One sentence summary :
Particular bacteria from the gut microbiota metabolize Levodopa (L-dopa), reducing bioavailability of the drug for treating Parkinson’s disease.
Parkinson’s disease (PD) is a prevalent neurodegenerative disorder, afflicting over 1 million people in the United States and 1% of the global population over 60 years of age. A hallmark of PD is the loss of dopamine-producing neurons in the striatum, which results in debilitating motor problems. Over the last 50 years, the mainstay treatment for motor symptoms in PD has been the drug Levodopa (L-dopa). L-dopa crosses the blood brain barrier and is metabolized in brain by the enzyme aromatic amino acid decarboxylase (AADC) to produce dopamine, the active therapeutic agent. Despite its widespread use, there are many adverse side effects associated with L-dopa treatment, and its efficacy varies highly across patients. This is largely due to the ability for AADC in peripheral organs to decarboxylate L-dopa to dopamine before it can enter the brain5. As such, L-dopa is often co-prescribed with enzyme inhibitors such as carbidopa in attempt to minimize its peripheral metabolism. Given that L-dopa is commonly administered orally or enterally, scientists have proposed that the gut microbiome may also impact treatment efficacy6,7. Indeed, individual bacterial species were known to express enzymes for catechol metabolism8, but whether indigenous bacteria from the gut microbiome actually influence L-dopa metabolism has been unclear.
In the present study, Rekdal and colleagues uncover effects of gut microbes on L-dopa metabolism. They first mined genomic databases to identify candidate bacterial species that contain sequences similar to tyrosine decarboxylases (tyrDC) previously shown to have promiscuous activity towards L-dopa8. They then tested these candidates for their activity in culture and found that only one species, Enterococcous faecalis, was able to completely metabolize L-dopa. When they generated mutations that rendered the tyrDC enzyme inactive, the bacteria were no longer able to decarboxylate L-dopa to dopamine. This demonstrated that bacterial tyrDCs can metabolize L-dopa and suggested that the gut microbiome could reduce peripheral availability of L-dopa.
The authors next examined the conversion of dopamine to m-tyramine by dehydroxylation because this activity is thought to contribute to the adverse side effects associated with peripheral L-dopa metabolism9. Since dehydroxylation of dopamine had not previously been reported for any bacterial isolate, they turned to enrichment culturing, whereby bacteria are grown in select media to the favor the growth of particular microorganisms over others. By adding dopamine to cultures, the authors identified a single bacterium called Eggerthella lenta that enriched and converted dopamine to m-tyramine. In order to identify the dopamine-dehydroxylating enzyme (Dadh) in E. lenta, the authors profiled which genes increased expression in response to dopamine. Three genes were upregulated, which together encode a bis-molybdopterin guanine dinucleotide cofactor (moco)-containing enzyme. Based on this, they identified 26 additional bacterial species that harbor these genes and screened them for dehydroxylation activity in culture. Of the 26 species, 10 were capable of converting dopamine to m-tyramine despite all of them harboring the dadh gene. To better understand what may differentiate metabolizers from non-metabolizers, the authors profiled gene expression of bacteria from each category. Surprisingly, dadh was upregulated in the presence of dopamine all tested species, suggesting that differences in metabolic activity did not arise from differences in transcription. Instead, they found that a single nucleotide difference in the dadh gene almost perfectly predicted metabolizer status. Overall, these data were the first to identify bacteria that dehydroxylate dopamine.
Having identified two bacterial enzymes capable of carrying out sequential steps in L-dopa metabolism, the authors next asked whether E. faecalis and E. lenta could together convert L-dopa to m-tyramine. Indeed, when the two species were co-cultured, L-dopa was completely converted to m-tyramine in a tyrDC-dependent manner. To gain insight into whether similar microbial interactions may occur in humans, they found that levels of E. faecalis and tyrDC in human microbiota samples correlated with differences in L-dopa metabolism. When they added E. faecalis to cultures of bacteria from non-metabolizers, L-dopa was fully depleted. To assess the clinical relevance of these findings, they obtained fecal samples from PD patients with varying levels of L-dopa metabolism. Indeed, abundance of E. faecalis and tyrDC again correlated with metabolic status in patient samples. Together these data raise the prospect that levels of E. faecalis and tyrDC in the gut microbiome could serve as biomarkers for the efficacy of L-dopa treatment in PD patients.
These findings also suggest that drugs that inhibit microbial enzymes for L-dopa metabolism could increase the amount of L-dopa available to enter the brain. To determine whether the identified bacterial pathways could be inhibited with small molecules, the authors tested (S)-a-Fluoromethyltyrosine (AFMT) as a potential decarboxylase inhibitor based on their findings from a survey of potential substrates. When they added AFMT to human microbiota samples in culture, L-dopa levels were maintained at higher levels. To test whether a similar effect would be seen in animals, germ-free mice were colonized with E. faecalis and treated with L-dopa plus AFMT or a vehicle control. AFMT significantly increased peak circulating concentrations of L-dopa, suggesting that it effectively inhibited metabolism of L-dopa by E. faecalis. These findings warrant continued study into whether AFMT could increase L-dopa efficiency in PD patients who exhibit high peripheral L-dopa metabolism. Overall, the study adds to the growing body of work describing microbial effects on drug metabolism10,11 and underscore the importance of examining the role of microbial metabolic activity during drug treatment to reduce inter-patient variability and enhance drug efficacy. Importantly, the study uncovers fundamental pathways for bacterial metabolism of L-dopa that could inform novel approaches for treating PD.
Figure 1:
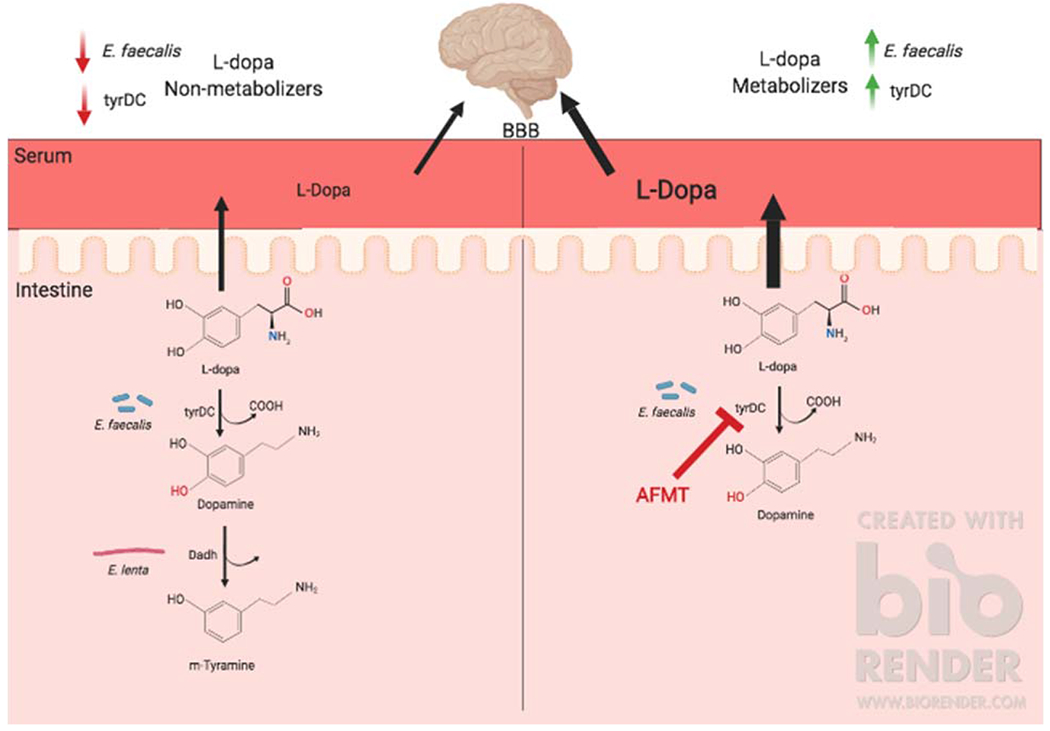
Redkal et al. identify a novel pathway for microbial metabolism of L-dopa. Left: Tyrosine decarboxylases (tyrDC) from E. faecalis convert L-dopa to dopamine, which can subsequently be metabolized to m-tyramine by dopamine dehydroxylases (Dadh) from E. lenta. Right: Inhibition of E. faecalis tyrDC by (S)-a-Fluoromethyltyrosine (AFMT) leads to increased bioavailability of L-dopa, which has the potential to be absorbed into the bloodstream and cross the blood brain barrier (BBB) into the brain to protect against motor symptoms of Parkinson’s disease.
References:
- 1.Sampson TR et al. Cell 167, 1469–1480.e12 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sun MF et al. Brain, Behavior, and Immunity 70, 48–60 (2018). [DOI] [PubMed] [Google Scholar]
- 3.Bedarf JR et al. Genome Med. 9, 39 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Minato T et al. PLOS ONE. 12, e0187307 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Papavasiliou PS et al. N. Engl. J. Med 286, 8–14 (1972). [DOI] [PubMed] [Google Scholar]
- 6.Hashim H et al. PLOS ONE. 9, e112330 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fasano A et al. Mov. Disord 28, 1241–1249 (2013). [DOI] [PubMed] [Google Scholar]
- 8.Zhu H et al. Sci. Rep 6, 27779 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Whitfield AC et al. ACS Chem. Neurosci. 5, 1192–1197 (2014). [DOI] [PubMed] [Google Scholar]
- 10.Zimmermann M et al. Nature. 570, 462–467 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zimmermann M et al. Science. 363, 6427 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]


