Abstract
The mechanisms underlying the release of interleukin-1 (IL-1) family cytokines from phagocytes have been the subject of intense investigations for more than 30 years. The absence of an N-terminal secretion signal from members of this family suggests a previously unknown mechanism of protein secretion that transfers cytosolic IL-1 directly across the plasma membrane into the extracellular space. The pore-forming protein gasdermin D (GSDMD) has emerged as the conduit for IL-1 secretion from the cytosol, serving to induce the release of IL-1 from living (hyperactive) or dead (pyroptotic) cells. In this Review, we discuss the mechanism by which GSDMD pore formation is regulated by the activity of inflammatory caspases, which are commonly associated with inflammasomes. We discuss how GSDMD promotes IL-1 release from hyperactive or pyroptotic cells, with a specific focus on defining how these distinct cell fates associated with GSDMD activity can be regulated. Last, the physiological consequences of GSDMD activity and therapeutic potential of targeting this pore-forming protein are discussed, which highlight the abundance of questions that remain to be answered by the community.
One sentence summary
This review summarizes our current understanding of the functions of Gasdermin D.
Introduction
The process by which mammalian cells express and secrete cytokines has long been the subject of investigation. Most cytokines are not present in resting cells and are encoded by genes that are inducible by inflammatory transcription factors, such as AP-1, nuclear factor–κB, and interferon regulatory factors (IRFs) (1). Consequently, it is generally accepted that various environmental stimuli induce intracellular signaling pathways that stimulate cytokine synthesis. Upon synthesis, most cytokines are translocated into the endoplasmic reticulum (ER), where transport through the biosynthetic pathway begins. Ultimately, these factors are released (secreted) into the extracellular space, where they bind cognate receptors and promote inflammatory and immunoregulatory activities. This general model applies to cytokines that contain N-terminal secretion sequences that mediate their cotranslational insertion into the ER.
One group of cytokines does not follow the path outlined above, members of which comprise some of the first cytokines identified: the interleukin-1 (IL-1) family (2). There are nine members of the IL-1 family, with the best characterized being IL-1α, IL-1β, and IL-18. These proteins do not contain N-terminal secretion signal sequences and are therefore not released into the extracellular space through the conventional secretory pathway. Rather, these proteins are secreted by a mechanism that (in many instances) depends on a pore-forming protein called gasdermin D (GSDMD). In this Review, we discuss the recent work on the biology of GSDMD and other GSDM family members, which all share the common ability to form pores within lipid bilayers (3–5). In the case of GSDMD, the pores formed operate as size-restricted conduits for the transport of IL-1 and other small molecules from the cytosolic space into the extracellular environment (6). If these GSDMD pores are formed in high abundance at the plasma membrane, a lytic form of cell death called pyroptosis occurs, which results in the release of numerous intracellular contents (including IL-1) (3–5). Pyroptosis should release intracellular (cytosolic) proteins regardless of whether they are small enough to pass through the pores formed by GSDMD. If GSDMD pores are formed in low abundance, plasma membrane pores form within living cells that serve as channels for IL-1 secretion (6). Thus, depending on context, GSDMD mediates the release of IL-1 from living or dead cells. In this Review, we describe recent cell biological, immunological, and structural analyses that have provided an increasingly clear view of the mechanisms and consequences of GSDMD activities in the immune system.
Genetic analysis of GSDMD and other GSDM family members
In 2015, three laboratories reported the identification of GSDMD as a regulator of IL-1 release from macrophages (7–9). All three studies focused their efforts on understanding inflammasome biology. Inflammasomes are members of a family of supramolecular organizing centers (SMOCs), which are receptor-proximal or cytosolic oligomeric protein complexes that operate as the principal subcellular sites of innate immune signal transduction (10). In this regard, SMOCs are considered the signaling organelles of the innate immune system. Of the known SMOCs, inflammasomes are unusual in their ability to drive immediate (transcription- independent) inflammatory responses because these organizing centers serve as the site of signals that induce inflammatory caspase activation, most notably caspase-1. Active caspase-1 can then cleave its cytosolic substrates, which include GSDMD and members of the IL-1 family. For the purpose of this Review, an extensive discussion of inflammasome biology is not required. We refer the reader to several recent reviews on this topic (11–14). However, some discussion of inflammasomes is necessary to best understand the regulation of GSDMD.
Like all SMOCs, inflammasomes are protein complexes that have two major components. One component is a “scaffold” protein with oligomerizing potential (classically referred to as an adaptor) and a second component consisting of effector enzymes. Upstream of these proteins is a factor we refer to as a “seed.” Interactions between the adaptor and the seed converts adaptor oligomerizing potential into activity, creating the scaffold for the recruitment and activation of effector enzymes within the inflammasome. The seed proteins are found in substoichiometric amounts within inflammasomes because a single seed can promote oligomerization of many adaptors, resulting in effector enzyme recruitment (15, 16). Numerous environmental stresses activate inflammasome seed proteins, such as microbial infections or cellular or subcellular injury. As such, proteins that seed inflammasome assembly include receptors that detect microbial products or other indicators of cellular dysfunction (11). The first studies that identified GSDMD as a regulator of inflammasome activities focused on cellular responses induced by cytosolic bacterial lipopolysaccharide (LPS) (8, 9), which binds directly to the mouse receptor caspase-11 (or human caspase-4 and −5) (17). After binding LPS, caspase-11 is activated to cleave GSDMD to form plasma membrane pores (3, 4). Disruption of the cell membrane then activates the protein NLRP3, which senses membrane disruption and serves as the seed to oligomerize the adaptor ASC. In this context, oligomerized ASC serves as the scaffold to recruit the downstream effector enzyme caspase-1 (18). This NLRP3-ASC– caspase-1 complex represents an inflammasome, which serves as the subcellular site of caspase-1 activation. Other inflammasomes activate caspase-1, with the best-defined being those seeded by the proteins NLRP1, AIM2, Pyrin, and several members of the NAIP family of NLRs (19). Different mechanisms drive activation of these receptors, but their activities culminate in a common outcome: the seeding of the oligomerizing unit that serves as a scaffold for caspase-1 recruitment, dimerization, and activation.
Caspase-1 has long been recognized as an enzyme that cleaves pro–IL-1β and other IL- 1 family members in the cytosol of mammalian cells and causes the release of IL-1 from cells. But the mechanism responsible for IL-1 release was perplexing until the discovery of GSDMD. Two independent forward genetic screens identified Gsdmd as a gene required for IL-1 release or pyroptosis in response to cytosolic LPS (8, 9). Shortly after that work was published, a third study identified GSDMD as a protein activated by the NLRP3 inflammasome that was necessary for IL-1 release and pyroptosis (7). Since these initial discoveries, GSDMD research has diverged into two areas: genetic analysis and mechanistic analysis. In the following sections, we will explore both of these areas of GSDMD biology.
Mechanisms of GSDMD activity
The human genome encodes six GSDM family members, whereas mice encode 10 members (9). Within these families, each member is ~45% homologous at the amino acid level. The best characterized and the subject of this Review is GSDMD, whose expression is positively regulated by the transcription factor interferon regulatory factor–2 (IRF2) (20). GSDMD is a 480–amino acid protein that contains two defined domains separated by a linker region. When inflammasomes are assembled, the GSDMD linker region is cleaved by caspase-1, which releases the N terminus from the C terminus (8, 9). Other enzymes can also cleave GSDMD, including mouse caspase-11, caspase-8, and human caspases-4 and −5 (Fig. 1) (8, 9, 21, 22). As will be described later, other enzymes can also cleave GSDMD. The cleaved N terminus is capable of auto-oligomerization on membranes when encountering phosphoinositides or other acidic lipids, resulting in the formation of a large circular pore (3, 4). Interactions with these lipids are thought to promote the assembly and insertion of the pore into membranes, which creates a conduit through which small molecules can traverse a lipid bilayer. Of the lipids bound to the GSDMD N terminus, several are found at the inner leaflet of the plasma membrane, including phosphatidylinositol 4,5-bisphosphate [PI(4,5)P2] and phosphatidylserine (PS) (3, 4). None are found on the outer leaflet of the plasma membrane of live cells. Cardiolipin is also bound by the GSDMD N terminus; this lipid is located exclusively on mitochondrial and bacterial membranes. On the basis of these observations, a model emerged in which in resting cells, GSDMD is auto-inhibited and resident in the cytosol. Within cells that contain active inflammatory caspases, GSDMD is cleaved within the linker region to create a noncovalent complex of N terminus and the auto-inhibitory C terminus. Interactions between the N terminus and acidic phospholipids may then release the C terminus, promote N terminus oligomerization, and facilitate membrane insertion, resulting in a functional pore.
Fig. 1. Activating and inhibitory cleavage sites within GSDMD.
Schematic of the domain structure of human GSDMD. The N-terminal pore-forming and C- terminal auto-inhibitory domains are indicated, along with an intervening linker region. The arrows above the schematic indicate the enzyme that cleaves GSDMD, and the arrows below the schematic indicate the amino acid that is targeted by each enzyme. The cleavage events mediated by caspase-3 and −7 are inactivating, which prevent pore formation. The cleavage mediated by ELANE (neutrophil elastase) and caspase-1, −8, and −11 promote pore-forming activity. Credit: A. Kitterman/Science Immunology
Evidence supporting this model is ample because caspase-dependent cleavage of GSDMD correlates with pore-forming activity within cells that contain active inflammatory caspases (8, 9). Moreover, cell-free systems demonstrated that full-length GSDMD can be cleaved by recombinant caspase-11 and that this cleavage event leads to the insertion of GSDMD pores into liposomes that contain acidic phospholipids (3, 4). Functionally, genetic ablation of inflammasome components renders macrophages unable to activate caspase-1 and therefore unable to cleave GSDMD (8, 9). Similarly, under conditions in which caspase-11 is the primary inflammatory caspase activated, genetic ablation of caspase-11 abolishes the cleavage of GSDMD (8, 9). Under these conditions, no plasma membrane pores are formed. Further support for the cleaved GSDMD pore model comes from an experiment in which the caspase-1 cleavage site within GSDMD was converted into a caspase-3 cleavage site (9). When this engineered GSDMD variant was expressed in cells, it was cleaved by caspase-3 in response to tumor necrosis factor receptor signaling, resulting in pore formation at the plasma membrane. Thus, cleavage of GSDMD is necessary and sufficient to stimulate the formation of plasma membrane pores that are classically associated with inflammasome activity. GSDMD can naturally be cleaved by caspase-3 within its N-terminal domain, an event that is thought to ablate its ability to form pores (23). In addition, the GSDMD homolog GSDME can be cleaved by caspase-3 to trigger pore formation (24). This process converts slow, noninflammatory apoptotic death to inflammatory pyroptosis (24, 25).
Structural perspectives of lipid binding, oligomerization, and pore formation
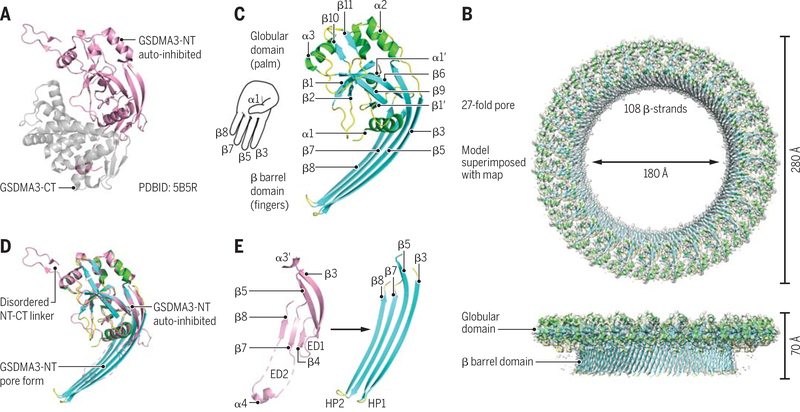
The auto-inhibited conformation of a full-length GSDM was revealed by the crystal structure of mouse GSDMDA3 (3), in which the C-terminal domain interacts closely with the N- terminal domain to suppress the toxic function of the N-terminal domain (Fig. 2A). When mapped to homologous structure models, many human genetic mutations in GSDM family members were shown to disrupt the auto-inhibited conformation, resulting in a gain of function (3). Recently, a crystal structure of human GSDMD was reported, confirming the overall structural architecture of GSDM proteins (26).
Fig. 2. GSDM structures and mechanisms.
(A) Crystal structure of auto-inhibited GSDMA3. (B) Cryo-EM map (gray) superimposed onto the atomic model of the 27-fold symmetric GSDMA3 N-terminal domain pore at 3.8-Å resolution. (C) Ribbon diagram of GSDMA3 N-terminal domain in the pore conformation. (D) Superposition of the auto-inhibited form and the pore form of GSDMA3 N-terminal domain. (E) Structural transitions that accompany the formation of the two β-hairpins HP1 and HP2 in the pore conformation. ED1 and ED2, extension domains 1 and 2, respectively. [Adapted from (27).] Credit: A. Kitterman/Science Immunology
To reveal the structure of the pore form of a GSDM, the cleaved N-terminal domain of mouse GSDMA3 was reconstituted to form pores in cardiolipin-containing liposomes, which were subsequently solubilized in detergent and subjected to cryo–electron microscopy (cryo-EM) structure determination (27). Using data collected on a Titan Krios microscope and a K2 direct electron detector, the three-dimensional structure of GSDMA3 pore with 27-fold symmetry was reconstructed to 3.8-Å resolution (Fig. 2B). GSDMA3 pores with 26- and 28-fold symmetry were also observed, but the main population of the reconstituted pores had 27-fold symmetry. The size of the pore is large, ~180 Å in the inner diameter. This diameter should permit the release of IL-1 family cytokines and other small proteins but not the enzymatically active tetramer of lactate dehydrogenase (LDH), which is often used for detection of cell death. Therefore, IL-1 secretion may be separated from cell death, with the former involving pore formation on the cytoplasmic membrane and the latter also requiring bursting of the cytoplasmic membrane after pore formation (6).
The subunit structure of the GSDMA3 N-terminal domain in the pore form resembles the shape of a left hand, with a globular palm domain, a positively charged thumb helix (α1), and four membrane-inserted fingers from two pairs of β-hairpins (Fig. 2C). The active conformation of the N-terminal domain displays prominent conformational changes in comparison with the auto-inhibited conformation, especially at the β-hairpin region (Fig. 2, D and E). By contrast, the palm and the thumb regions of the structures mainly retain their conformations during membrane insertion. The cryo-EM density revealed the bound cardiolipin head group, which is negatively charged and situated adjacent to the positively charged α1 thumb helix. In a full-length GSDM structure, this helix is buried at the interface with the C-terminal domain and not exposed to acidic lipids, explaining at least part of the mechanism of auto-inhibition. The interface for subunit interaction and oligomerization involves the palm region, the thumb helix, and the inserted β-hairpins.
Consequences of GSDMD activity within cells
The consequences of GSDMD activity were originally suggested to be terminal, resulting in pyroptosis or the lysis of bacterial cells containing exposed cardiolipin (8, 9). In mammalian cells, this conclusion was derived from studies that used stimuli that represent a variety of threats to the host. These threats can be of microbial or host origin. Well-characterized threats that stimulate GSDMD cleavage activate proteins that seed the assembly of inflammasomes. These stimuli include (i) ionic imbalances or organelle disruption (which activate NLRP3), (ii) bacterial proteases that induce the functional degradation of NLRP1, (iii) cytosolic double-stranded DNA (which stimulates AIM2 in mice and NLRP3 in humans), and bacterial toxins that alter Rho guanosine triphosphatase activity (which stimulate Pyrin) (11–13). Each of these threats leads to the assembly of an inflammasome and the consequential caspase-1–dependent pore formation. The cleavage of GSDMD results in pore formation at the cell surface and the death of the cell by means of pyroptosis (4, 5, 8, 9). For this reason, pyroptosis can be defined as GSDM-mediated lytic cell death (28). Because these stimuli were known to promote pyroptosis, the genetic identification of GSDMD as a regulator of pyroptosis led to the belief that pore formation led to the immediate lysis of the cell. However, subsequent work demonstrated that GSDMD pore formation does not necessarily lead to death (6, 29). Cell death after GSDMD pore formation is regulated and can be delayed or even avoided.
Evidence that suggests that pore-forming activity after inflammasome activation may not immediately kill cells derived from work that predated the discovery of the GSDMD pore. These studies were focused on defining how cytosolic LPS can promote pyroptosis and IL-1 release (18). Experimentally, there are several means by which LPS is delivered into the cytosol, with the simplest models being those that transfect or electroporate cells with this bacterial product (30, 31). Exposure to living gram-negative bacteria or bacteria-derived outer-membrane vesicles also delivers LPS to the cytosol (32, 33). Additional strategies of LPS delivery to the cytosol include infection with virulent gram-negative bacteria that encode type III or type IV secretion systems (30). All of these approaches are now recognized to activate the mouse LPS receptor caspase-11 (or caspase-4 and −5 in humans), resulting in GSDMD cleavage, pore formation, and pyroptosis (34). However, this minimal pathway of LPS–caspase-11–GSDMD does not involve caspase-1. This point is critical because caspase-11 cannot cleave pro–IL-1β (35). In fact, caspase-11 has no other known substrates besides GSDMD. Caspase-1, by contrast, can cleave GSDMD (8, 9) and pro–IL-1β (36, 37). Moreover, pro-IL-1β is cleaved by caspase-1 within cells transfected with LPS (30, 31), suggesting a mechanism to somehow activate caspase-1 after LPS detection by caspase-11. Caspase-11–dependent pores cause an efflux of K+ from the cell (18), an ionic imbalance that promotes the assembly of an NLRP3 inflammasome that activates caspase-1 (38). These conditions place GSDMD at two critical places: at the end of a short LPS-triggered pathway (LPS sensing activates caspase-11, −4, and −5 to cleave GSDMD) that directly causes membrane permeabilization and subsequent NLRP3 inflammasome assembly. The NLRP3 inflammasome stimulates caspase-1 to unleash a second wave of GSDMD activity and the sole wave of pro-IL1 processing. It is likely that NLRP3 activation occurs secondarily as a consequence of caspase-11–dependent K+ efflux when the membrane is damaged by GSDMD pores. The fact that wild-type cells release active IL-1 after detection of cytosolic LPS suggests that these cells must survive for at least a short time after pore formation in order to stimulate NLRP3. Thus, GSDMD pore formation does not instantaneously kill all cells.
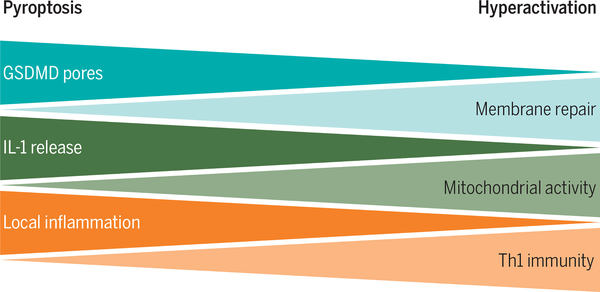
In response to certain stimuli, phagocytes can tolerate the presence of active inflammasomes and release IL-1 while maintaining viability for extended periods of time (39–41). The ability to add IL-1 to the repertoire of cytokines secreted renders these cells immunologically “hyperactive” (41). The term hyperactive is used to distinguish this activation state from that of traditionally activated macrophages (such as those stimulated with extracellular LPS), which cannot release IL-1. This distinction is physiologically important because immunizations that hyperactivate dendritic cells (DCs) stimulate a more robust T helper cell type I (TH1) adaptive immune response than immunizations that merely activate DCs (41). Cells that die from pyroptosis after inflammasome activation are poor stimulators of TH1immunity but induce strong local inflammatory responses (Fig. 3) (42). Recent work has demonstrated that GSDMD pores serve as conduits for the release of IL-1 from living (hyperactive) cells (6). This finding raises the question of how cells can tolerate the presence of GSDMD pores while maintaining viability.
Fig. 3. A working model to explain the cellular and physiological consequences of inflammasome-dependent GSDMD activity.
Within cells that contain inflammasomes, GSDMD is cleaved to form membrane pores. If these pores are formed in high abundance, membrane repair pathways are unable to repair them, and pyroptosis results. Pyroptotic cells release a large bolus of IL-1β at the time of lysis, which results in a strong local inflammatory response. However, the death of the responding cell limits its ability to participate in later immunological events, and consequently, pyroptosis-inducing stimuli are poor at inducing T cell–based adaptive immunity. When GSDMD pores are formed in low abundance, membrane repair pathways can remove them through exocytosis, endocytosis, or both. Some pores persist over time, resulting in the long-term release of IL-1β and the viability of the responding cell. The ability to survive GSDMD pores renders responding cells capable of participating in subsequent immunological events, which render these cells hyperactive, in terms of the ability to stimulate adaptive immunity. Credit: A. Kitterman/Science Immunology
When the plasma membrane is damaged by mechanical disruption or formation of large non–ion-selective pores, the concentration of ions that normally differs between the cytosol and extracellular fluids rapidly equalizes; not only is K+ released, which activates the NLRP3 inflammasome, but there is also an influx of Ca++ and Na+. All cells have the capacity to trigger a rapid mechanism to repair plasma membrane damage; this repair process is initiated when intracellular Ca++ levels rise above ~100 μM (43). This response has been called the “cellular wound-healing response” because it is activated by mechanical trauma to the cell membrane. However, it is also activated in response to bacterial pore-forming toxins and immune pore- forming proteins, including the killer lymphocyte cytotoxic granule protein, perforin, the pore- forming protein that delivers the granzymes into target cells to induce noninflammatory programmed cell death, and MLKL, the pore-forming protein responsible for necroptosis (44–46). Perforin forms a non–ion-selective β-barrel shaped pore of similar size and structure as GSDMD (~160-Å inner diameter) (47). The membrane repair response involves three processes: (i) Intracellular vesicles, including endosomes, lysosomes, and multivesicular bodies, are mobilized to the damaged membrane to donate their membranes to patch and reseal the damaged membrane; (ii) accelerated endocytosis pinches off and internalizes the damaged membrane; and (iii) the damaged membrane is removed into extracellular blebs or vesicles (43, 44, 48). Many of the proteins that target to membranes contain Ca++-sensing C2 domains, which regulate their trafficking to membranes. Consequently, cellular membrane repair can be inhibited by incubating cells with a cell-permeable Ca++-chelator. Rapid cell-membrane repair also depends on the endosomal sorting complex required for transport (ESCRT), particularly ESCRT-III, which is recruited to the damaged membrane (49). Knockdown of its components, such as CHMP4B and Vps4, can also be used to investigate whether membrane repair occurs.
Membrane repair plays a critical role in regulating cell death caused by immune pore- forming proteins. In killer cell cytotoxic granule-mediated cytotoxicity, repair of perforin plasma membrane damage is critical for preventing lytic cell death upon perforin-mediated delivery of the granzymes into cells, which is intended to activate noninflammatory death, so that only the targeted infected or cancerous cell is harmed without damaging bystander cells (45, 50). In necroptosis, activation of MLKL can be slowed down or averted by shedding damaged membrane bubbles, which can give the damaged cell time to function (present antigen and secrete chemokines) and even survive (46). Recently, Ca++ and ESCRT-III–dependent cell membrane repair was shown to be mobilized in response to GSDMD pores to reduce pyroptotic cell death by bone marrow–derived macrophages that were activated by Salmonella enterica serovar typhimurium infection or LPS transfection (51). Further work is needed to identify under what circumstances membrane repair is able to prevent GSDMD-mediated cell death and how these events influence the release of IL-1β and other inflammatory mediators. In some cells, the levels of GSDMD or the levels or activation of the inflammatory caspases that cleave it may be low enough that membrane repair overcomes membrane damage, whereas in other cells, membrane damage is too severe to be repaired (Fig. 3). So far, we do not know of cellular mechanisms that regulate how cleaved GSDMD forms pores, but phosphorylation of GSDME is a mechanism that influences pore-forming activity (52). Discovery of processes that regulate pore formation will undoubtedly shed light on the situations in which pyroptosis is triggered, but cell death is averted through membrane repair and other types of regulation. A recent study implicated the Toll–IL-1R protein SARM in macrophages in regulating how much pyroptosis versus IL-1 release occurs after NLRP3 inflammasome activation of macrophages (53). SARM deficiency increased IL-1β production and release but reduced pyroptosis, and increasing SARM had the opposite effect. SARM suppressed IL-1β by directly restraining the NLRP3 inflammasome and, hence, caspase-1 activation. It is likely that membrane repair is also triggered when other gasdermins are activated and regulates whether cells survive, although this has not been shown. Activation of membrane repair is likely the most rapid and immediate consequence of GSDMD pore formation at the cell surface.
Although studies of pyroptosis have focused on plasma membrane damage by activated GSDMD, GSDMD (and GSDME and possibly other gasdermins) bind avidly to cardiolipin that is on mitochondrial membranes (3, 4, 25). Although most cardiolipin is on the inner mitochondrial membrane, which is not accessible to cytosolic GSDMD, cardiolipin also shuttles to the outer membrane, where it could serve as a docking site for pore formation. Moreover, other acidic phospholipids that N-terminal GSDMD bind in the inner leaflet of the plasma membrane are also contained on the outer leaflet of endosomes, phagosomes, and lysosomes (54); these membranes could potentially be damaged during pyroptosis. Recent studies suggest that activated gasdermins induce mitochondrial damage, which amplifies pyroptosis. Single-cell imaging of macrophages undergoing pyroptosis showed that activated GSDMD causes loss of mitochondrial transmembrane potential and lysosomal disruption before the plasma membrane is permeabilized (55). This study also showed that cells undergoing pyroptosis release into their culture supernatants proteins normally found within mitochondria (cytochrome c), lysosomes (cathepsin B), and nuclei (HMGB1), suggesting that organelle membranes are also damaged. Mitochondrial reactive oxygen species (ROS) has been shown to be induced by Shiga toxin 2 plus LPS in a caspase-4– and GSDMD-dependent, but NLRP3-independent, manner in THP-1 cells (56). Moreover, in this system, ROS scavengers suppress IL-1 release and pyroptosis. SARM, which increases pyroptosis, localizes to mitochondria and, after LPS priming and nigericin treatment of macrophages, clusters on mitochondria (53). Treatment of macrophages with peptidoglycan, which causes IL-1 release without pyroptosis (“hyperactivation”) (6, 57), did not cause SARM clustering or much mitochondrial depolarization (53). How SARM does this and whether SARM plays a role in other inflammasome-mediated pathways is unclear. Taken together, these studies suggest that activated GSDMD might bind rapidly to mitochondrial membranes to damage them and cause mitochondrial ROS and loss of transmembrane potential that promote the commitment of cells to pyroptosis. However, further studies are needed to understand how mitochondria are damaged, how mitochondrial damage is regulated, and how it drives pyroptosis. In a recent study, an ectopically expressed N-terminal GSDME fusion protein also appeared to localize to mitochondria and cause mitochondrial release of mitochondrial intermembrane space proteins (cytochrome c and HtrA2) (52), suggesting that other gasdermins also trigger mitochondrial damage and possibly activate apoptotic caspases.
The scenarios described above indicate that pore formation at the cell surface may not be sufficient for cells to commit to pyroptosis. In some settings, mitochondrial disruption may be needed for pyroptosis to occur, and/or membrane damage may be repaired to allow the cell to survive. Cells that lack mitochondrial damage, with low levels of GSDMD pores or with exuberant membrane repair, may avoid pyroptosis but rather use plasma membrane–localized GSDMD pores as conduits for the secretion of IL-1 family cytokines while maintaining viability (Fig. 3).
An increasing diversity of contexts have been identified in which living cells release IL-1. In most instances in which the function of GSDMD has been examined, this pore-forming protein is necessary for the rapid release of IL-1 (6, 29). However, even in cells where GSDMD is necessary for the rapid release of IL-1, a delayed release of this cytokine can occur in the absence of GSDMD (58). GSDMD is therefore considered a regulator of cell hyperactivation as well as pyroptosis. Evidence supporting this claim derives from studies that have demonstrated the correlation between GSDMD pore formation in cellular or liposomal membranes and the release of IL-1 across these same lipid bilayers. This correlation has been observed with several stimuli that hyperactivate macrophages, including the oxidized phospholipids PGPC or POVPC, bacterial peptidoglycan or its N-Acetylglucosamine (NAG) fragment, and mutant Staphylococcus aureus that contain easily degradable peptidoglycans (6, 29). There is no evidence to support the idea that IL-1 is specifically selected as cargo for release by means of GSDMD pores. The release of IL-1 may simply be because (i) its small size (diameter 4.5 nm), which would increase its diffusion rate and ability to translocate through the GSDMD pore; (ii) its abundance in cells after TLR activation; and (iii) its proximity to the pore itself. On this latter point, a recent study demonstrated that cleaved IL-1 localizes to the plasma membrane (58), perhaps through interaction with lipids, poising this cytokine for rapid secretion upon pore formation. The full spectrum of factors released by GSDMD pores from living cells in unclear, but K+ ions represent factors of interest. Immediately after pore formation occurs, K+ efflux via GSDMD can occur, which (as described above) can drive NLRP3 inflammasome assembly. In addition, the efflux of K+ inactivates the interferon-inducing DNA sensor cyclic guanosine monophosphate–adenosine monophosphate synthase (cGAS) (59).
Within neutrophils, GSDMD appears to have additional activities. Like DCs and macrophages, neutrophils can be induced to release IL-1 while maintaining viability (39). This resistance to cell death is likely linked to cell type–specific differences in the kinetics and robustness of caspase-1 activity (60). When using stimuli that promote pyroptosis in macrophages, robust caspase-1 activity is observed within minutes of inflammasome assembly. Side-by-side stimulations of neutrophils revealed that caspase-1 activity occurs less robustly and with slower kinetics (60). Consequently, the rates of GSDMD pore formation differs between these cell types, which may result in macrophages experiencing an abundance of GSDMD pores that overwhelm the repair machinery and result in pyroptosis. Neutrophils, by contrast, may be able to repair the smaller pool of GSDMD pores before lysis occurs, allowing these cells to achieve a hyperactive state in which IL-1 is released from viable cells.
In aged neutrophils, caspase-1 is not responsible for GSDMD cleavage. Rather, neutrophil elastase (ELANE) and cathepsin G are reportedly capable of GSDMD cleavage (61, 62). These two studies differ in their conclusion on the ability of ELANE to cleave GSDMD, suggesting that further work is necessary to clarify the role these enzymes in GSDMD activities in neutrophils. Neutrophils produce neutrophil extracellular traps (NETs), which are antibacterial webs of nuclear DNA that are released from these cells during various infections (63). GSDMD pores appear to play a critical role in NET formation and release because GSDMD appears to be necessary for nuclear envelope disruption and genomic release into the cytosol and for plasma membrane disruption that releases DNA into the extracellular environment (64, 65). The necessity of GSDMD for disruption of the nuclear envelope provides additional evidence that the plasma membrane is not the only site of pore formation.
Consequences of GSDMD activity within mice
Although much of our mechanistic insight into GSDMD activities has been obtained from in vitro studies, several functions of GSDMD in mice have been identified. GSDMD-deficient mice are more susceptible than their wild-type counterparts to a variety of infections, such as Burkholderia thailandensis and Francisella tularensis subspecies novicida (66, 67).
GSDMD has also been implicated in the regulation of the inflammatory responses within mice that harbor active alleles of NLRP3 or Pyrin. These NLRP3 alleles are associated with neonatal-onset multisystem inflammatory disease (NOMID) and result in IL-1–dependent inflammation (68). GSDMD is necessary for the inflammatory responses in mice that harbor NOMID-associated NLRP3 mutants (69). Similar findings were made with mice that harbor active alleles of Pyrin, which also induce inflammasome-dependent IL-1–associated inflammation (70). In these mice, which contain Pyrin mutants associated with the human disease Familial Mediterranean Fever (FMF), GSDMD is necessary for inflammation-associated pathology. Whether the GSDMD-dependent IL-1 that is released from these patients (or mice) occurs from pyroptotic or hyperactive cells is unknown, but FMF mice do not have reduced numbers of inflammatory phagocytes (71).
GSDMD deficiency is not uniformly associated with increased susceptibility to infection because the lethality associated with mouse norovirus infections is delayed in the absence of this pore-forming protein (72). The reason for this delay is likely linked to a decrease in intestinal inflammation in GSDMD-deficient mice. In the absence of GSDMD-dependent inflammation, mice live longer and have fewer infection-associated symptoms (72). Studies of how the NAIP-NLRC4 inflammasome induces pyroptosis of intestinal epithelial cells have also implicated GSDMD in this process because GSDMD-deficient small intestinal epithelia are resistant to inflammasome-induced pyroptosis (73). Gsdmd−/− mice also control Escherichia coli better than wild-type mice (61). In this case, the paradoxical improvement in host defense was linked to more neutrophils at the site of infection and a longer neutrophil half-life because there was no ELANE-triggered pyroptosis.
Because of the increasing importance of GSDMD-dependent pore formation in inflammation, pharmacological inhibitors of this activity have begun to emerge. For example, necrosulpfonamide was identified as a molecule that bind directly to GSDMD and prevents pyroptosis (74). Consequently, this compound suppresses inflammatory responses associated with murine models of sepsis. Genetic GSDMD deficiency also protects mice from LPS-induced or polymicrobial sepsis-induced inflammation (75).
Perspectives
The discussions offered above were designed to offer a perspective on the mechanisms and consequences of GSDMD activity. Although the inflammatory functions for GSDMD were necrosulfonamide was identified as a molecule that binds directly to GSDMD and prevents pyroptosis (74). Consequently, this compound suppresses inflammatory responses associated with mouse models of sepsis. Genetic GSDMD deficiency also protects mice from LPS-induced or polymicrobial sepsis–induced inflammation (75).
Only recently defined, research into this protein has proceeded at a rapid pace. Today, we have some understanding of how GSDMD pores are regulated and the importance of this regulation for a variety of physiological responses. However, large gaps remain in our understanding of GSDMD and the pathways that regulate this protein. For example, why some cells and stimuli use GSDMD pores to promote pyroptosis or cell hyperactivation is still not completely understood, yet the physiological distinction between these two cell fates (life verses death) is huge. The upstream regulators of inflammasome activity that influence GSDMD cleavage are only beginning to be defined, as well as the cell types that naturally use GSDMD activities. The spectrum of factors that can be secreted by means of GSDMD pores also remains undefined.
When the discussion shifts to the in vivo consequences and therapeutic potential of GSDMD activity and manipulations, even more questions arise. What is the fate of GSDMD pores once assembled in the plasma membrane? The N-terminal domain is released into culture supernatants in vitro and is active (4), but is it released in membrane bound vesicles and active in vivo, and is it degraded in the extracellular space or in lysosomes? Does the ability of GSDMD to add IL-1 to the repertoire of factors secreted by DCs render these hyperactive cells more potent stimulators of protective immunity? oxPAPC has been shown to synergize with LPS to promote stronger antigen-specific T cell responses in mice than does LPS alone, but the role of GSDMD in these activities is undefined. Last, the emerging focus on the development of GSDMD inhibitors raises the question of whether these therapeutics will provide different benefits than current those of strategies that block IL-1. We envision a bright future for GSDMD research, which will be focused on answering the questions outlined above and raising new ones.
Acknowledgments
We thank members of the laboratories of J.C.K, J.L., and H.W. for helpful discussions.
Funding: J.C.K. is supported by NIH grants AI093589, AI116550, AI133524, and P30 DK34854 and an Investigators in the Pathogenesis of Infectious Disease Award from the Burroughs Wellcome Fund. H.W. is supported by NIH grants HD087988, AI139914, Al124491, AI050872, and AI125535.
References and Notes
- 1.Smale ST, Natoli G, Transcriptional control of inflammatory responses. Cold Spring Harbor perspectives in biology 6, a016261 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Garlanda C, Dinarello CA, Mantovani A, The interleukin-1 family: back to the future. Immunity 39, 1003–1018 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ding J et al. , Pore-forming activity and structural autoinhibition of the gasdermin family. Nature 535, 111–116 (2016). [DOI] [PubMed] [Google Scholar]
- 4.Liu X et al. , Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores. Nature 535, 153–158 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sborgi L et al. , GSDMD membrane pore formation constitutes the mechanism of pyroptotic cell death. The EMBO journal 35, 1766–1778 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Evavold CL et al. , The Pore-Forming Protein Gasdermin D Regulates Interleukin-1 Secretion from Living Macrophages. Immunity 48, 35–44 e36 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.He WT et al. , Gasdermin D is an executor of pyroptosis and required for interleukin- 1beta secretion. Cell Res 25, 1285–1298 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kayagaki N et al. , Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature 526, 666–671 (2015). [DOI] [PubMed] [Google Scholar]
- 9.Shi J et al. , Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 526, 660–665 (2015). [DOI] [PubMed] [Google Scholar]
- 10.Kagan JC, Magupalli VG, Wu H, SMOCs: supramolecular organizing centres that control innate immunity. Nature reviews. Immunology, (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brewer SM, Brubaker SW, Monack DM, Host inflammasome defense mechanisms and bacterial pathogen evasion strategies. Current opinion in immunology 60, 63–70 (2019). [DOI] [PubMed] [Google Scholar]
- 12.Place DE, Kanneganti TD, Recent advances in inflammasome biology. Current opinion in immunology 50, 32–38 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Swanson KV, Deng M, Ting JP, The NLRP3 inflammasome: molecular activation and regulation to therapeutics. Nature reviews. Immunology, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shen C, Sharif H, Xia S, Wu H, Structural and mechanistic elucidation of inflammasome signaling by cryo-EM. Curr Opin Struct Biol 58, 18–25 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tenthorey JL et al. , The structural basis of flagellin detection by NAIP5: A strategy to limit pathogen immune evasion. Science 358, 888–893 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang L et al. , Cryo-EM structure of the activated NAIP2-NLRC4 inflammasome reveals nucleated polymerization. Science 350, 404–409 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shi J et al. , Inflammatory caspases are innate immune receptors for intracellular LPS. Nature 514, 187–192 (2014). [DOI] [PubMed] [Google Scholar]
- 18.Ruhl S, Broz P, Caspase-11 activates a canonical NLRP3 inflammasome by promoting K(+) efflux. European journal of immunology 45, 2927–2936 (2015). [DOI] [PubMed] [Google Scholar]
- 19.Broz P, Dixit VM, Inflammasomes: mechanism of assembly, regulation and signalling. Nature reviews. Immunology 16, 407–420 (2016). [DOI] [PubMed] [Google Scholar]
- 20.Kayagaki N et al. , IRF2 transcriptionally induces GSDMD expression for pyroptosis. Science signaling 12, (2019). [DOI] [PubMed] [Google Scholar]
- 21.Sarhan J et al. , Caspase-8 induces cleavage of gasdermin D to elicit pyroptosis during Yersinia infection. Proceedings of the National Academy of Sciences of the United States of America 115, E10888–E10897 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Orning P et al. , Pathogen blockade of TAK1 triggers caspase-8-dependent cleavage of gasdermin D and cell death. Science 362, 1064–1069 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Taabazuing CY, Okondo MC, Bachovchin DA, Pyroptosis and Apoptosis Pathways Engage in Bidirectional Crosstalk in Monocytes and Macrophages. Cell Chem Biol 24, 507–514 e504 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rogers C et al. , Cleavage of DFNA5 by caspase-3 during apoptosis mediates progression to secondary necrotic/pyroptotic cell death. Nature communications 8, 14128 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang Y et al. , Chemotherapy drugs induce pyroptosis through caspase-3 cleavage of a Gasdermin. Nature, (2017). [DOI] [PubMed] [Google Scholar]
- 26.Liu Z et al. , Crystal Structures of the Full-Length Murine and Human Gasdermin D Reveal Mechanisms of Autoinhibition, Lipid Binding, and Oligomerization. Immunity, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ruan J, Xia S, Liu X, Lieberman J, Wu H, Cryo-EM structure of the gasdermin A3 membrane pore. Nature 557, 62–67 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shi J, Gao W, Shao F, Pyroptosis: Gasdermin-Mediated Programmed Necrotic Cell Death. Trends Biochem Sci 42, 245–254 (2017). [DOI] [PubMed] [Google Scholar]
- 29.Heilig R et al. , The Gasdermin-D pore acts as a conduit for IL-1beta secretion in mice. European journal of immunology, (2017). [DOI] [PubMed] [Google Scholar]
- 30.Hagar JA, Powell DA, Aachoui Y, Ernst RK, Miao EA, Cytoplasmic LPS activates caspase-11: implications in TLR4-independent endotoxic shock. Science 341, 1250–1253 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kayagaki N et al. , Noncanonical inflammasome activation by intracellular LPS independent of TLR4. Science 341, 1246–1249 (2013). [DOI] [PubMed] [Google Scholar]
- 32.Rathinam VA et al. , TRIF licenses caspase-11-dependent NLRP3 inflammasome activation by gram-negative bacteria. Cell 150, 606–619 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vanaja SK et al. , Bacterial Outer Membrane Vesicles Mediate Cytosolic Localization of LPS and Caspase-11 Activation. Cell 165, 1106–1119 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kovacs SB, Miao EA, Gasdermins: Effectors of Pyroptosis. Trends in cell biology 27, 673–684 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang S et al. , Identification and characterization of Ich-3, a member of the interleukin- 1beta converting enzyme (ICE)/Ced-3 family and an upstream regulator of ICE. The Journal of biological chemistry 271, 20580–20587 (1996). [DOI] [PubMed] [Google Scholar]
- 36.Thornberry NA et al. , A novel heterodimeric cysteine protease is required for interleukin-1 beta processing in monocytes. Nature 356, 768–774 (1992). [DOI] [PubMed] [Google Scholar]
- 37.Cerretti DP et al. , Molecular cloning of the interleukin-1 beta converting enzyme. Science 256, 97–100 (1992). [DOI] [PubMed] [Google Scholar]
- 38.Gurcel L, Abrami L, Girardin S, Tschopp J, van der Goot FG, Caspase-1 activation of lipid metabolic pathways in response to bacterial pore-forming toxins promotes cell survival. Cell 126, 1135–1145 (2006). [DOI] [PubMed] [Google Scholar]
- 39.Chen KW et al. , The neutrophil NLRC4 inflammasome selectively promotes IL-1beta maturation without pyroptosis during acute Salmonella challenge. Cell reports 8, 570–582 (2014). [DOI] [PubMed] [Google Scholar]
- 40.Gaidt MM et al. , Human Monocytes Engage an Alternative Inflammasome Pathway. Immunity 44, 833–846 (2016). [DOI] [PubMed] [Google Scholar]
- 41.Zanoni I et al. , An endogenous caspase-11 ligand elicits interleukin-1 release from living dendritic cells. Science 352, 1232–1236 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Evavold CL, Kagan JC, How Inflammasomes Inform Adaptive Immunity. J Mol Biol 430, 217–237 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McNeil PL, Terasaki M, Coping with the inevitable: how cells repair a torn surface membrane. Nature cell biology 3, E124–129 (2001). [DOI] [PubMed] [Google Scholar]
- 44.Idone V et al. , Repair of injured plasma membrane by rapid Ca2+-dependent endocytosis. The Journal of cell biology 180, 905–914 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Keefe D et al. , Perforin triggers a plasma membrane-repair response that facilitates CTL induction of apoptosis. Immunity 23, 249–262 (2005). [DOI] [PubMed] [Google Scholar]
- 46.Gong YN et al. , ESCRT-III Acts Downstream of MLKL to Regulate Necroptotic Cell Death and Its Consequences. Cell 169, 286–300 e216 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Law RH et al. , The structural basis for membrane binding and pore formation by lymphocyte perforin. Nature 468, 447–451 (2010). [DOI] [PubMed] [Google Scholar]
- 48.Reddy A, Caler EV, Andrews NW, Plasma membrane repair is mediated by Ca(2+)- regulated exocytosis of lysosomes. Cell 106, 157–169 (2001). [DOI] [PubMed] [Google Scholar]
- 49.Jimenez AJ et al. , ESCRT machinery is required for plasma membrane repair. Science 343, 1247136 (2014). [DOI] [PubMed] [Google Scholar]
- 50.Thiery J et al. , Perforin pores in the endosomal membrane trigger the release of endocytosed granzyme B into the cytosol of target cells. Nature immunology 12, 770–777 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ruhl S et al. , ESCRT-dependent membrane repair negatively regulates pyroptosis downstream of GSDMD activation. Science 362, 956–960 (2018). [DOI] [PubMed] [Google Scholar]
- 52.Rogers C et al. , Gasdermin pores permeabilize mitochondria to augment caspase-3 activation during apoptosis and inflammasome activation. Nature communications 10, 1689 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Carty M et al. , Cell Survival and Cytokine Release after Inflammasome Activation Is Regulated by the Toll-IL-1R Protein SARM. Immunity, (2019). [DOI] [PubMed] [Google Scholar]
- 54.Leventis PA, Grinstein S, The distribution and function of phosphatidylserine in cellular membranes. Annu Rev Biophys 39, 407–427 (2010). [DOI] [PubMed] [Google Scholar]
- 55.de Vasconcelos NM, Van Opdenbosch N, Van Gorp H, Parthoens E, Lamkanfi M, Single-cell analysis of pyroptosis dynamics reveals conserved GSDMD-mediated subcellular events that precede plasma membrane rupture. Cell Death Differ 26, 146–161 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Platnich JM et al. , Shiga Toxin/Lipopolysaccharide Activates Caspase-4 and Gasdermin D to Trigger Mitochondrial Reactive Oxygen Species Upstream of the NLRP3 Inflammasome. Cell reports 25, 1525–1536 e1527 (2018). [DOI] [PubMed] [Google Scholar]
- 57.Shimada T et al. , Staphylococcus aureus evades lysozyme-based peptidoglycan digestion that links phagocytosis, inflammasome activation, and IL-1beta secretion. Cell host and microbe 7, 38–49 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Monteleone M et al. , Interleukin-1beta Maturation Triggers Its Relocation to the Plasma Membrane for Gasdermin-D-Dependent and -Independent Secretion. Cell reports 24, 1425–1433 (2018). [DOI] [PubMed] [Google Scholar]
- 59.Banerjee I et al. , Gasdermin D Restrains Type I Interferon Response to Cytosolic DNA by Disrupting Ionic Homeostasis. Immunity 49, 413–426 e415 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Boucher D et al. , Caspase-1 self-cleavage is an intrinsic mechanism to terminate inflammasome activity. The Journal of experimental medicine 215, 827–840 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kambara H et al. , Gasdermin D Exerts Anti-inflammatory Effects by Promoting Neutrophil Death. Cell reports 22, 2924–2936 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Burgener SS et al. , Cathepsin G Inhibition by Serpinb1 and Serpinb6 Prevents Programmed Necrosis in Neutrophils and Monocytes and Reduces GSDMD-Driven Inflammation. Cell reports 27, 3646–3656 e3645 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sollberger G, Tilley DO, Zychlinsky A, Neutrophil Extracellular Traps: The Biology of Chromatin Externalization. Developmental cell 44, 542–553 (2018). [DOI] [PubMed] [Google Scholar]
- 64.Sollberger G et al. , Gasdermin D plays a vital role in the generation of neutrophil extracellular traps. Sci Immunol 3, (2018). [DOI] [PubMed] [Google Scholar]
- 65.Chen KW et al. , Noncanonical inflammasome signaling elicits gasdermin D-dependent neutrophil extracellular traps. Sci Immunol 3, (2018). [DOI] [PubMed] [Google Scholar]
- 66.Wang J, Deobald K, Re F, Gasdermin D Protects from Melioidosis through Pyroptosis and Direct Killing of Bacteria. J Immunol 202, 3468–3473 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhu Q, Zheng M, Balakrishnan A, Karki R, Kanneganti TD, Gasdermin D Promotes AIM2 Inflammasome Activation and Is Required for Host Protection against Francisella novicida. J Immunol 201, 3662–3668 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Manthiram K, Zhou Q, Aksentijevich I, Kastner DL, The monogenic autoinflammatory diseases define new pathways in human innate immunity and inflammation. Nature immunology 18, 832–842 (2017). [DOI] [PubMed] [Google Scholar]
- 69.Xiao J et al. , Gasdermin D mediates the pathogenesis of neonatal-onset multisystem inflammatory disease in mice. PLoS Biol 16, e3000047 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kanneganti A et al. , GSDMD is critical for autoinflammatory pathology in a mouse model of Familial Mediterranean Fever. The Journal of experimental medicine 215, 1519–1529 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chae JJ et al. , Gain-of-function Pyrin mutations induce NLRP3 protein-independent interleukin-1beta activation and severe autoinflammation in mice. Immunity 34, 755–768 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dubois H et al. , Nlrp3 inflammasome activation and Gasdermin D-driven pyroptosis are immunopathogenic upon gastrointestinal norovirus infection. PLoS pathogens 15, e1007709 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rauch I et al. , NAIP-NLRC4 Inflammasomes Coordinate Intestinal Epithelial Cell Expulsion with Eicosanoid and IL-18 Release via Activation of Caspase-1 and −8. Immunity 46, 649–659 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rathkey JK et al. , Chemical disruption of the pyroptotic pore-forming protein gasdermin D inhibits inflammatory cell death and sepsis. Sci Immunol 3, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kang R et al. , Lipid Peroxidation Drives Gasdermin D-Mediated Pyroptosis in Lethal Polymicrobial Sepsis. Cell host and microbe 24, 97–108 e104 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]