Abstract
Aims and Objectives
Dipeptidyl peptidase‐4 inhibitor (DPP4i) is widely used for the treatment of type 2 diabetes (T2DM) in several countries such as Japan, whereas biguanide (BG; mostly metformin) is recommended as a first‐line antidiabetic medication in many countries according to evidence mainly from Western countries. Although previous studies reported that DPP4i may be more efficacious for East Asians, direct comparisons of effectiveness and cost between DPP4i and BG have never been conducted in East Asia.
Methods
We extracted claims and medical check‐up data (observation period from January 2010 to March 2016) of adult patients under 70 years old with T2DM who received DPP4i or BG as first‐line antidiabetic drugs. Changes in HbA1c and BMI before and 2 years after the first prescription and annual cost of antidiabetic medication during the second year were compared between the DPP4i and BG groups.
Results
We extracted 1034 patients who received DPP4i and 365 patients who received BG as the first antidiabetic medication (male sex, 83.0% and 84.9%; HbA1c (mean [SD]), 7.7 [1.4]% and 7.9 [1.4]%; BMI, 26.6 [4.5] kg/m2 and 28.1 [4.3] kg/m2). After propensity score matching, changes in HbA1c and BMI were not significantly different between the groups (HbA1c, −0.67% vs −0.80% [P = .28]; BMI, −0.3 kg/m2 vs −0.4 kg/m2 [P = .42]). Annual cost of antidiabetic drugs was significantly higher in the DPP4i group (US $458.7 vs 273.3 [P < .001]). Many patients continued each medication at the follow‐up visit (78.3% of the DPP4i group and 73.7% of the BG groups).
Conclusions
The first antidiabetic prescription for the patient was mostly continued thereafter. BG may be recommendable as the first‐line medication for patients with T2DM, especially for middle‐aged, male population with greater BMI. It is worth addressing the discrepancy between practice in Japan and that recommended in international guidelines.
Keywords: dipeptidyl peptidase‐4 inhibitor, metformin, type 2 diabetes
Abbreviations
- BG
biguanide
- DPP4i
dipeptidyl peptidase‐4 inhibitor
- SGLT2i
sodium glucose transporter 2 inhibitor
- T2DM
type 2 diabetes
- UKPDS
the UK Prospective Diabetes Study
1. INTRODUCTION
Diabetes is one of the fastest‐growing diseases in patients worldwide, and the cost of treatment has become a social burden. An ideal medical treatment for diabetes should have proven efficacy, safety, and cost‐effectiveness. According to treatment guidelines in many Western countries, biguanide (BG, especially metformin) is recommended as an initial medical therapy for type 2 diabetes (T2DM),1 because of its efficacy, effectiveness, and superior cost advantage. Metformin's cardiovascular benefits, however, have been reappraised recently, especially since its evidence was mainly derived from the UK Prospective Diabetes Study (UKPDS) results.2 As for Asian countries, priority of metformin use is different. In some East Asian countries, for example, Japan and Korea, metformin is not ranked as first‐line antidiabetic medicine based on the paucity of evidence among East Asian patients. On the other hand, in other East Asian countries, such as China and Malaysia, metformin is ranked as first‐line medicine probably following the recommendations of the Western and International Diabetes Federation guidelines.3, 4, 5, 6
Compared with Caucasians, studies indicated that the characteristics of patients with diabetes in East Asian populations included lower BMI and less insulin secretion7, 8; and it was also suggested that DPP4i are more efficacious for lowering glucose levels in East Asian populations.9 Presumably due to their perceived efficacy and effectiveness in glycaemic control and safety profiles, DPP4i's currently are prescribed for initial treatment of T2DM in Japan much more frequently than metformin.10 This prescription tendency might be justified since the treatment guidelines for T2DM in Japan recommend selecting an appropriate oral or injectable medication based on the pathophysiological background of and desired treatment goals for a given patient, not specifying any oral antidiabetic medication for first‐line use.6 Nonetheless, whether DPP4i is the most suitable first‐line medication for Japanese patients with T2DM as metformin is in Western countries remains unclear.
Evidence regarding comparisons between these two drugs with respect to effectiveness and cost in clinical practice is scarce. Moreover, studies comparing between BG and newly approved drugs, such as dipeptidyl peptidase‐4 inhibitor (DPP4i) or sodium glucose transporter 2 inhibitor (SGLT‐2i), have been sparse and be projected not to be performed since metformin is regarded as a de facto initial medical treatment for T2DM in many countries. It is important to confirm which drug is more appropriate as first‐line oral medication in Japan and other East Asian countries as in the Western countries.
We investigated the difference in effectiveness and cost between BG and DPP4i using claims and medical check‐up data in Japan. This information may be useful for revising current guidelines and may be applicable for other East Asian countries.
2. METHODS
This is a secondary data analysis using claims and medical check‐up data in Japan provided by the JMDC Inc. Japan has a system of universal health coverage, with almost 3500 insurers. The characteristics of a person (eg, age, region, and job) almost uniquely determine the insurance to participate. Employees and their dependents are insured by employer‐sponsored health insurances. JMDC contracted with these insurers and collected their claims data to develop the JMDC Claims Database.11 Employers are required to conduct medical check‐ups for all employees to maintain employees' health. In addition, the Japanese government asks insurers to conduct the annual “special” health check‐ups in order to screen and prevent metabolic syndrome for those insured (both employees and their dependents) aged 40 to 74 years, at health care centre or hospitals/clinics. These two types of health check‐ups are often combined when both are applicable. Results of medical check‐ups are captured by each insurer. According to the JMDC, participation rates of medical check‐ups among employees aged 40 or older were quite higher (approximately 80%), whereas those among all of those insured including dependents and employees aged less than 40 were about 37%.
The design of data collection has been described previously.11 In brief, the JMDC collected claims and medical check‐up data for more than three million persons safely and anonymously. Because the subjects were employees or employers and their dependents, they included more men, healthy people, and young people (<75 years old).
2.1. Study design
The design of the current study is shown in Figure 1. The specific aim of this retrospective longitudinal study was to investigate the influence of the strategies of first‐line antidiabetic drug on the effectiveness and cost of antidiabetic drugs thereafter, specifically compared between patients who were prescribed DPP4i or BG for T2DM. As Japanese guideline does not specify a first‐line antidiabetic drug and the inclination of the prescription is varied, in the beginning, we showed the share of the first antidiabetic prescription to understand the background. Next, we included patients who had started their antidiabetic medication only with DPP4i or BG. In order to compare the two strategies about the first‐line antidiabetic prescription (starting with DPP4i or BG), we included patients who had started with DPP4i or BG regardless of their medication change or interruption thereafter, as the inclusion criteria of an intention‐to‐treat randomized control trial should include all patients who started an allocated intervention regardless of whether or not they accomplished their treatment regimen or they discontinued on the way. We assessed the changes in HbA1c as the primary effectiveness and those in BMI as the secondary effectiveness, respectively, from the time of medical check‐up before the first prescription to the time of medical check‐up approximately 2 years after the first prescription. We defined “cost” as the approximate annual cost of all antidiabetic medications used during the second year since the first prescription. We also investigated the difference in the type (single or multiple) of antidiabetic medication approximately 2 years after the first prescription compared with the drug type at the first antidiabetic prescription. For these purposes, we extracted information as explained below.
Figure 1.

Study Protocol. (1) First prescription: the date on which first antidiabetic medication was prescribed. (2) Baseline medical check‐up: the latest medical check‐up that was performed within 1 year from the first prescription of antidiabetic medications. (3) Follow‐up medical check‐up: the medical check‐up that was performed between 1.5 and 2.5 years after the first prescription and the nearest one from the first prescription. (4) Follow‐up prescription: the latest prescription of an antidiabetic drug that was prescribed within 3 months before follow‐up examination. (5) Annual cost of antidiabetic drugs before follow‐up medical check‐up
First, we extracted patients who received antidiabetic medications at least once between January 2010 and March 2016. Antidiabetic drugs were determined by the code of A10 (drugs used in diabetes) in the anatomical therapeutic chemical (ATC) classification,12, 13 except for voglibose 0.2‐mg tablets and epalrestat due to the possible use for diabetes prevention and its efficacy for diabetic neuropathy (not an anti‐hyperglycaemic effect), respectively. We then excluded patients whose first antidiabetic medication observed in the database was prescribed within a year after the entrance to the insurance because they might have been previously prescribed antidiabetic drug. Among the rest, the type of the firstly prescribed antidiabetic medication was designated and used as the main exposure variable. Second, for patients who received the first prescription, the latest medical check‐up before the prescription (and also within 1 y before the prescription), including information on HbA1c and BMI, was identified as the baseline medical check‐up. A follow‐up medical check‐up closest to the first prescription was identified during the period 1.5 to 2.5 years after the first prescription. HbA1c values and BMI measured at baseline and at follow‐up medical check‐ups were used for analyses.
Next, annual drug costs for all antidiabetic medications were measured by summing the cost of antidiabetic drugs used prescribed during 1 year (365 d) before the follow‐up medical check‐up (thus, approximately during the second year since the first prescription). The cost of antidiabetic drugs was calculated from governmental price lists to investigate the degree of the economic burden for antidiabetic drugs in Japan. Because the drug cost just after the first prescription should be strongly affected by the price of the firstly prescribed drug, we focused on the annual cost of antidiabetic drugs 1 year before the follow‐up examination, instead of the average cost of antidiabetic drugs from the first prescription before the follow‐up examination. Lastly, the type of subsequent antidiabetic medication (single or multiple) was identified according to the kind of prescription(s) that were prescribed last (within 3 mo) before the follow‐up medical check‐up.
This study was approved by the ethics committee of the National Center of Global Health and Medicine Center Hospital (NCGM‐G‐002096‐00) and The Research Ethics Committee of the Graduate School of Medicine, the University of Tokyo (11520).
2.2. Participants
Patients who had information about their first prescription of antidiabetic medications, and baseline and follow‐up medical check‐ups were included in this study. Patients less than 20 years or 70 years or older at the first prescription were excluded. Based on the results of baseline medical check‐ups, patients who had a history of diabetes, low HbA1c level (<6.5%), admission history during the observation period, possibility of renal dysfunction (high serum creatinine level [male, 1.3 mg/dL or higher; female, 1.2 mg/dL or higher] or a history of chronic renal dysfunction) also was excluded (Figure 2). In addition, we excluded patients who had received the prescription for antidiabetic medications within 1 year since the coverage of their insurance (and/or its observation) had started, because the prescription might not have been the “first” prescription of antidiabetic medications for the patient (ie, some may have received a prescription before the start of the insurance coverage).
Figure 2.
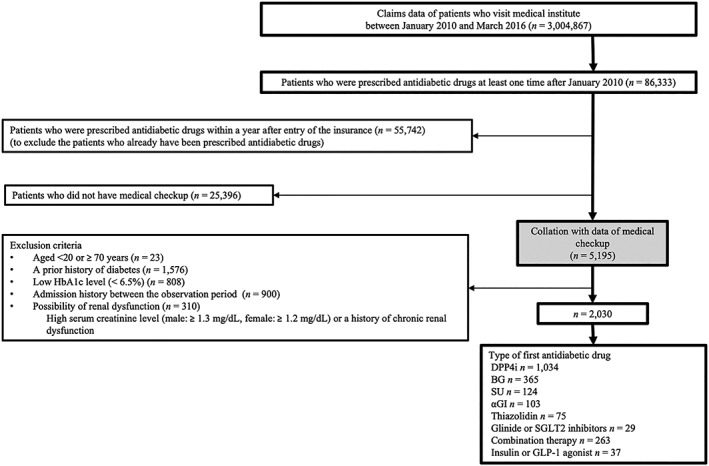
Flow chart of the study patient. HbA1c, haemoglobin A1c; DPP4i, dipeptidyl peptidase‐4 inhibitor; BG, biguanide; SU, sulfonylurea; αGI, alpha glucosidase inhibitor; SGLT2 inhibitors, sodium glucose cotransporter 2 inhibitor; GLP‐1 agonist, glucagon‐like peptide‐1 receptor agonist
2.3. Measurements
Timing of major measurements has been described in the study design section. Here, we briefly illustrate each variable used in the analyses.
Outcome variables were changes in HbA1c and BMI between baseline and follow‐up medical check‐ups and annual drug cost for all antidiabetic medications. For the drug cost, we converted Japanese yen into US$ according to the rate of 113 JPY per US$ based on December 14, 2017, exchange rates.
The type of the first antidiabetic medication prescribed was used as the main exposure variable. Among the patients, those who used DPP4i only (DPP4i group) and BG only (BG group) were included in the main analyses, while information on other prescription types was used only for descriptive analysis.
Covariates needed for propensity score matching were age on the day of the first prescription, sex, size of the medical facility where the first prescription was ordered, baseline HbA1c, baseline BMI, and calendar year of the first prescription. The type of subsequent antidiabetic medication (single or multiple) was also prepared as a secondary outcome information.
Regarding the source of information, prescription information and patients' characteristics were extracted from medical claims data, while only HbA1c and BMI values were derived from medical check‐up data.
2.4. Statistical analyses
We first described the characteristics of patients who received BG only or DPP4i only as the first antidiabetic prescription. Likewise, the characteristics of patients who received other types of drugs as the first prescription (categorized as sulfonylurea only, alpha‐glucosidase inhibitor only, thiazolidine only, glinide only, SGLT‐2i only, multiple oral antidiabetic drugs, or fixed‐dose oral combination drugs, and prescriptions, including at least a type of injection, such as insulin or glucagon‐like peptide‐1 agonist [GLP‐1 agonist]) also were described for the purpose of reference.
Then types of DPP4i and BG and their dose were described at baseline. The type of subsequent antidiabetic medications (follow‐up prescription) was also described according to the type of the first prescription (DPP4i or BG) and was categorized as follows: no antidiabetic drug prescribed; one, two, three, or more oral antidiabetic drugs prescribed (without insulin or GLP‐1 analog); and insulin or GLP‐1 analog used. The proportions of patients who had continued original antidiabetic drugs (DPP4i or BG) as the follow‐up prescription also were calculated according to the type of the first prescription (DPP4i or BG).
Next, the difference in change of HbA1c and BMI between baseline and follow‐up and the annual cost of antidiabetic drugs was compared between the DPP4i and BG groups, with and without covariates adjustment. The drug cost was calculated using the official prices for drugs regulated by the government in Japan. First, unadjusted comparisons were conducted between the two groups using the χ 2 test for categorical valuables and Student t test for continuous variables, including all patients whose first antidiabetic prescription was DPP4i only or BG only. Next, as the main analysis, we conducted propensity score matching using the following variables: age, age2 (the quadratic term), age3 (the cubic term), sex, baseline HbA1c (b_HbA1c), b_HbA1c2 (the quadratic term), baseline BMI (b_BMI), b_BMI2 (the quadratic term), b_BMI3 (the cubic term), size of medical facilities (categorical), and the calendar year (categorical) when the antidiabetic prescription had started. These possible confounders were chosen because of their potential association with the outcome of interest and the choice of medication based on clinical knowledge; the quadratic terms and the cubic terms were inserted to achieve better balance of each variable between the groups (observed as smaller size of % bias). The setting of propensity score matching was nearest neighbour matching without replacement with the calliper of 0.01. After performing 1:1 matching, we compared changes in HbA1c and BMI with Student t test and the χ 2 test between the groups. We repeated the same analyses stratified by BMI category (BMI ≥ 25 kg/m2 and < 25 kg/m2). The patients who did not have information of BMI and HbA1c, including those who did not participate in a couple of medical check‐ups, were excluded in this analysis. We also illustrated the changes in unmatched (crude) analyses because these changes should be close to the effectiveness that physicians in clinics actually perceived when prescribing drugs based on patients' characteristics and knowledge about these drugs.
As additional information, we also described characteristics of patients who did or did not have medical check‐ups and details of antidiabetic drugs in the two groups at follow‐up.
Data on the descriptive tables were presented as the mean ± standard deviation (SD) or the number of patients (%). All tests were considered significant at P < .05. All statistical analyses were performed using Stata 15.0 software (StataCorp, College Station, Texas).
3. RESULTS
Of 3 004 867 beneficiaries of insurances between January 2010 and March 2016, 86 333 received antidiabetic medications. Among these patients, 5195 began antidiabetic medication use 1 year or later from the date of insurance entry and also had information on medical check‐ups (characteristics of the patients who had or did not have a couple of medical check‐up data were shown in Table S1). We excluded patients who met the following criteria: age < 20 or ≥ 70 years (n = 23), history of diabetes (n = 1576), HbA1c < 6.5% (n = 808), hospitalized during the observation period (n = 900), and possible renal dysfunction (n = 310). In total, 2030 patients were included in this study, among which 1034 and 365 received DPP4i and BG as their first antidiabetic prescription, respectively (Figure 2).
3.1. HbA1c and BMI
Baseline characteristics are shown in Table 1. Patients who began antidiabetic medication with DPP4i were older and had lower HbA1c and lower BMI values than those who began with BG (male sex, 83.0% and 84.9%; age (mean [SD]), 51.2 [7.0] years and 48.2 [7.7] years; HbA1c, 7.7 [1.4]% and 7.9 [1.4]%; BMI, 26.6 [4.5] kg/m2 and 28.1 [4.3] kg/m2, respectively). DPP4i was prescribed more frequently at small medical facilities than was BG. Characteristics of patients who received antidiabetic drugs other than DPP4i and BG are shown in Table S2. Of patients who began diabetic treatment with DPP4i, 345 were propensity score matched to those who began with BG. The covariate balance in the matched cohort was improved considerably (Table 1). The proportion of patients who had high HbA1c level was described in Table S5.
Table 1.
Baseline characteristics of DPP4i and BG groups in unmatched and PS matched patients
| Variables | Unmatched Patients | PS‐matched Patients | |||||
|---|---|---|---|---|---|---|---|
| DPP4i Group (n = 1034) | BG Group (n = 365) | P Value | DPP4i Group (n = 345) | BG Group (n = 345) | P Value | %bias | |
| Age, y | 51.2 ± 7.0 | 48.2 ± 7.7 | <.001 | 48.2 ± 7.3 | 48.7 ± 7.5 | .375 | −6.7 |
| Male sex, % | 83.0 | 84.9 | .388 | 85.5 | 84.1 | .596 | −3.9 |
| HbA1c, % | 7.7 ± 1.4 | 7.9 ± 1.4 | .004 | 7.9 ± 1.4 | 7.9 ± 1.4 | .647 | 3.6 |
| BMI, kg/m2 | 26.6 ± 4.5 | 28.1 ± 4.3 | <.001 | 28.0 ± 4.0 | 27.9 ± 4.3 | .718 | 2.6 |
| The number of beds of medical facilities in which patients were first prescribed, % | |||||||
| 0‐19 | 76.1 | 69.9 | .002 | 73.6 | 72.2 | .894 | – |
| 20‐199 | 10.6 | 9.0 | 8.7 | 9.6 | −2.9 | ||
| 200‐ | 13.3 | 21.1 | 17.7 | 18.3 | −1.5 | ||
| Calendar year of prescription, % | |||||||
| 2011 | 14.2 | 21.9 | .006 | 20.6 | 19.7 | .970 | – |
| 2012 | 39.5 | 36.2 | 38.8 | 38.0 | 1.8 | ||
| 2013 | 36.6 | 34.3 | 33.3 | 34.5 | −2.4 | ||
| 2014 | 9.8 | 7.7 | 7.3 | 7.8 | −2.1 | ||
Note. Mean (95% CI) or %.
Abbreviations: BG, biguanide; BMI, body mass index; DPP4i, dipeptidyl peptidase‐4 inhibitor; HbA1c, haemoglobin A1c; PS, propensity score.
3.2. Baseline and follow‐up prescription
Regarding types of DPP4i received by patients, 53.8% received sitagliptin, 20.9% alogliptin, and 13.7% vildagliptin. Only two of the 365 patients in the BG group received buformin, while the others received metformin. Among the metformin prescriptions, most were less than 1000 mg per day (90.1%; Table 2). At follow‐up, 17.5% of patients in the DPP4i group and 16.7% in the BG group did not continue any antidiabetic drugs, while 78.3% and 73.7%, respectively, continued their original oral antidiabetic mediations. Among the patients who received any antidiabetic medication at follow‐up, 95.0% and 88.5%, respectively, received the same type of drug as firstly prescribed. Precise follow‐up prescriptions of DPP4i and BG are shown in Table S3.
Table 2.
Prescription at baseline and follow‐up
| DPP4i Group (n = 1034) | BG group (n = 365) | |
|---|---|---|
| Baseline | ||
| Type of DPP4i | N/A | |
| Sitagliptin | 556 (53.8) | |
| 100 mg | 6 | |
| 50 mg | 496 | |
| ≤25 mg | 54 | |
| Alogliptin | 216 (20.9) | |
| 25 mg | 188 | |
| ≤12.5 mg | 28 | |
| Vildagliptin | 142 (13.7) | |
| 100 mg | 70 | |
| 50 mg | 72 | |
| Other type of DPP4i | 120 (11.6) | |
| Dose of metformin | N/A | |
| ≥2000 mg | 0 (0.0) | |
| ≥1000 mg, <2000 mg | 34 (9.3) | |
| <1000 mg | 329 (90.1) | |
| Buformin | 2 (0.6) | |
| Follow‐up | ||
| None of DM drug | 181 (17.5) | 61 (16.7) |
| Only OAD | ||
| Number of type of OAD | ||
| 1 | 525 (50.8) | 159 (43.6) |
| 2 | 237 (22.9) | 106 (29.0) |
| 3‐ | 83 (8.0) | 32 (8.8) |
| Insulin (or Insulin combined with OAD) | 5 (0.5) | 3 (0.8) |
| GLP‐1 analog (or GLP‐1 combined with OAD) | 3 (0.3) | 4 (1.1) |
| Patients continuing first kinds of OAD | 810 (78.3) | 269 (73.7) |
Note. N (%). There are no patients who received both insulin and GLP‐1 analog at the follow‐up prescription.
Abbreviations: BG, biguanide; DM, diabetes mellitus; DPP4i, dipeptidyl peptidase‐4 inhibitors; OAD, oral antidiabetic drug.
3.3. Effectiveness and cost
Before propensity score matching, the change in HbA1c was significantly less and that in BMI was equivalent in the DPP4i group compared with BG group (HbA1c, −0.57% vs –0.77%, P = .02; BMI, −0.26 kg/m2 vs −0.41 kg/m2, P = .10). Annual cost of antidiabetic drugs was higher in the DPP4i than in the BG groups (US $453.2 vs US $277.9, P < .001; Figure 3). Changes in HbA1c and BMI from baseline to follow‐up in the matched DPP4i group were not significantly different from those in the BG group (HbA1c, −0.67% vs –0.79%, P = .28; BMI: −0.30 kg/m2 vs −0.39 kg/m2, P = .42). Conversely, annual cost of antidiabetic drugs before the follow‐up medical check‐up remained significantly higher in the DPP4i group compared with that in the BG group (US $458.7 vs US $273.3, P < .001; Figure 3). In the stratified analyses by BMI category, the changes in HbA1c and BMI were not different between the two groups after propensity score matching. Annual cost for antidiabetic drugs was significantly higher in the DPP4i than in the BG groups; these results were almost the same as those of unstratified analyses and no apparent interaction was observed (Figure S1).
Figure 3.
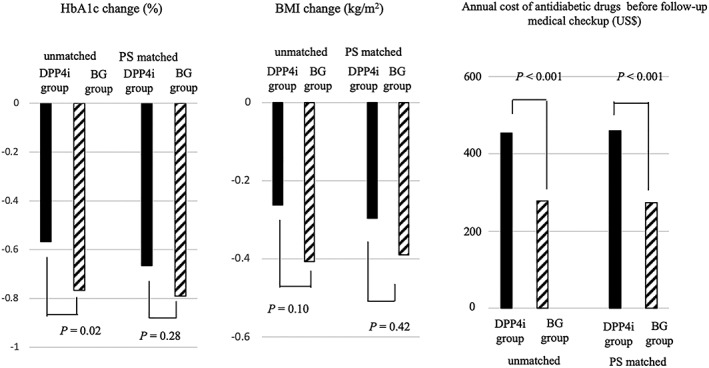
Therapeutic effectiveness and antidiabetic drug cost comparing the DPP4i and BG groups. Black bar indicates DPP4i group, and stripes bar indicates BG group. Of each bar graph, the left two and right two bars show the result before and after propensity score matching, respectively. DPP4i, dipeptidyl peptidase‐4 inhibitor; BG, biguanide; HbA1c, haemoglobin A1c; BMI, body mass index; PS, propensity score
4. DISCUSSION
Our results demonstrated that DPP4i and BG had no difference in terms of effectiveness, whereas the cost was much higher in the DPP4i compared with the BG groups in Japan. The study results suggested that BG may be recommendable for patients among Japanese working generations with T2DM as the first‐line treatment. To the best of our knowledge, this is the first study to evaluate the effect and cost between DPP4i and BG as first‐line antidiabetic medications.
Among new antidiabetic drugs launched recently, DPP4i has been used in a certain proportion for the treatment of T2DM in the world.10, 14, 15, 16, 17, 18, 19 Compared with BG, the first‐line antidiabetic medication in many Western countries, few studies have investigated the efficacy or effectiveness of DPP4i for glycaemic control and prevention of diabetic comorbidities. In clinical studies, DPP4i has been reported to have an equivalent or inferior effect for decreasing HbA1c and BMI compared with metformin.20, 21, 22 Regarding the prevention of complications induced by diabetes, it has been unclear whether DPP4i has superior effect on comorbidity prevention compared with BG.23, 24, 25 Especially considering that East Asians may have a pathology of diabetes and responsiveness to antidiabetic drugs different from Caucasian,8, 9, 26 further investigation is needed for first‐line antidiabetic medication to treat patients with T2DM in Japan and other East Asian countries. We believe that the results of the current real‐world analyses would help the decision for first‐line antidiabetic medications in these countries. In addition, direct comparisons of effectiveness and costs between biguanide and other antidiabetic medications in the real‐world setting have been scarce in other countries where metformin has been recommended as the first‐line medication. Future similar studies in other countries may help re‐evaluate the position of each drug.
Our results also demonstrated a high retention rate of the antidiabetic medication used first in the patient. Actually, it also has been reported that the first‐line antidiabetic medication, once prescribed, remains to be prescribed thereafter in England.27 Evaluating influences on long‐time cost is important to determine guidance regarding the first‐line prescription as well. Our observational study used claims and medical check‐up data and attempted to increase comparability using propensity score matching. Another characteristic of this study is that we classified patients according to the first antidiabetic medication prescribed to generate evidence about the first‐line prescription. This type of study may be useful for generating real‐world evidence about the first‐line prescription. Conversely, the study results may not be generalizable for other countries or long‐term future because the price of the drug may change over time and the amount of medication used may change by time and/or country.
This study has several limitations. First, because these claims data were gathered from patients working for relatively large companies, most patients were male workers who did not change their companies during the observation periods. Also, we included neither patients who had renal dysfunction nor those with a recent history of admission. In the main analyses, we matched patients in the DPP4i group to those in the BG group using propensity score; the obtained effects should be applicable for population who tended to use metformin for the first‐line antidiabetic medication (eg, younger age and greater BMI, shown in Table 1). As described above, we focus on patients who had information about their medical check‐ups; in other words, we did not consider missingness for those who started diabetic medication but did not have their information about their medical check‐ups. These may induce selection bias and/or reduce generalizability. We also conducted a multivariable regression analysis for sensitivity analysis, which indicated almost the same result as that of the propensity score matching. HbA1c decrease in the DPP4i group was slightly smaller compared with that in the BG group, although the difference was not a clinically important difference (0.14%, P = .043). The BMI change was not different level between the two groups; annual costs of antidiabetic drugs before the follow‐up medical check‐up was higher in the DPP4i group compared with the BG group (Table S4). Although the result of the multivariable regression analysis may be applicable to more general population than that of the PS matching (only generalizable to those who were more likely to receive BG, that is, more male, middle‐aged, and heavier in body weight), future studies would need to evaluate whether results of the present study are generalizable to other population. Second, HbA1c and BMI values were not measured just before the first prescription. Because these baseline data were extracted within 1 year before the first prescription (mean interval, 137 ± 102 d), there was a possibility to slightly underestimate or overestimate baseline HbA1c and BMI. Third, we could not evaluate adverse events, such as gastrointestinal symptoms, hypoglycaemia, and admissions, because we did not have detailed clinical information of side effects and could not verify the main cause of admission among multiple disease names as well. Therefore, it should be noted that only relatively healthy patients were analysed. In addition, we investigated neither effect nor cost of these antidiabetic drugs on preventing diabetic comorbidities. Lastly, the average duration of observation in the present study was 1.9 years. To investigate the effectiveness and/or cost‐effectiveness of the first‐line antidiabetic medications in terms of preventing comorbidities, longer observation period would be warranted in the future study.
In conclusion, these results indicated that the annual cost for antidiabetic drugs in the BG group is nearly half that in the DPP4i group at approximately 2 years after starting diabetes treatment, while the effectiveness of the two medications did not differ. BG may be recommendable for patients with T2DM as the first‐line treatment in Japan, especially for middle‐aged, more male population with greater BMI. Although Japanese guideline does not recommend a specific first‐line antidiabetic medication, it is worth addressing the discrepancy between practice in Japan and that recommended in international guidelines, especially now that effectiveness and drug costs have attracted more attention. This type of observational study that satisfies comparability may be applicable to consideration of first‐line medication for diabetes in other countries and also for other diseases.
AUTHORS' CONTRIBUTION
All authors have contributed significantly. N.I.S. and T.S. conceptualized the idea for the study, analysed the data, and drafted the article. H.T. participated in the design and the discussion and supported the analysis. K.U., Y.K., and M.O. participated in the design of the study and supervised the work. All authors have read and approved the final manuscript.
The authors would like to thank Enago (http://www.enago.jp/) for the English language review.
CONFLICT OF INTEREST
The authors have no conflicts of interest directly relevant to the content of this article. K.U. received honoraria for lectures from MSD K.K., Takeda Pharmaceutical Co, Ltd, Mitsubishi Tanabe Pharma Corporation, Kyowa Hakko Kirin Co, Ltd, Novo Nordisk Pharma Ltd, Eli Lilly Japan K.K., Boehringer Ingelheim Japan, Inc, and Astellas Pharma Inc; clinical research grants from Astellas Pharma Inc; scholarship grants from Takeda Pharmaceutical Co, Ltd, Sanofi K.K., Astellas Pharma Inc, Mitsubishi Tanabe Pharma Corporation, Kyowa Hakko Kirin Co, Ltd, Novo Nordisk Pharma Ltd, Eli Lilly Japan K.K., Boehringer Ingelheim Japan, Inc, Daiichi Sankyo Co, Ltd, and Sumitomo Dainippon Pharma Co, Ltd. M.O. received Honoraria for lectures from MSD K.K., Takeda Pharmaceutical Co, Sanofi K.K., Mitsubishi Tanabe Pharma Corporation, Novo Nordisk Pharma Ltd, and Novartis Pharma K.K.; clinical research grants from Novartis Pharma K.K. and Tanabe Pharma Corporation; scholarship grants from Sumitomo Dainippon Pharma Co, Ltd. These grants were not received for this research.
ETHICAL APPROVAL
This study was approved by the ethics committee of the National Center of Global Health and Medicine Center Hospital (NCGM‐G‐002096‐00) and The Research Ethics Committee of the Graduate School of Medicine, the University of Tokyo (11520).
Supporting information
Fig. S1. Therapeutic effectiveness and antidiabetic drug cost comparing DPP4i and BG groups stratified by BMI25. Black bar indicates DPP4i group and stripes bar indicates BG group. Of each bar graph, the left two and right two bars show the results before and after propensity score matching, respectively. DPP4i, dipeptidyl peptidase‐4 inhibitor; BG, biguanide; HbA1c, hemoglobin A1c; BMI, body mass index; PS, propensity score.
Table S1. Characteristics of patients who had or did not have information of medical checkup.
Table S2. Characteristics of 2,030 patients by types of the first anti‐diabetic medication.
Table S3. Follow‐up prescription of DPP4i and BG groups.
Table S4. Result from multivariate analysis for influences of DPP4i first‐line group compared with BG.
Table S5. The proportion of patients who had a higher HbA1c level at the follow‐up period.
ACKNOWLEDGEMENTS
This study was partly supported by the Grant of National Center or Global Health and Medicine (26‐D‐002, PI: Dr Kohjiro Ueki) and the Health and Labour Sciences Research Grant (Comprehensive Research on Life‐Style Related Diseases including Cardiovascular Diseases and Diabetes Mellitus, H29‐Cardiovascular‐General‐004, PI: Dr Takashi Kadowaki).
Ihana‐Sugiyama N, Sugiyama T, Tanaka H, Ueki K, Kobayashi Y, Ohsugi M. Comparison of effectiveness and drug cost between dipeptidyl peptidase‐4 inhibitor and biguanide as the first‐line anti‐hyperglycaemic medication among Japanese working generation with type 2 diabetes. J Eval Clin Pract. 2020;26:299–307. 10.1111/jep.13171
REFERENCES
- 1. American Diabetes Association . 8. Pharmacologic approaches to glycemic treatment. Diabetes Care. 2017;40(Supplement 1):S64‐S74. [DOI] [PubMed] [Google Scholar]
- 2. Griffin SJ, Leaver JK, Irving GJ. Impact of metformin on cardiovascular disease: a meta‐analysis of randomised trials among people with type 2 diabetes. Diabetologia. 2017;60(9):1620‐1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ministry of Health Malaysia . Clinical Practice Guidelines Management of Type 2 Diabetes Mellitus (5th Edition). 2015. Available at: http://www.acadmed.org.my/view_file.cfm?fileid=763 [acccessed 02. 05. 2019]
- 4. Ko SH, Kim SR, Kim DJ, et al. 2011 clinical practice guidelines for type 2 diabetes in Korea. Diabetes Metab J. 2011;35(5):431‐436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Weng J, Ji L, Jia W, et al. Standards of care for type 2 diabetes in China. Diabetes Metab Res Rev. 2016;32(5):442‐458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. The Japan Diabetes Society. Japanese Clinical Practice Guideline for Diabetes. 2016. Available at: http://www.jds.or.jp/modules/en/index.php?content_id=44 [acccessed 02. 05. 2019]
- 7. Moller JB, Pedersen M, Tanaka H, et al. Body composition is the main determinant for the difference in type 2 diabetes pathophysiology between Japanese and Caucasians. Diabetes Care. 2014;37(3):796‐804. [DOI] [PubMed] [Google Scholar]
- 8. Kodama K, Tojjar D, Yamada S, Toda K, Patel CJ, Butte AJ. Ethnic differences in the relationship between insulin sensitivity and insulin response: a systematic review and meta‐analysis. Diabetes Care. 2013;36(6):1789‐1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kim YG, Hahn S, Oh TJ, Kwak SH, Park KS, Cho YM. Differences in the glucose‐lowering efficacy of dipeptidyl peptidase‐4 inhibitors between Asians and non‐Asians: a systematic review and meta‐analysis. Diabetologia. 2013;56(4):696‐708. [DOI] [PubMed] [Google Scholar]
- 10. Kohro T, Yamazaki T, Sato H, et al. Trends in antidiabetic prescription patterns in Japan from 2005 to 2011. Int Heart J. 2013;54(2):93‐97. [DOI] [PubMed] [Google Scholar]
- 11. Kimura S, Sato T, Ikeda S, Noda M, Nakayama T. Development of a database of health insurance claims: standardization of disease classifications and anonymous record linkage. J Epidemiol. 2010;20(5):413‐419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. WHO Collaborating Centre for Drug Statistics Methodology. ATC/DDD Index 2017. Available at: https://www.whocc.no/atc_ddd_index/ [acccessed 02. 05. 2019]
- 13. European Pharmaceutical Market Research Association . EphMRA anatomical classification guidelines. 2016. Available at: http://www.ephmra.org/media/1090/atcguidelines2016final.pdf [acccessed 02. 05. 2019] [Google Scholar]
- 14. Hampp C, Borders‐Hemphill V, Moeny DG, Wysowski DK. Use of antidiabetic drugs in the U.S., 2003‐2012. Diabetes Care. 2014;37(5):1367‐1374. [DOI] [PubMed] [Google Scholar]
- 15. Montvida O, Shaw J, Atherton JJ, Stringer F, Paul SK. Long‐term trends in antidiabetes drug usage in the U.S.: real‐world evidence in patients newly diagnosed with type 2 diabetes. Diabetes Care. 2018;41(1):69‐78. [DOI] [PubMed] [Google Scholar]
- 16. Arnaud M, Bezin J, Begaud B, Pariente A, Salvo F. Trends in the incidence of use of noninsulin glucose‐lowering drugs between 2006 and 2013 in France. Fundam Clin Pharmacol. 2017;31(6):663‐675. [DOI] [PubMed] [Google Scholar]
- 17. Ko SH, Kim DJ, Park JH, et al. Trends of antidiabetic drug use in adult type 2 diabetes in Korea in 2002‐2013: nationwide population‐based cohort study. Medicine (Baltimore). 2016;95(27):e4018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sliwczynski A, Brzozowska M, Jacyna A, et al. Drug‐class‐specific changes in the volume and cost of antidiabetic medications in Poland between 2012 and 2015. PLoS ONE. 2017;12(6):e0178764 10.1371/journal.pone.0178764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Christensen DH, Rungby J, Thomsen RW. Nationwide trends in glucose‐lowering drug use, Denmark, 1999‐2014. Clin Epidemiol. 2016;8:381‐387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Aschner P, Katzeff HL, Guo H, et al. Efficacy and safety of monotherapy of sitagliptin compared with metformin in patients with type 2 diabetes. Diabetes Obes Metab. 2010;12(3):252‐261. [DOI] [PubMed] [Google Scholar]
- 21. Amori RE, Lau J, Pittas AG. Efficacy and safety of incretin therapy in type 2 diabetes: systematic review and meta‐analysis. JAMA. 2007;298(2):194‐206. [DOI] [PubMed] [Google Scholar]
- 22. Karagiannis T, Paschos P, Paletas K, Matthews DR, Tsapas A. Dipeptidyl peptidase‐4 inhibitors for treatment of type 2 diabetes mellitus in the clinical setting: systematic review and meta‐analysis. BMJ. 2012;344(mar12 1):e1369. [DOI] [PubMed] [Google Scholar]
- 23. Scheller NM, Mogensen UM, Andersson C, Vaag A, Torp‐Pedersen C. All‐cause mortality and cardiovascular effects associated with the DPP‐IV inhibitor sitagliptin compared with metformin, a retrospective cohort study on the Danish population. Diabetes Obes Metab. 2014;16(3):231‐236. [DOI] [PubMed] [Google Scholar]
- 24. Kim SC, Glynn RJ, Liu J, Everett BM, Goldfine AB. Dipeptidyl peptidase‐4 inhibitors do not increase the risk of cardiovascular events in type 2 diabetes: a cohort study. Acta Diabetol. 2014;51(6):1015‐1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ou HT, Chang KC, Li CY, Wu JS. Risks of cardiovascular diseases associated with dipeptidyl peptidase‐4 inhibitors and other antidiabetic drugs in patients with type 2 diabetes: a nation‐wide longitudinal study. Cardiovasc Diabetol. 2016;15(1):41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fukushima M, Suzuki H, Seino Y. Insulin secretion capacity in the development from normal glucose tolerance to type 2 diabetes. Diabetes Res Clin Pract. 2004;66(Suppl 1):S37‐S43. [DOI] [PubMed] [Google Scholar]
- 27. Datta‐Nemdharry P, Thomson A, Beynon J, Donegan K. Patterns of anti‐diabetic medication use in patients with type 2 diabetes mellitus in England and Wales. Pharmacoepidemiol Drug Saf. 2017;26(2):127‐135. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Therapeutic effectiveness and antidiabetic drug cost comparing DPP4i and BG groups stratified by BMI25. Black bar indicates DPP4i group and stripes bar indicates BG group. Of each bar graph, the left two and right two bars show the results before and after propensity score matching, respectively. DPP4i, dipeptidyl peptidase‐4 inhibitor; BG, biguanide; HbA1c, hemoglobin A1c; BMI, body mass index; PS, propensity score.
Table S1. Characteristics of patients who had or did not have information of medical checkup.
Table S2. Characteristics of 2,030 patients by types of the first anti‐diabetic medication.
Table S3. Follow‐up prescription of DPP4i and BG groups.
Table S4. Result from multivariate analysis for influences of DPP4i first‐line group compared with BG.
Table S5. The proportion of patients who had a higher HbA1c level at the follow‐up period.


