Abstract
Macrophage activation syndrome (MAS) is the name given to secondary hemophagocytic lymphohistiocytosis (sHLH) associated with rheumatic diseases. Previously, MAS has been best studied in children with systemic juvenile idiopathic arthritis (sJIA), who are at high risk of developing MAS. MAS/sHLH is a cytokine storm that results in multi-organ system failure and is frequently fatal. Early diagnosis and treatment is critical for improving survival. Various diagnostic tools have been developed for identifying MAS in the setting of sJIA, as well as for all forms of MAS/sHLH. These are largely based on clinical (e.g., fever) and laboratory features (e.g., cytopenias). None are perfectly sensitive and specific, however, increasing awareness of this condition is also paramount in making the diagnosis. Rare familial forms of HLH can also be diagnosed based on homozygous mutation in genes largely involved in perforin-mediated cytolytic function of lymphocytes (natural killer cells and CD8 T cells). Intriguingly, heterozygous defects in these same genes are frequently identified in patients with sHLH and MAS. Decreased cytolytic function results in prolonged interaction of the lytic lymphocytes and their target antigen presenting cells, thus resulting in the pro-inflammatory cytokine storm believed responsible for the multi-organ failure. Novel cytokine-targeted therapies are currently being explored for a less toxic yet effective alternative to chemotherapeutic approaches to treating children with sHLH/MAS. As increased recognition and diagnosis of MAS is on the rise, an earlier and cytokine-targeted approach to therapy will likely save many lives of children with this disorder.
Keywords: Macrophage activation syndrome, hemophagocytic lymphohistiocytosis, systemic juvenile idiopathic arthritis, cytokine storm, interleukin-1 receptor antagonist
Introduction
Macrophage activation syndrome (MAS), a term often used interchangeably with secondary hemophagocytic lymphohistiocytosis (sHLH), describes a severe hyperinflammatory reaction, which can be idiopathic or triggered by underlying systemic illness (e.g., autoimmune disease, malignancy, infection) that frequently leads to abnormal hemophagocytic macrophages with associated hypercytokinemia, otherwise known as a “cytokine storm.” Unlike primary or familial HLH, which commonly presents during infancy and results from homozygous or compound heterozygous mutations in genes involved in the perforin-mediated pathway of cytolysis shared by both the innate (i.e., natural killer (NK) cells) and adaptive (i.e., cytotoxic CD8 T cells) immune systems (1), MAS can occur at any age and often complicates an underlying systemic inflammatory disorder, most commonly systemic juvenile idiopathic arthritis (sJIA) and its adult equivalent, adult onset Still’s disease (AOSD). If unrecognized and untreated, MAS can lead to multi-organ failure and ultimately death (2–4).
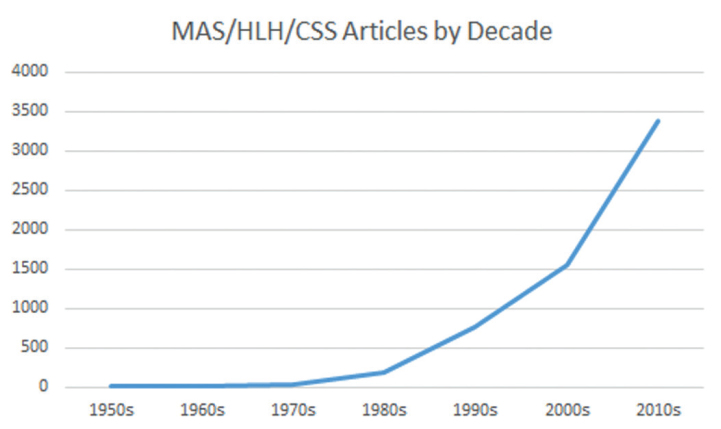
Clinical and laboratory features of MAS include sustained fever, hyperferritinemia, pancytopenia, consumptive coagulopathy mimicking disseminated intravascular coagulation, central nervous system dysfunction, and elevated liver enzymes. Many of these features complicate co-existing systemic inflammatory disease, thus making a diagnosis of MAS difficult (5–9). A majority of clinical data available describes MAS as a complication of sJIA with the prevalence of fulminant MAS in patients with sJIA reported to be about 10%. Subclinical MAS, however, may be present in as many as 30%–40% of children with known or suspected sJIA (2, 8–10). As MAS becomes more clinically recognized, an increasing frequency of occurrence in other systemic inflammatory disorders [i.e., systemic lupus erythematosus (SLE), Kawasaki disease, and periodic fever syndromes] has been reported (Figure 1) (11–14). However, we are likely just beginning to recognize the tip of the iceberg, as many febrile and hyperferritinemic pediatric and adult hospitalized patients with multi-organ failure and systemic inflammation may indeed be suffering from sHLH/MAS, including those with frank sepsis (Figure 2) (15–17).
Figure 1.
Macrophage Activation Syndrome (MAS), Hemophagocytic Lymphohistiocytosis (HLH), Cytokine Storm Syndrome publications excluding review articles, as cited in PUBMED and grouped by decade.
Figure 2.
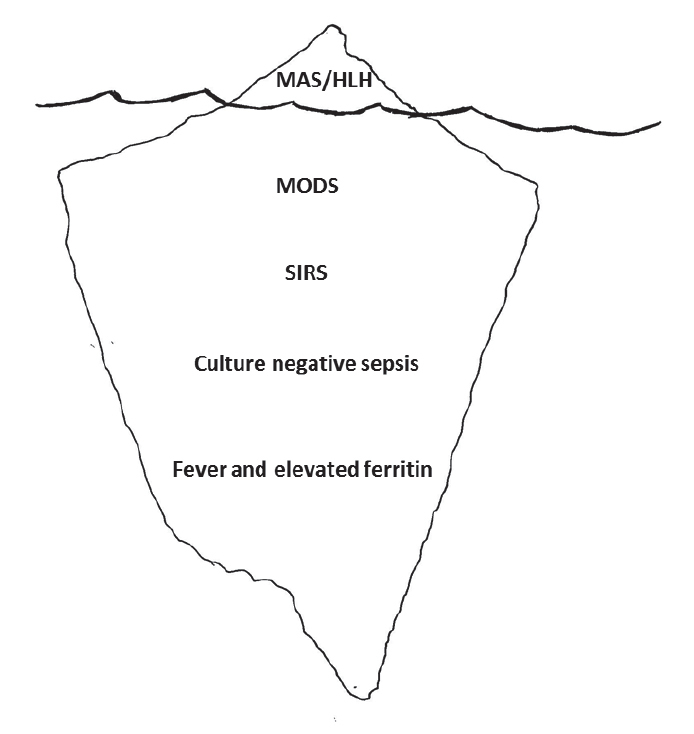
Recognizing the tip of the HLH/MAS iceberg which may include many febrile, hyperferritinemic, hospitalized patients with multi-organ dysfunction syndrome, systemic inflammatory response syndrome, and negative and positive sepsis cultures.
Diagnostic criteria
Early identification remains diagnostically challenging as there is no single pathognomonic feature of MAS or even a set of universal diagnostic criteria. The clinical similarity of MAS and secondary HLH has led some clinicians to use the longer-standing HLH-2004 diagnostic guidelines, which require 5 of the following 8 criteria to be met for diagnosis: fever, splenomegaly, cytopenias (affecting ≥2 of 3: hemoglobin< 90 g/L, platelets<100×109/L, neutrophils< 1.0×109/L), hypertriglyceridemia (≥265 mg/dL) and/or hypofibrinogenemia (≤1.5 g/L), hemophagocytosis in bone marrow or spleen or lymph nodes, low or absent NK cell activity, and ferritin 500 μg/L, and sCD25≥2400 units/mL (Table 1) (18). While specific but insensitive for identifying MAS, strict usage of HLH-2004 criteria may delay diagnosis in patients with a less severe initial presentation (5).
Table 1.
Comparison of diagnostic criteria for Macrophage Activation Syndrome (MAS)/Secondary Hemophagocytic Lymphohistiocytosis (sHLH)
| Parameter | HLH-2004 | 2016 sJIA/MAS | MAS/sJIA Score | H score |
|---|---|---|---|---|
| Fever °C | ≥38.5 | Degree not specified | --- | 0 (<38.4), 33 (38.4–39.4), of 49 (>39.4) |
| Ferritin | ≥ 500 μg/L | > 684 ng/mL | 0.0001*serum level | 0 (<2,000), 2,000–6,000), or 50 (>6,000) |
| Organomegaly | Splenomegaly | --- | --- | 0 (no), 23 (hepato- or splenomegaly), 38 (both) |
| Hematology | affecting ≥ 2 of 3# | platelets≤181×109/L | −0.003*platelet count | 0 (one lineage), 24 (2 lineages), or 34 (3 lineages)% |
| Hemorrhagic Manifestations | --- | --- | 1.54*1(yes) or *0(no) | --- |
| Triglyceride Level | ≥ 265 mg/dL | > 156 mg/dL | --- | 0 (<1.5 mmol/L), 44 (1.5–4 mmol/L), or 64 (>4 mmol/L) |
| Fibrinogen Level | ≤ 1.5 g/L | ≤ 360 mg/dL | −0.004*serum level | 0 (>2.5 g/L) or 30 (≤2.5 g/L) |
| Lactate Dehydrogenase Level | --- | --- | 0.001*serum level | --- |
| Aspartate Aminotransferase (AST) | --- | > 48 units/L | --- | 0 (<30 IU/L) or 19 (≥30 IU/L) |
| CNS Involvement | --- | --- | 2.44*1(yes) or *0(no) | --- |
| Active Arthritis | --- | --- | −1.3*1(yes) or *0(no) | --- |
| Known immunosuppression | --- | --- | --- | 0 (no) or 18 (yes) |
| Histopathology | hemophagocytosis in bone marrow or spleen or lymph nodes | --- | --- | Hemophagocytosis in bone marrow: 0 (no) or 35 (yes) |
| Natural killer (NK) cell activity | low or absent | --- | --- | --- |
| sCD25 | ≥ 2400 units/mL | --- | --- | --- |
| Diagnosis | 5 of 8 criteria met | Fever in known or suspected sJIA + Ferritin + 2 of the remaining 4 | Sum of parameters ≥ −2.1 | Sum of parameters ≥169 |
hemoglobin<90 g/L, platelets<100×109/L, neutrophils <1.0×109/L;
hemoglobin<92 g/L, platelets<110×109/L, leukocytes <5.0×109/L;
MAS, macrophage activation syndrome; HLH, hemophagocytic lymphohistiocytosis; sJIA, systemic juvenile idiopathic arthritis;
multiplication (e.g., 0.0001 times platelet count).
In 2016, an expert consensus panel published a set of validated classification criteria to help distinguish a sJIA flare from MAS. The identification of MAS can be made in a febrile patient with sJIA or suspected sJIA, who has a serum ferritin level >684 ng/mL plus any 2 of the following: platelet count ≤181×109/L, aspartate aminotransferase (AST)>48 units/L, triglyceride concentration >156 mg/dL, or fibrinogen ≤360 mg/dL (Table 1) (8, 9). These relatively few total criteria are routinely readily available and timely. While the final MAS criteria for children with sJIA proved to have a sensitivity of 0.73 and specificity of 0.99, emerging clinical practice data suggest that patients with known sJIA treated with anti-IL-1 and anti-IL-6 biologic agents may have alterations in laboratory findings and possibly remain afebrile, which subsequently results in a missed diagnosis of MAS (19). To date, these criteria are yet to be proven to have diagnostic value for other autoimmune diseases and remain limited to children with known or suspected sJIA, with the possible exception of AOSD (3).
The inadequate performance of the MAS classification criteria in daily clinical practice led to a validated, weighted MAS/sJIA (MS) scoring system using the original data set from the 2016 classification criteria. The newer MS scoring system excluded the control sample with systemic infection, which had less pronounced systemic inflammation and subsequently laboratory values, thus creating an inflation effect on the laboratory abnormalities. Central nervous system (CNS) involvement (β-coefficient 2.44), hemorrhagic manifestations (β-coefficient 1.54), active arthritis (β-coefficient −1.30), platelet count (β-coefficient −0.003), lactate dehydrogenase (LDH) (β-coefficient 0.001), fibrinogen (β-coefficient −0.004), and ferritin (β-coefficient 0.0001) are included in the MS score calculation. Each clinical variable is given a binary constant of “1” or “0” based on the presence or absence of the feature and multiplied by the respective β-coefficient. Absolute laboratory values are multiplied by the respective β-coefficient, and all variables are added for a final MS score (Table 1). The sum of the values ranges from −8.4 to 41.8 with a cutoff value of ≥−2.1, yielding a sensitivity of 0.85 and specificity of 0.95 in discriminating MAS from an active sJIA flare (20). While the newer MS scoring system potentially may prove applicable to AOSD, it is not intended to be used in other pediatric rheumatic diseases.
MAS complicated by other rheumatic diseases is less commonly reported than sJIA. Comparison of clinical and laboratory data from 38 juvenile SLE patients with definite or probable MAS to controls suggests that with the exception of fever, the other clinical features (i.e., CNS involvement, hemorrhage, hepatomegaly, splenomegaly) have better specificity than sensitivity in distinguishing MAS from an active SLE flare. Preliminary diagnostic guidelines for MAS as a complication of juvenile SLE requires 1 clinical feature (i.e., fever, CNS involvement, hepatomegaly, splenomegaly, hemorrhage) and 2 laboratory criteria, which includes cytopenia affecting≥2 cell lines (i.e., hemoglobin ≤90 g/L, platelets≤150×109/L, white blood cells (WBC)≤4.0×109/L), hypertriglyceridemia (≥178 mg/dL) and/or hypofibrinogenemia (≤1.5 g/L), AST>40 units/L, increased LDH>567 units/L, hyperferritinemia ≥500 μg/L, or evidence of macrophage hemophagocytosis in the bone marrow aspirate (Table 2) (14). These proposed guidelines are based on a small sample size with a limited control group and have not been validated. The clinical utility of the Parodi et al. (14) diagnostic criteria for MAS in juvenile SLE remains unclear.
Table 2.
Proposed diagnostic criteria for Macrophage Activation Syndrome complicating Systemic Lupus Erythematosus
| Clinical Criteria | Fever (>38°C) |
| Hepatomegaly (≥3 cm below the costal arch) | |
| Splenomegaly (≥3 cm below the costal arch) | |
| Hemorrhagic manifestations (purpura, easy bruising, or mucosal bleeding) | |
| Central nervous system dysfunction (irritability, disorientation, lethargy, headache, seizures, or coma) | |
| Laboratory Criteria | 2 of 3: white blood cell count ≤4.0×109/L, hemoglobin ≤90 g/L, or platelet ≤150×109/L) |
| Aspartate aminotransferase (AST) (>40 units/L) | |
| Lactate dehydrogenase (LDH) (>567 units/L) | |
| Fibrinogen ≤1.5 g/L | |
| Triglycerides >178 mg/dL | |
| Ferritin >500 μg/L | |
| Diagnosis of MAS if 1 Clinical + 2 Laboratory | |
| OR | |
| Histopathologic criteria | Evidence of macrophage hemophagocytosis in the bone marrow aspirate |
Hemophagocytosis, defined as the engulfment of blood cells [e.g., red blood cells (RBC), WBC, platelets] by macrophages has been widely associated with the development of MAS in patients with sJIA and other rheumatologic diseases (8, 9, 14, 21, 22). Histopathology may reveal characteristic increased hemophagocytic activity in the bone marrow, liver, and spleen with positive CD163 (histiocyte) staining, although hemophagocytosis may not be present in the initial stages and is neither sensitive nor specific for MAS (1, 23, 24). Detection of activated lymphocytes and hemophagocytosis by other means, including serum laboratory tests, includes soluble interleukin 2 receptor alpha chain (sCD25) and soluble CD163 (sCD163), a high affinity scavenger receptor for hemoglobin-haptoglobin complexes. Both of these parameters may be elevated, which suggests that sCD25 and sCD163 may be more sensitive in the detection of MAS. These tests are only performed at select sites, making them costly with a long turnaround time for results, thus leading to a delay in diagnosis and ultimately treatment (25, 26).
In the absence of a gold-standard diagnostic test and overlap of underlying disease manifestations and MAS, the HScore utilizes a scoring system comprised of 9 variables [i.e., 3 clinical (fever, known underlying immunosuppression, and organomegaly), 5 biologic (triglyceride level, ferritin, AST, fibrinogen, and cytopenia), and 1 histopathologic (i.e., hemophagocytosis on bone marrow aspirate)] (Table 1). Each variable is further stratified based on the level range, assigning a numerical value ranging from 0 to 64 to each variable for a maximum of 250. Fardet et al. (27) found that a score of 169 corresponded to a sensitivity of 93% and specificity of 86%, proving to be 90% accurate in correctly diagnosing sHLH. These criteria were developed in adults, many with oncologic conditions, and their ease and utility in pediatric sHLH/MAS is unknown. A simpler and timely set of criteria of sHLH in a broad array of disorders is needed.
With this in mind, a significant rise in serum ferritin (e.g., >10,000 ng/mL) in the setting of a hospitalized febrile patient is an inexpensive, rapid screening tool for MAS (28). With a cutoff value of ≥ 627 ng/mL for screening with a set sensitivity (0.95), the ferritin level alone had a specificity of 0.89 in identifying cases of all-cause MAS as compared to febrile hospitalized children (29). In combination with the erythrocyte sedimentation rate (ESR), the ferritin to ESR ratio has been shown to be both sensitive and specific in distinguishing MAS in sJIA from an active sJIA flare (29, 30). The ESR may initially be elevated but can drop rather quickly and be surprisingly low with MAS. Consumptive coagulopathy, a hallmark feature of MAS, leads to fibrinogen degradation and results in a drop in ESR (31–33). Unlike in other systemic inflammatory diseases, a combination of a high serum ferritin and low ESR may help confirm a diagnosis of MAS. Gorelik et al. (30) reported 100% sensitivity and specificity with a ratio of 80 in a small cohort of sJIA patients. Recently, Eloseily et al. (29) found, using 2 larger cohorts, a ferritin to ESR ratio of 21.5 (ng/mL divided by mm/hr) was 82% sensitive and 78% specific for diagnosing MAS in sJIA compared to active sJIA without MAS. The ferritin to ESR ratio shows promise as a generalizable, inexpensive, and rapid screening calculation that may lead to an earlier diagnosis and ultimately more timely initiation of treatment in MAS, thereby improving overall patient outcomes.
Genetics
The clinical and etiologic overlap between MAS and fHLH is significant, and includes an increased prevalence of heterozygous mutations in known fHLH genes found in MAS patients. Defects in the perforin-mediated cytolytic pathway result in an inability of cytolytic lymphocytes to lyse the infected antigen presenting cell (APC), which subsequently results in a prolonged cell-to-cell interaction causing a pro-inflammatory cytokine storm that ultimately leads to the clinical sequelae seen in MAS (34, 35). Heterozygous mutations in fHLH genes (e.g., PRF1, LYST, RAB27A, UNC13D, STXBP2, STX11) may be found in as high as 40% of patients with MAS (36, 37). This is likely significantly higher than the reported combined rates of these mutations (~15%) in the general population or disease control groups (38). As in adult onset HLH, heterozygous mutations in fHLH genes may also contribute to lymphoma development (39, 40). As in fHLH, these heterozygous hypomorphic and dominant-negative gene mutations can alter cytolytic function in NK cells and CD8 T cells (38). Using a threshold model of disease (41), a combination of a chronic inflammatory state, such as in sJIA or SLE, with a genetic predisposition, and/or a triggering infection may result in fatal MAS or sHLH as evidenced in the increased percentages of PRF1 and UNC13D heterozygous mutations in cohorts of sJIA patients who develop MAS (42, 43).
In addition to defects in the perforin-mediated cytolytic pathway, there are other mechanisms by which genetic mutations can trigger MAS and directly affect cells (e.g., macrophages and dendritic cells) of the innate immune system by altering cytokine production via the inflammasome complex (44). Gain of function mutations, as seen in Familial Mediterranean Syndrome (FMF), result in hyperactivation of the NLRC4 inflammasome which can in turn result in MAS. NLRC4 triggers the inflammasome, an innate immune complex that responds via caspase-1 activation and IL-1β and IL-18 secretion (45, 46). Moreover, rare activating mutations in NLRC4 itself can lead to an autoinflammatory disorder complicated by high IL-18 levels and clinical MAS (47). Although the mechanisms have not been worked out as clearly, there are other gene mutations associated with MAS/HLH. These include genes involved metabolism (e.g., SLC7A7), autophagy (e.g., NEMO), and viral control (e.g., CD27) (48). For many patients, the combination of a genetic predisposition, an underlying inflammatory state, and a triggering agent (e.g., infection) likely contribute to the cytokine storm seen in MAS (41).
Pathophysiology / Immunology
The acute phase of MAS is often associated with markedly elevated levels of pro-inflammatory cytokines like interferon-gamma (IFNγ), which are thought to be the primary drivers of pro-inflammatory (M1) macrophages (33, 49). The working hypothesis suggests that macrophages produce an array of cytokines, notably tumor necrosis factor (TNF) and various interleukins (i.e., IL-6, IL-1, and IL-18), which trigger a cascade of inflammatory pathways and ultimately create a cytokine storm (49). The pro-inflammatory cytokine environment, particularly IL-6, has been shown to decrease the cytolytic function of the NK cell (50). The inability of NK cells and cytolytic CD8 T cells to lyse infected and otherwise APCs results in prolonged cell-to-cell interactions and amplification of a pro-inflammatory cytokine cascade, which ultimately leads to the activation of macrophages, causing hemophagocytosis and multi-organ dysfunction. In contrast to the pro-inflammatory macrophages, some macrophages exhibit anti-inflammatory phenotype (M2) with upregulated CD163 receptors and likely serve to dampen the immune response through hemophagocytosis (51, 52).
Expression of TNF by hemophagocytic macrophages was reported in the liver of MAS patients (53). Elevated levels of TNF have been found in patients with other rheumatic diseases [e.g., rheumatoid arthritis (RA)] and are known to successfully modify disease activity in a milieu of rheumatic diseases (e.g., RA, JIA, uveitis) (54, 55). Like TNF, IL-6 producing macrophages have been found in the liver of MAS patients (53). Increased levels of IL-6 have also been reported in the serum of sJIA and in sepsis patients (56–58). Despite the association of IL-6 levels and MAS, the role of IL-6 in the pathogenesis of disease is not well-understood. It remains unknown whether macrophages are the main cellular sources of IL-6 in MAS patients.
As members of the IL-1 family of cytokines, IL-1β and IL-18 are potent inducers of IL-6 production in monocytes and macrophages (59, 60). Levels of IL-1β and IL-18 are frequently markedly increased in patients with active sJIA and MAS (61–66). Shimizu et al. (64) used the ratio of IL-18 to IL-6 to predict the development of MAS, noting higher IL-18 levels during the active phase of MAS. Patients within this cohort, who had higher levels of IL-18, were more likely to develop MAS following treatment with IL-6 blockade (i.e., tocilizumab), suggesting that IL-18, rather than IL-6, may play a dominant role in the pathogenesis of MAS. Likewise, while IL-18 is elevated in children with sJIA, the serum levels are significantly higher in sJIA that is complicated by active MAS (66). It is important to understand the mechanism behind the uncontrolled cytokine storm seen in MAS to target specific cytokines upstream and prevent further stimulation of the activated pro-inflammatory M1 macrophages (33).
Treatment
Historically, the treatment of MAS has been focused on controlling the underlying trigger, such as infection or sJIA treatment. However, not all cases present with a known pathogen or with a known etiology, making the treatment of the underlying trigger virtually impossible. Many rheumatologists have shifted toward cytokine-specific therapies in conjunction with treatment of the underlying triggering disease, if it is known. This differs from the HLH-2004 treatment protocol often recommended by oncologists, in which patients receive initial treatment with etoposide and dexamethasone (previously cyclosporine as well) for 8 weeks and possibly intrathecal methotrexate and prednisolone if CNS involvement is suspected. Patients who do not achieve remission are then bridged to receive bone marrow transplants (18). Mortality rates in patients treated using the HLH 2004 protocol remain high, with a 5-year survival rate of 64% in children with sHLH (67).
In addition to broadly immunosuppressive medications, such as corticosteroids and cyclosporine, cytokine-specific therapy (e.g., anakinra) may prove to be more effective in dampening the overly active immune system. Anakinra is a recombinant IL-1 receptor antagonist targeting both IL-1α and IL-1β cytokines used off-label in patients with sJIA and less commonly in patients with MAS, either in association with sJIA or other etiologies (68–70). Efficacy data in the treatment of MAS with anakinra is limited to retrospective data, but many patients achieve disease remission with normalization of lab abnormalities and fever despite the poor prior response to more traditional therapies (69, 71). Earlier initiation of anakinra within 5 days of hospitalization was associated a statistically significant reduction in mortality among patients with non-malignancy associated MAS (72).
Likewise, canakinumab is a monoclonal antibody that specifically targets only the IL-1β cytokine and is a common treatment target in patients with sJIA. Patients with sJIA treated with either anakinra or canakinumab remain at risk for MAS, suggesting that IL-1 receptor is not the sole contributor to the pathogenesis of MAS and that the increased risk may be dose dependent (19, 68). Treatment with recombinant IL-18 binding protein (IL-18bp) in combination with anakinra successfully improved life-threatening hyperinflammation in a patient with sJIA and refractory MAS, suggesting that IL-18 may also stimulate the inflammatory cascade leading to MAS in patients with sJIA (46). Similarly, IL-18bp has been known to effectively treat a child with an autoinflammatory disorder and refractory MAS (73).
Tocilizumab is a monoclonal antibody that targets the IL-6 receptor and is approved for use in RA, giant cell arteritis, polyarticular JIA, and sJIA (74). Despite its success in treating acute sJIA, patients with sJIA who are treated with tocilizumab remain at risk for MAS, which suggests that IL-6 blockade alone is insufficient to control the inflammatory cascade (75–77). These patients tend to be afebrile and had lower cell counts and ferritin levels with higher liver enzymes (19, 76). The mechanism of IL-6 in the pathogenesis of MAS remains controversial. Maude et al. (78) reported rapid resolution of HLH-like cytokine release syndrome (CRS) following the administration of tocilizumab in one patient with drug-induced (i.e., blinatumomab) cytokine storm. IL-6 blockade has similarly shown efficacy in CAR T cell therapy-triggered CRS (78). The utility of IL-6 blockade in other forms of sHLH/MAS remains unknown at present, but targeting other cytokines for treating sHLH/MAS are currently being explored.
While successful treatment of MAS with etanercept, a TNF receptor antagonist, has been reported (79), other studies have shown that it may trigger or worsen disease progression (80). Thus, the role of TNF and its blockade in MAS remains unclear. By comparison, targeting IFNγ with the monoclonal antibody emapalumab (81) has recently been approved by the FDA for treating fHLH; it’s role in sHLH/MAS is under exploration. Similarly, inhibition of cytokine signaling via JAK-STAT inhibitors may also have a future role in treating sHLH/MAS (82). Thus, the future of cytokine-targeted therapies looks bright for treating patients with frequently fatal disorders, such as sHLH and MAS.
Anecdotally, cytokine-specific therapies in combination with treatments for the underlying disease appear to be effective in reducing mortality rates and improving overall morbidity outcomes in children with MAS. Further studies and clinical trials are needed to better assess the role of various pro-inflammatory cytokines in the pathogenesis of MAS and to determine their clinical relevance. Ultimately, a personalized medicine approach with a variety of cytokine targeting therapeutics may be available for various forms of sHLH/MAS.
Conclusion
MAS is a potentially fatal inflammatory condition that can lead to multi-organ failure if it is treated inadequately. In the absence of generalizable validated diagnostic criteria, its recognition is often delayed. Clinical overlap with fHLH suggests that MAS is on one end of the same disease spectrum. Recognition of the pathogenesis of MAS can guide diagnosis and direct therapy toward target specific treatment. A common hypothesis to understand the pathophysiology of MAS proposes a defect in lymphocyte cytolytic activity. Normally, cytolytic cells induce cell apoptosis in infected or activated APCs. In an infected or inflammatory state, cytolytic cells may induce apoptosis in activated macrophages and dendritic cells and serve to control the inflammatory response. A defect in the cytolytic function may result in overstimulation of the immune system leading to the multi-organ failure seen in MAS. The cytokine storm (i.e., IL-1, IL-6, IL-18) results in activation of macrophages, causing hemophagocytosis, and contributes to multi-organ dysfunction. Specific heterozygous gene mutations in fHLH-associated cytolytic pathway genes (e.g., PRF1, UNC13D) have been linked to a substantial subset of MAS patients. These mutations cause defects in various proteins responsible for the production and transport of granules leading to the apoptosis of target cells. In addition, mutations activating the inflammasome complex lead to high IL-18 levels responsible for MAS pathophysiology. Early recognition of and prompt treatment with cytokine-specific therapy (e.g., anakinra, IL-18bp) in MAS is critical in maximizing outcomes with this potentially life-threatening disease. Future studies are needed to compare existing diagnostic criteria to develop a set of uniform criteria that may be applied across all rheumatic diseases and other forms of sHLH/MAS. Ultimately, tailored therapy for individual sHLH/MAS patients based on genetics, underlying disorders, and triggers (e.g., infections) will likely optimize outcomes (83).
Main Points.
Macrophage activation syndrome (MAS) and the related condition of secondary hemophagocytic lymphohistiocytosis (sHLH) are the result of “cytokine storms”, leading to multi-organ system failure and frequently death.
Novel diagnostic criteria are being developed for the timelier recognition of MAS/sHLH to allow for earlier treatment and improved outcomes.
Many children with MAS/sHLH possess heterozygous mutations in cytolytic pathway proteins present as homozygous defects in children with familial forms of HLH, thus sharing a similar pathophysiology in many cases.
Cytokine-targeted approaches (e.g., IL-1, IFNγ) are being explored for safer yet effective therapies for children with MAS/sHLH.
Footnotes
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - C.R., C.C.; Design - C.R., C.C.; Supervision - C.R., C.C.; Data Collection and/or Processing - C.R., C.C.; Analysis and/or Interpretation - C.R., C.C.; Literature Search - C.R., C.C.; Writing Manuscript - C.R., C.C.; Critical Review - C.R., C.C.
Conflict of Interest: The authors have no conflict of interest to declare.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Filipovich AH, Chandrakasan S. Pathogenesis of Hemophagocytic Lymphohistiocytosis. Hematol Oncol Clin North Am. 2015;29:895–902. doi: 10.1016/j.hoc.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 2.Behrens EM, Beukelman T, Paessler M, Cron RQ. Occult macrophage activation syndrome in patients with systemic juvenile idiopathic arthritis. J Rheumatol. 2007;34:1133–8. [PubMed] [Google Scholar]
- 3.Tada Y, Inokuchi S, Maruyama A, Suematsu R, Sakai M, Sadanaga Y, et al. Are the 2016 EULAR/ACR/PRINTO classification criteria for macrophage activation syndrome applicable to patients with adult-onset Still’s disease? Rheumatol Int. 2019;39:97–104. doi: 10.1007/s00296-018-4114-1. [DOI] [PubMed] [Google Scholar]
- 4.Crayne CB, Cron RQ. Weathering a Macrophage Storm. J Rheumatol. 2017;44:970–2. doi: 10.3899/jrheum.170370. [DOI] [PubMed] [Google Scholar]
- 5.Davi S, Minoia F, Pistorio A, Horne A, Consolaro A, Rosina S, et al. Performance of current guidelines for diagnosis of macrophage activation syndrome complicating systemic juvenile idiopathic arthritis. Arthritis Rheumatol. 2014;66:2871–80. doi: 10.1002/art.38769. [DOI] [PubMed] [Google Scholar]
- 6.Minoia F, Davi S, Horne A, Demirkaya E, Bovis F, Li C, et al. Clinical features, treatment, and outcome of macrophage activation syndrome complicating systemic juvenile idiopathic arthritis: a multinational, multicenter study of 362 patients. Arthritis Rheumatol. 2014;66:3160–9. doi: 10.1002/art.38802. [DOI] [PubMed] [Google Scholar]
- 7.Ravelli A, Grom AA, Behrens EM, Cron RQ. Macrophage activation syndrome as part of systemic juvenile idiopathic arthritis: diagnosis, genetics, pathophysiology and treatment. Genes Immun. 2012;13:289–98. doi: 10.1038/gene.2012.3. [DOI] [PubMed] [Google Scholar]
- 8.Ravelli A, Minoia F, Davi S, Horne A, Bovis F, Pistorio A, et al. 2016 Classification Criteria for Macrophage Activation Syndrome Complicating Systemic Juvenile Idiopathic Arthritis: A European League Against Rheumatism/American College of Rheumatology/Paediatric Rheumatology International Trials Organisation Collaborative Initiative. Ann Rheum Dis. 2016;75:481–9. doi: 10.1136/annrheumdis-2015-208982. [DOI] [PubMed] [Google Scholar]
- 9.Ravelli A, Minoia F, Davi S, Horne A, Bovis F, Pistorio A, et al. 2016 Classification Criteria for Macrophage Activation Syndrome Complicating Systemic Juvenile Idiopathic Arthritis: A European League Against Rheumatism/American College of Rheumatology/Paediatric Rheumatology International Trials Organisation Collaborative Initiative. Arthritis Rheumatol. 2016;68:566–76. doi: 10.1002/art.39332. [DOI] [PubMed] [Google Scholar]
- 10.Minoia F, Davi S, Horne A, Bovis F, Demirkaya E, Akikusa J, et al. Dissecting the heterogeneity of macrophage activation syndrome complicating systemic juvenile idiopathic arthritis. J Rheumatol. 2015;42:994–1001. doi: 10.3899/jrheum.141261. [DOI] [PubMed] [Google Scholar]
- 11.Borgia RE, Gerstein M, Levy DM, Silverman ED, Hiraki LT. Features, Treatment, and Outcomes of Macrophage Activation Syndrome in Childhood-Onset Systemic Lupus Erythematosus. Arthritis Rheumatol. 2018;70:616–24. doi: 10.1002/art.40417. [DOI] [PubMed] [Google Scholar]
- 12.Garcia-Pavon S, Yamazaki-Nakashimada MA, Baez M, Borjas-Aguilar KL, Murata C. Kawasaki Disease Complicated With Macrophage Activation Syndrome: A Systematic Review. J Pediatr Hematol Oncol. 2017;39:445–51. doi: 10.1097/MPH.0000000000000872. [DOI] [PubMed] [Google Scholar]
- 13.Rigante D, Emmi G, Fastiggi M, Silvestri E, Cantarini L. Macrophage activation syndrome in the course of monogenic autoinflammatory disorders. Clin Rheumatol. 2015;34:1333–9. doi: 10.1007/s10067-015-2923-0. [DOI] [PubMed] [Google Scholar]
- 14.Parodi A, Davi S, Pringe AB, Pistorio A, Ruperto N, Magni-Manzoni S, et al. Macrophage activation syndrome in juvenile systemic lupus erythematosus: a multinational multicenter study of thirty-eight patients. Arthritis Rheum. 2009;60:3388–99. doi: 10.1002/art.24883. [DOI] [PubMed] [Google Scholar]
- 15.Carcillo JA, Shakoory B, Simon D, Kernan K. Understanding Disseminated Intravascular Coagulation and Hepatobiliary Dysfunction Multiple Organ Failure in Hyperferritinemic Critical Illness. Pediatr Crit Care Med. 2018;19:1006–9. doi: 10.1097/PCC.0000000000001712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kernan KF, Ghaloul-Gonzalez L, Shakoory B, Kellum JA, Angus DC, Carcillo JA. Adults with septic shock and extreme hyperferritinemia exhibit pathogenic immune variation. Genes Immun. 2019;20:520–6. doi: 10.1038/s41435-018-0030-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shakoory B, Carcillo JA, Chatham WW, Amdur RL, Zhao H, Dinarello CA, et al. Interleukin-1 Receptor Blockade Is Associated With Reduced Mortality in Sepsis Patients With Features of Macrophage Activation Syndrome: Reanalysis of a Prior Phase III Trial. Crit Care Med. 2016;44:275–81. doi: 10.1097/CCM.0000000000001402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Henter JI, Horne A, Arico M, Egeler RM, Filipovich AH, Imashuku S, et al. HLH-2004: Diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer. 2007;48:124–31. doi: 10.1002/pbc.21039. [DOI] [PubMed] [Google Scholar]
- 19.Schulert GS, Minoia F, Bohnsack J, Cron RQ, Hashad S, Kon EPI, et al. Effect of Biologic Therapy on Clinical and Laboratory Features of Macrophage Activation Syndrome Associated With Systemic Juvenile Idiopathic Arthritis. Arthritis Care Res. 2018;70:409–19. doi: 10.1002/acr.23277. [DOI] [PubMed] [Google Scholar]
- 20.Minoia F, Bovis F, Davi S, Horne A, Fischbach M, Frosch M, et al. Development and initial validation of the MS score for diagnosis of macrophage activation syndrome in systemic juvenile idiopathic arthritis. Ann Rheum Dis. 2019;78:1357–62. doi: 10.1136/annrheumdis-2019-215211. [DOI] [PubMed] [Google Scholar]
- 21.Alkoht A, Hanafi I, Khalil B. Macrophage Activation Syndrome: A Report of Two Cases and a Literature Review. Case Rep Rheumatol. 2017;2017 doi: 10.1155/2017/5304180. 5304180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bennett TD, Fluchel M, Hersh AO, Hayward KN, Hersh AL, Brogan TV, et al. Macrophage activation syndrome in children with systemic lupus erythematosus and children with juvenile idiopathic arthritis. Arthritis Rheum. 2012;64:4135–42. doi: 10.1002/art.34661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goel S, Polski JM, Imran H. Sensitivity and specificity of bone marrow hemophagocytosis in hemophagocytic lymphohistiocytosis. Ann Clin Lab Sci. 2012;42:21–5. [PubMed] [Google Scholar]
- 24.Ho C, Yao X, Tian L, Li FY, Podoltsev N, Xu ML. Marrow assessment for hemophagocytic lymphohistiocytosis demonstrates poor correlation with disease probability. Am J Clin Pathol. 2014;141:62–71. doi: 10.1309/AJCPMD5TJEFOOVBW. [DOI] [PubMed] [Google Scholar]
- 25.Bleesing J, Prada A, Siegel DM, Villanueva J, Olson J, Ilowite NT, et al. The diagnostic significance of soluble CD163 and soluble interleukin-2 receptor alpha-chain in macrophage activation syndrome and untreated new-onset systemic juvenile idiopathic arthritis. Arthritis Rheum. 2007;56:965–71. doi: 10.1002/art.22416. [DOI] [PubMed] [Google Scholar]
- 26.Sakumura N, Shimizu M, Mizuta M, Inoue N, Nakagishi Y, Yachie A. Soluble CD163, a unique biomarker to evaluate the disease activity, exhibits macrophage activation in systemic juvenile idiopathic arthritis. Cytokine. 2018;110:459–65. doi: 10.1016/j.cyto.2018.05.017. [DOI] [PubMed] [Google Scholar]
- 27.Fardet L, Galicier L, Lambotte O, Marzac C, Aumont C, Chahwan D, et al. Development and validation of the HScore, a score for the diagnosis of reactive hemophagocytic syndrome. Arthritis Rheum. 2014;66:2613–20. doi: 10.1002/art.38690. [DOI] [PubMed] [Google Scholar]
- 28.Allen CE, Yu X, Kozinetz CA, McClain KL. Highly elevated ferritin levels and the diagnosis of hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer. 2008;50:1227–35. doi: 10.1002/pbc.21423. [DOI] [PubMed] [Google Scholar]
- 29.Eloseily EMA, Minoia F, Crayne CB, Beukelman T, Ravelli A, Cron RQ. Ferritin to Erythrocyte Sedimentation Rate Ratio: Simple Measure to Identify Macrophage Activation Syndrome in Systemic Juvenile Idiopathic Arthritis. ACR Open Rheumatol. 2019;1:345–9. doi: 10.1002/acr2.11048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gorelik M, Fall N, Altaye M, Barnes MG, Thompson SD, Grom AA, et al. Follistatin-like protein 1 and the ferritin/erythrocyte sedimentation rate ratio are potential biomarkers for dysregulated gene expression and macrophage activation syndrome in systemic juvenile idiopathic arthritis. J Rheumatol. 2013;40:1191–9. doi: 10.3899/jrheum.121131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ravelli A, Magni-Manzoni S, Pistorio A, Besana C, Foti T, Ruperto N, et al. Preliminary diagnostic guidelines for macrophage activation syndrome complicating systemic juvenile idiopathic arthritis. J Pediatr. 2005;146:598–604. doi: 10.1016/j.jpeds.2004.12.016. [DOI] [PubMed] [Google Scholar]
- 32.Grom AA, Mellins ED. Macrophage activation syndrome: advances towards understanding pathogenesis. Curr Opin Rheumatol. 2010;22:561–6. doi: 10.1097/01.bor.0000381996.69261.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Crayne CB, Albeituni S, Nichols KE, Cron RQ. The Immunology of Macrophage Activation Syndrome. Front Immunol. 2019;10:119. doi: 10.3389/fimmu.2019.00119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jenkins MR, Rudd-Schmidt JA, Lopez JA, Ramsbottom KM, Mannering SI, Andrews DM, et al. Failed CTL/NK cell killing and cytokine hypersecretion are directly linked through prolonged synapse time. J Exp Med. 2015;212:307–17. doi: 10.1084/jem.20140964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang M, Bracaglia C, Prencipe G, Bemrich-Stolz CJ, Beukelman T, Dimmitt RA, et al. A heterozygous RAB27A mutation associated with delayed cytolytic granule polarization and hemophagocytic lymphohistiocytosis. J Immunol. 2016;196:2492–503. doi: 10.4049/jimmunol.1501284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kaufman KM, Linghu B, Szustakowski JD, Husami A, Yang F, Zhang K, et al. Whole-exome sequencing reveals overlap between macrophage activation syndrome in systemic juvenile idiopathic arthritis and familial hemophagocytic lymphohistiocytosis. Arthritis Rheum. 2014;66:3486–95. doi: 10.1002/art.38793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang M, Behrens EM, Atkinson TP, Shakoory B, Grom AA, Cron RQ. Genetic defects in cytolysis in macrophage activation syndrome. Curr Rheumatol Rep. 2014;16:439. doi: 10.1007/s11926-014-0439-2. [DOI] [PubMed] [Google Scholar]
- 38.Schulert GS, Zhang M, Fall N, Husami A, Kissell D, Hanosh A, et al. Whole-Exome Sequencing Reveals Mutations in Genes Linked to Hemophagocytic Lymphohistiocytosis and Macrophage Activation Syndrome in Fatal Cases of H1N1 Influenza. J Infect Dis. 2016;213:1180–8. doi: 10.1093/infdis/jiv550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lofstedt A, Ahlm C, Tesi B, Bergdahl IA, Nordenskjold M, Bryceson YT, et al. Haploinsufficiency of UNC13D increases the risk of lymphoma. Cancer. 2019;125:1848–54. doi: 10.1002/cncr.32011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang K, Jordan MB, Marsh RA, Johnson JA, Kissell D, Meller J, et al. Hypomorphic mutations in PRF1, MUNC13–4, and STXBP2 are associated with adult-onset familial HLH. Blood. 2011;118:5794–8. doi: 10.1182/blood-2011-07-370148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Strippoli R, Caiello I, De Benedetti F. Reaching the threshold: a multilayer pathogenesis of macrophage activation syndrome. J Rheumatol. 2013;40:761–7. doi: 10.3899/jrheum.121233. [DOI] [PubMed] [Google Scholar]
- 42.Vastert SJ, van Wijk R, D’Urbano LE, de Vooght KM, de Jager W, Ravelli A, et al. Mutations in the perforin gene can be linked to macrophage activation syndrome in patients with systemic onset juvenile idiopathic arthritis. Rheumatology (Oxford) 2010;49:441–9. doi: 10.1093/rheumatology/kep418. [DOI] [PubMed] [Google Scholar]
- 43.Zhang K, Biroschak J, Glass DN, Thompson SD, Finkel T, Passo MH, et al. Macrophage activation syndrome in patients with systemic juvenile idiopathic arthritis is associated with MUNC13–4 polymorphisms. Arthritis Rheum. 2008;58:2892–6. doi: 10.1002/art.23734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schulert GS, Canna SW. Convergent pathways of the hyperferritinemic syndromes. Int Immunol. 2018;30:195–203. doi: 10.1093/intimm/dxy012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Duncan JA, Canna SW. The NLRC4 Inflammasome. Immunol Rev. 2018;281:115–23. doi: 10.1111/imr.12607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yasin S, Solomon K, Canna SW, Girard-Guyonvarc’h C, Gabay C, Schiffrin E, et al. IL-18 as therapeutic target in a patient with resistant systemic juvenile idiopathic arthritis and recurrent macrophage activation syndrome. Rheumatology (Oxford) 2019 Jul 29; doi: 10.1093/rheumatology/kez284. doi: 10.1093/rheumatology/kez284. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Canna SW, de Jesus AA, Gouni S, Brooks SR, Marrero B, Liu Y, et al. An activating NLRC4 inflammasome mutation causes autoinflammation with recurrent macrophage activation syndrome. Nat Genet. 2014;46:1140–6. doi: 10.1038/ng.3089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tesi B, Bryceson YT. HLH: genomics illuminates pathophysiological diversity. Blood. 2018;132:5–7. doi: 10.1182/blood-2018-05-845818. [DOI] [PubMed] [Google Scholar]
- 49.Canna SW, Behrens EM. Making sense of the cytokine storm: a conceptual framework for understanding, diagnosing, and treating hemophagocytic syndromes. Pediatr Clin North Am. 2012;59:329–44. doi: 10.1016/j.pcl.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cifaldi L, Prencipe G, Caiello I, Bracaglia C, Locatelli F, De Benedetti F, et al. Inhibition of Natural Killer Cell Cytotoxicity by Interleukin-6: Implications for the Pathogenesis of Macrophage Activation Syndrome. Arthritis Rheumatol. 2015;67:3037–46. doi: 10.1002/art.39295. [DOI] [PubMed] [Google Scholar]
- 51.Behrens EM. Macrophage activation syndrome in rheumatic disease: what is the role of the antigen presenting cell? Autoimmun Rev. 2008;7:305–8. doi: 10.1016/j.autrev.2007.11.025. [DOI] [PubMed] [Google Scholar]
- 52.Do T, Tan R, Bennett M, Medvedovic M, Grom AA, Shen N, et al. MicroRNA networks associated with active systemic juvenile idiopathic arthritis regulate CD163 expression and anti-inflammatory functions in macrophages through two distinct mechanisms. J Leukoc Biol. 2018;103:71–85. doi: 10.1002/JLB.2A0317-107R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Billiau AD, Roskams T, Van Damme-Lombaerts R, Matthys P, Wouters C. Macrophage activation syndrome: characteristic findings on liver biopsy illustrating the key role of activated, IFN-gamma-producing lymphocytes and IL-6-and TNF-alpha-producing macrophages. Blood. 2005;105:1648–51. doi: 10.1182/blood-2004-08-2997. [DOI] [PubMed] [Google Scholar]
- 54.Harris JG, Kessler EA, Verbsky JW. Update on the treatment of juvenile idiopathic arthritis. Curr Allergy Asthma Rep. 2013;13:337–46. doi: 10.1007/s11882-013-0351-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stoll ML, Cron RQ. Treatment of juvenile idiopathic arthritis: a revolution in care. Pediatr Rheumatol Online J. 2014;12:13. doi: 10.1186/1546-0096-12-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.de Benedetti F, Massa M, Robbioni P, Ravelli A, Burgio GR, Martini A. Correlation of serum interleukin-6 levels with joint involvement and thrombocytosis in systemic juvenile rheumatoid arthritis. Arthritis Rheum. 1991;34:1158–63. doi: 10.1002/art.1780340912. [DOI] [PubMed] [Google Scholar]
- 57.Lasiglie D, Traggiai E, Federici S, Alessio M, Buoncompagni A, Accogli A, et al. Role of IL-1 beta in the development of human T(H)17 cells: lesson from NLPR3 mutated patients. PLoS One. 2011;6:e20014. doi: 10.1371/journal.pone.0020014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xu XJ, Tang YM, Song H, Yang SL, Xu WQ, Zhao N, et al. Diagnostic accuracy of a specific cytokine pattern in hemophagocytic lymphohistiocytosis in children. J Pediatr. 2012;160:984–90.e1. doi: 10.1016/j.jpeds.2011.11.046. [DOI] [PubMed] [Google Scholar]
- 59.Musso T, Espinoza-Delgado I, Pulkki K, Gusella GL, Longo DL, Varesio L. Transforming growth factor beta downregulates interleukin-1 (IL-1)-induced IL-6 production by human monocytes. Blood. 1990;76:2466–9. doi: 10.1182/blood.V76.12.2466.bloodjournal76122466. [DOI] [PubMed] [Google Scholar]
- 60.Netea MG, Kullberg BJ, Verschueren I, Van Der Meer JW. Interleukin-18 induces production of proinflammatory cytokines in mice: no intermediate role for the cytokines of the tumor necrosis factor family and interleukin-1beta. Eur J Immunol. 2000;30:3057–60. doi: 10.1002/1521-4141(200010)30:10<3057::AID-IMMU3057>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 61.Fall N, Barnes M, Thornton S, Luyrink L, Olson J, Ilowite NT, et al. Gene expression profiling of peripheral blood from patients with untreated new-onset systemic juvenile idiopathic arthritis reveals molecular heterogeneity that may predict macrophage activation syndrome. Arthritis Rheum. 2007;56:3793–804. doi: 10.1002/art.22981. [DOI] [PubMed] [Google Scholar]
- 62.Ling XB, Park JL, Carroll T, Nguyen KD, Lau K, Macaubas C, et al. Plasma profiles in active systemic juvenile idiopathic arthritis: Biomarkers and biological implications. Proteomics. 2010;10:4415–30. doi: 10.1002/pmic.201000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shimizu M, Inoue N, Mizuta M, Nakagishi Y, Yachie A. Characteristic elevation of soluble TNF receptor II : I ratio in macrophage activation syndrome with systemic juvenile idiopathic arthritis. Clin Exp Immunol. 2018;191:349–55. doi: 10.1111/cei.13026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shimizu M, Yokoyama T, Yamada K, Kaneda H, Wada H, Wada T, et al. Distinct cytokine profiles of systemic-onset juvenile idiopathic arthritis-associated macrophage activation syndrome with particular emphasis on the role of interleukin-18 in its pathogenesis. Rheumatology (Oxford) 2010;49:1645–53. doi: 10.1093/rheumatology/keq133. [DOI] [PubMed] [Google Scholar]
- 65.Kaplanski G. Interleukin-18: Biological properties and role in disease pathogenesis. Immunol Rev. 2018;281:138–53. doi: 10.1111/imr.12616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yasin S, Fall N, Brown RA, Henderlight M, Canna SW, Girard-Guyonvarc’h C, et al. IL-18 as a biomarker linking systemic juvenile idiopathic arthritis and macrophage activation syndrome. Rheumatology (Oxford) 2019 Jul 20; doi: 10.1093/rheumatology/kez282. doi: 10.1093/rheumatology/kez282. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bergsten E, Horne A, Arico M, Astigarraga I, Egeler RM, Filipovich AH, et al. Confirmed efficacy of etoposide and dexamethasone in HLH treatment: long-term results of the cooperative HLH-2004 study. Blood. 2017;130:2728–38. doi: 10.1182/blood-2017-06-788349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nigrovic PA, Mannion M, Prince FH, Zeft A, Rabinovich CE, van Rossum MA, et al. Anakinra as first-line disease-modifying therapy in systemic juvenile idiopathic arthritis: report of forty-six patients from an international multicenter series. Arthritis Rheum. 2011;63:545–55. doi: 10.1002/art.30128. [DOI] [PubMed] [Google Scholar]
- 69.Sonmez HE, Demir S, Bilginer Y, Ozen S. Anakinra treatment in macrophage activation syndrome: a single center experience and systemic review of literature. Clin Rheumatol. 2018;37:3329–35. doi: 10.1007/s10067-018-4095-1. [DOI] [PubMed] [Google Scholar]
- 70.Miettunen PM, Narendran A, Jayanthan A, Behrens EM, Cron RQ. Successful treatment of severe paediatric rheumatic disease-associated macrophage activation syndrome with interleukin-1 inhibition following conventional immunosuppressive therapy: case series with 12 patients. Rheumatology (Oxford, England) 2011;50:417–9. doi: 10.1093/rheumatology/keq218. [DOI] [PubMed] [Google Scholar]
- 71.Rajasekaran S, Kruse K, Kovey K, Davis AT, Hassan NE, Ndika AN, et al. Therapeutic role of anakinra, an interleukin-1 receptor antagonist, in the management of secondary hemophagocytic lymphohistiocytosis/sepsis/multiple organ dysfunction/macrophage activating syndrome in critically ill children*. Pediatr Crit Care Med. 2014;15:401–8. doi: 10.1097/PCC.0000000000000078. [DOI] [PubMed] [Google Scholar]
- 72.Eloseily EMWP, Crayne CB, Haines H, Mannion M, Stoll ML, Beukelman T, Atkinson TP, Cron RQ. Benefit of Anakinra in Treating Pediatric Secondary Hemophagocytic Lymphohistiocytosis. Arthritis Rheum. 2019 Sep 12; doi: 10.1002/art.41103. doi: 10.1002/art.41103. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 73.Canna SW, Girard C, Malle L, de Jesus A, Romberg N, Kelsen J, et al. Life-threatening NLRC4-associated hyperinflammation successfully treated with IL-18 inhibition. J Allergy Clin Immunol. 2017;139:1698–701. doi: 10.1016/j.jaci.2016.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sterba Y, Ilowite N. Biologics in Pediatric Rheumatology: Quo Vadis? Curr Rheumatol Rep. 2016;18:45. doi: 10.1007/s11926-016-0593-9. [DOI] [PubMed] [Google Scholar]
- 75.De Benedetti F, Brunner HI, Ruperto N, Kenwright A, Wright S, Calvo I, et al. Randomized trial of tocilizumab in systemic juvenile idiopathic arthritis. N Engl J Med. 2012;367:2385–95. doi: 10.1056/NEJMoa1112802. [DOI] [PubMed] [Google Scholar]
- 76.Grom AA, Horne A, De Benedetti F. Macrophage activation syndrome in the era of biologic therapy. Nat Rev Rheumatol. 2016;12:259–68. doi: 10.1038/nrrheum.2015.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yokota S, Itoh Y, Morio T, Sumitomo N, Daimaru K, Minota S. Macrophage Activation Syndrome in Patients with Systemic Juvenile Idiopathic Arthritis under Treatment with Tocilizumab. J Rheumatol. 2015;42:712–22. doi: 10.3899/jrheum.140288. [DOI] [PubMed] [Google Scholar]
- 78.Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014;371:1507–17. doi: 10.1056/NEJMoa1407222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Flammiger A, Fiedler W, Bacher U, Bokemeyer C, Schneider M, Binder M. Critical imbalance of TNF-alpha and soluble TNF receptor 1 in a patient with macrophage activation syndrome: potential implications for diagnostics and treatment. Acta Haematol. 2012;128:69–72. doi: 10.1159/000338179. [DOI] [PubMed] [Google Scholar]
- 80.Buonuomo PS, Campana A, Insalaco A, Bracaglia C, Pardeo M, Cortis E. Necrotizing fasciitis in a pediatric patient treated with etanercept and cyclosporine for macrophage activation syndrome. Rheumatol Int. 2013;33:1097–8. doi: 10.1007/s00296-011-2319-7. [DOI] [PubMed] [Google Scholar]
- 81.Lounder DT, Bin Q, de Min C, Jordan MB. Treatment of refractory hemophagocytic lymphohistiocytosis with emapalumab despite severe concurrent infections. Blood Adv. 2019;3:47–50. doi: 10.1182/bloodadvances.2018025858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Albeituni S, Verbist KC, Tedrick PE, Tillman H, Picarsic J, Bassett R, et al. Mechanisms of action of ruxolitinib in murine models of hemophagocytic lymphohistiocytosis. Blood. 2019;134:147–59. doi: 10.1182/blood.2019000761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Behrens EM, Koretzky GA. Review: Cytokine Storm Syndrome: Looking Toward the Precision Medicine Era. Arthritis Rheumatol. 2017;69:1135–43. doi: 10.1002/art.40071. [DOI] [PubMed] [Google Scholar]