Abstract
Background
In a Phase 3 study, amyotrophic lateral sclerosis (ALS) patients experienced significantly less physical functional decline with 24‐week edaravone vs placebo, followed by open‐label treatment for an additional 24 weeks.
Methods
Outcome (the change in ALS Functional Rating Scale–Revised, ALSFRS‐R, from baseline) was projected for placebo patients through 48 weeks and compared with 48‐week edaravone or 24‐week edaravone after switching from placebo.
Results
A total of 123 patients received open‐label treatment (65 edaravone‐edaravone; 58 placebo‐edaravone). The projected ALSFRS‐R decline for placebo from baseline through week 48 was greater than for 48‐week edaravone (P < .0001). For patients switching from placebo to edaravone, ALSFRS‐R slope approached that of continued edaravone for 48 weeks. ALSFRS‐R decline did not differ between actual and projected edaravone through week 48.
Conclusions
Compared with placebo, these analyses suggest that edaravone is beneficial in ALS patients even after 6 mo of receiving placebo, and efficacy is maintained for up to 1 year.
Keywords: ALSFRS‐R, chronic, disease progression, functional decline, linear regression, oxidative stress
Short abstract
Abbreviations
- ALS
amyotrophic lateral sclerosis
- ALSAQ‐40
ALS Assessment Questionnaire
- ALSFRS‐R
ALS Functional Rating Scale–Revised
- FDA
United States Food and Drug Administration
- FVC
forced vital capacity
1. INTRODUCTION
Amyotrophic lateral sclerosis (ALS) is a progressive and fatal neuromuscular disease that affects approximately 5 of every 100,000 individuals.1, 2 The disease is characterized by the degeneration of motor neurons in the brain and spinal cord.1 Approximately half of ALS patients die within 30 mo of symptom onset and the majority do not survive beyond 5 years.3 Currently, riluzole and edaravone are the only United States Food and Drug Administration (FDA)–approved treatment options.4, 5, 6, 7 There are other therapeutic agents for symptom management, and devices and multidisciplinary care strategies are used with the goals of providing palliative care, prolonging survival, improving quality of life, and maintaining the patient's independence for as long as possible. In the absence of a cure for ALS, additional treatment options that have demonstrated long‐term benefits would be desirable.8
Edaravone is thought to function as a free‐radical scavenger that is hypothesized to protect motor neurons from free‐radical and oxidative‐stress damage.9 ALS patients taking edaravone had a significantly smaller decline in scores of the ALS Functional Rating Scale–Revised (ALSFRS‐R), as compared with placebo patients, in a phase 3, randomized, double‐blind study (Study 19; MCI186‐19).10 In the open‐label 24‐week extension period, which aimed to explore the longer‐term efficacy and safety of edaravone, patients receiving edaravone had maintained benefit throughout the 48 weeks, with no new or cumulative safety concerns.11 Secondary end points, including ALS assessment questionnaire (ALSAQ‐40), forced vital capacity (FVC), and Modified Norris Scale score, were significantly in favor of edaravone compared with placebo.
Given the proven efficacy and commercial availability of edaravone, practical and ethical issues limit the feasibility of conducting a prospective, randomized, placebo‐controlled assessment of the efficacy of edaravone over 48 weeks. Furthermore, previous longer‐term efficacy data were limited to patients who remained on edaravone for 48 weeks or 24 weeks after switching from placebo, ie, comparison with placebo during the additional 24 weeks was not possible. Therefore, to better understand the long‐term efficacy of edaravone therapy in ALS patients, a post‐hoc analysis from the open‐label study was conducted, comparing the effect of edaravone for 48 weeks with that of placebo for 24 weeks followed by edaravone for 24 weeks, or for projected placebo from baseline through week 48.
2. METHODS
2.1. Study 19 (MCI186‐19) study design
Study 19 was a randomized, double‐blind, parallel‐group, placebo‐controlled study. The details of study methodology, ethical study conduct, patient selection (inclusion and exclusion criteria), end points, and prospective statistical analyses have been previously described in detail (http://clinicaltrials.org: NCT01492686).10, 11 Briefly, patients were randomized to either edaravone (60 mg) or placebo for 24 weeks followed by a 24‐week open‐label extension period. The primary efficacy end point was the change in ALSFRS‐R score from baseline to the end of week 24.
2.2. Post‐hoc assessment
Multiple linear regression analysis was performed to project the results from the placebo‐controlled, double‐blind phase from baseline through week 48 for all treatment groups. Progression rate in the treatment group originally randomized to edaravone was estimated for the first 24 weeks and was used to predict progression for the next 24 weeks; this was compared with actual change in ALSFRS‐R in patients who initially received edaravone for 24 weeks and continued on open‐label edaravone from weeks 24 to 48 (edaravone‐edaravone group). For the group originally randomized to placebo, rate of progression for the first 24 weeks was used to predict progression for the following 24 weeks; this was compared with the actual progression in this group, who were switched to edaravone from weeks 24 to 48 (placebo‐edaravone group). Thus, at the 48‐week time point, projected placebo progression rate could be compared with the actual progression in the placebo‐edaravone group as well as the edaravone‐edaravone group, and the projected progression in the edaravone‐edaravone group compared with actual progression.
2.3. Post‐hoc analysis statistics
The relationship between the change in ALSFRS‐R and time was visualized with linear regression analysis for each treatment group. A linear mixed model with covariates of linear slope of time, treatment, baseline value, and interaction of time and treatment, and random intercept, was used to estimate the treatment difference in the change in ALSFRS‐R at week 48.
3. RESULTS
3.1. Patient disposition and baseline characteristics
A total of 137 patients were initially randomized to receive either edaravone (n = 69) or placebo (n = 68) in the double‐blind phase. The demographics and baseline characteristics were well balanced between treatment groups at baseline, except for male gender and Japan ALS Severity Classification.10, 11 Briefly, the mean age was approximately 60 years, and the mean duration of disease at study enrollment was 1 year. The mean (SD) ALSFRS‐R score for patients in the edaravone and placebo groups, respectively, was 41.9 (2.4) and 41.8 (2.2) at baseline. A total of 127 patients completed the double‐blind period, and 123 patients (65 patients from the edaravone group [edaravone‐edaravone] and 58 patients from the placebo group [placebo‐edaravone]) continued into the active‐treatment period. From these groups, 53 edaravone‐edaravone and 40 placebo‐edaravone patients completed the open‐label treatment period.11 Six patients died in the study; all were considered “not reasonably possible” with regard to a relationship to study drug.11
3.2. Post‐hoc analysis
The projected change in ALSFRS‐R score from baseline through week 48 was significantly greater for placebo than edaravone (Figure 1). This was also evident when measuring the rate of decline in ALSFRS‐R score through week 48 (slope of the graphs), which was significantly greater for the projected placebo than for the projected edaravone group (Figure 2). The projected placebo group also showed significantly greater change (decline) in ALSFRS‐R score from baseline through week 48 than the actual edaravone‐edaravone treatment group (patients originally randomized to edaravone and participating in the open‐label period) (Figure 1). The corresponding rates of decline in ALSFRS‐R are shown in Figure 2. The rate of decline in ALSFRS‐R score for placebo‐edaravone (originally randomized to placebo and received open‐label, 24‐week edaravone) was significantly less than that of projected placebo while appearing similar to that of edaravone patients (Figure 2). Comparing the actual edaravone‐edaravone group with the projected edaravone estimate, there was no difference in either the change in ALSFRS‐R scores from baseline through week 48 (Figure 1) or the rate of ALSFRS‐R decline (Figure 2).
Figure 1.
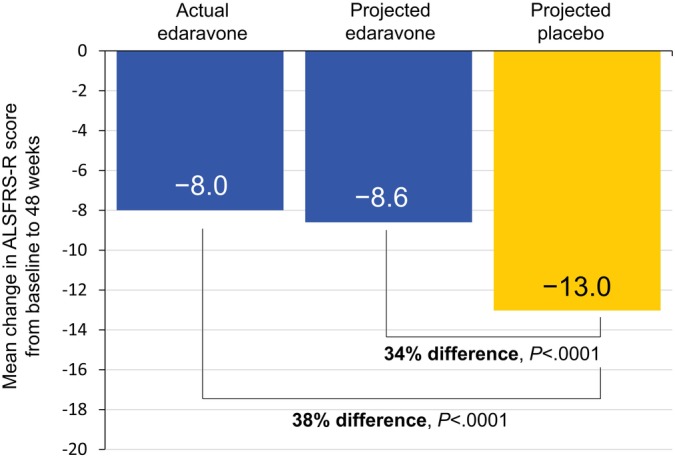
Changes in ALSFRS‐R scores from baseline to week 48. Mean change in ALSFRS‐R scores from baseline to week 48 for measured values from actual treatment as well as projected estimates [Color figure can be viewed at http://wileyonlinelibrary.com]
Figure 2.
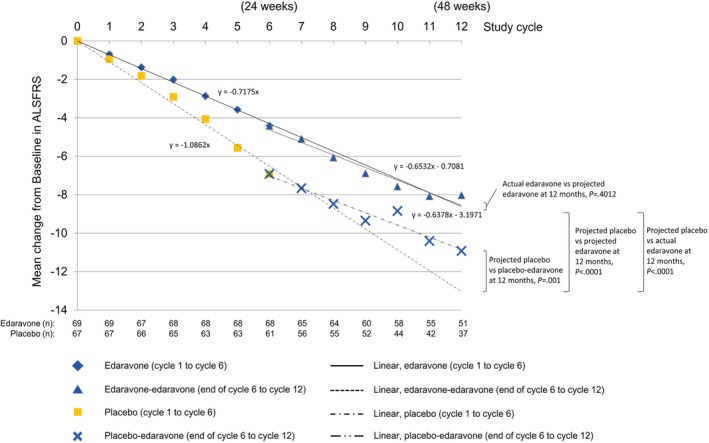
Change in ALSFRS‐R scores for actual treatment and projected estimates from baseline through week 48. Changes in ALSFRS‐R scores are shown for patients who received edaravone or placebo through week 24 and their estimated projection through week 48. Also shown are the changes in ALSFRS‐R score for patients who either continued on edaravone or switched from placebo to edaravone at the end of week 24 through week 48. The number of patients in each study arm at each time point is listed at the bottom of the graph. Linear regression equations are in ALSFRS‐R units/month [Color figure can be viewed at http://wileyonlinelibrary.com]
4. DISCUSSION
In this post‐hoc analysis, the change in ALSFRS‐R score was shown to remain significantly lower for the edaravone treatment group than for the placebo group for up to week 48, regardless of whether a comparison was made between projected estimates (a 34% difference) or between actual edaravone‐edaravone and projected placebo (a 38% difference). With a significant difference between actual edaravone and placebo already observed at week 24 (−5.0 vs −7.5, least‐mean square difference = 2.5, P = .0013; a 33% difference),10 these results suggested that the benefits of edaravone over placebo were maintained throughout the 48 weeks of treatment. Additionally, functional decline appeared to be lower for placebo patients who switched to edaravone at week 24 than for those who would have remained on placebo through week 48 (−10.9 vs −13.0).11 Notably, only approximately 15% of placebo patients met the original inclusion criteria (ALSFRS‐R item score of ≥2, FVC ≥80%, and disease duration ≤2 years) before starting open‐label edaravone at week 24 (unpublished data), suggesting potential benefits with edaravone in patients who may have more disease progression. The slope of decline in weeks 24 to 48 for the placebo‐edaravone group also appeared to be similar to patients who had been randomized to edaravone initially and who continued on open‐label edaravone for weeks 24 to 48 (edaravone‐edaravone), supporting treatment benefit of edaravone in ALS patients who begin receiving treatment later in their disease course.12
4.1. Limitations of the study
The linear regression model and linear mixed model were used to investigate the long‐term efficacy and safety of edaravone. While this approach showed consistent results between actual treatment vs projected treatment, careful consideration is needed when interpreting clinical implications from these post‐hoc analyses. The linear regression and linear mixed model also assumed that change from baseline in ALSFRS‐R linearly decreases with time. A previous report of the open‐label 24‐week extension study showed that the ALSFRS‐R score changed almost linearly from baseline through 48 weeks in the edaravone‐edaravone treatment group.11 With this linearity assumption, the change from baseline in ALSFRS‐R between week 24 and week 48 was predicted by extrapolating the results of week 4 to week 24. While this appeared to be a reasonable assumption, there is some evidence suggesting that functional decline may not be linear, with the early and late phases showing differing rates of decline.13, 14, 15 Further exploration of the clinical development program data, including other end points, is needed to better address this topic.
5. CONCLUSION
Findings from these post‐hoc analyses suggested a possible continued treatment effect of edaravone and maintenance of long‐term efficacy for up to 1 year. Ongoing and planned studies will further our understanding of the long‐term safety and efficacy of edaravone in patients with ALS.
CONFLICT OF INTEREST
J.S., T.H.P., E.P.P., and M.W.P. are consultants for MTPA. S.A. is an employee of MTPA. W.A. is a former employee of MTPA. S.L. is an employee of MTDA. J.Z. is under contract with MTPA.
ETHICAL PUBLICATION STATEMENT
We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
ACKNOWLEDGMENTS
p‐value communications provided support for technical writing, editing, and publication assistance, and was funded by Mitsubishi Tanabe Pharma America, Inc. (MTPA). Study 19 was funded by Mitsubishi Tanabe Pharma Corporation (MTPC) and this post‐hoc analysis was funded by MTPA. MTPA and MTPC did not have any input in the manuscript beyond the input provided by the authors listed here.
Shefner J, Heiman‐Patterson T, Pioro EP, et al. Long‐term edaravone efficacy in amyotrophic lateral sclerosis: Post‐hoc analyses of Study 19 (MCI186‐19). Muscle Nerve. 2020;61:218–221. 10.1002/mus.26740
Funding information Mitsubishi Tanabe Pharma America, Inc.
REFERENCES
- 1. Brown RH Jr, Al‐Chalabi A. Amyotrophic lateral sclerosis. N Engl J Med. 2017;377(16):1602. [DOI] [PubMed] [Google Scholar]
- 2. Mehta P, Kaye W, Raymond J, et al. Prevalence of amyotrophic lateral sclerosis ‐ United States, 2014. MMWR Morb Mortal Wkly Rep. 2018;67(7):216‐218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Talbot K. Motor neuron disease: the bare essentials. Pract Neurol. 2009;9(5):303‐309. [DOI] [PubMed] [Google Scholar]
- 4.Radicava® (edaravone injection) [package insert]. Jersey City, NJ: Mitsubishi Tanabe Pharma Corporation; August 2017.
- 5.Rilutek® (riluzole) [package insert]. Bridgewater, NJ: sanofi‐aventis U.S. LLC; November 2012.
- 6. U.S. Food and Drug Administration . Drugs@FDA: FDA Approved Drug Products. 2019. https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=020599. Accessed September 18, 2019.
- 7. U.S. Food and Drug Administration. FDA approves drug to treat ALS. 2017. https://www.fda.gov/news-events/press-announcements/fda-approves-drug-treat-als. Accessed September 18, 2019.
- 8. Zarei S, Carr K, Reiley L, et al. A comprehensive review of amyotrophic lateral sclerosis. Surg Neurol Int. 2015;6:171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Brooks BR, Jorgenson JA, Newhouse BJ, Shefner JM, Agnese W. Edaravone in the treatment of amyotrophic lateral sclerosis: efficacy and access to therapy ‐ a roundtable discussion. Am J Manag Care. 2018;24(9 suppl):S175‐S186. [PubMed] [Google Scholar]
- 10. Writing Group on Behalf of the Edaravone ALS 19 Study Group . Safety and efficacy of edaravone in well defined patients with amyotrophic lateral sclerosis: a randomised, double‐blind, placebo‐controlled trial. Lancet Neurol. 2017;16(7):505‐512. [DOI] [PubMed] [Google Scholar]
- 11. Writing Group on Behalf of the Edaravone ALS 19 Study Group . Open‐label 24‐week extension study of edaravone (MCI‐186) in amyotrophic lateral sclerosis. Amyotroph Lateral Scler Frontotemporal Degener. 2017;18(suppl 1):55‐63. [DOI] [PubMed] [Google Scholar]
- 12. Takei K, Tsuda K, Takahashi F, Palumbo J. Post‐hoc analysis of open‐label extension period of study MCI186‐19 in amyotrophic lateral sclerosis. Amyotroph Lateral Scler Frontotemporal Degener. 2017;18(suppl 1):64‐70. [DOI] [PubMed] [Google Scholar]
- 13. Thakore NJ, Lapin BR, Pioro EP, Pooled Resource Open‐Access ALSCTC . Trajectories of impairment in amyotrophic lateral sclerosis: insights from the Pooled Resource Open‐Access ALS Clinical Trials cohort. Muscle Nerve. 2018;57(6):937‐945. [DOI] [PubMed] [Google Scholar]
- 14. Proudfoot M, Jones A, Talbot K, Al‐Chalabi A, Turner MR. The ALSFRS as an outcome measure in therapeutic trials and its relationship to symptom onset. Amyotroph Lateral Scler Frontotemporal Degener. 2016;17(5–6):414‐425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gordon PH, Cheng B, Salachas F, et al. Progression in ALS is not linear but is curvilinear. J Neurol. 2010;257(10):1713‐1717. [DOI] [PubMed] [Google Scholar]


