Abstract
Background:
Understanding the impact of prevention programs on Clostridioides difficile infection rates is important, and decisions on future program changes, including how to use vaccines currently in development, requires a detailed understanding of the epidemiologic features of C. difficile infection. We analyzed Ontario health administrative data to determine incidence rates and medical costs of C. difficile infection, based on whether acquisition and onset occurred in acute care hospitals (ACHs), long-term care facilities or the community.
Methods:
We performed a retrospective analysis using individual-level data from Ontario health databases from Apr. 1, 2005, to Mar. 31, 2015, identifying rates of C. difficile infection requiring hospital admission per 100 000 person-years in adults aged 18 years or more for categories of acquisition and onset. We estimated health care system costs of infection 180 and 365 days after admission by matching patients with C. difficile infection with control patients with similar characteristics.
Results:
Over the study period, 33 909 people in Ontario were admitted to hospital with C. difficile infection; 17 272 cases (50.9%) were associated with ACHs. The number of cases per 100 000 person-years ranged from 27.7 in 2009/10 to 37.0 in 2012/13. Annually, the highest incidence of infection was for ACH-associated/ACH-onset. Community-associated infection became more prevalent over time, rising from 19.4% of cases in 2005/06 to 29.2% in 2014/15. Infection costs were mostly due to hospital admission within 180 days after hospital discharge. Infection associated with ACHs had the highest total costs and the largest cost attributable to C. difficile infection (median $38 953 for infected patients v. $13 542 for control patients). Median costs attributable to C. difficile infection were $1051 for that associated with long-term care facilities, $13 249 for community-associated infection and $11 917 for ACH-associated/community-onset infection.
Interpretation:
Community-associated C. difficile infection had similar health care cost implications as hospital-associated infection. With rates of community-associated C. difficile infection on the rise, family physicians should be supported to prevent this infection in their patients.
In Canada, Clostridioides difficile infection is a leading infectious cause of morbidity and mortality.1,2 A Quebec study identified an increase from 35.6 to 156.3 cases per 100 000 people between 1991 and 2003.3 More recent surveillance of the 10 provinces showed a 2011 incidence rate of 535 per 100 000 admissions.4 Clostridioides difficile infection is associated with considerable costs. An Ontario study showed that acquiring this infection in hospital increased the median length of stay by 6 days.5 This translates to a substantial economic burden: a Canadian model estimated a total of 37 900 C. difficile infection episodes (in hospital and in the community) in 2012 and a total societal cost of $2.8 million, of which 92% was in-hospital costs.1
Although the majority of the literature on the epidemiologic features of C. difficile infection is based on association with acute care hospital (ACH) settings, this infection is also associated with long-term care facilities (LTCFs) owing to residents’ advanced age, comorbidities and antibiotic exposure.6–8 In addition, rates of community-associated C. difficile infection appear to be increasing.9–11
Understanding both changes in epidemiologic features and cost associated with this infection is important for several reasons. Experience in Quebec in the early 2000s illustrated the impact of the introduction of a new and virulent strain.12 Early detection of similar changes will permit more effective prevention. If rates of community-associated infections are truly increasing, recognition of the risk factors for disease will be important to support diagnosis and management.
In Ontario hospitals, multiple measures, including a hand hygiene program, funded infection prevention and control practitioners, regional infection control networks, antimicrobial stewardship programs and mandatory reporting of hospital C. difficile infections beginning in 2008, have been implemented since 2003 to prevent infection.13,14 Understanding the impact of such programs on C. difficile infection rates is important.
Finally, several vaccines to prevent C. difficile infection are under development. Decisions about whether and how best to use vaccines requires a detailed understanding of the epidemiologic features of C. difficile infection, particularly since the emergence of the virulent NAP1 strain in Ontario.
Therefore, we used linked health administrative data from Ontario to assess the incidence and economic burden of in-hospital C. difficile infection in the province.
Methods
We conducted a retrospective cohort study to obtain provincial estimates on the incidence and cost of C. difficile infection in ACH inpatients, residents of LTCFs and community-dwelling people.
Data sources
We conducted analyses using data for Ontario. The ICES houses Ontario’s health administrative data on hospital and physician billings.15 Health card numbers were encrypted, converted into unique identifiers and linked to the following databases for analysis: the Ontario Health Insurance Plan physician billing claims database, which contains data for about 95% of physician visits in the province; the Canadian Institute for Health Information’s Discharge Abstract Database and the National Ambulatory Care Reporting System for data related to hospital admissions and emergency department visits, respectively; and the Registered Persons Database, which contains age, sex, postal code and date of death (where applicable) for all Ontario residents eligible for health care services.16
Setting
Our study was set in Ontario (estimated 2015 population 13.7 million17) and included people admitted to hospital with C. difficile infection in the province. As of 2017, there were 182 acute care hospitals and 626 LTCFs.
Cases
We used administrative data to identify cases of C. difficile infection between Apr. 1, 2005, and Mar. 31, 2015. Affected patients 1) had an International Statistical Classification of Diseases and Related Health Problems, 10th Revision, enhanced Canadian version (ICD-10-CA) diagnosis code for C. difficile infection (A04.7) during an inpatient hospital stay as the most responsible diagnosis, a preadmission comorbidity or a postadmission comorbidity of clinical significance, 2) were at least 18 years at diagnosis and 3) had no C. difficile infection diagnosis code in the previous 180 days (a second diagnosis after 180 d following discharge was considered a separate incident).
Incidence
To calculate the incidence of C. difficile infection necessitating hospital admission per 100 000 person-years, the denominator included the base population aged 18–104 years. Person-years were based on postal code, Ontario Health Insurance Plan eligibility, date of death and date of last contact with the health care system.
We stratified cases into 6 cohorts depending on location of association and onset (we used hospital admission date, since the databases did not capture when laboratory testing for C. difficile infection was conducted) (Table 1).
Table 1:
Definitions of Clostridioides difficile infection cohorts based on location of disease association and onset*
| Cohort | Definition |
|---|---|
| ACH-associated/ACH-onset | C. difficile infection was coded as postadmission comorbidity of clinical significance AND patient did not reside in LTCF in 12 wk before admission |
| ACH- or LTCF-associated†/ACH-onset | C. difficile infection was coded as postadmission comorbidity of clinical significance AND patient resided in LTCF in 12 wk before admission |
| LTCF-associated/LTCF-onset | C. difficile infection was coded as most responsible diagnosis or preadmission comorbidity AND patient resided in LTCF with no history of hospital admission in 12 wk before admission |
| LTCF- or ACH-associated†/LTCF-onset | C. difficile infection was coded as most responsible diagnosis or preadmission comorbidity AND patient resided in LTCF in 12 wk before admission AND patient had history of hospital admission during this time |
| Community-associated/community-onset | C. difficile infection was coded as most responsible diagnosis or preadmission comorbidity AND patient neither resided in LTCF nor was admitted to hospital in 12 wk before admission |
| ACH-associated/community-onset | C. difficile infection was coded as most responsible diagnosis or preadmission comorbidity AND patient did not reside in LTCF but was admitted to hospital in 12 wk before admission |
Note: ACH = acute care hospital, LTCF = long-term care facility.
Adapted from reference 18. Reproduced with permission.
Because the case involved a person who resided in an LTCF and was admitted to hospital in the 12 weeks before onset, it was not possible to determine whether the infection was acquired in an ACH or LTCF.
Cost
To estimate the health care system costs of C. difficile infection, we combined the 6 cohorts into 4 combined cohorts (Table 2) where possible and created control groups. Those who did not meet the definitions for any of the cohorts (n = 972) or could not be matched to control subjects (n = 10 355) were excluded from the analysis.
Table 2:
Definitions of Clostridioides difficile infection cohort and matched control groups*
| Cohort | Cohort definition | Control groups | |
|---|---|---|---|
| Hard-match criteria | Propensity-score–match criteria | ||
| ACH-associated/ACH-onset | ICD-10-CA diagnosis code for C. difficile infection (A04.7) during inpatient hospital stay, coded as postadmission comorbidity of clinical significance |
|
|
| LTCF-associated/LTCF-onset† | LTCF resident with ICD-10-CA diagnosis code for C. difficile infection (A04.7) during inpatient hospital stay, coded as most responsible diagnosis or preadmission comorbidity AND no hospital admission in 12 wk before onset |
|
|
| Community-associated/community-onset* | Community resident with ICD-10-CA diagnosis code for C. difficile infection (A04.7) during inpatient hospital stay, coded as most responsible diagnosis or preadmission comorbidity AND no hospital admission in 12 wk before onset |
|
|
| ACH-associated, community-onset | ICD-10-CA diagnosis code for C. difficile infection (A04.7) during inpatient hospital stay, coded as most responsible diagnosis or preadmission comorbidity AND did not reside in LTCF in 12 wk before onset |
|
|
Note: ACH = acute care hospital, ICD-10-CA = International Statistical Classification of Diseases and Related Health Problems, 10th Revision, enhanced Canadian version, LTCF = long-term care facility.
Adapted from reference 19 (Oxford University Press).
People in this control group had not necessarily been admitted to hospital at the index date.
Matched on the first 3 digits of the ICD-10-CA code.
A measure of a community’s rurality based on its population and population density, travel time to nearest basic referral centre and travel time to nearest advanced referral centre.20
Comorbidity score based on ICD-10-CA coding.21
Control groups
Control groups consisted of patients aged 18 years or more who resided in Ontario, were eligible for Ontario Health Insurance Plan benefits and did not have C. difficile infection (absence of ICD-10-CA diagnosis code A04.7) between Apr. 1, 2005, and Mar. 31, 2015. Members of the control groups were matched to cohort members at cohort members’ disease onset. Each cohort member was greedy-matched to 1–3 control patients based on hard-match and propensity-score–match criteria at the time of cohort member’s disease onset (Table 2).
For all 4 sets of matches, we used calipers of width equal to 0.2 of the standard deviation of the propensity score.
Outcomes
We collected costs using ICES’s GETCOST macro for 180 and 365 days after onset (hospital admission dates for cohort members) and after the index date (date of hospital admission for ACH-associated control patients, and date of matching for LTCF-associated, community-associated and ACH-associated/community-onset control patients) for hospital stays, same-day surgical procedures, emergency department visits, outpatient medications (all prescription drugs covered under the Ontario Drug benefit program for those aged ≥ 65 yr, those residing in LTCFs and those receiving social assistance), physician services, outpatient laboratory tests, complex continuing care stays and home care services. The periods were selected to capture all associated outcomes including those that do not manifest immediately. Costs were reported in 2015 Canadian dollars.
Statistical analysis
Given privacy rules regarding access to the data, all analyses were conducted by ICES staff. We used descriptive statistics to present unadjusted baseline characteristics and annual C. difficile infection incidence from fiscal years 2005/06 to 2014/15 and to characterize the C. difficile infection cohorts/control groups at baseline (i.e., index date). We estimated age-adjusted incidence rates per 100 person-years using the 2010 Ontario population as the standard.14 We compared categorical variables and continuous variables using generalized estimating equations to calculate the standardized differences.22
Ethics approval
Ethics approval was granted by Advarra’s Institutional Review Board Services, Canada’s largest central review board.
Results
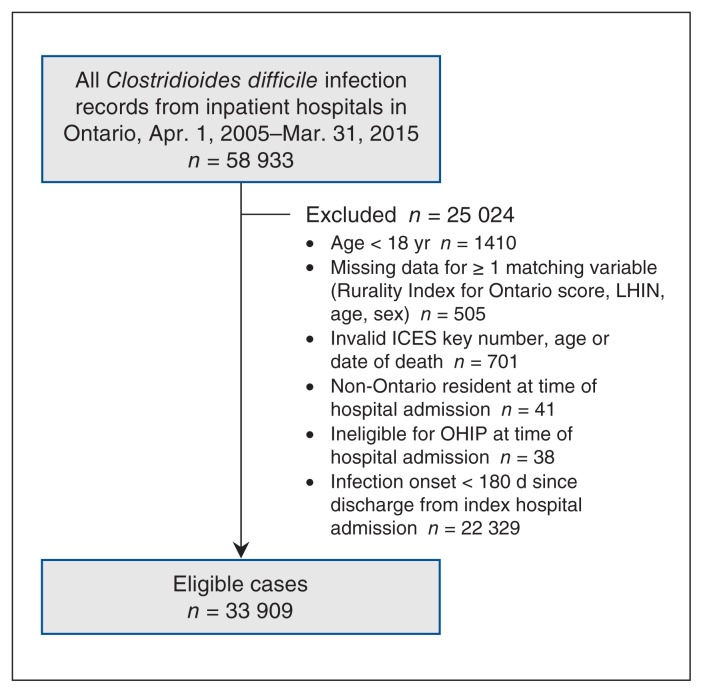
Between 2005/06 and 2014/15, there were 33 909 new cases of C. difficile infection in Ontario (Figure 1), of which 17 272 (50.9%) were ACH-associated/ACH-onset, 7216 (21.3%) were community-associated/community-onset, and 7098 (20.9%) were ACH-associated/community-onset (Table 3).
Figure 1:
Flow diagram showing case inclusion. Note: LHIN = Local Health Integration Network, OHIP = Ontario Health Insurance Plan.
Table 3:
Baseline characteristics of Clostridioides difficile infection cohorts in Ontario, 2005/06–2014/15, stratified by association and onset*
| Characteristic | No. (%) of patients† | ||||||
|---|---|---|---|---|---|---|---|
| ACH-associated/ACH-onset n = 17 272 |
ACH- or LTCF-associated/ACH-onset n = 842 |
LTCF-associated/LTCF-onset n = 544 |
LTCF- or ACH-associated/LTCF-onset n = 937 |
Community-associated/community-onset n = 7216 |
ACH-associated/community-onset n = 7098 |
All n = 33 909 |
|
| Patient days, mean ± SD | 49.00 ± 64.69 | 33.66 ± 46.46 | 13.89 ± 19.53 | 13.44 ± 14.68 | 20.63 ± 34.27 | 21.54 ± 38.35 | 35.29 ± 54.37 |
| Age at index date, yr, mean ± SD | 72.22 ± 15.29 | 81.43 ± 9.67 | 84.57 ± 8.48 | 82.14 ± 9.99 | 70.07 ± 17.78 | 71.48 ± 15.77 | 72.31 ± 15.90 |
| Age group, yr | |||||||
| 18–44 | 1005 (5.8) | 1–5‡ | 1–5‡ | 4–8‡ | 728 (10.1) | 486 (6.8) | 2227 (6.6) |
| 45–64 | 3516 (20.4) | 46–50‡ | 7–11‡ | 45–49‡ | 1578 (21.9) | 1485 (20.9) | 6687 (19.7) |
| 65–74 | 3576 (20.7) | 118 (14.0) | 48 (8.8) | 116 (12.4) | 1330 (18.4) | 1474 (20.8) | 6662 (19.6) |
| 75–84 | 5458 (31.6) | 296 (35.2) | 174 (32.0) | 337 (36.0) | 1946 (27.0) | 2197 (31.0) | 10 408 (30.7) |
| ≥ 85 | 3717 (21.5) | 377 (44.8) | 310 (57.0) | 431 (46.0) | 1634 (22.6) | 1456 (20.5) | 7925 (23.4) |
| Sex | |||||||
| Female | 8735 (50.6) | 504 (59.9) | 356 (65.4) | 585 (62.4) | 4305 (59.7) | 3880 (54.7) | 18 365 (54.2) |
| Male | 8537 (49.4) | 338 (40.1) | 188 (34.6) | 352 (37.6) | 2911 (40.3) | 3218 (45.3) | 15 544 (45.8) |
| Admitted within 90 d before onset | 5909 (34.2) | 481 (57.1) | 13 (2.4) | 937 (100.0) | 132 (1.8) | 7098 (100.0) | 14 570 (43.0) |
| Antibiotic use in 30 d before onset* | 4216 (24.4) | 342 (40.6) | 147 (27.0) | 512 (54.6) | 1570 (21.8) | 3212 (45.3) | 9999 (29.5) |
| Comorbidities | |||||||
| Cardiovascular disease | 10 771 (62.4) | 663 (78.7) | 378 (69.5) | 794 (84.7) | 3935 (54.5) | 5045 (71.1) | 21 586 (63.7) |
| Chronic obstructive pulmonary disease | 2057 (11.9) | 114 (13.5) | 77 (14.2) | 148 (15.8) | 771 (10.7) | 1104 (15.6) | 4271 (12.6) |
| Congestive heart failure | 3076 (17.8) | 205 (24.3) | 108 (19.9) | 286 (30.5) | 998 (13.8) | 1567 (22.1) | 6240 (18.4) |
| Diabetes | 1590 (9.2) | 100 (11.9) | 60 (11.0) | 92 (9.8) | 583 (8.1) | 775 (10.9) | 3200 (9.4) |
| Renal disease | 3682 (21.3) | 212 (25.2) | 98 (18.0) | 270 (28.8) | 1277 (17.7) | 1839 (25.9) | 7378 (21.8) |
| Liver disease | 1311 (7.6) | 52 (6.2) | 26 (4.8) | 55 (5.9) | 474 (6.6) | 686 (9.7) | 2604 (7.7) |
| Cancer | 3249 (18.8) | 76 (9.0) | 28 (5.1) | 84 (9.0) | 924 (12.8) | 1668 (23.5) | 6029 (17.8) |
| Pulmonary circulation disease | 634 (3.7) | 44 (5.2) | 20 (3.7) | 46 (4.9) | 197 (2.7) | 405 (5.7) | 1346 (4.0) |
| Valvular disease | 986 (5.7) | 53 (6.3) | 16 (2.9) | 73 (7.8) | 242 (3.4) | 462 (6.5) | 1832 (5.4) |
| Inflammatory bowel disease | 605 (3.5) | 16 (1.9) | 14 (2.6) | 35 (3.7) | 640 (8.9) | 537 (7.6) | 1847 (5.4) |
| Hospital characteristics | |||||||
| Location | |||||||
| Urban | 16 726 (96.8) | 823 (97.7) | 524 (96.3) | 916 (97.8) | 6802 (94.3) | 6624 (93.3) | 32 415 (95.6) |
| Rural | 546 (3.2) | 19 (2.3) | 20 (3.7) | 21 (2.2) | 414 (5.7) | 474 (6.7) | 1494 (4.4) |
| No. of beds | |||||||
| < 100 | 1682 (9.7) | 79 (9.4) | 87 (16.0) | 114 (12.2) | 1155 (16.0) | 1232 (17.4) | 4349 (12.8) |
| 100–299 | 7026 (40.7) | 409 (48.6) | 279 (51.3) | 430 (45.9) | 3118 (43.2) | 3037 (42.8) | 14 299 (42.2) |
| 300–499 | 6395 (37.0) | 282 (33.5) | 144 (26.5) | 312 (33.3) | 2221 (30.8) | 2130 (30.0) | 11 484 (33.9) |
| ≥ 500 | 2169 (12.6) | 72 (8.6) | 34 (6.3) | 81 (8.6) | 722 (10.0) | 699 (9.8) | 3777 (11.1) |
Note: ACH = acute care hospital, LTCF = long-term care facility, SD = standard deviation.
We excluded 22 329 cases from analysis because they occurred within 180 days of the index hospital admission date.
Except where noted otherwise.
Exact counts suppressed for privacy reasons.
Residents of LTCFs whose infection was associated with their facility, or with either the facility or an ACH contributed a smaller proportion of cases (1.6% and 2.8%, respectively). Patients in LTCF cohorts were older than those in other cohorts (mean age 84.6 yr for LTCF-associated/LTCF-onset, and 82.1 yr for LTCF- or ACH-associated/LTCF-onset).
More than 40% of the ACH- or LTCF-associated/ACH-onset, LTCF- or ACH-associated/LTCF-onset, and ACH-associated/community-onset cohort members had used antibiotics in the 30 days before onset of the infection.
A higher proportion of community-associated/community-onset cohort members than other cohort members had inflammatory bowel disease (8.9% v. 3.5% for ACH-associated/ACH-onset infection).
Incidence
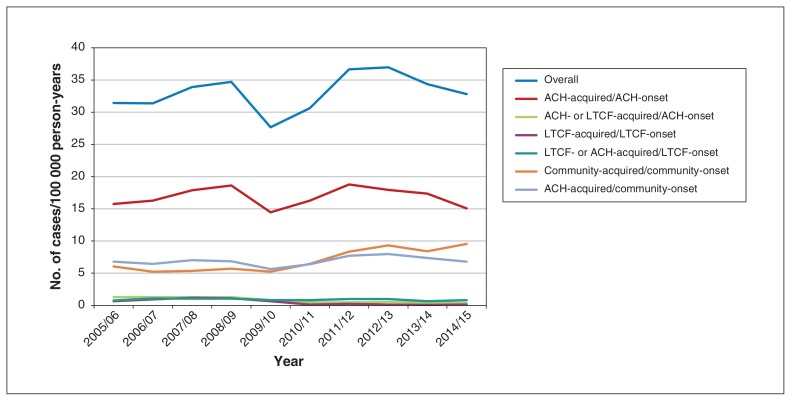
The overall annual number of cases of C. difficile infection per 100 000 person-years ranged from 27.7 (95% confidence interval 26.6–28.7) in 2009/10 to 37.0 (95% confidence interval 35.8–38.1) in 2012/13, with no obvious trends over time (Figure 2). The highest incidence was for ACH-associated/ACH-onset infection, and the second-highest was for ACH-associated/community-onset infection until 2009/10, when it was replaced with community-associated/community-onset infection.
Figure 2:
Number of cases of Clostridioides difficile infection per 100 000 person-years based on association and onset, 2005/06–2014/15.
Note: ACH = acute care hospital, LTCF = long-term care facility.
The number of cases of ACH-associated/ACH-onset C. difficile infection per 100 000 person-years declined from 18.8 in 2011/12 to 15.1 in 2014/15, a decrease of 19.6% (Figure 1; Appendix 1, available at www.cmajopen.ca/content/8/1/E16/suppl/DC1). There was a decline of 69.2% in ACH- or LTCF-associated/ACH-onset cases per 100 000 person-years from 2005/06 (1.30) to 2014/15 (0.40).
The highest incidence of LTCF-associated/LTCF-onset cases per 100 000 person-years was in 2008/09, at 1.20; it declined to 0.13 in 2014/15. Community-associated/community-onset cases increased by 36.3% between 2005/06 (6.09 cases per 100 000 person-years) and 2014/15 (9.56 cases per 100 000 person-years). For ACH-associated/community-onset cases, the incidence at the study period’s beginning and end was 6.8 cases per 100 000 person-years.
Comparison of cohort and control groups
Acute care hospital–associated/acute care hospital–onset infection
Compared to the control group, the infection cohort had a significantly lower proportion of LTCF residents and a longer hospital stay. Infected patients were more likely to have been admitted to hospital or have had health care exposure in the previous 12 weeks and up to 1 year prior, and were more likely to have used antibiotics in the 30 days before infection onset (Table 4). The infection cohort also had a higher prevalence of comorbidities, including cardiovascular disease and renal disease.
Table 4:
Baseline characteristics of Clostridioides difficile infection cohorts and matched control patients, stratified by association and onset*
| No. (%) of patients†‡ | ||||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| ACH-associated/ACH-onset | LTCF-associated/LTCF-onset | Community-associated/community-onset | ACH-associated/community-onset | |||||
|
|
|
|
|
|||||
| Characteristic | Cohort n = 13 152 |
Control group n = 33 058 |
Cohort n = 502 |
Control group n = 1407 |
Cohort n = 7116 |
Control group n = 21 127 |
Cohort n = 1847 |
Control group n = 3817 |
| Patient days, mean ± SD | 47.30 ± 59.70 | 12.11 ± 22.80 | 13.80 ± 17.80 | 0.96 ± 4.20 | 20.70 ± 34.40 | 0.85 ± 6.20 | 23.80 ± 33.40 | 13.80 ± 24.20 |
|
| ||||||||
| Age group, yr | ||||||||
|
| ||||||||
| 18–44 | 428 (3.3) | 943 (2.9) | 0 (0.0) | 0 (0.0) | 718 (10.1) | 2106 (10.0) | 61 (3.3) | 112 (2.9) |
|
| ||||||||
| 45–64 | 2238 (17.0) | 5263 (15.9) | 8 (1.6) | 18 (1.3) | 1552 (21.8) | 4491 (21.3) | 346 (18.7) | 621 (16.3) |
|
| ||||||||
| 65–74 | 2677 (20.4) | 6561 (19.8) | 41 (8.2) | 99 (7.0) | 1315 (18.5) | 3899 (18.5) | 436 (23.6) | 851 (22.3) |
|
| ||||||||
| 75–84 | 4558 (34.7) | 11 584 (35.0) | 166 (33.1) | 469 (33.3) | 1930 (27.1) | 5578 (26.4) | 628 (34.0) | 1393 (36.5) |
|
| ||||||||
| ≥ 85 | 3251 (24.7) | 8707 (26.3) | 287 (57.2) | 821 (58.4) | 1601 (22.5) | 5053 (23.9) | 376 (20.4) | 840 (22.0) |
|
| ||||||||
| Male sex | 6418 (48.8) | 16 015 (48.4) | 168 (33.5) | 455 (32.3) | 2876 (40.4) | 8533 (40.4) | 913 (49.4) | 1897 (49.7) |
|
| ||||||||
| LTCF resident | 660 (5.0) | 3088 (9.3) | 502 (100.0) | 1407 (100.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
|
| ||||||||
| Admitted in previous 12 wk | 4149 (31.5) | 7831 (23.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1847 (100.0) | 3817 (100.0) |
|
| ||||||||
| Admitted within 90 d before onset | 4246 (32.3) | 8043 (24.3) | 11 (2.2) | 16 (1.1) | 125 (1.8) | 101 (0.5) | 1847 (100.0) | 3817 (100.0) |
|
| ||||||||
| Admitted in year before onset | 6513 (49.5) | 13 429 (40.6) | 217 (43.2) | 403 (28.6) | 2303 (32.4) | 3631 (17.2) | 1847 (100.0) | 3817 (100.0) |
|
| ||||||||
| Comorbidities | ||||||||
|
| ||||||||
| Cardiovascular disease | 8287 (63.0) | 19 476 (58.9) | 343 (68.3) | 925 (65.7) | 3854 (54.2) | 9542 (45.2) | 1400 (75.8) | 2795 (73.2) |
|
| ||||||||
| Chronic obstructive pulmonary disease | 1498 (11.4) | 3597 (10.9) | 69 (13.7) | 147 (10.4) | 748 (10.5) | 1728 (8.2) | 310 (16.8) | 522 (13.7) |
|
| ||||||||
| Congestive heart failure | 2289 (17.4) | 5001 (15.1) | 93 (18.5) | 235 (16.7) | 954 (13.4) | 2305 (10.9) | 459 (24.9) | 900 (23.6) |
|
| ||||||||
| Diabetes | 1204 (9.2) | 2864 (8.7) | 57 (11.4) | 131 (9.3) | 569 (8.0) | 1503 (7.1) | 194 (10.5) | 408 (10.7) |
|
| ||||||||
| Renal disease | 2572 (19.6) | 4358 (13.2) | 83 (16.5) | 154 (10.9) | 1232 (17.3) | 2273 (10.8) | 512 (27.7) | 817 (21.4) |
|
| ||||||||
| Liver disease | 684 (5.2) | 1359 (4.1) | 20 (4.0) | 37 (2.6) | 433 (6.1) | 1501 (7.1) | 139 (7.5) | 261 (6.8) |
|
| ||||||||
| Cancer | 2115 (16.1) | 5550 (16.8) | 22 (4.4) | 69 (4.9) | 887 (12.5) | 2243 (10.6) | 444 (24.0) | 1118 (29.3) |
|
| ||||||||
| Pulmonary circulation disease | 408 (3.1) | 867 (2.6) | 16 (3.2) | 24 (1.7) | 179 (2.5) | 419 (2.0) | 99 (5.4) | 228 (6.0) |
|
| ||||||||
| Valvular disease | 696 (5.3) | 1540 (4.7) | 10 (2.0) | 49 (3.5) | 232 (3.3) | 704 (3.3) | 135 (7.3) | 280 (7.3) |
|
| ||||||||
| Inflammatory bowel disease | 414 (3.1) | 727 (2.2) | 14 (2.8) | 27 (1.9) | 634 (8.9) | 311 (1.5) | 127 (6.9) | 156 (4.1) |
|
| ||||||||
| Antibiotic use 30 d before onset | 3283 (25.0) | 6146 (18.6) | 141 (28.1) | 58 (4.1) | 1552 (21.8) | 949 (4.5) | 865 (46.8) | 1278 (33.5) |
|
| ||||||||
| Hospital location | ||||||||
|
| ||||||||
| Not admitted | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1252 (89.0) | 0 (0.0) | 19 475 (92.2) | 0 (0.0) | 0 (0.0) |
|
| ||||||||
| Rural | 449 (3.4) | 2694 (8.1) | 20 (4.0) | 7 (0.5) | 405 (5.7) | 127 (0.6) | 124 (6.7) | 360 (9.4) |
|
| ||||||||
| Urban | 12 703 (96.6) | 30 364 (91.9) | 482 (96.0) | 148 (10.5) | 6711 (94.3) | 1525 (7.2) | 1723 (93.3) | 3457 (90.6) |
|
| ||||||||
| No. of beds | ||||||||
|
| ||||||||
| Not admitted | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1252 (89.0) | 0 (0.0) | 19 475 (92.2) | 0 (0.0) | 0 (0.0) |
|
| ||||||||
| < 100 | 1380 (10.5) | 6813 (20.6) | 84 (16.7) | 18 (1.3) | 1138 (16.0) | 318 (1.5) | 308 (16.7) | 832 (21.8) |
|
| ||||||||
| 100–299 | 5652 (43.0) | 14 739 (44.6) | 259 (51.6) | 81 (5.8) | 3084 (43.3) | 674 (3.2) | 817 (44.2) | 1634 (42.8) |
|
| ||||||||
| 300–499 | 4665 (35.5) | 10 178 (30.8) | 130 (25.9) | 43 (3.1) | 2187 (30.7) | 484 (2.3) | 554 (30.0) | 1106 (29.0) |
|
| ||||||||
| ≥ 500 | 455 (11.1) | 1328 (4.0) | 29 (5.8) | 13 (0.9) | 707 (9.9) | 176 (0.8) | 168 (9.1) | 245 (6.4) |
Note: ACH = acute care hospital, LTCF = long-term care facility, SD = standard deviation.
Adapted from reference 19 (Oxford University Press).
Shaded cells denote the statistical significance (p ≤ 0.05) of the standardized difference between the infection cohort and matched control patients.
Except where noted otherwise.
Long-term care facility–associated/long-term care facility–onset infection
Compared to the control group, the infection cohort had a significantly longer hospital stay, and higher rates of renal disease, health care exposure in the previous year and antibiotic use in the previous 30 days (Table 4).
Community-associated/community-onset infection
There were multiple significant baseline differences between the cohort and control groups (Table 4). The former had a longer hospital stay, and a higher proportion of patients had had health care exposure in the previous year and antibiotic use in the previous 30 days. The infection cohort also had a higher prevalence of cardiovascular disease and renal disease. Acute care hospital–associated/community-onset infection Compared to the control group, those in the infection cohort had a significantly longer hospital stay, a higher rate of renal disease and a lower rate of cancer (Table 4).
Cost
For each cohort, costs were incurred primarily in the first 180 days after admission (Table 5), mainly owing to the hospital stay, followed by physician services, outpatient medications and emergency department visits. Clostridioides difficile infection associated with ACHs had the highest median cost for inpatient hospital stays ($36 370 v. $8270 for control patients), as well as the highest median overall cost compared to control patients ($48 593 v. $13 542). However, large differences in median cost between the infection cohort and control patients were also seen with community-associated infection ($20 258 v. $1144). The median cost of outpatient medications was highest in the LTCF group, although the infection cohort had a lower cost than the control group ($318 v. $1646).
Table 5:
Impact of hospital admission for Clostridioides difficile infection on costs (adjusted to 2015 Canadian dollars) 180 and 365 days after admission stratified by association and onset
| Item† | Cost, $* | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| ACH-associated | LTCF-associated | Community-associated | ACH-associated/community-onset | |||||
|
|
|
|
|
|||||
| Cohort | Control group | Cohort | Control group | Cohort | Control group | Cohort | Control group | |
| 180 d after admission | ||||||||
|
| ||||||||
| Inpatient hospital stays | ||||||||
|
| ||||||||
| Median (IQR) | 36 370 (19 700–72 050) | 8270 (4977–15 118) | 10 512 (5605–17 358) | 0 (0–0) | 13 249 (6106–28 465) | 0 (0–0) | 19 948 (10 793–40 147) | 8031 (4815–16 231) |
|
| ||||||||
| Mean ± SD | 60 634.46 ± 71 778.71 | 15 040.39 ± 26 077.94 | 16 204.58 ± 24 121.61 | 1686.52 ± 6180.19 | 26 078.24 ± 39 508.03 | 1521.96 ± 7627.97 | 34 061.46 ± 45 162.54 | 16 579.24 ± 29 053.97 |
|
| ||||||||
| Emergency department visits | ||||||||
|
| ||||||||
| Median (IQR) | 611 (0–902) | 559 (0–789) | 668 (0–829) | 0 (0–126) | 635 (167–958) | 0 (0–0) | 619 (0–915) | 548 (0–752) |
|
| ||||||||
| Mean ± SD | 666.89 ± 766.98 | 518.66 ± 495.71 | 617.35 ± 640.14 | 181.46 ± 420.58 | 708.21 ± 677.24 | 150.72 ± 423.95 | 724.86 ± 851.88 | 500.98 ± 478.89 |
|
| ||||||||
| Outpatient medications‡ | ||||||||
|
| ||||||||
| Median (IQR) | 192 (0–1038) | 146 (0–936) | 318 (42–1583) | 1646 (957–2434) | 278 (0–1224) | 332 (0–986) | 64 (0–1114) | 13 (0–683) |
|
| ||||||||
| Mean ± SD | 842.03 ± 2378.90 | 732.77 ± 1717.44 | 968.89 ± 1267.01 | 1781.81 ± 1152.34 | 1008.56 ± 2414.25 | 789.91 ± 2695.40 | 923.58 ± 2092.82 | 669.52 ± 1941.53 |
|
| ||||||||
| Physician services | ||||||||
|
| ||||||||
| Median (IQR) | 4579 (2592–8324) | 1788 (951–3234) | 1430 (832–2478) | 679 (515–988) | 2338 (1322–4465) | 310 (76–849) | 2808 (1412–5433) | 1586 (828–2802) |
|
| ||||||||
| Mean ± SD | 6827.81 ± 6945.65 | 577.23 ± 2830.52 | 2064.48 ± 2598.27 | 929.47 ± 1013.78 | 3667.64 ± 4133.96 | 776.38 ± 1437.33 | 4191.95 ± 4453.04 | 2367.13 ± 2772.78 |
|
| ||||||||
| Total costs | ||||||||
|
| ||||||||
| Median (IQR) | 48 593 (27 707–92 417) | 13 542 (8372–23 576) | 13 951 (8756–23 048) | 2995 (1942–4622) | 20 258 (10 658–41 263) | 1144 (300–3234) | 28 486 (16 058–53 697) | 13 557 (8100–25 203) |
|
| ||||||||
| Mean ± SD | 74 496.36 ± 78 111.95 | 21 833.49 ± 30 088.58 | 20 760.15 ± 27 362.89 | 5355.20 ± 9468.88 | 35 458.34 ± 45 345.38 | 4264.40 ± 11 330.13 | 44 738.49 ± 51 144.10 | 23 616.52 ± 33 062.58 |
|
| ||||||||
| 365 d after admission | ||||||||
|
| ||||||||
| Inpatient hospital stays | ||||||||
|
| ||||||||
| Median (IQR) | 38 832 (21 256–76 985) | 8391 (4999–15 430) | 10 829 (5605–18 865) | 0 (0–0) | 15 218 (6959–32 952) | 0 (0–0) | 21 765 (11 468–46 265) | 8159 (4862–16 938) |
|
| ||||||||
| Mean ± SD | 67 121.13 ± 90 699.65 | 15 446.71 ± 28 373.96 | 17 669.40 ± 32 455.65 | 2881.96 ± 8133.05 | 29 809.12 ± 47 241.81 | 3164.33 ± 11 986.56 | 39 015.27 ± 53 837.57 | 17 185.72 ± 32 413.07 |
|
| ||||||||
| Emergency department visits | ||||||||
|
| ||||||||
| Median (IQR) | 678 (0–1151) | 588 (0–814) | 692 (0–913) | 0 (0–533) | 723 (388–1211) | 0 (0–357) | 678 (0–1 228) | 578 (0–794) |
|
| ||||||||
| Mean ± SD | 867.96 ± 1118.86 | 577.73 ± 620.81 | 704.63 ± 735.94 | 330.35 ± 591.97 | 942.05 ± 987.16 | 307.38 ± 693.48 | 990.25 ± 1457.78 | 561.37 ± 610.01 |
|
| ||||||||
| Outpatient medications‡ | ||||||||
|
| ||||||||
| Median (IQR) | 396 (0–2153) | 215 (0–1709) | 332 (42–2729) | 3030 (1588–4666) | 518 (0–2432) | 685 (0–1980) | 96 (0–2084) | 25 (0–1078) |
|
| ||||||||
| Mean ± SD | 1674.50 ± 4784.24 | 1331.64 ± 3036.82 | 1645.95 ± 2354.53 | 3347.08 ± 2305.86 | 1940.65 ± 4352.58 | 1579.82 ± 5241.77 | 1675.95 ± 3684.43 | 1166.97 ± 3654.67 |
|
| ||||||||
| Physician services | ||||||||
|
| ||||||||
| Median (IQR) | 5298 (2969–9701) | 2128 (1079–3807) | 1729 (886–2943) | 1366 (1007–1969) | 3077 (1668–5733) | 743 (231–1877) | 3307 (1615–6535) | 1798 (914–3327) |
|
| ||||||||
| Mean ± SD | 7918.96 ± 8131.04 | 2967.15 ± 3144.92 | 2488.54 ± 3377.68 | 1711.19 ± 1539.40 | 4738.46 ± 5187.00 | 1538.04 ± 2393.55 | 5244.60 ± 5815.23 | 2726.24 ± 3317.25 |
|
| ||||||||
| Total costs | ||||||||
|
| ||||||||
| Median (IQR) | 54 169 (30 873–102 711) | 15 168 (9182–26 004) | 15 565 (9750–25 649) | 6232 (3732–9912) | 25 245 (12 873–51 066) | 2616 (721–7833) | 33 342 (17 487–65 334) | 14 837 (8710–28 267) |
|
| ||||||||
| Mean ± SD | 86 882.61 ± 101 953.16 | 24 224.39 ± 34 851.75 | 24 114.74 ± 38 520.25 | 9942.79 ± 16 397.21 | 44 047.52 ± 58 622.89 | 8564.23 ± 19 207.29 | 54 170.54 ± 65 160.54 | 26 175.76 ± 39 323.83 |
Note: ACH = acute care hospital, IQR = interquartile range, LTCF = long-term care facility, SD = standard deviation.
Shaded cells denote the statistical significance (p ≤ 0.05) of the standardized difference between the infection cohort and matched control patients.
Costs for other services such as home care and same-day surgical procedures were excluded from the table but included in the total costs.
Includes any prescription drugs purchased for outpatient use through the Ontario Drug Benefit plan. Costs are based on the total amount paid to the pharmacy (including pharmacy fees) from Ontario Ministry of Health and Long-Term Care.
Interpretation
In the last 15 years, Ontario hospitals have filled in important gaps toward improving antimicrobial stewardship, as well as stringent hand hygiene and cleaning practices.13,14,23 Our findings suggest that it is too early to assess the impact of these changes. Although the overall C. difficile infection rate appears to have declined between 2012 and March 2015, it follows an increase from 2005. Our data do confirm that community-associated C. difficile infection is increasing in incidence. This increase, and the considerable associated costs, have important implications for prevention strategies and practices.
Our data are compatible with those from other jurisdictions.24–26 A report from the sentinel hospitals of the Canadian Nosocomial Infection Surveillance Program also documented an increase in rates of hospital-associated C. difficile infection in central Canada from 2009 to 2012, followed by a decrease from 2012 to 2015. Those authors attributed the decline to improved infection control and prevention practices, and a regression in the NAP1 strain, which is associated with health care facility outbreaks.27
We observed low rates of LTCF-associated C. difficile infection, similar to a study of LTCFs in Alberta in 2012/13, which also showed infected patients to be older (≥ 85 yr) and female, likely owing to their higher prevalence in these facilities.6 The authors of a US study that also showed an annual decline in C. difficile infection rates in 10 LTCFs attributed the decline to the decreased use of fluoroquinolone.28
Conversely, we observed a 36% increase in the incidence of community-associated infection; similar increases have been observed in other North American studies.5,29–31 This trend may be due to increased exposure of community-dwelling people to outpatient health care settings, as well as greater clinician awareness of C. difficile infection as a potential cause of diarrhea, leading to more stool tests and diagnoses.
Patients whose C. difficile infection was associated with hospital admission had the highest median 180-day health care costs. The costs of community-associated and LTCF-associated infection were considerably lower, although still substantial. The control patients for both groups had much lower costs, mainly because many were not admitted to hospital. It is challenging to compare our costing results to those of previous studies, given that other investigators used a variety of time frames and typically focused on ACH-associated C. difficile infection only. Several systematic reviews have been conducted that reflect this variation but also validate our ACH-associated costs; estimated costs attributable to C. difficile infection have ranged from $10 861 to $36 960.32–35
The impact of community-associated C. difficile infection is not insignificant: up to 40% of patients require hospital admission, 20% experience a severe infection, and 28% have a recurrence.30 The continued education of physicians who may be the first point of health care contact for those with C. difficile infection (family physicians and emergency department physicians) is critical to identify patients at high risk quickly in order to permit successful treatment of the infection and to contain and limit transmission. Careful monitoring for C. difficile infection may include diagnostic testing of older patients with diarrhea who have recently been exposed to antibiotics and asking patients about recent hospital admissions and health care exposure. A 2018 Canadian analysis of isolates in community-acquired cases of C. difficile infection showed that all patients had been admitted in the previous year,36 which indicates that many, and perhaps all, community-associated cases actually have nosocomial origins.
Limitations
Although we strove to match infected patients to control patients with similar demographic characteristics and medical history in each association/onset group, infected patients had higher rates of several comorbidities, which may have contributed to their higher incurred health care costs, owing to both unrelated hospital admissions and difficulty recovering from C. difficile infection because of frailty.
Given that the health administrative databases did not include the actual date of symptom onset or laboratory test confirmation of C. difficile infection, we modified the Centers for Disease Control and Prevention case definitions;18 our adaptations have not been validated, and therefore a small proportion of cases may have been misclassified by location of association. Since we used ICD-10-CA codes to identify cases, any coding errors will have under- or overestimated the number of cases. However, we expect that this impact is minimal given that a Canadian study showed high sensitivity (88%) and specificity (100%) associated with the International Classification of Diseases, 9th Revision/ICD-10-CA code for C. difficile infection.37
We did not have access to outpatient data and could not include this information in our definition of “previous health care exposure,” so we could not assess whether recent exposure to outpatient health care settings was associated with C. difficile infection. We assumed that all ICD-10-CA codes for C. difficile infection within 180 days were from the same case and that codes 181 days or more from a prior code represented a new case; however, we did not have data on strain type to validate our assumptions.
We did not have access to prescription data for patients less than 65 years of age and those not receiving social assistance, which will have underestimated the total costs of C. difficile infection treatment and management. Data were not available for Ontario residents in whom C. difficile infection was diagnosed or who were admitted to hospitals outside the province. Given that only hospital costs and certain physician and drug costs are included in the databases, we were unable to estimate the total cost of C. difficile infection to the health care system.
Although the analysis included data up to March 2015 only, there have not been any substantial changes in antimicrobial stewardship or hospital reporting requirements since then, nor have there been changes in circulating strains of C. difficile. Finally, any incidence trends identified may have been confounded by changes in infection control practices within 1 or more hospital.
Conclusion
We have reported on the incidence and cost burden of C. difficile infection in Ontario using comprehensive provincial health administrative databases. Increases in incidence among community-dwelling people present a need to strengthen efforts to identify those at risk for this infectious disease, particularly those who have been prescribed antibiotics or have had recent health care exposure, including but not limited to hospital admission.
Supplementary Material
Acknowledgement
The authors gratefully acknowledge Dr. Shudong Li for carrying out analyses at ICES.
Footnotes
Competing interests: Jennifer Pereira and Allison McGeer acted as consultants to Sanofi Pasteur during the conduct of this study. Ayman Chit, Antigona Tomovici and Alex Selmani are employees of Sanofi Pasteur.
This article has been peer reviewed.
Contributors: Jennifer Pereira, Antigona Tomovici and Ayman Chit conceived and designed the study. Allison McGeer and Alex Selmani contributed to the study design and analysis plan. Jennifer Pereira drafted the manuscript. All of the authors interpreted the data, revised the manuscript critically for important intellectual content, approved the final version to be published and agreed to be accountable for all aspects of the work.
Funding: The study was funded by Sanofi Pasteur through the provision of salaries to Antigona Tomovici, Alex Selmani and Ayman Chit, and consulting fees to Jennifer Pereira and Allison McGeer, as well as research material.
Disclaimer: The authors had full control of study design, data collection and analysis, decision to publish and preparation of the manuscript. This study made use of deidentified data from the ICES Data Repository, which is managed by ICES with support from its funders and partners: Canada’s Strategy for Patient-Oriented Research (SPOR), the Ontario SPOR SUPPORT Unit, the Canadian Institutes of Health Research and the Government of Ontario. The opinions, results and conclusions reported are those of the authors. No endorsement by ICES or any of its funders or partners is intended or should be inferred. Parts of this material are based on data and information compiled and provided by the Canadian Institute for Health Information (CIHI). However, the analyses, conclusions, opinions and statements expressed herein are those of the authors and not necessarily those of CIHI. Parts of this material are based on data and information provided by Cancer Care Ontario (CCO). The opinions, results, view and conclusions reported in this paper are those of the authors and do not necessarily reflect those of CCO. No endorsement by CCO is intended or should be inferred.
Supplemental information: For reviewer comments and the original submission of this manuscript, please see www.cmajopen.ca/content/8/1/E16/suppl/DC1.
References
- 1.Levy AR, Szabo SM, Lozano-Ortega G, et al. Incidence and costs of Clostridium difficile infections in Canada. Open Forum Infect Dis. 2015;2:ofv076. doi: 10.1093/ofid/ofv076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kwong JC, Ratnasingham S, Campitelli MA, et al. The impact of infection on population health: results of the Ontario burden of infectious diseases study. PLoS One. 2012;7:e44103. doi: 10.1371/journal.pone.0044103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pépin J, Valiquette L, Alary ME, et al. Clostridium difficile-associated diarrhea in a region of Quebec from 1991 to 2003: a changing pattern of disease severity. CMAJ. 2004;171:466–72. doi: 10.1503/cmaj.1041104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.The Canadian Nosocomial Infection Surveillance Program (CNISP) Healthcare-associated-Clostridium difficile infection (HA-CDI) 2007–2011. Ottawa: Public Health Agency of Canada; [accessed 2016 Apr 4]. modified 2013 Nov 22. Available: www.phac-aspc.gc.ca/nois-sinp/projects/cdad-eng.php. [Google Scholar]
- 5.Forster AJ, Taljaard M, Oake N, et al. The effect of hospital-acquired infection with Clostridium difficile on length of stay in hospital. CMAJ. 2012;184:37–42. doi: 10.1503/cmaj.110543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lindeman C, Leal J, Rusk A, et al. Clostridium difficile infection in Alberta’s long-term care facilities. Can J Infect Control. 2017;32:87–92. [Google Scholar]
- 7.Simor AE. Diagnosis, management and prevention of Clostridium difficile infection in long-term care facilities: a review. J Am Geriatr Soc. 2010;58:1556–64. doi: 10.1111/j.1532-5415.2010.02958.x. [DOI] [PubMed] [Google Scholar]
- 8.Hunter JC, Mu Y, Dumyati GK, et al. Burden of nursing home-onset Clostridium difficile infection in the United States: estimates of incidence and patient outcomes. Open Forum Infect Dis. 2016;3:ofv196. doi: 10.1093/ofid/ofv196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Salaripour M, Johnstone J, Gardam M. Epidemiology of patients hospitalized with Clostridium difficile infection: a comparative analysis of community-associated and healthcare-associated Clostridium difficile infections. Can J Infect Control. 2018;33:96–101. [Google Scholar]
- 10.Gupta A, Khanna S. Community-acquired Clostridium difficile infection: an increasing public health threat. Infect Drug Resist. 2014;7:63–72. doi: 10.2147/IDR.S46780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lessa FC, Mu Y, Bamberg WM. Burden of Clostridium difficile infection in the United States. N Engl J Med. 2015;372:2368–9. doi: 10.1056/NEJMoa1408913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Loo VG, Poirier L, Miller MA, et al. A predominantly clonal multi-institutional outbreak of Clostridium difficile-associated diarrhea with high morbidity and mortality. N Engl J Med. 2005;353:2442–9. doi: 10.1056/NEJMoa051639. [DOI] [PubMed] [Google Scholar]
- 13.Daneman N, Stukel T, Ma X, et al. Impact of mandatory public reporting of hospital rates of Clostridium difficile in Ontario, Canada. PLoS Med. 2012;9:e1001268. doi: 10.1371/journal.pmed.1001268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clostridium difficile. Toronto: Ministry of Health and Long-Term Care; [accessed 2019 July 9]. Available: www.health.gov.on.ca/en/ccom/cdi/outbreak.aspx. [Google Scholar]
- 15.Types of ICES data. Toronto: ICES; [accessed 2016 Apr 5]. Available: www.ices.on.ca/Data-and-Privacy/ICES-data/Types-of-ICES-Data. [Google Scholar]
- 16.Data dictionary: data repository. Toronto: ICES; [accessed 2019 Dec 10]. Available: https://datadictionary.ices.on.ca/Applications/DataDictionary/Default.aspx. [Google Scholar]
- 17.Estimates of population, Canada, provinces, territories. Ottawa: Statistics Canada; [accessed 2017 Apr 2]. modified 2019 Nov 15. Available: http://www5.statcan.gc.ca/cansim/a26?lang=eng&id=510005. [Google Scholar]
- 18.McDonald LC, Coignard B, Dubberke E, et al. Ad Hoc Clostridium difficile Surveillance Working Group. Recommendations for surveillance of Clostridium difficile-associated disease. Infect Control Hosp Epidemiol. 2007;28:140–5. doi: 10.1086/511798. [DOI] [PubMed] [Google Scholar]
- 19.Pereira JA, McGeer A, Tomovici A, et al. The clinical burden of Clostridioides difficile in Ontario, Canada. Open Forum Infect Dis. 2019;6(12):ofz523. doi: 10.1093/ofid/ofz523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kralj B. Measuring rurality – RIO2008_BASIC: methodology and results. Toronto: Ontario Medical Association; 2009. [accessed 2019 Dec 10]. Available: www.oma.org/wp-content/uploads/2008rio-fulltechnicalpaper.pdf. [Google Scholar]
- 21.Van Walraven C, Austin PC, Jenings A, et al. A modification of the Elixhauser comorbidity measures into a point system for hospital death using administrative data. Med Care. 2009;47:626–33. doi: 10.1097/MLR.0b013e31819432e5. [DOI] [PubMed] [Google Scholar]
- 22.Austin PC. Assessing balance in measured baseline covariates when using many-to-one matching on the propensity-score. Pharmacoepidemiol Drug Saf. 2008;17:1218–25. doi: 10.1002/pds.1674. [DOI] [PubMed] [Google Scholar]
- 23.Gravel D, Gardam M, Taylor G, et al. Infection control practices related to Clostridium difficile infection in acute care hospitals in Canada. Am J Infect Control. 2009;37:9–14. doi: 10.1016/j.ajic.2008.07.012. [DOI] [PubMed] [Google Scholar]
- 24.Wilcox MH, Shetty N, Fawley WN, et al. Changing epidemiology of Clostridium difficile infection following the introduction of a national ribotyping-based surveillance scheme in England. Clin Infect Dis. 2012;55:1056–63. doi: 10.1093/cid/cis614. [DOI] [PubMed] [Google Scholar]
- 25.Giancola SE, Williams RD, Gentry CA. Prevalence of the Clostridium difficile BI/NAP1/027 strain across the United States Veterans Health Administration. Clin Microbiol Infect. 2018;24:877–81. doi: 10.1016/j.cmi.2017.11.011. [DOI] [PubMed] [Google Scholar]
- 26.Hensgens MP, Goorhuis A, Notermans DW, et al. Decrease of hypervirulent Clostridium difficile PCR ribotype 027 in the Netherlands. Euro Surveill. 2009;14 doi: 10.2807/ese.14.45.19402-en. pii=19402. [DOI] [PubMed] [Google Scholar]
- 27.Katz KC, Golding GR, Choi KB, et al. The evolving epidemiology of Clostridium difficile infection in Canadian hospitals during a postepidemic period (2009–2015) CMAJ. 2018;190:E758–65. doi: 10.1503/cmaj.180013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guh AY, Mu Y, Baggs J, et al. Trends in incidence of long-term-care facility onset Clostridium difficile infections in 10 US geographic locations during 2011–2015. Am J Infect Control. 2018;46:840–2. doi: 10.1016/j.ajic.2017.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Young-Xu Y, Kuntz JL, Gerding DN, et al. Clostridium difficile infection among Veterans Health Administration patients. Infect Control Hosp Epidemiol. 2015;36:1038–45. doi: 10.1017/ice.2015.138. [DOI] [PubMed] [Google Scholar]
- 30.Khanna S, Pardi DS, Aronson SL, et al. The epidemiology of community-acquired Clostridium difficile infection: a population-based study. Am J Gastroenterol. 2012;107:89–95. doi: 10.1038/ajg.2011.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Olsen MA, Young-Xu Y, Stwalley D, et al. The burden of Clostridium difficile infection: estimates of the incidence of CDI from U.S. administrative databases. BMC Infect Dis. 2016;16:177. doi: 10.1186/s12879-016-1501-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gabriel L, Beriot-Mathiot A. Hospitalization stay and costs attributable to Clostridium difficile infection: a critical review. J Hosp Infect. 2014;88:12–21. doi: 10.1016/j.jhin.2014.04.011. [DOI] [PubMed] [Google Scholar]
- 33.Ghantoji SS, Sail K, Lairson DR, et al. Economic healthcare costs of Clostridium difficile infection: a systematic review. J Hosp Infect. 2010;74:309–18. doi: 10.1016/j.jhin.2009.10.016. [DOI] [PubMed] [Google Scholar]
- 34.Zhang D, Prabhu VS, Marcella SW. Attributable healthcare resource utilization and costs for patients with primary and recurrent Clostridium difficile infection in the United States. Clin Infect Dis. 2018;66:1326–32. doi: 10.1093/cid/cix1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nanwa N, Kendzerska T, Krahn M. The economic impact of Clostridium difficile infection: a systematic review. Am J Gastroenterol. 2015;110:511–9. doi: 10.1038/ajg.2015.48. [DOI] [PubMed] [Google Scholar]
- 36.Thornton CS, Rubin JE, Greninger AL, et al. Epidemiological and genomic characterization of community-acquired Clostridium difficile infections. BMC Infect Dis. 2018;18:443. doi: 10.1186/s12879-018-3337-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Negron M, Barkema H, Rioux K, et al. Accuracy of ICD-9 and ICD-10 codes for Clostridium difficile among ulcerative colitis patients. Am J Gastroenterol. 2011;106:S481. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.