Abstract
The ASTRO-CM dose-finding pilot study investigated the role of adjunctive sertraline for the treatment of HIV-associated cryptococcal meningitis in HIV-infected Ugandan patients. The present study is a post-hoc pharmacokinetic-pharmacodynamic analysis of the ASTRO-CM pilot study to provide insight into sertraline exposure-response-outcome relationships. We performed a population pharmacokinetic analysis using sertraline plasma concentration data and correlated various predicted PK-PD indices with the percentage change in log10 CFU/mL from baseline. Sertraline clearance was 1.95-fold higher in patients receiving antiretroviral (ART), resulting in 49 % lower drug exposure To quantify the clinical benefit of sertraline, we estimated rates of fungal clearance from cerebrospinal fluid (CSF) of ASTRO-CM patients using Poisson model and compared the clearance rates to a historical control study (COAT) in which patients received standard Cryptococcus therapy of amphotericin B (0·7–1·0 mg/kg per day) and fluconazole (800 mg/day). Adjunctive sertraline significantly increased CSF fungal clearance rate compared to COAT trial and sertraline effect was dose-independent with no covariate found to affect fungal clearance including ART. Study findings suggest sertraline response could be mediated by different mechanisms than directly inhibiting the initiation of protein translation as previously suggested; this is supported by the prediction of unbound sertraline concentrations is unlikely to reach MIC concentrations in the brain. Study findings also recommend against the use of higher doses of sertraline, especially those greater than the maximum FDA-approved daily dose (200 mg/day), since they unlikely provide any additional benefits and come with greater costs and risk of adverse events.
Keywords: Sertraline, Pharmacokinetics, Pharmacodynamics, Fungal Clearance, Survival, HIV-Associated Cryptococcal Meningitis
INTRODUCTION
Cryptococcal meningitis (CM) is an opportunistic fungal infection of the central nervous system caused by the pathogenic encapsulated yeast Cryptococcus neoformans. Cryptococcus is one of the most common acquired immunodeficiency syndrome and (AIDS)-defining opportunistic infections accounting for 15% of AIDS-related deaths worldwide [1]. The mortality rate in low and middle income countries is up to 70% [1, 2]. Cerebral spinal fluid (CSF) sterilization by two weeks is achieved only in 50–70% of patients receiving standard amphotericin B deoxycholate therapy [3–6]. Current drug therapies are expensive, toxic and not readily accessible, especially in resource-poor settings. This highlights the critical unmet need for new safe, effective, affordable and readily accessible antifungal therapies for cryptococcosis.
Sertraline, a selective serotonin reuptake inhibitor commonly prescribed for the treatment of depression and other mental disorders, was shown to have an in vitro and in vivo fungicidal activity against Cryptococcus neoformans [7–9]. The sertraline effect was dose-dependent and believed to be mediated by the inhibition of protein synthesis of the fungus [7, 9, 10]. Other proposed sertraline mechanisms of antifungal activity include: non-specific lipophilicity-dependent cytotoxicity; membrane phospholipids disruption of acidic intracellular organelles; and elevation of plasma serotonin (5-HT) that is biological active against Candida and Aspergillus species [11–14]. The effect of sertraline in combination with fluconazole, a major component of standard induction, consolidation, and maintenance therapy for CM, was also synergistic against Cryptococcus [15–17].
Previous findings support the potential use of sertraline in treating HIV-related CM given its favorable physiochemical, pharmaceutical, and therapeutic properties. Sertraline has high lipophilic characteristics that enable sertraline to cross the blood brain barrier easily and concentrate in the brain at concentrations 10–57 times higher than in plasma [18]. Sertraline can also help with depression, a common co-morbidity in HIV-infected patients [19, 20]. More importantly, sertraline has a good safety profile: sertraline overdose are relatively safe and easily managed; sertraline drug-drug interactions are minimal providing an important advantage in this therapeutically complex setting; sertraline is available as a less-costly generic formulation; sertraline is orally bioavailable; and sertraline has long half-life that allows once-daily dosing [21].
Based on the above compelling evidence and therapeutic properties of sertraline, our group hypothesized that the addition of sertraline to Amphotericin B and fluconazole would result in faster rates of fungal clearance from CSF and better survival. To test this hypothesis, we conducted and authored the first-in human dose-escalating clinical trial to investigate the safety and efficacy of adjunctive sertraline for the treatment of HIV-associated CM (ASTRO-CM, ) [3]. The present study is a post-hoc analysis of the ASTRO-CM pilot study using a pharmacokinetic-pharmacodynamic (PK-PD) modeling approach to provide insight into sertraline exposure-response-outcome relationships. An older study, COAT (Cryptococcal Optimal ART Timing, ), was used as a control comparison to quantify sertraline added effect on CSF fungal clearance rate [4].
METHODS
Patients Cohort & Sampling Collection.
This study included a subset of HIV-infected Ugandans with CM from the ASTRO-CM pilot study, who had sertraline plasma concentrations measured [3]. Venous blood samples were collected on day 1, 3, 7, 10 and 14 mostly within 8 hours after dose administration and analyzed using a high-performance liquid chromatography (HPLC) method. Full details of study design and methods are outlined in our previously published manuscript [3].
Pharmacokinetic Analysis.
All sertraline plasma concentrations were fitted simultaneously using nonlinear mixed-effect regression to one- and two-compartment PK models with linear and nonlinear clearance. Between subject variability (BSV) was described by an exponential model and residual unexplained variability (RUV) was evaluated by proportional and combined error model. The effects of age, sex, weight, concomitant antiretroviral therapy (ART), parent-to-metabolite ratio (sertraline-to-desmethylsertraline ratio, SDR), serum creatinine (SCr) and liver function enzymes (AST and ALT) were visually screened against Empirical Bayes Estimates (EBEs) of the base model. Potential covariates were then tested as linear and power models for continuous covariates and as a fractional change for categorical ones. The statistical significance of covariates inclusion was tested using the likelihood ratio test (χ2, α =0.05, df = 1) which corresponds to at least a 3.84-point drop in objective function value (OFV). The criteria to select the PK model were OFV, plausibility of parameter estimates, diagnostic plots and the prospective applicability of the model. The performance of the selected model was assessed by prediction-corrected visual predictive check (pcVPC) [22] and parameter precision was evaluated by the sampling importance resampling (SIR) method [23].
There were missing data in weight, SDR and time of dose administration and blood draws. A single imputation was performed to replace missing weights with the median value of known weights based on sex (54 kg for male and 46 kg for female). Within a patient, SDR, SCr, AST, and ALT were imputed with the last observation carried forward then the last observation carried backward. Missing dosing times were guided by the frequency of usual dosing times of patients. Missing blood draw times were imputed by visit times when available or by the most frequent blood draw time for other patients on the same day. When there was no information to make reasonable imputation, records were excluded. Furthermore, the impact of the imputation on PK parameter estimates was assessed by sensitivity analysis to ensure the acceptability of the imputation.
Predictive PK-PD Index Exploration.
This was a visual exploratory correlation between various predicted PK-PD indices with the percent change in log10 CFU/mL from baseline and was limited to patients who had fungal count quantifications in CSF and sertraline plasma concentration measurements. Quantitative CSF cultures were obtained by therapeutic lumber punctures using manometers at diagnosis, and on day 3, 7, 10, 14 and as needed for signs of elevated intracranial pressure. A previously described method was used for determining quantitative CSF cultures measured as CFU/mL [24]. The EBEs from the selected PK model were used to simulate the sertraline brain concentration-time profiles using literature brain-to-plasma concentration ratios and to calculate standardized PK-PD indices (Cmax/MIC, AUC24/MIC and %T>MIC) [18].
Cmax is the highest (peak) concentration reached, MIC is the minimum inhibitory concentration of sertraline against Cryptococcus, AUC24 is the area under concentration-time profile over 24 hours, and %T>MIC is the cumulative percentage of 24-hr period that the concentration is above MIC. cAUC/MIC, the ratio of cumulative area under concentration-time profile to MIC, is not a commonly used PK-PD index but it was calculated to evaluate whether the drug effect was better associated with cumulative drug exposure. The sertraline MIC was measured by broth microdilution in RPMI1640 media per protocol [25]. When MICs of clinical isolates were undetermined, susceptibility was imputed as the median MIC value (4 μg/mL).
PK-PD indices are generally based on the unbound (free) plasma or tissue concentrations, and indicated by the prefix f. However, because unbound plasma concentrations were not measured in our patients, the prefix f was not used and total, rather than unbound, brain sertraline concentrations were predicted using the literature median value (16.5 fold) of total brain-to-plasma concentration ratios [18]. Assuming measured fungal CFU/mL was unlikely affected by the most recent dose, PK-PD indices were calculated after the dose before the most recent dose for Cmax/MIC, AUC24/MIC, %T>MIC and up to the dose before the most recent dose for cAUC/MIC. Drug effect was calculated as the percent change in log10 CFU/mL from the baseline culture and plotted against the predicted PK-PD indices to identify any general trend.
Fungal Count Analysis.
Fungal CSF counts from the ASTRO-CM pilot study and COAT trial were modeled together to determine the added benefit of sertraline on brain CSF fungal clearance rate when given with standard induction therapy for 2 weeks [3]. COAT is an earlier study that included similar patients from the same hospital, who were treated with the same induction regimen of amphotericin and fluconazole without sertraline [4]. All CSF fungal counts were log10-transformed, rounded to nearest integer and fitted to a Poisson model (eq. 1) [26].
| (1) |
The probability of observing Yij equal to n =0, 1, 2, … is determined by lambda (λij), the mean fungal count for individual i occurring at fixed time-interval j and the factorial function (!) of n. λij was further influenced by the preceding counts (f(λi(j-1))) and time (f(tj)) (eq. 2).
| (2) |
A three-state transition Markov model (MM) for an increase, decrease and no change in log10 CFU/mL from the previous count was used to account for the correlation of fungal counts within a patient. Time effect was best modeled by a mono-exponential decline function with a separate random effect for each study (eq. 3).
| (3) |
MM is a multiplier factor for each Markov model state. SER is an indicator variable; SER equals 0 for COAT trial and 1 for ASTRO-CM study. TE is an exponential constant for the daily decrease in fungal counts (day−1) and K is a TE multiplier that was fixed to 1 for the COAT trial and estimated for each dose arm (100, 200, 300, 400 mg/day) of the ASTRO-CM study. This model parameterization was purposely chosen to allow for easy comparison among different sertraline arms and historical control study.
Given the in vitro and in vivo animal evidence of sertraline dose-dependent effect against Cryptococccous, an exploratory sigmoidal Emax model was also used to understand what the dose-response relationship would be and to compare the results to the Poisson model (eq. 4) [7–9].
| (4) |
D50 is sertraline dose to MIC ratio when sertraline effect is half maximal and γ is a shape parameter for the dose-response curve. The final count model was assessed by VPC and parameters uncertainty was estimated by SIR.
Survival Analysis.
This analysis was limited to patients included in the fungal count analysis. Two-week survival rate of patients from both (COAT & ASTRO-CM) studies was modeled as time to death using a time-varying exponential hazard function (eq. 5).
| (5) |
λo is the baseline hazard and βo is the shape parameter of the hazard function. Based on whether covariates were time-invariant or time-varying, they were modeled, respectively, as follows [27]:
| (5) |
| (6) |
The selected model was validated internally by VPC and distributions of parameter uncertainty were calculated by SIR.
Software.
Nonlinear mixed-effect, count and survival modeling analyses were performed in NONMEM 7.3 (ICON Development Solutions, Ellicott City, MD) using various ADVANs and estimation methods. The PK analysis was performed with ADVAN2 TRANS2 and FOCEI method; count analysis was done using PREDPP with the FO Laplacian method; and survival rates were estimated using ADAVN13 with the FO likelihood method. Data manipulation, imputation and plotting were done in R (version 3.5.0). Perl-speaks-NONMEM (PsN) was utilized to perform VPC and SIR analyses [28]. The Pirana interface was used to maintain and compare NONMEM and PsN runs [29].
RESULTS
Pharmacokinetic Model.
The final analysis included 335 sertraline plasma concentrations from 137 patients whose characteristics are summarized in Table 1. Weights of 45 unique patients (32 males and 13 females), 94 dosing times and 19 blood draw times were imputed as described above. Nine observations below the lower limit of quantification (1 ng/mL) were excluded. PK parameter estimates were similar before and after including the imputed records in the analysis; therefore, imputation was considered acceptable and all imputed records were included that actually improved the precision of parameter estimates.
Table 1.
Characteristics of patients included in PK Analysis
| 100 mg | 200 mg | 300 mg | 400 mg | All Arms | |
|---|---|---|---|---|---|
| Subjects, n | 14 | 49 | 36 | 38 | 137 |
| Male, n (%) | 8 (57) | 33 (67) | 20 (56) | 26 (68) | 87 (64) |
| Age, mean ± SD | 38 ± 7 | 36 ± 8 | 38 ± 8 | 34 ± 8 | 36 ± 8 |
| Weight, mean ± SD | 53 ± 9 | 52 ± 11 | 51 ± 9 | 52 ± 10 | 52 ± 10 |
| ART, n (%) | 8 (57) | 23 (47) | 12 (33) | 14 (37) | 57 (42) |
Study arms were not statistically different from each other for characteristics
A 1-compartment PK model with first-order absorption and elimination adequately described the sertraline data. A combined proportional and additive error model better fit the RUV. Oral clearance (CL/F) and volume of distribution (V/F) were allometrically scaled to a standard 70-kg person. The selected PK model included ART as a covariate on CL/F, reducing the OFV by 24 points (p-value < 0.0001).
| (7) |
| (8) |
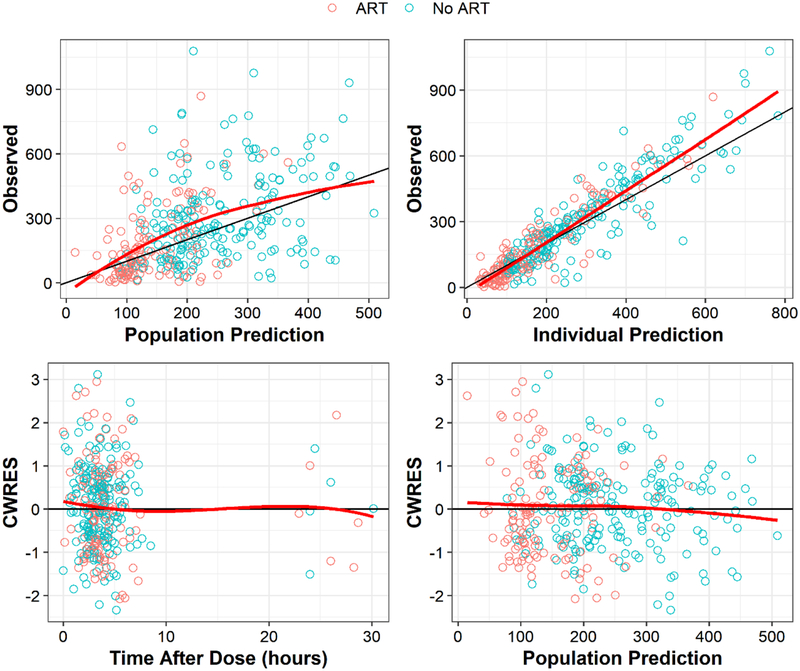
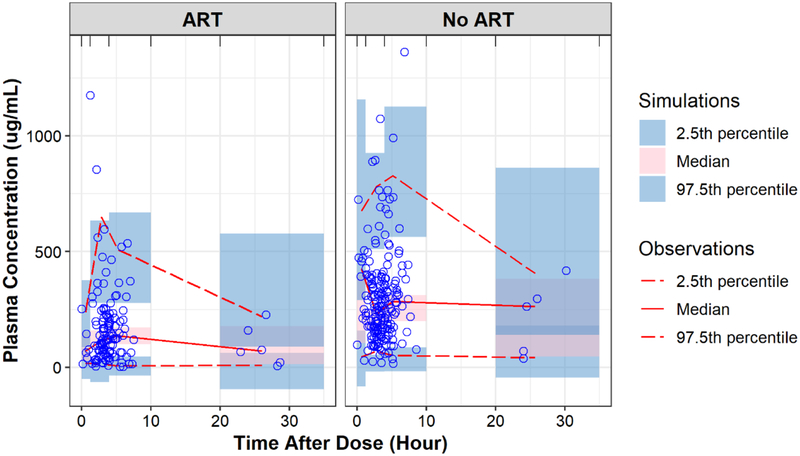
Sertraline clearance was 1.95-fold higher in patients on ART and the effect was indifferent between patients receiving efavirenz- and nevirapine-based ART. The diagnostic plots and pcVPC showed the model reasonably captured the observed data (Fig. 1 & 2). Table 2 summarizes the parameter estimates of the selected PK model their SIR-based uncertainties.
Fig. 1.
Diagnostic plots for the selected PK model
Fig. 2.
Prediction-corrected and observed sertraline concentrations vs time after dose by ART status.
Table 2.
Parameter estimates for the selected PK model
| Parameter | Definition | Estimate (RSE) | SIR median (95% CI) |
|---|---|---|---|
| CL/F* | Oral clearance (L/hr) | 52.7 (8) | 52.4 (42.7 – 62.2) |
| V/F* | Volume of distribution (L) | 1780 (18) | 1883 (1261 – 2361) |
| ART_CL | Fraction change in CL for ART patients | 0.948 (26) | 1.02 (0.48 – 1.65) |
| Ka | Absorption rate constant (hr−1) | 0.101 (31) | 0.110 (0.05 – 0.19) |
| BSV_CL | Between subjective variability in CL/F (%CV) | 70.3 (10) | 70.5 (58.9 – 91.4) |
| BSV_V | Between subjective variability in V/F (%CV) | 60.4 (21) | 63.2 (35.3 – 94.0) |
| RUV1 | Additive residual variability (SD) | 44.3 (53) | 44.6 (30.5 – 60.5) |
| RUV2 | Proportional residual variability (%CV) | 10.2 (29) | 10.3 (6.3 – 15.9) |
Parameters are allometrically scaled to a 70-kg person, RSE is relative standard of error, SIR is sampling importance resampling.
Predictive PK-PD Index.
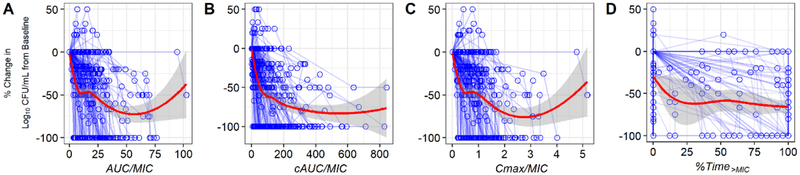
In vitro susceptibilities were determined for 151 Cryptococcus clinical isolates (81 from ASTRO-CM and 70 from COAT) obtained from baseline CSF cultures from participants with a first episode of cryptococcosis. The MIC distributions were comparable for both studies. The median (range) of sertraline MIC were 4 (1–8) and 4 (1–12) μg/mL for ASTRO-CM and COAT Cryptococcus isolates, respectively. The undetermined MIC levels for the remaining 138 clinical isolates (34 from ASTRO-CM and 104 from COAT) were assumed 4 μg/mL. Figure 3 shows the visual correlation between the percent change in log10 CFU/mL from baseline and the predicted PK-PD indices of ASTRO-CM patients. None of the PK-PD indices appears to correlate well with the daily reduction in fungal count.
Fig. 3.
Association between the percent change in log10 CFU/mL from baseline and PK-PD indices (A-D). Red line is lowess smoothing.
Rate of Fungal Clearance.
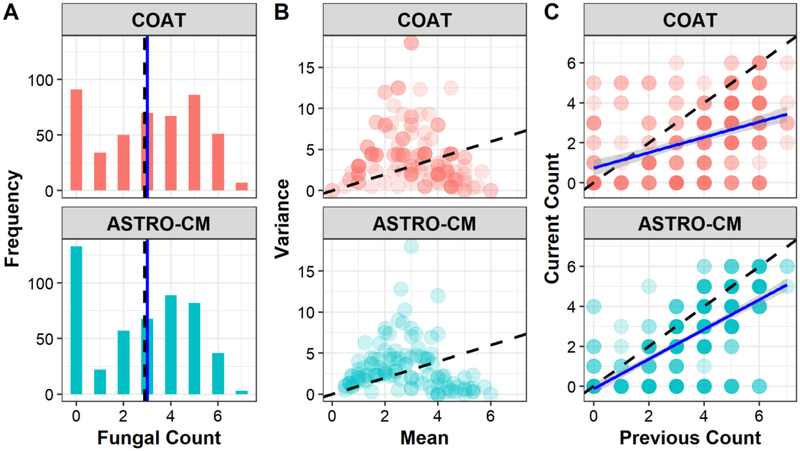
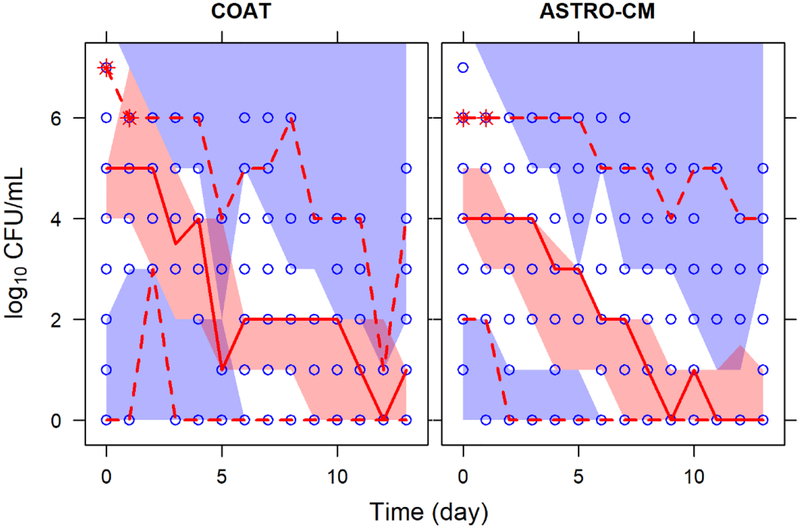
A total of 947 fungal count observations (491 from ASTRO-CM and 456 from COAT) from 289 patients (115 from ASTRO-CM and 174 from COAT) were included in the final analysis. The distribution of fungal counts and individual mean-variance relationships were similar in the two studies, but ASTRO-CM had a slightly stronger correlation of counts than COAT (Fig. 4). ASTRO-CM treatment arms were comparable but fewer patients were initially assigned the 100 mg dose compared to the other arms since the 100 mg dose was less likely to produce therapeutic concentrations.
Fig. 4.
Characteristics of log-transformed fungal counts by study. (A) distribution of counts; (B) variance (of individual counts) vs mean (of individual counts); (C) Correlation of counts. Dash black line represents the mean in A and identity line in B & C. Solid blue line is the median in A and is a linear regression line in C whose slope indicates the strength correlation of counts.
No covariate was found to affect fungal counts. The selected model (eq. 3) found the addition of sertraline to standard induction antifungal therapy significantly increased CSF fungal clearance by 34 to 48%. The sertraline effect was similar irrespective of the daily dose our patients received (Table 3). The adequacy of the selected model was supported by the VPC in which model predictions reasonably captured the observed data (Fig. 5 & 6). The exploratory sigmoidal Emax model with fewer parameters (eq. 4) produced similar results obtained by the Poisson model. Despite being inferior with unreliable estimate of D50, the sigmoidal Emax model had comparable VPC plots (results not shown). Modeling results were also similar when un-rounded log10-transformed were fitted to Poisson and sigmoidal Emax model, indicating minimum effect of rounding (results not shown).
Table 3.
Parameter estimates for the selected count model
| Parameter | Definition | Estimate (RSE) | SIR median (95% CI) |
|---|---|---|---|
| λ | Population mean fungal count (log10 CFU/mL) | 4.43 (2) | 4.42 (4.20 – 4.67) |
| MM 1 | Fractional increase in mean fungal count from the previous one | 1.55 (4) | 1.55 (1.36 – 1.75) |
| MM -1 | Fractional decrease in mean fungal count from the previous one | 1.14 (4) | 1.14 (1.02 – 1.27) |
| TE | Rate of daily decrease in fungal count (day−1) | 0.156 (8) | 0.154 (0.131 – 0.176) |
| K-100 | Estimated multiplier for 100 mg arm of ASTRO-CM | 1.41 (28) | 1.42 (0.68 – 2.19) |
| K-200 | Estimated multiplier for 200 mg arm of ASTRO-CM | 1.42 (15) | 1.45 (1.06 – 1.90) |
| K-300 | Estimated multiplier for 300 mg arm of ASTRO-CM | 1.34 (18) | 1.35 (0.96 – 1.82) |
| K-400 | Estimated multiplier for 400 mg arm of ASTRO-CM | 1.48 (18) | 1.51 (1.07 – 1.98) |
| BSV1 in TE | Between subjective variability in TE for COAT (SD) | 0.0744 (14) | 0.0749 (0.0562 – 0.0953) |
| BSV2 in TE | Between subjective variability in TE for ASTRO-CM (SD) | 0.135 (13) | 0.142 (0.111 – 0.175) |
RSE is relative standard of error and SIR is sampling importance resampling.
Fig. 5.
Predicted and observed fungal counts over time by study. Blue open circles are the observed counts. Solid and dash red lines represent the median, 5th and 95th percentiles of the observed data. The shaded red and purple areas are simulated 95% CI for the median, 5th and 95th percentiles.
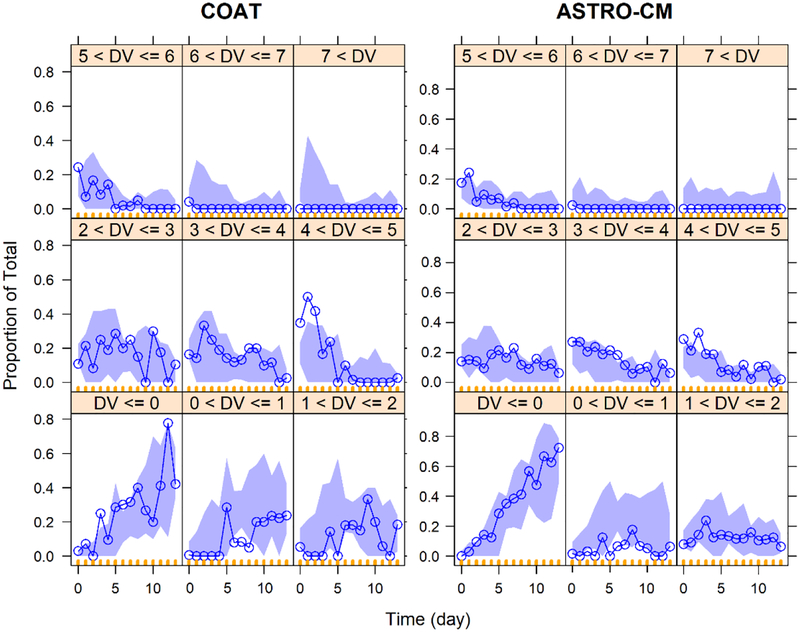
Fig. 6.
Visual predictive checks of proportion of counts vs. time by study. The blue line and purple shaded area are the proportion of observed and simulated 95% CI at each count level, respectively.
Survival Rate.
The selected survival model found hazard of death was significantly influenced by sex and sertraline daily dose as follows:
| (9) |
SEX equals 0 for female and 1 for male. DOSE100/400 is 1 for those patients receiving 100 or 400 mg and 0 otherwise. Inclusion of sex reduced OFV by 13.9 points (p-value < 0.0005) and subsequent inclusion of DOSE100/400 resulted in additional drop of 6.24 points in OFV (p-value < 0.02). The exponentiation of β’s coefficients revealed that the hazard ratio of male to female is 0.25 and patients receiving sertraline 100 or 400 mg a day to those receiving no sertraline, 200 or 300 mg daily is 2.92 (Table 4). The internal validation by VPC confirmed the model adequate (Fig. 7).
Table 4.
Parameter estimates for the selected survival model
| Parameter | Definition | Estimate (RSE) | SIR median (95% CI) |
|---|---|---|---|
| λo | Baseline hazard | 0.0367 (17) | 0.0365 (0.0241 – 0.0473) |
| βo | Shape parameter of the hazard function | 2.32 (16) | 2.30 (1.61 – 3.25) |
| SEXmale | Effect of being male on the baseline hazard | −1.38 (29) | −1.38 (−2.20 – −0.695) |
| DOSE100/400 | Effect of receiving 100 or 400 mg of sertraline daily on the baseline hazard | 1.07 (38) | 1.03 (0.25 – 1.82) |
RSE is relative standard of error and SIR is sampling importance resampling.
Fig. 7.
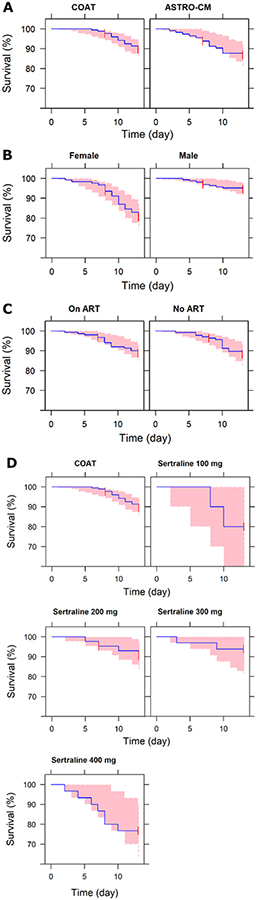
Kaplan-Meier plots for time to death by (A) study, (B) sex (female = 0 and male =1), (C) ART (no ART = 0 and ART = 1) and (D) sertraline daily dose (DOSE = 0 for COAT). This is comparison of observed data (blue line) to the 95% prediction interval of the simulated data (pink area) with the time-to-event (TTE) model.
DISCUSSION
We found the ART co-administration increased sertraline oral clearance by 1.95-fold, resulting in 49% lower drug exposures. This has a broad implication for sertraline use as an antidepressant in HIV-infected populations. Yet, ART did not affect fungal clearance from CSF or survival of patients. Of the PK-PD indices explored, none appeared to correlate well with the percent change in log10 CFU/mL from baseline. The addition of sertraline significantly increased the rate of CSF fungal clearance with a similar effect across dose arms. We also found that female patients and those receiving 100 or 400 mg of sertraline daily had lower 2-week survival rates.
Estimated sertraline clearance was similar to what was previously published, but the volume of distribution was 2–3 fold lower even after adjusting for body weight [30, 31]. The difference in volume of distribution could possibly be explained by the disease state and/or race given that our patients are HIV-infected and sub-Saharan Africans. Metabolism is the main route of sertraline elimination and its clearance was expected to increase with ART co-administration because our patients received non-nucleotide reverse transcriptase inhibitors (NNRTI) (e.g. efavirenz, nevirapine) that are known inducers of cytochrome P450 metabolizing enzymes responsible for sertraline metabolism [32–34].
It is challenging to separate out a predictive PD index of sertraline effect in the current study due to variability and confounders. This may explain why we did not find a PD index that correlates well with the daily reduction in fungal count. Even if we did find an index, it would be unreliable PD metric of sertraline because our patients were on the CM standard therapy and the observed reduction in log10 CFU/ml cannot be solely attributed to sertraline. A reliable PD target of drug effect is usually determined from in vitro, ex vivo, or in vivo animal studies that can control for host and fungal factors in a clinical setting. Findings from these studies can then be used to propose a rational dosage regimen of sertraline for confirmatory testing in humans. Our correlation analysis was exploratory and strictly visual that neither accounts for correlation of counts with a patient nor the effect of other covariates. Therefore, we did not expect to find a correlation, Rather, it was an exploratory exercised aimed to identify any general trend and more importantly to select relevant sertraline exposure metric for formal testing in subsequent analyses.
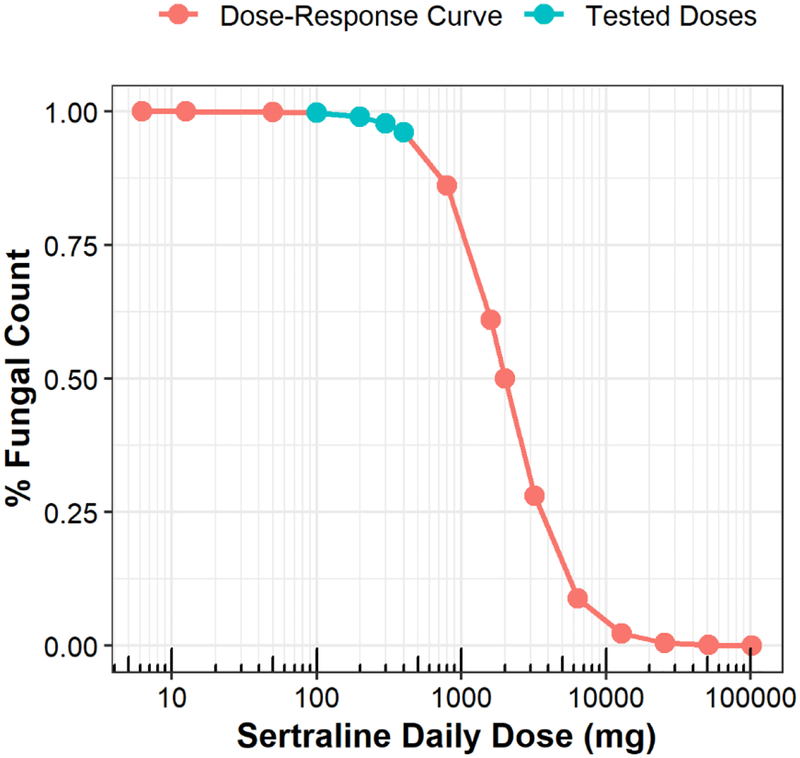
Although ART decreased sertraline exposure, ART did not influence the rate of CSF fungal clearance. The sertraline effect was also similar across doses. Together, this suggests the observed clinical effect of sertraline is dose- and concentration-independent which contradicts previous evidence from in vitro and animal studies[7–9]. Possible explanations are unbalanced arms, large variability or a narrow range of tested sertraline doses. The range of studied sertraline doses were only 4-fold and seem to fall at the lower range of the sertraline dose-response curve (Fig. 8) assuming the sertraline dose-response curve is true. At least 10-fold range between the lowest and highest studied doses is recommended by the European Medicines Agency to sufficiently estimate dose-response relationships [35]. The dose to MIC ratio at which sertraline effect is half maximal (D50) was estimated 500 mg. With MIC is 4 μg/mL, the sertraline daily dose required to achieve a 50% reduction in fungal counts would be 2000 mg. This is 10-fold greater than the maximum daily dose approved by the Food & Drug Administration (FDA) [36].
Fig. 8.
Dose-response curve of sertraline. This is the exploratory sigmoidal Emax function describing the sertraline effect in equation 4 assuming sertraline has a dose-dependent effect with MIC of 4 μg/mL.
Sertraline effect in human could possibly be mediated by different mechanisms than directly inhibiting protein synthesis of fungi because predicted unbound brain sertraline concentration is unlikely to reach MIC concentrations [9, 11]. Sertraline is highly-protein bound in plasma and only unbound concentration is able to cross tissue membranes (e.g. blood-brain barrier and fungal cell wall), bind to therapeutic targets, and elicit pharmacological action [37]. The highest steady-state total sertraline plasma concentration observed in our patients was 1.078 μg/mL. With 98% protein binding and median of 16.5-fold higher concentration in brain tissue than in blood, the unbound brain sertraline concentration is predicted to be 0.356 μg/mL. This is lower than the lowest determined MIC level (1 μg/mL) for our clinical isolates. Despite consensus that unbound concentration is the driver of drug action in vivo, there is unfortunately still a persistent inclination to report total tissue concentrations, arguing total concentrations are better related to drug efficacy. In fact, total tissue concentrations are shown to be poor surrogates for drug efficacy because total concentrations are determined after tissue homogenization that are unlikely representative of concentrations at the site of action [38].
Enhancing fluconazole effect through beneficial pharmacokinetic interaction is another plausible mechanism for the observed clinical effect of sertraline. Fluconazole is a substrate for the efflux transport P-glycoprotein that is widely expressed in gut, blood-brain barrier, renal tubules and other human tissues [39–41]. Sertraline, on the other hand, is a substrate and inhibitor of P-glycoprotein [42, 43]. In theory, the inhibition of P-glycoprotein (P-gp) by sertraline would increase gut absorption, central nervous system (CNS) penetration producing higher brain concentrations and decrease renal clearance of fluconazole. Ultimately, sertraline would increase fluconazole exposure particularly in the brain which could possibly explain our results as pointed out by Veringa and his colleagues [44]. This was confirmed in preclinical species and recently in humans despite the skepticism about the clinical relevance of P-gp-mediated drug-drug interaction at blood brain barrier [45, 46].
Increasing 5-HT in plasma could also be the driver of the sertraline observed effect. 5-HT has direct and indirect anti-microbial effects against invading pathogens [47, 48]. 5-HT possesses antimicrobial properties by itself and it is a master regulator of innate and adaptive immune response through its widely expressed receptors on immune cells and via receptor independent signaling so-called serotonylation [49]. Depletion of 5-HT in blood and platelets is associated with impaired immune responses as in HIV-infected individuals [50]. On the other hand, elevated circulating level of 5-HT is documented in multiple autoimmune inflammatory diseases, such as rheumatoid arthritis, asthma, Crohn’s disease and ulcerative colitis due to either over-production of 5-HT or/and reduced function of 5-HT transporter (SERT) [47]. Just like in the brain, SERT in the periphery functions to tightly regulate 5-HT signaling via the reuptake 5-HT back into the cell, terminate and prevent off-target 5-HT effect, protect 5-HT from degradation by monoaminoxidases and store 5-HT for later release upon subsequent stimulation [51, 52]. With that, it is plausible that SERT inhibition by sertraline could be the underlying mechanism for the improved fungal clearance in the ASTRO-CM patients.
Survival was not a primary outcome and the study was not powered to find survival difference between sertraline dose arms. However, there was statistical evidence that female patients (p-value <0.0005) and those receiving sertraline 100 or 400 mg daily (p-value < 0.02) had lower survival rates. Either this finding represents an immunomodulatory effect of sertraline, which we are currently investigating, or this is biased finding. Analyzed patients represented a nonrandom sample since the analysis only included patients who have plasma measurements and fungal counts (115 out of 137). Results could also be biased by differential co-morbidities or unadjusted confounders. The first 60 patients were assigned non-randomly and it could possibly be that patients receiving 100 or 400 mg of sertraline daily were prognostically different than the rest. Though, results should be taken into considerations for further studies to balance patients by sex and test at least two different sertraline doses (low and high) to further investigate these findings.
The current study has several limitations. The ASTRO-CM study was not designed for the pharmacometric analyses presented here. Considerable data were missing and needed to be imputed to complete the analyses. Pharmacokinetic data were also sparse and not optimally sampled. Sertraline concentrations were measured mostly in the absorption phase on different days. Moreover, our patients were diagnosed with multiple medical conditions and on poly-drug therapy. This created a challenging situation to account for all probable drug-drug interactions but did reflect reality. Lastly, sertraline arms were not balanced with fewer patients in the 100 mg arm. With these limitations, our findings should be interpreted cautiously. Nonetheless, these post-hoc pharmacometric analyses illustrate the utility of leveraging data from previous studies to gain insight into exposure-response relationships.
CONCLUSIONS
Sertraline increased the fungal clearance rate from CSF when added to the standard combination therapy of amphotericin B and fluconazole compared to a prior study with no survival benefit. The sertraline effect is dose-independent, indicating higher doses, especially those greater than the maximum FDA-approved daily dose (200 mg/day), may not provide any additional benefits and come with greater costs and risk of adverse events. Observed clinical effect of sertraline unlikely to be mediated by the inhibition of fungal protein synthesis since unbound sertraline concentrations do not reach MIC concentrations. Further studies are in need to confirm or refine current findings.
AKNOWLEDGEMENTS
This work was supported in part by the National Center for Advancing Translational Sciences of the National Institute of Health Award (UL1TR000114), Fogarty International Center and National Institute of Neurologic Disorder and Stroke (R01NS086312), and coin foundation fellowship supporting PhD students at Experimental and Clinical Pharmacology conducting research in infectious diseases.
REFERENCES
- 1.Rajasingham R, Smith RM, Park BJ, Jarvis JN, Govender NP, Chiller TM, Denning DW, Loyse A, Boulware DR (2017) Global burden of disease of HIV-associated cryptococcal meningitis: an updated analysis Lancet Infect Dis 17:873–881. 10.1016/S1473-3099(17)30243-8 Elsevier; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Park BJ, Wannemuehler KA, Marston BJ, Govender N, Pappas PG, Chiller TM (2009) Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS. Aids 23:525–30. 10.1097/QAD.0b013e328322ffac [DOI] [PubMed] [Google Scholar]
- 3.Rhein J, Morawski BM, Hullsiek KH, Nabeta HW, Kiggundu R, Tugume L, Musubire A, Akampurira A, Smith KD, Alhadab A, Williams DA, Abassi M, Bahr NC, Velamakanni SS, Fisher J, Nielsen K, Meya DB, Boulware DR, Ndyetukira JF, Ahimbisibwe C, Kugonza F, Sadiq A, Kandole TK, Luggya T, Kaboggoza J, Laker E, Butler EK, Dyal J, Neborak JM, King AM, Fujita AW, Yueh N, Namudde A, Halupnick R, Jawed B, Vedula P, Peterson M, Bohjanen PR, Kambugu A (2016) Efficacy of adjunctive sertraline for the treatment of HIV-associated cryptococcal meningitis: An open-label dose-ranging study Lancet Infect Dis 16:809–818. 10.1016/S1473-3099(16)00074-8Elsevier Ltd; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boulware DR, Meya DB, Muzoora C, Rolfes MA, Huppler Hullsiek K, Musubire A, Taseera K, Nabeta HW, Schutz C, Williams DA, Rajasingham R, Rhein J, Thienemann F, Lo MW, Nielsen K, Bergemann TL, Kambugu A, Manabe YC, Janoff EN, Bohjanen PR, Meintjes G (2014) Timing of Antiretroviral Therapy after Diagnosis of Cryptococcal Meningitis. N Engl J Med 370:2487–2498. 10.1056/NEJMoa1312884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jarvis JN, Bicanic T, Loyse A, Namarika D, Jackson A, Nussbaum JC, Longley N, Muzoora C, Phulusa J, Taseera K, Kanyembe C, Wilson D, Hosseinipour MC, Brouwer AE, Limmathurotsakul D, White N, Van Der Horst C, Wood R, Meintjes G, Bradley J, Jaffar S, Harrison T (2014) Determinants of mortality in a combined cohort of 501 patients with HIV-associated cryptococcal meningitis: Implications for improving outcomes Clin Infect Dis 58:736–745. 10.1093/cid/cit794Oxford University Press; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Day JN, Chau TTH, Wolbers M, Mai PP, Dung NT, Mai NH, Phu NH, Nghia HD, Phong ND, Thai CQ, Thai LH, Chuong LV., Sinh DX, Duong VA, Hoang TN, Diep PT, Campbell JI, Sieu TPM, Baker SG, Chau NVV, Hien TT, Lalloo DG, Farrar JJ (2013) Combination Antifungal Therapy for Cryptococcal Meningitis N Engl J Med 368:1291–1302. 10.1056/NEJMoa1110404 Massachusetts Medical Society; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Trevino-Rangel RDJ, Villanueva-Lozano H, Hernandez-Rodriguez P, Martinez-Resendez MF, Garcia-Juarez J, Rodriguez-Rocha H, Gonzalez GM (2016) Activity of sertraline against Cryptococcus neoformans: In vitro and in vivo assays. Med Mycol 54:280–286. 10.1093/mmy/myv109 [DOI] [PubMed] [Google Scholar]
- 8.Smith KD, Achan B, Hullsiek KH, Mcdonald TR, Okagaki LH, Alhadab AA, Akampurira A, Rhein JR, Meya DB, Boulware DR, Nielsen K (2015) Increased Antifungal Drug Resistance in Clinical Isolates of Cryptococcus neoformans in Uganda. Antimicrob Agents Chemother 59:7197–7204. 10.1128/AAC.01299-15.Address [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhai B, Wu C, Wang L, Sachs MS, Lin X (2012) The antidepressant sertraline provides a promising therapeutic option for neurotropic cryptococcal infections. Antimicrob Agents Chemother 56:3758–3766. 10.1128/AAC.00212-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin C-JJ, Robert F, Sukarieh R, Michnick S, Pelletier J (2010) The antidepressant sertraline inhibits translation initiation by curtailing mammalian target of rapamycin signaling Cancer Res 70:3199–208. 10.1158/0008-5472.CAN-09-4072American Association for Cancer Research; [DOI] [PubMed] [Google Scholar]
- 11.Young TJ, Oliver GP, Pryde D, Perros M, Parkinson T (2003) Antifungal activity of selective serotonin reuptake inhibitors attributed to non-specific cytotoxicity. J Antimicrob Chemother 51:1045–7. 10.1093/jac/dkg184 [DOI] [PubMed] [Google Scholar]
- 12.Rainey MM, Korostyshevsky D, Lee S, Perlstein EO (2010) The antidepressant sertraline targets intracellular vesiculogenic membranes in yeast. Genetics 185:1221–33. 10.1534/genetics.110.117846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mayr A, Hinterberger G, Dierich MP, Lass-Flörl C (2005) Interaction of serotonin with Candida albicans selectively attenuates fungal virulence in vitro. Int J Antimicrob Agents 26:335–337. 10.1016/j.ijantimicag.2005.07.006Europe PMC Funders [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heller I, Leitner S, Dierich MP, Lass-Flörl C (2004) Serotonin (5-HT) enhances the activity of amphotericin B against Aspergillus fumigatus in vitro. Int J Antimicrob Agents 24:401–404. 10.1016/j.ijantimicag.2004.03.021 [DOI] [PubMed] [Google Scholar]
- 15.Nayak R, Xu J (2010) Effects of sertraline hydrochloride and fluconazole combinations on Cryptococcus neoformansand Cryptococcus gattii. Mycology 1:99–105. 10.1080/21501203.2010.487054 [DOI] [Google Scholar]
- 16.Perfect JRR, Dismukes WEE, Dromer F, Goldman DLL, Graybill JRR, Hamill RJJ, Harrison TSS, Larsen RA a, Lortholary O, Nguyen MM-H, Pappas PGG, Powderly WGG, Singh N, Sobel JDD, Sorrell TCC (2010) Clinical Practice Guidelines for the Management of Cryptococcal Disease: 2010 Update by the Infectious Diseases Society of America. Clin Infect Dis 50:291–322. 10.1086/649858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spitzer M, Griffiths E, Blakely KM, Wildenhain J, Ejim L, Rossi L, De Pascale G, Curak J, Brown E, Tyers M, Wright GD (2014) Cross-species discovery of syncretic drug combinations that potentiate the antifungal fluconazole. Mol Syst Biol 7:499–499. 10.1038/msb.2011.31Nature Publishing Group [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lewis RJ, Angier MK, Williamson KS, Johnson RD (2013) Analysis of sertraline in postmortem fluids and tissues in 11 aviation accident victims. J Anal Toxicol 37:208–216. 10.1093/jat/bkt014 [DOI] [PubMed] [Google Scholar]
- 19.Orlando M, Burnam MA, Beckman R, Morton SC, London AS, Bing EG, Fleishman JA, Health R (2002) Re-estimating the prevalence of psychiatric disorders in a nationally representative sample of persons receiving care for HIV: results from the HIV Cost and Services Utilization Study. Int J Methods Psychiatr Res 11:75–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eller LS, Bunch EH, Wantland DJ, Portillo CJ, Reynolds NR, Nokes KM, Coleman CL, Kemppainen JK, Kirksey KM, Corless IB, Hamilton MJ, Dole PJ, Nicholas PK, Holzemer WL, Tsai YF (2010) Prevalence, correlates, and self-management of HIV-related depressive symptoms. AIDS Care - Psychol Socio-Medical Asp AIDS/HIV 22:1159–1170. 10.1080/09540121.2010.498860 [DOI] [PubMed] [Google Scholar]
- 21.DeVane CL, Liston HL, Markowitz JS (2002) Clinical Pharmacokinetics of Sertraline. Clin Pharmacokinet 41:1247–1266. 10.2165/00003088-200241150-00002 [DOI] [PubMed] [Google Scholar]
- 22.Bergstrand M, Hooker AC, Wallin JE, Karlsson MO (2011) Prediction-corrected visual predictive checks for diagnosing nonlinear mixed-effects models. AAPS J 13:143–51. 10.1208/s12248-011-9255-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dosne A-G, Bergstrand M, Harling K, Karlsson MO (2016) Improving the estimation of parameter uncertainty distributions in nonlinear mixed effects models using sampling importance resampling. J Pharmacokinet Pharmacodyn 43:583–596. 10.1007/s10928-016-9487-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dyal J, Akampurira A, Rhein J, Morawski BM, Kiggundu R, Nabeta HW, Musubire AK, Bahr NC, Williams DA, Bicanic T, Larsen RA, Meya DB, Boulware DR (2016) Reproducibility of CSF quantitative culture methods for estimating rate of clearance in cryptococcal meningitis. Med Mycol 54:361–369. 10.1093/mmy/myv104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.National Committee for Clinical Laboratory Standards (1997) Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts ; Approved Standard. Wayne, PA: Natl Comm Clin Lab Stand NCCLS:M27-A [Google Scholar]
- 26.Plan EL (2014) Modeling and simulation of count data. CPT Pharmacometrics Syst Pharmacol 3:. 10.1038/psp.2014.27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Holford N (2013) A time to event tutorial for pharmacometricians. CPT Pharmacometrics Syst. Pharmacol 2:1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lindbom L, Ribbing J, Jonsson EN (2004) Perl-speaks-NONMEM (PsN) - A Perl module for NONMEM related programming. Comput Methods Programs Biomed 75:85–94. 10.1016/j.cmpb.2003.11.003 [DOI] [PubMed] [Google Scholar]
- 29.Keizer RJ, Karlsson MO, Hooker A (2013) Modeling and simulation workbench for NONMEM: Tutorial on Pirana, PsN, and Xpose CPT Pharmacometrics Syst Pharmacol 2:e50 10.1038/psp.2013.24 Wiley-Blackwell; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cooper JM, Duffull SB, Saiao AS, Isbister GK (2015) The pharmacokinetics of sertraline in overdose and the effect of activated charcoal. Br J Clin Pharmacol 79:307–15. 10.1111/bcp.12500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li CH, Pollock BG, Lyketsos CG, Vaidya V, Drye LT, Kirshner M, Sorisio D, Bies RR (2012) Population Pharmacokinetic Modeling of Sertraline Treatment in Patients With Alzheimer Disease: The DIADS-2 Study. J Clin Pharmacol XX: 10.1177/0091270012445793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Michaud V, Ogburn E, Thong N, Aregbe AO, Quigg TC, Flockhart DA, Desta Z (2012) Induction of CYP2C19 and CYP3A activity following repeated administration of efavirenz in healthy volunteers. Clin Pharmacol Ther 91:475–482. 10.1038/clpt.2011.249NIH Public Access [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kobayashi K, Ishizuka T, Shimada N, Yoshimura Y, Kamijima K, Chiba K (1999) Sertraline N-demethylation is catalyzed by multiple isoforms of human cytochrome P-450 in vitro. Drug Metab Dispos 27:763–766 [PubMed] [Google Scholar]
- 34.Obach RS, Cox LM, Tremaine LM (2005) Sertraline is metabolized by multiple cytochrome p450 enzymes, monoamine oxidases, and glucuronyl transferases in human: an in vitro study. 33:262–270. 10.1124/dmd.104.002428.chrome [DOI] [PubMed] [Google Scholar]
- 35.EMA; (2014) Report from dose finding workshop. London, UK [Google Scholar]
- 36.Zoloft (sertraline HCl) [package Insert] (2019) Zoloft (sertraline HCl) [package Insert]. New York, NY Pfizer: 1–35 [Google Scholar]
- 37.Lepak AJ, Andes DR (2014) Antifungal pharmacokinetics and pharmacodynamics. Essentials Clin Mycol Second Ed 121–134. 10.1007/978-1-4419-6640-7_8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Toutain PL, Del Castillo JRE, Bousquet-Mélou A (2002) The pharmacokinetic-pharmacodynamic approach to a rational dosage regimen for antibiotics. Res. Vet. Sci 73:105–114 [DOI] [PubMed] [Google Scholar]
- 39.Thiebaut F, Tsuruot T, Hamadat H, Gottesman MM, Pastan IRA (1987) Cellular localization of multidrug-resistance gene product P-glycoprotein in normal human tissues. 84:7735–7738. 10.1073/pnas.84.21.7735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schinkel AH (1999) P-Glycoprotein, a gatekeeper in the blood-brain barrier. Adv Drug Deliv Rev 36:179–194. 10.1016/S0169-409X(98)00085-4 [DOI] [PubMed] [Google Scholar]
- 41.Wang EJ, Lew K, Casciano CN, Clement RP, Johnson WW (2002) Interaction of common azole antifungals with P glycoprotein. Antimicrob Agents Chemother 46:160–165. 10.1128/AAC.46.1.160-165.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kapoor A, Iqbal M, Petropoulos S, Ho HL, Gibb W, Matthews SG (2013) Effects of Sertraline and Fluoxetine on P-Glycoprotein at Barrier Sites: In Vivo and In Vitro Approaches. PLoS One 8:3–8. 10.1371/journal.pone.0056525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weiss J, Dormann S-MG, Martin-Facklam M, Kerpen CJ, Ketabi-Kiyanvash N, Haefeli WE (2003) Inhibition of P-glycoprotein by newer antidepressants. J Pharmacol Exp Ther 305:197–204. 10.1124/jpet.102.046532 [DOI] [PubMed] [Google Scholar]
- 44.Veringa A, van der Elst KCM, Day JN, Thwaites GE, Alffenaar JWC (2016) Sertraline for HIV-associated cryptococcal meningitis. Lancet Infect. Dis 16:1111. [DOI] [PubMed] [Google Scholar]
- 45.Kalvass JC, Polli JW, Bourdet DL, Feng B, Huang S-M, Liu X, Smith QR, Zhang LK, Zamek-Gliszczynski MJ (2013) Why Clinical Modulation of Efflux Transport at the Human Blood–Brain Barrier Is Unlikely: The ITC Evidence-Based Position. Clin Pharmacol Ther 94:80–94. 10.1038/clpt.2013.34 [DOI] [PubMed] [Google Scholar]
- 46.Eyal S, Hsiao P, Unadkat JD (2009) Drug interactions at the blood-brain barrier: fact or fantasy? Pharmacol Ther 123:80–104. 10.1016/j.pharmthera.2009.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shajib MS, Khan WI (2015) The role of serotonin and its receptors in activation of immune responses and inflammation. Acta Physiol (Oxf) 213:561–74. 10.1111/apha.12430 [DOI] [PubMed] [Google Scholar]
- 48.Speth C, Rambach G, Lass-Flörl C (2014) Platelet immunology in fungal infections. Thromb Haemost 112:632–639. 10.1160/TH14-01-0074 [DOI] [PubMed] [Google Scholar]
- 49.Mercado CP, Ziu E, Kilic F (2011) Communication between 5-HT and small GTPases. Curr Opin Pharmacol 11:23–28. 10.1016/j.coph.2011.01.006NIH Public Access [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Larsson M, Hagberg L, Norkrans G, Forsman A (1989) Indole amine deficiency in blood and cerebrospinal fluid from patients with human immunodeficiency virus infection J Neurosci Res 23:441–446. 10.1002/jnr.490230410Wiley Subscription Services, Inc., A Wiley Company; [DOI] [PubMed] [Google Scholar]
- 51.Meredith EJ, Chamba A, Holder MJ, Barnes NM, Gordon J (2005) Close encounters of the monoamine kind: Immune cells betray their nervous disposition. Immunology 115:289–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Baganz NL, Blakely RD (2013) A dialogue between the immune system and brain, spoken in the language of serotonin. ACS Chem. Neurosci 4:48–63 [DOI] [PMC free article] [PubMed] [Google Scholar]