Abstract
Background
Food allergy is an abnormal immunological response following exposure (usually ingestion) to a food. Elimination of the allergen is the principle treatment for food allergy, including allergy to fruit. Accidental ingestion of allergenic foods can result in severe anaphylactic reactions. Allergen‐specific immunotherapy (SIT) is a specific treatment, when the avoidance of allergenic foods is problematic. Recently, studies have been conducted on different types of immunotherapy for the treatment of food allergy, including oral (OIT) and sublingual immunotherapy (SLIT).
Objectives
To determine the efficacy and safety of oral and sublingual immunotherapy in children and adults with food allergy to fruits, when compared with placebo or an elimination strategy.
Search methods
The Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, CINAHL, and AMED were searched for published results along with trial registries and the Journal of Negative Results in BioMedicine for grey literature. The date of the most recent search was July 2015.
Selection criteria
Randomised controlled trials (RCTs) comparing OIT or SLIT with placebo or an elimination diet were included. Participants were children or adults diagnosed with food allergy who presented immediate fruit reactions.
Data collection and analysis
We used standard methodological procedures expected by the Cochrane Collaboration. We assessed treatment effect through risk ratios (RRs) for dichotomous outcomes.
Main results
We identified two RCTs (N=89) eligible for inclusion. These RCTs addressed oral or sublingual immunotherapy, both in adults, with an allergy to apple or peach respectively. Both studies enrolled a small number of participants and used different methods to provide these differing types of immunotherapy. Both studies were judged to be at high risk of bias in at least one domain. Overall, the quality of evidence was judged to be very low due to the small number of studies and participants and possible bias. The studies were clinically heterogeneous and hence we did not pool the results. A study comparing SLIT with placebo for allergy to peach did not detect a significant difference between the number of patients desensitised at six months following a double‐blind placebo‐controlled food challenge (RR 1.16, 95% confidence interval (CI) 0.49 to 2.74). The second study, comparing OIT versus no treatment for apple allergy, found an effect on desensitisation in favour of the intervention using an oral provocation test at eight months, but results were imprecise (RR 17.50, 95% CI 1.13 to 270.19). Neither study reported data on evidence of immunologic tolerance. In both studies, the incidence of mild and moderate adverse events was higher in the intervention groups than in the controls. In the study comparing SLIT with placebo, patients in the intervention group experienced significantly more local adverse reactions than participants in the control group (RR 3.21, 95% CI 1.51 to 6.82), though there was not a significant difference in the number of participants experiencing systemic adverse reactions (RR 0.81, 95% CI 0.22 to 3.02). In the study of OIT, two of the 25 participants in the intervention group reported relevant side effects, whereas no participants in the control group reported relevant side effects.
Authors' conclusions
There is insufficient evidence for using OIT or SLIT to treat allergy to fruit, specifically related to peach and apple. Mild or moderate adverse reactions were reported more frequently in people receiving OIT or SLIT. However, these reactions could be treated successfully with medications.
Keywords: Adult; Humans; Desensitization, Immunologic; Desensitization, Immunologic/methods; Food Hypersensitivity; Food Hypersensitivity/etiology; Food Hypersensitivity/therapy; Fruit; Fruit/adverse effects; Malus; Malus/adverse effects; Pyrus; Pyrus/adverse effects; Randomized Controlled Trials as Topic; Sublingual Immunotherapy; Sublingual Immunotherapy/methods
Plain language summary
Oral and sublingual immunotherapy (giving people small amounts of things they are allergic to via their mouth or under their tongue) for fruit allergy
Review question and background
We reviewed the evidence about the effect of oral and sublingual immunotherapy in people with a food allergy to fruit. Food allergy is an abnormal response to a food, usually after eating it. The main treatment for food allergy is to avoid the food, but in people with food allergy, accidentally eating the food can cause severe reactions.
Immunotherapy is a possible long‐term treatment for food allergy, including for food allergy to fruit. Immunotherapy is a process where increasing doses of the thing someone is allergic to (the allergen) are given over a period of time to help the person be less sensitive to the allergen. In oral immunotherapy, a small amount of the allergen is given to someone to eat. In sublingual immunotherapy, an extract from the allergen is put under the tongue.
Study characteristics
We found two studies with 89 participants in total. One looked at oral immunotherapy in people with an allergy to apple, comparing it to no treatment, and one looked at sublingual immunotherapy in people with an allergy to peach, comparing it to a placebo. Both studies were in adults. The evidence is current to July 2015.
Key results
The study of oral immunotherapy for apple allergy found participants who received the intervention were less sensitive to apple than people who did not receive the intervention at eight months. The study of sublingual immunotherapy for peach allergy did not find a difference between the groups in terms of sensitivity at six months. In both studies, there were more side effects in people receiving the treatment, but these were not serious. Overall, the quality of evidence is very low because both studies were small, results were not similar between studies, and there were issues with study design. More research is needed before we can tell if oral and sublingual therapy works for food allergy to fruits.
Summary of findings
Summary of findings for the main comparison. Summary of Findings Table.
| Oral or sublingual immunotherapy compared with placebo or no treatment for food allergy to fruits | ||||
|
Patient or population: adults with food allergy to fruits Settings: Outpatients Intervention: Oral or sublingual immunotherapy Comparison: placebo or no treatment | ||||
| Main Outcomes | Impact | No of Participants (studies) | Quality of the evidence (GRADE)* | Comments |
| Evidence of desensitization |
Fernandez‐Rivas 2009: RR 1.16 (95% CI 0.49 to 2.74) after 6 months of SLIT Kopac 2012: RR 17.50 (95% CI 1.13 to 270.19) after 8 months of OIT |
49 (1) 40 (1) |
⊕⊝⊝⊝ very low | The studies were not pooled as the interventions were not sufficiently similar in design. |
| Evidence of immunologic tolerance | Not applicable | Not applicable | Not applicable | This planned outcome was not measured by either of the included studies, providing no evidence of the effect of immunotherapy on immunologic tolerance. |
| Adverse events |
Fernandez‐Rivas 2009: Local 1328 events in 33/37 intervention participants (89.2%) versus 9 in 5/8 control participants (27.8%); systemic 16 in 5/37 intervention participants (13.5%), 3 in 3/18 control participants (16.7%) Kopac 2012: (did not separate into local and systemic) events in 2/28 intervention participants (8%) and 0/13 control participants |
As above | ⊕⊝⊝⊝ very low | The studies were not pooled as the interventions were not sufficiently similar in design. Neither study reported anaphylaxis. |
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||
*The certainty of the evidence for both outcomes is very low due imprecision and high risk of bias in both studies.
Background
Description of the condition
The National Institute of Allergy and Infectious Diseases (NIAID) defines food allergy as an "adverse immune response that occurs reproducibly on exposure to a given food and is distinct from other adverse responses to food, such as food intolerance, pharmacologic reactions, and toxin‐mediated reactions" (Chafen 2010). This definition encompasses immune responses that are Immunoglobin E (IgE) mediated (immediate), non–IgE mediated (delayed), or a combination of both, and is in agreement with other international guidelines (Burks 2012a). There are multiple types of food allergy, each with distinct clinical and pathophysiologic features. Clinical syndromes characterized by pollinosis and allergic reactions to plant foods are caused by cross‐reacting molecules present in different allergen sources. An example of these clinical syndromes are: oral allergy syndrome (OAS); pollen‐food allergy syndrome (PFAS); and lipid transfer protein syndrome (LTP syndrome). Clinical manifestations of these food allergies syndromes can be of varying severity and either generalized (anaphylaxis) or localized; for example restricted to the oropharyngeal area, skin or gastrointestinal tract (Pastorello 2004). Proteins in fruits and vegetables may cause these reactions because they are similar to those allergy‐causing proteins found in certain pollens. Clinically relevant cross‐reactivity between plant‐foods and pollens has been documented. For instance, multiple plant‐food sensitization is a common feature of LTP‐allergic patients, who are often considered to have LTP syndrome. In some places, such as the Mediterranean area, tree nuts (mainly hazelnuts) and fruits, especially Rosaceae and kiwi, stand out as the most important allergens and their relevance is limited to this area (Pascal 2012).
Food allergy is a disease on the increase, and affects around 8% of young children in the US and 3 to 4% of adults in the UK (Gupta 2011). According to The National Center for Health Statistics, 3.9% of US children in 2007 reported a food allergy (Kim 2011; Beyer 2012), with an 18% increase in prevalence from 1997 to 2007 (Branum 2008). There are several hypotheses for this increase, of which the 'hygiene hypothesis' has received attention, but does not provide a sufficient immunological explanation (Zeiger 2003). Other hypotheses describe associations between environmental and genetic factors, and also include food allergens (Mousallem 2012). There is a lack of accurate data on the prevalence of food allergies with regards to particular food groups, such as fruits, vegetables, nuts, and other edible plants. The prevalence of allergy to fruits has been estimated to be between 0.1 to 4.3% (Zuidmeer 2008). However, if prevalence is measured only by skin tests, this figure may be closer to 1% (Dalal 2002; Rance 2005). The prevalence of allergy to fruits as diagnosed by the patient's perception is between 0.4% to 3.5% in adults, and in children under three years can be up to 11.5% (Eggesbo 1999). In this latter age group, Zuidmeer 2008 found the prevalence of allergy dependant on fruit species as 8.5% for apple, and 6.8% for orange and/or lemon.
The treatment for food allergy, including to fruit, is the elimination of the allergen (Cox 2011). Unfortunately, many patients accidentally ingest allergenic foods, which can result in severe anaphylactic reactions (Bock 1989). While it is advisable to use intramuscular adrenaline as emergency treatment in cases of accidental ingestion of allergenic food (Kim 2011), allergen‐specific immunotherapy (SIT) has also been studied as a longer‐term treatment option in cases where avoidance of allergenic foods may prove difficult (Enrique 2005).
Description of the intervention
The concept of 'allergen immunotherapy' refers to a modulation of the immune system (Krishna 2011), which is expected to perform an allergen involved hyposensitization; in this case, to a food allergen (Scott‐Taylor 2005). Recently, studies have been conducted on different types of immunotherapy for the treatment of food allergies, including oral immunotherapy (OIT) and sublingual immunotherapy (SLIT). OIT involves the ingestion of small amounts of the allergen (milligrams to grams); while SLIT involves the administration of micrograms or milligrams of allergen extract under the tongue.
In 2009, Jones et al. (Jones 2009) reported a case series of 39 subjects with peanut allergy who received OIT. Twenty‐nine subjects completed all three phases of the OIT protocol and 27 reached the total peanut dose of 3.9 g with mild symptoms. Desensitization to peanut protein in these subjects suggested treatment was successful. In the same year, Clark et al. (Clark 2009) published another case series of four children with allergy to peanut protein. Similar to Jones 2009, children received OIT to peanut, during three phases, which was well tolerated. At the end of the study, OIT conferred protection against 2.38 g peanut protein in all children. In terms of randomized controlled trials, Varshney et al. (Varshney 2011) published the first randomized controlled trial to investigate the effectiveness of OIT in subjects between 1 to 16 years with peanut allergy. Twenty‐eight subjects were enrolled. Nineteen were exposed to the peanut protein and nine to placebo. After one year, 16 of the 19 subjects in the active group reached the goal maintenance doses of 4000 mg. This result indicated that OIT can induce tolerance in subjects with peanut allergy. In 2012, Burks et al. (Burks 2012b) published the first randomized controlled trial of egg OIT. Fifty‐five children between five and eleven years of age with egg allergy were enrolled, 40 received egg OIT and 15 placebo. Similar to other studies, the protocol for OIT consisted of three phases. After 10 and 25 months of egg OIT, children were exposed to oral food challenge (OFC) with 5 and 10 g of egg‐white powder respectively. Fifty‐five percent (22 of 40 children) and 75% (30 of 40 children) of children were considered to be desensitized. Children who passed the OFC at 22 months concluded the OIT protocol and were instructed not to consume any egg for four to six weeks. Twenty‐nine of the 30 children who passed the OFC at 22 months underwent additional OFC with 10 g of egg‐white powder at 24 months. Eleven children passed this OFC and were considered to be sustained unresponsive (28% according to the intention‐to‐treat analysis). Thus, egg OIT appears to produce desensitization in a considerable proportion of patients with egg allergy.
SLIT offers an alternative to OIT, although evidence suggests it could be less effective than OIT in inducing desensitization. Nonetheless, SLIT appears to be superior to OIT in terms of safety, specifically in the treatment of peanut allergy (Le 2014). In addition, a variety of food allergens have been treated using SLIT, such as kiwi, milk, peach, hazelnut and peanut (Le 2014). In 2011, Kim et al. (Kim 2011) published a double‐blind, placebo‐controlled study to investigate the reaction threshold to peanut ingestion after 12 months of peanut SLIT. Eighteen subjects aged between one and eleven years of age were randomized; eleven to peanut SLIT and seven to placebo. Children were instructed to have a diet free of peanut during the study. After 12 months of SLIT or placebo, subjects were exposed to a double‐blind, placebo‐controlled food challenge (DBPCFC) with peanut. The eleven subjects in the active group ingested a median cumulative dose of 1710 mg compared with 85 mg in the placebo group. Therefore, SLIT was able to produce desensitization in subjects with peanut allergy. Subsequently, Feischer et al. (Fleischer 2013) published a randomized, double‐blind, placebo‐controlled multicenter trial to investigate the clinical effects and safety of peanut SLIT. Forty subjects were randomized to two groups: 20 to peanut SLIT and 20 to placebo. After 44 weeks, a 5 g OFC was performed and the results were compared to the baseline 2 g OFC. Fourteen subjects in the SLIT group versus three in the placebo group consumed 5 g or at least 10 fold more peanut powder than at baseline, with a median successfully consumed dose of 371 mg (responders). At 68 weeks, fifteen subjects underwent 10 g OFC and the median successfully consumed dose increased to 996 mg. The results support the concept that peanut SLIT induced desensitization in subjects with peanut allergy compared with placebo.
SLIT and OIT have been compared in one open‐label randomized controlled trial. Keet et al. (Keet 2012) compared the efficacy of SLIT versus OIT in 30 children with cow´s milk allergy (CMA). At the start of the trial, all subjects received SLIT when CMA was confirmed by DBPCFC. After a minimum of four weeks with SLIT, all subjects were randomized into three different groups: one group continued with SLIT with a target goal of 7 mg daily (SLIT/SLIT), and the other two groups were crossed over with OIT goal doses of either 1000 mg (SLIT/OITB) or 2000 mg (SLIT/OITA). When the target dose was reached the interventions were maintained for 12 weeks and then all subjects were exposed to DBPCFC again. All subjects underwent 48 weeks of intervention (or 60 weeks of maintenance) and were subsequently exposed to open OFC with 8 g of cow´s milk protein (OFC‐1). Another OFC was carried out a week later (OFC‐2). Finally, five weeks after OFC‐2, the last OFC (OFC‐3) was carried out only in subjects who passed OFC‐2. One of the ten subjects in the SLIT/SLIT group, six of the ten subjects in the SLIT/OITB group, and eight of the ten subjects in the SLIT/OITA group were not reactive to the entire 8 g challenge after 60 weeks of treatment. After six weeks off treatment one of the 10 subjects in the SLIT/SLIT group, three of the ten subjects in the SLIT/OITB group, and five of the ten subjects in the SLIT/OITA group were considered tolerant. In summary, desensitization with OIT was more efficacious than SLIT in subjects with CMA. However, clinical desensitization could not be maintained in some subjects after one week off therapy. Based on the existing evidence, OIT and SLIT can induce desensitization; however further studies are needed to establish long term efficacy and safety (Le 2014).
How the intervention might work
Desensitization is defined as the ability to increase the amount of food protein required to induce a clinical reaction, while still on regular immunotherapy (Le 2014). Sustained unresponsiveness or tolerance is the ability to consume large amounts of the food protein after treatment cessation. Thus food allergy immunotherapy aims to establish a permanent state of tolerance. While the mechanism of mucosal immunotherapy (OIT or SLIT) is unclear, immunotherapy appears to alter the T cell responses to the allergen by skewing the Th2 response to a Th1 response via the induction of regulatory T cells (Tregs). These Tregs can be natural (thymus derived) or inducible (antigen‐specific), and both can suppress the immune responses by different mechanisms, including secretion of Interleukin‐10 (IL‐10) and transforming growth factor‐b (TGF‐b) (Shevach 2009). Both these cytokines have been found to be important in food allergies (Chehade 2005; Maggi 2010; Mousallem 2012; Perez‐Machado 2003).
Why it is important to do this review
OIT and SLIT are approaches to treating food allergies. Existing evidence suggests that OIT appears to be more effective than SLIT (Scott‐Taylor 2005; Keet 2012). However, the effectiveness and safety of these interventions in fruit allergy is still unclear. This review analyses the available evidence investigating the efficacy associated with OIT and SLIT for the management of allergy to fruits.
Objectives
To determine the efficacy and safety of oral and sublingual immunotherapy in children and adults with food allergy to fruits, when compared with placebo or an elimination strategy.
Methods
Criteria for considering studies for this review
Types of studies
We included only randomised controlled trials (RCTs) comparing OIT or SLIT with placebo or an elimination diet.
Types of participants
We included studies of children and adults diagnosed with food allergies who presented 'immediate fruit' reactions. 'Immediate' allergic reactions are IgE mediated and defined as: 1) a suggestive history and positive skin prick test to fruit, represented by a wheal size of ≥ 3 mm, compared with saline control or with an elevation of serum IgE specific to fruit (cut point defined by each centre); 2) positive oral challenges: open and simple or double‐blind placebo‐controlled trial. The methodology, application and interpretation of provocation tests in patients with serum IgE‐mediated reactions have been established by the European Academy of Allergology and Clinical Immunology (Bindslev‐Jensen 2004).
Types of interventions
We included oral or sublingual immunotherapies with fruits, which were administered through any protocol and compared to a placebo or continuous elimination diet control, with or without carriage of an epinephrine auto‐injector.
Types of outcome measures
Primary outcomes
Evidence of desensitization: an increase in the amount of fruit that can be tolerated while receiving immunotherapy (oral or sublingual)
Evidence of immunologic tolerance: a complete recovery from allergy to fruits after completion of immunotherapy (oral or sublingual), or after a period of not having eaten the fruit involved
Secondary outcomes
Number of days free of symptoms
Changes in quality of life related to health, assessed by generic and specific instruments for food allergy
Local adverse reactions: Oral Allergy Syndrome (OAS), angioedema, rash, gastrointestinal symptoms
Systemic Adverse Reactions: commitment of two or more systems, including anaphylaxis
Immunological Changes:
Decrease in the size of the wheal obtained through the prick test
Decrease in the level of specific serum IgE for the fruit
Increased levels of specific IgG4 for the fruit.
Search methods for identification of studies
Electronic searches
The Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, CINAHL, and AMED were searched for published results along with the following trial registries: Current Controlled Trials (www.controlled‐trials.com); The U.S. National Institutes of Health Ongoing Trials Register (www.clinicaltrials.gov); The Australian and New Zealand Clinical Trials Registry (www.anzctr.org.au); and The World Health Organization International Clinical Trials Registry platform (www.who.int/trialsearch). In addition the Journal of Negative Results in BioMedicine (http://www.jnrbm.com/) was searched for grey literature. The date of the most recent search was July 2015. Restrictions on language of publication were not imposed.
See Appendix 1 for the MEDLINE search strategy, Appendix 2 for the EMBASE search strategy, Appendix 3 for the CENTRAL search strategy, Appendix 4 for the CINAHL search strategy, and Appendix 5 for the AMED search strategy.
Searching other resources
We checked reference lists of all primary studies and reviewed articles for additional references. We contacted authors of identified trials and asked them to identify other published and unpublished studies. We also contacted manufacturers and experts in the field.
Data collection and analysis
Selection of studies
Two independent evaluators (JJYN and YZ) screened the titles and abstracts, obtained through the electronic searches, to identify studies to include in the review. If a study was deemed potentially relevant we obtained the full text and both authors also assessed this to make a final eligibility decision. In the case of any disagreement this would have been discussed with a third reviewer (JMR). If additional information or any clarification was needed about any study, we contacted article authors.
Data extraction and management
Two independent evaluators (JJYN and YZ) read all reports of eligible studies in detail and summarised the pertinent details in a standard data extraction sheet (which included the type of study; methodology; characteristics of participants, results and outcome measurements; as well as an evaluation of the quality of methodology). We discussed any disagreements, and aimed to reach agreement by consensus with a third reviewer (MR).
Assessment of risk of bias in included studies
Two review authors (JJYN and YZ) independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreement by discussion, or by involving a third assessor. We assessed risk of bias according to the following domains:
Sequence generation (selection bias). For each included study, we described in detail the methodology used to generate the allocation sequence, and we evaluated the methodology to determine if it was likely to produce comparable groups. We rated sequence generation according to the following criteria: low risk of bias (any truly random process, e.g. random number table, computer random number generator); high risk of bias (any non‐random process, e.g. odd or even date of birth, hospital or clinic record number); or unclear risk of bias.
Allocation concealment (selection bias). For each included study, we described in detail the methodology used to conceal the allocation sequence and we evaluated the methodology to determine whether intervention allocation could have been foreseen in advance, during recruitment, or changed after assignment. We evaluated allocation concealment using the following criteria: low risk of bias (e.g. telephone or central randomisation, consecutively numbered sealed opaque envelopes); high risk of bias (e.g. open random allocation, unsealed or non‐opaque envelopes, alternation, date of birth); or unclear risk of bias.
Blinding (performance bias). For each included study, we described the methodology used, if any, to blind study participants and personnel from knowing the intervention that a participant received. We also provided information on whether the intended blinding was effective. Where blinding was not possible, we assessed whether the lack of blinding was likely to have introduced bias. We assessed blinding separately for different outcomes or classes of outcomes. We evaluated blinding as: low risk of bias; high risk of bias; or unclear risk of bias for participants, and outcome evaluators.
Incomplete outcome data (attrition bias through withdrawals, dropouts, or protocol deviations). For each included study and for each outcome or class of outcomes, we included a description of data completeness, including attrition and exclusions from the analysis, as well as an assessment of the reasons for attrition or data exclusion (if available). We recorded the number of attrition and exclusions, as well as the number of patients included in the analysis at each stage (compared with the total randomised participants).
Selective outcome reporting. We assessed selective outcome reporting for each included study. We evaluated selective outcome reporting based on the following criteria: low risk of bias (where it was clear that all of the study’s prespecified outcomes and all expected outcomes of interest to the review had been reported); high risk of bias (where not all of the study’s prespecified outcomes had been reported, one or more reported primary outcome(s) was/were not prespecified, outcomes of interest were reported incompletely and so could not be used, study failed to include results of a key outcome that would have been expected to have been reported); or unclear risk of bias.
Measures of treatment effect
We assessed the treatment effect through risk ratios (RRs) for dichotomous outcomes for individual studies. We presented all measures with 95% confidence intervals (CIs). We carried out all statistical analyses using Review Manager 5.3 (RevMan 2011). We planned to use standardized mean differences (SMDs) for continuous outcomes, had appropriate data been available.
Unit of analysis issues
The unit of analysis was the patient for dichotomous outcomes such as presence or absence of tolerance, partial tolerance and adverse effects.
Dealing with missing data
We contacted authors where details about study design or descriptive statistics for outcomes were not presented in original papers. We conducted the main analysis of dichotomous and continuous outcomes on the resulting available study data.
We also planned to conducted sensitivity analyses by conducting an intention‐to‐treat analysis for dichotomous outcomes, imputing missing data on bases that were considered to be clinically plausible by the authors: for the outcome 'evidence desensitization', we planned to assume that all lost patients did not improve and thus did not present with the event. For adverse effects, both local and systemic, we planned to assume a worst case scenario where all patients lost from immunotherapy presented adverse effects, but none of the lost patients in the control group presented with them. However, the current data did not warrant such an approach.
Assessment of heterogeneity
We assessed clinical heterogeneity by examining the differences and similarities between trials in terms of patient characteristics, interventions, controls and definition of results.
We evaluated statistical heterogeneity through the I2 statistic. A cut‐off point of I2 > 40% to indicate relevant statistical heterogeneity was used (Higgins 2002). We planned to investigate potential causes of heterogeneity through sensitivity and subgroup analyses.
Assessment of reporting biases
We planned to create a funnel plot if ten or more studies were available, to investigate the likelihood of any publication bias existing (Egger 1997).
Data synthesis
We planned to perform meta‐analyses using a random‐effects model and using the inverse variance method. We also planned to present forest plots for each outcome for which data was available. However, meta‐analyses were not appropriate.
Subgroup analysis and investigation of heterogeneity
We planned subgroup analyses according to the immunotherapy regimen (oral vs sublingual), type of fruit allergenic: peach, apple, banana, kiwi, melon, strawberry and citrus fruits, and according to age (children and adults).
Sensitivity analysis
As mentioned above we planned sensitivity analyses to investigate the impact of carrying out analyses based on an intention‐to‐treat approach, imputing missing data. We also planned analyses to determine the effect of the following parameters on treatment effect estimates: risk of bias (including only low risk of bias studies), and meta‐analysis model used (applying a fixed‐effect model compared to a random effects model).
Summary of findings
We summarized the findings of our primary outcomes and adverse events in a summary of findings table, using the GRADE approach to assess the quality of the body of evidence, according to standard Cochrane methodology.
Results
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies.
Results of the search
Our search of the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, CINAHL, AMED and other resources (trial registries and grey literature) yielded 703 records, after duplicates were removed. Screening of titles and abstracts by two review authors led to the exclusion of 683 references. We reviewed the full text of 20 publications, (reporting 18 studies) in detail, resulting in the inclusion of two randomized clinical trials (Fernandez‐Rivas 2009; Kopac 2012) reported by four publications in total. None of the studies found in the grey literature search presented sufficient information about population or the characteristics of the allergen used in immunotherapy to be judged eligible. Figure 1 is a flowchart illustrating the selection of eligible studies, following PRISMA guidance.
1.
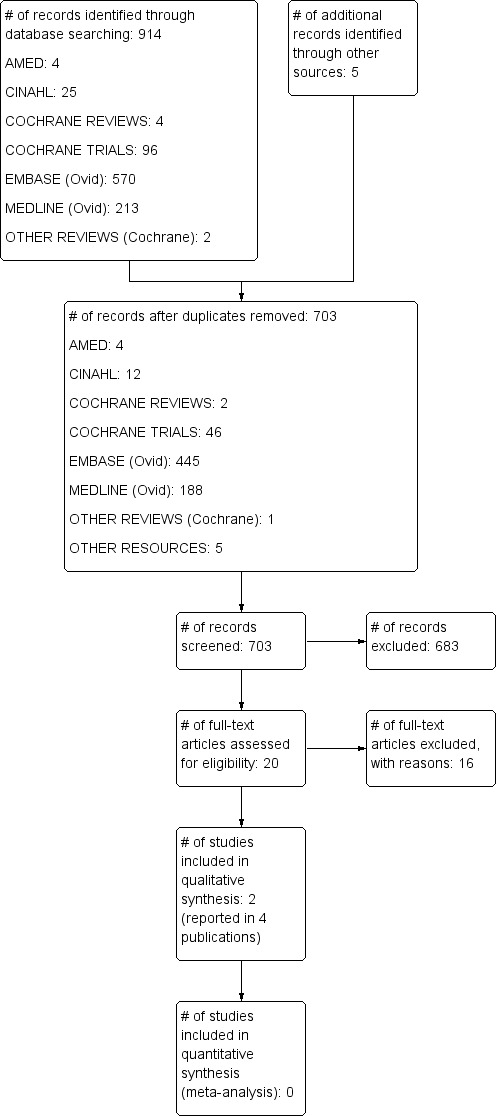
Study flow diagram.
Included studies
We included two randomized controlled trials in this review. One study compared sublingual immunotherapy, using Pru p 3 (peach extract), with placebo (Fernandez‐Rivas 2009), and the other compared regular apple consumption with no treatment (Kopac 2012).
Fernandez‐Rivas 2009
Fernandez‐Rivas 2009 was a randomised double blind placebo controlled trial, which was conducted in two centres: at the Servicio de Alergia, Hospital Clinico San Carlos, Madrid, Spain, and at the Unidad de Alergia, Hospital de Fuenlabrada, Fuenlabrada, Spain. The participants were 56 adults (32 female, 24 male) aged between 18 and 65 years, with immediate reactions to peach ingestion, specific IgE to peach proven by positive skin prick test, and/or by a peach CAP ≥ 0.70 KU/l and positive DBPCFC with peach. Randomization was to either SLIT with peach extract of Pru p 3 or placebo. Peach extract was obtained from fresh peelings and quantified in micrograms of Pru p 3. The treatment was administered sublingually (sublingual‐swallow technique) and comprised four vials containing 0.4, 2, 10 and 50 ug/ml of Pru p 3 or placebo. After a rush build phase with SLIT, participants had six months of home maintenance. During the maintenance phase participants completed diary cards. Efficacy of the intervention was assessed by the change in the response to a DBPCFC at baseline and after 6 months of treatment.
Kopac 2012
Kopac 2012 was an open, randomized controlled parallel group trial which was conducted during eight months at the Division of Allergology, Inselspital, University Hospital of Bern, Switzerland. The participants consisted of 40 adults (28 females and 12 males) with a mean age of 37 years (range 18 to 61) with birch pollen allergy and associated oral allergy syndrome to apple (OAS). The study started and concluded outside birch pollen season. Sensitization was confirmed by positive skin prick test to birch pollen, positive intradermal test with Bet v1 and Mal d 1, specific IgE to Bet v 1 (≥ 0.70 KU/l), and positive oral challenge provocation with fresh apple. Randomization was to either oral tolerance induction with small amounts of apple (active group) or no treatment control. The oral induction tolerance consisted of two phases: a build phase and a maintenance phase. Efficacy was assessed as the proportion of participants that tolerated at least 128 g of apple after eight months of treatment in the active treatment versus control group.
For further details of included studies, see the Characteristics of included studies table.
Excluded studies
After full text review 16 studies were excluded. Most were excluded because they were not randomized controlled trials. However, one study was excluded because the intervention treatment was made with pollen extract instead of fruit extract (Hansen 2004). Individual reasons for study exclusion are given in the Characteristics of excluded studies table.
Risk of bias in included studies
Both of the included studies were judged to be at low risk of bias for the selective reporting and 'other bias' domains. This judgment was made because published reports included all expected outcomes, including those that were pre‐specified. Neither of the studies reported intention‐to‐treat analyses. In the case of the tolerance assessment outcome both Kopac 2012 and Fernandez‐Rivas 2009, used the "last observation carried forward" (LOCF) method to impute missing data. Kopac 2012 was judged to be at high risk of bias in most domains, except allocation concealment, selective reporting, and other bias. No specific information about statistical issues was provided by the authors. Fernandez‐Rivas 2009 described their random sequence generation clearly and did not have differential dropout; however, it was not clear how concealment and blinding of participants and personnel was carried out.
Study‐specific risk of bias assessments are presented in Figure 2 for each criteria, and these judgements are summarized by risk of bias criteria in Figure 3. See below and the Characteristics of included studies table for justifications of each judgement.
2.
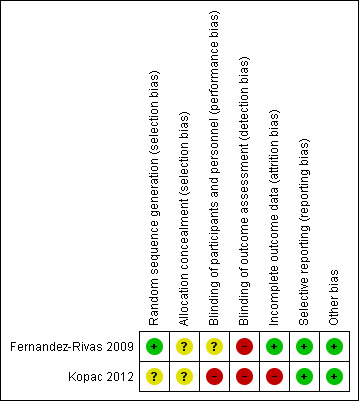
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
3.
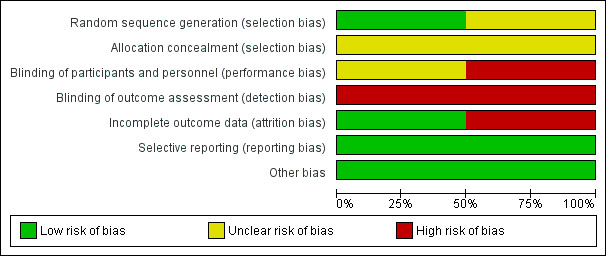
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
The Fernandez‐Rivas 2009 trial had a low risk of allocation bias (selection bias) as they used stratified block randomization, with an allocation ratio of 2:1. In Kopac 2012, the method of sequence generation was not described and was therefore judged to be at unclear risk of bias, though the authors did state that randomization was based on sex, age, dose of apple‐tolerated, intradermal tests (IDT), and ratio of specific IgE to Bet v1/total IgE. Finally, the method of allocation concealment was not described in either of these studies which produced insufficient information to permit judgments of ‘Low risk’ or ‘High risk’. Therefore risk of bias was deemed 'unclear'.
Blinding
The Kopac 2012 study had an open‐label design and for this reason was judged as "high risk". Fernandez‐Rivas 2009 did not describe if participants and personnel were blinded or not, so this resulted in a unclear risk of performance bias. In addition, detection bias was high in both studies because not all of the outcomes were blinded.
Incomplete outcome data
In the Fernandez‐Rivas 2009 study, two different approaches were taken to analyses, dependent on outcome. For the efficacy outcome the analysis was carried out according to trial completion (per‐protocol analysis), whereas all participants who had received at least one dose of immunotherapy were included in the tolerance assessment. Authors used "last observation carried forward" (LOCF) to impute the missing data. This latter approach was also used in Kopac 2012 for measuring the primary endpoint, which was the proportion of patients who achieved tolerance of 128 grams of apple.
In Fernandez‐Rivas 2009 researchers randomly assigned 56 participants (37 active and 19 placebo control). Four patients withdrew from the active group: two for unknown reasons, one for personal reasons and one due to allergic symptoms not related to the treatment. In the placebo group, three patients withdrew: two patients for personal reasons, and one patient was lost to follow‐up. Therefore the trial was completed by 49 patients, 33 in the active group and 16 in the placebo group. This represented 11% of the intervention and 16% of the control group, which we did not judge to be differential attrition, and therefore we judged this study to be at low risk of attrition bias. In Kopac 2012 researchers randomly assigned 40 participants (27 active and 13 no treatment control). Five of the 27 participants in the active group (19%) and none of the 13 patients in the control group withdrew. Two patients withdrew because of side‐effects; two patients withdrew because of personal reasons; and one patient forgot to eat apples whilst on holiday for one month during the study period. We judged this study to be at high risk of attrition bias due to differential attrition between study arms.
In conclusion, these studies did not conduct intention‐to‐treat analyses but reported clearly the numbers of participants randomly assigned and withdrawn from the study.
Selective reporting
Study protocols were not available for either trial, however published reports included all outcomes expected, allowing us to make a judgment of "low risk".
Other potential sources of bias
Neither Fernandez‐Rivas 2009 or Kopac 2012 were stopped early for reasons which may have biased the results. No other sources of bias were apparent.
Effects of interventions
See: Table 1
See Table 1 for a summary of the following outcomes: evidence of desensitization; evidence of immunologic tolerance; and adverse reactions.
Evidence of desensitization
Evidence of desensitization is one of the most important outcomes in patients with food allergy. In the Fernandez‐Rivas 2009 study, the evidence of desensitization was reported as the change in the response to a double‐blind, placebo controlled, food challenge (DBPCFC) with peach, performed at baseline and after six months of SLIT, using a Log Rank test. Through direct communication with the principal author, we were also provided with additional information for this outcome (number of patients desensitized by group). In the active group, 12 of 33 patients did not present a reaction (either local or systemic) at DBPCFC after six months with SLIT. In addition, 5 of 11 patients in the placebo control group did not present any type of reaction (RR 1.16, 95% CI 0.49 to 2.74, Analysis 1.1, Figure 4). Kopac 2012 measured the tolerance to apple through an oral provocation test (OPT). Data from this study reported that tolerance of at least 128 grams of apple (the highest amount at OPT) after eight months of treatment was achieved in 17 of 27 patients in the active group and none of 13 patients in the no treatment control group (RR 17.50, 95% CI 1.13 to 270.19, Analysis 1.1). The 17 responders reached the maintenance dose after an average time interval of 20 weeks (7 to 30 weeks) of OIT. They continued to eat on average four to seven apples per week. Studies were not pooled due to significant clinical heterogeneity.
1.1. Analysis.
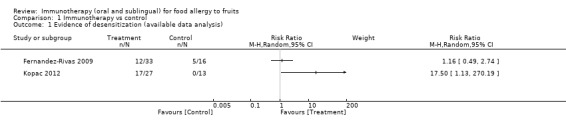
Comparison 1 Immunotherapy vs control, Outcome 1 Evidence of desensitization (available data analysis).
4.

Forest plot of comparison: 1 Immunotherapy vs control, outcome: 1.1 Evidence of desensitization (available data analysis).
Evidence of immunologic tolerance
Immunologic tolerance, defined as complete recovery from allergy to fruit after completion of immunotherapy (oral or sublingual), or after a period of not having eaten the fruit involved, was not measured by either of the included studies.
Adverse reactions
Adverse reactions were reported in both studies. Fernandez‐Rivas 2009 split reactions into local and systemic, whereas Kopac 2012 did not differentiate between the two. None of these studies reported anaphylaxis reactions with oral or sublingual immunotherapy (note: Kopac 2012 excluded participants with a history of anaphylaxis from fruit or nut ingestion).
During the six weeks that participants were treated with sublingual immunotherapy in the Fernandez‐Rivas 2009 study, they experienced a greater rate of local adverse reactions than participants in the control group, with 1328 events in 33/37 (89.2%) participants versus 9 events in 5/18 (27.8%) participants (RR 3.21, 95% CI 1.51 to 6.82; analysis not shown). Most adverse reactions were located on the oropharynx, and the remaining ones consisted of transient gastric complaints. Local reactions were more frequently recorded during the build‐up phase and the first maintenance week, whereas during the home maintenance period up to 40.5% of patients did not present with any complaints. Nineteen systemic reactions were reported, 16 in 5/37 (13.5%) participants in the active group and three in 3/18 (16.7%) participants in the control group (RR 0.81, 95% CI 0.22 to 3.02; analysis not shown). In the active group, 14 of the reactions were recorded during the build‐up phase, one during the hospital maintenance week and one during the home maintenance period. Systemic reactions consisted of skin reactions (six patients), rhinoconjunctivitis (one patient), and digestive symptoms (seven patients). Two reactions were observed in the placebo group in the first maintenance week (one angioedema and one diarrhoea) and one in the build‐up phase (cutaneous itching). Finally, all the systematic reactions had a mild intensity and most resolved spontaneously or decreased after treatment with oral antihistamines, antacids and/or omeprazole.
The Kopac 2012 study reported fewer adverse reactions in the active treatment group than the Fernandez‐Rivas 2009 study. Only two of 25 (8.0%) participants presented relevant side‐effects in the OIT group ‐ one patient reported severe rhinoconjunctivitis and an increase in oral allergy symptoms and another patient reported diarrhoea after three weeks of continuous apple exposure. None of the 13 patients in the control group reported relevant side effects. Given the clinical heterogeneity among comparator conditions, it was not appropriate to pool data from the two trials.
Change in the size of the wheal obtained through the prick test
Both studies reported on the change of skin test wheal size before and after OIT or SLIT, using different techniques. The Fernandez‐Rivas 2009 study performed skin prick tests (SPT) at the beginning of the study (T0), one month later (T1) and at the end of the study (six months ‐ T6), with four peach extracts containing Pru p 3. Changes in skin prick tests were studied by parallel‐line assay and were expressed as the cutaneous tolerance index (CTI). The intradermal test (IDT) was used in the Kopac 2012 study with recombinant apple extract (rMal d 1) at the beginning of the study (T0), and at the end (eight months ‐ T8), and the difference between these two measures was calculated (Δ T0‐T8). According to Fernandez‐Rivas 2009, the SLIT for peach allergy resulted in a decrease in the SPT result, meaning that after six months of treatment a higher concentration of peach extract was needed to induce the same wheal area as at T0. Non significant changes in IDT and CTI were reported in Kopac 2012. Due to the difference in measurement techniques we thought it inappropriate to combine the results from the two studies in a meta‐analysis and instead describe the results in Table 2.
1. Change in the size of the wheal obtained through the prick test.
| Study ID | Result |
| Fernandez‐Rivas 2009 | After 6 months the patients in the active group had a significant decrease in skin prick test (SPT) to the Pru p 3 (peach extract). The cutaneous tolerance index (CTI) was 1.83 (95% CI: 1.16–2.87, P < 0.05), meaning that after 6 months of SLIT, a concentration of peach extract 1.83 times higher was needed to induce the same wheal area as in T0. Patients in the placebo group did not show changes in the SPT response (CTI 0.87, 95% CI: 0.48–1.55, P > 0.05). |
| Kopac 2012 | Wheal size at IDT with different amounts of rMal d 1 at T0 and T8 reported as Δ T0‐T8 did not reveal a statistical significance in active vs control group (Mann‐Whitney‐U‐Test). |
Change in the level of specific serum IgE (sIgE) for the fruit
In Fernandez‐Rivas 2009 recombinant sIgE to peach (rPru p3) was measured at the beginning of the study and then at one month (T1), and at six months (T6) using the ADVIA Centaur system (Bayer Health‐ Care Diagnostics Division). sIgE to apple (Mal d1) was measured at the beginning of the study (T0) and at eight months (T8) using the ImmunoCAP system (Phadia, Uppsala, Sweden) in Kopac 2012. Again differences in measurement across studies meant these data could not be combined in a meta‐anaysis; however we describe the results at the end of each treatment (T6 and T8) for both studies in Table 3.
2. Change in the level of sIgE.
| Study ID | Result |
| Fernandez‐Rivas 2009 | sIgE to rPru p 3 showed a significant increase both in the active [T0: 3.08 (1.73–5.48), T1: 4.94 (2.63–9.28), and T6: 4.23 (2.17–8.23; P < 0.001] and placebo [T0: 3.42 (1.55–7.57), T1: 4.78 (2.00–11.4), and T6: 4.04 (1.61–10.13) ; P = 0.025] groups, although the increase remained only significant at T6 in the former (active 4.23, P < 0.001; placebo 4.04, P = 0.079, T‐test). However, no significant inter‐group differences were observed (P = 0.456). None of the patients with negative IgE to rPru p 3 at T0 converted to positive at T1 or T6. |
| Kopac 2012 | There was no significant change in Δ T0‐T8 in sIgE level to Mal d 1 in active vs control group [active 5.12 (3.5–25.2), control 3.50 (1.6–21.7), P= 0.52, Mann‐Whitney‐U‐Test]. |
Change levels of specific IgG4 for the fruit
Data for IgG4 were presented in both studies. Specific IgG4 to nPru p 3 was determined by means of ELISA in Fernandez‐Rivas 2009, and IgG4 antibodies to Mal d1 were determined using the ImmunoCAP system (Phadia, Uppsala, Sweden) in Kopac 2012, at the same time as specific serum IgE was measured. No significant differences in IgG4 levels in response to peach (n Pru p 3) or apple (Mal d 1) were observed after treatment. We describe the results at the end of each treatment (T6 and T8) for both studies in Table 4. Again data was not combined in a meta‐analysis due to measurement differences, which could not be standardised.
3. Change in the level of specific serum IgG4.
| Study ID | Result |
| Fernandez‐Rivas 2009 | IgG4 to nPru p 3 showed a different evolution between groups (P = 0.022) with a significant increase in the active arm [T0: 0.11 (0.06–0.18), T1: 0.12 (0.07–0.20), and T6: 0.13 (0.07–0.24); P = 0.007] not observed in the placebo one [T0: 0.10 (0.05–0.22), T1: 0.10 (0.05–0.21), and T6: 0.09 (0.04–0.18); P = 0.185]. |
| Kopac 2012 | There was no significant change in Δ T0‐T8 in IgG4 level to Mal d 1 in active vs control group [active 0.11 (0.02–0.31), control 0.04 (0.00–0.22), P= 0.06, Mann‐Whitney‐U‐Test]. |
Other outcomes
No other outcomes specified in the protocol were reported in the included studies.
Discussion
Summary of main results
This review identified two randomised controlled trials (RCTs) which fit our inclusion criteria. However, we identified high clinical, methodological and statistical heterogeneity between studies, and therefore we were unable to present pooled estimates of outcome effects. As a result findings should be interpreted with caution.
Evidence of desensitization was mixed, with one study demonstrating an imprecise positive effect of treatment for apple allergy, and another showing no effect of treatment on desensitization to peach allergy. There was very limited evidence that patients treated with oral or sublingual immunotherapy for allergy to fruit have an increase in the presentation of local and systemic adverse reactions, principally during the build‐up phase, which subsided either spontaneously or was treated successfully with oral antihistamines, antiacids and/or omeprazole. The most frequent local reactions presented were oral allergy syndrome symptoms (itching and hoarseness) and transient gastric complaints such as diarrhoea. Skin symptoms such as urticaria and erythema, were the main systemic reactions reported. The presentation of these reactions have been reported as common effects with oral or sublingual immunotherapy for patients with food allergy to milk (Yeung 2012), peanut (Nurmatov 2012), egg (Burks 2012b), kiwi (Kerz 2007), and hazelnut (Enrique 2008). Although anaphylactic reactions have been reported in response to this type of treatment for other allergens, in these cases (of peach and apple), studies did not replicate this finding, and participants did not require use of adrenaline.
Immunological changes were reported as change in the size of the wheal obtained through a prick test, change in the level of specific serum IgE for the fruit, and change in levels of specific IgG4 for the fruit after treatment, in both studies. Data could not be pooled, and individual data from each study provided through cutaneous tests such as SPT and IDT produced contradictory results. In Fernandez‐Rivas 2009 the active group showed a decrease in SPT with Pru p and IgG4 to peach showed an increase in the active group which was not observed in the control group; however no serological changes in sIgE to peach were reported after SLIT. Similarly, no changes in wheal size were present after OIT with apple, and the Kopac 2012 treatment did not appear to result in changes in sIgE and IgG4.
We were unable to carry out any subgroup or sensitivity analyses to explore effects further due to the minimal studies found to be eligible for inclusion in the review. This also made the exploration of publication bias, using funnel plots, impossible.
Overall completeness and applicability of evidence
The included studies were identified through an extensive, systematic search of the published and unpublished literature. Therefore, we believe that this review represents a summary of the best evidence available on the efficacy and safety of oral or sublingual immunotherapy in patients with allergy to fruit. Required outcomes were reported in both studies, including measurements of efficacy and adverse outcomes, as well as antibody test results. However, our conclusions are limited by the quality, heterogeneity and applicability of the findings. The Fernandez‐Rivas 2009 study was a small (N= 56) exploratory randomized clinical trial. The Kopac 2012 study was an open, parallel‐controlled trial of only 40 patients. Both studies were carried out in very specific, heterogenous patient groups (those with allergy to apple and those with allergy to peach). Unfortunately neither study produced data on the evidence of immunologic tolerance.
Quality of the evidence
Conclusions from both studies should be interpreted and generalised with caution given the small sample size and the high specificity of the interventions used. No definite conclusion can be made on the efficacy of immunotherapy for fruit allergy. According to the GRADE criteria, the overall quality of evidence was very low for the primary and secondary outcomes due to serious risk of bias, as well as serious imprecision. Fernandez‐Rivas 2009 clearly described the random sequence generation but it was not clear whether and how allocation concealment and the blinding of participants and personnel was conducted. For the efficacy evaluation, the analysis was carried out according to patients who completed the trial (per‐protocol analysis) rather than using an intention‐to‐treat analysis; however a sensitivity analysis was planned to account for this. Kopac 2012 was an open label study which was subject to high risk of bias due to selection bias and lack of blinding. The authors used the "last observation carried forward" (LOCF) to account for missing data. No details of random sequence generation or data analysis were provided. Overall the quality of evidence was very low for all outcomes, which suggests that the conclusions of this review are very likely to be impacted by the inclusion of future studies.
Potential biases in the review process
We do not believe that there were any potential sources of bias during the systematic review process, on account of the comprehensive search strategy, independent screening and data extraction by two review authors and the restriction of inclusion criteria to RCTs only.
Agreements and disagreements with other studies or reviews
To our knowledge, this is the first systematic review to summarize the evidence available for OIT and SLIT for treatment of food allergy to fruit. Systematic reviews have been carried out to assess these types of treatment for other food allergies, including milk (Yeung 2012), peanut (Nurmatov 2012) and egg (Romantsik 2014). However, these also had small numbers of patients and/or low quality evidence included, and demonstrated a high rate of adverse effects; principally local. This is in agreement with the limited findings of this review, and we also agree that OIT and SLIT treatments require further study before routine implementation for the management of food allergies.
Authors' conclusions
Implications for practice.
The only evidence we were able to identify referred to immunotherapy treatment for allergy to peach and apple. Though one study did find a statistically significant effect on desensitisation, the quality of the evidence is very low and the other study did not detect an effect. The two included studies included small numbers of patients and no children, and were judged to be at risk of bias. Mild or moderate adverse reactions were presented more frequently in people receiving OIT or SLIT. However, these reactions could be treated successfully with ambulatory medications.
Implications for research.
Further research must be done in order to increase the size of the evidence base and improve the precision and generalization of the results. Additional RCTs should be conducted in other fruits that produce food allergy, as well as apple and peach. New studies should also be carried out in relevant paediatric populations. New studies should also take into account the potential occurrence of anaphylactic reactions. Finally, new RCTs should report their results according to international recommendations such as The CONSORT Statement in order to improve the quality of research used in decision‐making for fruit allergy treatment.
What's new
| Date | Event | Description |
|---|---|---|
| 6 February 2020 | Amended | Note on declarations of interest added to review |
Notes
This review is currently not compliant with Cochrane Commercial Sponsorship policy. The next updated version of the review will be compliant.
Acknowledgements
Many thanks to the authors of individual studies who provided additional data and to Thomas Fanshawe (University of Oxford) for his statistical support. Many thanks to Matthew Greenhawt at the University of Michigan for subject‐specific editorial support.
Appendices
Appendix 1. MEDLINE search strategy
1. exp Fruit/ (56180)
2. Fruit$ protein$.mp. (54)
3. (fruit$ and allerg$).mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier] (1395)
4. Peach$.mp. (2281)
5. Apple$.mp. (10088)
6. Banana$.mp. (2912)
7. Kiwi$.mp. (918)
8. Strawberry.mp. (2027)
9. Citrus fruit$.mp. or Citrus/ (5402)
10. Melon$.mp. (1913)
11. exp Food Hypersensitivity/ (14622)
12. Food allerg$.mp. (7591)
13. 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 (90747)
14. exp Immunotherapy/ (207996)
15. exp Desensitization, Immunologic/ (8324)
16. Oral immunotherapy.mp. (266)
17. Oral desensitization.mp. (116)
18. OIT.mp. (159)
19. Oral induction.mp. (32)
20. Oral tolerance.mp. (1523)
21. exp Sublingual Immunotherapy/ (8)
22. SLIT.mp. (12687)
23. 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 (221483)
24. 13 and 23 (1547)
25. Randomized controlled trial.pt. (371712)
26. randomized.mp. (560596)
27. placebo.mp. (158272)
28. 25 or 26 or 27 (609669)
29. 24 and 28 (174)
Appendix 2. EMBASE search strategy
1. exp Fruit/ (77955)
2. Fruit$ protein$.mp. (82)
3. (fruit$ and allerg$).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword] (2604)
4. Peach$.mp. (2758)
5. Apple$.mp. (12730)
6. Banana$.mp. (3876)
7. Kiwi$.mp. (1405)
8. Strawberry.mp. (2861)
9. Citrus fruit$.mp. or Citrus/ (5477)
10. Melon$.mp. (2451)
11. exp Food Hypersensitivity/ (22294)
12. Food allerg$.mp. (22097)
13. 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 (114610)
14. exp Immunotherapy/ (127076)
15. exp Desensitization, Immunologic/ (16686)
16. Oral immunotherapy.mp. (607)
17. Oral desensitization.mp. (206)
18. OIT.mp. (326)
19. Oral induction.mp. (55)
20. Oral tolerance.mp. (2126)
21. exp Sublingual Immunotherapy/ (848)
22. SLIT.mp. (19974)
23. 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 (163299)
24. 13 and 23 (2808)
25. random:.tw. (874242)
26. placebo:.mp. (327962)
27. double‐blind:.tw. (145397)
28. 25 or 26 or 27 (1086262)
29. 24 and 28 (481)
Appendix 3. COCHRANE (CENTRAL) search strategy
1. MeSH descriptor: [Fruit] explode all trees (1255)
2. Fruit$ protein$.mp. (5)
3. (fruit$ and allerg$) .mp. (3)
4. "peach":ti,ab,kw (Word variations have been searched) (54)
5. "apple":ti,ab,kw (Word variations have been searched) (308)
6. Banana:ti,ab,kw (Word variations have been searched) (73)
7. Kiwi:ti,ab,kw (Word variations have been searched) (29)
8. Strawberry:ti,ab,kw (Word variations have been searched) (81)
9. Citrus fruit:ti,ab,kw (Word variations have been searched) (114)
10. MeSH descriptor: [Citrus] explode all trees (337)
11. Melon:ti,ab,kw (Word variations have been searched) (16)
12. MeSH descriptor: [Food Hypersensitivity] explode all trees (597)
13. Fruit protein:ti,ab,kw (Word variations have been searched) (238)
14. #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 (2549)
15. MeSH descriptor: [Immunotherapy] explode all trees (6763)
16. MeSH descriptor: [Desensitization, Immunologic] explode all trees (735)
17. Oral immunotherapy:ti,ab,kw (Word variations have been searched) (312)
18. Oral desensitization:ti,ab,kw (Word variations have been searched) (176)
19. OIT:ti,ab,kw (Word variations have been searched) (28)
20. Oral induction:ti,ab,kw (Word variations have been searched) (2425)
21. Oral tolerance:ti,ab,kw (Word variations have been searched) (4046)
22. MeSH descriptor: [Sublingual Immunotherapy] explode all trees (0)
23. Sublingual Immunotherapy:ti,ab,kw (Word variations have been searched) (514)
24. SLIT:ti,ab,kw (Word variations have been searched) (934)
25. #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 (14227)
26. #14 and #25 (123)
27. randomized controlled trial:pt (Word variations have been searched) (346349)
28. controlled clinical trial:pt (Word variations have been searched) (300103)
29. randomized:ti,ab,kw (Word variations have been searched) (325318)
30. placebo:ti,ab,kw (Word variations have been searched) (145510)
31. MeSH descriptor: [Clinical Trials as Topic] this term only (34469)
32. randomly:ti,ab,kw (Word variations have been searched) (101007)
33. "trial":ti (Word variations have been searched) (137016)
34. #27 or #28 or #29 or #30 or #31 or #32 or #33 (596189)
35. MeSH descriptor: [Animals] explode all trees (6411)
36. MeSH descriptor: [Humans] explode all trees (768)
37. #35 not #36 (5643)
38. #34 not #37 (590680)
39. #26 and #38 (93)
Appendix 4. CINAHL search strategy
S1. (MH "Fruit+") (9,663)
S2. TX fruit* and allerg* (152) View Details Edit
S3. TX Fruit* and protein* (765)
S4. TX Peach* (562)
S5. TX Apple* (2,988)
S6. TX Banana* (237)
S7. TX Kiwi* (121)
S8. TX Strawberry* (198)
S9. TX Citrus fruit* (90)
S10. TX Melon* (312)
S11. (MH "Citrus+") (737)
S12. (MH "Food Hypersensitivity+") (2,722)
S13. TX Food allerg* (1,124)
S14. S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 (16,363)
S15. (MH "Immunotherapy+") (16,786)
S16. (MH "Desensitization, Immunologic") (504)
S17. TX Oral immunotherapy (56)
S18. TX Oral desensitization (21)
S19. TX OIT (35)
S20. TX Oral induction (2)
S21. TX Oral tolerance (54)
S22. (MM "Administration, Sublingual") (87)
S23. TX Sublingual Immunotherapy (131)
S24. TX SLIT (277)
S25. S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23 OR S24 (17,137)
S26. S14 AND S25 (219)
S27. TX randomized (71,987)
S28. MW treatment outcomes (112,443)
S29. PT clinical trial (51,312)
S30. S27 OR S28 OR S29 (191,285)
S31. S26 AND S30 (21)
Appendix 5. AMED search strategy
1. exp Fruit/ (786)
2. (fruit$ and allerg$).mp. [mp=abstract, heading words, title] (23)
3. Peach$.mp. (11)
4. Apple$.mp. (77)
5. Banana$.mp. (24)
6. Kiwi$.mp. (15)
7. Strawberry.mp. (10)
8. Citrus fruit$.mp. (15)
9. Citrus.mp. (131)
10. Melon$.mp. (24)
11. exp Food hypersensitivity/ (126)
12. (Food$ and allerg$).mp. [mp=abstract, heading words, title] (182)
13. 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 (1252)
14. exp Immunotherapy/ (85)
15. exp Desensitization/ (17)
16. OIT.mp. (1)
17. Oral tolerance.mp. (6)
18. Sublingual.mp. (47)
19. Sublingual Immunotherapy.mp. (1)
20. SLIT.mp. (17)
21. 14 or 15 or 16 or 17 or 18 or 19 or 20 (171)
22. 13 and 21 (4)
Data and analyses
Comparison 1. Immunotherapy vs control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Evidence of desensitization (available data analysis) | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Fernandez‐Rivas 2009.
| Methods |
Design: Randomised controlled trial Participating centres: * Servicio de Alergia, Hospital Clinico San Carlos, Madrid, Spain. * Unidad de Alergia, Hospital de Fuenlabrada, Fuenlabrada, Spain. |
|
| Participants |
Inclusion criteria: * 18 to 65 years old, * Immediate reaction to peach ingestion: ‐ Specific IgE to peach proven by positive (≥ 3 mm) SPT either to a peach extract or fresh peach (prick–prick technique) and/or by a peach CAP ≥ 0.70 kU/l (Phadia, Uppsala, Sweden), and positive double‐blind, placebo‐controlled food challenge (DBPCFC) with peach. Exclusion criteria: * Placebo reaction in the DBPCFC with peach, previous history of food allergic reactions with hypotension, a case history of allergy to coconut, pollen immunotherapy within the previous 2 years and any clinical condition that contraindicates immunotherapy according to the European Academy of Allergy and Clinical Immunology (EAACI) guidelines or that the investigators judged that inclusion might hamper the patients safety or the study outcomes. No. of randomly assigned participants: 56 (37 SLIT and 19 placebo) Age [mean (SD)]: SLIT group: 29.1 (6.1); control group: 29.7 (7.8). Sex [Nº(% male)]: SLIT group: 15 (40.6%); control group: 9 (47.4%). |
|
| Interventions |
Intervention: SLIT treatment was administered sublingually (sublingual‐swallow technique) and comprised four vials containing 0.4, 2, 10 and 50 μg/ ml of Pru p 3. Control: Placebo administered in the same way to treatment. |
|
| Outcomes |
Primary outcome: Change in response to a double blind placebo controlled food challenge (DBPCFC) with peach. Secondary outcome: Changes in skin prick test (SPT), and in specific immunoglobulin E (IgE) and IgG4 to Pru p 3. Tolerance was assessed with a careful recording of adverse events. |
|
| Notes | The clinical trial was supported by ALK‐Abelló S.A. (Madrid, Spain). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were randomized to receive either active treatment or placebo in a 2:1 proportion, respectively, over 6 months. A stratified block randomization was carried out to ensure a similar distribution of patients presenting with systemic reactions. |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not specified |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Only the "serum samples" outcome was described as blinded to investigator. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4 intervention and 3 control participants lost to follow‐up (11% versus 16%) |
| Selective reporting (reporting bias) | Low risk | All outcomes related to peach allergy treatment were measured and reported. |
| Other bias | Low risk | The trial was not stopped early for benefit and no other bias appeared to be present in this study. |
Kopac 2012.
| Methods |
Design: Open, parallel group, randomized controlled clinical trial. Participating centres: The study was conducted in the Division of Allergology, Inselspital, University Hospital of Bern. |
|
| Participants |
Inclusion criteria: Positive skin prick test to birch pollen, positive IDT with Bet v 1 and Mal d 1, sIgE to Bet v 1 ≥ 0.7 kU/l, and positive oral provocation test (OPT) with fresh apple (threshold amount ≤ 64 g). Exclusion criteria: Anaphylactic reactions to fruits and nuts, sIgE to Pru p 3 ≥ 0.35 kU/l, severe asthma, and immunotherapy within last 3 years. No. of randomly assigned participants: 40 (27 Oral tolerance induction (OTI); 13 control group) Age [mean]: OTI group: 36; control group: 42. Sex [Nº(% male)]: OTI group: 9 (33%); control group: 3 (23%). |
|
| Interventions |
Intervention: Oral tolerance induction (OTI). Patients consumed small amounts of apple daily. The starting dose was the largest amount tolerated in the preceding Oral Provocation Test (OPT). The daily amount of apple was doubled every 2–3 weeks if the current amount was well tolerated. When the whole apple (150–200 g) was tolerated without symptoms, the subject continued eating at least three apples per week to maintain tolerance. Control: No treatment. |
|
| Outcomes |
Primary outcome: Proportion of patients that achieved tolerance of 128g of apple. For missing values, the last observed value was carried forward. Secondary outcome: Clinical questionnaire about cross‐tolerance to other PR‐10 protein‐containing fruits/nuts and a symptom diary concerning pollen allergy symptoms, wheal size at IDT, and the symptom score at CPT. Laboratory exploratory endpoints were levels of tIgE, sIgE, and IgG4 to Mal d 1 and Bet v 1, and basophil reactivity to Mal d 1 and Bet v 1. |
|
| Notes | This study was supported by a research grant from Ulrich Muller‐Gierok Fundation, and P. Kopac received a scholarship from The Slovene Human Resources and Scholarship Fund. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Although investigators reported that patients were randomized, the sequence generation process was not specified (based on sex, age, dose of apple‐tolerated, intradermal tests (IDT), and ratio of specific IgE to Bet v1/total IgE) . |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The trial is described as an open randomized trial and therefore was not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | OPT assessment was unblinded as the processing which would be needed for blinding could alter the allergenic extract‐ Mal d 1. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 5 intervention (19%) and 0 control participants lost to follow‐up, risk of bias judged to be high due to differential attrition |
| Selective reporting (reporting bias) | Low risk | All outcomes relating to the treatment of apple allergy were measured. |
| Other bias | Low risk | The trial was not stopped early for benefit and no other bias appeared to be present in this study. |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Akdis 2011 | 1. Invited speaker presentation, not original contribution (not RCT). |
| Asero 1998 | 1. Observational study (not RCT) and authors used SCIT. |
| Asero 2007 | 1. Systematic review, not RCT. |
| Bolhaar 2005 | 1. Paper is case report (not RCT). |
| Boyano‐Martínez 2013 | 1. Cross‐sectional study. |
| Conference Review 2009 | 1. Congress report. |
| Conference Review 2013 | 1. Congress report. |
| Cromwell 2011 | 1. Narrative review, not original contribution. |
| Hansen 2004 | 1. Authors used SLIT and SCIT with birch pollen extract, not fruit extract. |
| Kinaciyan 2007 | 1. Not RCT. 2. Authors used SLIT with birch pollen extract. |
| Lamotte 2013 | 1. Systematic review, not RCT. |
| Nurmatov 2013 | 1. Systematic review, not RCT. 2. Focus is peanut immunotherapy. |
| Nurmatov 2014 | 1. Systematic review, not RCT. |
| Pereira 2009 | 1. Case report study (not RCT). 2. Did not evaluate an immunotherapy intervention. |
| Schnoor 2011 | 1. Not RCT. |
| Wisniewski 2012 | 1. Narrative review, not original contribution. |
Differences between protocol and review
Following protocol publication we decided to only include RCT studies to maximise the quality of the evidence. Studies with other types of design were excluded.
Contributions of authors
Conceptualized and designed the study: Ernesto Enrique, Joan Bartra, Juan Jose Yepes‐Nuñez
Eligibility of studies: Juan Jose Yepes‐Nuñez, Yuan Zhang, Juan Manuel Reyes
Data extraction: Juan Jose Yepes‐Nuñez, Yuan Zhang
Risk of bias assessment: Juan Jose Yepes‐Nuñez, Yuan Zhang, Marta Roqué i Figuls
Summary of findings tables: Juan Jose Yepes‐Nuñez, Yuan Zhang, Marta Roqué i Figuls
Data analysis and interpretation of results: Juan Jose Yepes‐Nuñez, Yuan Zhang, Marta Roqué i Figuls
Manuscript Drafting: Juan Jose Yepes‐Nuñez, Yuan Zhang
Critically reviewed the protocol and manuscript as submitted: Juan Jose Yepes‐Nuñez, Yuan Zhang, Marta Roqué i Figuls, Juan Manuel Reyes, Joan Bartra Tomas, Fernando Pineda de la Losa, Ernesto Enrique.
Sources of support
Internal sources
Department of Clinical Epidemiology and Biostatistics, McMaster University, Hamilton, Canada, Canada.
External sources
-
Colciencias, Colombia.
Colciencias has granted a loan‐scholarship to Dr. Juan José Yepes Nuñez, to finance his Doctorate in Heatlh Research Methodology at McMaster University in Canada
-
Programa Enlaza‐Mundos. Alcaldía de Medellín 2013, Colombia.
Enlaza‐Mundos program has granted a loan‐scholarship to Dr. Juan José Yepes Nuñez, to finance his Doctorate in Heatlh Research Methodology at McMaster University in Canada
Declarations of interest
JBT has done consultancy for Faes Farma, Novartis and Hal Allergy. He has also received payments for lectures from Grupo Uriach and Thermo Fisher Scientific. None of these activities are related to the submitted work
EE has provided expert testimony for ALK‐Abelló, Hal (Spain) and Menarini regarding polisensitization immunotherapy, pollen and mites specific immunotherapy and anti‐histaminics respectively. He has also received funds from Glaxo Smith Kline to lecture on anti‐histaminics. None of these activities are relevant to the current work.
FP is an employee of Diater Laboratories, which specializes in the manufacture of specific immunotherapy with allergens. Diater Laboratories does not currently have any programmes related to fruit immunotherapy.
JMR, MR, JJYN & YZ have no known conflicts of interest.
Edited (no change to conclusions)
References
References to studies included in this review
Fernandez‐Rivas 2009 {published data only}
- Fernandez‐Rivas M, Garrido Fernandez S, Nadal JA, Diaz de Durana MD, AlonsoGarcia BE, Gonzalez‐Mancebo E, et al. Randomized double‐blind, placebo‐controlled trial of sublingual immunotherapy with a Pru p 3 quantified peach extract. Allergy 2009;64(6):876‐83. [PUBMED: PMID: 19183164] [DOI] [PubMed] [Google Scholar]
- Garcia BE, Gonzalez‐Mancebo, E, Barber D, Martin S, Tabar A, Durana MD, et al. Sublingual immunotherapy in peach allergy: monitoring molecular sensitizations and reactivity to apple fruit and Platanus pollen. Journal of Investigational Allergology and Clinical Immunology 2010;20(6):514‐520. [PUBMED: PMID: 2010688413] [PubMed] [Google Scholar]
- Garrido‐Fernández S, García BE, Sanz ML, Echechipía E, Lizaso MT, Tabar AI. Are basophil activation and sulphidoleukotriene determination useful tests for monitoring patients with peach allergy receiving sublingual immunotherapy with a pru p 3‐enriched peach extract?. Journal of Investigational Allergology and Clininal Immunology 2014;24(2):106‐13. [Pubmed: PMID: 24834773] [PubMed] [Google Scholar]
Kopac 2012 {published data only}
- Kopac P, Rudin M, Gentinetta T, Gerber R, Pichler CH, Hausmann O, et al. Continuous apple consumption induces oral tolerance in birch‐pollen‐associated apple allergy. Allergy 2012;67(2):280‐5. [PUBMED: PMID: 22070352] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Akdis 2011 {published data only}
- Akdis M. Applications of novel vaccines to immunotherapy of food allergy. Clinical and Translational Allergy 2011;1(Suppl 1):S79. [PUBMED: PMID: 71388016] [Google Scholar]
Asero 1998 {published data only}
- Asero R. Effects of birch pollen‐specific immunotherapy on apple allergy in birch pollen‐hypersensitive patients. Clinical and Experimental Allergy 1998;28(11):1368‐73. [Pubmed: PMID: 9824409] [DOI] [PubMed] [Google Scholar]
Asero 2007 {published data only}
- Asero R. Allergen‐specific immunotherapy for food allergy: Current knowledge and (possible) future directions. Allergy and Clinical Immunology International 2007;19(3):104‐107. [PUBMED: PMID: 2007509382] [Google Scholar]
Bolhaar 2005 {published data only}
- Bolhaar ST, Zuidmeer L, Ma Y, Ferreira F, Bruijnzeel‐Koomen CA, Hoffmann‐Sommergruber K, et al. A mutant of the major apple allergen, Mal d 1, demonstrating hypo‐allergenicity in the target organ by double‐blind placebo‐controlled food challenge. Clinical and Experimental Allergy 2005;35(12):1638‐1644. [PUBMED: PMID: 2006295760] [DOI] [PubMed] [Google Scholar]
Boyano‐Martínez 2013 {published data only}
- Boyano‐Martínez T, Pedrosa M, Belver T, Quirce S, García‐Ara C. Peach allergy in Spanish children: tolerance to the pulp and molecular sensitization profile. Pediatric allergy and immunology 2013;24(2):168‐72. [Pubmed: PMID: 23506291] [DOI] [PubMed] [Google Scholar]
Conference Review 2009 {published data only}
- 28th Congress of the European Academy of Allergy and Clinical Immunology Abstract Book. 28th Congress of the European Academy of Allergy and Clinical Immunology Abstract Book. Allergy: European Journal of Allergy and Clinical Immunology 2009 June;64:Conference review. [PUBMED: PMID: 70021496] [Google Scholar]
Conference Review 2013 {published data only}
- 2013 Annual Meeting of the American College of Allergy, Asthma, Immunology. 2013 Annual Meeting of the American College of Allergy, Asthma and Immunology. Annals of Allergy, Asthma and Immunology 2013;1):Conference review. [PUBMED: PMID: 71307226] [Google Scholar]
Cromwell 2011 {published data only}
- Cromwell O, Niederberger V, Horak F, Fiebig H. Clinical experience with recombinant molecules for allergy vaccination. Current Topics in Microbiology and Immunology. Vol. 352, 2011:27‐42. [Pubmed: PMID: 21562972] [DOI] [PubMed] [Google Scholar]
Hansen 2004 {published data only}
- Hansen KS, Khinchi MS, Skov PS, Bindslev‐Jensen C, Poulsen LK, Malling HJ. Food allergy to apple and specific immunotherapy with birch pollen. Molecular Nutrition & Food Research 2004;48(6):441‐8. [PUBMED: PMID: 15508179] [DOI] [PubMed] [Google Scholar]
Kinaciyan 2007 {published data only}
- Kinaciyan T, Jahn‐Schmid B, Radakovics A, Zwölfer B, Schreiber C, Francis JN, et al. Successful sublingual immunotherapy with birch pollen has limited effects on concomitant food allergy to apple and the immune response to the Bet v 1 homolog Mal d 1. Journal of Allergy and Clinical Immunology 2007;119(4):937‐43. [PUBMED: PMID: 17204315] [DOI] [PubMed] [Google Scholar]
Lamotte 2013 {published data only}
- Iliescu C, Lamotte C, Preda C. Specific oral immunotherapy versus allergen avoidance for food allergy in children: systematic review and meta‐analysis (update). Clinical and Translational Allergy 2013;3(Suppl 3):P2. [Doi: 10.1186/2045‐7022‐3‐S3‐P2; PUBMED: PMID: 71069134] [Google Scholar]
Nurmatov 2013 {published data only}
- Nurmatov U, Venderbosch I, Devereux G, Simons FE, Sheikh A. Allergen‐specific oral immunotherapy for peanut allergy: A Cochrane systematic review. Cochrane Database of Systematic Reviews 2012, (9):CD009014. [PUBMED: PMID: 22972130] [DOI] [PMC free article] [PubMed] [Google Scholar]
Nurmatov 2014 {published data only}
- Nurmatov U, Devereux G, Worth A, Healy L, Sheikh A. Effectiveness and safety of orally administered immunotherapy for food allergies: A systematic review and meta‐analysis. British Journal of Nutrition 2013;111(1):12‐22. [PUBMED: PMID: 23945022] [DOI] [PubMed] [Google Scholar]
Pereira 2009 {published data only}
- Pereira C, Bartolomé B, Asturias JA, Ibarrola I, Tavares B, Loureiro G, et al. Sublingual specific immunotherapy with peach LTP (Pru p 3). One year treatment: a case report. Cases Journal 2009;12(1):6553. [PUBMED: PMID: 70021139] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schnoor 2011 {published data only}
- Schnoor HJ, Witten M, Ree R, Montserrat FR, Poulsen LK. Blinding of freeze‐dried cod – a recipe developed for the FAST project. Clinical and Translational Allergy 2011;1(Suppl1):P84. [PMCID: PMC3354218] [Google Scholar]
Wisniewski 2012 {published data only}
- Wisniewski JA, Li XM. Alternative and complementary treatment for food allergy. Immunology and Allergy Clinics of North America 2012;32(1):135‐150. [PUBMED: PMID: 2012034585] [DOI] [PubMed] [Google Scholar]
Additional references
Beyer 2012
- Beyer K. A European perspective on immunotherapy for food allergies. Journal of Allergy and Clinical Immunology 2012;129(5):1179‐84. [DOI] [PubMed] [Google Scholar]
Bindslev‐Jensen 2004
- Bindslev‐Jensen C, Ballmer‐Weber BK, Bengtsson U, Blanco C, Ebner C, Hourihane J, et al. Standardization of food challenges in patients with immediate reactions to foods‐‐position paper from the European Academy of Allergology and Clinical Immunology. Allergy 2004;59(7):690‐7. [DOI] [PubMed] [Google Scholar]
Bock 1989
- Bock SA, Atkins FM. The natural history of peanut allergy. Journal of Allergy and Clinical Immunology 1989 May;83(5):900‐4. [DOI] [PubMed] [Google Scholar]
Branum 2008
- Branum A, Lukacs S. Food allergy among U.S. children: trends in prevalence and hospitalizations. NCHS Data Brief 2008;10:1‐8. [PubMed] [Google Scholar]
Burks 2012a
- Burks AW, Tang M, Sicherer S, Muraro A, Eigenmann PA, Ebisawa M, et al. ICON: food allergy. Journal of Allergy and Clinical Immunology 2012 Apr;129(4):906‐20. [DOI] [PubMed] [Google Scholar]
Burks 2012b
- Burks AW, Jones SM, Wood RA, Fleischer DM, Sicherer SH, Lindblad RW, et al. Oral Immunotherapy for Treatment of Egg Allergy in Children. New England Journal of Medicine 2012;367(3):233‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chafen 2010
- Chafen JJ, Newberry SJ, Riedl MA, Bravata DM, Maglione M, Suttorp MJ, et al. Diagnosing and managing common food allergies: a systematic review. JAMA 2010;303(18):1848‐56. [DOI] [PubMed] [Google Scholar]
Chehade 2005
- Chehade M, Mayer L. Oral tolerance and its relation to food hypersensitivities. Journal of Allergy and Clinical Immunology 2005;115(1):3‐12; quiz 3. [DOI] [PubMed] [Google Scholar]
Clark 2009
- Clark AT, Islam S, King Y, Deighton J, Anagnostou K, Ewan PW. Successful oral tolerance induction in severe peanut allergy. Allergy 2009;64(8):1218‐20. [DOI] [PubMed] [Google Scholar]
Cox 2011
- Cox L, Nelson H, Lockey R, Calabria C, Chacko T, Finegold I, et al. Allergen immunotherapy: a practice parameter third update. Journa of Allergy and Clinical Immunology 2011;127(1 Suppl):S1‐55. [DOI] [PubMed] [Google Scholar]
Dalal 2002
- Dalal I, Binson I, Reifen R, Amitai Z, Shohat T, Rahmani S, et al. Food allergy is a matter of geography after all: sesame as a major cause of severe IgE‐mediated food allergic reactions among infants and young children in Israel. Allergy 2002;57:362‐365. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ (Clinical research ed.) 1997;315(7109):629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Eggesbo 1999
- Eggesbo M, Halvorsen R, Tambs K, Botten G. Prevalence of parentally perceived adverse reactions to food in young children. Pediatric Allergy and Immunology 1999;10:122‐132. [DOI] [PubMed] [Google Scholar]
Enrique 2005
- Enrique E, Pineda F, Malek T, Bartra J, Basagaña M, Tella R, et al. Sublingual immunotherapy for hazelnut food allergy: a randomized, double‐blind, placebo‐controlled study with a standardized hazelnut extract. Journal of Allergy and Clinical Immunology 2005;116(5):1073‐9. [DOI] [PubMed] [Google Scholar]
Enrique 2008
- Enrique E, Malek T, Pineda F, Palacios R, Bartra J, Tella R, et al. Sublingual immunotherapy for hazelnut food allergy: a follow‐up study. Annals of Allergy, Asthma & Immunology 2008;100(3):283‐4. [DOI] [PubMed] [Google Scholar]
Fleischer 2013
- Fleischer DM, Burks AW, Vickery BP, Scurlock AM, Wood RA, Jones SM, et al. Sublingual immunotherapy for peanut allergy: a randomized, double‐blind, placebo‐controlled multicenter trial. Journal of Allergy and Clinical Immunology 2013;131(1):119‐27. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gupta 2011
- Gupta RS, Springston EE, Warrier MR, Smith B, Kumar R, Pongracic J, et al. The prevalence, severity, and distribution of childhood food allergy in the United States. Pediatrics 2011;128(1):e9‐17. [DOI] [PubMed] [Google Scholar]
Higgins 2002
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21(11):1539‐58. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Jones 2009
- Jones SM, Pons L, Roberts JL, Scurlock AM, Perry TT, Kulis M, et al. Clinical efficacy and immune regulation with peanut oral immunotherapy. Journal of Allergy and Clinical Immunology 2009;124(2):292‐300. [DOI] [PMC free article] [PubMed] [Google Scholar]
Keet 2012
- Keet CA, Frischmeyer‐Guerrerio PA, Thyagarajan A, Schroeder JT, Hamilton RG, Boden S, et al. The safety and efficacy of sublingual and oral immunotherapy for milk allergy. Journal of Allergy and Clinical Immunology 2012;129(2):448‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kerz 2007
- Kerzl R, Simonowa A, Ring J, Ollert M, Mempel M. Life‐threatening anaphylaxis to kiwi fruit: protective sublingual allergen immunotherapy effect persists even after discontinuation. Journal of Allergy and Clinical Immunology 2007;119:506‐7. [DOI] [PubMed] [Google Scholar]
Kim 2011
- Kim EH, Bird JA, Kulis M, Laubach S, Pons L, Shreffler W, et al. Sublingual immunotherapy for peanut allergy: clinical and immunologic evidence of desensitization. Journal of Allergy and Clinical Immunology 2011;127(3):640‐6, e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Krishna 2011
- Krishna MT, Huissoon AP. Clinical immunology review series: an approach to desensitization. Clinical & Experimental Immunology 2011;163(2):131‐46. [DOI] [PMC free article] [PubMed] [Google Scholar]
Le 2014
- Le U, Burks W. Oral and sublingual immunotherapy for food allergy. World Allergy Organization Journal 2014;7(35). [DOI] [PMC free article] [PubMed] [Google Scholar]
Maggi 2010
- Maggi E. T‐cell responses induced by allergen‐specific immunotherapy. Clinical & Experimental Immunology 2010;161(1):10‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mousallem 2012
- Mousallem T, Burks AW. Immunology in the Clinic Review Series; focus on allergies: immunotherapy for food allergy. Clinical & Experimental Immunology 2012;167(1):26‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nurmatov 2012
- Nurmatov U, Venderbosch I, Devereux G, Simons FER, Sheikh A. Allergen‐specific oral immunotherapy for peanut allergy. Cochrane Database of Systematic Reviews 2012, (9):CD009014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pascal 2012
- Pascal M, Muñoz‐Cano R, Reina Z, Palacín A, Vilella R, Picado C, et al. Lipid transfer protein syndrome: clinical pattern, cofactor effect and profile of molecular sensitization to plant‐foods and pollens. Clinical and Experimental Allergy 2012;42(10):1529‐39. [DOI] [PubMed] [Google Scholar]
Pastorello 2004
- Pastorello EA, Robino AM. Clinical role of lipid transfer proteins in food allergy. Molecular Nutrition & Food Research 2004;48:356–62. [DOI] [PubMed] [Google Scholar]
Perez‐Machado 2003
- Pérez‐Machado MA, Ashwood P, Thomson MA, Latcham F, Sim R, Walker‐Smith JA, et al. Reduced transforming growth factor‐beta1‐producing T cells in the duodenal mucosa of children with food allergy. European Journal of Immunology 2003;33(8):2307‐15. [DOI] [PubMed] [Google Scholar]
Rance 2005
- Rance F, Grandmottet X, Grandjean H. Prevalence and main characteristics of schoolchildren diagnosed with food allergies in France. Clinical & Experimental Immunology 2005;35:167‐172. [DOI] [PubMed] [Google Scholar]
RevMan 2011 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan) [Review Manager (RevMan)]. Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Romantsik 2014
- Romantsik O, Bruschettini M, Tosca MA, Zappettini S, Della Casa Alberighi O, Calevo MG. Oral and sublingual immunotherapy for egg allergy. Cochrane Database of Systematic Reviews 2014, Issue 11. [DOI: 10.1002/14651858.CD010638.pub2] [DOI] [PubMed] [Google Scholar]
Scott‐Taylor 2005
- Scott‐Taylor TH, Hourihane JB, Harper J, Strobel S. Patterns of food allergen‐specific cytokine production by T lymphocytes of children with multiple allergies. Clinical & Experimental Allergy 2005;35(11):1473‐80. [DOI] [PubMed] [Google Scholar]
Shevach 2009
- Shevach EM. Mechanisms of foxp3+ T regulatory cell‐mediated suppression. Immunity 2009;30(5):636‐45. [DOI] [PubMed] [Google Scholar]
Varshney 2011
- Varshney P, Jones SM, Scurlock AM, Perry TT, Kemper A, Steele P, et al. A randomized controlled study of peanut oral immunotherapy: clinical desensitization and modulation of the allergic response. Journal of Allergy and Clinical Immunology 2011;127(654‐660). [DOI] [PMC free article] [PubMed] [Google Scholar]
Yeung 2012
- Yeung JP, Kloda LA, McDevitt J, Ben‐Shoshan M, Alizadehfar R. Oral immunotherapy for milk allergy. Cochrane Database of Systematic Reviews 2011, (11):CD009542. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zeiger 2003
- Zeiger, R. Food Allergen Avoidance in the Prevention ofFood Allergy in Infants and Children. Pediatrics 2003;11(6):1662‐1671. [PubMed] [Google Scholar]
Zuidmeer 2008
- Zuidmeer L, Goldhahn K, Rona RJ, Gislason D, Madsen C, Summers C. The prevalence of plant food allergies: A systematic review. Journal of Allergy and Clinical Immunology 2008;121:1210‐8. [DOI] [PubMed] [Google Scholar]


