Abstract
Introduction:
Electroencephalography can also be used to monitor long-term recovery of the patient after acute phase of the disease. Impaired neurocognitive function after infection, similar to brain injury, may present a transient but also prolonged problem for the functioning of an individual. Some studies have shown that importance of EEG may not be significant in sequel monitoring, because the extensive changes in EEG seen with severe forms of CNS infection do not necessarily imply a longer-term poor outcome.
Aim:
To examine the predictive potential of electroencephalography (EEG) in regard to the emergence of neurological and cognitive sequelae of acute central nervous system (CNS) infection.
Methods:
The study included 62 patients treated at the Clinic for Infectious Diseases, Clinical Center of Sarajevo University, who were diagnosed with acute CNS infection. The EEG record was characterized as: normal, non-specific changes of mild, moderate and severe degree and specific changes. The sequelae (headache, cognitive dysfunction, neurological and neurophysiological disorders, audiological and behavioral disorders) was evaluated by combining neurological, psychiatric, pediatric, otolaryngological, ophthalmic and infectological examination in the Neuroinfective Counseling Department for up to 6 months after discharge.
Results:
After a treatment of an acute CNS infection 25 (40.3%) patients had no sequelae and 37 (59.7%) were with sequelae. The EEG in the initial stage of the disease (Wald’s coefficient = 12.8), followed by the age of the patients (Wald = 6.4), had the greatest influence on the prediction of sequela (p=0.0001). For each additional degree of verified pathological changes in the EEG, the risk of sequelae was increased by 5 degrees (OR = 5.3), respectively. There was no statistically significant association between changes in cerebrospinal fluid (CSF) findings, meningeal symptoms, and signs with sequelae development.
Conclusion:
Younger age, as well as severe clinical status of a patient, which implies a disorder of consciousness and seizures on admission, are associated with irreversible consequences on a previously mentally healthy individual. Pathological changes (Delta and Theta waves, spike slow complex wave) on the EEG finding significantly predicted presence of sequelae. .
Keywords: cognitive manifestation, CNS diseases, EEG
1. INTRODUCTION
Acute central nervous system (CNS) infections are ethiologically diverse diseases, characterized by complex pathogenesis, specific clinical symptomatology, and often severe and even life-threatening events. The most common causative agents are viruses and bacteria, and potential, microorganisms known as mycoplasmas, rickettsia, parasites and fungi (1). Infection of CNS is medical emergency, and there is a need of early recognition, effective decision making, and treatment (2). Precise etiologic diagnosis of acute CNS infections is difficult to determine, because diagnostic capacity of individual hospital centers is rarely sufficiently developed, which, together with lack of standardized clinical protocols, usually leads to imprecise characterization of these infections as “aseptic” or “purulent” (3). The most important etiologic pathogens of purulent meningitis are Haemophylus influenzae, Streptococcus pneumoniae and Neisseria meningitidis (4). With successfully implemented vaccination, economically developed countries have reduced the prevalence of purulent CNS infections, while middle and developing countries are still having a high rate (5). We know today that aseptic CNS infections can be infectious and non-infectious, but the etiological spectrum of is still unknown. Herpesviruses are the most significant cause of aseptic acute CNS infection (6). Meningeal syndrome involves a variety of neurological symptoms, from fever and headache, to altered quality of consciousness, to lethargy or coma (6). According to the current protocols, diagnosis is established by clinical finding and analysis of CSF, which is the gold standard of diagnosis for these infections. Aseptic infections may have a discretely altered CSF (7).
The persistence of various neurological and cognitive symptoms after CNS infection was first described in 1923 (8). The effects of acute CNS infections on patients’ cognitive ability can be eliminated and treated if the etiologic and predisposing factors and extent of brain damage were detected during the infection. Patients with severe impairment on the electroencephalography (EEG), or those with severe cognitive or neurological sequelae on discharge, should be treated by physical therapy or under the supervision of a psychiatrist, speech therapist or neurologist as soon as possible (9). Electroencephalography and various neuroimaging methods, in particular magnetic resonance imaging (MR), can be useful to confirm the diagnosis and to identify focal complications (10).
Electroencephalography can also be used to monitor long-term recovery of the patient after acute phase of the disease. Impaired neurocognitive function after infection, similar to brain injury, may present a transient but also prolonged problem for the functioning of an individual (11). Some studies have shown that importance of EEG may not be significant in sequel monitoring, because the extensive changes in EEG seen with severe forms of CNS infection do not necessarily imply a longer-term poor outcome (12). However, such studies were rarely done in resource-poor settings of developing countries.
2. AIM
The aim of this study was to examine the predictive potential of electroencephalography (EEG) in regard to the emergence of neurological and cognitive sequelae of acute central nervous system (CNS) infection.
3. METHODS
Patients and study design
This retrospective study included 62 patients treated at the Infectious Diseases Clinic over a four-year period (January 2014 - January 2018). After discharge from the Clinic, for the patients were re-examined 6 months later. Data collection did not interfere with the care and treatment of patients in any way, and statistical analyzes were processed retrospectively and anonymously. Ethical approval was obtained from the Ethics Committee of the Clinical Center of the University of Sarajevo. The reasons for hospitalization were acute CNS infections, which were diagnosed by CSF examination on the day of admission.
Data from case histories, temperature lists, and specialist opinion findings stored in the Hospital Information System (BIS) of the Infectious Diseases Clinic were used. Patients were treated according to current local clinical standards and were included in the study if they had completed: CSF, clinical, laboratory, neuroimaging and neurophysiological diagnostics. The age, gender, length of hospitalization, clinical parameters on the day of admission with special reference to neurological status (fever, headache, and state of consciousness, neck pain, malaise, meningeal signs, tone and muscular motility, reflexes) were analyzed for each patient who matched the sample.
The CSF examination including the number of cells, proteins, and glucose was made immediately after raising suspicion of CNS infection. Electroencephalography was performed at the Pediatric Clinic and the Neurology Clinic using 32 channel techniques for 20 min. Electroencephalography was performed at the Pediatric Clinic and the Neurology Clinic using 32 channel techniques for 20 min.
Methods
The EEG record was characterized as: physiological, pathological changes of mild grade, medium-severe grade, severe grade and specifically modified. The sequelae were evaluated by combining neurological, psychiatric, pediatric, otolaryngological, ophthalmic and infectological examination in a Neuroinfective Disease Counseling Center. Sequelae included: headache, cognitive dysfunction (loss of concentration and memory), neurological and neurophysiological disorders, audiological and behavioral disorders (disorientation, psychosis, personality disorder, agitation, etc.).
Statistical analysis
The data were analyzed at the level of descriptive statistics by measuring central tendency (arithmetic mean and median) and variability (standard deviation and standard error). The univariate binary logistic regression examined the individual impact of independent variables on the binary dependent variable survival (presence / absence of sequelae) in patients treated for acute CNS infection. Multivariate binary regression analysis examined the influence of independent predictors (model 1), which showed a significant impact on the dependently variable “presence / absence of sequela”. All analyzes were estimated at the level of statistical significance of p <0.05.
4. RESULTS
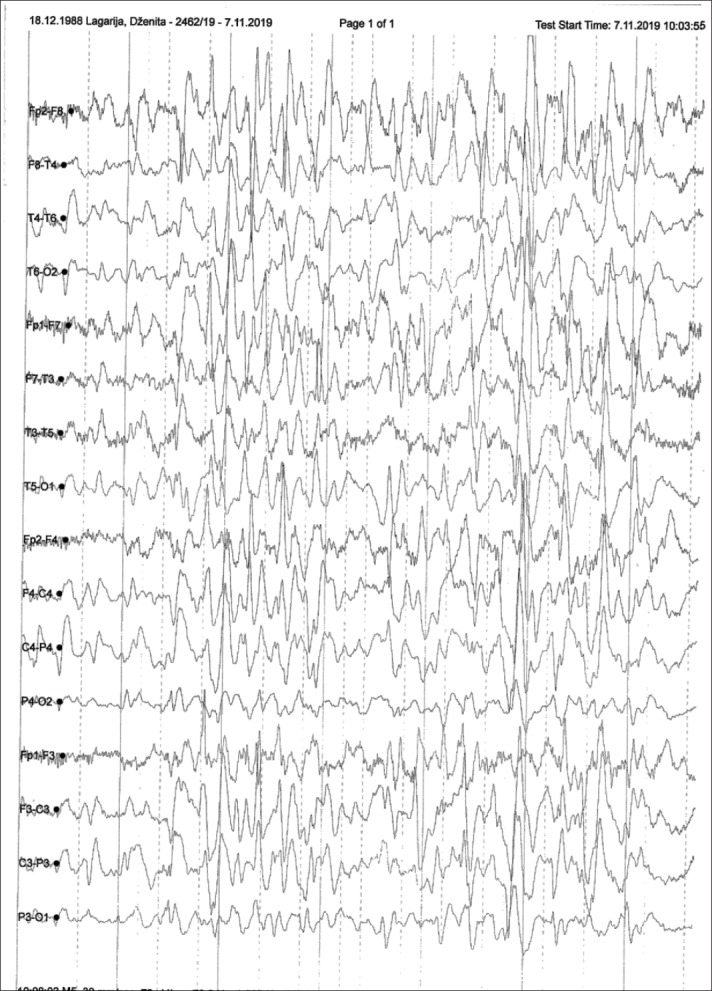
The study included 62 patients, of whom 25 (40.3%) had no sequelae and 37 (59.7%) were with sequelae after a treatment of an acute CNS infection. The male patients dominated, 43 (69.4%) with average age of 17 (12-24) years, 19 (30.6%) were female patients with average age of 35 (14-55) years. The average length of illness before hospitalization was 1 (1-4) days. A total of 37 (59.7%) patients had severe clinical status at admission; fever, headache and malaise were dominant symptoms in 58 (93.5%), 52 (83.9%), and 39 (62.9%) patients, respectively. Meningeal signs were positive in 39 (62.9%) patients, while 18 (29%) patients had neurological disturbance of tone, motor and reflex. Organoleptic turbidity was found in 37 (59.7%) CSF samples, while the others had clear CSF. The average number of inflammatory cells was 352 (53-2510) with an average proteinorachia of 1.2 (0.5-3.2). Neuroimaging showed that MR was more sensitive method in detecting pathological brain changes than CT and standard radiography. EEG findings with moderate (delta-theta slowdown) changes were dominant, with 7 (11.3%) patients having a specifically modified EEG (spike-slow wave) (Figure 1 and 2).
Figure 1. Specific changes (spike-slow wave pattern).
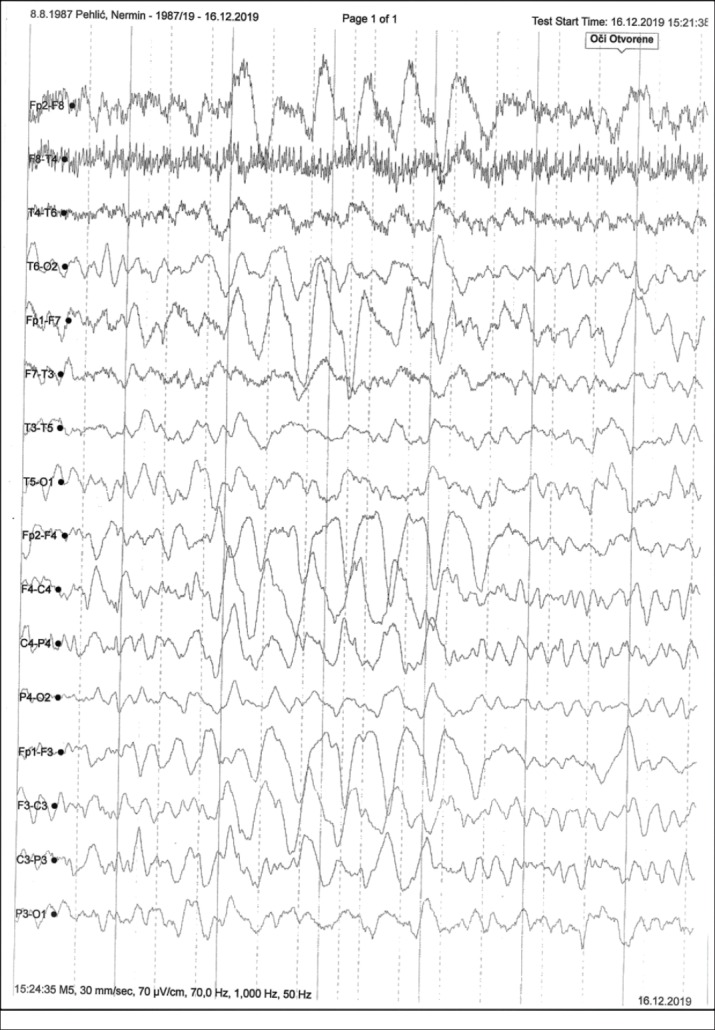
Figure 2. Diffuse Theta-Delta slow waves.
The following independent parameters did not significantly affect development of sequela after acute CNS infection: sex, length of disease before treatment, symptoms (fever, headache, pain in the back of the spine and weakness), meningeal signs and state of consciousness, overall CSF, and standard head radiography (Table 1) However, the age of the subjects proved to be a significant predictor of the presence of sequela (p = 0.021) as the odds (Odds ratio) of having a patient with sequela for each year increased by 4%, with an interval of 1 to 8%. Patients who were unconscious on admission were 4 times (OR = 4.4) more likely to develop sequelae, as were patients with severe general admission status compared to medium severity, about 3 times (OR = 3, 01).
Table 1. Univariate regression analysis: The influence of independent predictors on outcome of patients with CNS infection (presence or absence of sequela). B-coefficient; Wald-coefficient; df- number of degrees of freedom, p- probability; EXP (B) - odds ratio; 95.0% CI.for EXP (B) - 95% confidence interval for odds ratio; lower - lower limit; Upper- upper limit. * EEG (EEG findings are numbered in ascending order, monitoring the severity of: 1. physiological; 2.pathological changes of mild grade; 3. medium-severe grade; 4. severe grade; and 5. specifically modified.
| 95.0% C.I.for EXP(B) | |||||||
|---|---|---|---|---|---|---|---|
| B | Wald | df | Sig. | Exp(B) | Lower | Upper | |
| Age (years) | .039 | 5.310 | 1 | .021 | 1.040 | 1.009 | 1.075 |
| Gender (male / female) | .890 | 2.170 | 1 | .141 | 2.435 | ||
| Length of illness (days) | .114 | 1.263 | 1 | .261 | 1.121 | ||
| Elevated Body Temperature (yes / no) | -.750 | .401 | 1 | .527 | .472 | ||
| Headache (yes / no) | -.537 | .520 | 1 | .471 | .584 | ||
| Non-contactability (yes / no) | 1.496 | 4.533 | 1 | .033 | 4.464 | 1.126 | 17.692 |
| Back pain (yes / no) | -.303 | .232 | 1 | .630 | .739 | ||
| General weakness (yes / no) | .493 | .850 | 1 | .357 | 1.637 | ||
| Confusion (yes / no) | .693 | 1.723 | 1 | .189 | 2.000 | ||
| Clinical status at admission (moderate / severe) | 1.101 | 4.158 | 1 | .041 | 3.008 | 1.044 | 8.671 |
| Meningeal signs (Negative / positive) | -.079 | .022 | 1 | .883 | .924 | ||
| Consciousness (unconscious / conscious) | .576 | 1.208 | 1 | .272 | 1.780 | ||
| Muscle tone, Muscle Motor, Reflexes (Present / Absent) | 1.609 | 5.277 | 1 | .022 | 5.000 | 1.266 | 19.741 |
| Organoleptic CSF (clear / blurry) | .022 | .002 | 1 | .966 | 1.023 | ||
| Number of cells (cel / mm3) | .000 | .013 | 1 | .910 | 1.000 | ||
| Proteinorrachia (g/L) | .117 | 1.133 | 1 | .287 | 1.124 | ||
| Glycorrachia (mmol/L) | -.038 | .047 | 1 | .828 | .963 | ||
| MR of the head (physiological / pathological) | 1.172 | 7.328 | 1 | .007 | 3.227 | 1.063 | 6.553 |
| CT of the head (physiological / pathological) | .901 | 7.196 | 1 | .007 | 2.461 | 1.030 | 4.143 |
| Pyramid and mastoid radiogram (physiological / pathological) | .474 | 1.264 | 1 | .261 | 1.607 | ||
| *EEG | 1.673 | 13.164 | 1 | .000 | 5.326 | 2.158 | 13.146 |
Absence of muscle motor on admission was significantly influenced the presence of sequela (p = 0.021). Absence of a reflex on admission increased the risk of sequelae 5 times (OR = 5.0) (from 1 to 19 times). Pathological changes observed on MR and CT of the head significantly affected presence of sequelae (p = 0.007), increasing the risk, MR (OR = 3.2) and CT (OR = 2 , 2). Pathological changes (Delta and Theta waves, spike-slow complex wave) on the EEG finding significantly predicted presence of sequelae (p = 0.0001).
Using the 6-step Backaer Wald method on all statistically significant variables (p <0.05), the EEG findings from the initial stage of the disease (Wald’s coefficient = 12.8), followed by the patients’ age (Wald = 6,4) had the greatest impact to the development of sequelae. (Table 2). Model 1 had values (Cox & Snell) R2 = 0.435 and (Nagelkerke) R2 = 0.0.587, indicating that the set of sixth step variables explains the deviation between 43.5% and 58.7% of the variance. Furthermore, the results of the Hosmer and Lemeshow test supported the claim that the model was good (χ2 = 7.5; p = 0.479) (Table 2).
Table 2. Multivariate regression analysis. Impact of independent predictors on the outcome of CNS infection (presence or absence of sequelae). * EEG (EEG findings are numbered in ascending order, monitoring the severity of: 1. physiological; 2.pathological changes of mild grade; 3. medium-severe grade; 4. severe grade; and 5. specifically modified.
| 95.0% C.I.for EXP(B) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| B | S.E. | Wald | df | p | Exp(B) | Lower | Upper | ||
| Step 1 | Age (years) | .063 | .031 | 4.192 | 1 | .041 | 1.065 | 1.003 | 1.131 |
| Consciousness (yes / no) | 1.788 | 1.141 | 2.454 | 1 | .117 | 5.975 | .638 | 55.940 | |
| Clinical status at admission (moderate / severe) | -.955 | .918 | 1.081 | 1 | .298 | .385 | .064 | 2.328 | |
| Muscle tone, Muscle Motor Reflexes (Present / Absent) | -.610 | 1.261 | .234 | 1 | .628 | .543 | .046 | 6.430 | |
| MR of the head (physiological / pathological) | .010 | .662 | .000 | 1 | .988 | 1.010 | .276 | 3.697 | |
| CT of the head (physiological / pathological) | .632 | .510 | 1.536 | 1 | .215 | 1.881 | .693 | 5.110 | |
| *EEG | 2.154 | .686 | 9.871 | 1 | .002 | 8.622 | 2.249 | 33.056 | |
| Constant | -4.992 | 2.520 | 3.923 | 1 | .048 | .007 | |||
| Step 6 | Age (years) | .068 | .027 | 6.475 | 1 | .011 | 1.070 | 1.016 | 1.128 |
| *EEG | 1.975 | .550 | 12.884 | 1 | .0001 | 7.203 | 2.451 | 21.172 | |
| Constant | -6.923 | 1.896 | 13.332 | 1 | .000 | .001 |
5. DISCUSSION
The study shows that pathological changes in EEG registration and the patient’s younger age are significant predictive factors for various neurological and cognitive sequelae after acute neuroinfectious disease such as meningitis and encephalitis.
In the acute stage of the disease, patients with CNS infection have significant neurophysiological disorders seen with EEG, and a lot of number of these disorders correlate with a decrease in cognitive function. At young age, when congenital cognitive status is still incomplete, the EEG help assessing initial severity of the disease and predicting long-term outcome. Research by other authors pointed to the importance of EEG in recognizing non-convulsive seizures that may ultimately be the cause of cognitive long-term disorders (13).
Neurobehavioral disorders consist of a spectrum of neuropsychiatric and somatic symptoms present after brain inflammation, and may include cognitive and behavioral / psychiatric disorders (14). Comparison of rate of sequelae with other studies is difficult because the settings are different: length of follow-up, outcome of disease designated as “good” or “bad”, and different research methodologies. True incidence of acute CNS infections is controversial, due to the low rate of ethiologically confirmed cases, but in Western countries it is estimated to be 0.6–4 / 100,000 per year for bacterial meningitis, 5.2–7.6 / 100,000 for viral meningitis, and for encephalitis of 2.73-8.66 / 100,000 (15, 16, 17). Cases suspected of having acute neuroinfection do not tolerate delaying the therapy for more than 48 hours of clinical presentation due to high morbidity and mortality rates in untreated patients, and gold standard of diagnosis during this period is LP (18). According to published studies, if antibiotic therapy is not included in the first hours after admission of patients with bacterial meningitis, mortality increases from 7% to 26% (19).
In our study, 59.7% of patients had neuropsychological sequelae after treatment of an acute CNS infection, which when compares with the general clinical status at the day of admission, could be characterized as “severe”. Sequelae presented in the range from general weakness and headache to severe neurological and cognitive disorders, which persisted in the six-month period after treatment, and were estimated by infectious disease specialists after consulting other specialists (otolaryngologist, ophthalmologist and neuropsychiatrist). In large French study of long-term outcomes of patients with encephalitis of different etiology, the rate of patients who had sequelae was significantly lower (39%), because only general neuropsychological variables, moderate and severe instability, and vegetative status were searched for (20). In the retrospective cohort study by Jouan et al., the incidence of disability after Herpes simplex virus (HSV) encephalitis was high, and only 50% of patients showed complete neurological recovery at one-year follow-up, while the remaining became a burden for their families and society (21). Pillai et al had approximately the same rate of sequelae (49%) in patients after 5.8 years of follow-up, both with HSV as the detected cause, and with cases that were not etiologically differentiated. Epileptic status and changes in MRI at the initial stage of the disease were stressed as predictors of sequelae, which is not in accordance with our study, since status epilepticus is a pathological condition suggesting a difficult clinical status of the patient on admission (22). Studies addressing sequelae after meningitis or encephalitis of different ages have found no prognostic correlation with CSF bio-markers or laboratory parameters, but correlated longer-term poor outcome with severe clinical status of patients who required treatment in an intensive care unit (23, 24). We came to the same conclusions, that there was no correlation between the CSF findings and the development of sequelae, as well as meningeal symptoms and signs at the initial stage of the disease. Clinical and laboratory status upon admission of patients to hospital should be used by physicians to raise suspicion of acute CNS infection and start empirical therapy as early as possible. Studies that had only a pediatric population found a link between severe proteinorrhagia in bacterial meningitis and neurological sequelae, which raised the question of morbidity in the younger population according to the etiology of the infection (25). Structural changes (reduction) of brain gray mass observed by neuroimaging method were correlated with cognitive status of patients suffering from tuberculous meningitis, according to studies of other authors (26). Our study also showed that changes in the observed CT and MR, were significant predictors for the development of sequelae during the six-month follow-up of patients after CNS infection, regardless of etiology. Similar to other authors, MRI proved to be a more significant neuroimaging scan to predict sequelae (OR = 3.2) than CT (OR = 2.2). Computed tomography is more used in terms of identifying an alternative diagnosis such as intracranial hemorrhage or hydrocephalus (17, 27, 28).
6. CONCLUSION
Younger age, as well as severe clinical status of a patient, which implies a disorder of consciousness and seizures on admission, are associated with irreversible consequences on a previously mentally healthy individual. Studies have also shown the importance of more recent neuroimaging methods, such as functional MR or MR spectroscopy, that can predict impaired cognition based on metabolic or functional changes in the brain. Pathological changes (Delta and Theta waves, spike-slow complex wave) on the EEG finding significantly predicted presence of sequelae.
Author’s contribution:
All authors were included in all steps of preparation this article. Final proof reading was made by the first author.
Conflict of interests:
There are no conflicts of interest.
Financial support and funding:
Nil.
REFERENCES
- 1.Dando SJ, Mackay-Sim A, Norton R, Currie BJ, et al. Pathogens penetrating the central nervous system: infection pathways and the cellular and molecular mechanisms of invasion. Clinical microbiology reviews. 2014 Oct;27(4):691–726. doi: 10.1128/CMR.00118-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Michael BD, Sidhu M, Stoeter D, et al. Acute central nervous system infections in adults-a retrospective cohort study in the NHS North West region. QJM: An International Journal of Medicine. 2010 Oct;103(10):749–758. doi: 10.1093/qjmed/hcq121. [DOI] [PubMed] [Google Scholar]
- 3.Mawuntu AH, Bernadus JB, Dhenni R, Wiyatno A, et al. Detection of central nervous system viral infections in adults in Manado, North Sulawesi, Indonesia. PloS one. 2018 Nov;13(11):e0207440. doi: 10.1371/journal.pone.0207440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Akhvlediani T, Bautista CT, Shakarishvili R, Tsertsvadze T, et al. Etiologic agents of central nervous system infections among febrile hospitalized patients in the country of Georgia. PloS one. 2014 Nov;9(11):e111393. doi: 10.1371/journal.pone.0111393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Griffiths UK, Clark A, Shimanovich V, Glinskaya I, Tursunova D, et al. Comparative Economic Evaluation of Haemophilus influenzae Type b Vaccination in Belarus and Uzbekistan. PLoS ONE. 2011 Jun;6(6):e21472. doi: 10.1371/journal.pone.0021472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Babamahmoodi F, Davoudi A, Babamahmoodi A, et al. Epidemiologic characteristics of patients treated in a referral center with the diagnosis of central nervous system infection in North of Iran, from March 2008 to March 2012. Arch of Neurosc. 2014 Sep;1(2):82–87. doi: 10.5812/archneurosci.13894. [DOI] [Google Scholar]
- 7.Gaston I, Muruzabal J, Quesada P, Maravi E. Infections of the central nervous system in emergency department. An Sist Sanit Navar. 2008 Jan;31(1):99–113. [PubMed] [Google Scholar]
- 8.Ebaugh FG. Neuropsychiatric sequelae of acute epidemic encephalitis in children. 1923. J Atten Disord. 2007 Feb;11:339–340. doi: 10.1001/archpedi.1923.01920020006002. [DOI] [PubMed] [Google Scholar]
- 9.Nordholm AC, Søborg B, Andersson M, Hoffmann S, Skinhøj P, Koch A. CNS infections in Greenland: A nationwide register- based cohort study. PloS one. 2017 Feb;12(2):e0171094. doi: 10.1371/journal.pone.0171094. eCollection 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fitch MT, Abrahamian FM, Moran GJ, Talan DA. Emergency department management of meningitis and encephalitis. Infect Dis Clin North Am. 2008 Mar;22(1):33–52. doi: 10.1016/j.idc.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 11.Safavynia SA, Goldstein PA. The role of neuroinflammation in postoperative cognitive dysfunction: moving from hypothe-sis to treatment. Frontiers in psychiatry. 2018 Jan 9;752 doi: 10.3389/fpsyt.2018.00752. eCollection 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dorsett M, Liang SY. Diagnosis and treatment of central nervous system infections in the emergency department. Emergency Medicine Clinics. 2016 Nov;34(4):917–942. doi: 10.1016/j.emc.2016.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gold JJ, Crawford JR, Glaser C, Sheriff H, Wang S, Nespeca M. The role of continuous electroencephalography in childhood encephalitis. Pediatric Neurology. 2014 Apr;50(4):318–323. doi: 10.1016/j.pediatrneurol.2013.12.014. Epub 2013 Dec 19. [DOI] [PubMed] [Google Scholar]
- 14.Riggio S. Traumatic brain injury and its neurobehavioral sequelae. Neurologic Clinics. 2011 Feb;29(1):35–47. doi: 10.1016/j.ncl.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 15.Kramer AH. Viral encephalitis in the ICU. Crit Care Clin. 2013 Jul;29:621–650. doi: 10.1016/j.ccc.2013.03.011. [DOI] [PubMed] [Google Scholar]
- 16.Granerod J, Cousens S, Davies NWS, et al. New estimates of the incidence of encephalitis in England. Emerg Infect Dis. 2013 Sep;19:1455–1462. doi: 10.3201/eid1909.130064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Solomon T, Michael BD, Smith PE, et al. On behalf of the National Encephalitis Guidelines Development and Stakeholder Groups. Management of suspected viral encephalitis in adults. J Infect. 2012 Aprr;64:347–373. doi: 10.1016/j.jinf.2011.11.014. [DOI] [PubMed] [Google Scholar]
- 18.Stoeter DJ, Michael BD, Solomon T, Poole L. Managing acute central nervous system infections in the UK adult intensive care unit in the wake of UK encephalitis guidelines. Journal of the Intensive Care Society. 2015 Nov;16(4):330–338. doi: 10.1177/1751143715587927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dellinger RP, Levy MM, Rhodes A, et al. Surviving Sepsis Campaign: International Guidelines for Management of Severe Sepsis and Septic Shock: 2012. Crit Care Med. 2013 Feb;41:580–637. doi: 10.1097/CCM.0b013e31827e83af. [DOI] [PubMed] [Google Scholar]
- 20.Mailles A, De Broucker T, Costanzo P, et al. Long-term outcome of patients presenting with acute infectious encephalitis of various causes in France. Clinical infectious diseases. 2012 May;54(10):1455–1464. doi: 10.1093/cid/cis226. Epub 2012 Mar 28. [DOI] [PubMed] [Google Scholar]
- 21.Jouan Y, Grammatico-Guillon L, Espitalier F, Cazals X, François P, Guillon A. Long-term outcome of severe herpes simplex encephalitis: a population-based observational study. Critical Care. 2015 Sep;19(1):345. doi: 10.1186/s13054-015-1046-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pillai SC, Hacohen Y, Tantsis E, Prelog K, Merheb V, Kesson A, et al. Infectious and autoantibody-associated encephalitis: clinical features and long-term outcome. Pediatrics. 2015 Apr;135(4):e974–984. doi: 10.1542/peds.2014-2702. [DOI] [PubMed] [Google Scholar]
- 23.Tsukahara H, Fujii Y, Matsubara K, Yamada M, Nagaoka Y, et al. Prognostic value of brain injury biomarkers in acute encephalitis/encephalopathy. Pediatrics International. 2013 Aug;55(4):461–464. doi: 10.1111/ped.12094. [DOI] [PubMed] [Google Scholar]
- 24.Als LC, et al. Neuropsychologic function three to six months following admission to the PICU with meningoencephalitis, sepsis, and other disorders: a prospective study of school-aged children. Crit Care Med. 2013 Apr;41(4):1094–1103. doi: 10.1097/CCM.0b013e318275d032. [DOI] [PubMed] [Google Scholar]
- 25.Hsu MH, Hsu JF, Kuo HC, Lai MY, Chiang MC, Lin YJ, Huang HR, Chu SM, Tsai MH. Neurological complications in young infants with acute bacterial meningitis. Frontiers in neurology. 2018 Oct;9 doi: 10.3389/fneur.2018.00903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen HL, Lu CH, Chang CD, Chen PC, Chen MH, Hsu NW, Chou KH, Lin WM, Lin CP, Lin WC. Structural deficits and cognitive impairment in tuberculous meningitis. BMC infectious diseases. 2015 Jul;15(1):279. doi: 10.1186/s12879-015-1011-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ullman H, Almeida R, Klingberg T. Structural maturation and brain activity predict future working memory capacity during childhood development. Journal of Neuroscience. 2014 Jan;34(5):1592–1598. doi: 10.1523/JNEUROSCI.0842-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bookstaver PB, Mohorn PL, Shah A, Tesh LD, et al. Management of viral central nervous system infections: a primer for clinicians. Journal of central nervous system disease. 2017 May;9:1179573517703342. doi: 10.1177/1179573517703342. eCollection 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]


