Abstract
Background
This is an update of a review that was first published in 2002. Female sterilisation is the most popular contraceptive method worldwide. Several techniques exist for interrupting the patency of fallopian tubes, including cutting and tying the tubes, damaging the tube using electric current, applying clips or silicone rubber rings, and blocking the tubes with chemicals or tubal inserts.
Objectives
To compare the different tubal occlusion techniques in terms of major and minor morbidity, failure rates (pregnancies), technical failures and difficulties, and women's and surgeons' satisfaction.
Search methods
For the original review published in 2002 we searched MEDLINE and the Cochrane Central Register of Controlled Trials (CENTRAL). For this 2015 update, we searched POPLINE, LILACS, PubMed and CENTRAL on 23 July 2015. We used the related articles feature of PubMed and searched reference lists of newly identified trials.
Selection criteria
All randomized controlled trials (RCTs) comparing different techniques for tubal sterilisation, irrespective of the route of fallopian tube access or the method of anaesthesia.
Data collection and analysis
For the original review, two review authors independently selected studies, extracted data and assessed risk of bias. For this update, data extraction was performed by one author (TL) and checked by another (RK). We grouped trials according to the type of comparison evaluated. Results are reported as odds ratios (OR) or mean differences (MD) using fixed‐effect methods, unless heterogeneity was high, in which case we used random‐effects methods.
Main results
We included 19 RCTs involving 13,209 women. Most studies concerned interval sterilisation; three RCTs involving 1632 women, concerned postpartum sterilisation. Comparisons included tubal rings versus clips (six RCTs, 4232 women); partial salpingectomy versus electrocoagulation (three RCTs, 2019 women); tubal rings versus electrocoagulation (two RCTs, 599 women); partial salpingectomy versus clips (four RCTs, 3627 women); clips versus electrocoagulation (two RCTs, 206 women); and Hulka versus Filshie clips (two RCTs, 2326 women). RCTs of clips versus electrocoagulation contributed no data to the review.
One year after sterilisation, failure rates were low (< 5/1000) for all methods.There were no deaths reported with any method, and major morbidity related to the occlusion technique was rare.
Minor morbidity was higher with the tubal ring than the clip (Peto OR 2.15, 95% CI 1.22 to 3.78; participants = 842; studies = 2; I² = 0%; high‐quality evidence), as were technical failures (Peto OR 3.93, 95% CI 2.43 to 6.35; participants = 3476; studies = 3; I² = 0%; high‐quality evidence).
Major morbidity was significantly higher with the modified Pomeroy technique than electrocoagulation (Peto OR 2.87, 95% CI 1.13 to 7.25; participants = 1905; studies = 2; I² = 0%; low‐quality evidence), as was postoperative pain (Peto OR 3.85, 95% CI 2.91 to 5.10; participants = 1905; studies = 2; I² = 0%; moderate‐quality evidence).
When tubal rings were compared with electrocoagulation, postoperative pain was reported significantly more frequently for tubal rings (OR 3.40, 95% CI 1.17 to 9.84; participants = 596; studies = 2; I² = 87%; low‐quality evidence).
When partial salpingectomy was compared with clips, there were no major morbidity events in either group (participants = 2198, studies = 1). The frequency of minor morbidity was low and not significantly different between groups (Peto OR 7.39, 95% CI 0.46 to 119.01; participants = 193; studies = 1, low‐quality evidence). Although technical failure occurred more frequently with clips (Peto OR 0.18, 95% CI 0.08 to 0.40; participants = 2198; studies = 1; moderate‐quality evidence); operative time was shorter with clips than partial salpingectomy (MD 4.26 minutes, 95% CI 3.65 to 4.86; participants = 2223; studies = 2; I² = 0%; high‐quality evidence).
We found little evidence concerning women's or surgeon's satisfaction. No RCTs compared tubal microinserts (hysteroscopic sterilisation) or chemical inserts (quinacrine) to other methods.
Authors' conclusions
Tubal sterilisation by partial salpingectomy, electrocoagulation, or using clips or rings, is a safe and effective method of contraception. Failure rates at 12 months post‐sterilisation and major morbidity are rare outcomes with any of these techniques. Minor complications and technical failures appear to be more common with rings than clips. Electrocoagulation may be associated with less postoperative pain than the modified Pomeroy or tubal ring methods. Further research should include RCTs (for effectiveness) and controlled observational studies (for adverse effects) on sterilisation by minimally‐invasive methods, i.e. tubal inserts and quinacrine.
Plain language summary
A review of techniques for tubal sterilisation (blocking the fallopian tubes)
Background
This is an update of a Cochrane Review that was first published in 2002 and previously updated in 2011.
Tubal sterilisation prevents pregnancy by stopping the woman's unfertilised eggs from passing through the fallopian tubes to be fertilised by sperm. Techniques to close the tubes include cutting and tying them (partial salpingectomy), blocking them mechanically by using clips or rings, or by applying electric current (electrocoagulation) to damage and block them, and blocking them by using chemicals or tubal inserts (inserted via the mouth of the womb) that cause tubal scarring.
Methods
We, the Cochrane researchers, wanted to compare the different techniques for tubal sterilisation in terms of:
‐ how unwell they made women feel in the short and long term, including pain experienced (major and minor morbidity);
‐ failure rates (pregnancies);
‐ technical failures and difficulties encountered during the sterilisation procedure; and
‐ women's and surgeons' satisfaction.
We searched the medical literature up to 23 July 2015 for randomised controlled trials (RCTs) that compared any methods of closing the fallopian tubes; RCTs produce the most reliable results.
Findings
We included 19 RCTs involving 13,209 women of childbearing age. The trials compared:
‐ tubal rings versus clips (six RCTs, 4232 women);
‐ partial salpingectomy versus electrocoagulation (three RCTs, 2019 women);
‐ tubal rings versus electrocoagulation (two RCTs, 599 women);
‐ partial salpingectomy versus clips (four RCTs, 3827 women);
‐ clips versus electrocoagulation (two RCTs, 206 women); and
‐ two types of clips, i.e. Hulka clips versus Filshie clips (two RCTs, 2326 women).
We found no RCTs that investigated sterilisation by chemicals or tubal inserts, so all the included studies involved an abdominal operation.
There were no deaths reported with any method, and major and minor morbidity were rare. Pregnancy rates were less than 5/1000 procedures one year after surgery. Complicationrates (problems after surgery/minor morbidity) were very low for all methods compared. Minor complications, including pain, and technical failures were more common with rings than clips. Major morbidity and postoperative pain were more common with partial salpingectomy than with electrocoagulation. Postoperative pain was reported twice as often by women sterilised by tubal rings than those sterilised by electrocoagulation.Technical failures were more common with clips than cutting and tying techniques, but operating time was shorter for clips.
We found little evidence concerning women's or surgeon's satisfaction.
Conclusions
Tubal sterilisation by cutting and tying the tubes, or using electric current, clips or rings, is an effective method of contraception with few problems. The choice of method will depend upon the surgeon's experience, availability of equipment, setting, and cost. More research is needed about methods for tubal sterilisation that do not require an abdominal operation.
Summary of findings
Summary of findings for the main comparison. Summary of findings: ring versus clip.
| Tubal ring compared with tubal clip for interval sterilisation | ||||||
|
Patient or population: women > 6 weeks postpartum requesting tubal sterilisation Settings: any Intervention: tubal ring Comparison: tubal clip | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Clip | Ring | |||||
| Major morbidity: total | Low risk population |
OR 0.14 (0.00 to 7.05) |
545 (1) |
⊕⊕⊝⊝ low1,2 | Only one event occurred in the clip group | |
| 4 per 1000 | 1 per 1000 (0 to 28) |
|||||
| Minor morbidity: total | Low risk population |
OR 2.15 (1.22 to 3.78) |
842 (2) | ⊕⊕⊕⊕ high | ||
| 57 per 1000 | 123 per 1000 (70 to 215) | |||||
| Minor morbidity: details ‐ procedure‐related injuries | Low risk population |
OR 1.95 (1.36 to 2.78) |
3575 (3) | ⊕⊕⊕⊕ high | ||
| 21 per 1000 | 41 per 1000 (29 to 58) | |||||
| Technical failures | Low risk population |
OR 3.93 (2.43 to 6.35) |
3476 (3) |
⊕⊕⊕⊕ high | ||
| 10 per 1000 | 39 per 1000 (24 to 63) | |||||
|
Failure rate: details (12 to 24 months) |
Low risk population |
OR 0.72 (0.33 to 1.57) |
3822 (4) | ⊕⊕⊕⊕ high | ||
| 4 per 1000 | 3 per 1000 (1 to 6) | |||||
|
Complaints: Postoperative pain (24 hours) |
Low risk population |
OR 1.14 (0.88 to 1.48) |
922 (3) |
⊕⊕⊕⊕ high | ||
| 477 per 1000 | 544 per 1000 (420 to 706) | |||||
| *The basis for the assumed risk is the median control group (clip) risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; OR: odds ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Downgraded due to imprecision.
2 Downgraded due to sparse data.
Summary of findings 2. Summary of findings: modified Pomeroy partial salpingectomy versus electrocoagulation.
| Modified Pomeroy partial salpingectomy compared with tubal electrocoagulation for interval sterilisation | ||||||
|
Patient or population: women > 6 weeks postpartum requesting tubal sterilisation Settings: any Intervention: modified Pomeroy partial salpingectomy Comparison: electrocoagulation | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Electrocoagulation | Modified Pomeroy | |||||
| Major morbidity: total | Low risk population |
OR 2.87 (1.13 to 7.25) |
1905 (2) |
⊕⊕⊝⊝ low1,2 | ||
| 10 per 1000 | 29 per 1000 (11 to 73) | |||||
| Major morbidity: procedure‐related injuries requiring additional operation or blood transfusion | 10 per 1000 |
19 per 1000 (19 to 190) |
OR 1.90 (0.19 to 18.96) |
1905 (2) |
⊕⊕⊝⊝ low 1,2 | |
| Major morbidity: rehospitalisation as a consequence of the operation | 20 per 1000 |
115 per 1000 (15 to 900) |
OR 5.74 (0.73 to 45.09) |
295 (1) |
⊕⊝⊝⊝ very low1,2 | |
| Minor morbidity: total | Low risk population |
OR 1.60 (1.10 to 2.33) |
1905 (2) |
⊕⊕⊝⊝ low 1,4 | The WHO study reported significantly more wound infections in the modified Pomeroy group, where participants underwent minilaparotomy, compared with the electrocoagulation group where laparoscopy was used) | |
| 38 per 1000 | 61 per 1000 (42 to 89) | |||||
| Minor morbidity: procedure‐related injuries with no additional operation | Low risk population |
OR 0.53 (0.06 to 5.11) |
1610 (1) | ⊕⊕⊕⊝ moderate1 | ||
| 2 per 1000 | 1 per 1000 0 to 10) | |||||
|
Failure rate: total (12 months) |
Low risk population | OR 4.47 (0.07 to 286.78) | 295 (1) |
⊕⊕⊝⊝ low1,3 | ||
| 0.5 per 1000 |
2 per 1000 (0 to 143) |
|||||
|
Complaints ‐ postoperative pain (24 hours) |
Low risk population |
OR 3.85 (2.91 to 5.10) |
1905 (2) |
⊕⊕⊕⊝ moderate4 | ||
| 95 per 1000 | 366 per 1000 (276 to 485) | |||||
| Complaints ‐ persistent pain at follow‐up visit | Low risk population |
OR 1.09 (0.88 to 1.47) |
1610 (1) | ⊕⊕⊕⊝ moderate4 | ||
| 117 per 1000 | 128 per 1000 (95 to 172) | |||||
| *The basis for the assumed risk is the median control group (electrocoagulation) risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; OR: odds ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Downgraded due to imprecision.
2 Downgraded due to inconsistency.
3 Sparse data.
4 Downgraded due to indirectness (this effect may be due to the abdominal approach (minilaparotomy versus laparoscopy) rather than the tubal technique).
Summary of findings 3. Summary of findings: tubal ring versus electrocoagulation.
| Tubal ring compared with electrocoagulation for interval sterilisation | ||||||
|
Patient or population: women > 6 weeks postpartum requesting tubal sterilisation Settings: any Intervention: tubal ring Comparison: electrocoagulation | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Electrocoagulation | Ring | |||||
| Major morbidity: total | Low risk population |
OR 0.14 0.00 to 7.01 |
596 (2) | ⊕⊕⊝⊝ low1,2 |
Unipolar electrocoagulation stated in one study and not specified in the other. Only one event reported in total | |
| 0.5 per 1000 |
0 per 1000 (0 to 4) |
|||||
| Minor morbidity: total | Low risk population |
OR 0.97 (0.50, 1.87) |
596 (2) | ⊕⊕⊕⊝ moderate1 | ||
| 66 per 1000 | 64 per 1000 (33 to 123) | |||||
| Technical failures: total | Low risk population |
OR 3.42 (0.59 to 19.81) |
596 (2) | ⊕⊕⊕⊝ moderate1 | ||
| 3 per 1000 | 10 per 1000 (2 to 60) | |||||
| Failure rate: total | not estimable | not estimable | Not estimable due to insufficient data | 160 (1) | ‐ | No pregnancies reported in one study |
|
Complaints ‐ postoperative pain (24 hours) |
Low risk population |
OR 3.40 (1.17 to 9.84) |
596 (2) | ⊕⊕⊝⊝ low1,3 |
||
| 176 per 1000 | 598 per 1000 (206 to 1000) | |||||
| Complaints ‐ persistent pain at follow‐up visit | Low risk population |
OR 1.22 (0.75 to 1.97) |
594 (2) | ⊕⊕⊕⊝ moderate1 | ||
| 140 per 1000 | 171 per 1000 (105 to 276) | |||||
| *The basis for the assumed risk is the median control group (electrocoagulation) risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; OR: odds ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Downgraded due to imprecision.
2 Downgraded due to sparse data.
3 Downgraded due to inconsistency.
Summary of findings 4. Summary of findings: partial salpingectomy versus clip.
| Partial salpingectomy compared with tubal clips for tubal sterilisation | ||||||
|
Patient or population: women requesting postpartum or interval sterilisation Settings: any Intervention: partial salpingectomy Comparison: tubal clips | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Clips | Partial salpingectomy | |||||
| Major morbidity: total | Low risk population | not estimable | 2198 (1) | ‐ | No deaths or major morbidity events reported in one large trial | |
| 0 per 1000 | 0 per 1000 | |||||
| Minor morbidity: total | Low risk population |
OR 7.39 (0.46 to 119.01) |
193 (1) | ⊕⊕⊝⊝ low1,2 | ||
| 0.5 per 1000 | 4 per 1000 (0 to 60) | |||||
| Technical failures | Low risk population | OR 0.18 (0.08 to 0.40) |
2198 (1) | ⊕⊕⊕⊝ moderate3 | ||
| 20 per 1000 | 4 per 1000 (2 to 8) | |||||
|
Failure rate: total (12 months) |
Low risk population | OR 0.21, 95% CI 0.05 to 0.84 | 3537 (2) | ⊕⊕⊕⊝ moderate 4 | In this analysis, we grouped studies according to whether sterilisation was performed on a postpartum (1) or interval basis (1). Results were similar across these subgroups (Test for subgroup differences: P value 0.58, I² = 0%) | |
| 2 per 1000 | 0.4 per 1000 (0 to 2) | |||||
|
Complaints (12 months) |
Low risk population | OR 1.30 (0.92 to 1.82) | 2137 (1) |
⊕⊕⊕⊝ moderate1 | This single study reported data on 'chief complaints' at 3, 6, and 12 months and rates were similar between groups at all assessment points | |
| 59 per 1000 | 77 per 1000 (54 to 107) |
|||||
| *The basis for the assumed risk is the median control group (clips) risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; OR: odds ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Downgraded due to imprecision.
2 Downgraded due to sparse data.
3 Downgraded due to indirectness (unclear whether silver clips and Filshie clips are similarly effective).
4 Downgraded due to risk of bias.
Summary of findings 5. Summary of findings: Hulka clip versus Filshie clip.
| Hulka clips compared with Filshie clips for interval sterilisation | ||||||
|
Patient or population: women requesting sterilisation Settings: any Intervention: Hulka clips Comparison: Filshie clips | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Filshie clip | Hulka clip | |||||
| Minor morbidity: total | Low risk population |
OR 0.14 (0.00 to 7.32) |
197 (1) | ⊕⊕⊝⊝ low1,2 | ||
| 10 per 1000 | 1 per 1000 (0 to 70) | |||||
| Minor morbidity: procedure‐related injuries | Low risk population |
OR 1.55 (0.73 to 3.26) |
2322 (2) | ⊕⊕⊕⊝ moderate1 | ||
| 10 per 1000 | 16 per 1000 (7 to 33) | |||||
| Technical failures | Low risk population |
OR 1.04 (0.10 to 11.33) |
2325 (2) |
⊕⊕⊝⊝ low1,3 | ||
| 7 per 1000 |
7 per 1000 (1 to 79) |
|||||
|
Failure rate: total (12 months) |
Low risk population |
OR 6.20 (0.75 to 51.66) |
1441 (1) |
⊕⊕⊕⊝ moderate1 | ||
| 1 per 1000 |
6 per 1000 (1 to 52) |
|||||
|
Complaints: postoperative pain (24 hours) |
Low risk population | OR 1.74 (0.99 to 3.03) |
197 (1) | ⊕⊕⊝⊝ low1,4 | ||
| 45 per 1000 | 78 per 1000 (45 to 136) | |||||
| *The basis for the assumed risk is the median control group (Filshie clips) risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; OR: odds ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Downgraded due to imprecision.
2 Downgraded due to sparse data.
3Downgraded due to inconsistency.
4 Downgraded due to risk of bias.
Background
This is an updated version of this review. The original version of the review was published in 2002 and the last update was published in The Cochrane Database of Systematic Reviews, Issue 2, 2011.
Female sterilisation, also called tubal ligation or tubal occlusion, is the most widely used contraceptive method in the world. Globally, in 2011, sterilisation accounted for approximately 19% of all contraceptive methods used by women between the ages of 15 and 49 years who were married or in a union, with the highest prevalences occurring in developing region (21%), and the lowest prevalences occurring in the least developed countries (3%) (UN 2013). Female sterilisation is most prevalent in Latin America and the Carribean (26%) (UN 2013). Figures published by the United Nations Population Division estimate prevalence rates for various other countries as follows: India 35.8%, China 28.7%, North America 22%, South Africa 14.3%, Germany 8.3%, United Kingdom 8%, France 3.8%, and Nigeria 0.3% (UN 2013). The increased efficacy and acceptability of long‐acting reversible contraceptive methods (LARCs) has contributed in a trend towards declining sterilisation rates in some regions, e.g. the United Kingdom, in favour of LARCs.
Description of the intervention
Female sterilisation prevents pregnancy by occluding or disrupting tubal patency so that the ovum cannot reach the uterus. In the 1930s, Pomeroy made tubal sterilisation well known, however it was considered a major procedure (Bhiwandiwala 1980). From 1950 to 1982 voluntary sterilisation increased thirty‐fold worldwide, the increase partly being attributed to surgical innovations that made sterilisation a safe and effective outpatient procedure (Bhiwandiwala 1980).
Sterilisation failures (pregnancies) in the first year post‐sterilisation of five per 1000 procedures are comparable with pregnancy rates for women using LARCs; however, tubal sterilisation appears to be a more effective contraceptive method over time (Peterson 2008). This is probably due to high continuation rates compared with LARCs. Sterilisation failures may result from conception occurring before the procedure (so‐called luteal phase pregnancy), incomplete tubal occlusion, or the formation of fistulas, and may occur several years after the procedure (Gupta 1980; Peterson 1996; Peterson 2008).
Tubal sterilisation is traditionally achieved by an abdominal operation (either via laparotomy or laparoscopy). Tubal sterilisation techniques employed via the abdominal route include surgically cutting and tying the fallopian tubes (with or without a section of tube being removed), mechanically blocking the tubes using clips or rings, and electrically coagulating the tubes. Tubal sterilisation can also be achieved via the vaginal route by means of chemicals or mechanical tubal inserts that block the tubes by inducing a fibrotic reaction. Interventions such as hysterectomy or ovariectomy also lead to female sterility, but are not considered in this review as these operations are usually performed primarily for other medical reasons.
Surgical methods
There are a number of surgical techniques employed for interrupting tubal patency. Possibly the most common method of surgical sterilisation is a partial salpingectomy using the Pomeroy or 'modified' Pomeroy technique in which a chromic tie is placed around a loop of fallopian tube, and a 1 cm to 2 cm portion is then excised. The Parkland method involves separating a mid‐portion of the tube from mesosalpinx and twice ligating the tube; the intervening segment between the ties is then resected, achieving immediate separation of the tubal ends. Alternatively, the Irving method double ties and divides the tube, then buries the proximal stump of the tube into the myometrium through an incision in the posterior uterine wall near the utero‐tubal junction. The Uchida method involves infiltration of the serosa of the tube with a vasoconstricting solution with subsequent dissection of the subserosa and resection of a 2 cm portion of the muscular part of the tube; the proximal stump retracts into the mesosalpinx, which is closed, and the distal stump is exteriorised to the peritoneal cavity (Peterson 2008). Other methods and modifications include fimbriectomies and salpingectomies, e.g. Kroener, Madlener and Aldrich techniques. In a large, prospective cohort study (CREST) conducted in the United States between 1978 and 1992, interval (not within 42 days of pregnancy) and postpartum partial salpingectomy were associated with cumulative 10‐year probabilities of pregnancy of 20.1 per 1000 and 7.5 per 1000 procedures, respectively (Peterson 1996).
Mechanical methods
Bands or rings made of silicone and rubber (e.g. Yoon, Falope) are placed around a loop of fallopian tube, using a cone‐shaped applicator. When the ring is released onto the loop of tube, it contracts and constricts the base of the loop, thereby blocking the tube. The 2 cm to 3 cm loop undergoes necrosis and the healthy ends of the tube separate. Hinged clips (e.g. Filshie, Hulka) can also be used to block the fallopian tubes mechanically. Filshie clips are made of titanium and silicone rubber, while Hulka clips are made of plastic with a gold spring lock. Only a small portion of the tube is damaged when these devices are used (Chi 1994; Kaplan 1990; Lipscomb 1992), therefore their use might increase the chance of reversibility among women who experience regret (Hillis 1999). In the CREST study, tubal rings and clips were associated with cumulative 10‐year probabilities of pregnancy of 17.7 per 1000 and 36.5 per 1000 procedures, respectively (Peterson 1996).
Electrical methods
The standard laparoscopic technique for tubal occlusion by electrocoagulation originally used unipolar forceps, however, since the risk of burns to the bowel and other organs is decreased with the use of bipolar forceps, the latter are preferred (Kessel 1976). With bipolar coagulation, the tube is grasped with the forceps, and electrical current passes between the two ends of the forceps, damaging the tube. To achieve successful occlusion it is recommended that at least 3 cm of the isthmic portion of the tube is coagulated (Peterson 2008). Unipolar coagulation damages a wider segment of tube, which is often cut after coagulation. In the CREST study, unipolar and bipolar electrocoagulation were associated with cumulative 10‐year probabilities of pregnancy of 7.5 per 1000 and 24.7 per 1000 procedures, respectively (Peterson 1996).
Chemical methods
Licensed as an antimalarial and in use for more than 70 years, quinacrine's use in sterilisation in low‐ and middle‐income countries has been fraught with ethical issues (Bhattacharyya 2003). However, a report on 40,252 cases of quinacrine sterilisation (QS) from Chile, Indonesia, Pakistan, India, Egypt, Libya, Syria, China, Costa Rica and the USA concluded that this is a safe and effective method (IJOG 2003). The method, involving the interuterine device‐like insertion of quinacrine pellets trans‐cervically into the uterus, leads to chemical irritation and scarring of the fallopian tubes (Suhadi 1998). QS does not immediately result in sterilisation, which can take up to 12 weeks, and failure rates appear to vary depending on dosage and the number of insertions (Agoestina 2003). Two insertions one month apart seems to be the most common and effective method of QS, and results in reported gross pregnancy rates of 1.2% to 4.3% in 10 years (Lu 2003; Suhadi 2003).
Tubal inserts
Essure® inserts are 4 cm devices consisting of a stainless steel inner coil coated with PET (polyethylene tenephterate), and a nickel titanium outer coil. According to the manufacturer, approximately 750,000 women have undergone Essure® sterilisation to date (Bayer 2015). To achieve sterilisation, these inserts are introduced bilaterally into the proximal fallopian tubes via hysteroscopy and expand on insertion. The PET fibres induce a tissue response that causes fibrosis of the tubes (Valle 2001). Bilateral occlusion must be verified, usually by hysterosalpingogram (HSG), three months postinsertion (Veersema 2015). Although other inserts have been developed (Adiana, Ovabloc), Essure® is currently the only tubal insert on the market. Successful bilateral placement varies from between 80% to 99% of first attempts (Arjona 2008; Connor 2009; Cooper 2003; Duffy 2005; Panel 2010; Savage 2009; Shavell 2009), and placement failure has been attributed mainly to related to poor visualisation of the tubal ostia or tubal spasm/stenosis on hysteroscopy, and operator experience (Mino 2007). Sterilisation failures may be mainly attributed to misinterpretation of the HSG and non‐adherence to the follow‐up protocol (Veersema 2015), although evidence from controlled studies is lacking.
Why it is important to do this review
Contraception plays a vital role in reducing maternal morbidity and mortality, and the acceptability and satisfaction of women with contraceptive methods is increased when users are well‐informed (Blumenthal 2011). This review considers the different techniques for tubal interruption, regardless of the method used to access the fallopian tubes, and evaluates them for their safety and effectiveness. Previous versions of this review identified no eligible studies of chemical or hysteroscopic methods that could be included in the review. Given the evolving nature of sterilisation methods, and contraception in general, it is important that we keep this review updated.
Objectives
To compare the different tubal occlusion techniques in terms of major and minor morbidity, failure rates (pregnancies), technical failures and difficulties, and women's and surgeons' satisfaction.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) comparing different occlusion techniques for tubal sterilisation. Quasi‐RCTs are excluded.
Types of participants
Women requesting tubal sterilisation.
Types of interventions
Interventions include interrupting tubal patency by partial salpingectomy, clips, silicone rings, electrocoagulation, chemicals and tubal inserts.
Interventions may be performed as:
postpartum sterilisation: sterilisation performed during caesarean section or within 42 days of delivery (it is usually performed during the first 48 to 72 hours postpartum);
postabortion sterilisation: sterilisation performed immediately after termination of pregnancy; or
interval sterilisation: sterilisation performed at least six weeks after delivery.
Types of outcome measures
Primary outcomes
Failure rate (yearly incidence of unintended pregnancy) including extrauterine pregnancy.
Operative mortality, major and minor morbidity (procedure‐related intestinal, vascular or bladder injuries, injury to other pelvic organ, blood transfusion, re‐admission).
Failure of technical approach (e.g. clip converted to partial salpingectomy).
Other outcomes included:
operative time;
changes in menstrual bleeding pattern;
postoperative pain: pain scores or use of analgesics;
postoperative complications: wound infection, reoperation, urinary tract infection, pelvic inflammatory disease;
length of hospital stay;
difficulty of procedure;
persistent pain;
women's satisfaction;
surgeons' satisfaction.
Definitions
Postoperative pain: defined whenever possible as localised physical suffering related to the tubal occlusion technique. Postoperative complication: any disease or condition developed as a direct consequence of the procedure. Changes in menstrual pattern: any changes in frequency or quantity of menstrual bleeding. Major morbidity: any morbidity occurring as a result of the intervention that lead to an additional intervention (e.g. additional surgical procedure, blood transfusion) or to re‐admission. Minor morbidity: any morbidity occurring as a result of the intervention and which does not lead to major additional interventions. Technical failure or failure of technical approach: failure to apply the intended method with the consequent need to switch to another technique. Technical difficulties: any difficulty in applying the selected method and which does not lead to change to another procedure.
Search methods for identification of studies
For the original review, the Cochrane Central Register of Controlled Trials (CENTRAL) and MEDLINE were searched; the electronic search strategy included the following terms: (tubal OR female OR contracep*) AND (sterilis* OR steriliz* OR laparo* OR culdoscopy OR colpotomy OR Filshie OR Hulka OR Yoon OR Pomeroy OR Irving OR Parkland OR (Rocket and Clips) OR (tubal and ring) OR (silastic and ring) OR (Quinacrine AND tubal) OR (chemical AND instillation AND tubal)).
For the 2010 and 2015 updates, PubMed, POPLINE and LILACS were also searched and the following search strategy was used:
PubMed: sterilisation, tubal AND (technique* OR method OR methods OR methodology OR procedure*) AND clinical trial.
POPLINE: (female sterilisation/female sterilisation/((tubal & (ligat*/occlud*/occlus*)) & female)) & clinical trial.
LILACS: sterilisation, tubal or esterilizacion tubaria or esterilizacao tubaria [Words] AND method OR metodo OR methods OR metodos OR technique OR techniques OR technica OR technicas OR procedure OR procedures OR procedimiento.
Searches were conducted by Carol Manion of FHI 360 (formerly Family Health International). In addition, we searched reference lists of identified trials and used the 'related articles' feature of PubMed to search for other possible trials.
Data collection and analysis
Selection of studies
For the original 2002 review, two reviewers (RK, JMN) selected the trials for inclusion (Nardin 2002; Nardin 2003). For the update, TL and RK sifted the searches and selected trials.
Data extraction and management
For this update, we designed a Microsoft Excel® spreadsheet based on a Microsoft Word® form that was previously designed and used for this review. Data extraction for the original review was performed by RK and JMN. For the updates, this was performed by TL and checked by RK. TL entered data into Review Manager software and RK checked them (RevMan 2014).
In addition to outcome data, we also extracted information about the following:
setting (country, level of the healthcare institution, year);
details of surgery: type of surgical procedure, type of anaesthesia, timing of procedure (postpartum, interval, postabortion);
interventions compared;
types and numbers of participants;
risk of bias criteria including, method of randomisation, concealment of allocation, loss to follow‐up and postrandomisation exclusions.
Whenever possible, we extracted outcome data according to 'intention to treat'.
Assessment of risk of bias in included studies
The 2002 and 2010 versions of this review utilised older Cochrane methodology for assessing risk of bias that involved an A, B, C system of assessing bias and excluded studies with poor allocation concealment (Appendix 1). For this updated review we reviewed previous exclusions and, where possible, updated the 'Risk of bias' assessment of previously included studies. For this version of the review (and future versions), risk of bias has been assessed according to the following criteria:
1. Random sequence generation (checking for possible selection bias)
For each study we assessed the method used to generate the allocation sequence as:
low risk of bias (any truly random process, e.g. random‐number table; computer random‐number generator);
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number); though quasi‐randomised studies are not eligible for inclusion in the review;
unclear risk of bias (if the process of sequence generation was not described).
2. Allocation concealment (checking for possible selection bias)
For each included study, we assessed the method used to conceal allocation to interventions prior to assignment and assessed whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment. We assessed the methods as:
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear risk of bias (if the process of was not described).
3. Blinding of participants, personnel and outcome assessors (checking for possible performance and detection bias)
For each included study, we assessed the methods used, if any, to blind study participants, personnel and outcome assessors from knowledge of which intervention a participant received. We considered that studies were at low risk of bias if they were blinded.
We assessed the methods as:
low, high or unclear risk of bias for participants;
low, high or unclear risk of bias for personnel;
low, high or unclear risk of bias for outcome assessors.
4. Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
We assessed the completeness of data including attrition and exclusions from the analyses. We recorded whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomized participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. We assessed methods as:
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomisation);
unclear risk of bias (e.g. withdrawals not stated, denominators not given).
5. Selective reporting (checking for reporting bias)
We assessed the possibility of selective outcome reporting bias as:
low risk of bias (where it was clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
high risk of bias (where not all the study’s pre‐specified outcomes have been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest were reported incompletely and so could not be used; study failed to include results of a key outcome that would have been expected to have been reported);
unclear risk of bias.
6. Other bias (checking for bias due to problems not covered by 1 to 5 above)
We assessed whether there were other possible sources of bias, for example, imbalances in important baseline characteristics, and judged these to be at low, high or unclear risk.
7. Overall risk of bias
We made explicit judgements about whether studies were at high risk of bias, according to the criteria given in theCochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). With reference to points 1 to 6 above, we attempted to assess the likely magnitude and direction of the bias and whether we considered it likely to impact on the findings. We explored the impact of the level of bias through undertaking sensitivity analyses ‐ seeSensitivity analysis.
Measures of treatment effect
Dichotomous data
For dichotomous data, we used summary odds ratios (OR) with 95% confidence intervals (CI).
Continuous data
We used the mean difference (MD) with 95% CIs if outcomes were measured in the same way between trials, which was the case for this version of the review. Had they not been measured in the same way, we would have used the standardised mean difference (SMD), provided pooling these data was considered meaningful.
Unit of analysis issues
We did not anticipate unit of analysis issues.
Dealing with missing data
For included studies, we noted levels of attrition. We did not impute data. For all outcomes, as far as possible, we performed analyses on an intention‐to‐treat basis, that is, we attempted to include all participants randomized to each group in the analyses. The denominator for each outcome in each trial was the number randomized minus any participants whose outcomes were known to be missing.
Assessment of heterogeneity
We assessed statistical heterogeneity in each meta‐analysis using the Tau², I² and Chi² statistics. We regarded heterogeneity as substantial if I² was greater than 30% and, either Tau² was greater than zero, or there was a low P value (less than 0.10) in the Chi² test for heterogeneity.
Assessment of reporting biases
There were insufficient studies to assess publication bias using funnel plots; however, in future versions, this may be possible if there are 10 or more studies in a meta‐analysis.
Data synthesis
We carried out statistical analysis using Review Manager software (RevMan 2014). We used fixed‐effect methods to produce an overall summary of effect if heterogeneity was low (I² < 30%), otherwise we used random‐effects methods. The random‐effects summary was treated as the average of the range of possible treatment effects.
Quality of evidence
The quality of the evidence was assessed using the GRADE approach for the following key outcomes (GRADE 2014):
failure rate (yearly incidence of unintended pregnancy);
major morbidity;
minor morbidity;
failure of technical approach;
postoperative pain.
We considered evidence from RCTs to be high quality in the first instance, and downgraded the evidence quality for imprecision, inconsistency, indirectness, risk of bias, and publication bias when present. We also downgraded for sparse data when few events occurred (equivalent to downgrading twice for imprecision).
Sensitivity analysis
If there were sufficient trials, we carried out sensitivity analyses to explore the effect of trial quality by excluding studies at high risk of bias from the analyses in order to assess whether this made any difference to the overall results.
Results
Description of studies
Results of the search
We included nine RCTs in the original 2002 review, and 12 trials in the 2011 version. For this latest review, we ran the searches on 23 July 2015, which produced a list of 62 references. After screening these references for title and abstract, we identified three eligible studies; two of which we included (Qui 2011; Rodriguez 2013), and one that we excluded (Chapa 2015). We also identified two additional studies that we included using the 'related articles' feature of PubMed (Dominik 2000; Siegle 2005).
We reviewed all previously excluded RCTs according to our updated methodology, and included three that had previously been excluded due to risk of bias concerns, bringing the total number of included RCTs in the review to 19.
Included studies
Trials evaluated the following comparisons.
Sterilisation (interval) using tubal ring compared with tubal clip: six trials, including a total of 4232 women (Aranda 1985; Argueta 1980; Geirsson 1985; Pymar 2004; Sokal 2000; Stovall 1991).
Sterilisation (interval) with partial salpingectomy (modified Pomeroy technique) compared with electrocoagulation: three trials, including 2019 women (Siegle 2005; Sitompul 1984; WHO 1982).
Sterilisation (interval) using tubal ring compared with electrocoagulation: two trials, including a total of 599 women (Aranda 1976; Koetsawang 1978).
Postpartum sterilisation by partial salpingectomy (Pomeroy and Modified Pomeroy techniques) compared with Filshie clip: three trials, including 1629 women (Kohaut 2004; Rodriguez 2013; Yan 1990).
Interval or postabortion sterilisation by partial salpingectomy (modified Uchida technique) compared with silver clip: one trial, including 2198 women (Qui 2011).
Interval sterilisation by Hulka clip compared with Filshie clip: two trials, including 2326 women (Dominik 2000; Toplis 1988).
Interval sterilisation by clip compared to electrocoagulation: two trials, including 206 women (Gentile 2006; Goynumer 2009).
Electrocoagulation was specified as unipolar in Koetsawang 1978, and bipolar in Gentile 2006, Goynumer 2009 and Siegle 2005, but type was not specified in three other trials that used electrocoagulation.
Design and settings
Most of the studies were single‐centre RCTs, with six exceptions: WHO 1982 involved eight centres, four in industrialised countries and four in non‐industrialised countries; Aranda 1976 was conducted in three low‐ and middle‐income country centres (Costa Rica, El Salvador, Egypt); Qui 2011 was conducted in 20 clinics in China; Rodriguez 2013 was conducted in centres in Thailand, Taiwan, Panama and the Phillipines; Sokal 2000 was conducted in centres in Panama, Peru, Kenya, Brazil, Mexico, Indonesia,Thailand and the Dominican Republic; and Dominik 2000 was conducted in centres in Malaysia, Panama, the Dominican Republic, Mexico, Venezuela, Guatamala, and Haiti.
Surgical approach
Access to the abdomen was achieved by different approaches. Ten studies used laparoscopy (Aranda 1976; Argueta 1980; Geirsson 1985; Gentile 2006; Goynumer 2009; Koetsawang 1978; Pymar 2004; Siegle 2005; Stovall 1991; Toplis 1988); three used laparotomy (Aranda 1985; Qui 2011; Yan 1990); two used minilaparotomy (Kohaut 2004; Rodriguez 2013); three used minilaparotomy or laparoscopy (Dominik 2000; Sokal 2000; WHO 1982), and one study compared three different approaches to enter the abdominal cavity (Sitompul 1984).
Procedures were performed by experienced surgeons in five trials (Dominik 2000; Sitompul 1984; Sokal 2000; Toplis 1988; WHO 1982), and by trainee third year residents in two trials (Siegle 2005; Stovall 1991); in the remainder, the surgeon's experience was not explicitly stated.
The type of anaesthesia used varied among participating institutions according to institutional standards or at the surgeons' discretion for certain multicentre studies (Rodriguez 2013; Sokal 2000; WHO 1982). For other studies, procedures were performed under general anaesthesia (Geirsson 1985; Goynumer 2009; Siegle 2005), local anaesthesia (Aranda 1976; Argueta 1980; Koetsawang 1978; Qui 2011; Sitompul 1984) epidural anaesthesia (Yan 1990), general or local (Aranda 1985), general or spinal (Kohaut 2004), or was not clearly stated (Dominik 2000; Gentile 2006; Pymar 2004; Stovall 1991; Toplis 1988).
Participants and outcomes
1. Tubal ring versus clip trials
Aranda 1985 randomized 663 women to tubal ring or Rocket clip. Women had similar socio‐demographic characteristics, and a similar percentage of interval and post‐spontaneous abortion procedures (about 55% and 45% respectively) was performed in each group. Main outcomes were major and minor morbidity, technical failures and difficulties, failure rates and complaints.
Argueta 1980 randomized 299 women to interval sterilisation by tubal ring or spring‐loaded clip. Selected socio‐demographic characteristics of the subjects were similar in both groups. Main outcomes were operative morbidity, technical failures and difficulties, failure rates, and complaints. A total of 114 women were lost to follow‐up at 24 months; 54 from the clip group (36% of group) and 60 from the ring group (40% of group).
Stovall 1991 randomized 365 women to interval sterilisation by tubal ring (189 women) or the spring‐loaded clip (176 women). All women had urine tests for human chorionic gonadotropin (hCG) 72 hours before their planned surgical procedure. Both groups had similar socio‐demographic characteristics. The primary outcome was failure rate. An average of 16 months (range, 6 to 24 months) of follow‐up was reported. Chromopertubation was performed on all the women after application of the occluding devicesand confirmed successful tubal occlusion in all women.
Geirsson 1985 randomized 79 women to interval sterilisation by tubal ring or Filshie clip. Mean age and parity were similar between the two groups. Primary outcomes were postoperative pain and analgesic requirements.
Pymar 2004 included 40 women who had a Filshie clip and a ring applied to opposite tubes. The side of application and type of device was randomized. Pain during the first 24 hours postoperatively was the primary outcome based on evidence that women can discriminate between pain on each side of the abdomen. The method of anaesthesia was not stated.
Sokal 2000 randomized 2746 women to a Filshie clip (1381 women) or tubal ring (1365 women). The report combined data from two studies, one utilising a minilaparotomy approach, the other utilising laparoscopy. Outcomes evaluated included pregnancy, adverse events, hospital admissions, and further surgery with follow‐up conducted at one, six, and 12 months.
2. Partial salpingectomy versus electrocoagulation
Sitompul 1984 randomized 300 women to interval sterilisation in three groups (100 each for minilaparotomy, laparoscopy and culdoscopy). The modified Pomeroy technique was performed for all women in the minilaparotomy and the culdoscopy group, while electrocoagulation was the sterilisation method used in the laparoscopy group. Outcomes included operative time, hospitalisation, postoperative complications, and failure rates.
WHO 1982 randomized 1827 women to interval sterilisation by Pomeroy partial salpingectomy via minilaparotomy (912 women) or electrocoagulation via laparoscopy (915 women). Main outcomes were major and minor morbidity, technical failures, and postoperative complaints.
Siegle 2005 randomized 109 women to interval partial salpingectomy (Pomeroy) or bipolar electrocoagulation. The primary outcome was postoperative pain up to two weeks after surgery. There was little usable data from this study.
3. Tubal ring versus electrocoagulation
In Aranda 1976, 299 women who were at least six weeks postpartum were randomly assigned to electrocoagulation or tubal ring groups via laparoscopy (interval sterilisation). Women in the two groups were similar with respect to socio‐demographic characteristics. Outcomes included surgical and early postoperative complications, and complaints.
Koetsawang 1978 randomized 300 women in equal numbers to electrocoagulation (unipolar) or the tubal ring. All operations were performed on an outpatient basis for women who had not recently been pregnant (interval sterilisation). The two groups had similar socio‐economic characteristics. All women completed the six month follow‐up. Outcomes included operative morbidity, technical failures and difficulties, failure rates, operative time, and complaints.
4. Postpartum partial salpingectomy versus clip
Yan 1990 randomized 200 women postpartum: 100 to Pomeroy partial salpingectomy and 100 to Filshie clip, and followed them up for 24 months after sterilisation. Socio‐demographic characteristics (age, total live births and previous contraceptive use) were reported to be similar between groups.
Rodriguez 2013 randomized 1400 postpartum women to partial salpingectomy or Filshie clip. All women had undergone a vaginal delivery. Follow‐up was performed at one, six, 12, and 24 months following sterilisation. This report includes the 200 particpants in Yan 1990 (but reports fewer outcomes).
Kohaut 2004 randomized 32 women to postpartum or intraoperative (after caesarean section) sterilisation by the Filshie clip or the Pomeroy method. Main outcomes concerned the ease of procedure and the surgeons' satisfaction. There was little usable data from this study.
5. Interval partial salpingectomy versus clip
Qui 2011 randomized 2198 women to partial salpingectomy (Uchida technique) or silver clips. The participants were mostly more than six weeks postpartum (interval sterilisation), with less than 2% being postabortion. Approximately 63% of sterilisations were performed in lactating women in whom menses had not resumed. Outcomes were pregnancy rates, morbidity, operative time, and women's satisfaction. Women were followed up at one week, and three, six, and 12 months following sterilisation.
6. Comparison of different clip methods for interval sterilisation
Toplis 1988 randomized 200 women to Filshie clip or Hulka clip (spring‐loaded clip). Main outcomes were operative morbidity, operative time, and complaints.
Dominik 2000 reported the combined results of two multicentre RCTs comparing Filshie and Hulka clips, one using a minilaparotomy approach (878 women), the other using a laparoscopic approach (1248 women). Outcomes were failure rates, technical failure and difficulties, and morbidity.
7. Clips versus electrocoagulation
Gentile 2006 randomized 118 women to Hulka clips or bipolar electrocautery for interval sterilisation and conducted a series of urine and serum oestradiol and progesterone tests for two years poststerilisation. Unpublished data on secondary outcomes, including women's satisfaction, were not available.
Goynumer 2009 randomized 88 women to a mechanical clip or bipolar electrocoagulation for interval sterilisation. Outcomes were ovarian reserve indicators (hormones and ovarian volume). These two trials contributed no data that could be used in the review analyses.
Risk of bias in included studies
The risk of bias of included studies is summarised in Figure 1. Most studies were older studies with an unclear risk of bias as information about study methods was often missing from trial reports. Randomisation and allocation concealment was inadequately described in 50% of the studies. Blinding of the outcome assessor was described in nine studies (Aranda 1976; Aranda 1985; Argueta 1980; Gentile 2006; Koetsawang 1978; Pymar 2004; Rodriguez 2013; Sokal 2000; Yan 1990).
1.
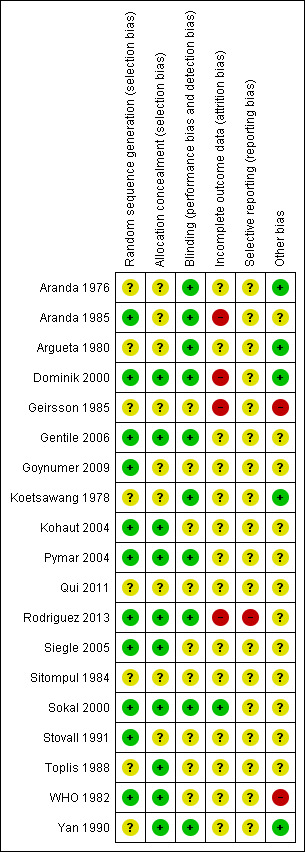
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Attrition bias was serious in two studies: in Geirsson 1985, nine women were excluded post‐procedure "due to intra‐operative difficulties" that were not described in detail, while in Aranda 1985, 30 cases of technical failure (5% of total) were excluded from the analyses. It was not clear from which groups these women came, and we were unable to include these data on technical failures in the review. Postrandomisation exclusions due to protocol violations occurred with similar frequency in the WHO 1982 trial (about 12% in the Pomeroy group and about 10% in the electrocoagulation group); however, there were also important differences in baseline characteristics and methods of accessing the tubes between arms of this trial, which may have had an impact on the results. We took the assessment of risk of bias into consideration when grading the quality of the evidence. For more details, see Characteristics of included studies.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5
1. Tubal ring versus clip
Six trials evaluated this comparison for interval sterilisation. Only one trial reported major morbidity (one event in the clip arm; Peto OR 0.14, 95% CI 0.00 to 7.05; participants = 545; studies = 1; Analysis 1.1) and no deaths were reported in any of the trials. Overall minor morbidity was more frequent in the ring group (Peto OR 2.15, 95% CI 1.22 to 3.78; participants = 842; studies = 2; I² = 0%; Analysis 1.2) and there were more procedure‐related injuries in the ring group compared with the clip group (Peto OR 1.95, 95% CI 1.36 to 2.78; participants = 3575; studies = 3; I² = 0%; Analysis 1.3.1). Failure of technical approach occurred more often in the ring group (Peto OR 3.93, 95% CI 2.43 to 6.35; participants = 3476; studies = 3; I² = 0%; Analysis 1.4). Technical difficulties were not very different between tubal ring and clip groups (Peto OR 1.13, 95% CI 0.87 to 1.46; participants = 3590; studies = 3; I² = 28%; Analysis 1.5).
1.1. Analysis.
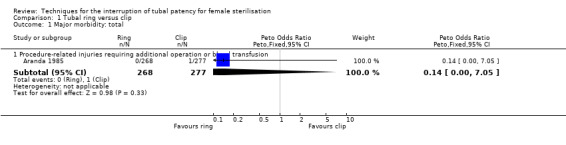
Comparison 1 Tubal ring versus clip, Outcome 1 Major morbidity: total.
1.2. Analysis.
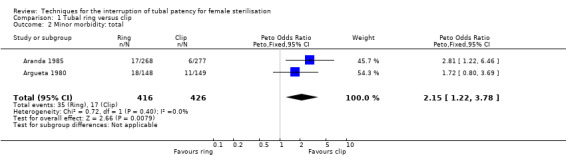
Comparison 1 Tubal ring versus clip, Outcome 2 Minor morbidity: total.
1.3. Analysis.
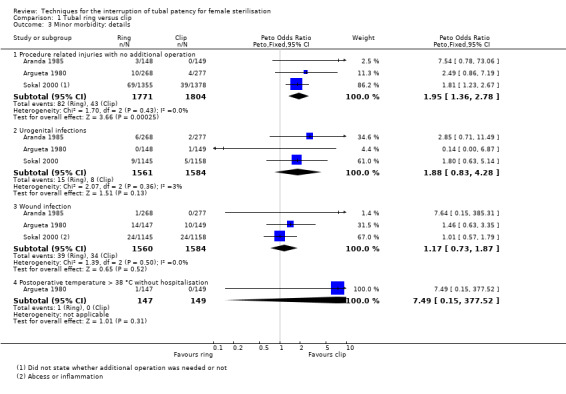
Comparison 1 Tubal ring versus clip, Outcome 3 Minor morbidity: details.
1.4. Analysis.
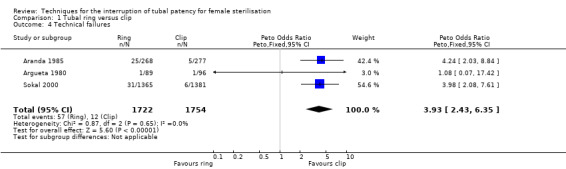
Comparison 1 Tubal ring versus clip, Outcome 4 Technical failures.
1.5. Analysis.
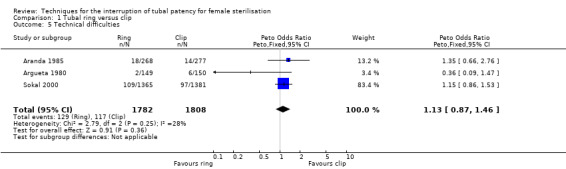
Comparison 1 Tubal ring versus clip, Outcome 5 Technical difficulties.
There was no clear difference in failure (pregnancy) rates between the tubal ring and clip groups (Peto OR 0.72, 95% CI 0.33 to 1.57; participants = 3822; studies = 4; I² = 0%; Analysis 1.6). Follow‐up in these studies was between 12 and 24 months. There were no clear differences in postoperative pain complaints (Peto OR 1.14, 95% CI 0.88 to 1.48; participants = 922; studies = 3; I² = 0%; Analysis 1.10.1) or analgesic use; however, one study reported more complaints of cramping pain during the first week after surgery with the tubal ring compared with the clip (Peto OR 5.24, 95% CI 1.52 to 18.00; participants = 70; studies = 1; Analysis 1.10.3). There was no difference in the frequency of menstrual irregularities between groups (Peto OR 1.61, 95% CI 0.75 to 3.49; participants = 612; studies = 2; I² = 0%; Analysis 1.11).
1.6. Analysis.
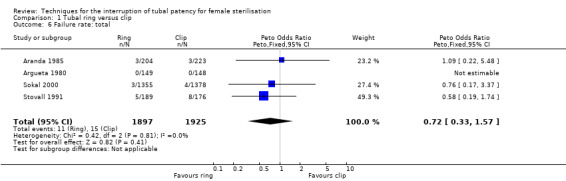
Comparison 1 Tubal ring versus clip, Outcome 6 Failure rate: total.
1.10. Analysis.
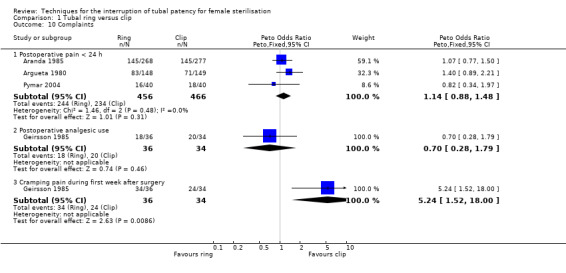
Comparison 1 Tubal ring versus clip, Outcome 10 Complaints.
1.11. Analysis.
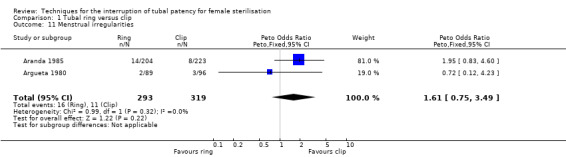
Comparison 1 Tubal ring versus clip, Outcome 11 Menstrual irregularities.
2. Modified Pomeroy partial salpingectomy versus electrocoagulation
Three trials evaluated this comparison for interval sterilisation. There were no cases of operative mortality in the one study that reported this outcome (WHO 1982). Major morbidity was more frequent in the Pomeroy group than the electrocoagulation group (Peto OR 2.87, 95% CI 1.13 to 7.25; participants = 1905; studies = 2; I² = 0%; Analysis 2.2); with one case of a burn to the small bowel reported in the electrocoagulation group. Minor morbidity was also more frequent in the Pomeroy group (Peto OR 1.60, 95% CI 1.10 to 2.33; participants = 1905; studies = 2; I² = 0%; Analysis 2.4), mainly due to wound infections. There were no data on technical failures and difficulties. One pregnancy was reported (in the Pomeroy group) in the only trial that reported this outcome (Sitompul 1984; Peto OR 4.47, 95% CI 0.07 to 286.78; participants = 295; studies = 1; Analysis 2.6). This intrauterine pregnancy occurred between one and two years of follow‐up (Analysis 2.7). There was no clear difference in the proportion of women hospitalised for more than 24 hours (OR 0.48, 95% CI 0.08 to 2.74; participants = 108; studies = 1; Analysis 2.8). More women in the Pomeroy group reported postoperative abdominal pain (Peto OR 3.85, 95% CI 2.91 to 5.10; participants = 1905; studies = 2; I² = 0%; Analysis 2.9). Single studies found no clear difference in rates of analgesic use (Peto OR 2.05, 95% CI 0.40 to 10.56; participants = 109; studies = 1; Analysis 2.9.2), or rates of persistent pain at follow‐up visit between the groups (Peto OR 1.09, 95% CI 0.81 to 1.47; participants = 1610; studies = 1; Analysis 2.9.3).
2.2. Analysis.
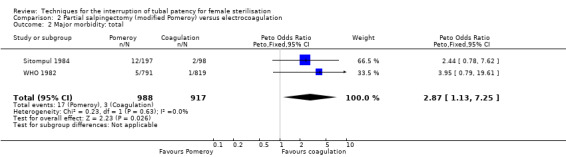
Comparison 2 Partial salpingectomy (modified Pomeroy) versus electrocoagulation, Outcome 2 Major morbidity: total.
2.4. Analysis.
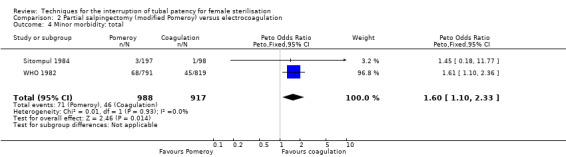
Comparison 2 Partial salpingectomy (modified Pomeroy) versus electrocoagulation, Outcome 4 Minor morbidity: total.
2.6. Analysis.
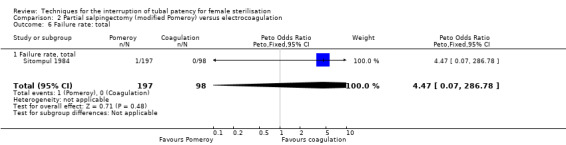
Comparison 2 Partial salpingectomy (modified Pomeroy) versus electrocoagulation, Outcome 6 Failure rate: total.
2.7. Analysis.
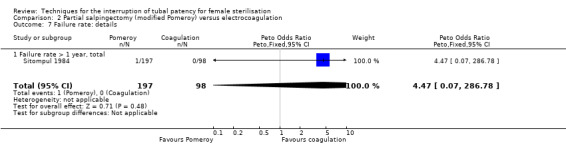
Comparison 2 Partial salpingectomy (modified Pomeroy) versus electrocoagulation, Outcome 7 Failure rate: details.
2.8. Analysis.
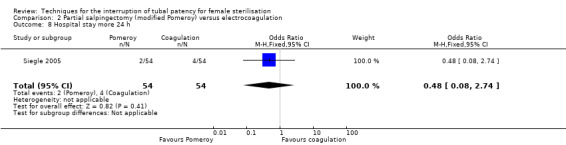
Comparison 2 Partial salpingectomy (modified Pomeroy) versus electrocoagulation, Outcome 8 Hospital stay more 24 h.
2.9. Analysis.
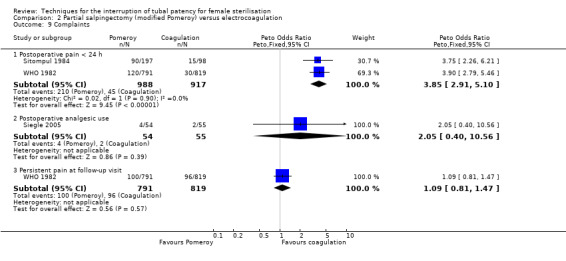
Comparison 2 Partial salpingectomy (modified Pomeroy) versus electrocoagulation, Outcome 9 Complaints.
3. Tubal ring versus electrocoagulation
Two trials evaluated this comparison for laparoscopic interval sterilisation (Aranda 1976; Koetsawang 1978). Electrocoagulation was unipolar in Koetsawang 1978 and not specified in Aranda 1976. No deaths were reported. Major morbidity was not significantly different between the groups, with only Aranda 1976 reporting an adverse event due to a burn of the small intestine in the electrocoagulation group (Peto OR 0.14, 95% CI 0.00 to 7.01; participants = 596; studies = 2; I² = 0%; Analysis 3.1 and Analysis 3.2). There were no clear differences in minor morbidity (Peto OR 0.97, 95% CI 0.50 to 1.87; participants = 596; studies = 2; I² = 0%; Analysis 3.3), technical failures (Peto OR 3.42, 95% CI 0.59 to 19.81; participants = 596; studies = 2; I² = 0%; Analysis 3.5) or technical difficulties (Peto OR 0.14, 95% CI 0.01 to 1.33; participants = 298; studies = 1; Analysis 3.6) between the groups. No pregnancies were reported. More women in the ring group reported postoperative abdominal pain (OR 3.40, 95% CI 1.17 to 9.84; participants = 596; studies = 2; I² = 87%; Analysis 3.9). There was no difference between groups in either operative time (Analysis 1.8) or menstrual irregularities (Analysis 1.11).
3.1. Analysis.
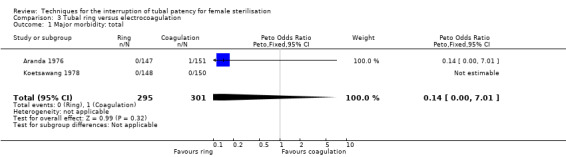
Comparison 3 Tubal ring versus electrocoagulation, Outcome 1 Major morbidity: total.
3.2. Analysis.
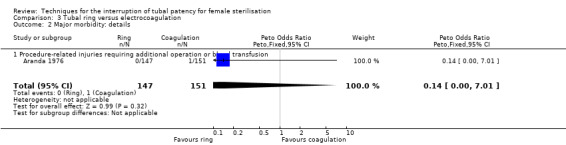
Comparison 3 Tubal ring versus electrocoagulation, Outcome 2 Major morbidity: details.
3.3. Analysis.
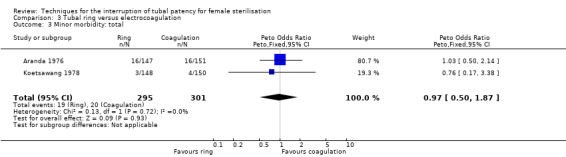
Comparison 3 Tubal ring versus electrocoagulation, Outcome 3 Minor morbidity: total.
3.5. Analysis.
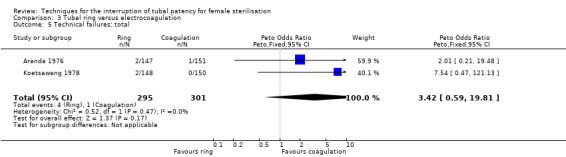
Comparison 3 Tubal ring versus electrocoagulation, Outcome 5 Technical failures: total.
3.6. Analysis.
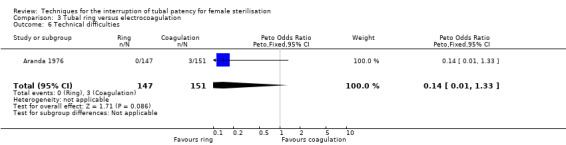
Comparison 3 Tubal ring versus electrocoagulation, Outcome 6 Technical difficulties.
3.9. Analysis.
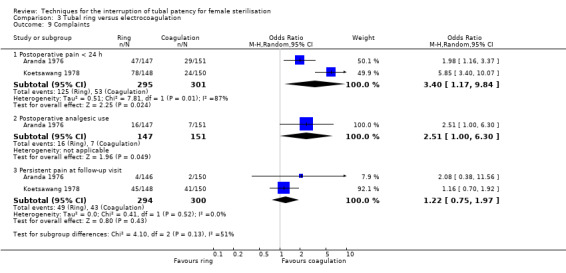
Comparison 3 Tubal ring versus electrocoagulation, Outcome 9 Complaints.
1.8. Analysis.
Comparison 1 Tubal ring versus clip, Outcome 8 Operative time.
4. Partial salpingectomy versus clip
Four trials evaluated this comparison, three used the modified Pomeroy technique for partial salpingectomy (Kohaut 2004; Rodriguez 2013; Yan 1990) versus titanium (Filshie) clips, one using the modified Uchida technique for interval sterilisation versus silver clips (Qui 2011). Two studies contributed little data (Kohaut 2004; Rodriguez 2013). Yan 1990 was one of the study centres included in Rodriguez 2013 and reported more outcomes than Rodriguez 2013. There were no cases of operative mortality or major morbidity in the only study that reported these outcomes (Qui 2011; Analysis 4.1 and Analysis 4.2). Minor morbidity was not significantly different between treatment groups (Peto OR 7.39, 95% CI 0.46 to 119.01; participants = 193; studies = 1; Analysis 4.3). Technical failures were more common in the clip group (Peto OR 0.18, 95% CI 0.08 to 0.40; participants = 2198; studies = 1; Analysis 4.5), but not technical difficulties (Peto OR 0.97, 95% CI 0.42 to 2.24; participants = 2198; studies = 1; Analysis 4.6). Pregnancies by 12 months occurred more frequently in the clip group (OR 0.21, 95% CI 0.05 to 0.84; participants = 3537; studies = 2; I2 = 0%; Analysis 4.7).
4.1. Analysis.
Comparison 4 Partial salpingectomy (PS) versus clip, Outcome 1 Operative mortality.
4.2. Analysis.
Comparison 4 Partial salpingectomy (PS) versus clip, Outcome 2 Major morbidity: total.
4.3. Analysis.
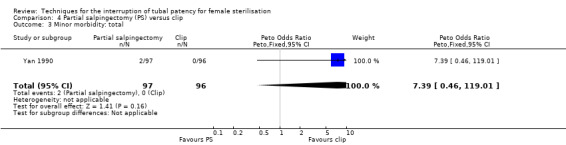
Comparison 4 Partial salpingectomy (PS) versus clip, Outcome 3 Minor morbidity: total.
4.5. Analysis.
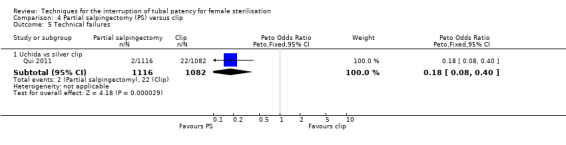
Comparison 4 Partial salpingectomy (PS) versus clip, Outcome 5 Technical failures.
4.6. Analysis.
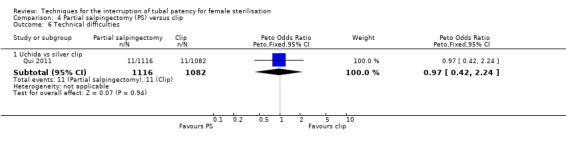
Comparison 4 Partial salpingectomy (PS) versus clip, Outcome 6 Technical difficulties.
4.7. Analysis.
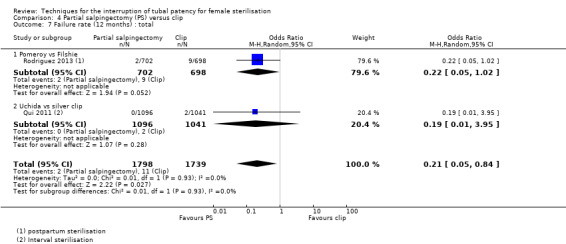
Comparison 4 Partial salpingectomy (PS) versus clip, Outcome 7 Failure rate (12 months) : total.
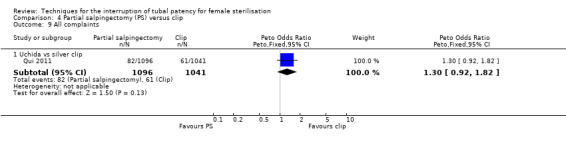
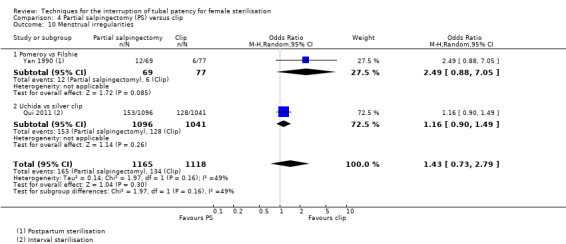
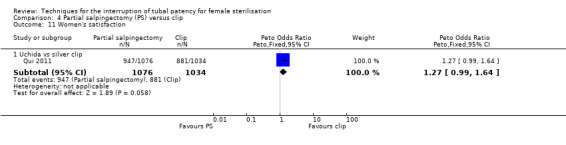
Operative time was longer for partial salpingectomy procedures than for clips (MD 4.26, 95% CI 3.65 to 4.86; participants = 2223; studies = 2; I² = 0%; Analysis 4.8). Neither patient complaints (Peto OR 1.30, 95% CI 0.92 to 1.82; participants = 2137; studies = 1; Analysis 4.9) nor menstrual irregularities were significantly different between groups (OR 1.43, 95% CI 0.73 to 2.79; participants = 2283; studies = 2; I² = 49%; Analysis 4.10). Patient complaints were reported by Qui 2011 at three, six, and 12 months and rates were similar at all assessment points. Women's satisfaction, reported in this study favoured the partial salpingectomy group (Peto OR 1.27, 95% CI 0.99 to 1.64; participants = 2110; studies = 1; Analysis 4.11); authors of this Chinese trial linked this to historical preferences.
4.8. Analysis.
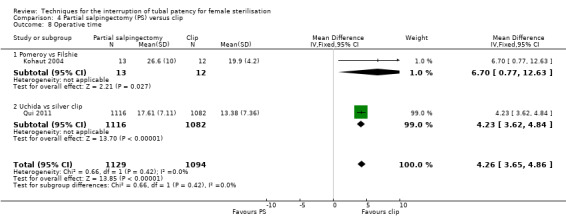
Comparison 4 Partial salpingectomy (PS) versus clip, Outcome 8 Operative time.
4.9. Analysis.
Comparison 4 Partial salpingectomy (PS) versus clip, Outcome 9 All complaints.
4.10. Analysis.
Comparison 4 Partial salpingectomy (PS) versus clip, Outcome 10 Menstrual irregularities.
4.11. Analysis.
Comparison 4 Partial salpingectomy (PS) versus clip, Outcome 11 Women's satisfaction.
5. Filshie clip versus Hulka clip
Two trials evaluated this comparison (Dominik 2000; Toplis 1988). There was no clear difference in minor morbidity overall (Peto OR 0.14, 95% CI 0.00 to 7.32; participants = 197; studies = 1; Analysis 5.1), or in procedure‐related injuries (OR 1.57, 95% CI 0.73 to 3.36; participants = 2322; studies = 2; I² = 0%; Analysis 5.2.1), urogenital tract infections (OR 2.40, 95% CI 0.62 to 9.30; participants = 1910; studies = 1; Analysis 5.2.2), or minor wound complications (OR 0.86, 95% CI 0.63 to 1.17; participants = 1910; studies = 1; Analysis 5.2.3). There was no clear difference in technical failures (OR 1.04, 95% CI 0.10 to 11.33; participants = 2325; studies = 2; I² = 55%; Analysis 5.3); however, technical difficulties occurred more frequently with the Hulka clip (Peto OR 1.51, 95% CI 1.09 to 2.10; participants = 2323; studies = 2; I² = 0%; Analysis 5.4). There was no clear difference between groups in the failure rate at one year poststerilisation (OR 6.20, 95% CI 0.75 to 51.66; participants = 1441; studies = 1; Analysis 5.5). Cumulative two‐year failures rates in the largest study, Dominik 2000, were 11.7 and 28.1 pregnancies per 1000 procedures for Filshie and Hulka clips, respectively.
5.1. Analysis.

Comparison 5 Hulka versus Filshie clip, Outcome 1 Minor morbidity: total.
5.2. Analysis.
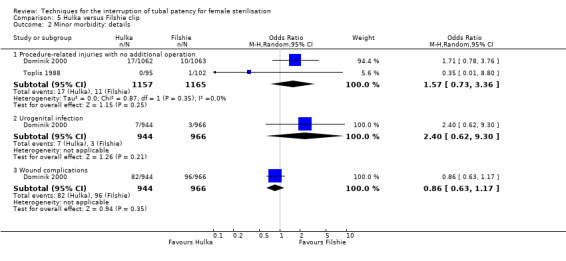
Comparison 5 Hulka versus Filshie clip, Outcome 2 Minor morbidity: details.
5.3. Analysis.
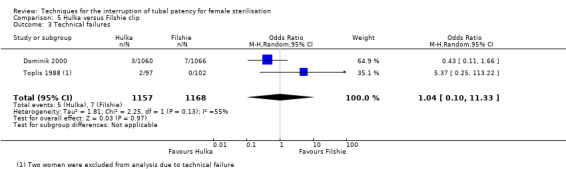
Comparison 5 Hulka versus Filshie clip, Outcome 3 Technical failures.
5.4. Analysis.
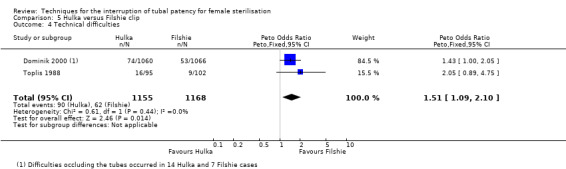
Comparison 5 Hulka versus Filshie clip, Outcome 4 Technical difficulties.
5.5. Analysis.

Comparison 5 Hulka versus Filshie clip, Outcome 5 Failure rate: total.
Discussion
Summary of main results
Altogether we included 19 RCTs involving 13,209 women requesting sterilisation. Sterilisation was performed on an interval basis in most trials, apart from three RCTs of postpartum sterilisation involving 1632 women. RCTs compared tubal rings versus clips (six RCTs, 4232 women), partial salpingectomy versus electrocoagulation (three RCTs, 2019 women), tubal rings versus electrocoagulation (two RCTs, 599 women), partial salpingectomy versus clips (four RCTs, 3627 women), clips versus electrocoagulation (two RCTs, 206 women) and Hulka versus Filshie clips (2 RCTs, 2326 women). The RCTs of clips versus electrocoagulation contributed no data to analyses. Studies of postpartum sterilisation compared partial salpingectomy with clips. No RCTs compared tubal inserts (hysteroscopic sterilisation) to other methods.
The main findings are summarised in Table 1, Table 2; Table 3; Table 4; and Table 5. One year after sterilisation, failure rates were comparable for tubal rings and clips (high‐quality evidence), partial salpingectomy and clips (high‐quality evidence), and for partial salpingectomy and electrocoagulation (low‐quality evidence). Estimates of failure rates for these methods were less than five pregnancies per 1000 procedures in the first year post‐sterilisation, and longer‐term pregnancy rates were generally not reported.
No deaths were reported as a results of the procedures in any of the studies. Major morbidity was rare with 22 events reported in three trials (Aranda 1976; Aranda 1985; WHO 1982), 17 events occurred with partial salpingectomy, four with electrocoagulation, and one with a clip procedure.
Minor morbidity occurred twice as often with tubal rings than with tubal clips (high‐quality evidence) and technical failures were also significantly more common with rings than clips (high‐quality evidence). There was no significant difference in postoperative pain between these groups (see Table 1).
Major and minor morbidity occurred more frequently with partial salpingectomy than with electrocoagulation for interval sterilisation (low‐ to moderate‐quality evidence; Table 2). Postoperative pain (up to 24 hours) was also significantly more common in the partial salpingectomy group than with electrocoagulation (moderate‐quality evidence).
There was no significant difference in major or minor morbidity when tubal rings were compared with electrocoagulation for interval sterilisation (low‐ to moderate‐quality evidence; Table 3). Evidence relating to technical failures was of a low quality for this comparison. Significantly more women undergoing sterilisation by tubal ring complained of postoperative pain in the first 24 hours compared with those in the electrocoagulation group (low‐quality evidence); however, this difference did not persist to the follow‐up visit.
For partial salpingectomy versus tubal clips, one large study reported no major morbidity with either method (Table 4). We found limited data on minor morbidity suggesting that there may be little difference between groups (low‐quality evidence). Evidence suggested that technical failures were more frequent with clip sterilisation (moderate‐quality evidence). There was no significant difference in patients' complaints at follow‐up in the one large study that reported this outcome (moderate‐quality evidence). Pooled data from two studies indicated that operative time was shorter on average with the clip technique than with partial salpingectomy (high‐quality evidence).
Hulka and Filshie clips were comparable in most outcomes for which there were data (Table 5), except that technical difficulties occurred more frequently in the Hulka clip group (moderate‐quality evidence).
We found little evidence about women's and surgeons' satisfaction for any of the comparisons.
Overall completeness and applicability of evidence
We found a fairly substantial body of evidence indicating that various established techniques for interrupting tubal patency, including partial salpingectomy, electrocoagulation, and use of tubal clips and rings, are safe and effective methods. As studies utilising electrocoagulation did not always state whether unipolar or bipolar coagulation was used, we were unable to draw differential conclusions regarding these methods; however, major morbidity attributed to electrocoagulation in the included studies was very low. We found no RCTs that compared sterilisation by tubal inserts (hysteroscopic sterilisation) with other methods, so more evidence on the safety and efficacy of this relatively new method is needed.
The short duration of follow‐up in the RCTs included in this review, which was usually one or two years, limits the evidence on failure (pregnancy) rates. In addition, failure rates were possibly underestimated due to high losses to follow‐up in those RCTs that reported a two‐year follow‐up.Thus, data on longer‐term failure rates may best be derived from the CREST study (Peterson 1996). Cumulative evidence from this prospective cohort study found that the 10‐year probability of pregnancy was highest after clip sterilisation (36.5/1000 procedures) and lowest for postpartum partial salpingectomy (7.5/1000) and unipolar coagulation (7.5/1000). Tubal ring was the most common sterilisation technique in the CREST cohort and was associated with a 10‐year probability of pregnancy of 17.7/1000 procedures. The one‐year and 10‐year probabilities of pregnancy with any procedure was 5.5 /1000 and 18.5/1000 procedures, respectively. Younger women (18 to 27 years) undergoing sterilisation by bipolar coagulation were at greatest risk of sterilisation failure within ten years of the procedure (54.3/1000 procedures).
We did not try to determine the relative effects of different types of anaesthetic methods (local, spinal, general anaesthesia and other) on women's sterilisation experience, including postoperative pain, and this could be the subject of a separate review.
Quality of the evidence
We graded the quality of the main findings of the review using the GRADE (Grading of Recommendations Assessment, Development and Evaluation) approach. The quality of the evidence relating to tubal rings versus clips was mainly high. For the evidence related to partial salpingectomy compared with electrocoagulation, we downgraded the evidence quality to low or moderate due to imprecision or indirectness, as the findings may have been due to the access approach rather than the tubal occlusion technique (partial salpingectomy was mainly performed via minilaparotomy, whereas electrocoagulation was performed via laparoscopy). We downgraded the quality of the evidence relating to tubal rings versus electrocoagulation for most outcomes, most frequently due to imprecision of the effect.
We graded the evidence relating to partial salpingectomy versus tubal clips mainly as moderate due to imprecision or risk of bias. Evidence on minor morbidity for this comparison was very sparse and imprecise, hence we downgraded it to low quality evidence. For the comparison of Filshie and Hulka clips, evidence was mainly of a low to moderate quality due to imprecision, with or without other factors.
Potential biases in the review process
The original review was performed in 2002 using old Cochrane methods for classifying studies and assessing risk of bias. For this update, we revised the methodology to conform with current Cochrane methods for 'Risk of bias' assessment, resulting in the inclusion of three RCTs that had been excluded from previous versions of the review. Two of these studies contributed little (Geirsson 1985), or no (Goynumer 2009), data. In addition, we identified two RCTs using the related articles feature of PubMed, which should have been included in earlier versions of the review (Dominik 2000; Siegle 2005). Due to resource constraints we did not re‐extract the missing risk of bias details from previously included studies for this update.
For the comparison 'partial salpingectomy versus clips', we pooled data from two RCTs that used different methods for partial salpingectomy (modified Pomeroy and Uchida methods) and different clip methods (titanium and silver clips; see Included studies) and performed subgroup analysis to compare these findings. The test for subgroup differences indicated no difference, however, these subgroup analyses were not pre‐specified in the protocol. This subgrouping also served to distinguish between studies according to the timing of the procedure, i.e. postpartum and interval sterilisation, which similarly indicated no significant difference in findings according to the procedure timing.
Although we noted when studies were at moderate or high risk of bias for specific outcomes, we did not perform sensitivity analysis, because few studies contributed to most analyses; however, we downgraded results accordingly in the 'Summary of findings' tables.
We found no RCTs comparing types of tubal inserts or comparing tubal inserts with other methods of interrupting tubal patency; however, we found one RCT comparing two methods of accessing the fallopian tubes (vaginoscopy compared with hysteroscopy) to insert Essure inserts (Chapa 2015). Studies comparing methods of accessing the tubes are not included in this review. Abdominal approaches (minilaparotomy versus laparoscopy) for female sterilisation are the subject of a separate Cochrane review (Kulier 2004) and, similarly, studies comparing methods of vaginal approaches to hysteroscopic sterisation should be considered in a separate Cochrane review.
Agreements and disagreements with other studies or reviews
Data from newly included studies support the previous findings of this review, that tubal sterilisation by most established methods is an effective and safe procedure. Evidence relating to postoperative pain was limited in our review; however, for the comparison of partial salpingectomy versus electrocoagulation, and tubal rings versus electrocoagulation, moderate‐quality evidence indicated that there was less postoperative pain with electrocoagulation. A recent review of RCTs of local anaesthesia to reduce postoperative pain following interval laparoscopic sterilisation found that the intraoperative application of local anaesthetic to the tubes significantly reduced postoperative pain for ring and clip sterilisation (Harrison 2014). In addition, a protocol for a Cochrane Review to evaluate the effectiveness of interventions for pain relief in women undergoing postpartum mini‐laparotomy tubal sterilisation has recently been published (Lumbiganon 2015). This review should help further towards improving the experience of women requesting this popular form of contraception.
We found no RCTs of hysteroscopic sterilisation compared with other methods of sterilisation. Hysteroscopic sterilisation with tubal inserts has several advantages over older techniques in that the procedure avoids the peritoneal cavity, requires no incisions, usually requires no anaesthesia, may be well tolerated, and is usually performed as an outpatient procedure (Peterson 2008). In the USA, hysteroscopic sterilisation with Essure® is reported to be rapidly replacing laparoscopic sterilisation and is potentially associated with lower cost (Connor 2009; Kraemer 2009). User satisfaction rates are reported to be high (Arjona 2008; Chudnoff 2015; Connor 2009; Sinha 2007), however, placement failure rates vary and long‐term efficacy has still to be established. Two systematic reviews of non‐randomised studies were unable to calculate the cumulative probability of pregnancy with Essure® due to limitations in available data (Cleary 2013; la Chapelle 2015). One found 'fair‐quality evidence' that pregnancy was rare in women with documented bilateral tubal occlusion at three months after the procedure (Cleary 2013); the other reported a cumulative three‐year probability of pregnancy with Adiana (no longer available) of 16/1000 procedures (la Chapelle 2015). Gariepy 2014 used an evidence‐based Markov model to estimate the relative probability of pregnancy over 10 years following sterilisation with three methods ‐ Essure®, the silicone ring, and bipolar coagulation ‐ and estimated pregnancy probabilities of 96, 24 and 30 per 1000 women, respectively, with the highest risk of pregnancy after Essure® sterilisation occurring in the first year post‐procedure (57 per 1000 women). This estimate considered the complete clinical pathways of the procedures, taking into account uncertainties regarding placement success, hysterosalpingogram (HSG) follow‐up, and successful tubal occlusion, and therefore possibly reflects the 'real‐life' situation. Other recent reports of Essure® sterilisation include a prospective study that reported no pregnancies in 449 women with successful bilateral placement and confirmed occlusion who completed the five‐year follow‐up (71% of cohort; Chudnoff 2015), and a retrospective cohort study of 109,277 women who underwent sterilisation by Essure® (39,169 women) or laparoscopic tubal ligation procedures (70,108 women) in France between 2006 and 2010 reporting pregnancy rates of 0.36% and 0.45%, respectively (Fernandez 2014).
More data are needed on the short‐ and long‐term side‐effects of hysteroscopic sterilisation relative to other methods. In Chudnoff 2015, intermenstrual bleeding occurred among 23.6% of women during the first three months postprocedure, 20% of women (74/386) reported heavier menses at the five‐year follow‐up visit; 5.3% (25/473) experienced recurrent pelvic pain, and 15 women underwent hysterectomy during the five‐year follow‐up period (apparently only two were possibly attributable to Essure®). Ouzounelli 2015 conducted a review of hysteroscopic sterilisation compared with laparoscopic tubal ligation and found that both had low rates of complications, although complications related to Essure® procedures were "more likely to be minor in nature". Another review concluded that "the incidence and severity of complications with hysteroscopic sterilisation has not been adequately studied and remains unclear" (la Chapelle 2015). A review by Cooper 2010 found that the vaginoscopic approach (whereby hysteroscopy is performed without a vaginal speculum or cervical instruments to grasp the cervix) may be associated with less pain than traditional hysteroscopic sterilisation techniques with no difference in the number of failed procedures.
One of the obvious limitations of hysteroscopic sterilisation at present is the delay in achieving its occlusive effect and the need for confirmatory testing at three months. A new iteration of the Essure® insert designed to achieve immediate tubal occlusion appears to show promise in this regard, and may reduce pre‐HSG pregnancies (Thiel 2014). A search of clinical trial registries at the time of writing (31 July 2015) found no registered or ongoing RCTs comparing hysteroscopic methods with more widely used methods.
No RCTs investigating the safety and effectiveness of quinacrine sterilisation, which is used unlicensed in some regions, have been conducted or registered. Due to its low cost, easy application, apparent safety, and comparable effectiveness, a call has been made recently for the use of quinacrine in low‐ and middle‐income countries to be reconsidered (Lippes 2015). Concerns about the mutagenicity of quinacrine have not been supported by epidemiological evidence from extensive human studies (Lippes 2015), with at least two large studies finding no association between quinacrine sterilisation (QS) and the risk of gynaecological cancer (Sokal 2010a; Sokal 2010b). Furthermore, studies conducted in China and Iran have reported that QS is more acceptable to women than surgical sterilisation (Lu 2003; Soroodi‐Moghaddam 2003). A large, well‐conducted RCT of this inexpensive, minimally‐invasive method could be important.
Authors' conclusions
Implications for practice.
Failure rates at 12 months post‐sterilisation and major morbidity were found to be rare outcomes with sterilisation by partial salpingectomy, electrocoagulation, clips, and tubal rings. Technical failures were more common with tubal rings compared with clips, and more common with clips compared with partial salpingectomy. The choice of the tubal occlusion technique should include consideration of the costs, equipment availability, the setting and the surgeon's experience. We were unable to draw any conclusions about the relative efficacy and safety of hysteroscopic sterilisation and more research is needed.
Implications for research.
RCTs comparing hysteroscopic sterilisation methods, e.g. Essure®, with laparoscopic (and other) methods are needed. To assess adverse effects, controlled observational studies of sterilisation by minimally invasive methods would also be of value. Women's satisfaction, side‐effects, and cost are important outcomes for future RCTs. Women in their forties without hormonal treatment may experience more dysfunctional uterine bleeding, which is often treated with hormonally‐impregnated intrauterine systems (French 2004); studies evaluating differences in long‐term effectiveness and adverse effects between hysteroscopic sterilisation methods and IUSs, especially among women over 40 years of age, are therefore of interest. Given that quinacrine sterilisation is cheap, minimally invasive, and in use in some countries (Lippes 2015), a trial of quinacrine compared with hysteroscopic sterilisation would be very informative. Further comparative trials of abdominally accessed sterilisation methods are not considered to be a high priority for research.
What's new
| Date | Event | Description |
|---|---|---|
| 22 July 2016 | Amended | Data from Yan 2009 was removed from Analysis 4.7 after it was determined that these study data were probably included in the Rodriguez 2013 study data for this outcome. |
| 10 August 2015 | New citation required but conclusions have not changed | Review updated. |
| 31 July 2015 | New search has been performed | Seven additional studies included. Risk of bias assessment methods updated. |
History
Protocol first published: Issue 2, 2001 Review first published: Issue 4, 2002
| Date | Event | Description |
|---|---|---|
| 27 August 2010 | New citation required but conclusions have not changed | Update submitted. |
| 15 May 2010 | New search has been performed | Three additional studies included (Kohaut 2004, Gentile 2006 and Pymar 2004). |
| 3 March 2010 | New search has been performed | Search updated. |
| 15 April 2008 | Amended | Converted to new review format. |
| 20 July 2002 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
We thank the Co‐ordinating and Managing Editors of the Fertility Regulation Group, Frans and Anja Hemerhorst, for their support and advice. We also thank Alan Pinol, WHO Geneva, for his help in clarifying methodological issues regarding the WHO 1982 trial for the original version of this review. M Boulvain and HB Peterson contributed to the text of the original review.
Appendices
Appendix 1. Risk of bias assessment used in earlier versions of this review
A quality score for concealment of allocation was assigned to each trial, using the following criteria: A = adequate concealment of allocation B = unclear whether concealment of allocation is adequate C = inadequate concealment of allocation, quasi‐randomisation
Only studies scoring A or B were included in the review originally.
For withdrawals, studies were classified as follows: a = less than 3% of participants withdrawn; b = between 3% to 9.9% of participants withdrawn; c = between 10% to 19.9% of participants withdrawn. Trials were excluded if it was not possible to enter data on an intention‐to‐treat basis and/or 20% or more participants were excluded.
Data and analyses
Comparison 1. Tubal ring versus clip.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Major morbidity: total | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 1.1 Procedure‐related injuries requiring additional operation or blood transfusion | 1 | 545 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.14 [0.00, 7.05] |
| 2 Minor morbidity: total | 2 | 842 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.15 [1.22, 3.78] |
| 3 Minor morbidity: details | 3 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 3.1 Procedure related injuries with no additional operation | 3 | 3575 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.95 [1.36, 2.78] |
| 3.2 Urogenital infections | 3 | 3145 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.88 [0.83, 4.28] |
| 3.3 Wound infection | 3 | 3144 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.17 [0.73, 1.87] |
| 3.4 Postoperative temperature > 38 °C without hospitalisation | 1 | 296 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 7.49 [0.15, 377.52] |
| 4 Technical failures | 3 | 3476 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.93 [2.43, 6.35] |
| 5 Technical difficulties | 3 | 3590 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.13 [0.87, 1.46] |
| 6 Failure rate: total | 4 | 3822 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.72 [0.33, 1.57] |
| 7 Failure rate: details | 2 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 7.1 Failure rate ≤ 1 year, total | 2 | 2629 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.85 [0.23, 3.14] |
| 7.2 Failure rate ≤ 1 year, extrauterine pregnancy | 1 | 2202 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.3 Failure rate > 1 year, extrauterine pregnancy | 1 | 427 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 8.11 [0.16, 410.33] |
| 8 Operative time | 1 | 297 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Hospital stay > 24 h | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 10 Complaints | 4 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 10.1 Postoperative pain < 24 h | 3 | 922 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.14 [0.88, 1.48] |
| 10.2 Postoperative analgesic use | 1 | 70 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.70 [0.28, 1.79] |
| 10.3 Cramping pain during first week after surgery | 1 | 70 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 5.24 [1.52, 18.00] |
| 11 Menstrual irregularities | 2 | 612 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.61 [0.75, 3.49] |
1.7. Analysis.
Comparison 1 Tubal ring versus clip, Outcome 7 Failure rate: details.
1.9. Analysis.
Comparison 1 Tubal ring versus clip, Outcome 9 Hospital stay > 24 h.
Comparison 2. Partial salpingectomy (modified Pomeroy) versus electrocoagulation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Operative mortality | 1 | 1610 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Major morbidity: total | 2 | 1905 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.87 [1.13, 7.25] |
| 3 Major morbidity: details | 2 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Procedure‐related injuries requiring additional operation or blood transfusion | 2 | 1905 | Odds Ratio (M‐H, Random, 95% CI) | 1.90 [0.19, 18.96] |
| 3.2 Rehospitalisation as a consequence of operation | 1 | 295 | Odds Ratio (M‐H, Random, 95% CI) | 5.74 [0.73, 45.09] |
| 4 Minor morbidity: total | 2 | 1905 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.60 [1.10, 2.33] |
| 5 Minor morbidity: details | 2 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 5.1 Procedure‐related injuries with no additional operation | 1 | 1610 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.53 [0.06, 5.11] |
| 5.2 Urogenital infections | 1 | 1610 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.81 [0.43, 1.50] |
| 5.3 Wound infection | 1 | 1610 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.49 [1.54, 4.04] |
| 5.4 Postoperative temperature > 38 °C without hospitalisation | 1 | 295 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.45 [0.18, 11.77] |
| 6 Failure rate: total | 1 | 295 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 4.47 [0.07, 286.78] |
| 6.1 Failure rate, total | 1 | 295 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 4.47 [0.07, 286.78] |
| 7 Failure rate: details | 1 | 295 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 4.47 [0.07, 286.78] |
| 7.1 Failure rate > 1 year, total | 1 | 295 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 4.47 [0.07, 286.78] |
| 8 Hospital stay more 24 h | 1 | 108 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.08, 2.74] |
| 9 Complaints | 3 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 9.1 Postoperative pain < 24 h | 2 | 1905 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.85 [2.91, 5.10] |
| 9.2 Postoperative analgesic use | 1 | 109 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.05 [0.40, 10.56] |
| 9.3 Persistent pain at follow‐up visit | 1 | 1610 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.09 [0.81, 1.47] |
2.1. Analysis.
Comparison 2 Partial salpingectomy (modified Pomeroy) versus electrocoagulation, Outcome 1 Operative mortality.
2.3. Analysis.
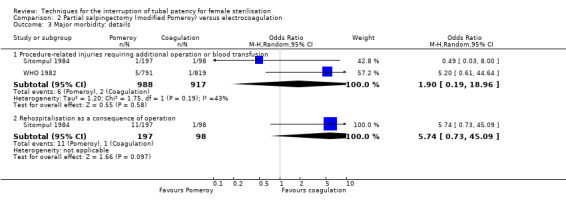
Comparison 2 Partial salpingectomy (modified Pomeroy) versus electrocoagulation, Outcome 3 Major morbidity: details.
2.5. Analysis.
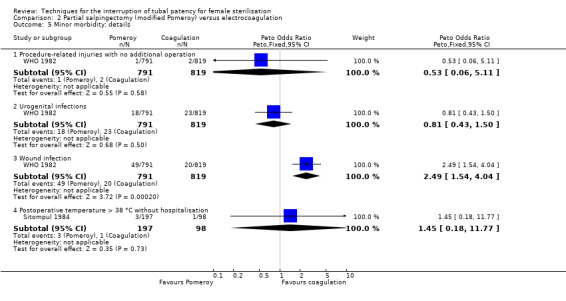
Comparison 2 Partial salpingectomy (modified Pomeroy) versus electrocoagulation, Outcome 5 Minor morbidity: details.
Comparison 3. Tubal ring versus electrocoagulation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Major morbidity: total | 2 | 596 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.14 [0.00, 7.01] |
| 2 Major morbidity: details | 1 | 298 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.14 [0.00, 7.01] |
| 2.1 Procedure‐related injuries requiring additional operation or blood transfusion | 1 | 298 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.14 [0.00, 7.01] |
| 3 Minor morbidity: total | 2 | 596 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.97 [0.50, 1.87] |
| 4 Minor morbidity: details | 2 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 4.1 Procedure‐related injuries with no additional operation | 2 | 596 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.76 [0.17, 3.38] |
| 4.2 Urogenital infections | 1 | 296 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.03 [0.14, 7.37] |
| 4.3 Wound infection | 1 | 296 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.93 [0.38, 2.25] |
| 4.4 Postoperative temperature > 38 °C without hospitalisation | 2 | 594 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.37 [0.31, 6.06] |
| 5 Technical failures: total | 2 | 596 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.42 [0.59, 19.81] |
| 6 Technical difficulties | 1 | 298 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.14 [0.01, 1.33] |
| 7 Failure rate: total | 1 | 160 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.1 Failure rate, total | 1 | 160 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Operative time | 1 | 298 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Complaints | 2 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 9.1 Postoperative pain < 24 h | 2 | 596 | Odds Ratio (M‐H, Random, 95% CI) | 3.40 [1.17, 9.84] |
| 9.2 Postoperative analgesic use | 1 | 298 | Odds Ratio (M‐H, Random, 95% CI) | 2.51 [1.00, 6.30] |
| 9.3 Persistent pain at follow‐up visit | 2 | 594 | Odds Ratio (M‐H, Random, 95% CI) | 1.22 [0.75, 1.97] |
| 10 Menstrual irregularities | 1 | 296 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.90 [0.56, 1.45] |
3.4. Analysis.
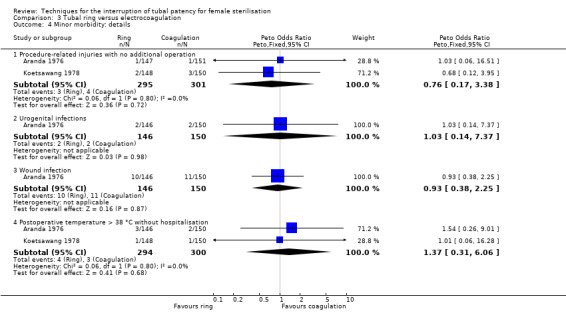
Comparison 3 Tubal ring versus electrocoagulation, Outcome 4 Minor morbidity: details.
3.7. Analysis.
Comparison 3 Tubal ring versus electrocoagulation, Outcome 7 Failure rate: total.
3.8. Analysis.
Comparison 3 Tubal ring versus electrocoagulation, Outcome 8 Operative time.
3.10. Analysis.
Comparison 3 Tubal ring versus electrocoagulation, Outcome 10 Menstrual irregularities.
Comparison 4. Partial salpingectomy (PS) versus clip.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Operative mortality | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 1.1 Uchida vs silver clip | 1 | 2198 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Major morbidity: total | 1 | 2198 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.1 Uchida vs silver clip | 1 | 2198 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Minor morbidity: total | 1 | 193 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 7.39 [0.46, 119.01] |
| 4 Minor morbidity: details | 1 | 193 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 7.39 [0.46, 119.01] |
| 4.1 Procedure related injuries with no additional operation | 1 | 193 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 7.39 [0.46, 119.01] |
| 5 Technical failures | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 5.1 Uchida vs silver clip | 1 | 2198 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.18 [0.08, 0.40] |
| 6 Technical difficulties | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 6.1 Uchida vs silver clip | 1 | 2198 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.97 [0.42, 2.24] |
| 7 Failure rate (12 months) : total | 2 | 3537 | Odds Ratio (M‐H, Random, 95% CI) | 0.21 [0.05, 0.84] |
| 7.1 Pomeroy vs Filshie | 1 | 1400 | Odds Ratio (M‐H, Random, 95% CI) | 0.22 [0.05, 1.02] |
| 7.2 Uchida vs silver clip | 1 | 2137 | Odds Ratio (M‐H, Random, 95% CI) | 0.19 [0.01, 3.95] |
| 8 Operative time | 2 | 2223 | Mean Difference (IV, Fixed, 95% CI) | 4.26 [3.65, 4.86] |
| 8.1 Pomeroy vs Filshie | 1 | 25 | Mean Difference (IV, Fixed, 95% CI) | 6.70 [0.77, 12.63] |
| 8.2 Uchida vs silver clip | 1 | 2198 | Mean Difference (IV, Fixed, 95% CI) | 4.23 [3.62, 4.84] |
| 9 All complaints | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 9.1 Uchida vs silver clip | 1 | 2137 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.30 [0.92, 1.82] |
| 10 Menstrual irregularities | 2 | 2283 | Odds Ratio (M‐H, Random, 95% CI) | 1.43 [0.73, 2.79] |
| 10.1 Pomeroy vs Filshie | 1 | 146 | Odds Ratio (M‐H, Random, 95% CI) | 2.49 [0.88, 7.05] |
| 10.2 Uchida vs silver clip | 1 | 2137 | Odds Ratio (M‐H, Random, 95% CI) | 1.16 [0.90, 1.49] |
| 11 Women's satisfaction | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 11.1 Uchida vs silver clip | 1 | 2110 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.27 [0.99, 1.64] |
| 12 Surgeon's satisfaction | Other data | No numeric data |
4.4. Analysis.
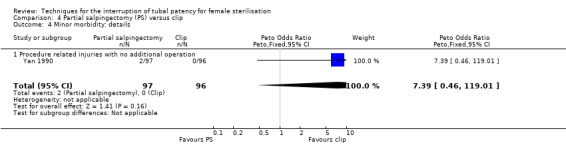
Comparison 4 Partial salpingectomy (PS) versus clip, Outcome 4 Minor morbidity: details.
4.12. Analysis.
Comparison 4 Partial salpingectomy (PS) versus clip, Outcome 12 Surgeon's satisfaction.
| Surgeon's satisfaction | ||
|---|---|---|
| Study | . | . |
| Kohaut 2004 | Seven out of 10 surgeons performing a total of 29 sterilisations preferred the Filshie clip method to the Pomeroy method. | |
Comparison 5. Hulka versus Filshie clip.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Minor morbidity: total | 1 | 197 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.14 [0.00, 7.32] |
| 2 Minor morbidity: details | 2 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Procedure‐related injuries with no additional operation | 2 | 2322 | Odds Ratio (M‐H, Random, 95% CI) | 1.57 [0.73, 3.36] |
| 2.2 Urogenital infection | 1 | 1910 | Odds Ratio (M‐H, Random, 95% CI) | 2.40 [0.62, 9.30] |
| 2.3 Wound complications | 1 | 1910 | Odds Ratio (M‐H, Random, 95% CI) | 0.86 [0.63, 1.17] |
| 3 Technical failures | 2 | 2325 | Odds Ratio (M‐H, Random, 95% CI) | 1.04 [0.10, 11.33] |
| 4 Technical difficulties | 2 | 2323 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.51 [1.09, 2.10] |
| 5 Failure rate: total | 1 | 1441 | Odds Ratio (M‐H, Fixed, 95% CI) | 6.20 [0.75, 51.66] |
| 6 Operative time | 1 | 197 | Mean Difference (IV, Fixed, 95% CI) | 0.70 [‐0.04, 1.44] |
| 7 Complaints | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 7.1 Postoperative pain < 24 h | 1 | 197 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.74 [0.99, 3.03] |
5.6. Analysis.
Comparison 5 Hulka versus Filshie clip, Outcome 6 Operative time.
5.7. Analysis.
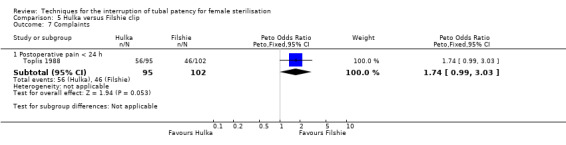
Comparison 5 Hulka versus Filshie clip, Outcome 7 Complaints.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Aranda 1976.
| Methods | Randomisation not specified. Concealment of allocation by sealed envelopes containing a card that specified the technique for tubal occlusion | |
| Participants | 299 women requesting sterilisation for family planning reasons, at least 6 weeks postpartum Conducted at the Hospital Mexico, San Jose, Costa Rica | |
| Interventions | Electrocoagulation versus tubal ring, all laparoscopy. All under local anaesthesia and intravenous sedation | |
| Outcomes | Surgical and early postoperative complications and complaints | |
| Notes | Blinding of postoperative evaluation | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly assigned" |
| Allocation concealment (selection bias) | Unclear risk | Concealment of allocation by sealed envelopes containing a card which specified the technique of tubal occlusion. Assessed as a 'B' study (unclear allocation concealment) in original review |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Outcome assessor was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | ‐ |
| Selective reporting (reporting bias) | Unclear risk | Unable to determine |
| Other bias | Low risk | Women had similar socio‐demographic characteristics |
Aranda 1985.
| Methods | Multicenter study. Randomisation by computer‐generated labels. Concealment of allocation by sealed opaque envelopes. Not stated whether sequentially numbered | |
| Participants | 663 women requesting sterilisation to limit family size and free of major systemic and pelvic abnormalities. Interval (55%) and postspontaneous abortion (45%). Conducted in San Jose, San Salvador and Cairo | |
| Interventions | Tubal ring versus Rocket clip via minilaparotomy. Under general anaesthesia (55%) or local anaesthesia and intravenous sedation | |
| Outcomes | Major and minor morbidity, technical failures and difficulties, failure rates and complaints | |
| Notes | Blinding of postoperative evaluation. About 90% of women in both groups remained hospitalised for at least 1 night. The operations were performed with general anaesthesia in 55% of cases and with analgesia and/or sedation plus local anaesthesia in 45% of procedures | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation by computer‐generated labels |
| Allocation concealment (selection bias) | Unclear risk | Concealment of allocation by sealed opaque envelopes. Not stated if sequentially numbered. Assessed as a 'B' study (unclear allocation concealment) in original review |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Blinding of postoperative evaluation |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 30 cases of technical failure (5% of total) were excluded from the analyses |
| Selective reporting (reporting bias) | Unclear risk | ‐ |
| Other bias | Unclear risk | ‐ |
Argueta 1980.
| Methods | RCT | |
| Participants | 299 women requesting sterilisation at the Asociacion Demografica Salvadorena, San Salvador | |
| Interventions | Spring‐loaded clip versus tubal ring all laparoscopy. All under local anaesthesia and intravenous sedation | |
| Outcomes | Operative morbidity, technical failures and difficulties, failure rates, complaints | |
| Notes | Participant and postoperative evaluation blinding 1 surgeon performed all surgical procedures on an outpatient basis |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not specified |
| Allocation concealment (selection bias) | Unclear risk | Assessed as a 'B' study (unclear allocation concealment) in original review |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Participants and postoperative evaluators were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | In the clip group 54 women (36%) and 60 (40%) in the ring group were lost to follow‐up at 24 months |
| Selective reporting (reporting bias) | Unclear risk | Unable to determine |
| Other bias | Low risk | Women had similar socio‐demographic characteristics |
Dominik 2000.
| Methods | 2 multicentre RCTs conducted by Family Health International in Malaysia, Panama, Dominican Republic, Mexico, Venezuela, Guatamala, Haiti. 1 RCT involved a minilaparotomy approach, the other involved a laparoscopic approach | |
| Participants | 2126 women were included if they were at least 21 years old, had no pregnancy within 42 days, and no chronic medical conditions 878 participants were enrolled in the minilap study and 1248 enrolled in the laparoscopy study |
|
| Interventions | Filshie clip (1066 women) vs Hulka clip (1060 women) | |
| Outcomes | Failure rates, technical failure and difficulties, morbidity Assessed at 1, 6, and 12 months after sterilisation. A subset of women were assessed at 24 months |
|
| Notes | Article reports the combined results of 2 RCTs, 1 of sterilisation via minilaparotomy, the other by laparoscopy Surgeons were experienced. Cumulative failure rates were 11.7 for Filshie and 28.1 per 1000 for Hulka at 24 months |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer‐generated randomisation scheme" |
| Allocation concealment (selection bias) | Low risk | Sealed sequentially numbered opaque envelope opened at the time of surgery |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Outcome assessor was blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Loss to follow‐up at 12 months was high (31% and 34% for the laparoscopy and minilaparotomy studies, respectively) but balanced across the groups. Loss to follow‐up at 24 months in a subset of participants was 43%. Protocol deviations were low |
| Selective reporting (reporting bias) | Unclear risk | Not able to determine. ITT and per protocol analyses performed |
| Other bias | Low risk | Baseline characteristics were similar. Mean age was 31 years and average parity was 4.2 children |
Geirsson 1985.
| Methods | RCT conducted in Scotland | |
| Participants | 79 women requesting sterilisation; excluded if postpartum or postabortion | |
| Interventions | Falope rings (36 women) vs Filshie clips (34 women) | |
| Outcomes | Day 1‐6 postoperative pain, analgesic requirements, additional medical assistance | |
| Notes | Interval sterilisation via laparoscopy | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "a prospective randomized comparison" |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 9 post‐procedure exclusions due to intra‐operative difficulties, subsequent UTI, incomplete follow‐up and anaesthetic complications. Protocol deviations and ITT analysis not described |
| Selective reporting (reporting bias) | Unclear risk | Unable to determine |
| Other bias | High risk | Post‐randomisation exclusions of women with complications occurred in this study of morbidity |
Gentile 2006.
| Methods | RCT | |
| Participants | 118 women requesting sterilisation | |
| Interventions | 62 Hulka clips and 56 electrocautery | |
| Outcomes | Urinary and serum oestradiol and progesterone levels; participants' satisfaction and regret | |
| Notes | Secondary outcomes relating to women’s satisfaction/regret have never been published. The authors were contacted and provided some additional information regarding method of randomisation/allocation concealment but were unable to find the unpublished data regarding women's satisfaction. Included but no pertinent outcome data available Anaesthesia not specified |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table |
| Allocation concealment (selection bias) | Low risk | Consecutively numbered sealed opaque envelopes (unpublished information) |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Participants blinded throughout the 2‐year follow‐up (unpublished information) |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not able to determine |
| Selective reporting (reporting bias) | Unclear risk | Secondary outcomes were never reported |
| Other bias | Unclear risk | Not able to determine |
Goynumer 2009.
| Methods | RCT | |
| Participants | 88 women Inclusion criteria: regular menses, no risk of ovarian failure in personal or family history, ovarian reserve on transvaginal ultrasound (TVU) in normal range Exclusion criteria: perimenopausal symptoms, abnormal BMI, ovarian cysts > 25 mm on TVU, pelvic surgery in previous year |
|
| Interventions | Laparoscopic interval sterilisation via electrocoagulation or mechanical clips | |
| Outcomes | Post‐operative 10th month mean values of ovarian reserve i.e. serum FSH, LH, estradiol, inhibin‐B, antimullerian hormone. Total ovarian volume and anthral follicle count on TVU | |
| Notes | General anaesthesia used No review outcomes reported, therefore this study contributed no data to the review |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information on loss to follow‐up or postrandomisation exclusions |
| Selective reporting (reporting bias) | Unclear risk | Not able to determine |
| Other bias | Unclear risk | Not able to determine |
Koetsawang 1978.
| Methods | Did not specify method of randomisation | |
| Participants | 300 women requesting sterilisation for family planning purposes at the Siriraj Hospital in Bangkok | |
| Interventions | Unipolar electrocoagulation versus tubal ring via laparoscopy. All performed under local anaesthesia and intravenous sedation | |
| Outcomes | Operative morbidity, technical failures and difficulties, failure rates, operative time, complaints | |
| Notes | Postoperative evaluation blinding, prophylactic antibiotics for 5 days Up to 54% loss to follow‐up at 12 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Unclear risk | Assessed as a 'B' study (unclear allocation concealment) in original review |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Outcome assessor was blinded |
| Other bias | Low risk | The 2 groups had similar socio‐economic characteristics |
Kohaut 2004.
| Methods | RCT pilot study of postpartum sterilisation techniques. Computer‐generated randomisation; sealed opaque envelope opened immediately before sterilisation | |
| Participants | 32 pregnant patients requesting sterilisation after vaginal delivery (25) or Caesarean section (4). Inclusion criteria: ≥ 21 years | |
| Interventions | 14 Filshie clip vs 15 Pomeroy method | |
| Outcomes | Time from skin incision to closure, technical difficulties, surgeon's satisfaction, surgeon's preference (7/10 preferred Filshie to Pomeroy) | |
| Notes | Baseline characteristics similar in the 2 groups. 2/32 study questionnaires lost = missing data (1 in each group). 1 post‐randomisation exclusion from the Filshie group as the woman had had a previous failed Filshie sterilisation and so Pomeroy was performed when surgeon saw old clips ‐ no other details provided General or spinal anaesthesia used |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelope opened immediately before sterilisation |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | ‐ |
| Selective reporting (reporting bias) | Unclear risk | ‐ |
| Other bias | Unclear risk | Baseline characteristics similar in the 2 groups |
Pymar 2004.
| Methods | RCT. Women received a Filshie clip on 1 fallopian tube and a ring on the opposite side; site allocation was randomized. Randomisation (via random number table) was performed following laparoscopic abdominal examination after excluding adhesions, endometriosis and pelvic masses. Allocation concealment by sequentially numbered sealed opaque envelopes. Group assignment determined the device that would be applied first and the side to which the first occlusion method would be placed. The subject was blind to her group, as were the monitoring and research staff | |
| Participants | 40 women in Pittsburg, USA, requesting sterilisation Inclusion criteria: > 21years, literate and with telephone access Exclusion criteria: allergy to local anaesthetic, morbid obesity > 100 kg, > 2 previous laparotomies, history of moderate to severe endometriosis, pelvic mass, current chronic pelvic pain, use of pain medications for any indication regularly during the past 2 weeks, history of depression or anxiety or other psychiatric disorder, allergy/intolerance to analgesics, plan for open laparoscopic procedure, previous tubal surgery, in‐operative discovery of dense adhesions, endometriosis or pelvic mass that required concurrent surgery |
|
| Interventions | Sterilisation with Filshie clip and fallopian ring on opposite fallopian tubes following topical bupivicaine application to the tubes (abdominal entry via laparoscopy) | |
| Outcomes | Postoperative pain in first 24 h by visual analogue scale at 1 h, 2 h and 24 h | |
| Notes | Rationale behind design was that women are apparently able to discriminate pain on each side of their abdomen after tubal occlusion (Kaplan 1990). ITT analysis; no significant difference in results when reported major (8) and minor (9); deviations from protocol were excluded | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table |
| Allocation concealment (selection bias) | Low risk | Allocation concealment was by sequentially numbered sealed opaque envelopes |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Participants were blinded to group allocation, as were the monitoring and research staff |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Minimal loss to follow‐up. 8 women had major deviations from protocol and 17 women had minor deviations from protocol. Results were reported according to ITT |
| Selective reporting (reporting bias) | Unclear risk | Unable to determine |
| Other bias | Unclear risk | None noted |
Qui 2011.
| Methods | Multicentre RCT conducted in 20 clinics in China between June 2007 and August 2008 | |
| Participants | 2198 women requiring sterilisation Inclusion criteria: 20‐40 years old, at least 2 children, married Exclusion criteria: history of major abdominal surgery, epilepsy, neurosis, chronic pelvic inflammatory disease, acute infectious disease, body temperature > 37.5 °C, noted to have severe adhesions in previous operations |
|
| Interventions | 1116 women sterilised via Uchida technique 1082 via silver clips |
|
| Outcomes | Pregnancy rates, morbidity, operative time, satisfaction Follow‐up at 1 week, 3, 6, 12 months following sterilisation |
|
| Notes | Mostly interval sterilisation but < 2% were performed postabortion. Approximately 63% were performed in lactating women in whom menses had not resumed Operations were performed under local anaesthetic and women were monitored for 2‐4 h after the operation Surgical duration was significantly longer and amount of bleeding (classified as small, moderate or large amount) was greater in the Uchida arm |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "participants were randomly assigned" |
| Allocation concealment (selection bias) | Unclear risk | "this information was placed in an envelope and delivered to the surgeon" |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Blinding of assessors was not described Possibly high risk of performance bias for personnel |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 61 lost to follow‐up altogether with 20 lost in Uchida group and 41 lost in the clip group |
| Selective reporting (reporting bias) | Unclear risk | Did not report morbidity or postoperative/persistent pain. However, reported 'chief complaints' which were not significantly different between the study arms. Also, investigators only included data from women who attended all 3 follow‐up visits |
| Other bias | Unclear risk | Unable to determine. Baseline characteristics were similar |
Rodriguez 2013.
| Methods | Multicentre RCT conducted by Family Health International in Thailand, Taiwan, Panama and the Phillipines with recruitment from April 1984 to June 1989 | |
| Participants | 1400 postpartum women Inclusion criteria: able to consent, within 42 days postpartum, ≥ 21 years old, normal physical and pelvic examination Exclusion criteria: incapable of consenting, severe pre‐exisiting systemic disease, profound anaemia, anticipated concurrent surgery, limited accessibility for follow‐up, caesarean section |
|
| Interventions | Sterilisation within 72 h of delivery by: Titanium clip (Filshie; 698 women) or partial salpingectomy (Pomeroy; 702 women) All procedures were by infra‐umbilical mini‐laparotomy incision of 1 cm‐2 cm in length |
|
| Outcomes | Failure rate. Follow‐up at 1, 6, 12, and 24 months following sterilisation | |
| Notes | Loss to follow‐up was 51% at end of study. Method of anaesthesia was "local, epidural or spinal at the surgeon's discretion" Cumulative failure rates at one year were reported as 11 per 1000 for Filshie versus 2 per 1000 for partial salpingectomy; at two years, cumulative failure rates in this study were reported as 17 per 1000 versus 4 per 1000, respectively | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer‐generated code" |
| Allocation concealment (selection bias) | Low risk | "assignment was performed immediately before surgery after consent using an off‐site computer‐generated code that was unavailable to study staff" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "the investigator in charge of follow‐up was" blinded to the procedure |
| Incomplete outcome data (attrition bias) All outcomes | High risk | High attrition at 6 months with approximately 30% lost to follow‐up; at 1 year approximately 42% were lost to follow‐up, and at 2 years 51% were lost to follow‐up. 348 women were present in each group at the end of the study. 13 technical failures and random allocation errors occurred (but these did not result in pregnancy) ‐ it is not clear in which study groups these occurred |
| Selective reporting (reporting bias) | High risk | Only the primary outcome was reported |
| Other bias | Unclear risk | Unable to determine but "demographic variables were comparable between groups at baseline" |
Siegle 2005.
| Methods | RCT conducted in the USA with recruitment between July 1999 and June 2001 | |
| Participants | 109 women requesting sterilisation. Inclusion/exclusion criteria not stated | |
| Interventions | Salpingectomy after bipolar coagulation (55 women) versus Pomeroy partial salpingectomy (54 women) | |
| Outcomes | Pain at 6 h and 14 days postoperatively | |
| Notes | Sterilisation via micro‐laparoscopy. Timing not clear, but probably interval sterilisation Third‐year residents performed the procedure |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer‐generated randomisation code" |
| Allocation concealment (selection bias) | Low risk | "Technique assignment was written on a card placed in the sealed opaque envelope"; the "next consecutively numbered envelope" was allocated |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Numbers analyzed/loss to follow‐up not described |
| Selective reporting (reporting bias) | Unclear risk | Not able to determine |
| Other bias | Unclear risk | Coagulation group was heavier than ligation group (180 vs 160 pounds) and had had more previous abdominal operations (12 vs 8) |
Sitompul 1984.
| Methods | Not specified method of randomisation | |
| Participants | 300 women requesting sterilisation at the University Hospital in Medan, Indonesia Exclusion criteria: heart, pulmonary, endocrine or other systemic illness, PID or vulvovaginal infections | |
| Interventions | Modified Pomeroy technique (via minilaparotomy or culdoscopy) versus electrocoagulation (via laparoscopy). All under local anaesthesia and 10 mg intravenous valium | |
| Outcomes | Operative time, hospitalisation, postoperative complications, failure rates | |
| Notes | Interval sterilisation | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 5 women were excluded after randomisation (3 Pomeroy, 2 electrocoagulation) |
| Selective reporting (reporting bias) | Unclear risk | Unable to determine |
| Other bias | Unclear risk | Unable to determine |
Sokal 2000.
| Methods | Multicentre RCT conducted by FHI from 1984 to 1990 in centres in Panama, Peru, Kenya, Brazil, Mexico, Indonesia, Thailand and the Dominican Republic | |
| Participants | 2746 women requiring interval sterilisation Inclusion criteria: at least 21 years old, legally able to consent, normal physical and pelvic examination Exclusion criteria: pregnant within last 42 days, pre‐existing chronic disease, no conconmitant surgical procedures needed except curettage |
|
| Interventions | Titanium clip (Filshie;1381 women) or tubal ring (1365 women) Randomisation was in 2 groups: 1) access via minilaparotomy (5 centres) 482 Filshie, 453 ring; 2) access via laparoscopy (7 centres) 919 Filshie, 912 ring |
|
| Outcomes | Pregnancy, adverse events, hospital admissions, further surgery Follow‐up conducted at 1, 6, and 12 months following sterilisation. A 24‐month follow‐up "was planned for a subset" of women |
|
| Notes | Report combined data from 2 studies, one utilising a minilapraotomy approach, the other utilising laparoscopy. 'Experienced surgeons' performed the procedures There were more tubal injuries in the ring group (e.g. tubal transections, haematomas) and also more surgical difficulties and failures Filshie clip expulsions occurred at 10, 30 and 34 months after sterilisation in 3 women |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer‐generated randomisation scheme" |
| Allocation concealment (selection bias) | Low risk | "sealed, sequentially numbered opaque envelope" provided by FHI |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Outcome assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up was relatively low, approximately 7% (low) for the early follow‐up visit and 18% at 12 months. 30 and 41 protocol violations of inclusion/exclusion criteria in Filshi and tubal ring groups respectively. Characteristics of women lost to follow‐up were similar in both groups |
| Selective reporting (reporting bias) | Unclear risk | Reported treated population data mainly (i.e. not ITT data) |
| Other bias | Unclear risk | One centre used its own randomisation schedule and randomized 68 women (34 to each group), therefore assignment was not according to FHI randomisation schedule |
Stovall 1991.
| Methods | Randomisation by computer‐generated schedule | |
| Participants | 365 women at the University of Tennessee, Memphis | |
| Interventions | Spring‐loaded clip (Hulka‐Clemens) versus tubal ring (Falope ring). All procedures via laparoscopy | |
| Outcomes | Failure rates | |
| Notes | All procedures performed by third‐year residents. Urine hCG within 72 h before procedure. Methylene‐blue test with no spillage recorded | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated schedule |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No post‐randomisation exclusion or losses to follow‐up were reported |
| Selective reporting (reporting bias) | Unclear risk | Unable to determine |
| Other bias | Unclear risk | Both groups had similar socio‐demographic characteristics |
Toplis 1988.
| Methods | Randomisation not specified. Concealment of allocation by envelope opened immediately before operation | |
| Participants | 200 non pregnant women at the Churchill Hospital, Oxford | |
| Interventions | Spring‐loaded clip (Hulka‐Clemens) versus Filshie clip (titanium clip) via laparoscopy | |
| Outcomes | Operative morbidity, operative time, complaints | |
| Notes | Interval sterilisation Authors as the only surgeons |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Low risk | Concealment of allocation by envelope opened immediately before operation |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Two women from the Hulka clip group were excluded from the study because of technical failure |
| Selective reporting (reporting bias) | Unclear risk | Unable to determine |
| Other bias | Unclear risk | Unable to determine. Women in the Filshie group were slightly heavier than those in the Hulka clip group |
WHO 1982.
| Methods | Multicenter, multinational randomized study. Randomisation centrally generated by WHO. Concealment of allocation by sealed, sequentially numbered opaque envelopes | |
| Participants | 1827 healthy women with at least one child and eligible for both interventions. Exclusion criteria: pelvic pathologies, history of previous PID or peritonitis, scar below the umbilicus or any condition which would increase the risk of any surgical procedure Conducted in Bangkok, Havana, London, Los Angeles, Santiago, Seoul, Singapore, Sydney | |
| Interventions | Modified Pomeroy method via minilaparotomy versus electrocoagulation via laparoscopy | |
| Outcomes | Major and minor morbidity, technical failures, postoperative complaints | |
| Notes | Interval sterilisation Anaesthesia standardised within individual centres according to routine practice in the institution. In the 3 high‐income country centres (London, Los Angeles, Sydney) all operations were performed under general anaesthesia, whereas in 2 middle‐ or low‐income country centres (Bangkok, Seoul) local anaesthesia was used for both procedures. In Havana and Singapore all women in the electrocoagulation group received general anaesthesia and most Pomeroy procedures were done under spinal/epidural anaesthesia. In Santiago all Pomeroy procedures were performed under spinal anaesthesia, and all electrocoagulation procedures under local anaesthesia. All centres used sedatives for pre‐medication were used All procedures performed by experienced surgeons |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation generated centrally by WHO |
| Allocation concealment (selection bias) | Low risk | Concealment of allocation by sealed, sequentially numbered opaque envelopes. Assessed as an 'A' study) in original review |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | ‐ |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The post‐randomisation exclusion rate was about 12% (121 women) in the Pomeroy group and about 10% (96 women) in the electrocoagulation group due to protocol violations |
| Selective reporting (reporting bias) | Unclear risk | Unable to determine |
| Other bias | High risk | There were important differences in baseline characteristics mainly due to 1 centre (Bangkok) where women in the electrocoagulation group were older, had more living children and had been married longer. Also, women in the Pomeroy group were lighter and had a lower ponderal index, mainly due to the contribution of 2 centres (Bangkok and Havana).These differences were statistically significant for the Bangkok centre |
Yan 1990.
| Methods | Randomisation not specified. Concealment of allocation by sealed preprinted labels | |
| Participants | 200 women postpartum at the Tri‐Service General Hospital, Taipei, Taiwan | |
| Interventions | Pomeroy method versus Filshie clip, all via subumbilical minilaparotomy. 88% under epidural anaesthesia and the remainder under local anaesthesia | |
| Outcomes | Complications, menstrual irregularities, failure rates | |
| Notes | Postpartum sterilisation with 24 month follow‐up. Pregnancy rates at 24 months were 1/70 and 0/78 in the PS and clip arms, respectively. Blinding of postoperative evaluation. All procedures were performed by one of the authors This trial is a subset of the Rodriguez 2013 report but includes more outcomes |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Low risk | Concealment of allocation by sealed preprinted labels |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Outcome assessor blinded. |
| Selective reporting (reporting bias) | Unclear risk | Unable to determine |
| Other bias | Low risk | Selected socio‐demographic characteristics (age, total live births and previous contraceptive use) were found to be similar between groups |
Abbreviations
BMI: body mass index FSH: follicle‐stimulating hormone h: hour(s) hCG: human chorionic gonadotropin ITT: intention‐to‐treat analysis LH: lutenising hormone PID: pelvic inflammatory disease RCT: randomized controlled trial
TVU: transvaginal ultrasound UTI: urinary tract infection WHO: World Health Organization
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Alvarez 1989 | RCT (uncertain whether quasi‐randomised) of post‐tubal sterilisation hormone levels following Pomeroy or Uchida techniques 17/38 completed the protocol and only 17 were included in analyses |
| Bordahl 1984 | Quasi‐RCT with about 40% postrandomisation exclusions |
| Chapa 2015 | RCT of methods of access (vaginoscopy vs hysteroscopy) for hysteroscopic sterilisation (Essure), not of techniques for interrupting tubal patency |
| Dueholm 1986 | Not an RCT |
| Lee 1991 | Women were 'randomized' (no details provided) before surgery to Hulka clips or modified Pomeroy technique, but at the time of surgery, those found to have tubal disease underwent sterilisation with standard modified Pomeroy technique and were then analyzed in that group |
| Lipscomb 1994 | An RCT of chromotubation vs no chromotubation to confirm poststerilisation tubal occlusion. Although women were apparently also randomized to the sterilisation method (tubal ring, electrocautery, or Hulka clips), comparisons of these methods were not the objective of the study and outcomes and losses to follow‐up were not described separately for each method |
| Madrigal 1977 | ITT analysis was not performed. 1 participant from the clip group was changed to the electrocoagulation group due to a technical problem and was included in the latter for the further analysis |
| Murray 1992 | Quasi‐RCT |
| Rivera 1989 | Quasi‐RCT. The groups were divided into equal numbers of women. In addition, a fourth group was taken as a control group |
| Sahwi 1989 | Quasi‐RCT. The groups were divided into equal numbers of women |
Abbreviations
ITT: intention‐to‐treat analysis RCT: randomized controlled trial
Differences between protocol and review
The protocol and previous versions of this review used an older Cochrane method for assessment of the risk of bias of studies (see Appendix 1). We updated the methodology for the 2015 review to reflect current Cochrane 'Risk of bias' assessment methods.
Contributions of authors
JM Nardin and R Kulier wrote the original version of the review, performed the methodological assessment of studies, and performed the data extraction. TL and RK updated the review in 2010 and 2015. All listed authors reviewed the draft manuscript and approved the final version.
Sources of support
Internal sources
Department of Obstetrics and Gynaecology, University Hospital of Geneva (HUG), Switzerland.
Centro Rosarino de Estudios Perinatales (CREP), Rosario, Argentina.
External sources
Department of Reproductive Health and Research. World Health Organization (WHO), Geneva, Switzerland.
-
National Institute for Health Research, UK.
“This project was supported by the National Institute for Health Research, via Cochrane Incentive Award funding to the Fertility Regulation Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.”
New Source of support, Other.
Declarations of interest
TL: none known RK: none known JMN: none known
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Aranda 1976 {published data only}
- Aranda C, Broutin A, Edelman DA, Goldsmith A, Mangel T, Prada C, et al. A comparative study of electrocoagulation and tubal ring for tubal occlusion at laparoscopy. International Journal of Gynaecology and Obstetrics 1976;14:411‐5. [DOI] [PubMed] [Google Scholar]
Aranda 1985 {published data only}
- Aranda C, Badia D, Mahran M, Feldblum PJ. A comparative clinical trial of the tubal ring versus the Rocket clip for female sterilisation. American Journal of Obstetrics and Gynecology 1985;153:755‐9. [DOI] [PubMed] [Google Scholar]
Argueta 1980 {published data only}
- Argueta G, Henriquez E, Amador MN, Gardner SD. Comparison of laparoscopic sterilisation via spring‐loaded clip and tubal ring. International Journal of Gynaecology and Obstetrics 1980;18:115‐8. [DOI] [PubMed] [Google Scholar]
Dominik 2000 {published data only}
- Dominik R, Gates D, Sokal D, Cordero M, Lasso de la Vega J, Remes Ruiz A, et al. Two randomized controlled trials comparing the Hulka and Filshie Clips for tubal sterilisation. Contraception 2000;62(4):169‐75. [DOI] [PubMed] [Google Scholar]
Geirsson 1985 {published data only}
- Geirsson RT, Currie J, Redpath A. Prospective comparison of postoperative morbidity after laparoscopic sterilisation with rings and clips. British Journal of Family Planning 1985;11:55‐9. [Google Scholar]
Gentile 2006 {published and unpublished data}
- Gentile GP, Helbiga DW, Zacurb H, Park T, Lee YJ, Westhoff CL. Hormone levels before and after tubal sterilisation. Contraception 2006;73:507‐11. [DOI] [PubMed] [Google Scholar]
Goynumer 2009 {published and unpublished data}
- Goynumer G, Kayabasoglu F, Aydogdu S, Wetherilt L. The effect of tubal sterilisation through electrocoagulation on the ovarian reserve. Contraception 2009;80:90‐4. [DOI] [PubMed] [Google Scholar]
Koetsawang 1978 {published data only}
- Koetsawang S, Srisupandit S, Painter Cole L. Laparoscopic electrocoagulation and tubal ring techniques for sterilisation: a comparative study. International Journal of Gynaecology and Obstetrics 1978;15:455‐8. [DOI] [PubMed] [Google Scholar]
Kohaut 2004 {published data only}
- Kohaut BA, Musselman BL, Sanchez‐Ramos L, Kaunitz AM. Randomized trial to compare perioperative outcomes of Filshie clip vs.Pomeroy technique for postpartum and intraoperative cesarean tubal sterilisation: a pilot study. Contraception 2004;69:267‐70. [DOI] [PubMed] [Google Scholar]
Pymar 2004 {published data only}
- Pymar HC, Creinin MD, Vallejo MC. Prospective randomized, controlled study of postoperative pain after titanium silicone rubber clip or silastic ring tubal occlusion. Contraception 2004;69:145‐50. [DOI] [PubMed] [Google Scholar]
Qui 2011 {published data only}
- Qiu H, Li L, Wu S, Liang H, Yuan W, He Y. A comparative study of female sterilisation via modified Uchida and silver clip techniques in rural China. International Journal of Gynaecology and Obstetrics 2011;112(3):190‐4. [DOI] [PubMed] [Google Scholar]
Rodriguez 2013 {published data only}
- Rodriguez MI, Seuc A, Sokal DC. Comparative efficacy of postpartum sterilisation with the titanium clip versus partial salpingectomy: a randomised controlled trial. BJOG 2013;120(1):108‐12. [DOI] [PubMed] [Google Scholar]
Siegle 2005 {published data only}
- Siegle JC, Bishop LJ, Rayburn WF. Randomized comparison between two microlaparoscopic techniques for partial salpingectomy. JSLS: Journal of the Society of Laparoendoscopic Surgeons 2005;9(1):30‐4. [PMC free article] [PubMed] [Google Scholar]
Sitompul 1984 {published data only}
- Sitompul H, Lun KC, Lumbanraja M, Kaban RM, Albar E, Simanjuntak P, et al. Comparison of three types of tubal sterilisation: the Medan experience. Contraception 1984;29:55‐63. [DOI] [PubMed] [Google Scholar]
Sokal 2000 {published data only}
- Sokal D, Gates D, Amatya R, Dominik R. Two randomized controlled trials comparing the tubal ring and Filshie clip for tubal sterilisation. Fertility and Sterility 2000;74:525‐33. [DOI] [PubMed] [Google Scholar]
Stovall 1991 {published data only}
- Stovall TG, Ling FW, Henry GM, Ryan GM. Method failures of laparoscopic tubal sterilisation in a residency training program. Journal of Reproductive Medicine 1991;36:283‐6. [PubMed] [Google Scholar]
Toplis 1988 {published data only}
- Toplis PJ, Newman MRB, Gillmer MDG, Tingey WR, Sellers S. Laparoscopic sterilisation ‐ a comparison of Hulka‐Clemens and Filshie clips. British Journal of Family Planning 1988;14:43‐5. [Google Scholar]
WHO 1982 {published data only}
- WHO Task Force on Female Sterilization. Minilaparotomy or laparoscopy for sterilisation: a multicenter, multinational randomized study. American Journal of Obstetrics and Gynecology 1982;143:645‐52. [PubMed] [Google Scholar]
Yan 1990 {published data only}
- Yan J‐S, Hsu J, Yin CS. Comparative study of Filshie clip and Pomeroy method for postpartum sterilisation. International Journal of Gynaecology and Obstetrics 1990;33:263‐7. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Alvarez 1989 {published data only}
- Alvarez F, Faundes A, Brache V, Tejada AS, Segal S. Prospective study of the pituitary‐ovarian function after tubal sterilisation by Pomeroy or Uchida techniques. Fertility and Sterility 1989;51:604‐8. [DOI] [PubMed] [Google Scholar]
Bordahl 1984 {published data only}
- Bordahl PE, Bergsjo P, Solberg M. A controlled comparison of the Pomeroy resection technique and laparoscopic electrocoagulation of the tubes. Annales Chirugiae et Gynaecologiae 1984;73:288‐292. [PubMed] [Google Scholar]
Chapa 2015 {published data only}
- Chapa HO, Venegas G. Vaginoscopy compared to traditional hysteroscopy for hysteroscopic sterilisation. A randomized trial. Journal of Reproductive Medicine 2015;60(1‐2):43‐7. [PubMed] [Google Scholar]
Dueholm 1986 {published data only}
- Dueholm S, Zingenberg HJ, Sandgren G. Late sequelae following laparoscopic sterilisation employing electrocoagulation and tubal ring techniques: a comparative study. Annales Chirugiae et Gynaecologiae 1986;75:285‐289. [PubMed] [Google Scholar]
Lee 1991 {published data only}
- Lee SH, Stephen Jones J. Postpartum tubal sterilisation. A comparative study of the Hulka clip and modified Pomeroy technique. Journal of Reproductive Medicine 1991;36:703‐6. [PubMed] [Google Scholar]
Lipscomb 1994 {published data only}
- Lipscomb GH, Stovall TG, Summitt RL, Ling FW. Chromopertubation at laparoscopic tubal occlusion. Obstetrics and Gynecology 1994;83:725‐8. [PubMed] [Google Scholar]
Madrigal 1977 {published data only}
- Madrigal V, Edelman DA, Henriquez E, Goldsmith A. A comparative study of spring‐loaded clips and electrocoagulation for female sterilisation. Journal of Reproductive Medicine 1977;18:41‐5. [PubMed] [Google Scholar]
Murray 1992 {published data only}
- Murray JE, Hibbert ML, Heth SR, Letterie GS. A technique for laparoscopic Pomeroy tubal ligation with endoloop sutures. Obstetrics and Gynecology 1992;80:1053‐5. [PubMed] [Google Scholar]
Rivera 1989 {published data only}
- Rivera R, Gaitan JR, Ruiz R, Hurley DP, Arenas M, Flores C, et al. Menstrual patterns and progesterone levels following different procedures of tubal occlusion. Contraception 1989;40:157‐67. [DOI] [PubMed] [Google Scholar]
Sahwi 1989 {published data only}
- Sahwi S, Toppozada SS, Kamel M, Anwar MY, Ismail AAA. Changes in menstrual blood after four methods of female tubal sterilisation. Contraception 1989;40:387‐98. [DOI] [PubMed] [Google Scholar]
Additional references
Agoestina 2003
- Agoestina T. 8‐year follow‐up in a randomised trial of one vs two trans‐cervical insertions of quinacrine pellets for sterilisation in Indonesia. International Journal of Gynaecology and Obstetrics 2003;83(Suppl 2):S129‐31. [PubMed] [Google Scholar]
Arjona 2008
- Arjona JE, Mino M, Cordon J, Povedano B, Pelegrin B, Castelo‐Branco C. Satisfaction and tolerance with office hysteroscopic tubal sterilisation. Fertility and Sterility 2008;90:1182‐6. [DOI] [PubMed] [Google Scholar]
Bayer 2015
- Bayer. Essure: what is Essure?. www.essure.com/what‐is‐essure Accessed 14 July 2015.
Bhattacharyya 2003
- Bhattacharyya S. Quinicrine sterilisation (QS): the ethical issues. International Journal of Gynaecology and Obstetrics 2003;83(Suppl 2):S13‐21. [DOI] [PubMed] [Google Scholar]
Bhiwandiwala 1980
- Bhiwandiwala PP, Mumford SD, Feldblum PJ. A comparison of different laparoscopic techniques in 24439 procedures. American Journal of Obstetrics and Gynecology 1982;144:319‐31. [DOI] [PubMed] [Google Scholar]
Blumenthal 2011
- Blumenthal PD, Voedisch A, Gemzell‐Danielsson K. Strategies to prevent unintended pregnancy: increasing use of long‐acting reversible contraception. Human Reproduction Update 2011;17(1):121‐37. [DOI] [PubMed] [Google Scholar]
Chi 1994
- Chi IC. Use of multiple clips for tubal occlusion in interval laparoscopic sterilisation: circumstances and consequences. Contraception 1994;50:409‐16. [DOI] [PubMed] [Google Scholar]
Chudnoff 2015
- Chudnoff SG, Nichols JE Jr, Levie M. Hysteroscopic Essure inserts for permanent contraception‐extended follow‐up results of a phase III, multicenter, international study. Journal of Minimally Invasive Gynecology 2015;Epub:[Epub ahead of print]. [DOI: 10.1016/j.jmig.2015.04.017] [DOI] [PubMed] [Google Scholar]
Cleary 2013
- Cleary TP, Tepper NK, Cwiak C, Whiteman MK, Jamieson DJ, Marchbanks PA, et al. Pregnancies after hysteroscopic sterilisation: a systematic review. Contraception 2013;87(5):539‐48. [DOI] [PubMed] [Google Scholar]
Connor 2009
- Connor VF. Essure: a review six years later. Journal of Minimally Invasive Gynecology 2009;16(3):282‐90. [DOI] [PubMed] [Google Scholar]
Cooper 2003
- Cooper JM, Carignan CS, Cher D, Kerin JF. Microinsert non incisional hysteroscopic sterilisation. Obstetrics and Gynecology 2003;102:59‐67. [DOI] [PubMed] [Google Scholar]
Cooper 2010
- Cooper NA, Smith P, Khan KS, Clark TJ. Vaginoscopic approach to outpatient hysteroscopy: a systematic review of the effect on pain. British Journal of Obstetrics and Gynaecology 2010;117(5):532‐9. [DOI] [PubMed] [Google Scholar]
Duffy 2005
- Duffy S, Marsh F. Female sterilisation: a cohort controlled comparative study of ESSURE versus laparoscopic sterilisation. BJOG 2005;112(11):1522‐8. [DOI] [PubMed] [Google Scholar]
Fernandez 2014
- Fernandez H, Legendre G, Blein C, Lamarsalle L, Panel P. Tubal sterilisation: pregnancy rates after hysteroscopic versus laparoscopic sterilisation in France, 2006‐2010. European Journal of Obstetrics, Gynecology, and Reproductive Biology 2014;180:133‐7. [DOI] [PubMed] [Google Scholar]
French 2004
- French R, Vliet H, Cowan F, Mansour D, Morris S, Hughes D, et al. Progestogen‐releasing intrauterine systems versus other forms of reversible contraceptives for contraception. Cochrane Database of Systematic Reviews 2004, Issue 3. [DOI: 10.1002/14651858.CD001776.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gariepy 2014
- Gariepy AM, Creinin MD, Smith KJ, Xu X. Probablility of pregnancy after sterilisation: a comparison of hysteroscopic versus laparoscopic sterilisation. Contraception 2014;90(2):174‐81. [DOI] [PubMed] [Google Scholar]
GRADE 2014 [Computer program]
- McMaster University. GRADEpro. [Computer program on www.gradepro.org]. Version 2014. McMaster University, 2014.
Gupta 1980
- Gupta I, Rodrigues S, Jain S, Gupta AN, Devi PK. Comparative morbidity following tubal fulguration by abdominal and vaginal routes. Indian Journal of Medicine 1980;72:231‐5. [PubMed] [Google Scholar]
Harrison 2014
- Harrison MS, DiNapoli MN, Westhoff CL. Reducing postoperative pain after tubal ligation with rings or clips: a systematic review and meta‐analysis. Obstetrics and Gynecology 2014;124(1):68‐75. [DOI] [PubMed] [Google Scholar]
Hillis 1999
- Hillis SD, Marchbanks PA, Tylor LR, Peterson HB for the US Collaborative Review of Sterilization Working Group. Poststerilisation regret: findings from the United States Collaborative Review of Sterilization. Obstetrics and Gynecology 1999;93:889‐95. [DOI] [PubMed] [Google Scholar]
IJOG 2003
- No authors listed. Quinacrine sterilisation (QS): reports on 40,252 cases. International Journal of Gynaecology and Obstetrics 2003;83(Suppl 2):S1‐148. [PubMed] [Google Scholar]
Kaplan 1990
- Kaplan P, Freund R, Squires J, Herz M. Control of immediate postoperative pain with topical bupivacaine hydrochloride for laparoscopic Falope ring tubal ligation. Obstetrics and Gynecology 1990;76:798‐802. [DOI] [PubMed] [Google Scholar]
Kessel 1976
- Kessel E, Pachauri S, McCann MF. A comparison of laparoscopic tubal occlusion by cautery, spring‐loaded clip and tubal ring. In: Advances in Female Sterilization Techniques. Hagerstown, MD: Harper & Row, c1976, 1976:69‐90. [Google Scholar]
Kraemer 2009
- Kraemer DF, Yend P, Nicholse M. An economic comparison of female sterilisation of hysteroscopic tubal occlusion with laparoscopic bilateral tubal ligation. Contraception 2009;80:254‐60. [DOI] [PubMed] [Google Scholar]
Kulier 2004
- Kulier R, Boulvain M, Walker DM, Candolle G, Campana A. Minilaparotomy and endoscopic techniques for tubal sterilisation. Cochrane Database of Systematic Reviews 2004, Issue 3. [DOI: 10.1002/14651858.CD001328.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
la Chapelle 2015
- Chapelle CF, Veersema S, Brölmann HA, Jansen FW. Effectiveness and feasibility of hysteroscopic sterilisation techniques: a systematic review and meta‐analysis. Fertility and Sterility 2015;103(6):1516‐25. [DOI] [PubMed] [Google Scholar]
Lippes 2015
- Lippes J. Quinacrine sterilisation (QS): time for reconsideration. Contraception 2015;92(2):91‐5. [DOI: 10.1016/j.contraception.2015.06.005] [DOI] [PubMed] [Google Scholar]
Lipscomb 1992
- Lipscomb GH, Stovall TG, Ramanathan JA, Ling FW. Comparison of Silastic rings and electrocoagulation for laparoscopic tubal ligation under local anaesthesia. Obstetrics and Gynecology 1992;80:645‐9. [PubMed] [Google Scholar]
Lu 2003
- Lu W, Zhu J, Zhong C, Zhao Y. A comparison of quinacrine sterilisation and surgical sterilisation (QS) in 600 women in Ghizhou province, China. International Journal of Gynaecology and Obstetrics 2003;83(Suppl 2):S51‐8. [DOI] [PubMed] [Google Scholar]
Lumbiganon 2015
- Lumbiganon P, Werawatakul Y, Laopaiboon M, Sothornwit J. Interventions for intra‐operative pain relief during postpartum mini‐laparotomy tubal ligation. Cochrane Database of Systematic Reviews 2015, Issue 7. [DOI: 10.1002/14651858.CD011807] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mino 2007
- Minö M, Arjona J, Cordón J, Pelegrin B, Povedano B, Chacon E. Success rate and patient satisfaction with the EssureTM sterilisation in an outpatient setting: a prospective study of 857 women. British Journal of Obstetrics and Gynaecology 2007;114:763‐6. [DOI] [PubMed] [Google Scholar]
Ouzounelli 2015
- Ouzounelli M, Reaven NL. Essure hysteroscopic sterilisation versus interval laparoscopic bilateral tubal ligation: a comparative effectiveness review. Journal of Minimally Invasive Gynecology 2015;22(3):342‐52. [DOI] [PubMed] [Google Scholar]
Panel 2010
- Panel P, Grosdemouge I. Predictive factors of Essure implant placement failure: prospective, multicenter study of 495 patients. Fertility and Sterility 2010;93(1):29‐34. [DOI] [PubMed] [Google Scholar]
Peterson 1996
- Peterson HB, Zhisen X, Hughes JM, Wilcox LS, Tylor LR, Trussel J for the US Collaborative Review of Sterilization Working Group. The risk of pregnancy after tubal sterilisation: findings from the US Collaborative Review of Sterilization. American Journal of Obstetrics and Gynecology 1996;174:1161‐70. [DOI] [PubMed] [Google Scholar]
Peterson 2008
- Peterson HB. Sterilization. Obstetrics and Gynecology 2008;111(1):189‐203. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Savage 2009
- Savage UK, Masters SJ, Smid MC, Hung Y, Jacobson GF. Hysteroscopic sterilisation in a large group practice: experience and effectiveness. Obstetrics and Gynecology 2009;114(6):1227‐31. [DOI] [PubMed] [Google Scholar]
Shavell 2009
- Shavell VI, Abdallah ME, Diamond MP, Berman JM. Placement of a permanent birth control device at a university medical centre. Journal of Reproductive Medicine 2009;54:218‐22. [PubMed] [Google Scholar]
Sinha 2007
- Sinha D, Kalathy V, Gupta JK, Clark TJ. The feasibility, success and patient satisfaction associated with outpatient hysteroscopic sterilisation. British Journal of Obstetrics and Gynaecology 2007;114:676‐83. [DOI] [PubMed] [Google Scholar]
Sokal 2010a
- Sokal DC, Trujillo V, Guzman SC, Guzman‐Serani R, Wheeless A, Hubacher D. Cancer risk after sterilisation with transcervical quinacrine: updated findings from a Chilean cohort. Contraception 2010;81(1):75‐8. [DOI] [PubMed] [Google Scholar]
Sokal 2010b
- Sokal DC, Vach TH, Nanda K, Mccann MF, Weiner DH, Drobnes C, et al. Quinacrine sterilisation and gynecologic cancers: a case‐control study in northern Vietnam. Epidemiology 2010;21(2):164‐71. [DOI] [PubMed] [Google Scholar]
Soroodi‐Moghaddam 2003
- Soroodi‐Moghaddam S. Quinacrine sterilisation (QS) in Iran and the use of HSG as a measure of success. International Journal of Gynaecology and Obstetrics 2003;83(Supp 2):S93‐6. [DOI] [PubMed] [Google Scholar]
Suhadi 1998
- Suhadi A, Anwar M, Soejoenoes A. Four year clinical evaluation of quinacrine pellets for non‐surgical female sterilisation. Advances in Contraception 1998;14:69‐77. [DOI] [PubMed] [Google Scholar]
Suhadi 2003
- Suhadi A, Anwar M, Soejoenoes A. 10‐year follow up of women who elected quinacrine sterilisation (QS) in Wonosobo, Central Java, Indonesia. International Journal of Gynaecology and Obstetrics 2003;83(Suppl 2):S137‐9. [DOI] [PubMed] [Google Scholar]
Thiel 2014
- Thiel J, Rattray D, Cher DJ. Pre‐hysterectomy assessment of immediate tubal occlusion with the third‐generation ESSURE insert (ESS505). Journal of Minimally Invasive Gynecology 2014;21(6):1055‐60. [DOI] [PubMed] [Google Scholar]
UN 2013
- United Nations, Department of Economic and Social Affairs, Population Division. World Contraceptive Patterns 2013. http://www.un.org/en/development/desa/population/publications/pdf/family/worldContraceptivePatternsWallChart2013.pdf 2013:Accessed 14/07/2015.
Valle 2001
- Valle RF, Carignan CS, Wright TC. Tissue response to the STOP microcoil transcervical permanent contraceptive device: results from a prehysterectomy study. Fertility and Sterility 2001;76:974‐80. [DOI] [PubMed] [Google Scholar]
Veersema 2015
- Veersema S. Hysteroscopy and contraception. Best Practice & Research. Clinical Obstetrics & Gynaecology 2015;Epub:[Epub ahead of print]. [DOI: ] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Lawrie 2011
- Lawrie T, Nardin JM, Kulier R, Boulvain M. Techniques for the interruption of tubal patency for female sterilisation. Cochrane Database of Systematic Reviews 2011, Issue 2. [DOI: 10.1002/14651858.CD003034.pub2] [DOI] [PubMed] [Google Scholar]
Nardin 2002
- Nardin JM, Kulier R, Boulvain M, Peterson HB. Techniques for the interruption of tubal patency for female sterilisation. Cochrane Database of Systematic Reviews 2002, Issue 4. [DOI: 10.1002/14651858.CD003034.pub2] [DOI] [PubMed] [Google Scholar]
Nardin 2003
- Nardin JM, Kulier R, Boulvain M. Techniques for the interruption of tubal patency for female sterilisation. Cochrane Database of Systematic Reviews 2003, Issue 1. [DOI: 10.1002/14651858.CD003034.pub2] [DOI] [PubMed] [Google Scholar]


