Abstract
Connective tissues within the synovial joints are characterized by their dense extracellular matrix and sparse cellularity. With injury or disease, however, tissues commonly experience an influx of cells owing to proliferation and migration of endogenous mesenchymal cell populations, as well as invasion of the tissue by other cell types, including immune cells. Although this process is critical for successful wound healing, aberrant immune-mediated cell infiltration can lead to pathological inflammation of the joint. Importantly, cells of mesenchymal or haematopoietic origin use distinct modes of migration and thus might respond differently to similar biological cues and microenvironments. Furthermore, cell migration in the physiological microenvironment of musculoskeletal tissues differs considerably from migration in vitro. This Review addresses the complexities of cell migration in fibrous connective tissues from three separate but interdependent perspectives: physiology (including the cellular and extracellular factors affecting 3D cell migration), pathophysiology (cell migration in the context of synovial joint autoimmune disease and injury) and tissue engineering (cell migration in engineered biomaterials). Improved understanding of the fundamental mechanisms governing interstitial cell migration might lead to interventions that stop invasion processes that culminate in deleterious outcomes and/or that expedite migration to direct endogenous cell-mediated repair and regeneration of joint tissues.
Cell migration is critical for numerous physiological and pathophysiological processes, including embryogenesis, tissue morphogenesis, immune surveillance and inflammation, wound healing and cancer metastasis1. The efficacy and mode of migration are governed by a multifaceted set of biochemical and biophysical factors that are dependent on both cellular and extracellular matrix (ECM) properties. Although the mechanisms of migration have been studied extensively on planar substrates, these 2D systems might not reflect the in vivo environment, where most cells exist within a complex, interactive and a sometimes physically confining 3D matrix2–4. These characteristics introduce several additional factors that might affect cell locomotion, such as ECM composition, stiffness and structure. Cells can dynamically respond to these factors by adapting their shape, cytoplasmic or nuclear properties, actomyosin machinery and migration strategy5. Furthermore, cells are sensitive to mechanical and biochemical gradients in their microenvironment, which can potentiate motility and directed movement6,7.
Understanding the mechanisms that control cell migration in native tissue environments might provide important insights for the development of new strategies for treating immune-mediated disease or enhancing tissue repair and regeneration in synovial joints. In the first two sections of this Review, we independently consider the basic cellular and environmental factors that affect 3D migration in connective tissues. In the third section, we discuss factors that affect interstitial migration during rheumatic diseases, such as rheumatoid arthritis (RA) and osteoarthritis (OA), and dense connective tissue repair in the synovial joint. For example, signalling pathways that promote and sustain leukocyte and synovial cell migration might indirectly contribute to the destruction of intra-articular tissues and could be promising therapeutic targets. Conversely, damaged dense connective tissues might require interventions to enhance endogenous cell migration to expedite repair. Finally, current methods of modulating cell migration into biomaterial scaffolds are discussed with an emphasis on the implications of the material design of such scaffolds for musculoskeletal tissue engineering and regenerative medicine.
Cellular factors affecting migration
Interstitial migration involves the coordinated orchestration of various processes including cellular adhesion, dynamic rearrangement of the cytoskeleton, deformation of the cell body and its intracellular constituents and matrix remodelling (BOX 1). Furthermore, cells of mesenchymal origin (for example, fibroblasts) or haematopoietic origin (for example, leukocytes) migrate using different strategies (BOX 2).
Box 1 |. Mechanisms of cell migration.
Cell migration relies on an internal molecular assembly to generate force and motion. A net protrusive force generated by cytoskeletal contraction enables the cell to overcome the frictional and adhesive resistance of the surrounding environment and move forward20.
Integrin engagement with extracellular matrix (ECM) ligands results in the formation of focal adhesions, enabling the cell to generate traction
The assembly of filamentous actin (F-actin) from actin monomers (globular actin (G-actin)) results in the formation of actin-rich protrusions at the leading edge and cell polarization
Force on the focal adhesion activates the RHOA–RHO-associated protein kinase (ROCK) pathway, whose downstream effectors function to promote stress fibre formation and increase contractility by modulating non-muscle myosin II activity9
Contraction of the actomyosin cytoskeleton (stress fibres) at the leading edge produces tension between the leading and trailing edges, resulting in the detachment of adhesions and forward movement

Box 2 |. Modes of cell migration.
The mode of migration is classically based on cell morphology and is primarily dictated by the cell type. However, multiple cellular and extracellular factors interdependently determine the migration strategy of an individual cell5.
Mesenchymal movement, used by spindle-shaped cells with stiff nuclei, (such as fibroblasts), is associated with a slow migration speed, is dependent on focal adhesions and contractile stress fibres and generates a high traction force
Amoeboid movement, used by ellipsoid-shaped cells with highly deformable nuclei, (such as leukocytes), is associated with a rapid migration speed, involves transient adhesion and low contractility and generates a low traction force
Alternative migration mechanisms include the nuclear piston16 and water permeation (osmotic engine) models17

ECM, extracellular matrix. Part of this figure has been adapted from REF.25.
Cell adhesion and mechanotransduction.
Cell adhesion to the ECM occurs when transmembrane receptors such as integrins engage with ECM components. Integrins are a family of heterodimeric transmembrane receptors that consist of α and β subunits, which bind to various ligands in the ECM and can function as both mechanosensors (BOX 3) and bi-directional signalling receptors8. When integrins bind to their respective ligands, various structural and signalling molecules are recruited to the cell membrane to form focal adhesions that join with actin filaments to mechanically link the ECM and cytoskeleton (BOX 1). Focal adhesions anchor the cell to its environment, enabling the transmission of mechanical forces from the ECM to the cell. Activation of the RHOA-RHO-associated protein kinase (ROCK) pathway facilitates the formation of stress fibre bundles and modulates myosin motor activity, leading to stress fibre contraction (via the sliding of non-muscle myosin II and the actin filaments) to increase cytoskeletal tension9. This tension is transmitted to the ECM to pull the cell forward. In addition, stress fibre contraction can reinforce focal adhesions by recruiting proteins such as vinculin, which anchors actin filaments to integrins. Blocking integrin-mediated adhesion or ROCK-mediated phosphorylation of the downstream effector myosin light chain reduces migration speed in a dose-dependent manner10. Indeed, the actomyosin machinery is so important for embryonic development that total knockout of proteins related to the actin cytoskeleton (such as actin or myosin II), integrins (such as either the α or β subunit) or focal adhesions (such as vinculin, paxillin, talin or focal adhesion kinase) results in embryonic or early postnatal death11.
Box 3 |. Cell mechanotransduction.
Integrins enable cells to sense and respond to the physical forces transmitted by the extracellular matrix in a process known as mechanotransduction9
Cells in stiff microenvironments exhibit more stress fibre formation and generate greater contractile forces than cells in soft microenvironments
Cellular contractile forces feedback to reinforce focal adhesions, further activating downstream mechanotransduction pathways such as the RHOA-RHO-associated protein kinase (ROCK) pathway

ECM, extracellular matrix; F-actin, filamentous actin; G-actin, globular actin.
In contrast to mesenchymal cells, leukocytes such as neutrophils, T cells, B cells, monocytes and dendritic cells can also use amoeboid locomotion, which is characterized by a high migration speed, diffuse cytoskeletal organization and minimal interaction with the surrounding substrate12,13. Cells employing amoeboid motility lack discrete focal adhesions and instead utilize low-affinity binding to form transient adhesions12,13. Indeed, the migration velocity of CD4+ T cells is largely independent of β1 integrin-mediated adhesion and focal adhesion kinase, molecules that are important for the formation of focal adhesions13. Unlike the focal contact-dependent, adhesive migration mode of mesenchymal fibroblast-like cells, the amoeboid migration strategy enables immune cells to quickly adapt to different microenvironments to reach the site of inflammation. Interestingly, mesenchymal cells can transition to using the amoeboid migration strategy in states of low cell adhesion14 or high cortical contractility15, potentially facilitating the rapid migration of mesenchymal cells during embryogenesis and/or cancer metastasis. In an alternative model of cell propulsion, the nucleus might act as a piston to compartmentally increase hydrostatic pressure, pushing the cell forward16. Similarly, localized water permeation can lead to cell movement, even in the absence of cytoskeletal contraction17.
Cell-mediated matrix degradation.
The native ECM provides shape and structure to tissues, offers binding sites for cells and growth factors and regulates cell behaviour, intercellular communication and mechanical load transmission18. Interstitial migration through dense or impenetrable matrices is often made possible by cell-produced matrix metalloproteinases (MMPs), which cleave ECM molecules at specific peptide sequences to generate gaps that are wide enough for the cell to pass through19. Cells can remodel the matrix by contact-dependent, membrane-bound MMPs or by the secretion of MMPs into the pericellular space20. Localization of MMPs to the cell surface, which is common for MMP2, MMP9 and membrane-type MMPs, restricts proteolysis to the cell periphery such that tube-like trails are generated behind the migrating cell21. By contrast, the secretion of other MMPs results in a diffuse proteolysis that reduces biophysical matrix resistance at distances beyond the cell membrane, functioning to soften the tissue around pre-existing gaps in the ECM to facilitate cell deformation during passage. This method is commonly used in large-scale tissue remodelling events, such as morphogenesis and wound healing, although dysregulated overexpression of matrix-degrading enzymes can lead to the catabolic breakdown of articular cartilage in RA and OA22.
Nuclear mechanics.
In confined passages, cells must physically deform to move forward. Although cells can rapidly remodel their cytoskeleton, the nucleus is the rate-limiting organelle in cell migration because of its large size and stiffness, the latter of which is 2–4 times higher than the surrounding cytoplasm23. When the nuclear cross-sectional area is >4-fold the area of the constriction, cells stall, and the overall migration speed considerably declines24,25. The nucleus can reduce in diameter to 10% of its original cross-sectional area10, with some cells achieving a minimal diameter of 3 μm26. Indeed, cell translocation is severely limited when the constriction area is <25 μm2 (REFS10,26–28). This limitation is partly a function of the chromatin structure, such that a high degree of chromatin condensation reduces nuclear deformability25,29. Of equal importance in determining nuclear deformability are lamins, which are type V intermediate filament proteins that provide structure and stability to the nuclear envelope; in particular, lamins A and C (lamin A/C) are major contributors to nuclear mechanics in cells of mesenchymal origin30. Hence, the nuclear deformability of these cells can be modulated by controlling the expression of lamin A/C. For example, the overexpression of lamin A hinders the migratory capacity of a cell24,28,31, whereas knockdown of lamin A expression increases 3D cell migration through small pores27,32. However, lamin depletion can also lead to stress-induced cell death, whereby the act of squeezing the nucleus through a narrow (≤3 μm wide) constriction results in nuclear envelope rupture and DNA damage26,27. In tumour cells, nuclear confinement can also trigger a nuclear–cytoskeletal feedback mechanism that ultimately leads to pericellular proteolysis to widen the small pores in the ECM ahead of the cell33.
Although cells with stiff nuclei have limited mobility inside dense collagen gels, cells with compliant nuclei with low levels of lamin A/C remain highly mobile10,25,28. Some leukocytes, such as mononuclear CD4+ T lymphoblasts and polynuclear neutrophils, can navigate through small interstitial spaces (pores ranging from 2 μm2 to 5 μm2) in collagen lattices by deforming the nucleus to match the pore size of the matrix10. By comparison, nuclear deformation is more difficult, and passage through in vitro microfluidic constrictions is slower for leukocytes that contain higher levels of lamin A/C, such as macrophages34,35. In addition, the lobulated shape of the neutrophil nucleus enables reversible geometric changes, such as compact configurations or pearl chainlike unfolding, permitting further nuclear flexibility5. Although the nucleus might physically hinder mobility, removing the nucleus greatly reduces the ability of the cell to generate traction stress and consequently migration speed, indicating that the nucleus is an integral component of the cellular migration machinery36.
Environmental factors affecting migration
Within the complex 3D environment of connective tissues, cells must translate extracellular stimuli into intracellular signals that ultimately affect downstream behaviours, including migration. Insoluble and soluble biochemical cues, such as adhesive and chemotactic signals, are essential for cell homing and directional migration, as in the case of immune cell infiltration. Biophysical properties, such as matrix micromechanics and microstructure, affect dense connective tissue function, injury and repair.
Biochemical cues.
The ECM contains an array of components (BOX 4) including insoluble signalling molecules. Of the insoluble signalling molecules, the most important for mesenchymal migration are adhesive ligands found on matrix molecules such as fibronectin and collagens; integrins on the cell surface bind to ligands in these molecules via adhesive peptide motifs, which enables direct coupling of the cytoskeleton to the environment. In general, cell adhesion and contractility are higher and 3D cell migration speed is lower in the ECMs of high adhesive ligand density than in the ECMs of intermediate adhesive ligand density24,37. Conversely, too low an adhesive ligand density in the ECM promotes cell detachment and hinders contractile force generation, leading to even slower migration by mesenchymal cells. The most prevalent adhesive peptide motif in the ECM is arginine–glycine–aspartic acid (RGD)8. However, damaged ECM molecules can also expose previously hidden epitopes. For example, proteolytic degradation of collagen exposes RGD sequences that can bind integrins38. Similarly, fragmented fibronectin in degenerated cartilage might bind to integrins on chondrocytes and synovial fibroblasts, resulting in upregulated production of pro-inflammatory cytokines (for example, IL-1, IL-6 and TNF) and various MMPs39. Indeed, the increased levels of expression of adhesive ligands and integrins during RA might contribute to enhanced adhesion and infiltration of immune and synovial cells38.
Box 4 |. Extracellular matrix components.
Structural components in the extracellular matrix (ECM) of joint tissues are important for load bearing on the macroscale and for cell migration on the microscale. Although this structure-function relationship is essential for resisting cyclic loads within the musculoskeletal system, the microenvironment of connective tissues, especially dense regular connective tissues, might pose a challenging barrier to migrating cells.
Collagen fibres: the mechanical properties of dense connective tissues are dictated by the concentration and organization of their ECM (primarily type I collagen, although type II collagen is also prevalent in articular cartilage and the inner region of the meniscus), such that the density and degree of alignment of collagen fibres correlate with increased tensile strength but decreased pore size that can impede migration47, 106.
Glycosaminoglycans (such as chondroitin sulfate) and proteoglycans (such as aggrecan): these molecules contain hydrophilic polysaccharides with a high density of negative charges, resulting in osmotic swelling of the tissue that imparts resistance to compressive forces along with impediments to migration at the microscale8.
Small leucine-rich proteoglycans (such as decorin and biglycan): these proteoglycans regulate collagen fibrillogenesis, assembly and organization and are important for growth factor sequestration41,42.
Glycoproteins (such as fibronectin): these proteins reinforce the structural ECM network and provide a connection between cells and the ECM by binding to cellular integrins8.
Matrix components also interact with soluble signalling molecules that are produced by cells and embedded within the matrix over the course of tissue formation, which can modulate the bioavailability and concentration gradients of the signalling molecules. For example, fibronectin and small leucine-rich proteoglycans (SLRPs) sequester a number of chemoattractive growth factors, including latent transforming growth factor-β1 (TGF β1), platelet-derived growth factor (PDGF), insulin-like growth factor (IGF) and fibroblast growth factor 2 (FGF2)40–42. In addition, SLRPs can immobilize the pro-inflammatory cytokine TNF43. The rapid release of soluble factors from the ECM during matrix degradation, inflammation and/or injury might induce chemotaxis44. Directional sensing is accomplished via binding of the soluble chemoattractant to cell surface receptors on one side of the cell, which activates signalling pathways that promote cell polarization and the formation of protrusions, adhesions and contractile forces in a particular direction. Cells might also preferentially migrate towards a matrix-bound gradient (for example, fibronectin45) in a phenomenon known as haptotaxis. In this manner, both soluble and insoluble biochemical signals in the ECM can modulate cell adhesion, motility and recruitment towards a target location and are especially important for directing collective migration during embryonic development7,11,44. Chemotaxis is critical for immune system function, especially for leukocyte recruitment to the site of disease or injury. Pro-inflammatory cytokines, including IL-1, IL-6 and TNF, activate specific cell populations (for example, neutrophils, monocytes and/or macrophages, T cells, B cells and fibroblasts), which in turn produce chemokines to recruit additional cells. For example, in response to microbial infection, the upregulation of TNF induces chemokine expression and migration by lympho-cytes46. Unfortunately, autoimmune dysregulation might lead to undesirable accumulation of immune cells in otherwise healthy tissues. Indeed, migration of leukocytes into the synovium is an important contributor to the pathogenesis and persistence of RA and OA. Likewise, overstimulation of the foreign body response by a biomaterial scaffold might promote colonization by immune cells rather than regenerative cell types, leading to implant failure. Therefore, immune cell migration could be an important therapeutic target for both chronic inflammatory diseases and tissue engineering applications.
Matrix micromechanics.
The biochemical composition and organization of ECM molecules in different tissues are linked to the biological and mechanical functions of the tissue. For example, joint tissues range from thin, loose vascular connective tissue lining the intra-articular space that serves as a host for cells (such as the synovium and fat pad) to dense irregular connective tissue surrounding the joint that functions as a structural element (such as the fibrous capsule) to dense regular connective tissues with highly organized collagen fibres that are designed to withstand mechanical stress (such as cartilage, menisci, tendons and ligaments) (FIG. 1). The mechanical properties (such as the tensile modulus, compressive modulus and shear modulus) of these tissues vary because of the heterogeneity and hierarchical nature of the tissue building blocks. In general, increasing the concentration, density and/or degree of alignment of collagen increases the load-bearing capacity of a tissue and results in higher ECM mechanical properties (that is, the Young’s modulus)47,48. Because of the heterogeneity in tissue mechanical properties, more homogeneous collagen-based hydrogels or synthetic hydrogels have most often been used to assess cell migration in different 3D microenvironments.
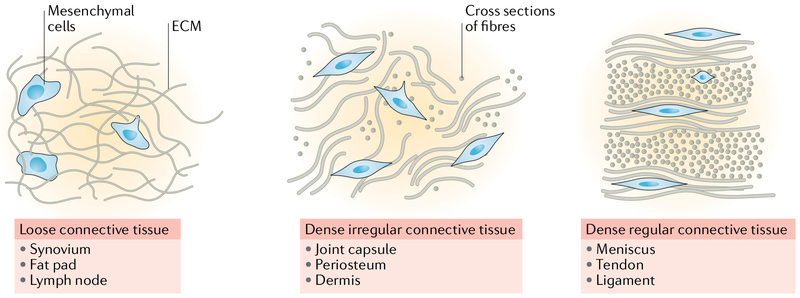
Fig. 1 |. Connective tissue type and migration capacity.
Fibrous tissues can be grouped into three major categories: loose connective tissue, dense irregular connective tissue and dense regular connective tissue. Steric barriers to migration increase with matrix density and organization, such that mesenchymal cell mobility becomes severely restricted in dense regular connective tissues. ECM, extracellular matrix.
Cells can sense the matrix mechanical properties of their surrounding environment via integrin-mediated adhesions. In stiff environments, integrin-mediated signalling results in the generation of high traction forces by the actomyosin contractile machinery (BOX 3), which might promote cell migration49. However, if the matrix stiffness is too high, cells cannot deform the surrounding ECM and so cannot pass through the confined spaces in the microenvironment (for example, between collagen fibres). Cells can partly overcome the steric hindrance of stiff environments by cell body deformation and/or by degrading the surrounding matrix via MMP secretion. Similar to 2D cell migration50, a bimodal relationship exists between matrix stiffness and 3D cell migration, such that maximal migration speed is achieved in environments of intermediate stiffness37,51–53, although the exact level of stiffness required for maximal speed is affected by the level of cell–ECM adhesion37,51 and matrix pore size52,53. Nonetheless, decoupling the effect of ECM stiffness on cell migration from that of pore size and/or adhesivity is difficult given that hydrogel mechanics are often directly related to matrix density (and in the case of ECM-derived materials, adhesive ligand concentration). Moreover, as the collagen concentration, stiffness and fibril organization of most gels used in 3D assays are extremely low (~100 times lower concentration than that of dense connective tissues47), to what extent these findings apply to migration in native dense connective tissues requires further investigation.
Matrix microstructure.
The microstructure of connective tissues is dependent on the composition of the matrix (primarily collagen composition), fibre alignment and inter-fibre porosity and pore size. Cells in a loose ECM with large pores are generally round in shape, whereas cells in a dense ECM with small pores are elongated and spindle-shaped and hence have a reduced cell diameter47. The physical properties of the ECM might affect cell migration. For example, in one study, cell migration speed through collagen gels decreased with pore size, an effect that was accentuated with MMP inhibition10. Even a high level of cell-mediated matrix deformation (50%) cannot compensate for a decrease in pore size below a certain limit54. The rate of cells translocating through nondegradable microporous membranes decreases with pore size10. Together, these results suggest that pore size, and not stiffness, is the true limiting physical factor. As with ECM stiffness, a bimodal relationship exists between pore size and cell migration speed24,37,52–55, although this relationship also depends on the concentration of adhesive ligands24,53,55. As contact guidance is diminished in wide channels, migration is optimal in environments with pore diameters that match or are slightly less than the diameter of migrating cells5. Migrating cells can also adapt to the spatial confinement of their environment by travelling along the path of least resistance5. For example, T cells avoid areas of dense ECM and preferentially travel along fibrillar strands12. Similarly, in collagen gels, cells preferentially align and migrate along fibres, and migration speed increases with collagen alignment47,56–58. This directional migration is mediated by RHOA–ROCK-mediated contractility, which preferentially aligns new cellular protrusions along the fibre length, increasing migration persistence57. Conversely, migration perpendicular to the fibre direction is slower than migration parallel with the fibre59,60. Thus, given the fibrous, interconnected nature of most ECMs, contact guidance has a central role in interstitial migration.
Migration in joint disease and repair
For diseases such as RA and OA, immune cell infiltration is a pathological process stimulated by biochemical cues such as pro-inflammatory cytokines and chemokines (FIG. 2). In fibrous tissue repair, the migration of reparative cells to the wound site is hindered by biophysical barriers of the ECM. Thus, treating these conditions will require vastly different strategies. In the following section, we explore cell migration in the context of immune-mediated joint disease and fibrous tissue repair, with a focus on the environmental factors that affect cell migration.
Fig. 2 |. Cell migration during joint development, disease and repair.
Cell migration is important during development, repair in response to injury and disease of various tissues in the knee joint. Meniscus progenitor cells migrate through loose mesenchyme along chemotactic gradients during tissue morphogenesis. After a meniscal tear, mature meniscal cells migrate through dense, aligned collagen fibres to initiate repair at the wound site. In rheumatoid arthritis (RA), immune cells enter via the blood vessels and cross the endothelium to invade the synovium in response to inflammatory cytokines. Migrating cells secrete matrix-degrading enzymes to facilitate passage, but uncontrolled enzyme production in RA might damage intra-articular tissues. ECM, extracellular matrix.
Immune-mediated joint disease.
Synovial joints are affected by several immune-mediated disorders, the most common being RA and OA. Although the mechanisms of disease progression differ considerably, both RA and OA are chronic inflammatory joint diseases that result in severe joint swelling, pain and reduced mobility, with cartilage and/or bony destruction at end-stage disease. The central site of inflammation for both diseases is the synovium, which includes a cellular surface layer of macrophages and fibroblast-like synoviocytes (the synovial intima) and an underlying tissue layer that contains fibroblasts, blood vessels and lymphatics arrayed within a loose collagenous matrix (the synovial subintima)61. The initial stages of immune-mediated joint diseases are characterized by the influx of immune cells, including macrophages, neutrophils, B cells and T cells, into the synovial compartment62–64. Local activation of these cells in the synovial vasculature enables their transendothelial migration into inflamed tissues, with leukocyte accumulation further potentiated by the upregulation of pro-inflammatory cytokines, notably IL-1, IL-6, TNF and IFNγ, and various chemokines (CXCL8, CXCL10, CXCL11, CXCL12, CCL2, CCL3, CCL5, CCL19 and CCL21) in the synovium46,62,65–67. Blocking the inflammatory cascade with TNF inhibitors is a highly successful treatment for RA, but partial or non-responsiveness to this treatment, and even loss of treatment efficacy over time, is an issue for some patients46. Furthermore, various chemokines are thought to regulate immune cell infiltration and retention in the synovium68, as well as drive the production of inflammatory mediators in other cell populations. Thus, researchers have targeted immune cell migration and infiltration through chemokine inhibition as a potential RA therapy. Efforts to antagonize specific chemokines, such as CXCL8, CCL2 and CCL5, had promising results in mouse models of inflammatory disease, but so far, such therapies have failed in clinical trials67. The cell surface expression of specific chemokine receptors on these immune cells might change with treatment, rendering the drug ineffective over time69. Furthermore, immune cells might express a variety of different chemokine receptors and respond to multiple chemokines, and hence, a combination of these chemokine antagonists might be required. As such, a better understanding of the mechanisms and functions of signalling pathways that regulate immune cell trafficking, recruitment and invasion is necessary for the development of effective therapeutics that target this aspect of rheumatic disease.
Inflammatory mediators of the arthritic joint environment also activate resident synovial fibroblasts, which secrete pro-inflammatory cytokines and other factors that contribute to tissue degradation70. For example, TNF and IL-18 (secreted by leukocytes) stimulate the production of IL-1 (REF46), and CXCL12 and CCL2 (chemokines involved in leukocyte homing)71, respectively, by synovial fibroblasts. Furthermore, exposure of synovial fibroblasts to IL-18, CCL19 and CCL21 increases the secretion of vascular endothelial growth factor (VEGF), which might promote angiogenesis in the synovial tissue and exacerbate immune infiltration71,72. Synovial fibroblasts also respond to the chemokines CXCL12 and CCL2, which promote their proliferation and migration73, as well as their production of IL-6 and IL-8 (REF74). An enhanced proliferative and migratory state of synovial fibroblasts in RA might result in synovial hyperplasia and synovial cell infiltration into adjacent intra-articular tissues as well as the formation of a fibrovascular pannus that damages the articular surface. The highly invasive nature of synovial fibroblasts correlates with their expression of growth factors75 and proteases, including MMP1, MMP3 (also known as stromelysin-1) and MMP10 (also known as stromelysin-2)76 and a disintegrin and metalloproteinase domain-containing protein 10 (ADAM10)77, indicating that cell-mediated matrix degradation might be an important facilitator of synovial cell migration. By-products of matrix degradation, including ECM components and matrix-bound growth factors (such as TGFβ), increase synovial fibroblast adhesion to cartilage, further enabling cell migration across the joint surface75. Notably, the degree of synovial cell invasiveness correlates with the severity of joint destruction in RA78. Such a pro-inflammatory state might also directly contribute to bone resorption and heterotopic ossification that occurs with osteophyte formation in ankylosing spondylitis (AS)79. For instance, IL-22, a cytokine that is increased in patients with inflammatory arthritis or AS, promotes the proliferation, migration and osteogenic differentiation of mesenchymal stem cells (MSCs)79,80. High levels of CCL19 and CCL21 induce expression of bone markers in fibroblasts81 and promote the migration and bone resorption activity of osteoclasts82. Exposed to this chronically inflamed environment, other intra-articular tissues are at risk of degeneration in joint diseases, such as the articular cartilage83, meniscus83–85, tendons of the rotator cuff (shoulder)86 and stabilizing ligaments of the upper cervical spine87,88. Therefore, an additional target for treating joints affected by rheumatic diseases might involve fibrous tissue repair.
Fibrous tissue repair.
Understanding fibrous tissue repair is important for considering how to regenerate tissues of the joint that have been damaged owing to trauma or secondary to immune-mediated disease. In the case of an acute traumatic injury, effective migration is critical for the initial inflammatory phase (hours to days), during which immune cells clear dead cells, pathogens and debris from the site of injury. Effective migration is also important for the later proliferative phase, wherein reparative cells actively divide and migrate into the wound bed to deposit a disorganized provisional matrix tissue and promote wound contraction (days to weeks)89. This stage is followed by a period of matrix remodelling (weeks to months), during which the tissue is organized into mature, crosslinked type I collagen fibres. In most adult tissues, this newly formed tissue does not match the native tissue in terms of the matrix content or mechanics but rather represents scar tissue. In the case of repairing an immune-mediated or degenerative injury, overcoming the pro-inflammatory environment is crucial, as cytokines such as IL-1 and TNF might inhibit matrix synthesis and tissue repair90,91.
Although tissues with a well-established vascular supply might partly depend on circulating progenitor cells to promote repair mechanisms, most dense connective tissues in the mature state are hypovascular92,93 and thus must rely on cells intrinsic to the tissue and/or from nearby extrinsic sources to regulate repair (FIG. 2). For example, in tendon injuries, both tenocytes from the endotenon and epitenon and fibroblasts from the tendon sheath and synovium contribute to the proliferative and remodelling phases94. Although intrinsic repair by native tenocytes results in superior functional outcomes, extrinsic cells often dominate the repair process, leading to scar tissue and adhesion formation95,96. Cells derived from the peritenon migrate more quickly than those derived from the tendon core, suggesting that an enhanced ability to reach the wound site might increase the propensity of a cell for extrinsic repair97. This concept suggests that regulation of the ability of endogenous and extrinsic cells to reach the wound interface could be a potential therapeutic approach. In addition to endogenous primary cells, the adult knee meniscus harbours a stem cell-like population that is capable of migration and fibrochondrogenesis in vitro98,99. However, degenerative tears in the inner avascular meniscus of the knee remain hypocellular and fail to self-repair in the long term100. Although a thin fibrovascular scar, likely produced by migrating synovial fibroblasts101, might eventually physically bridge the wound gap in the meniscus, this type of scar is mechanically inferior to native tissue and prone to re-injury102. Therefore, strategies that enhance the interstitial migration and proliferation of resident differentiated cells and/or tissue-specific progenitors are needed.
Several age-related factors might exacerbate healing of adult joint tissues. During development, the collagen concentration and degree of collagen alignment in the ECM increase with load-bearing use of the joint, whereas endogenous cell density declines103–105, resulting in higher mechanical properties (such as the Young’s modulus) on the microscale47,105,106 and at bulk tissue level107. The compressive forces on the inner meniscus that occur during normal joint load bearing also lead to substantial accumulation of proteoglycans, most notably of aggrecan, with age107–109. The dense network of aligned collagen bundles of the mature meniscal ECM, coupled with a highly pressurized, proteoglycan-rich inner zone, probably prevents a sufficient population of endogenous progenitor cells from migrating to an injury site to initiate repair110. As the ECM becomes stiffer and denser, cells become deformed (elongated and flattened) within the narrow spaces between adjacent collagen bundles96. In addition to reducing ECM porosity and pore size, the densely packed collagen might also increase the concentration of adhesive ligands, resulting in overly strong adhesive and contractile forces that impede forward movement. To study this phenomenon, investigators have developed an ex vivo platform to examine interstitial cell migration through native meniscal tissue47,111. Results using this platform indicate that the micromechanics and microstructure of the adult meniscus ECM sterically hinder meniscal cell mobility47 and that modulation of these ECM attributes via an exogenous matrix-degrading enzyme permits cell migration through this otherwise impenetrable network111,112. Similarly, enzymatic digestion of proteoglycans on the surface of defects in articular cartilage transiently enhanced the areas that could be reached by endogenous repair cells (probably chondrocytes from the surface zone) in a rabbit model113. Thus, by addressing the inherent limitations to repair imposed by the mature ECM, these studies might define new clinical strategies to promote repair of damaged dense connective tissues in adults.
Migration in engineered materials
Tissue engineering is a rapidly growing field in which cells, scaffolds and biochemical and/or mechanical signals are used to generate functional tissue replacements as a therapeutic approach to restore irreversibly damaged intra-articular tissues. Biomaterial scaffolds can provide 3D templates for tissue regeneration, directing cell ingress, proliferation and differentiation into a phenotype that culminates in the formation of a neo-tissue. Achieving sufficient cell density and a homogeneous distribution within the scaffold is a challenging but vital step towards this goal114–116. Similarly, attracting the correct cell type (for example, endogenous progenitor cells) while restricting immune cell infiltration might be required for long-term implant survival. In the remainder of this section, we focus on techniques that can enhance cell migration into scaffolds, either after cell seeding in vitro or implantation in vivo, by modulating biochemical and biophysical cues (BOX 5; FIG. 3). Furthermore, biomaterial-mediated strategies to modulate the immune system are explored.
Box 5 |. Considerations for scaffold design in tissue engineering.
An appropriate scaffold design is critical for engineering dense connective tissues. Specifically, scaffolds need to support tissue function and organization.
Functional support: the scaffold should resist cyclic tensile, compressive and/or shear forces. The mechanical properties of acellular constructs are determined by the intrinsic material properties of the polymer, as well as the fibre geometry and organization within the scaffold. For example, the uniaxial tensile strength of a scaffold is increased when fibres are deposited parallel to the loading direction, as it is in native tissue141.
Organizational support: the scaffold should provide an instructive macrostructure and microstructure that fosters organized matrix deposition and maturation. Macroscopically, the scaffold shape dictates the boundaries of tissue formation, whereas microscopically, the scaffold structural framework controls cell ingress, neo-tissue organization and nutrient diffusion.
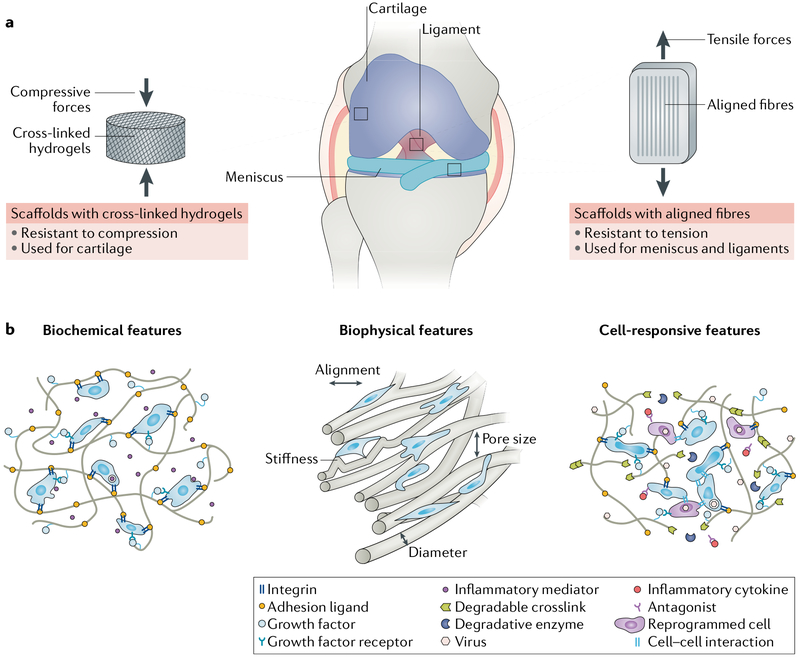
Fig. 3 |. Designing engineered scaffolds to promote cell migration and tissue repair.
a | Engineered replacements for intra-articular tissues require cells and scaffolds to withstand compressive and/or or tensile forces during joint loading. As such, crosslinked hydrogels (which resist compression) and aligned fibres (which resist tension) are often used for tissue engineering of cartilage and dense regular connective tissues such as meniscus and ligament, respectively. b | For all scaffolds, successful formation of functional tissues is achieved in part by considering the design of the biomaterial (such as the biochemical and biophysical aspects) to control cell behaviours such as adhesion, migration and differentiation. Furthermore, localized delivery of anti-inflammatory molecules might reduce the foreign body response and promote matrix synthesis by cells within the scaffold. To fine-tune the cellular response, biomaterials can be further modified to enable in situ cell reprogramming and/or cell-mediated scaffold remodelling (cell-responsive scaffolds).
Biochemical cues.
Cellular sensing of the biochemical and mechanical microenvironment depends on the ability of the cell to adhere to and exert forces on its surroundings. Ligands present within the microenvironment enable cells to adhere to and probe the microenvironment and can initiate signalling cascades, the strength and types of which are dependent on the ligand type and concentration. Small oligopeptide sequences within ECM proteins (such as RGD) can be conjugated to the backbone molecules of a scaffold and can function as adhesive ligands within the 3D matrix, as well as generate haptotactic gradients to encourage cell infiltration117. Chemoattractants can also be incorporated into the biomaterial scaffold to affect directional cell movement. Cells isolated from the meniscus118, tendon119 and ligament120 can migrate towards a wide variety of soluble chemical gradients, including PDGF-AB and PDGF-BB118–120, hepatocyte growth factor (HGF)118,120, bone morphogenetic protein 2 (BMP2)118,120 and IL-1 (REF.118). Additionally, meniscal progenitors98 and MSCs121 home towards CXCL12. Although immobilized chemoattractants can increase cell infiltration into scaffolds122, this method limits gradient sensing to cells that are already in contact with the scaffold. By contrast, biomaterial-mediated delivery of soluble chemoattractants into the surrounding tissue can affect cells farther afield. The large surface area-to-volume ratio of scaffolds that have nanoscale features is particularly well suited for providing an initial burst delivery of incorporated biomolecules followed by sustained release as the material degrades over time (as the release of such molecules is proportional to the surface area). For instance, in one study, interstitial migration of meniscal cells into nanofibrous scaffolds was higher in scaffolds engineered to release PDGF-AB than in scaffolds containing no PDGF-AB111; in such scaffolds, PDGF-AB was delivered in a sustained fashion over a course of 6 weeks with degradation of the hyaluronic acid (HA) nanofibres to improve in vivo migration.
Once the appropriate cells have colonized the scaffold, the long-term therapeutic success of engineered tissues is dictated by the host immune response123. Although infiltration by endogenous reparative cells is beneficial for the formation of tissue that matches the native tissue, infiltration by inflammatory cells often results in fibrosis or immune rejection. To promote regenerative tissue microenvironments, the emerging field of immunoengineering124 seeks to design scaffolds that can affect cells of the immune system, such as dendritic cells, macrophages, B cells and T cells. For example, by conjugating protein antigens to the scaffold, these antigens can be delivered to T cells to tune their tolerance, memory and cytotoxic response125–127. Materials can also be designed to release immunomodulatory cytokines (for example, IL-4 and IL-10) to drive macrophage polarization towards the pro-healing M2 phenotype128–130. A promising approach is the combined delivery of anabolic growth factors and anti-inflammatory molecules, which is more effective for tissue regeneration than single factor delivery131,132. These techniques could be applied for the treatment of rheumatic diseases, where biomaterial-mediated release of immunomodulatory factors could prevent the infiltration and retention of leukocytes in the joint.
Biophysical cues.
Systems that enable simultaneous control of the scaffold micromechanics, microstructure and adhesivity have gained considerable interest in tissue engineering. An attractive strategy for engineering tissues is to use scaffolds that are based on crosslinkable hydrogels, in which synthetic polymers, such as polyethylene glycol (PEG), and natural polysaccharides, such as HA, are modified with functional groups to form hydrophilic crosslinked networks that are stable in physiological environments133. The mechanical properties of the scaffold (such as the tensile, compressive and shear moduli) depend on how many modifications are present along the polymer backbone and the concentration of the polymer chains, both of which regulate how many crosslinks can form. Hydrogel-based scaffolds are commonly used for cartilage tissue engineering, where resistance to compressive loading is critical. By contrast, fibre-based scaffolds are better suited to recapitulate the structural and mechanical properties of dense regular connective tissues such as meniscus and ligaments, which are primarily subjected to tensile forces. The final microstructure of a scaffold can be engineered using micromoulding134, photolithography37,135, electrospinning135,136 or rapid prototyping137.
A major objective of musculoskeletal tissue engineering is to recapitulate the bulk mechanical properties of native tissues (for example, Young’s modulus), which can be up to several hundred MPa along the primary collagen fibre direction138–140. A widely used method for constructing biomimetic scaffolds is electrospinning, a technique that utilizes an electrical potential difference to draw polymers into highly aligned nanofibrous networks (fibre networks in which the fibres are less than a micrometre in diameter). The resulting material can replicate the organization of dense connective tissues that have bulk Young’s moduli typically in the MPa range (such as the meniscus)141. Cells sense and respond to microscale and nanoscale topography, such as ridges, grooves and channels142,143. Anisotropic features, such as those provided by aligned electrospun fibres, induce cell polarization and de novo collagen alignment along the fibre direction, leading to a higher tensile modulus than in non-aligned scaffolds144,145.
Although these organized scaffolds promote ordered matrix deposition, densely packed nanofibres could present a formidable physical barrier to cell ingress116,144, limiting matrix accumulation in the interior of the scaffold as well as integration of the scaffold with native tissue146. Additionally, although scaffold alignment might increase migration speed and persistence along the fibre length58,60, contact guidance also hinders migration perpendicular to the fibre axis59. Introducing porogens such as salt particles147, ice crystals148 or fibres with faster degradative rates116,149,150, can increase porosity and cell infiltration. For example, a composite scaffold of aligned, slow-degrading polycaprolactone fibres can be interspersed with water-soluble polyethylene oxide (PEO) fibres116. Rapid removal of the ‘sacrificial’ PEO fraction via aqueous hydration generates a highly porous scaffold that after long-term cell culture results in increased collagen content (via cell-mediated matrix deposition) and tensile properties151. Furthermore, high porosity scaffolds enable more cell infiltration than scaffolds without PEO, which improves the integration of these scaffolds with the surrounding tissue111,146. Alternatively, instead of removing scaffold components, adding an interpenetrating collection of fibres152–154 can provide vertical avenues for cellular ingress into the scaffold.
3D bioprinting, a technique in which bioinks are deposited layer by layer in a precise manner, can build biomimetic systems that recapitulate the structural heterogeneity of native joint tissues155. Similar to electrospinning, a sacrificial material can be included to encourage cell invasion into void channels156. By adjusting the mechanical, biochemical and adhesive properties of the bioink material157,158 and the deposition patterns at the microscale, 3D bioprinting enables greater spatial control than electrospinning. Cells and biomolecules can also be incorporated into bioinks before printing to generate bioactive regions where factors are locally released to control cells within the construct. Bioprinted matrices have been used to study cell migration in the context of cancer metastasis159 and to construct complex musculoskeletal tissues with zonal architecture, such as articular cartilage155.
Cell-responsive scaffolds.
Integrating cues that permit enzymatic and/or mechanical remodelling of the microenvironment might further expedite cell infiltration of dense, stiff scaffolds (FIG. 3). For example, a common approach is to introduce hydrolysable and/or MMP-sensitive moieties into the polymer backbone or crosslinker111,160–163. Cell secretion of proteolytic enzymes results in localized polymer degradation over time, softening the pericellular environment to enable cell mobility164,165. For example, cell migration is faster in MMP-degradable PEG hydrogels than in MMP-insensitive hydrogels, a difference that is further accentuated in gels of high stiffness161. As such, the presence of MMP-cleavable linkages improves cell infiltration into stiff hydrogels in vivo161. In addition to promoting migration, cell-mediated remodelling encourages cell proliferation162 and matrix synthesis166 in stiff environments. Additional modulations to the scaffold that consider cell mechanosensing after ingress into scaffolds might also be used to direct cells towards the appropriate lineage. For instance, although RGD ligands promote MSC adhesion and migration, prolonged adhesion might inhibit chondrogenic differentiation in the long term. Some developed hydrogels contain MMP-degradable sequences combined with RGD peptides, in which cells can self-regulate the amount of adhesive ligands in the environment to support a chondrogenic phenotype167. An alternative strategy for promoting cell-mediated remodelling of the microenvironment is to adjust the viscoelastic properties of the material, such that stress relaxation of the material occurs as the cells exert traction forces on it168. Notably, chondrocytes cultured in fast-relaxing gels produced more interconnected cartilage matrix and lower levels of inflammatory cytokines than those in slow-relaxing gels169.
Finally, endogenous cells must respond to dynamic environmental cues in a manner that maintains a pro-regenerative microenvironment. Advances in genome engineering, particularly the CRISPR–Cas9 genome editing system, have made it easier to reprogramme the intrinsic signalling pathways of cells. For example, mouse induced pluripotent stem cells can be genetically manipulated to not respond to cytokines such as IL-1 (REF.170) or to produce an IL-1 receptor antagonist or soluble TNF type 1 receptor in the presence of IL-1-mediated or TNF-mediated inflammation, generating a rapidly responsive and autoregulated system to modulate the inflammatory microenvironment171. This technology can be combined with virus-mediated gene delivery techniques to manipulate cells in situ in a spatially controlled manner, thereby promoting regenerative cell colonization while suppressing immune cell invasion172,173 (FIG. 3).
Conclusion
Cell migration has a central role in the initiation and/or maintenance of joint disease, repair and biomaterial-mediated regeneration. Interstitial migration is governed by a complex set of cellular and extracellular variables, including cell mechanics and force transduction, biochemical cues and ECM properties. Importantly, several factors that potentiate immune-mediated disorders could be promising therapeutic targets and/or exploited to promote fibrous tissue repair and regeneration. For example, intra-articular injection of small molecules that inhibit chemotactic cell homing could reduce synovial inflammation, whereas localized release of chemoattractants might recruit reparative cells to the wound site or scaffold. Similarly, although excessive enzymatic activity is associated with cell infiltration and joint tissue destruction in a disease context, promoting matrix remodelling by modulating MMP activity and/or biomaterial degradability can enhance cell migration into dense tissues and scaffolds. By designing smart, dynamic scaffolds that reflect the optimal microenvironmental niche for tissue growth and maintenance over time, we might ultimately recapitulate, and perhaps even augment, the natural biological cues that direct tissue formation to advance joint repair and regeneration.
Key points.
Interstitial cell migration in the fibrous microenvironments of intra-articular tissues is regulated by biophysical and biochemical factors.
Immune cells are recruited to and retained within the synovium by inflammatory cytokines and chemokines in rheumatic disorders.
High matrix density and stiffness of adult dense connective tissues restrict the mobility of endogenous cells, impeding wound healing after injury.
Early cell migration into biomaterial scaffolds is a critical but challenging step towards engineering functional musculoskeletal tissues.
Targeted strategies that limit inflammatory cell invasion while promoting the migration of endogenous reparative cells might enhance joint tissue formation and regeneration.
Stress fibre.
Contractile bundles in non-muscle cells composed of actin filaments and non-muscle myosin II; myosin motor activity results in contraction of the actomyosin bundles.
Micromechanics.
The mechanical properties of a material assessed at a local level (that is, at the micrometre scale). This approach can identify heterogeneities in materials or tissues that are indicative of the constituent materials and their properties at that location.
Microstructure.
The microscopic structure of a material or tissue.
Chemotaxis.
Directional cell movement along a soluble biochemical gradient.
Haptotaxis.
Directional cell movement along a substrate-bound insoluble gradient.
Collective migration.
The process by which a group of cells move together while maintaining cell–cell contact.
Tensile modulus.
Young’s modulus of a material evaluated in tension (that is, a measurement of tensile strength, which is the ability of a material to withstand being stretched).
Compressive modulus.
Young’s modulus of a material evaluated in compression (that is, a measurement of compressive strength, which is the ability of a material to withstand being compressed).
Shear modulus.
Young’s modulus of a material evaluated in shear (that is, a measurement of the shear strength, which is the ability of a material to withstand forces that can cause the internal structure of the material to slide against itself).
Young’s modulus.
A mechanical property that defines the relationship between stress (force per unit area) and strain (proportional deformation) of a linearly elastic material during uniaxial deformation (also referred to as the elastic modulus; measured in MPa). Although commonly referred to as tissue stiffness or rigidity, these two terms are actually structural properties (that is, dependent on the size and shape of the tissue) and are not inherent material properties.
Contact guidance.
The response of cells to topographic cues; the direction of cell alignment and migration is affected by geometrical patterns such as grooves or fibres.
Pannus.
An abnormal layer of fibrovascular tissue, which can occur in rheumatoid arthritis.
Heterotopic ossification.
The presence of bone in soft tissue where bone does not normally exist.
Scar tissue.
Dense fibrous tissue that replaces original tissue during wound healing; the scar tissue is generally disordered and does not match the original tissue in terms of the biochemical content or mechanical properties.
Adhesion.
Abnormal formation of scar tissue after injury that connects normally separated tissues and impedes joint motion.
Micromoulding.
A technique for generating microstructures using moulds with micrometre-scale features.
Photolithography.
A technique that uses light to transfer geometric patterns from a photomask (an opaque plate that enables light to shine through in a defined pattern) to a light-sensitive chemical on a substrate.
Electrospinning.
A technique that produces nanofibres by charging and pulling a polymer solution through a spinneret under a high-voltage electrical field.
Rapid prototyping.
A group of techniques for constructing a 3D product using computer-aided design (for example, 3D printing).
Anisotropic.
Describes mechanical and physical properties that vary on the basis of the testing direction.
Porogens.
Space-filling particles used to create porous materials, which are later removed to generate voids.
Bioinks.
Extrudable polymer solutions used in 3D bioprinting that can contain cells and/or other biologics that can solidify after printing.
Stress relaxation.
A time-dependent decrease in stress of a material in response to the same level of strain.
Acknowledgements
The work of F.Q., F.G. and R.L.M. is supported by the US National Institutes of Health (AR060719, AR056624, EB008722, AR50245, AR48852, AG46927, AG15768, AR48182, AR067467 and AR057235), the US Department of Veterans’ Affairs (I01 RX000174), the Arthritis Foundation, the Nancy Taylor Foundation for Chronic Diseases and the Collaborative Research Center of the AO Foundation in Davos.
Footnotes
Competing interests
The authors declare no competing interests.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Reviewer information
Nature Reviews Rheumatology thanks D. Grande and the other anonymous reviewer(s) for their contribution to the peer review of this work.
References
- 1.Franz CM, Jones GE & Ridley AJ Cell migration in development and disease. Dev. Cell 2, 153–158 (2002). [DOI] [PubMed] [Google Scholar]
- 2.Cukierman E, Pankov R, Stevens DR & Yamada KM Taking cell-matrix adhesions to the third dimension. Science 294, 1708–1712 (2001). [DOI] [PubMed] [Google Scholar]
- 3.Baker BM & Chen CS Deconstructing the third dimension: how 3D culture microenvironments alter cellular cues. J. Cell Sci 125, 3015–3024 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pathak A & Kumar S Biophysical regulation of tumor cell invasion: moving beyond matrix stiffness. Integr. Biol 3, 267–278 (2011). [DOI] [PubMed] [Google Scholar]
- 5.Friedl P & Wolf K Plasticity of cell migration: a multiscale tuning model. J. Cell Biol 188, 11–19 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lo CM, Wang HB, Dembo M & Wang YL Cell movement is guided by the rigidity of the substrate. Biophys. J 79, 144–152 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stoker M & Gherardi E Regulation of cell movement: the motogenic cytokines. Biochim. Biophys. Acta 1072, 81–102 (1991). [DOI] [PubMed] [Google Scholar]
- 8.Walters NJ & Gentleman E Evolving insights in cell-matrix interactions: elucidating how non-soluble properties of the extracellular niche direct stem cell fate. Acta Biomater 11, 3–16 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Petrie RJ & Yamada KM At the leading edge of three-dimensional cell migration. J. Cell Sci 125, 5917–5926 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wolf K et al. Physical limits of cell migration: control by ECM space and nuclear deformation and tuning by proteolysis and traction force. J. Cell Biol 201, 1069–1084 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kurosaka S & Kashina A Cell biology of embryonic migration. Birth Defects Res. C Embryo Today 84, 102–122 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Friedl P, Zanker KS & Brocker EB Cell migration strategies in 3D extracellular matrix: differences in morphology, cell matrix interactions, and integrin function. Microsc. Res. Tech 43, 369–378 (1998). [DOI] [PubMed] [Google Scholar]
- 13.Friedl P & Brocker EB T cell migration in three-dimensional extracellular matrix: guidance by polarity and sensations. Dev. Immunol 7, 249–266 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu YJ et al. Confinement and low adhesion induce fast amoeboid migration of slow mesenchymal cells. Cell 160, 659–672 (2015). [DOI] [PubMed] [Google Scholar]
- 15.Ruprecht V et al. Cortical contractility triggers a stochastic switch to fast amoeboid cell motility. Cell 160, 673–685 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Petrie RJ, Koo H & Yamada KM Generation of compartmentalized pressure by a nuclear piston governs cell motility in a 3D matrix. Science 345, 1062–1065 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stroka KM et al. Water permeation drives tumor cell migration in confined microenvironments. Cell 157, 611–623 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mouw JK, Ou G & Weaver VM Extracellular matrix assembly: a multiscale deconstruction. Nat. Rev. Mol. Cell. Biol 15, 771–785 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lu P, Takai K, Weaver VM & Werb Z Extracellular matrix degradation and remodeling in development and disease. Cold Spring Harb. Perspect. Biol 3, a005058 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Friedl P & Brocker EB The biology of cell locomotion within three-dimensional extracellular matrix. Cell. Mol. Life Sci 57, 41–64 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Friedl P & Wolf K Proteolytic interstitial cell migration: a five-step process. Cancer Metastasis Rev 28, 129–135 (2009). [DOI] [PubMed] [Google Scholar]
- 22.Guilak F, Nims R, Dicks A, Wu C-L & Meulenbelt I Osteoarthritis as a disease of the cartilage pericellular matrix. Matrix Biol 71–72, 40–50 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guilak F, Tedrow JR & Burgkart R Viscoelastic properties of the cell nucleus. Biochem. Biophys. Res. Commun 269, 781–786 (2000). [DOI] [PubMed] [Google Scholar]
- 24.Lautscham LA et al. Migration in confined 3D environments is determined by a combination of adhesiveness, nuclear volume, contractility, and cell stiffness. Biophys. J 109, 900–913 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Friedl P, Wolf K & Lammerding J Nuclear mechanics during cell migration. Curr. Opin. Cell Biol 23, 55–64 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Denais CM et al. Nuclear envelope rupture and repair during cancer cell migration. Science 352, 353–358 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harada T et al. Nuclear lamin stiffness is a barrier to 3D migration, but softness can limit survival. J. Cell Biol 204, 669–682 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rowat AC et al. Nuclear envelope composition determines the ability of neutrophil-type cells to passage through micron-scale constrictions. J. Biol. Chem 288, 8610–8618 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pajerowski JD, Dahl KN, Zhong FL, Sammak PJ & Discher DE Physical plasticity of the nucleus in stem cell differentiation. Proc. Natl Acad. Sci. USA 104, 15619–15624 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lammerding J et al. Lamins A and C but not lamin B1 regulate nuclear mechanics. J. Biol. Chem 281, 25768–25780 (2006). [DOI] [PubMed] [Google Scholar]
- 31.Booth-Gauthier EA et al. Hutchinson-Gilford progeria syndrome alters nuclear shape and reduces cell motility in three dimensional model substrates. Integr. Biol 5, 569–577 (2013). [DOI] [PubMed] [Google Scholar]
- 32.Greiner AM et al. Multifunctional polymer scaffolds with adjustable pore size and chemoattractant gradients for studying cell matrix invasion. Biomaterials 35, 611–619 (2014). [DOI] [PubMed] [Google Scholar]
- 33.Infante E et al. LINC complex-Lis1 interplay controls MT1-MMP matrix digest-on-demand response for confined tumor cell migration. Nat. Commun 9, 2443 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Olins AL et al. Nuclear envelope and chromatin compositional differences comparing undifferentiated and retinoic acid- and phorbol ester-treated HL-60 cells. Exp. Cell Res 268, 115–127 (2001). [DOI] [PubMed] [Google Scholar]
- 35.Ekpenyong AE et al. Viscoelastic properties of differentiating blood cells are fate- and function-dependent. PLOS ONE 7, e45237 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Graham DM et al. Enucleated cells reveal differential roles of the nucleus in cell migration, polarity, and mechanotransduction. J. Cell Biol 217, 895–914 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Singh SP, Schwartz MP, Lee JY, Fairbanks BD & Anseth KS A peptide functionalized poly(ethylene glycol) (PEG) hydrogel for investigating the influence of biochemical and biophysical matrix properties on tumor cell migration. Biomater. Sci 2, 1024–1034 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lowin T & Straub RH Integrins and their ligands in rheumatoid arthritis. Arthritis Res. Ther 13, 244 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Loeser RF Integrins and chondrocyte-matrix interactions in articular cartilage. Matrix Biol 39, 11–16 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hinz B The extracellular matrix and transforming growth factor-β1: tale of a strained relationship. Matrix Biol 47, 54–65 (2015). [DOI] [PubMed] [Google Scholar]
- 41.Ni GX, Li Z & Zhou YZ The role of small leucine-rich proteoglycans in osteoarthritis pathogenesis. Osteoarthr. Cartil 22, 896–903 (2014). [DOI] [PubMed] [Google Scholar]
- 42.Merline R, Schaefer RM & Schaefer L The matricellular functions of small leucine-rich proteoglycans (SLRPs). J. Cell Commun. Signal 3, 323–335 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tufvesson E & Westergren-Thorsson G Tumour necrosis factor-alpha interacts with biglycan and decorin. FEBS Lett 530, 124–128 (2002). [DOI] [PubMed] [Google Scholar]
- 44.Dormann D & Weijer CJ Chemotactic cell movement during development. Curr. Opin. Genet. Dev 13, 358–364 (2003). [DOI] [PubMed] [Google Scholar]
- 45.Wu J et al. Gradient biomaterials and their influences on cell migration. Interface Focus 2, 337–355 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Turner MD, Nedjai B, Hurst T & Pennington DJ Cytokines and chemokines: at the crossroads of cell signalling and inflammatory disease. Biochim. Biophys. Acta 1843, 2563–2582 (2014). [DOI] [PubMed] [Google Scholar]
- 47.Qu F et al. Maturation state and matrix microstructure regulate interstitial cell migration in dense connective tissues. Sci. Rep 8, 3295 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Swift J et al. Nuclear lamin-A scales with tissue stiffness and enhances matrix-directed differentiation. Science 341, 1240104 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vogel V & Sheetz M Local force and geometry sensing regulate cell functions. Nat. Rev. Mol. Cell. Biol 7, 265–275 (2006). [DOI] [PubMed] [Google Scholar]
- 50.Peyton SR & Putnam AJ Extracellular matrix rigidity governs smooth muscle cell motility in a biphasic fashion. J. Cell. Physiol 204, 198–209 (2005). [DOI] [PubMed] [Google Scholar]
- 51.Zaman MH et al. Migration of tumor cells in 3D matrices is governed by matrix stiffness along with cell-matrix adhesion and proteolysis. Proc. Natl Acad. Sci. USA 103, 10889–10894 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pathak A & Kumar S Independent regulation of tumor cell migration by matrix stiffness and confinement. Proc. Natl Acad. Sci. USA 109, 10334–10339 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Peyton SR et al. Marrow-derived stem cell motility in 3D synthetic scaffold is governed by geometry along with adhesivity and stiffness. Biotechnol. Bioeng 108, 1181–1193 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zaman MH, Matsudaira P & Lauffenburger DA Understanding effects of matrix protease and matrix organization on directional persistence and translational speed in three-dimensional cell migration. Ann. Biomed. Eng 35, 91–100 (2007). [DOI] [PubMed] [Google Scholar]
- 55.Kim MC et al. Integrating focal adhesion dynamics, cytoskeleton remodeling, and actin motor activity for predicting cell migration on 3D curved surfaces of the extracellular matrix. Integr. Biol 4, 1386–1397 (2012). [DOI] [PubMed] [Google Scholar]
- 56.Provenzano PP, Inman DR, Eliceiri KW, Trier SM & Keely PJ Contact guidance mediated three-dimensional cell migration is regulated by Rho/ROCK-dependent matrix reorganization. Biophys. J 95, 5374–5384 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Petrie RJ, Doyle AD & Yamada KM Random versus directionally persistent cell migration. Nat. Rev. Mol. Cell. Biol 10, 538–549 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fraley SI et al. Three-dimensional matrix fiber alignment modulates cell migration and MT1-MMP utility by spatially and temporally directing protrusions. Sci. Rep 5, 14580 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dickinson RB, Guido S & Tranquillo RT Biased cell migration of fibroblasts exhibiting contact guidance in oriented collagen gels. Ann. Biomed. Eng 22, 342–356 (1994). [DOI] [PubMed] [Google Scholar]
- 60.Riching KM et al. 3D collagen alignment limits protrusions to enhance breast cancer cell persistence. Biophys. J 107, 2546–2558 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Smith MD The normal synovium. Open Rheumatol. J 5, 100–106 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nevius E, Gomes AC & Pereira JP Inflammatory cell migration in rheumatoid arthritis: a comprehensive review. Clin. Rev. Allergy Immunol 51, 59–78 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tak PP et al. Analysis of the synovial cell infiltrate in early rheumatoid synovial tissue in relation to local disease activity. Arthritis. Rheum 40, 217–225 (1997). [DOI] [PubMed] [Google Scholar]
- 64.Mulherin D, Fitzgerald O & Bresnihan B Synovial tissue macrophage populations and articular damage in rheumatoid arthritis. Arthritis Rheum 39, 115–124 (1996). [DOI] [PubMed] [Google Scholar]
- 65.Mellado M et al. T cell migration in rheumatoid arthritis. Front. Immunol 6, 384 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Iwamoto T, Okamoto H, Toyama Y & Momohara S Molecular aspects of rheumatoid arthritis: chemokines in the joints of patients. FEBS J 275, 4448–4455 (2008). [DOI] [PubMed] [Google Scholar]
- 67.Koelink PJ et al. Targeting chemokine receptors in chronic inflammatory diseases: an extensive review. Pharmacol. Ther 133, 1–18 (2012). [DOI] [PubMed] [Google Scholar]
- 68.Buckley CD Why does chronic inflammatory joint disease persist? Clin. Med 3, 361–366 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Solari R, Pease JE & Begg M Chemokine receptors as therapeutic targets: why aren’t there more drugs? Eur. J. Pharmacol 746, 363–367 (2015). [DOI] [PubMed] [Google Scholar]
- 70.Hitchon CA & El-Gabalawy HS The synovium in rheumatoid arthritis. Open Rheumatol. J 5, 107–114 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Amin MA et al. Interleukin-18 induces angiogenic factors in rheumatoid arthritis synovial tissue fibroblasts via distinct signaling pathways. Arthritis Rheum 56, 1787–1797 (2007). [DOI] [PubMed] [Google Scholar]
- 72.Bruhl H et al. Functional expression of the chemokine receptor CCR7 on fibroblast-like synoviocytes. Rheumatology 47, 1771–1774 (2008). [DOI] [PubMed] [Google Scholar]
- 73.Garcia-Vicuna R et al. CC and CXC chemokine receptors mediate migration, proliferation, and matrix metalloproteinase production by fibroblast-like synoviocytes from rheumatoid arthritis patients. Arthritis Rheum 50, 3866–3877 (2004). [DOI] [PubMed] [Google Scholar]
- 74.Nanki T, Nagasaka K, Hayashida K, Saita Y & Miyasaka N Chemokines regulate IL-6 and IL-8 production by fibroblast-like synoviocytes from patients with rheumatoid arthritis. J. Immunol 167, 5381–5385 (2001). [DOI] [PubMed] [Google Scholar]
- 75.Lefevre S et al. Disease-specific effects of matrix and growth factors on adhesion and migration of rheumatoid synovial fibroblasts. J. Immunol 198, 4588–4595 (2017). [DOI] [PubMed] [Google Scholar]
- 76.Tolboom TC et al. Invasive properties of fibroblast-like synoviocytes: correlation with growth characteristics and expression of MMP-1, MMP-3, and MMP-10. Ann. Rheum. Dis 61, 975–980 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Li D, Xiao Z, Wang G & Song X Knockdown of ADAM10 inhibits migration and invasion of fibroblast-like synoviocytes in rheumatoid arthritis. Mol. Med. Rep 12, 5517–5523 (2015). [DOI] [PubMed] [Google Scholar]
- 78.Tolboom TC et al. Invasiveness of fibroblast-like synoviocytes is an individual patient characteristic associated with the rate of joint destruction in patients with rheumatoid arthritis. Arthritis Rheum 52, 1999–2002 (2005). [DOI] [PubMed] [Google Scholar]
- 79.El-Zayadi AA et al. Interleukin-22 drives the proliferation, migration and osteogenic differentiation of mesenchymal stem cells: a novel cytokine that could contribute to new bone formation in spondyloarthropathies. Rheumatology 56, 488–493 (2017). [DOI] [PubMed] [Google Scholar]
- 80.Justa S, Zhou X & Sarkar S Endogenous IL-22 plays a dual role in arthritis: regulation of established arthritis via IFN-gamma responses. PLOS ONE 9, e93279 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Qin Y et al. Increased CCL19 and CCL21 levels promote fibroblast ossification in ankylosing spondylitis hip ligament tissue. BMC Musculoskelet. Disord 15, 316 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lee J et al. Stimulation of osteoclast migration and bone resorption by C-C chemokine ligands 19 and 21. Exp. Mol. Med 49, e358 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Meng XH et al. Quantitative evaluation of knee cartilage and meniscus destruction in patients with rheumatoid arthritis using T1rho and T2 mapping. Eur. J. Radiol 96, 91–97 (2017). [DOI] [PubMed] [Google Scholar]
- 84.Fuhrmann IK, Steinhagen J, Ruther W & Schumacher U Comparative immunohistochemical evaluation of the zonal distribution of extracellular matrix and inflammation markers in human meniscus in osteoarthritis and rheumatoid arthritis. Acta Histochem 117, 243–254 (2015). [DOI] [PubMed] [Google Scholar]
- 85.Lopez-Franco M et al. Meniscal degeneration in human knee osteoarthritis: in situ hybridization and immunohistochemistry study. Arch. Orthop. Trauma Surg 136, 175–183 (2016). [DOI] [PubMed] [Google Scholar]
- 86.van de Sande MA, de Groot JH & Rozing PM Clinical implications of rotator cuff degeneration in the rheumatic shoulder. Arthritis Rheum 59, 317–324 (2008). [DOI] [PubMed] [Google Scholar]
- 87.Meyer C et al. Rheumatoid arthritis affecting the upper cervical spine: biomechanical assessment of the stabilizing ligaments. Biomed. Res. Int 2017, 6131703 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Puttlitz CM et al. Biomechanical rationale for the pathology of rheumatoid arthritis in the craniovertebral junction. Spine 25, 1607–1616 (2000). [DOI] [PubMed] [Google Scholar]
- 89.Yang G, Rothrauff BB & Tuan RS Tendon and ligament regeneration and repair: clinical relevance and developmental paradigm. Birth Defects Res. C Embryo Today 99, 203–222 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.McNulty AL, Moutos FT, Weinberg JB & Guilak F Enhanced integrative repair of the porcine meniscus in vitro by inhibition of interleukin-1 or tumor necrosis factor alpha. Arthritis Rheum 56, 3033–3042 (2007). [DOI] [PubMed] [Google Scholar]
- 91.Mohanraj B et al. Chondrocyte and mesenchymal stem cell derived engineered cartilage exhibits differential sensitivity to pro-inflammatory cytokines. J. Orthop. Res 36, 2901–2910 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Arnoczky SP & Warren RF The microvasculature of the meniscus and its response to injury. An experimental study in the dog. Am. J. Sports Med 11, 131–141 (1983). [DOI] [PubMed] [Google Scholar]
- 93.Hiraki Y & Shukunami C Angiogenesis inhibitors localized in hypovascular mesenchymal tissues: chondromodulin-I and tenomodulin. Connect. Tissue Res 46, 3–11 (2005). [DOI] [PubMed] [Google Scholar]
- 94.Manske PR, Lesker PA, Gelberman RH & Rucinsky TE Intrinsic restoration of the flexor tendon surface in the nonhuman primate. J. Hand Surg. Am 10, 632–637 (1985). [DOI] [PubMed] [Google Scholar]
- 95.Sharma P & Maffulli N Biology of tendon injury: healing, modeling and remodeling. J. Musculoskelet. Neuronal Interact 6, 181–190 (2006). [PubMed] [Google Scholar]
- 96.Lo IK, Chi S, Ivie T, Frank CB & Rattner JB The cellular matrix: a feature of tensile bearing dense soft connective tissues. Histol. Histopathol 17, 523–537 (2002). [DOI] [PubMed] [Google Scholar]
- 97.Cadby JA, Buehler E, Godbout C, van Weeren PR & Snedeker JG Differences between the cell populations from the peritenon and the tendon core with regard to their potential implication in tendon repair. PLOS ONE 9, e92474 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Shen W et al. Intra-articular injection of human meniscus stem/progenitor cells promotes meniscus regeneration and ameliorates osteoarthritis through stromal cell-derived factor-1/CXCR4-mediated homing. Stem Cells Transl Med 3, 387–394 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mauck RL, Martinez-Diaz GJ, Yuan X & Tuan RS Regional multilineage differentiation potential of meniscal fibrochondrocytes: implications for meniscus repair. Anat. Rec 290, 48–58 (2007). [DOI] [PubMed] [Google Scholar]
- 100.Mesiha M et al. Pathologic characteristics of the torn human meniscus. Am. J. Sports Med 35, 103–112 (2007). [DOI] [PubMed] [Google Scholar]
- 101.de Albornoz PM & Forriol F The meniscal healing process. Muscles Ligaments Tendons J 2, 10–18 (2012). [PMC free article] [PubMed] [Google Scholar]
- 102.Newman AP, Anderson DR, Daniels AU & Dales MC Mechanics of the healed meniscus in a canine model. Am. J. Sports Med 17, 164–175 (1989). [DOI] [PubMed] [Google Scholar]
- 103.Russo V et al. Cellular and molecular maturation in fetal and adult ovine calcaneal tendons. J. Anat 226, 126–142 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Clark CR & Ogden JA Development of the menisci of the human knee joint. Morphological changes and their potential role in childhood meniscal injury. J. Bone Joint Surg. Am 65, 538–547 (1983). [PubMed] [Google Scholar]
- 105.Marturano JE, Arena JD, Schiller ZA, Georgakoudi I & Kuo CK Characterization of mechanical and biochemical properties of developing embryonic tendon. Proc. Natl Acad. Sci. USA 110, 6370–6375 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Li Q et al. Impacts of maturation on the micromechanics of the meniscus extracellular matrix. J. Biomech 72, 252–257 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ionescu LC et al. Maturation state-dependent alterations in meniscus integration: implications for scaffold design and tissue engineering. Tissue Eng. Part A 17, 193–204 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Melrose J, Smith S, Cake M, Read R & Whitelock J Comparative spatial and temporal localisation of perlecan, aggrecan and type I, II and IV collagen in the ovine meniscus: an ageing study. Histochem. Cell Biol 124, 225–235 (2005). [DOI] [PubMed] [Google Scholar]
- 109.Di Giancamillo A, Deponti D, Addis A, Domeneghini C & Peretti GM Meniscus maturation in the swine model: changes occurring along with anterior to posterior and medial to lateral aspect during growth. J. Cell. Mol. Med 18, 1964–1974 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Morales TI Chondrocyte moves: clever strategies? Osteoarthr. Cartil 15, 861–871 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Qu F, Holloway JL, Esterhai JL, Burdick JA & Mauck RL Programmed biomolecule delivery to enable and direct cell migration for connective tissue repair. Nat. Commun 8, 1780 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Qu F et al. Repair of dense connective tissues via biomaterial-mediated matrix reprogramming of the wound interface. Biomaterials 39, 85–94 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hunziker EB & Kapfinger E Removal of proteoglycans from the surface of defects in articular cartilage transiently enhances coverage by repair cells. J. Bone Joint Surg. Br 80, 144–150 (1998). [DOI] [PubMed] [Google Scholar]
- 114.Kim M, Farrell MJ, Steinberg DR, Burdick JA & Mauck RL Enhanced nutrient transport improves the depth-dependent properties of tri-layered engineered cartilage constructs with zonal co-culture of chondrocytes and MSCs. Acta Biomater 58, 1–11 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Cheng NC, Estes BT, Young TH & Guilak F Genipin-crosslinked cartilage-derived matrix as a scaffold for human adipose-derived stem cell chondrogenesis. Tissue Eng. Part A 19, 484–496 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Baker BM et al. The potential to improve cell infiltration in composite fiber-aligned electrospun scaffolds by the selective removal of sacrificial fibers. Biomaterials 29, 2348–2358 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sundararaghavan HG & Burdick JA Gradients with depth in electrospun fibrous scaffolds for directed cell behavior. Biomacromolecules 12, 2344–2350 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bhargava MM et al. The effect of cytokines on the proliferation and migration of bovine meniscal cells. Am. J. Sports Med 27, 636–643 (1999). [DOI] [PubMed] [Google Scholar]
- 119.Caliari SR & Harley BA The effect of anisotropic collagen-GAG scaffolds and growth factor supplementation on tendon cell recruitment, alignment, and metabolic activity. Biomaterials 32, 5330–5340 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hannafin JA, Attia ET, Warren RF & Bhargava MM Characterization of chemotatic migration and growth kinetics of canine knee ligament fibroblasts. J. Orthop. Res 17, 398–404 (1999). [DOI] [PubMed] [Google Scholar]
- 121.Kucia M et al. CXCR4-SDF-1 signalling, locomotion, chemotaxis and adhesion. J. Mol. Histol 35, 233–245 (2004). [DOI] [PubMed] [Google Scholar]
- 122.Moore K, Macsween M & Shoichet M Immobilized concentration gradients of neurotrophic factors guide neurite outgrowth of primary neurons in macroporous scaffolds. Tissue Eng 12, 267–278 (2006). [DOI] [PubMed] [Google Scholar]
- 123.Wiles K, Fishman JM, De Coppi P & Birchall MA The host immune response to tissue-engineered organs: current problems and future directions. Tissue Eng. Part B Rev 22, 208–219 (2016). [DOI] [PubMed] [Google Scholar]
- 124.Swartz MA, Hirosue S & Hubbell JA Engineering approaches to immunotherapy. Sci. Transl Med 4, 148rv149 (2012). [DOI] [PubMed] [Google Scholar]
- 125.Stano A, Scott EA, Dane KY, Swartz MA & Hubbell JA Tunable T cell immunity towards a protein antigen using polymersomes versus solid-core nanoparticles. Biomaterials 34, 4339–4346 (2013). [DOI] [PubMed] [Google Scholar]
- 126.Nembrini C et al. Nanoparticle conjugation of antigen enhances cytotoxic T cell responses in pulmonary vaccination. Proc. Natl Acad. Sci. USA 108, E989–E997 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.McCarthy DP et al. An antigen-encapsulating nanoparticle platform for TH1/17 immune tolerance therapy. Nanomedicine 13, 191–200 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Spiller KL et al. Sequential delivery of immunomodulatory cytokines to facilitate the M1-to-M2 transition of macrophages and enhance vascularization of bone scaffolds. Biomaterials 37, 194–207 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Boehler RM et al. Lentivirus delivery of IL-10 to promote and sustain macrophage polarization towards an anti-inflammatory phenotype. Biotechnol. Bioeng 111, 1210–1221 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Sridharan R et al. Biomaterial based modulation of macrophage polarization: a review and suggested design principles. Mater. Today 18, 313–325 (2015). [Google Scholar]
- 131.Ratanavaraporn J, Furuya H & Tabata Y Local suppression of pro-inflammatory cytokines and the effects in BMP-2-induced bone regeneration. Biomaterials 33, 304–316 (2012). [DOI] [PubMed] [Google Scholar]
- 132.Chen WC et al. Controlled dual delivery of fibroblast growth factor-2 and Interleukin-10 by heparin-based coacervate synergistically enhances ischemic heart repair. Biomaterials 72, 138–151 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Geckil H, Xu F, Zhang X, Moon S & Demirci U Engineering hydrogels as extracellular matrix mimics. Nanomedicine 5, 469–484 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kim D-H, Provenzano PP, Smith CL & Levchenko A Matrix nanotopography as a regulator of cell function. J. Cell Biol 197, 351–360 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wade RJ, Bassin EJ, Gramlich WM & Burdick JA Nanofibrous hydrogels with spatially patterned biochemical signals to control cell behavior. Adv. Mater 27, 1356–1362 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kim IL, Khetan S, Baker BM, Chen CS & Burdick JA Fibrous hyaluronic acid hydrogels that direct MSC chondrogenesis through mechanical and adhesive cues. Biomaterials 34, 5571–5580 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Billiet T, Vandenhaute M, Schelfhout J, Van Vlierberghe S & Dubruel P A review of trends and limitations in hydrogel-rapid prototyping for tissue engineering. Biomaterials 33, 6020–6041 (2012). [DOI] [PubMed] [Google Scholar]
- 138.Bullough PG, Munuera L, Murphy J & Weinstein AM The strength of the menisci of the knee as it relates to their fine structure. J. Bone Joint Surg. Br 52, 564–567 (1970). [PubMed] [Google Scholar]
- 139.Proctor CS, Schmidt MB, Whipple RR, Kelly MA & Mow VC Material properties of the normal medial bovine meniscus. J. Orthop. Res 7, 771–782 (1989). [DOI] [PubMed] [Google Scholar]
- 140.LeRoux MA & Setton LA Experimental and biphasic FEM determinations of the material properties and hydraulic permeability of the meniscus in tension. J. Biomech. Eng 124, 315–321 (2002). [DOI] [PubMed] [Google Scholar]
- 141.Mauck RL et al. Engineering on the straight and narrow: the mechanics of nanofibrous assemblies for fiber-reinforced tissue regeneration. Tissue Eng. Part B Rev 15, 171–193 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lim JY & Donahue HJ Cell sensing and response to micro-and nanostructured surfaces produced by chemical and topographic patterning. Tissue Eng 13, 1879–1891 (2007). [DOI] [PubMed] [Google Scholar]
- 143.Gilchrist CL, Ruch DS, Little D & Guilak F Micro-scale and meso-scale architectural cues cooperate and compete to direct aligned tissue formation. Biomaterials 35, 10015–10024 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Baker BM & Mauck RL The effect of nanofiber alignment on the maturation of engineered meniscus constructs. Biomaterials 28, 1967–1977 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Orr SB et al. Aligned multilayered electrospun scaffolds for rotator cuff tendon tissue engineering. Acta Biomater 24, 117–126 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ionescu LC & Mauck RL Porosity and cell preseeding influence electrospun scaffold maturation and meniscus integration in vitro. Tissue Eng. Part A 19, 538–547 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Nam J, Huang Y, Agarwal S & Lannutti J Improved cellular infiltration in electrospun fiber via engineered porosity. Tissue Eng 13, 2249–2257 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Simonet M, Schneider OD, Neuenschwander P & Stark WJ Ultraporous 3D polymer meshes by low-temperature electrospinning: use of ice crystals as a removable void template. Polym. Eng. Sci 47, 2020–2026 (2007). [Google Scholar]
- 149.Lee BL-P et al. Synovial stem cells and their responses to the porosity of microfibrous scaffold. Acta Biomater 9, 7264–7275 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Phipps MC, Clem WC, Grunda JM, Clines GA & Bellis SL Increasing the pore sizes of bone-mimetic electrospun scaffolds comprised of polycaprolactone, collagen I and hydroxyapatite to enhance cell infiltration. Biomaterials 33, 524–534 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Baker BM et al. Sacrificial nanofibrous composites provide instruction without impediment and enable functional tissue formation. Proc. Natl Acad. Sci. USA 109, 14176–14181 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Cai S, Xu H, Jiang Q & Yang Y Novel 3D electrospun scaffolds with fibers oriented randomly and evenly in three dimensions to closely mimic the unique architectures of extracellular matrices in soft tissues: fabrication and mechanism study. Langmuir 29, 2311–2318 (2013). [DOI] [PubMed] [Google Scholar]
- 153.Moutos FT, Freed LE & Guilak F A biomimetic three-dimensional woven composite scaffold for functional tissue engineering of cartilage. Nat. Mater 6, 162–167 (2007). [DOI] [PubMed] [Google Scholar]
- 154.Lee J, Jang J, Oh H, Jeong YH & Cho D-W Fabrication of a three-dimensional nanofibrous scaffold with lattice pores using direct-write electrospinning. Mater. Lett 93, 397–400 (2013). [Google Scholar]
- 155.Daly AC et al. 3D bioprinting for cartilage and osteochondral tissue engineering. Adv. Healthc. Mater 6, 1700298 (2017). [DOI] [PubMed] [Google Scholar]
- 156.Miller JS et al. Rapid casting of patterned vascular networks for perfusable engineered three-dimensional tissues. Nat. Mater 11, 768–774 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Freeman FE & Kelly DJ Tuning alginate bioink stiffness and composition for controlled growth factor delivery and to spatially direct MSC fate within bioprinted tissues. Sci. Rep 7, 17042 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Skardal A et al. A hydrogel bioink toolkit for mimicking native tissue biochemical and mechanical properties in bioprinted tissue constructs. Acta Biomater 25, 24–34 (2015). [DOI] [PubMed] [Google Scholar]
- 159.Albritton JL & Miller JS 3D bioprinting: improving in vitro models of metastasis with heterogeneous tumor microenvironments. Dis. Model. Mech 10, 3–14 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Miller JS et al. Bioactive hydrogels made from step-growth derived PEG-peptide macromers. Biomaterials 31, 3736–3743 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Ehrbar M et al. Elucidating the role of matrix stiffness in 3D cell migration and remodeling. Biophys. J 100, 284–293 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Bott K et al. The effect of matrix characteristics on fibroblast proliferation in 3D gels. Biomaterials 31, 8454–8464 (2010). [DOI] [PubMed] [Google Scholar]
- 163.Wade RJ, Bassin EJ, Rodell CB & Burdick JA Protease-degradable electrospun fibrous hydrogels. Nat. Commun 6, 6639 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Schultz KM, Kyburz KA & Anseth KS Measuring dynamic cell-material interactions and remodeling during 3D human mesenchymal stem cell migration in hydrogels. Proc. Natl Acad. Sci. USA 112, E3757–E3764 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Lee SH, Miller JS, Moon JJ & West JL Proteolytically degradable hydrogels with a fluorogenic substrate for studies of cellular proteolytic activity and migration. Biotechnol. Prog 21, 1736–1741 (2005). [DOI] [PubMed] [Google Scholar]
- 166.Feng Q, Zhu M, Wei K & Bian L Cell-mediated degradation regulates human mesenchymal stem cell chondrogenesis and hypertrophy in MMP-sensitive hyaluronic acid hydrogels. PLOS ONE 9, e99587 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Salinas CN & Anseth KS The enhancement of chondrogenic differentiation of human mesenchymal stem cells by enzymatically regulated RGD functionalities. Biomaterials 29, 2370–2377 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Chaudhuri O et al. Substrate stress relaxation regulates cell spreading. Nat. Commun 6, 6364 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Lee HP, Gu L, Mooney DJ, Levenston ME & Chaudhuri O Mechanical confinement regulates cartilage matrix formation by chondrocytes. Nat. Mater 16, 1243–1251 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Brunger JM, Zutshi A, Willard VP, Gersbach CA & Guilak F CRISPR/Cas9 editing of murine induced pluripotent stem cells for engineering inflammation-resistant tissues. Arthritis Rheumatol 69, 1111–1121 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Brunger JM, Zutshi A, Willard VP, Gersbach CA & Guilak F Genome engineering of stem cells for autonomously regulated, closed-loop delivery of biologic drugs. Stem Cell Rep 8, 1202–1213 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Brunger JM et al. Scaffold-mediated lentiviral transduction for functional tissue engineering of cartilage. Proc. Natl Acad. Sci. USA 111, E798–806 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Glass KA et al. Tissue-engineered cartilage with inducible and tunable immunomodulatory properties. Biomaterials 35, 5921–5931 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]