Abstract
It is unclear whether use of electronic nicotine delivery systems (ENDS) precedes cigarette smoking initiation, relapse, and/or quitting. Healthcare systems with electronic health records (EHRs) provide unique data to examine ENDS use and changes in smoking.
We examined the incidence of ENDS use (2012-2015) based on clinician documentation and tested whether EHR documented ENDS use is associated with twelve-month changes in patient smoking status using a matched retrospective cohort design. The sample was Kaiser Permanente Northern California (KPNC) patients aged ≥12 with documented ENDS use (N=7926); 57% were current smokers, 35% former smokers, and 8% never-smokers. ENDS documentation incidence peaked in 2014 for current and former smokers and in 2015 for never-smokers. We matched patients with documented ENDS use to KPNC patients without documented ENDS use (N=7926) on age, sex, race/ethnicity, and smoking status.
Documented ENDS use predicted the likelihood of smoking in the following year. Among current smokers, ENDS use was associated with greater odds of quitting smoking (OR=1.17, 95%CI=1.05-1.31). Among former smokers, ENDS use was associated with greater odds of smoking relapse (OR=1.53, 95%CI=1.22-1.92). Among never-smokers, ENDS use was associated with greater odds of initiating smoking (OR=7.41, 95%CI=3.14-17.5). The overall number of current smokers at 12 months was slightly higher among patients with (N=3,931) versus without (N=3,850) documented ENDS use.
Results support both potential harm reduction of ENDS use (quitting combustibles among current smokers) and potential for harm (relapse to combustibles among former smokers, initiation for never-smokers).
INTRODUCTION
Cigarette smoking, including exposure to secondhand smoke, is linked to more than 520,000 deaths in the US each year, and smoking-related illnesses result in nearly $170 billion in direct medical costs and $156 billion attributable to lost work productivity annually.1 Alternatives to smoking, such as electronic nicotine delivery systems (ENDS), which include electronic cigarettes, have become increasingly popular in recent years.2–5 Regulated as a tobacco product by the U.S. Food and Drug Administration (FDA) since May 2016,6 data on the potential of ENDS for harm enhancement and harm reduction are limited. Given the substantial harms of combustible cigarettes, ENDS are thought to be safer nicotine delivery products. Similarly, there is some evidence of health benefits among smokers who fully switch to vaping ENDS.7–10 Further, ENDS may help some smokers cut down on or quit cigarette smoking, although dual use remains common.11 There is concern, however, that these products may serve as a gateway to smoking initiation12–14 and encourage relapse among those who have recently quit smoking.15,16 Simulation-based models indicate that the potential net effects of ENDS use on population health depend on a number of factors, including the impact of ENDS on cigarette smoking initiation and cessation, ENDS toxicity, and patterns of use.17,18
As patients increasingly turn to their healthcare providers for information about ENDS,19–22 research is critically needed to increase the surveillance of their use in healthcare settings.23 To fill this gap in the literature, we analyzed data from a large, integrated healthcare delivery system to describe the incidence of patients’ ENDS use based on clinician documentation in the EHR from 2012-2015 and to test whether ENDS use was associated with changes in patients’ smoking status (i.e., starting, quitting, relapsing) in the subsequent year using a matched retrospective cohort design.
METHODS
Setting
Kaiser Permanente Northern California (KPNC) is a nonprofit, multi-specialty healthcare delivery system providing comprehensive health services to >4 million members24 and covering ~40% of the region’s commercially insured population.25 KPNC provides integrated medical and behavioral health treatment and is a recognized leader in establishing tobacco treatment quality-of-care standards.26 Members are racially and socio-economically diverse, and highly representative of the population in the geographic catchment area.27 KPNC institutional review board approval was obtained for this study.
Study Participants
Our study population comprised 3,680,549 patients aged ≥12 with KPNC membership and ≥1 clinical contact between January 1, 2012 and December 31, 2015. Within this population, N = 8,256 patients had ≥1 valid instance of documented ENDS use in the EHR during this timeframe.
For the matched-case analyses examining changes in smoking status over the subsequent year, we matched each patient with documented ENDS use to a patient without documented ENDS use on age, sex, race/ethnicity, and smoking status in the same month and year as the first documented ENDS use. For matched patients without documented ENDS use who had multiple recorded smoking statuses at different times during the study period, we randomly sampled one smoking status before employing the matching algorithm to ensure that each patient was matched only once. Of documented ENDS users, 646 of the 664 never-smokers (97%), 2,752 of the 2,857 former smokers (96%), and 4,528 of the 4,735 current smokers (96%) were successfully matched to patients without documented ENDS use, resulting in samples of n=7,926 documented ENDS users and n=7,926 matched patients without documented ENDS use.
Measures
Identification of Documented ENDS Use.
We used natural language processing techniques to identify instances of ENDS use, based on clinicians’ documentation in the tobacco-use free text field within the social history section of the Epic EHR. We created a set of specific “shorthand” text strings (i.e., a series of characters one would expect to find within the keywords, such as “e-cig,” “electronic,” or “vape”) and used the SAS INDEX function (substring matches) to capture suspected variations of ENDS keywords in the tobacco comments. When we found new, potential flags, we manually reviewed the full comments for inclusion, alternative candidate strings, and exclusionary criteria (Appendix 1). We included only keywords that referenced ENDS (e.g., electronic cigarette but not electronic signature). We included the first (earliest) documented ENDS reference for each patient to estimate the number of new documented ENDS users in each year (2012-2015).
Smoking Status.
KPNC has several systems in place to ensure that smoking status is routinely asked about and documented, including an EHR prompt that triggers staff to ask about patients’ smoking status and management oversite of staff documentation of patient smoking. Overall, tobacco screening rates are about 90%, with the highest rates in primary care. We obtained patient-reported smoking status (i.e., current, former, or never-smoker) from the EHR.26 Prior studies support the validity of EHR-based smoking status data.28–30 KPNC clinicians began consistently documenting “former smoking” status in 2012, and our study included years 2012-2015. We included the smoking status from the same encounter as the earliest ENDS documentation. If no smoking status was associated with that encounter (19%), we included the last recorded smoking status preceding ENDS documentation.
We categorized current smokers as “quitting” if they had ≥1 “former smoker” status during the year following ENDS documentation. Quitting did not have to be sustained in future records to be coded as “quitting.” We categorized former smokers and never-smokers as starting smoking if they had ≥1 “current smoker” status during the year following ENDS documentation.
Demographic Variables.
Data on patient sex, race/ethnicity, age, and median household income were from the EHR. Neighborhood median household income was geocoded from census data using patients’ addresses and was dichotomized as 1 (≤ median household salary) or 0 (>median household salary).
Comorbidity Diagnoses.
We identified the most common psychiatric disorders (depressive disorders, anxiety disorders, attention deficit hyperactivity disorders, bipolar spectrum disorders, substance use disorders and psychotic disorders) in our sample based on current ICD-9 and ICD-10 diagnoses recorded in the EHR during year after ENDS use documentation.
Tobacco Cessation Medications.
Use of tobacco cessation medication was determined by dispensation of any FDA-approved tobacco cessation medication (i.e., nicotine replacement therapy (NRT) gum, lozenge, inhaler, patch, nasal spray, or varenicline) from a KPNC pharmacy in the year following earliest recorded ENDS use, or the date of the matched recorded smoking status. Because bupropion is commonly prescribed to treat depressive disorders and not solely as a smoking cessation aid, it was not included in analyses. Data were extracted from the KPNC Pharmacy Information Management System database, which contains all data related to prescriptions dispensed at a KPNC pharmacy.
Analysis
Analyses were conducted in 2016 using SAS© software, version 9.3. We first calculated the annual incidence rate of ENDS-use documentation in the EHR, defined as the number of newly documented ENDS users per 1,000 KPNC patients with a documented clinical encounter in a given year, from 2012-2015. We plotted annual incidence rates by smoking status to visualize the relative increases in documentation among current smokers, former smokers, and never-smokers.
We calculated the percentage of ENDS users and matched non-users of each smoking status with a 12-month change in smoking status (i.e., quit smoking, initated smoking, or relapsed) and used chi-square tests to assess statistical significance. We then estimated the association between ENDS-use documentation and a 12-month change in smoking status using matched odds ratios from conditional logistic regression models, stratified by smoking status. We also computed the total number of current smokers at 12 months among ENDS users and matched non-users.
For all analyses, we adjusted for median income and the presence of a psychiatric or substance use disorder in the year following documented ENDS use. For analyses with current and former smokers, we also adjusted for use of tobacco cessation medications in the year following documented ENDS use.
For analyses with former smokers, we adjusted for “time since quitting smoking” in the event that newly documented ENDS users quit cigarette smoking more recently than those without documented ENDS use and may be at greater risk of relapsing to smoking. For documented ENDS users, we calculated time since quitting as the difference between their most recent quit date (i.e., date of the most recent “former smoker” status that was immediately preceded by a “current smoker” status) and the date of their first documented ENDS use. For patients without documented ENDS use, we calculated time since quitting as the difference between their most recent quit date and the date of their reported status as a former smoker that was used to match each patient with an ENDS user. We dichotomized this variable into “quit within a 6-month period” or “quit more than 6 months ago.”
If a patient did not have a recorded smoking status in the year following the index date, we considered his or her smoking status to be unchanged. As this was a potential cause of outcome misclassification, we conducted a probabilistic bias analysis to assess the degree to which this misclassification could affect our estimates and conclusions31,32 (Appendix 2).
RESULTS
Each year, current smokers, and former smokers to a lesser extent, had higher rates of ENDS-use documentation relative to never-smokers. The number of patients with ENDS-use documentation peaked in 2014 for current (n = 1,723; 9.21 per 1,000 current smokers) and former smokers (n = 1,043; 2.19 per 1,000 former smokers) and in 2015 for never-smokers (n = 242; 0.18 per 1,000 never-smokers) (Figure 1). At the time of first ENDS-use documentation, 57% of patients were current smokers, 35% were former smokers, and 8% were never-smokers. The majority of patients with ENDS-use documentation were non-Hispanic White and male. Compared with current and former smokers, never-smokers with documented ENDS use were younger and more likely to be Hispanic (Table 1).
Figure 1.
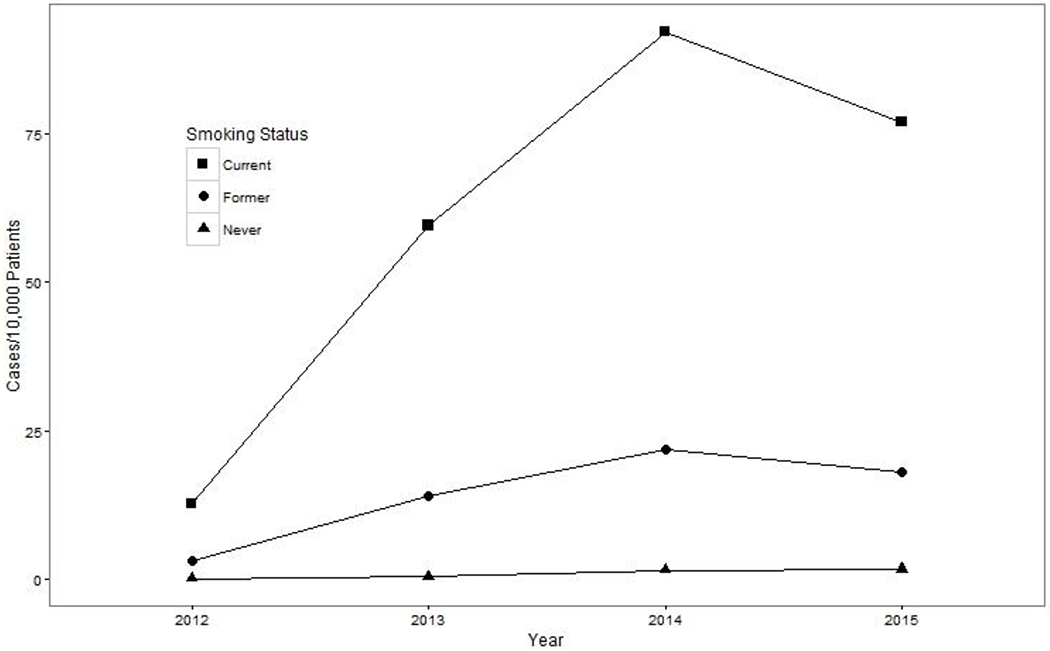
Unadjusted Annual Incidence Rate of ENDS-Use Documentation by Patient Smoking Status, Kaiser Permanente Northern California, 2012-2015
Table 1.
Characteristics of ENDS Users at the Time of First ENDS-Use Documentation (N = 7,926), Kaiser Permanente Northern California, 2012-2015
| Variable | Smoking status at first documented ENDS use | |||
|---|---|---|---|---|
| Current | Former | Never | ||
| N (%) | ||||
| Total | 4528 | 2752 | 646 | |
| Age | ||||
| 12-17 | 16 (0.35) | 10 (0.36) | 22 (3.4) | |
| 18-24 | 665 (15) | 250 (9.1) | 228 (35) | |
| 25-44 | 1885 (42) | 1280 (47) | 300 (46) | |
| 45-64 | 1545 (34) | 959 (35) | 81 (13) | |
| 65+ | 417 (9.2) | 253 (9.2) | 15 (2.3) | |
| Sex | ||||
| Male | 2543 (56) | 1478 (54) | 342 (53) | |
| Female | 1985 (44) | 1274 (46) | 304 (47) | |
| Race | ||||
| White | 3015 (67) | 1929 (70) | 390 (60) | |
| Hispanic | 479 (11) | 237 (8.6) | 113 (17) | |
| Black | 270 (6.0) | 148 (5.4) | 31 (4.8) | |
| Asian/Hawaiian Pacific Islander | 557 (12) | 306 (11) | 80 (12) | |
| Multiracial/American Indian | 207 (4.6) | 132 (4.8) | 32 (5.0) | |
| Median Household Income | 68,333 | 68,690 | 73,298 | |
| Tobacco Cessation Medication Use1 | 415 (9.2) | 160 (5.8) | NA | |
| Year of first documented use | ||||
| 2012 | 221 (4.9) | 134 (4.9) | 26 (4.0) | |
| 2013 | 1073 (24) | 647 (24) | 81 (13) | |
| 2014 | 1723 (38) | 1043 (38) | 242 (37) | |
| 2015 | 1511 (33) | 928 (34) | 297 (46) | |
| Psychiatric Disorders1 | ||||
| Depression | 972 (21) | 589 (21) | 93 (14) | |
| Anxiety | 917 (20) | 572 (21) | 114 (18) | |
| Bipolar Disorder | 233 (5.2) | 154 (5.6) | 28 (4.3) | |
| Psychotic Disorders | 161 (3.6) | 60 (2.2) | 9 (1.4) | |
| ADHD2 | 128 (2.8) | 96 (3.5) | 28 (4.3) | |
| Any | 1,611 (36) | 971 (35) | 176 (27) | |
| Substance Use Disorders1 | ||||
| Alcohol | 306 (6.8) | 148 (5.4) | 31 (4.8) | |
| Drug | 336 (7.4) | 182 (6.6) | 33 (5.1) | |
| Any | 537 (12) | 283 (10) | 52 (8.1) | |
Notes.
Recorded in the year following ENDS use documentation;
ADHD = Attention Deficit Hyperactivity Disorder.
ENDS Use and Changes in Smoking Status among Matched Patients
Results from bivariate matched case analyses (using Chi-square tests) comparing patients with and without documented ENDS use (N=7926 each group) indicated that among initially identified current smokers, 23% (n = 1,028) of ENDS users and 19% (n = 863) of matched non-users (based on EHR documentation) reported quitting smoking during the following year (p<.0001 for group comparison). Among the initially identified former smokers, 14% (n = 382) of ENDS users and 7% (n =179) of matched non-users reported returning to smoking in the following year (p<.0001). Among initially identified never-smokers, 8% (n = 49) of ENDS users and 1% (n = 6) of non-users reported initiating smoking in the next year (p<.0001). Current smokers with documented ENDS use (9%) did not differ from matched smokers without documented ENDS use (8%) in their utilization of tobacco cessation medications during the subsequent year. However, among former smokers, those with documented ENDS use (6%) had significantly greater prevalence of tobacco cessation medication use during the subsequent year relative to matched former smokers without documented ENDS use (2%) (p<.01).
Results from multivariable conditional logistic regression analyses demonstrated that among current smokers, ENDS users had 16% greater odds of quitting smoking in the next year relative to matched current smokers who were non-users during the same time period (OR=1.16, 95% CI =1.04-1.29). Among former smokers, ENDS users had 53% greater odds of relapsing to smoking in the following year compared to non-users during the same time period (OR=1.53, 95% CI=1.22-1.92). Finally, among never-smokers, ENDS users had greater than seven times the odds of becoming current smokers in the next year relative to non-users during the same time period (OR = 7.41, 95% CI=3.14-17.5). Odds ratios and 95% confidence intervals for the covariates for these regression analyses are provided in Appendix 3. Greater median household income and psychiatric or substance use disorders were associated with a greater likelihood of initiating smoking among never-smokers. Greater median household income, having a psychiatric disorder, tobacco cessation medication use, and being quit for less than 6 months were associated with a greater likelihood of smoking relapse among former smokers. Psychiatric and substance use disorders and tobacco cessation medication use were associated with greater odds of quitting smoking among current smokers. Probabilistic bias analysis showed that the effects of misclassification due to imperfect follow-up data were likely to be minimal, resulting in odds ratio changes of 2% or less (Appendix 2).
At 12 months, there were 3,931 current smokers among the patients with documented ENDS use compared with 3,850 current smokers among matched patients without documented ENDS use: a difference of 83 current smokers with documented ENDS use.
DISCUSSION
Incidence rates of patients’ ENDS use based on clinician documentation in the EHR have increased dramatically in recent years, likely reflecting both surges in patient uptake of ENDS and greater patient-provider discussions about ENDS.23,33 As expected, current smokers—and former smokers, to a lesser extent—had higher incidence rates of ENDS-use documentation each year than never-smokers. This finding likely reflects both greater use of ENDS and greater self-disclosure or patient-clinician discussion about ENDS use among current and former smokers relative to never-smokers. From 2012 to 2015, the number of patients with ENDS-use documentation peaked in 2014 for current and former smokers, and in 2015 for never-smokers. The peak among current and former smokers in 2014 may be explained by the simultaneous implementation of the Affordable Care Act and the influx of high-priority populations for tobacco control (e.g., men, adults aged 19-34, low-income enrollees) into the healthcare system in 2014 via the California insurance exchange and Medicaid expansion.34–37 While our text-based assessment of ENDS-use documentation is novel and represents how the incidence of ENDS documentation has increased over time, results substantially understimate the true incidence of ENDS use.23 Standard ENDS documentation practices and discrete EHR fields for mandatory ENDS documentation that would allow for an enhanced estimate of ENDS incidence and prevalence are recommended.
Notably, among current smokers, documented ENDS use was associated with increased odds of quitting smoking in the next year, even after adjusting for utilization of tobacco cessation medications. ENDS use may directly help some smokers quit smoking.11 In the literature, there are observational findings that smokers who use e-cigarettes are more likely to make a quit attempt and to succeed in quitting,38,39 particularly with a greater number of days of ENDS use in the past month.39 There also are studies indicating that ENDS use is associated with a lower likelihood of quitting.40 The potential for harm reduction may depend on patterns of ENDs use (e.g., intensity/frequency of use). Clinician inquiry and ENDS documentation in the EHR should quantify ENDs use. Most existing research on ENDS for quitting smoking is observational and, therefore, lacking the empirical strength of randomization that controls for inherent differences between exposure groups. ENDS users tend to have greater motivation to quit smoking and report more recent quit attempts.41,42 It may be that ENDS use (and discussion of ENDS with a healthcare provider) is a relevant marker for motivation to quit as well as receptivity to clinical intervention. Because quitting did not have to be sustained in future records to be coded as “quitting”, the potential for harm reduction in the long-run may be overestimated if patients did not quit for good. Notably, having a psychiatric or substance use disorder was also associated with greater odds of quitting smoking. These patients may have greater interactions with the healthcare system, providing more opportunities to receive clinician advice to quit and more opportunities to have a quit documented in the EHR.
Conversely, ENDS use was associated with increased odds of relapsing to smoking among former smokers, even after adjusting for tobacco cessation medication use. ENDS use may directly increase the risk for smoking relapse (e.g., via reinstating nicotine addiction, mimicking hand-to-mouth smoking behavior, re-normalizing smoking) or may be a marker for relapse risk. That is, former smokers who are at greater risk for smoking relapse may also be more likely to try ENDS or to talk to their healthcare providers about their ENDS use. ENDS use was associated with a greater likelihood of using tobacco cessation medication among former smokers, suggesting that this group may be fundamentally different and more actively attempting to stave off relapse. Randomized controlled trials are needed to further explore these associations.
Of concern, although the incidence of documented ENDS use was low among never-smokers, documented ENDS use was associated with more than eight times greater odds of initiating smoking in the next year. These findings are consistent with prior longitudinal survey research indicating that ENDS use is associated with subsequent initiation of cigarette smoking among youth and young adult never-smokers in the US.12,13,43–45 ENDS are the most commonly used tobacco product among US youth. Addressing this use, the US Surgeon General has issued a call to action to reduce ENDS use and related harms among young people.15 Interestingly, nearly two-thirds of never-smokers in our study with documented ENDS use were age 25 or older, suggesting that the increased risk for smoking initiation among ENDS users may not be limited to youth and young adults. Again, while the association could be causal, never-smokers who use ENDS, and those who choose to discuss ENDS use with their clinicians, may have already been at elevated risk for smoking initiation regardless of ENDS use. Additional research is needed to evaluate these possibilities and illuminate the potential harms and benefits of ENDS use.
Limitations and Strengths
Interpretations of study findings are limited by those data available in the EHR. Smoking status was self-reported and subject to social desirability bias. We are unable to determine the frequency or quantity with which patients used ENDS, duration of ENDS use, type of product used, level of nicotine, reasons for ENDS use, or motivation to initiate or quit smoking. These important details could be collected as part of more comprehensive routine ENDS inquiry and inclusion of discrete ENDS data elements in the EHR.46 The prevalence and quit rate of ENDS use cannot be reliably estimated from the EHR; thus, we focused on the earliest reference per person. It is possible that some matched patients without documented ENDS use were ENDS users; however, we would expect that patients with versus without documented ENDS use are more likely to be regular vs. single trial users. Further, if ENDS users are misclassified as non-users, this would bias results toward the null (i.e., no difference between groups). Our measure of quitting smoking focused on successful quit attempts in the short term. Patients may have returned to smoking over time. While ENDS documentation was associated with smoking status changes, we cannot determine the causal nature of these associations. Lastly, we lack data on ENDS use at 12 months, which prohibits our application of recently developed formulas17 to estimate the net health effects of ENDS use in our patient population. Major study strengths included examining smoking transitions in a large sample and wide age span of patients (ages 12+ years) that included current, former, and never smokers. This allowed us to examine cessation, relapse, and smoking initiation relative to ENDS use over a 12-month period and calculate changes in the number of smokers among patients with and without documented ENDS use. Notably, prior research has been more circumscribed, typically examining only adult current smokers or young never smokers. A novel use of KPNC’s rich longitudinal data, we developed a comprehensive matching algorithm and utilized a cohort design, thereby minimizing residual confounding in assessing smoking status changes. We also conducted a bias analysis to examine the expected validity of our findings despite outcome misclassification, increasing confidence in our results.
CONCLUSIONS
Patients’ use of ENDS, based on healthcare providers’ documentation in the EHR, has increased dramatically in recent years. Large, integrated healthcare delivery systems with EHRs collect longitudinal clinical data that can reveal novel insights in use patterns of emerging drug products over time. To achieve this, healthcare systems should implement more consistent and standardized documentation of the behavior (i.e., ENDS use) in the EHR with required discrete fields. Results from this matched retrospective cohort study corroborate earlier observational research and provide new evidence suggesting both the harm-reduction potential of ENDS among current smokers and the potential for harm (initiation and relapse to smoking) for never-smokers and former smokers. However, we cannot infer causality from these data.
The overall impact of ENDs on smoking behavior at a population level will depend on whether the number of current smokers who successfully quit smoking with ENDS is greater than the number of former and never-smokers who relapse or initiate smoking following ENDS use. In this study, the overall number of current smokers was slightly higher at 12 months among patients with versus without documented ENDS use.Given our study limitations in defining quits (not necessarily sustained) and detecting ENDS use (likely under-detected in young people, i.e., never smokers), we anticipate this differential may be even greater and believe further investigation is warranted. Worth considering, are the clinical, regulatory and policy strategies that could influence the balance e.g., by extending Tobacco 21 policies to ENDS, banning kid-friendly ENDS flavors, restricting marketing/advertising of ENDS to youth, and banning indoor use. Further research among nationally representative samples is needed to determine how safe ENDS are relative to cigarettes and whether the long-term effects of ENDS on smoking cessation, relapse, and initiation represent a net benefit or a net risk for population health.
Supplementary Material
Table 2.
Associations between documented ENDS use and change in smoking status within one-year follow-up
| Smoking Status at Baseline | Change in Smoking Status N (%) | Unadjusted OR (95% CI) | Adjusted OR (95% CI) |
|---|---|---|---|
| Current Smokers (N=9,056) | |||
| ENDS Users | 1028 (22.7) | 1.26 (1.13, 1.40) | 1.161,2 (1.04, 1.29) |
| Matched Non-Users | 863 (19.1) | ||
| Former Smokers (N=5,504) | |||
| ENDS Users | 382 (13.9) | 2.35 (1.94, 2.85) | 1.531,2,3 (1.22, 1.92) |
| Matched Non-Users | 179 (6.5) | ||
| Never-Smokers (N=1,292) | |||
| ENDS Users | 49 (7.6) | 8.17 (3.50, 19.06) | 7.411 (3.14-17.46) |
| Matched Non-Users | 6 (0.93) |
Notes. Change in smoking status is defined as quitting smoking for current smokers, and initiating smoking among former and never-smokers. ENDS users were matched to non-ENDS users on sex, race/ethnicity, exact age, and month and year of concordant smoking status documentation.
Adjusted for median household income and psychiatric/substance use disorder diagnoses in the year following ENDS use documentation.
Adjusted for use of tobacco cessation medication (Nicotine replacement therapy or varenicline).
Adjusted for time since quitting smoking.
Acknowledgments
We thank Alyce A. Adams for her feedback on an earlier version of this manuscript.
This study was supported by the Permanente Medical Group Delivery Science Rapid Analysis Program and a grant from the Tobacco-Related Disease Research Program (24XT-0008).
All authors contributed to this work. KCYW designed the study, drafted the manuscript and guided interpretation of the results. DK and BF participated in the data extraction, analysis and editing of the paper. RF, AT and JJP assisted in study design, advised on analysis methods, and assisted with editing of the paper.
Dr. Prochaska’s tobacco-related research is funded by the National Cancer Institute (R01CA204356), the National Heart, Lung and Blood Institute (R01HL117736), and the State of California Tobacco-Related Disease Research Program (24RT-0035 and 25IR-0032). She has consulted to Pfizer, which makes smoking cessation medications, and has been an expert witness for plaintiffs’ counsel in court cases against tobacco companies.
Footnotes
All other authors declare no conflict of interest.
REFERENCES
- 1.Xu X, Bishop EE, Kennedy SM, Simpson SA, Pechacek TF. Annual healthcare spending attributable to cigarette smoking: an update. Am J Prev Med. 2015;48(3):326–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McMillen RC, Gottlieb MA, Shaefer RM, Winickoff JP, Klein JD. Trends in electronic cigarette use among U.S. adults: use is increasing in both smokers and nonsmokers. Nicotine Tob Res. 2015;17(10):1195–1202. [DOI] [PubMed] [Google Scholar]
- 3.Pearson JL, Richardson A, Niaura RS, Vallone DM, Abrams DB. e-Cigarette awareness, use, and harm perceptions in US adults. Am J Public Health. 2012;102(9):1758–1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Regan AK, Promoff G, Dube SR, Arrazola R. Electronic nicotine delivery systems: adult use and awareness of the ‘e-cigarette’ in the USA. Tob Control. 2013;22(1):19–23. [DOI] [PubMed] [Google Scholar]
- 5.King BA, Patel R, Nguyen KH, Dube SR. Trends in awareness and use of electronic cigarettes among US adults, 2010–2013. Nicotine Tob Res. 2015;17(2):219–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deeming tobacco products to be subject to the Federal Food, Drug, and Cosmetic Act, as amended by the Family Smoking Prevention and Tobacco Control Act; restrictions on the sale and distribution of tobacco products and required warning statements for tobacco products. Fed. Regist 2016;81(90):28974–291106. https://www.govinfo.gov/content/pkg/FR-2016-05-10/pdf/2016-10685.pdf Accessed May 10 [PubMed] [Google Scholar]
- 7.Farsalinos KE, Polosa R. Safety evaluation and risk assessment of electronic cigarettes as tobacco cigarette substitutes: a systematic review. Ther Adv Drug Saf. 2014;5(2):67–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McRobbie H, Bullen C, Hartmann-Boyce J, Hajek P. Electronic cigarettes for smoking cessation and reduction. Cochrane Database Syst Rev. 2014;12:CD010216. [DOI] [PubMed] [Google Scholar]
- 9.Farsalinos K, Cibella F, Caponnetto P, et al. Effect of continuous smoking reduction and abstinence on blood pressure and heart rate in smokers switching to electronic cigarettes. Intern Emerg Med. 2016;11(1):85–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nolan M, Leischow S, Croghan I, et al. Feasibility of Electronic Nicotine Delivery Systems in Surgical Patients. Nicotine Tob Res. 2016;18(8):1757–1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hartmann-Boyce J, McRobbie H, Bullen C, Begh R, Stead LF, Hajek P. Electronic cigarettes for smoking cessation. Cochrane Database Syst Rev. 2016;9:CD010216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Primack BA, Soneji S, Stoolmiller M, Fine MJ, Sargent JD. Progression to Traditional Cigarette Smoking After Electronic Cigarette Use Among US Adolescents and Young Adults. JAMA Pediatr. 2015;169(11):1018–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leventhal AM, Strong DR, Kirkpatrick MG, et al. Association of electronic cigarette use with initiation of combustible tobacco product smoking in early adolescence. JAMA. 2015;314(7):700–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chatterjee K, Alzghoul B, Innabi A, Meena N. Is vaping a gateway to smoking: a review of the longitudinal studies. Int J Adolesc Med Health. 2016. [DOI] [PubMed] [Google Scholar]
- 15.U.S. Department of Health and Human Services. E-Cigarette Use Among Youth and Young Adults. A Report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion;2016. [Google Scholar]
- 16.Bhatnagar A, Whitsel LP, Ribisl KM, et al. Electronic cigarettes: a policy statement from the American Heart Association. Circulation. 2014;130(16):1418–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kalkhoran S, Glantz SA. Modeling the health effects of expanding e-cigarette sales in the United States and United Kingdom: A Monte Carlo analysis. JAMA Intern Med. 2015;175(10):1671–1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levy DT, Borland R, Villanti AC, et al. The application of a decision-theoretic model to estimate the public health impact of vaporized nicotine product initiation in the United States. Nicotine Tob Res. 2017;19(2):149–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nickels AS, Warner DO, Jenkins SM, Tilburt J, Hays JT. Beliefs, Practices, and Self-efficacy of US Physicians Regarding Smoking Cessation and Electronic Cigarettes: A National Survey. Nicotine Tob Res. 2016. [DOI] [PubMed] [Google Scholar]
- 20.Steinberg MB, Giovenco DP, Delnevo CD. Patient-physician communication regarding electronic cigarettes. Prev Med Rep. 2015;2:96–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.El-Shahawy O, Brown R, Elston Lafata J. Primary Care Physicians’ Beliefs and Practices Regarding E-Cigarette Use by Patients Who Smoke: A Qualitative Assessment. Int J Environ Res Public Health. 2016;13(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kandra KL, Ranney LM, Lee JG, Goldstein AO. Physicians’ attitudes and use of e-cigarettes as cessation devices, North Carolina, 2013. PLoS One. 2014;9(7):e103462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Young-Wolff KC, Klebaner D, Folck B, et al. Do you vape? Leveraging electronic health records to assess clinician documentation of electronic nicotine delivery system use among adolescents and adults. Prev Med. 2017;105:32–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaiser Permanente. Fast facts about Kaiser Permanente. Kaiser Permanente Share; 2011; http://xnet.kp.org/newscenter/aboutkp/fastfacts.html Accessed June 24, 2016. [Google Scholar]
- 25.Terhune C Report: Kaiser tops state health insurance market with 40% share Los Angeles Times January 29, 2013; http://articles.latimes.com/2013/jan/29/business/la-fi-mo-health-insure-market-20130129. [Google Scholar]
- 26.Goldstein A, Gee S, Mirkin R. Tobacco dependence program: a multifaceted systems approach to reducing tobacco use among kaiser permanente members in northern california. Perm J. 2005;9(2):9–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Selby JV, Smith DH, Johnson ES, Raebel MA, Friedman GD, McFarland BH. Kaiser Permanente Medical Care Program In: Strom BL, ed. Pharmacoepidemiology 4th ed New York: Wiley; 2005:241–259. [Google Scholar]
- 28.McGinnis KA, Brandt CA, Skanderson M, et al. Validating smoking data from the Veteran’s Affairs Health Factors dataset, an electronic data source. Nicotine Tob Res. 2011;13(12):1233–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McVeigh KH, Newton-Dame R, Chan PY, et al. Can electronic health records be used for population health surveillance? Validating population health metrics against established survey data. EGEMS (Wash DC). 2016;4(1):1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marston L, Carpenter JR, Walters KR, et al. Smoker, ex-smoker or non-smoker? The validity of routinely recorded smoking status in UK primary care: a cross-sectional study. BMJ Open. 2014;4(4):e004958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fox MP, Lash TL, Greenland S. A method to automate probabilistic sensitivity analyses of misclassified binary variables. Int J Epidemiol. 2005;34(6):1370–1376. [DOI] [PubMed] [Google Scholar]
- 32.Fox MP, Lash TL, Greenland S. Sensitivity Analysis SAS Macro. bias.analysis 2009; https://sites.google.com/site/biasanalysis/sensmac. [Google Scholar]
- 33.Brown-Johnson CG, Burbank A, Daza EJ, et al. Online patient-provider e-cigarette consultations: Perceptions of safety and harm. Am J Prev Med. 2016;51(6):882–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McAfee T, Babb S, McNabb S, Fiore MC. Helping smokers quit--opportunities created by the Affordable Care Act. N Engl J Med. 2015;372(1):5–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reichard J Survey shows drop in California’s uninsured, but with new cost concerns. Washington Health Policy Week in Review August 4, 2014; http://www.commonwealthfund.org/publications/newsletters/washington-health-policy-in-review/2014/aug/aug-4-2014/survey-shows-drop-in-californias-uninsured.
- 36.Satre DD, Altschuler A, Parthasarathy S, Silverberg MJ, Volberding P, Campbell CI. Implementation and operational research: Affordable Care Act implementation in a california health care system leads to growth in HIV-positive patient enrollment and changes in patient characteristics. J Acquir Immune Defic Syndr. 2016;73(5):e76–e82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Covered California. Covered California’s historic first open enrollment finishes with projections exceeded; agents, counselors, community organizations and county workers credited as reason for high enrollment in California. 2014; http://news.coveredca.com/2014/04/covered-californias-historic-first-open.html.
- 38.Zhu SH, Zhuang YL, Wong S, Cummins SE, Tedeschi GJ. E-cigarette use and associated changes in population smoking cessation: evidence from US current population surveys. BMJ. 2017;358:j3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Levy DT, Yuan Z, Luo Y, Abrams DB. The relationship of e-cigarette use to cigarette quit attempts and cessation: Insights from a large, nationally representative U.S. survey [published online August 31, 2017]. Nicotine & Tobacco Research; 2017;doi: ntx166, 10.1093/ntr/ntx166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kalkhoran S, Glantz SA. E-cigarettes and smoking cessation in real-world and clinical settings: a systematic review and meta-analysis. Lancet Respir Med. 2016;4(2):116–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Biener L, Hargraves JL. A longitudinal study of electronic cigarette use among a population-based sample of adult smokers: association with smoking cessation and motivation to quit. Nicotine Tob Res. 2015;17(2):127–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schoenborn CA, Gindi RM. Electronic cigarette use among adults: United States, 2014. NCHS Data Brief. 2015(217):1–8. [PubMed] [Google Scholar]
- 43.Wills TA, Knight R, Sargent JD, Gibbons FX, Pagano I, Williams RJ. Longitudinal study of e-cigarette use and onset of cigarette smoking among high school students in Hawaii. Tob Control. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barrington-Trimis JL, Urman R, Berhane K, et al. E-Cigarettes and Future Cigarette Use. Pediatrics. 2016;138(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Unger JB, Soto DW, Leventhal A. E-cigarette use and subsequent cigarette and marijuana use among Hispanic young adults. Drug Alcohol Depend. 2016;163:261–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Winden TJ, Chen ES, Wang Y, Sarkar IN, Carter EW, Melton GB. Towards the standardized documentation of e-cigarette use in the electronic health record for population health surveillance and research. AMIA Jt Summits Transl Sci Proc. 2015;2015:199–203. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


