma‐aminobutyric acid is released in the dorsal horn by electrical spinal cord stimulation: an in vivo microdialysis study in the rat. Neurosurgery. 1994;34:484–488; discussion 488–489. [DOI] [PubMed] [Google Scholar]
- 15. Stiller CO, Cui JG, O'Connor WT, Brodin E, Meyerson BA, Linderoth B. Release of gamma‐aminobutyric acid in the dorsal horn and suppression of tactile allodynia by spinal cord stimulation in mononeuropathic rats. Neurosurgery. 1996;39:367–374; discussion 374–375. [DOI] [PubMed] [Google Scholar]
- 16. Cui JG, O'Connor WT, Ungerstedt U, Linderoth B, Meyerson BA. Spinal cord stimulation attenuates augmented dorsal horn release of excitatory amino acids in mononeuropathy via a GABAergic mechanism. Pain. 1997;73:87–95. [DOI] [PubMed] [Google Scholar]
- 17. Cui JG, Meyerson BA, Sollevi A, Linderoth B. Effect of spinal cord stimulation on tactile hypersensitivity in mononeuropathic rats is potentiated by simultaneous GABA(B) and adenosine receptor activation. Neurosci Lett. 1998;247:183–186. [DOI] [PubMed] [Google Scholar]
- 18. Lind G, Schechtmann G, Winter J, Meyerson BA, Linderoth B. Baclofen‐enhanced spinal cord stimulation and intrathecal baclofen alone for neuropathic pain: long‐term outcome of a pilot study. Eur J Pain. 2008;12:132–136. [DOI] [PubMed] [Google Scholar]
- 19. Kriek N, Groeneweg JG, Stronks DL, de Ridder D, Huygen FJ. Preferred frequencies and waveforms for spinal cord stimulation in patients with complex regional pain syndrome: a multicentre, double‐blind, randomized and placebo‐controlled crossover trial. Eur J Pain. 2017;21:507–519. [DOI] [PubMed] [Google Scholar]
- 20. Ahmed S, Yearwood T, De Ridder D, Vanneste S. Burst and high frequency stimulation: underlying mechanism of action. Expert Rev Med Devices. 2018;15:61–70. [DOI] [PubMed] [Google Scholar]
- 21. De Ridder D, Lenders MW, De Vos CC, et al. A 2‐center comparative study on tonic versus burst spinal cord stimulation: amount of responders and amount of pain suppression. Clin J Pain. 2015;31:433–437. [DOI] [PubMed] [Google Scholar]
- 22. Tiede J, Brown L, Gekht G, Vallejo R, Yearwood T, Morgan D. Novel spinal cord stimulation parameters in patients with predominant back pain. Neuromodulation. 2013;16:370–375. [DOI] [PubMed] [Google Scholar]
- 23. Deer T, Slavin KV, Amirdelfan K, et al. Success using neuromodulation with BURST (SUNBURST) study: results from a prospective, randomized controlled trial using a novel burst waveform. Neuromodulation. 2018;21:56–66. [DOI] [PubMed] [Google Scholar]
- 24. De Ridder D, Vanneste S, Plazier M, van der Loo E, Menovsky T. Burst spinal cord stimulation: toward paresthesia‐free pain suppression. Neurosurgery. 2010;66:986–990. [DOI] [PubMed] [Google Scholar]
- 25. De Ridder D, Plazier M, Kamerling N, Menovsky T, Vanneste S. Burst spinal cord stimulation for limb and back pain. World Neurosurg. 2013;80:642.e1–649.e1. [DOI] [PubMed] [Google Scholar]
- 26. de Vos CC, Bom MJ, Vanneste S, Lenders MW, de Ridder D. Burst spinal cord stimulation evaluated in patients with failed back surgery syndrome and painful diabetic neuropathy. Neuromodulation. 2014;17:152–159. [DOI] [PubMed] [Google Scholar]
- 27. De Ridder D, Vanneste S. Burst and tonic spinal cord stimulation: different and common brain mechanisms. Neuromodulation. 2016;19:47–59. [DOI] [PubMed] [Google Scholar]
- 28. Crosby ND, Weisshaar CL, Smith JR, Zeeman ME, Goodman‐Keiser MD, Winkelstein BA. Burst and tonic spinal cord stimulation differentially activate GABAergic mechanisms to attenuate pain in a rat model of cervical radiculopathy. IEEE Trans Biomed Eng. 2015;62:1604–1613. [DOI] [PubMed] [Google Scholar]
- 29. Truin M, van Kleef M, Linderoth B, Smits H, Janssen SP, Joosten EA. Increased efficacy of early spinal cord stimulation in an animal model of neuropathic pain. Eur J Pain. 2011;15:111–117. [DOI] [PubMed] [Google Scholar]
- 30. Seltzer Z, Dubner R, Shir Y. A novel behavioral model of neuropathic pain disorders produced in rats by partial sciatic nerve injury. Pain. 1990;43:205–218. [DOI] [PubMed] [Google Scholar]
- 31. Truin M, Janssen SP, van Kleef M, Joosten EA. Successful pain relief in non‐responders to spinal cord stimulation: the combined use of ketamine and spinal cord stimulation. Eur J Pain. 2011;15: 1049.e1–1049.e9. [DOI] [PubMed] [Google Scholar]
- 32. Meuwissen KPV, Gu JW, Zhang TC, Joosten EAJ. Burst spinal cord stimulation in peripherally injured chronic neuropathic rats: a delayed effect. Pain Pract. 2018;18:988–996. [DOI] [PubMed] [Google Scholar]
- 33. Meuwissen KPV, Gu JW, Zhang TC, Joosten EAJ. Conventional‐SCS vs. burst‐SCS and the behavioral effect on mechanical hypersensitivity in a rat model of chronic neuropathic pain: effect of amplitude. Neuromodulation. 2018;21:19–30. [DOI] [PubMed] [Google Scholar]
- 34. Pluijms WA, van Kleef M, Honig WM, Janssen SP, Joosten EA. The effect of spinal cord stimulation frequency in experimental painful diabetic polyneuropathy. Eur J Pain. 2013;17:1338–1346. [DOI] [PubMed] [Google Scholar]
- 35. Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. [DOI] [PubMed] [Google Scholar]
- 36. Magoul R, Onteniente B, Geffard M, Calas A. Anatomical distribution and ultrastructural organization of the GABAergic system in the rat spinal cord. An immunocytochemical study using anti‐GABA antibodies. Neuroscience. 1987;20:1001–1009. [DOI] [PubMed] [Google Scholar]
- 37. Smits H, Ultenius C, Deumens R, et al. Effect of spinal cord stimulation in an animal model of neuropathic pain relates to degree of tactile “allodynia.” Neuroscience. 2006;143:541–546. [DOI] [PubMed] [Google Scholar]
- 38. Smits H, van Kleef M, Joosten EA. Spinal cord stimulation of dorsal columns in a rat model of neuropathic pain: evidence for a segmental spinal mechanism of pain relief. Pain. 2012;153:177–183. [DOI] [PubMed] [Google Scholar]
- 39. Malan TP, Mata HP, Porreca F. Spinal GABA(A) and GABA(B) receptor pharmacology in a rat model of neuropathic pain. Anesthesiology. 2002;96:1161–1167. [DOI] [PubMed] [Google Scholar]
- 40. van Beek M, van Kleef M, Linderoth B, van Kuijk SM, Honig WM, Joosten EA. Spinal cord stimulation in experimental chronic painful diabetic polyneuropathy: delayed effect of high‐frequency stimulation. Eur J Pain. 2017;21:795–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mills C, Leblond D, Joshi S, et al. Estimating efficacy and drug ED50's using von Frey thresholds: impact of weber's law and log transformation. J Pain. 2012;13:519–523. [DOI] [PubMed] [Google Scholar]
- 42. Hao JX, Xu XJ, Wiesenfeld‐Hallin Z. Intrathecal gamma‐aminobutyric acid B (GABAB) receptor antagonist CGP 35348 induces hypersensitivity to mechanical stimuli in the rat. Neurosci Lett. 1994;182:299–302. [DOI] [PubMed] [Google Scholar]
- 43. Sivilotti L, Woolf CJ. The contribution of GABAA and glycine receptors to central sensitization: disinhibition and touch‐evoked allodynia in the spinal cord. J Neurophysiol. 1994;72:169–179. [DOI] [PubMed] [Google Scholar]
- 44. Smits H, van Kleef M, Holsheimer J, Joosten EA. Experimental spinal cord stimulation and neuropathic pain: mechanism of action, technical aspects, and effectiveness. Pain Pract. 2013;13:154–168. [DOI] [PubMed] [Google Scholar]
- 45. Cui JG, Linderoth B, Meyerson BA. Effects of spinal cord stimulation on touch‐evoked allodynia involve GABAergic mechanisms. An experimental study in the mononeuropathic rat. Pain. 1996;66:287–295. [DOI] [PubMed] [Google Scholar]
- 46. Abejon D, Rueda P, del Saz J, Arango S, Monzón E, Gilsanz F. Is the introduction of another variable to the strength‐duration curve necessary in neurostimulation? Neuromodulation. 2015;18:182–190; discussion 190. [DOI] [PubMed] [Google Scholar]
- 47. Mogil JS. Animal models of pain: progress and challenges. Nat Rev Neurosci. 2009;10:283–294. [DOI] [PubMed] [Google Scholar]
- 48. Miller JP, Eldabe S, Buchser E, Johanek LM, Guan Y, Linderoth B. Parameters of spinal cord stimulation and their role in electrical charge delivery: a review. Neuromodulation. 2016;19:373–384. [DOI] [PubMed] [Google Scholar]
- 49. Holsheimer J, Buitenweg JR, Das J, de Sutter P, Manola L, Nuttin B. The effect of pulse width and contact configuration on paresthesia coverage in spinal cord stimulation. Neurosurgery. 2011;68:1452–1461; discussion 1461. [DOI] [PubMed] [Google Scholar]
- 50. Barchini J, Tchachaghian S, Shamaa F, et al. Spinal segmental and supraspinal mechanisms underlying the pain‐relieving effects of spinal cord stimulation: an experimental study in a rat model of neuropathy. Neuroscience. 2012;215:196–208. [DOI] [PubMed] [Google Scholar]
- 51. Song Z, Ultenius C, Meyerson BA, Linderoth B. Pain relief by spinal cord stimulation involves serotonergic mechanisms: an experimental study in a rat model of mononeuropathy. Pain. 2009;147:241–248. [DOI] [PubMed] [Google Scholar]
- 52. Linderoth B, Gazelius B, Franck J, Brodin E. Dorsal column stimulation induces release of serotonin and substance P in the cat dorsal horn. Neurosurgery. 1992;31:289–296; discussion 296–297. [DOI] [PubMed] [Google Scholar]
- 53. Saade NE, Jabbur SJ. Nociceptive behavior in animal models for peripheral neuropathy: spinal and supraspinal mechanisms. Prog Neurobiol. 2008;86:22–47. [DOI] [PubMed] [Google Scholar]
- 54. Foreman RD, Linderoth B. Neural mechanisms of spinal cord stimulation. Int Rev Neurobiol. 2012;107:87–119. [DOI] [PubMed] [Google Scholar]
- 55. Linderoth B, Foreman RD. Physiology of spinal cord stimulation: review and update. Neuromodulation. 1999;2:150–164. [DOI] [PubMed] [Google Scholar]
- 56. Piallat B, Chabardès S, Devergnas A, et al. Monophasic but not biphasic pulses induce brain tissue damage during monopolar high‐frequency deep brain stimulation. Neurosurgery. 2009;64:156–162; discussion 162–163. [DOI] [PubMed] [Google Scholar]
- 57. Meuwissen KPV, Gu JW, Zhang TC, Joosten EAJ. Response to: fundamental differences in burst stimulation waveform design: eliminating confusion in the marketplace. Neuromodulation. 2018;21:721–722. [DOI] [PubMed] [Google Scholar]
- 58. Falowski SM. Fundamental differences in burst stimulation waveform design: eliminating confusion in the marketplace. Neuromodulation. 2018;21:320. [DOI] [PubMed] [Google Scholar]
- 59. Saade NE, Tabet MS, Atweh SF, Jabbur SJ. Modulation of segmental mechanisms by activation of a dorsal column brainstem spinal loop. Brain Res. 1984;310:180–184. [DOI] [PubMed] [Google Scholar]


