Abstract
Ductal carcinoma in situ (DCIS) of the breast is a nonobligate precursor of invasive breast cancer, accounting for 20 % of screen‐detected breast cancers. Little is known about the natural progression of DCIS because most patients undergo surgery upon diagnosis. Many DCIS patients are likely being overtreated, as it is believed that only around 50 % of DCIS will progress to invasive carcinoma. Robust prognostic markers for progression to invasive carcinoma are lacking. In the past, studies have investigated women who developed a recurrence after breast‐conserving surgery (BCS) and compared them with those who did not. However, where there is no recurrence, the patient has probably been adequately treated. The present narrative review advocates a new research strategy, wherein only those patients with a recurrence are studied. Approximately half of the recurrences are invasive cancers, and half are DCIS. So‐called “recurrences” are probably most often the result of residual disease. The new approach allows us to ask: why did some residual DCIS evolve to invasive cancers and others not? This novel strategy compares the group of patients that developed in situ recurrence with the group of patients that developed invasive recurrence after BCS. The differences between these groups could then be used to develop a robust risk stratification tool. This tool should estimate the risk of synchronous and metachronous invasive carcinoma when DCIS is diagnosed in a biopsy. Identification of DCIS patients at low risk for developing invasive carcinoma will individualize future therapy and prevent overtreatment.
Keywords: ductal carcinoma in situ, recurrence, active surveillance, risk stratification, prognostic markers
Abbreviations
- BCS
breast‐conserving surgery
- COMET
comparison of operative to monitoring and endocrine therapy trial
- DCIS
ductal carcinoma in situ
- ER
estrogen receptor
- HER2
human epidermal growth factor receptor 2
- LORD
low‐risk DCIS trial
- LoRis
low‐risk DCIS trial
- NSABP
National Surgical Adjuvant Breast and Bowel Project
- PR
progesterone receptor
- TAM
tamoxifen
- UK/ANZ
United Kingdom/Australia, New Zealand
Introduction
Ductal carcinoma in situ (DCIS) of the breast is a nonobligate precursor of invasive breast cancer, representing a heterogeneous group of lesions in terms of morphology and genetics.1 DCIS is constituted by neoplastic epithelial cells which are confined within the ductal‐lobular system by myoepithelial cells and the basement membrane.1 Before the advent of screening mammography, DCIS was only diagnosed when symptomatic (i.e., due to nipple discharge and/or the presence of a palpable mass) and constituted less than 2 % of all diagnosed breast cancers.2 Since the widespread introduction of mammographic screening, DCIS detection rates substantially increased to approximately 20 % of all screen‐detected breast cancers diagnosed at present.3, 4, 5 Nowadays, around 9–19% of DCIS patients are symptomatic, whereas the majority has an occult screen‐detected lesion.5, 6, 7, 8
Whether this increased detection rate mainly represents overdiagnosis remains subject for debate. Early breast cancer diagnosis is considered as beneficial for patients since it is supposed to decrease the risk of both regional lymph node metastasis and distant metastasis. On the other hand, diagnosis of DCIS might also be considered as a negative side‐effect of mammography screening, as it is often questioned whether every DCIS would have become symptomatic in the absence of screening.9 It is currently unclear which DCIS lesions are able to progress to invasive cancer, and which DCIS lesions will remain indolent.10, 11 The identification of those indolent DCIS lesions remains a substantial challenge for future research. In the current narrative review, we discuss the available evidence on natural progression of DCIS, as well as the possible pitfalls of active surveillance trials. Additionally, we present a new research strategy which may serve as an efficient retrospective surrogate for active surveillance studies.
Current state‐of‐the‐art treatment of DCIS
Per definition, DCIS itself does not yield a risk of (lymph)angioinvasion and metastasis. Therefore, the cornerstone of current DCIS treatment is to prevent the development of invasive carcinoma.12 Patients with DCIS show excellent survival,13 with a 20‐year actuarial breast cancer‐specific mortality rate of 3.8%.14 However, women who develop ipsilateral invasive breast carcinoma after initial diagnosis of DCIS show reduced overall and breast cancer‐specific survival,15, 16, 17 as they are 18 times more likely to die of breast cancer than women who do not develop an ipsilateral invasive in‐breast recurrence.16 At present, most DCIS patients undergo surgery. Depending on the size of the lesion and patient preferences, surgical treatment consists either of breast‐conserving surgery (BCS; i.e., lumpectomy) or mastectomy.12 Adjuvant radiotherapy halves the overall recurrence risk after BCS, regardless DCIS size, grade and margin status.17, 18, 19 Despite its substantial influence on recurrence‐free survival, adjuvant radiotherapy does not significantly alter overall survival for DCIS patients.18 Adjuvant endocrine therapy with tamoxifen (TAM) was shown to be associated with both a reduced ipsilateral recurrence risk and a reduced risk of contralateral invasive and in situ carcinoma, although the United Kingdom/Australia, New Zealand (UK/ANZ) DCIS trial and National Surgical Adjuvant Breast and Bowel Project (NSABP) B‐24 trial did not discern estrogen receptor‐positive (ER) from ER‐negative DCIS patients.19, 20, 21 Post hoc analysis of the NSABP B‐24 cohort confirmed these results only for patients with ER‐positive DCIS treated with TAM.22 Despite these observations, adjuvant TAM did not significantly influence the all‐cause mortality risk for patients with ER‐positive DCIS.23 A recently published report on the UK Sloane Project, which prospectively studied a population‐based cohort of patients with screen‐detected DCIS, confirmed that both radiotherapy and endocrine therapy were associated with decreased ipsilateral recurrence risk.24 A retrospective population‐based analysis of endocrine treatment of DCIS patients in British Columbia showed similar results, thereby demonstrating the generalizability of these trial data at the population level.25
The gaps in our knowledge on natural progression
Since most DCIS patients are treated upon diagnosis, little is known about the natural course of progression to invasive breast cancer. Evidence on the prevalence and spontaneous course of DCIS is restricted. A systematic review on 13 autopsy studies reports a prevalence of undetected DCIS in 8.9% of adult women without a history of preexistent breast disease.26 Series of DCIS patients treated with biopsy only are scarce. Betsill and Rosen et al. observed that 8 of 15 DCIS patients, who were treated by biopsy only, developed an invasive carcinoma after a mean interval of 10 years.27, 28 Collins et al. identified a series of 13 DCIS patients who were initially diagnosed as having benign breast disease: 4–18 years later, six of these 13 untreated patients had developed invasive carcinoma, regardless DCIS grade.29 Sanders et al. reported on a cohort of 45 patients with low‐grade DCIS, initially diagnosed as having benign breast disease and therefore “treated” with biopsy only.30 Sixteen of these low‐grade DCIS patients developed invasive carcinoma in the same breast quadrant within three to 42 years after initial biopsy.30 More recently, Maxwell et al. reported a series of 89 DCIS patients who either declined or were unfit to undergo surgery.31 One in three patients developed invasive carcinoma after a median interval of 45 months, and high‐grade DCIS patients showed a significantly higher risk for developing invasive carcinoma (48%) than intermediate or low‐grade DCIS patients (32 and 18%, respectively).31
These retrospective series probably underestimate the risk of spontaneous progression of DCIS, as most reports mainly concern low‐grade DCIS. Despite these limitations, useful information can be deduced: both low‐grade and high‐grade DCIS show the ability to progress to invasive carcinoma, but this occurs more frequently and after a shorter time interval in high‐grade DCIS.13, 31 Patients with high‐grade DCIS also have a higher risk of breast cancer‐related death than patients with low‐ or intermediate‐grade DCIS.13 Notwithstanding the risk of progression, a significant number of unresected DCIS in the aforementioned retrospective series remained in situ, even among the patients with high‐grade DCIS. It is therefore generally accepted that a substantial number of DCIS patients is currently overtreated.
Is active surveillance a noninferior alternative?
At present, three active surveillance trials are conducted to investigate whether watchful waiting is a noninferior alternative strategy for low‐risk DCIS compared to conventional surgery with or without adjuvant irradiation and/or hormonal therapy, as per local protocol.32, 33 In the UK, the low‐risk DCIS (LoRis) trial is open to women with a vacuum‐assisted biopsy diagnosis of asymptomatic low‐ or intermediate‐grade DCIS without necrosis and with low‐mitotic rate.34 Upon central histopathological review of the biopsies, eligible patients are randomized between an active monitoring arm and a surgery arm with conventional surgical and adjuvant treatment.35 In mainland Europe, inclusion in the low‐risk DCIS (LORD) trial is limited to women aged over 45 with asymptomatic screen‐detected pure low‐grade DCIS.36 The LORD trial does not require central histopathological review and randomizes patients between active surveillance and standard treatment according to local policy.36 In the USA, the comparison of operative to monitoring and endocrine therapy (COMET) trial is open to women aged over 40 with newly diagnosed hormone receptor‐positive human epidermal growth factor receptor 2 (HER2)‐negative low‐ or intermediate‐grade DCIS.37 A fourth active surveillance study was announced in Australian and New Zealand with more stringent inclusion criteria than the aforementioned trials.38 This LARRIKIN trial will include women aged over 55 with screen‐detected or incidentally detected DCIS smaller than 25 mm on imaging. Additionally, only patients with hormone receptor‐positive, HER2 nonamplified low‐ or intermediate‐DCIS without comedonecrosis will be allowed to participate.38
Accrual for these trials seems challenging: a report on the first 22 months of the LoRis trial mentions randomization of 38 of only 55 eligible patients,39 whereas the required sample size amounts 932 patients.35 At that rate, accrual will take more than 40 years. The stringent inclusion criteria limit the number of eligible patients. It is likely that patients feel anxious upon being allocated to the watchful waiting arm. Collaboration between these trials for combined data analysis in case of lack of power will be arduous, as all trials apply slightly different inclusion criteria. Even if these active surveillance trials prove that watchful waiting is not inferior compared to standard treatment, it will take many years before these data will be available for routine clinical use. Moreover, only a minority of DCIS patients will benefit from these findings, since only 9–12% of DCIS are low grade.13, 24, 40, 41, 42 Additional inclusion criteria besides nuclear grade will further decrease the number of eligible patients. The overall impact of active surveillance trials on the population of DCIS patients might therefore be limited.43
Risk stratification and thus treatment allocation based on nuclear grade remains an additional challenge, since nuclear grade is characterized by considerable interobserver variability.42, 44 Pathologists disagree more often on the difference between low and intermediate grade, than on the difference between intermediate and high grade.45, 46 It would therefore be interesting to investigate the prognostic value of two‐tier grading as nonhigh grade vs. high grade instead of a three‐tier grading system.47 This two‐tier morphological grading is corroborated by several molecular and gene expression studies that indicate a low‐grade and high‐grade pathway in breast cancer development.48, 49, 50 The identification of alternative robust prognostic markers besides nuclear grade is of utmost importance, because it is likely that overtreatment of DCIS patients will continue despite the potential usage of active surveillance strategies in this limited subpopulation of “low‐risk” DCIS. Notwithstanding these drawbacks, active surveillance trials are presumed to teach us a lot about natural progression in this particular subgroup. This new knowledge should enable us to approach DCIS biology from a completely different perspective.
Risk assessment for synchronous invasion at the biopsy level
Active surveillance might be hazardous, as up to 24% of patients with a biopsy diagnosis of “low‐risk” DCIS present a synchronous invasive carcinoma component in the subsequent resection specimen.51, 52, 53, 54, 55 Five studies applied the inclusion criteria of one or more active surveillance studies on pure DCIS diagnosed in biopsies to investigate the risk of under‐treatment in case of a synchronous invasive carcinoma in the subsequent resection specimen (Table 1). Podoll et al. examined a series of 105 DCIS that were upstaged to invasive cancer in the subsequent resection specimen and applied the LORD and LoRis criteria on this cohort.56 Only three (3%) upgraded DCIS met the LORD criteria, but 20 (19%) upgraded DCIS met the LoRis criteria.56 A similar analysis by Alexander et al. reports 229 DCIS that were upstaged to invasive cancer, of which four (2%) met the LORD criteria, 37 (16%) met the LoRis criteria and 15 (7%) met the COMET criteria.57 A combination of the LORD, LoRis and COMET trial eligibility criteria was retrospectively applied on a subset of 37.544 patients in the National Cancer Database of the American College of Surgeons and the American Cancer Society, which revealed an upstaging rate to invasive carcinoma of 21.8% in this eligible subgroup.58
Table 1.
Overview of retrospective analyses of active surveillance trials’ eligibility criteria in relation to upstage rates to invasive cancer in the surgical resection specimen, after an initial diagnosis of pure DCIS at the biopsy level
| Ref. | Year of publication | Active surveillance eligibility criteria | Number of samples according to eligibility criteria n | Total upstage rate to invasive cancer n (%) | Upstage rate to invasive cancer according to nuclear grade n (%) |
|---|---|---|---|---|---|
| 51 | 2013 | LoRis | 31 | 0 (0) |
|
| 52 | 2016 | LoRis | 296 | 58 (20) |
|
| 53 | 2017 | LoRis | 74 | 5 (7) |
|
| LORD | 10 | 1 (10) |
|
||
| COMET | 81 | 5 (6) |
|
||
| 54 | 2018 | LoRis | 25 | 6 (24) | Not mentioned in this report |
| COMET | 23 | 5 (22) | Not mentioned in this report | ||
| 55 | 2017 | LoRis | 241 | 16 (7) | Not mentioned in this report |
Abbreviations: COMET, comparison of operative to monitoring and endocrine therapy trial; DCIS, ductal carcinoma in situ; LORD, low‐risk DCIS trial; LoRis, low‐risk DCIS trial; Ref, reference.
Overall, the prediction of synchronous invasive cancer when pure DCIS is diagnosed at the biopsy level remains challenging. Many studies have ascertained that upstaging is more frequent in high‐grade DCIS than in low‐ and intermediate‐grade DCIS.55, 59, 60, 61, 62 Nevertheless, a significant proportion of nonhigh‐grade pure DCIS shows synchronous invasive carcinoma in the subsequent resection specimen.57 Attempts have been made to identify additional histological and immunohistochemical features for prediction of concurrent invasive carcinoma. Among them, increased stromal inflammation seems perpetually associated with increased risk for (micro‐)invasive carcinoma,63, 64, 65 but this promising histopathological feature requires further validation in larger independent patient cohorts. Likewise, HER2 positivity in pure DCIS at the biopsy level seems to be associated with increased risk of synchronous invasive cancer in the subsequent resection specimen.66 This could be due to the fact that HER2‐positive invasive carcinomas have an extensive HER2‐positive in situ component,67 which is a risk factor for sampling error. However, further investigations are necessary as this upstage risk was not confirmed by others.63, 68 Of note, increased stromal inflammation is strongly correlated with HER2‐positivity in pure DCIS,69, 70 and stromal inflammation is more frequently observed in DCIS admixed with invasive carcinoma than in pure DCIS.65 It is currently unclear which feature is the most decisive factor in the progression of in situ to invasive carcinoma.
Innovation will discern indolence from agility in DCIS
Besides a lack of adequate markers to predict synchronous invasive carcinoma when pure DCIS is diagnosed at the biopsy level, there is also a need for reliable prognostic markers to assess recurrence risk after conventional treatment of DCIS patients. Despite decades of intensive research, adequate markers for the prediction of invasive recurrence after conventional treatment are lacking. This results in the current uniform treatment of DCIS patients: one size fits all. Why is this? Nearly all studies on prognostic markers in DCIS have applied the following strategy: all BCS‐treated patients diagnosed with pure DCIS in the lumpectomy specimen are investigated and clinicopathological characteristics are noted. Subsequently, the initial DCIS lesions of the patients who have developed a recurrence (designated as “cases”) are compared to the DCIS lesions of the patients who did not develop a recurrence (designated as “controls”). As a result, one or more clinicopathological features are significantly more or less often present in the DCIS lesions of the patients who developed a recurrence. Unfortunately, the prognostic power of these features often cannot be confirmed by others in independent patient cohorts.
This lack of validation is probably due to the fact that most DCIS patients who undergo surgery, will be adequately treated: if the initial DCIS lesion is completely removed, the patient will never develop a recurrence. Contrariwise, patients who do develop a so‐called “recurrence” should be considered as inadequately treated patients. These patients have residual, initially incompletely removed DCIS in their breasts. A so‐called “recurrence” can then be regarded as an outgrowth from this residual disease. Evidently, these “outgrowths” should be discerned from new, independent breast lesions that are not clonally related to the initial DCIS lesion. Only few studies have investigated the relationship between primary DCIS lesions and their recurrences. Although some studies were limited to histopathological and immunohistochemical features without molecular comparison, their results indicated that about 80–90% of recurrences are actually outgrowths from initially incompletely removed DCIS.71, 72 Other studies investigated copy number aberrations and loss of heterozygosity, which resulted in a similar high concordance between primary DCIS lesions and their recurrences.73, 74, 75, 76 To our opinion, retrospective studies should therefore only focus on these patients who recurred, and not on the entire initial patient cohort (Fig. 1).
Figure 1.
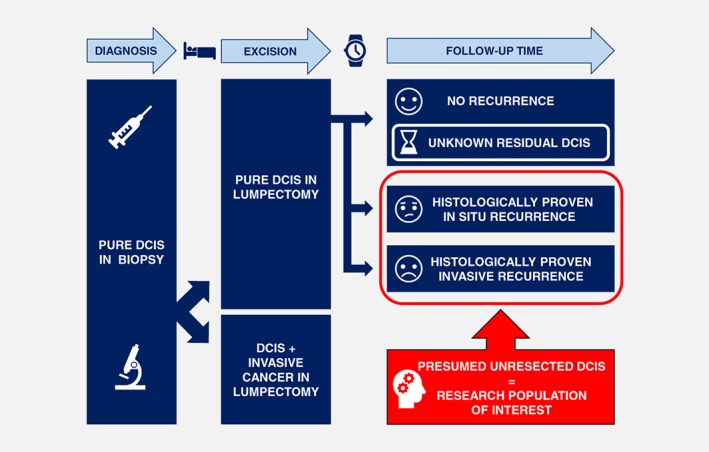
Schematic conceptualization of the novel research strategy. The characteristics of the primary DCIS lesions of patients with in situ and invasive recurrences after breast‐conserving surgery are compared to each other. The majority of patients without recurrences are considered to be adequately treated and are therefore not included in our study. [Color figure can be viewed at http://wileyonlinelibrary.com]
The key message is “pick the right cases and the right controls”. The correct “controls” are the patients with incompletely removed DCIS that remained in situ, that is, the patients who developed a so‐called “in situ recurrence”. Consequently, the right “cases” are not all patients who relapsed. Only those patients who had initially incompletely removed DCIS that has evolved into invasive carcinoma should be regarded as “cases”, that is, the patients who developed a so‐called “invasive recurrence” (Fig. 1). The patient group that has not developed a recurrence (yet) will probably contain a small but hitherto unidentifiable minority who does have incompletely removed DCIS. This small subgroup might either relapse somewhere in the future, or they might have initially incompletely removed DCIS that will remain clinically occult. However, this subgroup will not bias the study as it is not taken into account in the analysis. Such a comparison should enable us to answer the following question: why did some residual DCIS remain in situ, and why did other residual DCIS progress to invasive carcinoma? This novel approach can therefore be considered as a retrospective alternative for the current active surveillance trials: incomplete removal of DCIS allowed the residual DCIS lesion to “progress naturally,” enabling us to retrospectively compare the DCIS lesions that remained in situ with the DCIS lesions that have progressed to invasive cancer.
The advantage of this strategy is that it will include all BCS‐treated DCIS patients and not just the patients with low‐grade DCIS. This novel approach should enable the identification of unequivocal robust markers that predict progression to invasive cancer. Eventually, these markers might allow adequate risk stratification when pure DCIS is diagnosed in core biopsies, by discerning indolent DCIS lesions from aggressive DCIS lesions that have an invasive carcinoma component, either on the short term (i.e., synchronous invasive component) or on the long term (i.e., metachronous invasive component). The results of such a study will aid to individualize therapy for DCIS patients and are therefore expected to significantly reduce overtreatment.
Practical implementation of the alternative research strategy
To the best of our knowledge, this alternative strategy has been applied only once before. Zhou et al.77 investigated a series of 266 women with primary pure DCIS and a known ipsilateral breast event: 136 of these so‐called recurrences were invasive carcinoma and 130 were DCIS. Unfortunately, these authors did not investigate the clonal relationship between the primary DCIS lesion and its corresponding recurrence, since they regarded all ipsilateral new events as de facto recurrences.77 Establishing whether a “recurrence” is either a new second primary tumor or an outgrowth of the initial DCIS lesion is essential for the success of this research strategy. To determine the clonal relationship between the primary DCIS lesion and the second breast event, morphological features such as nuclear grade and DCIS growth pattern can be studied, complemented by immunohistochemistry to assess the expression of ER, progesterone receptor (PR) and HER2. However, these histopathological characteristics enable only a rough comparison, and they should therefore be complemented by molecular studies (i.e., next‐generation sequencing and/or copy number analyses). Such an approach seems feasible as it has been shown that there is a high degree of genomic concordance between both components in synchronous in situ and invasive carcinoma.78, 79, 80, 81, 82 Although genomic profiling requires larger financial resources, it allows a more refined comparison than mere immunohistochemistry. This is especially important as protein expression profiles (e.g., ER, PR and HER2 status) can change during breast cancer progression.72 An integrated approach of histopathological and molecular characteristics should enable to distinguish second primary breast lesions from true DCIS outgrowths with a higher degree of certainty than morphological and immunohistochemical features alone, and subsequent analyses can then be continued with the latter.
Despite the fact that Zhou et al.77 did not discern true DCIS outgrowths from new primary breast lesions, their findings remain very interesting: patients with ER‐negative HER2‐positive primary DCIS presented significantly more often with an in situ “recurrence,” whereas ER‐positive HER2‐negative primary DCIS presented significantly more often with an invasive “recurrence.”77 This might seem contradictory, as HER2 is associated with poor prognosis in invasive breast cancer. This apparent paradox is indirectly supported by other studies. For instance, HER2 protein overexpression in pure DCIS treated with BCS was associated with increased in situ recurrence risk, but not with invasive recurrence risk.83, 84, 85 HER2‐positive invasive breast cancers are more often associated with adjacent DCIS than HER2‐negative invasive breast cancers, and this adjacent DCIS shows more often a larger size and a higher rate of incomplete resection in HER2‐positive breast cancers.67 HER2 amplification and its concurrent protein overexpression might act as a driver for intraductal clonal proliferation, instead of being a driver of cancer cell invasion. This hypothesis may explain the observations of Zhou et al.,77 and it may also explain the paradoxical observation of HER2 overexpression being much more common in DCIS than in invasive carcinoma.86, 87 Nevertheless, a nested case–control study identified HER2 as a marker for progression to invasive carcinoma,88 and therefore additional investigations remain warranted to clarify the role of this intriguing receptor.87, 89
Practical implementation of the novel research strategy might be hampered by the low overall number of recurrences in a single center. A multicenter approach seems therefore mandatory, to enable the inclusion of a sufficiently large number of DCIS patients with subsequent recurrence. Patient recruitment might be facilitated by including patients of previously conducted randomized clinical trials and retrospective studies, of which a nonexhaustive selection is summarized in Table 2.17, 19, 20, 24, 41, 70, 90, 91, 92, 93, 94, 95, 96, 97 Inclusion of these patients in a study conducted according to the new research strategy would allow for correction of treatment effects, since a substantial number of patients are treated with radiotherapy and/or TAM after BCS. Additionally, it would be interesting to also include HER2‐positive DCIS patients with recurrences from the currently ongoing NSABP B‐43 trial, wherein the value of adjuvant trastuzumab is investigated.98 Inclusion of a sufficiently large number of patients does not only allow stratification according to type of adjuvant treatment, but it would also allow stratification according to DCIS grade and other clinicopathological parameters. It may therefore be possible to provide stronger evidence for the so‐called low‐grade and high‐grade pathway of breast cancer progression, wherein low‐grade DCIS gives rise to less aggressive low‐grade invasive carcinoma, and high‐grade DCIS gives rise to more aggressive high‐grade invasive carcinoma.50, 99 Implementation of this novel research strategy in a combined multicenter international effort could force a major breakthrough in the research on DCIS biology and its natural progression.
Table 2.
A selection of prospective trials and retrospective studies with a substantial number of ipsilateral local recurrences (either in situ or invasive) after breast‐conserving surgery for DCIS, with or without adjuvant radiotherapy and/or hormonal therapy
| Ref | Study /trial | FU time (months)1 Mean Median | Total number of patients n | Overall recurrence n (%) | In situ recurrence n (%) | Invasive recurrence n (%) | No radiotherapy, no TAM | Radiotherapy without TAM | Radiotherapy with TAM | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| In situ recurrence n (%) | Invasive recurrence n (%) | In situ recurrence n (%) | Invasive recurrence n (%) | In situ recurrence n (%) | Invasive recurrence n (%) | |||||||
| 20 | NSABP B‐17 | 207 | 813 | 222 (27) | 99 (12) | 123 (15) | 62 (15) | 79 (20) | 37 (9) | 44 (11) | NA | NA |
| 20 | NSABP B‐24 | 163 | 1,799 | 268 (15) | 128 (7) | 140 (8) | NA | NA | 68 (8) | 81 (9) | 60 (7) | 59 (7) |
| 90 | SweDCIS | 204 | 1,046 | 258 (25) | 129 (12) | 129 (12) | 91 (17) | 74 (14) | 38 (7) | 55 (11) | NA | NA |
| 19 | UK/ANZ DCIS2 | 152 | 1,694 | 376 (22) | 197 (12) | 163 (10) | 86 (16) | 52 (10) | 14 (5) | 10 (4) | 11 (3) | 11 (3) |
| 17 | EORTC10853 | 190 | 1,010 | 234 (23) | 110 (11) | 121 (12) | 74 (15) | 75 (15) | 37 (7) | 48 (10) | NA | NA |
| 24 | Sloane project | 64 | 7,007 | 368 (5) | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 |
| 70 | Pruneri et al. | 98 | 945 | 180 (19) | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 |
| 41 | Punglia et al. | 60 | 2,762 | 79 (3) | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 |
| 91 | E5194 cohort | 138 | 327 | 53 (16) | 26 (8) | 27 (8) | 3 | 3 | 3 | 3 | 3 | 3 |
| 91 | Ontario cohort | 118 | 446 | 65 (15) | 27 (6) | 38 (9) | 3 | 3 | 3 | 3 | 3 | 3 |
| 95 | Toss et al. | 103 | 776 | 83 (11) | 30 (4) | 53 (7) | 3 | 3 | 3 | 3 | 3 | 3 |
| 97 | Wai et al. | 113 | 460 | 60 (13) | 32 (7) | 28 (6) | 32 (7) | 28 (6) | NA | NA | NA | NA |
| 96 | Tunon‐de‐Lara et al. | 118 | 812 | 71 (9) | 24 (3) | 47 (6) | 3 | 3 | 3 | 3 | 3 | 3 |
| 92 | Butler‐Henderson et al. | 113 | 1,356 | 235 (17) | 86 (6) | 149 (11) | 3 | 3 | 3 | 3 | 3 | 3 |
| 93 | Collins et al. | 58 | 2,995 | 325 (11) | 172 (6) | 153 (5) | 3 | 3 | 3 | 3 | 3 | 3 |
| 94 | Rudloff et al. | 67 | 1,868 | 202 (11) | 122 (7) | 80 (4) | 3 | 3 | 3 | 3 | 3 | 3 |
Some study reports provided median follow‐up time in years; for these reports, the number of years was multiplied by 12 months to achieve median follow‐up in months to enable comparison.
The category “tamoxifen alone, without radiotherapy” from this report is not included in this table.
No details on different subgroups were provided in these study reports, and therefore data on different subgroups could not be provided in this table.
Abbreviations: DCIS, ductal carcinoma in situ; EORTC, European Organization for Research and Treatment of Cancer; FU, follow‐up; NA, not applicable; NSABP, National Surgical Adjuvant Breast and Bowel Project; Ref, reference number; TAM, tamoxifen; UK/ANZ, United Kingdom/Australia, New Zealand.
Bold values are means and values in italics are medians.
Conclusion
The breast is a rather unique organ regarding the clonality issue between DCIS and its recurrence, although a similar issue exists in the liver and the lungs, where intra‐organ metastases have to be discerned from new, metachronous and synchronous carcinomas.100, 101 Based on the currently available evidence regarding prognostic markers in DCIS (or the lack thereof), we propose a new model as a retrospective surrogate for active surveillance trials, which may provide useful data on the short term. This novel strategy is based on the comparison of the initial DCIS lesion between the patients who developed an in situ recurrence and the patients who developed an invasive recurrence. We hope this model will be included in future scientific studies on risk stratification of DCIS, as we believe this strategy will enable identification of robust markers for prediction of the natural course of DCIS. If this new approach succeeds in the development of a reliable risk stratification tool, the direct impact on clinical management of DCIS will be enormous, as it is likely that many patients will be treated less aggressively than they are now.
Conflict of interest: This work was supported by a bursary from the Mathilde Horlait‐Dapsens Foundation (Brussels, Belgium), received by M.R. Van Bockstal. All remaining authors have declared no conflicts of interest.
Mieke R. Van Bockstal's permanent address is: Department of Pathology, University Clinics St‐Luc, Brussels, Belgium
References
- 1. Pang JM, Gorringe KL, Fox SB. Ductal carcinoma in situ—update on risk assessment and management. Histopathology 2016;68:96–109. [DOI] [PubMed] [Google Scholar]
- 2. Rosner D, Bedwani RN, Vana J, et al. Noninvasive breast carcinoma: results of a national survey by the American College of Surgeons. Ann Surg 1980;192:139–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. van Steenbergen LN, Voogd AC, Roukema JA, et al. Screening caused rising incidence rates of ductal carcinoma in situ of the breast. Breast Cancer Res Treat 2009;115:181–3. [DOI] [PubMed] [Google Scholar]
- 4. Glover JA, Bannon FJ, Hughes CM, et al. Increased diagnosis and detection rates of carcinoma in situ of the breast. Breast Cancer Res Treat 2012;133:779–84. [DOI] [PubMed] [Google Scholar]
- 5. Kerlikowske K. Epidemiology of ductal carcinoma in situ. J Natl Cancer Inst 2010;2010:139–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sundara Rajan S, Verma R, Shaaban AM, et al. Palpable ductal carcinoma in situ: analysis of radiological and histological features of a large series with 5‐year follow‐up. Clin Breast Cancer 2013;13:486–91. [DOI] [PubMed] [Google Scholar]
- 7. Kerlikowske K, Molinaro AM, Gauthier ML, et al. Biomarker expression and risk of subsequent tumors after initial ductal carcinoma in situ diagnosis. J Natl Cancer Inst 2010;102:627–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Barnes NL, Dimopoulos N, Williams KE, et al. The frequency of presentation and clinico‐pathological characteristics of symptomatic versus screen detected ductal carcinoma in situ of the breast. Eur J Surg Oncol 2014;40:249–54. [DOI] [PubMed] [Google Scholar]
- 9. Esserman LJ, Thompson IM Jr, Reid B. Overdiagnosis and overtreatment in cancer: an opportunity for improvement. JAMA 2013;310:797–8. [DOI] [PubMed] [Google Scholar]
- 10. Agahozo MC, Hammerl D, Debets R, et al. Tumor‐infiltrating lymphocytes and ductal carcinoma in situ of the breast: friends or foes? Mod Pathol 2018;31:1012–25. [DOI] [PubMed] [Google Scholar]
- 11. Welch HG, Woloshin S, Schwartz LM. The sea of uncertainty surrounding ductal carcinoma in situ‐‐the price of screening mammography. J Natl Cancer Inst 2008;100:228–9. [DOI] [PubMed] [Google Scholar]
- 12. Bijker N, Donker M, Wesseling J, et al. Is DCIS breast cancer, and how do I treat it? Curr Treat Opt Oncol 2013;14:75–87. [DOI] [PubMed] [Google Scholar]
- 13. van Maaren MC, Lagendijk M, Tilanus‐Linthorst MMA, et al. Breast cancer‐related deaths according to grade in ductal carcinoma in situ: a Dutch population‐based study on patients diagnosed between 1999 and 2012. Eur J Cancer 2018;101:134–42. [DOI] [PubMed] [Google Scholar]
- 14. Sopik V, Sun P, Narod SA. Impact of microinvasion on breast cancer mortality in women with ductal carcinoma in situ. Breast Cancer Res Treat 2018;167:787–95. [DOI] [PubMed] [Google Scholar]
- 15. Elshof LE, Schmidt MK, Rutgers EJT, et al. Cause‐specific mortality in a population‐based cohort of 9799 women treated for ductal carcinoma in situ. Ann Surg 2018;267:952–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Narod SA, Iqbal J, Giannakeas V, et al. Breast cancer mortality after a diagnosis of ductal carcinoma in situ. JAMA Oncol 2015;1:888–96. [DOI] [PubMed] [Google Scholar]
- 17. Donker M, Litiere S, Werutsky G, et al. Breast‐conserving treatment with or without radiotherapy in ductal carcinoma in situ: 15‐year recurrence rates and outcome after a recurrence, from the EORTC 10853 randomized phase III trial. J Clin Oncol 2013;31:4054–9. [DOI] [PubMed] [Google Scholar]
- 18. Goodwin A, Parker S, Ghersi D, et al. Post‐operative radiotherapy for ductal carcinoma in situ of the breast. Cochrane Database Syst Rev 2013;11:CD000563. [DOI] [PubMed] [Google Scholar]
- 19. Cuzick J, Sestak I, Pinder SE, et al. Effect of tamoxifen and radiotherapy in women with locally excised ductal carcinoma in situ: long‐term results from the UK/ANZ DCIS trial. Lancet Oncol 2011;12:21–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wapnir IL, Dignam JJ, Fisher B, et al. Long‐term outcomes of invasive ipsilateral breast tumor recurrences after lumpectomy in NSABP B‐17 and B‐24 randomized clinical trials for DCIS. J Natl Cancer Inst 2011;103:478–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Staley H, McCallum I, Bruce J. Postoperative tamoxifen for ductal carcinoma in situ. Cochrane Database Syst Rev 2012;10:CD007847. [DOI] [PubMed] [Google Scholar]
- 22. Allred DC, Anderson SJ, Paik S, et al. Adjuvant tamoxifen reduces subsequent breast cancer in women with estrogen receptor‐positive ductal carcinoma in situ: a study based on NSABP protocol B‐24. J Clin Oncol 2012;30:1268–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Staley H, McCallum I, Bruce J. Postoperative Tamoxifen for ductal carcinoma in situ: Cochrane systematic review and meta‐analysis. Breast 2014;23:546–51. [DOI] [PubMed] [Google Scholar]
- 24. Thompson AM, Clements K, Cheung S, et al. Management and 5‐year outcomes in 9938 women with screen‐detected ductal carcinoma in situ: the UK Sloane project. Eur J Cancer 2018;101:210–9. [DOI] [PubMed] [Google Scholar]
- 25. Lo AC, Truong PT, Wai ES, et al. Population‐based analysis of the impact and generalizability of the NSABP‐B24 study on endocrine therapy for patients with ductal carcinoma in situ of the breast. Ann Oncol 2015;26:1898–903. [DOI] [PubMed] [Google Scholar]
- 26. Thomas ET, Del Mar C, Glasziou P, et al. Prevalence of incidental breast cancer and precursor lesions in autopsy studies: a systematic review and meta‐analysis. BMC Cancer 2017;17:808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Betsill WL Jr, Rosen PP, Lieberman PH, et al. Intraductal carcinoma. Long‐term follow‐up after treatment by biopsy alone. JAMA 1978;239:1863–7. [DOI] [PubMed] [Google Scholar]
- 28. Rosen PP, Braun DW Jr, Kinne DE. The clinical significance of pre‐invasive breast carcinoma. Cancer 1980;46:919–25. [DOI] [PubMed] [Google Scholar]
- 29. Collins LC, Tamimi RM, Baer HJ, et al. Outcome of patients with ductal carcinoma in situ untreated after diagnostic biopsy: results from the Nurses' health study. Cancer 2005;103:1778–84. [DOI] [PubMed] [Google Scholar]
- 30. Sanders ME, Schuyler PA, Simpson JF, et al. Continued observation of the natural history of low‐grade ductal carcinoma in situ reaffirms proclivity for local recurrence even after more than 30 years of follow‐up. Mod Pathol 2015;28:662–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Maxwell AJ, Clements K, Hilton B, et al. Risk factors for the development of invasive cancer in unresected ductal carcinoma in situ. Eur J Surg Oncol 2018;44:429–35. [DOI] [PubMed] [Google Scholar]
- 32. Toss M, Miligy I, Thompson AM, et al. Current trials to reduce surgical intervention in ductal carcinoma in situ of the breast: critical review. Breast 2017;35:151–6. [DOI] [PubMed] [Google Scholar]
- 33. Groen EJ, Elshof LE, Visser LL, et al. Finding the balance between over‐ and under‐treatment of ductal carcinoma in situ (DCIS). Breast 2017;31:274–83. [DOI] [PubMed] [Google Scholar]
- 34. Rea D, Francis A, Wallis M, et al. Confusion over differences in registration and randomization criteria for the LORIS (low‐risk DCIS) trial. Ann Surg Oncol 2017;24:566–7. [DOI] [PubMed] [Google Scholar]
- 35. Francis A, Thomas J, Fallowfield L, et al. Addressing overtreatment of screen detected DCIS; the LORIS trial. Eur J Cancer 2015;51:2296–303. [DOI] [PubMed] [Google Scholar]
- 36. Elshof LE, Tryfonidis K, Slaets L, et al. Feasibility of a prospective, randomised, open‐label, international multicentre, phase III, non‐inferiority trial to assess the safety of active surveillance for low risk ductal carcinoma in situ—the LORD study. Eur J Cancer 2015;51:1497–510. [DOI] [PubMed] [Google Scholar]
- 37. Hwang ES, Hyslop T, Lynch T, et al. The COMET (comparison of operative versus monitoring and endocrine therapy) trial: a phase III randomised controlled clinical trial for low‐risk ductal carcinoma in situ (DCIS). BMJ Open 2019;9:e026797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lippey J, Spillane A, Saunders C. Not all ductal carcinoma in situ is created equal: can we avoid surgery for low‐risk ductal carcinoma in situ? ANZ J Surg 2016;86:859–60. [DOI] [PubMed] [Google Scholar]
- 39. Thomas J, Hanby A, Pinder S, et al. LORIS trial of active monitoring for DCIS: how does the online pathology eligibility review process work? Cancer Res 2017;77:40. [Google Scholar]
- 40. Rakovitch E, Nofech‐Mozes S, Hanna W, et al. A population‐based validation study of the DCIS score predicting recurrence risk in individuals treated by breast‐conserving surgery alone. Breast Cancer Res Treat 2015;152:389–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Punglia RS, Jiang W, Lipsitz SR, et al. Clinical risk score to predict likelihood of recurrence after ductal carcinoma in situ treated with breast‐conserving surgery. Breast Cancer Res Treat 2018;167:751–9. [DOI] [PubMed] [Google Scholar]
- 42. van Dooijeweert C, van Diest PJ, Willems SM, et al. Significant inter‐ and intra‐laboratory variation in grading of ductal carcinoma in situ of the breast: a nationwide study of 4901 patients in The Netherlands. Breast Cancer Res Treat 2019;174:479–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Pilewskie M, Van Zee KJ, Morrow M. Confusion over differences in registration and randomization criteria for the LORIS (low‐risk DCIS) trial: a reply. Ann Surg Oncol 2017;24:568–9. [DOI] [PubMed] [Google Scholar]
- 44. Sneige N, Lagios MD, Schwarting R, et al. Interobserver reproducibility of the Lagios nuclear grading system for ductal carcinoma in situ. Hum Pathol 1999;30:257–62. [DOI] [PubMed] [Google Scholar]
- 45. Onega T, Weaver DL, Frederick PD, et al. The diagnostic challenge of low‐grade ductal carcinoma in situ. Eur J Cancer 2017;80:39–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Van Bockstal M, Baldewijns M, Colpaert C, et al. Dichotomous histopathological assessment of ductal carcinoma in situ of the breast results in substantial interobserver concordance. Histopathology 2018;73:923–32. [DOI] [PubMed] [Google Scholar]
- 47. Van Bockstal M, Lambein K, Smeets A, et al. Stromal characteristics are adequate prognosticators for recurrence risk in ductal carcinoma in situ of the breast. Eur J Surg Oncol 2019;45:550–9. [DOI] [PubMed] [Google Scholar]
- 48. Balleine RL, Webster LR, Davis S, et al. Molecular grading of ductal carcinoma in situ of the breast. Clin Cancer Res 2008;14:8244–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hannemann J, Velds A, Halfwerk JB, et al. Classification of ductal carcinoma in situ by gene expression profiling. Breast Cancer Res 2006;8:R61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lopez‐Garcia MA, Geyer FC, Lacroix‐Triki M, et al. Breast cancer precursors revisited: molecular features and progression pathways. Histopathology 2010;57:171–92. [DOI] [PubMed] [Google Scholar]
- 51. Soumian S, Verghese ET, Booth M, et al. Concordance between vacuum assisted biopsy and postoperative histology: implications for the proposed low risk DCIS trial (LORIS). Eur J Surg Oncol 2013;39:1337–40. [DOI] [PubMed] [Google Scholar]
- 52. Pilewskie M, Stempel M, Rosenfeld H, et al. Do LORIS trial eligibility criteria identify a ductal carcinoma in situ patient population at low risk of upgrade to invasive carcinoma? Ann Surg Oncol 2016;23:3487–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Grimm LJ, Ryser MD, Partridge AH, et al. Surgical upstaging rates for vacuum assisted biopsy proven DCIS: implications for active surveillance trials. Ann Surg Oncol 2017;24:3534–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Patel GV, Van Sant EP, Taback B, et al. Patient selection for ductal carcinoma in situ observation trials: are the lesions truly low risk? Am J Roentgenol 2018;211:712–3. [DOI] [PubMed] [Google Scholar]
- 55. Jakub JW, Murphy BL, Gonzalez AB, et al. A validated Nomogram to predict upstaging of ductal carcinoma in situ to invasive disease. Ann Surg Oncol 2017;24:2915–24. [DOI] [PubMed] [Google Scholar]
- 56. Podoll MB, Reisenbichler ES, Roland L, et al. Feasibility of the less is more approach in treating low‐risk ductal carcinoma in situ diagnosed on core needle biopsy: ten‐year review of ductal carcinoma in situ upgraded to invasion at surgery. Arch Pathol Lab Med 2018;142:1120–6. [DOI] [PubMed] [Google Scholar]
- 57. Alexander M, Beyda J, Nayak A, et al. Not all ductal carcinomas in situ are created IDLE (indolent lesions of epithelial origin). Arch Pathol Lab Med 2019;143:99–104. [DOI] [PubMed] [Google Scholar]
- 58. Chavez de Paz Villanueva C, Bonev V, Senthil M, et al. Factors associated with underestimation of invasive cancer in patients with ductal carcinoma in situ: precautions for active surveillance. JAMA Surg 2017;152:1007–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Al Nemer AM. Histologic factors predicting invasion in patients with ductal carcinoma in situ (DCIS) in the preoperative core biopsy. Pathol Res Pract 2017;213:429–34. [DOI] [PubMed] [Google Scholar]
- 60. Kim J, Han W, Lee JW, et al. Factors associated with upstaging from ductal carcinoma in situ following core needle biopsy to invasive cancer in subsequent surgical excision. Breast 2012;21:641–5. [DOI] [PubMed] [Google Scholar]
- 61. Brennan ME, Turner RM, Ciatto S, et al. Ductal carcinoma in situ at core‐needle biopsy: meta‐analysis of underestimation and predictors of invasive breast cancer. Radiology 2011;260:119–28. [DOI] [PubMed] [Google Scholar]
- 62. Huo L, Sneige N, Hunt KK, et al. Predictors of invasion in patients with core‐needle biopsy‐diagnosed ductal carcinoma in situ and recommendations for a selective approach to sentinel lymph node biopsy in ductal carcinoma in situ. Cancer 2006;107:1760–8. [DOI] [PubMed] [Google Scholar]
- 63. Doebar SC, de Monye C, Stoop H, et al. Ductal carcinoma in situ diagnosed by breast needle biopsy: predictors of invasion in the excision specimen. Breast 2016;27:15–21. [DOI] [PubMed] [Google Scholar]
- 64. Hoorntje LE, Schipper MEI, Peeters PHM, et al. The finding of invasive cancer after a preoperative diagnosis of ductal carcinoma‐in‐situ: causes of ductal carcinoma‐in‐situ underestimates with stereotactic 14‐gauge needle biopsy. Ann Surg Oncol 2003;10:748–53. [DOI] [PubMed] [Google Scholar]
- 65. Moriya T, Silverberg SG. Intraductal carcinoma (ductal carcinoma in situ) of the breast. A comparison of pure noninvasive tumors with those including different proportions of infiltrating carcinoma. Cancer 1994;74:2972–8. [DOI] [PubMed] [Google Scholar]
- 66. Mustafa RE, DeStefano LM, Bahng J, et al. Evaluating the risk of upstaging HER2‐positive DCIS to invasive breast cancer. Ann Surg Onco 2017;24:2999–3003. [DOI] [PubMed] [Google Scholar]
- 67. Doebar SC, van den Broek EC, Koppert LB, et al. Extent of ductal carcinoma in situ according to breast cancer subtypes: a population‐based cohort study. Breast Cancer Res Treat 2016;158:179–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Kondo T, Hayashi N, Ohde S, et al. A model to predict upstaging to invasive carcinoma in patients preoperatively diagnosed with ductal carcinoma in situ of the breast. J Surg Oncol 2015;112:476–80. [DOI] [PubMed] [Google Scholar]
- 69. Van Bockstal M, Lambein K, Denys H, et al. Histopathological characterization of ductal carcinoma in situ (DCIS) of the breast according to HER2 amplification status and molecular subtype. Virchows Arch 2014;465:275–89. [DOI] [PubMed] [Google Scholar]
- 70. Pruneri G, Lazzeroni M, Bagnardi V, et al. The prevalence and clinical relevance of tumor‐infiltrating lymphocytes (TILs) in ductal carcinoma in situ of the breast. Ann Oncol 2017;28:321–8. [DOI] [PubMed] [Google Scholar]
- 71. Bijker N, Peterse JL, Duchateau L, et al. Histological type and marker expression of the primary tumour compared with its local recurrence after breast‐conserving therapy for ductal carcinoma in situ. Br J Cancer 2001;84:539–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Karlsson E, Sandelin K, Appelgren J, et al. Clonal alteration of breast cancer receptors between primary ductal carcinoma in situ (DCIS) and corresponding local events. Eur J Cancer 2014;50:517–24. [DOI] [PubMed] [Google Scholar]
- 73. Gorringe KL, Hunter SM, Pang JM, et al. Copy number analysis of ductal carcinoma in situ with and without recurrence. Mod Pathol 2015;28:1174–84. [DOI] [PubMed] [Google Scholar]
- 74. Amari M, Moriya T, Ishida T, et al. Loss of heterozygosity analyses of asynchronous lesions of ductal carcinoma in situ and invasive ductal carcinoma of the human breast. Jpn J Clin Oncol 2003;33:556–62. [DOI] [PubMed] [Google Scholar]
- 75. Waldman FM, DeVries S, Chew KL, et al. Chromosomal alterations in ductal carcinomas in situ and their in situ recurrences. J Natl Cancer Inst 2000;92:313–20. [DOI] [PubMed] [Google Scholar]
- 76. Lininger RA, Fujii H, Man YG, et al. Comparison of loss heterozygosity in primary and recurrent ductal carcinoma in situ of the breast. Mod Pathol 1998;11:1151–9. [PubMed] [Google Scholar]
- 77. Zhou W, Johansson C, Jirstrom K, et al. A comparison of tumor biology in primary ductal carcinoma in situ recurring as invasive carcinoma versus a new in situ. Int J Breast Cancer 2013;2013:582134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Doebar SC, Krol NM, van Marion R, et al. Progression of ductal carcinoma in situ to invasive breast cancer: comparative genomic sequencing. Virchows Arch 2019;474:247–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Johnson CE, Gorringe KL, Thompson ER, et al. Identification of copy number alterations associated with the progression of DCIS to invasive ductal carcinoma. Breast Cancer Res Treat 2012;133:889–98. [DOI] [PubMed] [Google Scholar]
- 80. Rane SU, Mirza H, Grigoriadis A, et al. Selection and evolution in the genomic landscape of copy number alterations in ductal carcinoma in situ (DCIS) and its progression to invasive carcinoma of ductal/no special type: a meta‐analysis. Breast Cancer Res Treat 2015;153:101–21. [DOI] [PubMed] [Google Scholar]
- 81. Liao S, Desouki MM, Gaile DP, et al. Differential copy number aberrations in novel candidate genes associated with progression from in situ to invasive ductal carcinoma of the breast. Genes Chromosomes Cancer 2012;51:1067–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Iakovlev VV, Arneson NC, Wong V, et al. Genomic differences between pure ductal carcinoma in situ of the breast and that associated with invasive disease: a calibrated aCGH study. Clin Cancer Res 2008;14:4446–54. [DOI] [PubMed] [Google Scholar]
- 83. Han K, Nofech‐Mozes S, Narod S, et al. Expression of HER2neu in ductal carcinoma in situ is associated with local recurrence. Clin Oncol 2012;24:183–9. [DOI] [PubMed] [Google Scholar]
- 84. Rakovitch E, Nofech‐Mozes S, Hanna W, et al. HER2/neu and Ki‐67 expression predict non‐invasive recurrence following breast‐conserving therapy for ductal carcinoma in situ. Br J Cancer 2012;106:1160–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Curigliano G, Disalvatore D, Esposito A, et al. Risk of subsequent in situ and invasive breast cancer in human epidermal growth factor receptor 2‐positive ductal carcinoma in situ. Ann Oncol 2015;26:682–7. [DOI] [PubMed] [Google Scholar]
- 86. Lambein K, Van Bockstal M, Vandemaele L, et al. Comparison of HER2 amplification status among breast cancer subgroups offers new insights in pathways of breast cancer progression. Virchows Arch 2017;471:575–87. [DOI] [PubMed] [Google Scholar]
- 87. Lazzeroni M, Dunn BK, Pruneri G, et al. Adjuvant therapy in patients with ductal carcinoma in situ of the breast: the Pandora's box. Cancer Treat Rev 2017;55:1–9. [DOI] [PubMed] [Google Scholar]
- 88. Visser LL, Elshof LE, Schaapveld M, et al. Clinicopathological risk factors for an invasive breast cancer recurrence after ductal carcinoma in situ—a nested case‐control study. Clin Cancer Res 2018;24:3593–601. [DOI] [PubMed] [Google Scholar]
- 89. Martinez‐Perez C, Turnbull AK, Ekatah GE, et al. Current treatment trends and the need for better predictive tools in the management of ductal carcinoma in situ of the breast. Cancer Treat Rev 2017;55:163–72. [DOI] [PubMed] [Google Scholar]
- 90. Warnberg F, Garmo H, Emdin S, et al. Effect of radiotherapy after breast‐conserving surgery for ductal carcinoma in situ: 20 years follow‐up in the randomized SweDCIS trial. J Clin Oncol 2014;32:3613–8. [DOI] [PubMed] [Google Scholar]
- 91. Rakovitch E, Gray R, Baehner FL, et al. Refined estimates of local recurrence risks by DCIS score adjusting for clinicopathological features: a combined analysis of ECOG‐ACRIN E5194 and Ontario DCIS cohort studies. Breast Cancer Res Treat 2018;169:359–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Butler‐Henderson K, Lee AH, Lenzo NP, et al. Epidemiology of ductal carcinoma in situ in Western Australia: implications for surgical margins and management. Breast Cancer 2015;22:641–7. [DOI] [PubMed] [Google Scholar]
- 93. Collins LC, Achacoso N, Haque R, et al. Risk factors for non‐invasive and invasive local recurrence in patients with ductal carcinoma in situ. Breast Cancer Res Treat 2013;139:453–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Rudloff U, Jacks LM, Goldberg JI, et al. Nomogram for predicting the risk of local recurrence after breast‐conserving surgery for ductal carcinoma in situ. J Clin Oncol 2010;28:3762–9. [DOI] [PubMed] [Google Scholar]
- 95. Toss MS, Miligy IM, Gorringe KL, et al. Legumain is an independent predictor for invasive recurrence in breast ductal carcinoma in situ. Mod Pathol 2019;32:639–49. [DOI] [PubMed] [Google Scholar]
- 96. Tunon‐de‐Lara C, Andre G, Macgrogan G, et al. Ductal carcinoma in situ of the breast: influence of age on diagnostic, therapeutic, and prognostic features. Retrospective study of 812 patients. Ann Surg Oncol 2011;18:1372–9. [DOI] [PubMed] [Google Scholar]
- 97. Wai ES, Lesperance ML, Alexander CS, et al. Predictors of local recurrence in a population‐based cohort of women with ductal carcinoma in situ treated with breast conserving surgery alone. Ann Surg Oncol 2011;18:119–24. [DOI] [PubMed] [Google Scholar]
- 98. Siziopikou KP, Anderson SJ, Cobleigh MA, et al. Preliminary results of centralized HER2 testing in ductal carcinoma in situ (DCIS): NSABP B‐43. Breast Cancer Res Treat 2013;142:415–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Sanati S. Morphologic and molecular features of breast ductal carcinoma in situ. Am J Pathol 2019;189:946–55. [DOI] [PubMed] [Google Scholar]
- 100. Mansuet‐Lupo A, Barritault M, Alifano M, et al. Proposal for a combined histomolecular algorithm to distinguish multiple primary adenocarcinomas from intrapulmonary metastasis in patients with multiple lung tumors. J Thorac Oncol 2019;14:844–56. [DOI] [PubMed] [Google Scholar]
- 101. Portolani N, Coniglio A, Ghidoni S, et al. Early and late recurrence after liver resection for hepatocellular carcinoma: prognostic and therapeutic implications. Ann Surg 2006;243:229–35. [DOI] [PMC free article] [PubMed] [Google Scholar]


