Abstract
Objectives
The primary aim of the study was to compare environmental and external (cross‐) contamination of traces of cytostatics, during preparation of 5‐fluorouracil and cyclophosphamide using a robotic system (APOTECAchemo) or the conventional manual compounding procedure. The secondary aim was to validate the cleaning procedure of the robot.
Methods
Eighty ready‐to‐administer (RTA) infusion bags with 5‐fluorouracil, cyclophosphamide or sodium chloride were compounded using both techniques on 3–5 days. Wipe samples were taken from several locations in the compounding room before and after cleaning, and also from the technician’s gloves. These samples were analysed for 5‐fluorouracil and cyclophosphamide concentrations using GC/MS/MS.
Key findings
A total of 284 wipe samples were collected during the study (113 from the manual and 171 from the robotic process). External contamination on the outside of infusion bags was 3.75% for both manual and robotic compounding. For manual compounding, external cross‐contamination occurred on 2.5% of the prepared infusion bags. External cross‐contamination occurred on 1.25% of the infusion bags for the robotic procedure. Inside the compounding room, 9% of the environmental wipe samples were contaminated in case of manual production and 24% for robotic compounding. Since 50% of the contaminated environmental samples for the robotic system were taken after cleaning, the cleaning procedure was extended and parameter setting for cyclophosphamide handling was performed. After this, residual environmental or external contamination was no longer detectable.
Conclusion
Comparison of both preparation methods showed that external (cross‐)contamination of infusion bags was lower using the robotic system. An optimized cleaning procedure showed the best results in environmental contamination for the robot.
Keywords: automated compounding, cytotoxic drugs, environmental contamination, hazardous drugs, robotic preparation, robotic system, surface contamination, wipe sampling
Introduction
Preparing ready‐to‐administer (RTA) cytotoxic drug solutions has a risk of operator exposure to cytotoxic traces in the workplace.1 Contamination may occur directly by contact or indirect via formed aerosols. In case of contamination, dermal exposure to cytotoxic drugs is the most significant route of uptake in the human body.1 Cytotoxic drugs are known to have carcinogenic, mutagenic, and/or teratogenic properties.
To establish the exact relationship between occupational exposure to environmental levels of cytotoxic drugs and unwanted effects is challenging, since different cytotoxic drugs have variable physical properties. Consequently, both skin permeation and the potential of these drugs to vaporize from or to be wiped off surfaces of different materials, will vary. Since no clear threshold values for safe levels of exposure are defined, employers should aim to keep exposure as low as reasonably achievable (ALARA principle).2, 3
To protect healthcare workers against uptake due to contamination, the preparation is centralized in the hospital pharmacy. In general, preparation of RTA cytotoxic drugs is performed manually by pharmacy staff in biological safety cabinets (BSC) or isolators with laminar airflow. The staff wears protective clothing when compounding cytotoxic drugs. For healthcare workers working with cytotoxic drugs, personal protection equipment and working principles have to adhere to strict guidelines in Europe as well as in North‐America.2, 3
In the OLVG hospital in Amsterdam, the robotic system APOTECAchemo (Loccioni Humancare, Angeli di Rosora, Italy) is being introduced for preparing RTA products. RTA products are prefilled injectables that are produced by hospital pharmacy staff to avoid having the nurse manipulate drugs prior to administration. In the robotic system, all high‐risk manipulations are confined within a negative pressure closed chamber, which reduces the operator’s exposure risk as well as repetitive motions and needle stick injuries. When using this robot, healthcare workers may potentially be exposed to hazardous drugs during the cleaning procedure only or through final products that are externally contaminated.
This study was part of the validation of the robot. The robotic system will be used for preparing both cytotoxic drugs and monoclonal antibodies, for example rituximab and infliximab. After preparation, infliximab will be sent to the gastroenterology (internal medicine) department where there are less extensive protective measures against hazardous drugs for the healthcare staff as compared to the oncological wards. Therefore, external cross‐contamination should be assessed. External cross‐contamination is defined as the carry‐over of traces of cytotoxic drugs from one preparation to the outside of subsequent preparations. A list of definitions used in our contamination study is provided in Table 1.
Table 1.
Definitions used in contamination studies
| Term | Definition |
|---|---|
| Environmental contamination | Traces or residue of the compounded drugs are accidentally introduced in or around the compounding area, causing polluted surfaces and thus potential exposure of pharmacy staff |
| External contamination | Traces or residue of the compounded drugs are left on the outside of the final drug container (e.g. an infusion bag or syringe), causing potential exposure of healthcare workers handling the final containers |
| Cross‐contamination | Traces or residue of a compounded drug are transferred to the next or any other subsequent preparation, causing inadvertent exposure of a patient to this drug |
| External cross‐contamination | Traces or residue of a compounded drug are transferred to the outside of the final container of the next or any subsequent preparation. This phenomenon is also named carry‐over and causes potential exposure of healthcare workers handling the final containers |
The assessment of chemical contamination of the environment in preparing RTA cytotoxic drugs is a fundamental requirement to ensure safety for operator, nurse and patient. This is an especially important requirement, when non‐cytotoxic drugs are also prepared in the same system. Surface wiping is the established method for evaluating the extent of environmental contamination in the workplace.2, 3
A previous study by Schierl et al. compared contamination with cyclophosphamide prepared using a manual procedure in a BSC to robotic‐assisted compounding using APOTECAchemo by measuring traces on the outside of prepared infusions. This study concluded that the total cyclophosphamide contamination was lower when the production was performed by the closed and controlled robotic system.4 Another study carried out by Iwamoto et al. focussed on performance and accuracy of the APOTECAchemo. In addition, contamination was tested on four infusion bags. Two of the infusion bags prepared by the robotic system had contamination.5 Until now, no clinical study has been published concerning external cross‐contamination from one product to the next using a robotic system for iv cytotoxic drug preparation.
In the current study, we aimed to assess environmental chemical contamination and external (cross‐)contamination due to drug handling. Compounding by the APOTECAchemo was compared with manual compounding in this respect. The second objective was to validate the cleaning procedure, by determining the environmental contamination before and after cleaning.
Materials and methods
Study site
This study was conducted in the pharmacy of the OLVG hospital in Amsterdam, the Netherlands. The OLVG has 48 inpatient oncology beds and 17 outpatient daybeds (in the ambulatory department). A total number of 13 000 cytotoxic preparations are performed in the hospital pharmacy annually.
Compounding room
The robot is installed in the cleanroom separate from the BSC. The set‐up of the compounding room is shown in Figure 1. The compounding room is GMP class C with a negative pressure of 5Pa. The internal rooms of both the robotic system and the BSC have downward laminar airflow, characterized by a negative pressure gradient and grade A GMP air quality. This corresponds to ISO class 5 in ISO class 7 using ISO 14644‐1 classification. Manual compounding involved a negative pressure technique using spikes (Chemoprotect® spikes, Codan, Lensahn Germany) for both reconstitution and dilution. Spikes are devices that completely prevent aerosol formation and needle prick injuries.
Figure 1.
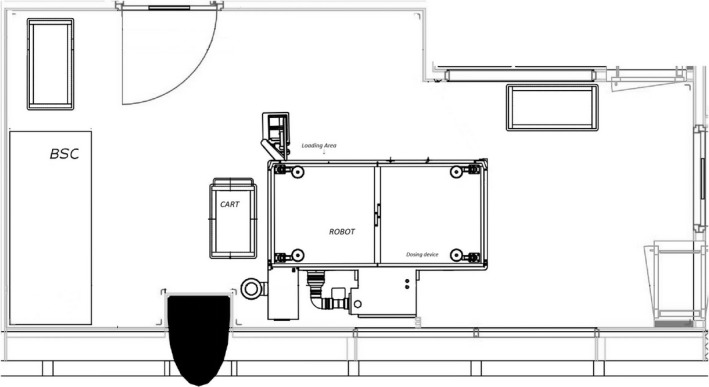
The set‐up of the compounding room with the biological safety cabinet (BSC) and the robotic system.
APOTECAchemo (Figure 2a,b) is a robotic system for automated compounding of sterile injectable drugs such as cytotoxic drugs. The pharmacy staff places the starting materials in the loading area of the robot. This loading area provides access to the rotating carousel for temporary storage of raw materials and finished products. The materials needed for compounding are transferred from the carousel to the working area by a robotic arm. The robotic arm compounds the cytotoxic drugs by handling vials, syringes and infusion bags, as well as reconstituting drugs and solvents with a dosing device. Gravimetric control and photograph recognition ensures the use of the correct drug and the correct dose.
Figure 2.
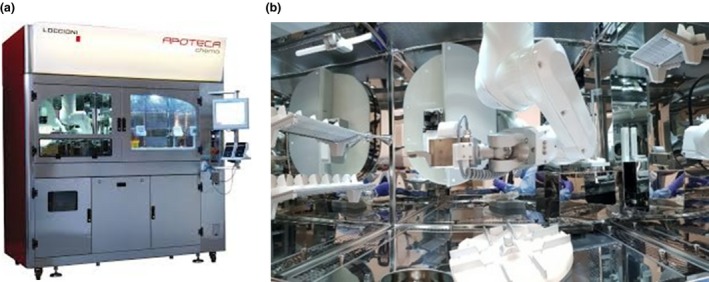
(a) The outside of the APOTECAchemo robot that is used for robotic compounding. (b) The working area of the robot with the robotic arm, the dosing device and gravimetric control. [Color figure can be viewed at http://www.wileyonlinelibrary.com]
Preparations
During the study, 20 5‐fluorouracil in 1000 ml and 20 cyclophosphamide in 50 ml drug products were compounded alternating with forty 250 ml normal saline infusions (as a proxy for infliximab), using APOTECAchemo and manual preparation in BSC’s by several different pharmacy staff members on 3 (manual) to 5 (robot) days. In order to obtain information about external cross‐contamination, the cytotoxic drugs and normal saline are compounded in the following order: 5‐fluorouracil infusion bag, NaCl 0.9% infusion bag, cyclophosphamide infusion bag and NaCl 0.9% infusion bag. Normal saline was chosen as a dummy rather than using infliximab because of the high costs of infliximab. The infliximab preparation was simulated with four empty vials, to each of which 10 ml solvent was added. Next, the content of these vials was diluted with 250 ml saline. Robotic reconstitution of cyclophosphamide was carried out both before compounding and during the preparation process on different days.
The hospital protocol for manual compounding of RTA products includes the use of a protective mat in the BSC. This mat is intended for use on work surfaces of isolators and safety cabinets when compounding and absorbs any spills. The preparations were performed on 3–5 days, representing a usual work week of routine cytotoxic drug preparation, in a crossover design with alternating pharmacy technicians.
Cleaning procedures
The BSC was cleaned according to the pharmacy internal protocol. This included cleaning with clinisteril (isopropylic alcohol) 80% every day. In addition, cleaning with sterile soapy water (Klercide neutral detergent, Ecolab) followed by biocide C (6% hydrogen peroxide) was carried out every week.
APOTECAchemo was cleaned according to the user manual with clinisteril (isopropylic alcohol) 80% at the end of each production day. After the first wipe sampling results were obtained, the cleaning procedure had to be expanded, as will be described in detail in the results section.
Sampling
All types of contamination were measured using wipe samples. Wipe sampling was performed following the validated method of the hospital pharmacy Midden‐Brabant in Tilburg. All samples were taken by a trained pharmacy professional following a validated protocol. The surface was wiped with two absorbent tissues, moistened with 5 ml sodium hydroxide 0.03 m (Avantor Performance Materials Poland S.A., Gliwice, Poland), which were then stored in plastic screw‐top containers. Samples were stored at −8°C before analysis. Surface areas were measured and noted in cm2.
This wipe sampling study was performed after the installation of the robot as part of the qualification process before the go‐live.
Surface sampling to asses environmental contamination
The same surfaces were wiped both before the daily routine cleaning procedure (at the end of production) and after the daily routine cleaning procedure. The sampling locations were selected so that the entire workflow was involved, from the preparation area to the unloading position of the compounded preparations.4
Three spots were chosen for each setting based on risk considerations (Figure 1):
APOTECAchemo.
Stainless steel surface area under the dosing device (compounding area).
Stainless steel surface of the robot, where drug vials and final products are briefly placed to load and unload (loading).
Plastic top of the table outside the system where the final products are kept, one single preparation at a time (table).
BSC
Stainless steel working area inside the BSC under the mat (BSC grill).
Plastic top of table located outside the BSC, where the final products are stored (table).
Plastic tray on which all the drug vials of the entire production cycle are kept, one single preparation at a time (tray).
Glove sampling to asses environmental contamination
The glove from the dominant hand of the pharmacy technician directly involved in drug preparation was sampled. For both manual and robotic compounding, the same brand of gloves (TouchNTuff 73‐701®, Ansell) were used, which are resistant to permeation and comply with NEN‐EN 374‐3:2003. During compounding, gloves were changed every 60 min according to the pharmacy protocol. In case of robotic compounding, gloves were used for loading the robot via the carousel with materials and unloading the carousel with the final product.
Infusion bags sampling to asses external contamination and external cross‐contamination
The total outer surface of prepared infusion bags from BSC and APOTECAchemo (n = 160) were wiped and analysed for the presence of cyclophosphamide and 5‐fluorouracil.
Sample analysis
Sample analysis was carried out by the laboratory of the hospital pharmacy Midden‐Brabant, Tilburg. This laboratory merely used validated laboratory chromatography methods that were sensitive to quantify the intended target values.
The analysis of 5‐fluorouracil and cyclophosphamide was performed using gas chromatography coupled to mass spectroscopy (5890 series II GC, 5971 A SD, Hewlett Packard). Both compounds are quantified in a single analytical procedure. Pre‐treatment of each sample occurred on an ultrasonic bath for 90 min with 45 ml sodium hydroxide (0.03 m). Thereafter, extraction was performed using 5 ml of ethyl acetate, and derivatization was carried out with trifluoroacetic acid (50 µl per 50 µl of extract). Separation was performed with an HP‐5 column (12 m, 0.22 mm internal diameter, 0.33 mm film). Samples were injected by splitless injection at a temperature of 225 °C) with helium as carrier gas (0.8 ml/min), and the temperature over the column had a fixed gradient. Detection took place at 280 °C with an electron ionization detector by selected ion monitoring. Identification was performed with masses of 150, 212, 307 and 309. All substances were of reagent grade. Lower levels of quantification were 0.00013 ng/cm2 for cyclophosphamide and 0.06 ng/cm2 for 5‐fluorouracil.
Criteria
The lower limit of detection (LLOD) during analysis was 10 ng per wipe sample with surfaces wiped between 88 and 1800 cm2.
In the context of the validation of the robotic system, the samples taken from the surfaces were judged according to the national consensus alert and action levels.6, 7 An alert level of 0.1 ng/cm2 is maintained. If the samples taken were above the action limit of 10 ng/cm2, an intervention was made in order to minimize the contamination followed by new sampling.
All detected contamination was included in the analysis.
Statistical analysis
The chemical contamination measured during this study was compared using descriptive statistics in Excel (Microsoft, Redmond, WA, USA).
Results
By each procedure, 20 cyclophosphamide (1300 mg in 50 ml), 20 5‐fluorouracil (900 mg in 1000 ml) and 40 dummy infusion bags (NaCl 0.9%) were compounded. This resulted in a total of 160 preparations, 80 by manual production and 80 by the robot, respectively.
BSC
A total of 113 wipe samples were taken during compounding in the BSC locations on 3 days (Table 2). This resulted in a total of six samples with contamination above the threshold value of 0.1 ng/cm2. Environmental contamination with cyclophosphamide was found on gloves on day 2 and on the cart after cleaning. There was no environmental contamination with 5‐fluorouracil. The total environmental contamination for BSC compounding was 9%. In the external contamination analysis, three of the 80 infusion bags showed detectable amounts of cyclophosphamide (3.75%). This contamination with cyclophosphamide occurred on the same bag (cyclophosphamide), but also external cross‐contamination on saline bags and 5‐fluorouracil bags was observed in 2.5% of the cases.
Table 2.
Results of wipe samples obtained from manual compounding in the biological safety cabinet (BSC)a
| BSC sample |
Day 1 no of contaminated items |
Day 2 no of contaminated items |
Day 3 no of contaminated items |
Total |
|---|---|---|---|---|
| BSC grill BC (n = 4) | – | – | – | – |
| BSC grill AC (n = 4) | – | – | – | – |
| Table BC (n = 4) | – | – | – | – |
| Table AC (n = 4) | 1a (0.64 ng/cm2) | – | 1a (0.73 ng/cm2) | 2/4 |
| Tray BC (n = 4) | – | – | – | – |
| Tray AC (n = 4) | – | – | – | – |
| Gloves (n = 9) | – | 1a (0.39 ng/cm2) | – | 1/9 |
|
Day 1 no of contaminated infusion bags |
Day 2 no of contaminated infusion bags |
Day 3 no of contaminated infusion bags |
Total | |
|---|---|---|---|---|
| Dummy bags (n = 40) | – |
1a (2.01 ng/cm2) |
– | 1/40 |
| 5‐fluorouracil bags (n = 20) | 1a (1.88 ng/cm2) | ‐ | – | 1/20 |
| Cyclophosphamide bags (n = 20) | – |
1a (>10 ng/cm2) |
– | 1/20 |
AC, after cleaning; BC, before cleaning.
Contaminated with cyclophosphamide.
APOTECAchemo
During the 5 days of sampling in the APOTECAchemo, 129 wipe samples were obtained (Table 3). In total, 15 samples were contaminated with cyclophosphamide above the LLOD. There was no contamination with 5‐fluorouracil on any of the wipe samples. For the environmental samples, 24% was contaminated. The highest contamination was recorded below the dosing device. The cyclophosphamide concentrations from these surfaces were generally lower after cleaning. However, substantial residues were still detected after daily routine cleaning under the dosing device. Of the 80 compounded infusion bags with APOTECAchemo, three showed external contamination with cyclophosphamide (3.75%). Among these were two infusion bags containing cyclophosphamide and one containing 5‐fluorouracil. The two infusion bags containing cyclophosphamide showed contamination above the alert limit. In total, external cross‐contamination was observed in 1.25% of the compounded bags.
Table 3.
Results of wipe samples obtained from compounding with APOTECAchemo
| APOTECAchemo sample |
Day 1 (no of contaminated items) |
Day 2 (no of contaminated items) |
Day 3 (no of contaminated items) |
Day 4 (no of contaminated items) |
Day 5 (no of contaminated items) |
Total |
|---|---|---|---|---|---|---|
| Loading area BC (n = 5) | – | – | – | – | – | – |
| Loading area AC (n = 5) | – | – | – | 1a (0.32 ng/cm2) | – | 1/5 |
| Compounding area BC (n = 5) | – | 1a (>2.25 ng/cm2) | 1a (0.67 ng/cm2) | 1a (>2.25 ng/cm2) | – | 3/5 |
| Compounding area AC (n = 5) | – | 1a (0.60 ng/cm2) | 1a (0.33 ng/cm2) | 1a (0.86 ng/cm2) | 1a (>2.25 ng/cm2) | 4/5 |
| Table BC (n = 5) | – | 1a (0.09 ng/cm2) | – | – | 1a (0.02 ng/cm2) | 2/5 |
| Table AC (n = 5) | – | – | – | – | 1a (0.02 ng/cm2) | 1/5 |
| Gloves (n = 19) | 1a (0.06) | – | – | – | – | 1/19 |
|
Day 1 (no of contaminated infusion bags) |
Day 2 (no of contaminated infusion bags) |
Day 3 (no of contaminated infusion bags) |
Day 4 (no of contaminated infusion bags) |
Day 5 (no of contaminated infusion bags) |
Total | |
|---|---|---|---|---|---|---|
| Dummy bags (n = 40) | – | – | – | – | – | – |
| 5‐fluorouracil bags (n = 20) | 1a (0.04 ng/cm2) | – | – | – | – | 1/20 |
| Cyclophosphamide bags (n = 20) | – | – | 1a (12.5 ng/cm2) | 1a (0.125 ng/cm2) | – | 2/20 |
AC, after cleaning; BC, before cleaning.
Contaminated with cyclophosphamide
Because manual preparation contained the use of a chemo mat and extra extensive weekly cleaning, the original parameters were in favour of manual compounding. Therefore, the cleaning procedure was altered and new manufacturing with the APOTECAchemo took place during 3 days. A mat was placed underneath the robotic arm and a weekly cleaning with sterile soapy water (Klercide neutral detergent, Ecolab), equal to the BSC cleaning protocol, was added. In addition, accurate setting of the parameters was performed to control the handling of cyclophosphamide. This ensured a more precise and safe preparation process.
APOTECAchemo after intervention
After the change in cleaning procedure, another 42 samples were obtained during three consecutive days of production with the APOTECAchemo (producing 18 infusion bags). Five samples (21%) from the compounding room were found to be contaminated with either cyclophosphamide or 5‐fluorouracil, demonstrating environmental contamination still occurred. Interestingly, no environmental contamination was found in the samples taken after cleaning. This resulted in a decline in environmental contamination remaining after cleaning, from 40% (6/15) to 0%. In addition, the wipe samples that were taken from the infusion bags showed that no external contamination or external cross‐contamination occurred after the intervention (Table 4).
Table 4.
Results of wipe samples obtained from compounding with APOTECAchemo after the intervention in the cleaning procedurea
| APOTECAchemo sample | Day 1 (no of contaminated items) | Day 2 (no of contaminated items) | Day 3 (no of contaminated items) | Total |
|---|---|---|---|---|
| Loading area BC (n = 3) | – | 1b (0.13 ng/cm2) | – | 1/3 |
| Loading area AC (n = 3) | – | – | – | – |
| Compounding area BC (n = 3) | 1a (5.97 ng/cm2) | – | 1a (8.09 ng/cm2) | 2/3 |
| Compounding area AC (n = 3) | – | – | – | – |
| Table BC (n = 3) | 1b (0.47 ng/cm2) | – | – | 1/3 |
| Table AC (n = 3) | – | – | – | – |
| Gloves (n = 6) | – | 1a (0.17 ng/cm2) | – | 1/6 |
|
Day 1 (no of contaminated infusion bags) |
Day 2 (no of contaminated infusion bags) |
Day 3 (no of contaminated infusion bags) |
Total | |
|---|---|---|---|---|
| Dummy bags (n = 9) | – | – | – | – |
| 5‐fluorouracil bags (n = 5) | – | – | – | – |
| Cyclophosphamide bags (n = 4) | – | – | – | – |
AC, after cleaning; BC, before cleaning.
Contaminated with cyclophosphamide.
Contaminated with 5‐fluorouracil.
Discussion
This study measured the external contamination on the outside of infusion bags and the environmental contamination on contacted surfaces, for both manual and robotic compounding of cytotoxic drugs. Our findings indicate that the rate of external contamination on infusion bags was equal when comparing manual compounding with robotic compounding. External cross‐contamination (from one preparation to the next) was lower for robotic compounding both in number of contaminated samples as well as in the observed amount of cyclophosphamide.
However, environmental wipe samples taken in the compounding room initially showed better results for manual compounding. Because the manual RTA compounding initially used a more extensive cleaning method, including a chemo mat, the initial conditions for manual compounding were better. For robotic RTA compounding, the initial cleaning procedure consisted of cleaning once daily using alcohol 80%. Since most environmental samples that showed contamination for robotic compounding (50%) were taken after cleaning, the cleaning procedure was extended. After this, environmental contamination following cleaning was no longer detectable.
Under the dosing device in the APOTECAchemo, high environmental contamination levels were found before cleaning (even higher than after manual preparation inside the BSC). Possibly this results from an inefficient or insufficient cleaning protocol but could also be caused by the transfer of the liquids by the robot in the preparation process itself. The very low level of external contamination and external cross‐contamination on the infusion bags, as well as the low level of environmental contamination on gloves and other surfaces outside of the robot, confirms the efficacy of the airflow system in the robot in removing aerosols generated during compounding.
Our results imply smaller contamination rates for both manual and robotic compounding than previous research did1, 4, 8, 9, 10, 11, 12. Earlier studies suggested that variability in surface contamination could be associated with different methods of drug preparation, which can influence work techniques, that is proximity of the storage area relative to the preparation area, as well as the size of the compounding area where drugs are handled. Also, the handling of externally contaminated drug vials is of influence.13, 14, 15 In OLVG, drug vials are wiped with ethanol to remove external cytotoxic residues before entering the robotic system or BSC. Furthermore, the fact that the pharmacy staff was aware of the undertaking of this study, even though they were instructed to conduct their work as usual, could have led to a more vigorous cleaning than on normal workdays and thus have biased the results in a positive way.
This is the first study that did not only investigate environmental contamination, but also carry over or external cross‐contamination. A crossover design was used to rule out a potential influence of the operating pharmacy technician. Moreover, the compounding of a large amount of infusion bags was carried out in order to rule out chance findings. However, some limitations of our study should be taken into account when interpreting the results. Firstly, only two cytotoxic drugs were measured. Cyclophosphamide and 5‐fluorouracil were chosen because of their high preparation numbers and known carcinogenicity, and because they consist of a concentrated solution and a freeze‐dried powder that has to be dissolved first, thus representing the two most common compounding activities. Theoretically, other cytotoxic drugs could vaporize more easily. However, these two drugs are commonly used in studies investigating contamination and are therefore appropriate for comparison with results from other studies.4, 5, 7, 8, 9, 10, 11, 12, 13
Secondly, it should be considered that the wipe samples taken from identical spots before and after cleaning, could possibly remove the compounds still present on the surface. The wipe samples taken from the APOTECAchemo before the change in cleaning procedure contradicts this hypothesis, as the spots with contamination were still found contaminated after cleaning.
In the OLVG hospital, spikes are used for manual compounding. This is the golden standard in the Netherlands. In other countries, the so‐called closed systems are used. In the manual compounding group, lower contamination rates were found compared to other studies, including studies with closed‐system devices. This suggests that the use of these spikes does not result in a positive bias for the robotic procedure.4, 8, 9, 10, 11, 12, 13, 14, 15, 16
Thirdly, this study was carried out in one hospital, so one should be careful extrapolating these results to other settings, especially, since the size and design of the working area is of high impact.
Finally, the recovery of the wipe samples is inherent to the work‐up, the used materials and the person performing the wipe sampling. As in other studies, this was not assessed. Wipe efficiencies from each sampled surface were not taken into consideration, a 100% wipe recovery was assumed; therefore, the concentrations reported in this study may under‐represent the actual amount of cyclophosphamide and 5‐fluorouracil on the sampled surfaces.9, 10, 14
Robotic compounding of parenterals is increasing rapidly in hospital pharmacies worldwide. Further research on contamination is needed studying other pharmaceutical forms than infusion bags, such as elastomeric pumps or syringes, and should also include a wider panel of preparation equipment.
Conclusions
This study shows environmental contamination risks for specific locations in the compounding room. These include the cart for the manual technique and the dosing area in the APOTECAchemo. Consequently, we recommend an extensive cleaning procedure in order to reflect a safe practice, as opposed to the initially recommended cleaning programme for the robot.
Furthermore, external contamination and external cross‐contamination on infusion bags were assessed comparing both the manual and robotic technique, showing better results for the robotic technique especially after optimization of the cleaning protocol. The findings of our study support the conclusion that (non‐) cytotoxic monoclonal antibodies can be prepared safely by the same robotic system used for cytotoxic drugs without a higher risk of external cross‐contamination.
Declarations
Conflict of interest
All authors have declared that no competing interests exist.
Funding
This research had not been funded by any specific grant from any funding agency in the public, commercial or not‐for‐profit sectors.
Acknowledgement
We are grateful to D. Paolucci for fruitful discussions and critically reading the manuscript.
Authors’ contributions
MC designed the study. AWB and THG were involved in planning and supervised the sample collection. AWB and THG processed the experimental data and performed the analysis of data. AWB drafted the manuscript with input from all authors. All authors had complete access to the study data that support the publication.
Ethical statement
This research article does not need ethical improvement from a medical ethical committee since it does not involve research in human subjects.
References
- 1. Fransman W, Vermeulen R, Kromhout H. Dermal exposure to cyclophosphamide in hospitals during preparation, nursing and cleaning activities. Int Arch Occup Environ Health 2005; 78: 403–412. [DOI] [PubMed] [Google Scholar]
- 2. Directive 2004/37/EC of the European parliament and of the council on the protection of workers from the risks related to exposure to carcinogens or mutagens at work (Sixth individual Directive within the meaning of Article 16(1) of Council Directive 89/391/EEC). December 2017. https://osha.europa.eu/en/legislation/directives/directive-2004-37-ec-carcinogens-or-mutagens-at-work (accessed 20 June 2018).
- 3. NIOSH (2004). Preventing occupational exposures to antineoplastic and other hazardous drugs in health care settings. Publication Number 2004‐165. https://www.cdc.gov/niosh/docs/2004-165/pdfs/2004-165.pdf (accessed 2 December 2018).
- 4. Schierl R et al Environmental contamination by cyclophosphamide preparation: comparison of conventional manual production in biological safety cabinet and robot‐assisted production by APOTECAchemo. J Oncol Pharm Pract 2016; 22: 37–45. [DOI] [PubMed] [Google Scholar]
- 5. Iwamoto T et al Performance evaluation of the compounding robot, APOTECAchemo, for injectable anticancer drugs in a Japanese hospital. J Pharm Health Care Sci 2017; 3: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Crul M, Simons‐Sanders K. Carry‐over of antineoplastic drug contamination in Dutch hospital pharmacies. J Oncol Pharm Pract 2018; 24: 483–489. [DOI] [PubMed] [Google Scholar]
- 7. van Balen P et al (2009) Meetstrategie en werkinstructie voor het nemen van veeg‐ proeven in ziekenhuizen (Dutch). NVvA, NVVK, NVZA. http://www.betermetarbo.nl (accessed 2 December 2018).
- 8. Sessink PJ et al Environmental contamination, product contamination and workers exposure using a robotic system for antineoplastic drug preparation. J Oncol Pharm Pract 2015; 21: 118–127. [DOI] [PubMed] [Google Scholar]
- 9. Yoshida J et al Use of a closed system device to reduce occupational contamination and exposure to antineoplastic drugs in the hospital work environment. Ann Occup Hyg 2009; 53: 153–160. [DOI] [PubMed] [Google Scholar]
- 10. Hon C, et al Antineoplastic drug contamination of surfaces throughout the hospital medication system in Canadian hospitals. J Occup Environ Hyg 2013; 10: 374–383. [DOI] [PubMed] [Google Scholar]
- 11. Siderov J, Kirsa S, McLauchlan R. Surface contamination of cytotoxic chemotherapy preparation areas in Australian hospital pharmacy departments. J Pharm Pract Res 2009; 39: 117. [Google Scholar]
- 12. Siderov J, Kirsa S, McLauchlan R. External surface contamination of cytotoxic admixtures: caveat emptor. J Pharm Pract Res 2011; 41: 181–82. [Google Scholar]
- 13. Touzin K et al Pilot study comparing the efficacy of two cleaning techniques in reducing environmental contamination with cyclophosphamide. Ann Occup Hyg 2010; 54: 351–359. [DOI] [PubMed] [Google Scholar]
- 14. Crauste‐Manciet S et al Environmental contamination with cytotoxic drugs in healthcare using positive air pressure isolators. Ann Occ Hyg 2005; 49: 619–628. [DOI] [PubMed] [Google Scholar]
- 15. Sugiura S et al Risks to health professionals from hazardous drugs in Japan: a pilot study of environmental and biological monitoring of occupational exposure to cyclophosphamide. J Oncol Pharm Pract 2011; 17: 14–19. [DOI] [PubMed] [Google Scholar]
- 16. Siderov J, Kirsa S, McLauchlan R. Reducing workplace cytotoxic surface contamination using a closed‐system drug transfer device. J Oncol Pharm Pract 2010; 16: 19–25. [DOI] [PubMed] [Google Scholar]


