Supplemental Digital Content is available in the text.
Keywords: atrial fibrillation, mortality, rivaroxaban, stroke, warfarin
Background and Purpose—
Oral anticoagulation therapy is standard of care for patients with nonvalvular atrial fibrillation to prevent stroke. This study compared rivaroxaban and warfarin for stroke and all-cause mortality risk reduction in a real-world setting.
Methods—
This retrospective cohort study (2011–2017) included de-identified patients from the Optum Clinformatics Database who started treatment with rivaroxaban or warfarin within 30 days following initial diagnosis of nonvalvular atrial fibrillation. Before nonvalvular atrial fibrillation diagnosis, patients had 6 months of continuous health plan enrollment and CHA2DS2-VASc score ≥2. Stroke severity was determined by the National Institutes of Health Stroke Scale, imputed based on machine learning algorithms. Stroke and all-cause mortality risks were compared by treatment using Cox proportional hazard regression, with inverse probability of treatment weighting to balance cohorts for baseline risk factors. Stratified analysis by treatment duration was also performed.
Results—
During a mean follow-up of 27 months, 175 (1.33/100 patient-years [PY]) rivaroxaban-treated and 536 (1.66/100 PY) warfarin-treated patients developed stroke. The inverse probability of treatment weighting model showed that rivaroxaban reduced stroke risk by 19% (hazard ratio [HR], 0.81 [95% CI, 0.73–0.91]). Analysis by stroke severity revealed risk reductions by rivaroxaban of 48% for severe stroke (National Institutes of Health Stroke Scale score, 16–42; HR, 0.52 [95% CI, 0.33–0.82]) and 19% for minor stroke (National Institutes of Health Stroke Scale score, 1 to <5; HR, 0.81 [95% CI, 0.68–0.96]), but no difference for moderate stroke (National Institutes of Health Stroke Scale score, 5 to <16; HR, 0.93 [95% CI, 0.78–1.10]). A total of 41 (0.31/100 PY) rivaroxaban-treated and 147 (0.44/100 PY) warfarin-treated patients died poststroke, 12 (0.09/100 PY) and 67 (0.20/100 PY) of whom died within 30 days, representing mortality risk reductions by rivaroxaban of 24% (HR, 0.76 [95% CI, 0.61–0.95]) poststroke and 59% (HR, 0.41 [95% CI, 0.28–0.60]) within 30 days.
Conclusions—
After the initial diagnosis of atrial fibrillation, patients treated with rivaroxaban versus warfarin had significant risk reduction for stroke, especially severe stroke, and all-cause mortality after a stroke. Findings from this observational study may help inform anticoagulant choice for stroke prevention in patients with nonvalvular atrial fibrillation.
Atrial fibrillation (AF) is the most common cardiac arrhythmia, affecting an estimated 3 to 6 million people in the United States.1,2 The prevalence of AF is expected to rise with the aging population and reach 12 million by 2030.1 AF is associated with a 4- to 5-fold increased risk of ischemic stroke, and the proportion of strokes attributable to AF increases with advancing age, ranging from ≈10% overall to 24% in those aged 80 to 89 years.1,3,4 The burden of stroke is substantial because it is a leading cause of functional impairment.4 Effective prevention remains the best approach to limit stroke burden. Oral anticoagulation therapy with direct-acting oral anticoagulants (DOACs) is the current standard of care for stroke prevention in patients with nonvalvular AF (NVAF).5
Patients with AF experience particularly high stroke-related disease burden. They are 3 to 4 times more likely to suffer severe strokes and have greater initial functional impairment compared with patients with normal sinus rhythm.6,7 For patients with AF, risks of 1-year disability and 1-year mortality after stroke are twice those of non-AF–related strokes; hospital stays are longer and acute and long-term costs are higher.6,7
Rivaroxaban, a factor Xa inhibitor used in clinical practice since its approval in November 2011,8 has increasing use in patients with existing and newly diagnosed AF.9 Results from clinical and observational studies support the efficacy and safety of rivaroxaban in this population, including better effectiveness than warfarin for preventing stroke or systemic embolism.9,10 In the noninferiority clinical trial, ROCKET-AF (Rivaroxaban Once Daily Oral Direct Factor Xa Inhibition Compared With Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation), secondary end points of all-cause mortality and stroke severity were favorably impacted by rivaroxaban versus warfarin.11 It is important to note, however, that treatment with warfarin in the clinical trial setting is well controlled, with medication reminders, coagulation testing, and associated dose adjustments. This contrasts with the real-world setting, in which studies show that international normalized ratio (INR) monitoring is not routinely performed in warfarin-treated patients and approximately two-thirds of patients have poor INR (<2) control.12–14 Warfarin’s narrow therapeutic range, broad dose-response variability, and food and drug interactions pose challenges to anticoagulation maintenance.15 Patients with NVAF and INR <2 have an increased risk of death or developing such cardiovascular-related events as acute coronary syndrome, ischemic stroke, transient ischemic attack, and systemic embolism compared with those with INR of 2 to 3.13,14 The need for constant INR monitoring and the potential consequences of poor anticoagulation maintenance in warfarin-treated patients pose a substantial burden.
Since the introduction of DOACs in 2010, few studies on the effectiveness of anticoagulation therapy have included stroke severity as an outcome measure. However, it is important to understand how anticoagulants protect patients not just from stroke but also from more severe strokes that lead to poor functional outcomes. Stroke severity, as assessed using the National Institutes of Health Stroke Scale (NIHSS), is a strong predictor of outcome. In the clinical trial setting, a score of 16 or higher (moderately severe to severe stroke) was found to predict a high probability of death or severe disability.16
Using integrated claims and electronic health record data, we found mortality risk to be significantly higher (≥100-fold) among patients with more severe stroke compared with patients without stroke.17 Although the absence altogether of anticoagulant therapy has been associated with higher NIHSS scores, few studies compare the effectiveness of specific anticoagulation therapies on the reduction in stroke risk by severity.
Because NIHSS scores are absent from administrative claims databases, machine learning models have been used to predict risks of stroke and stroke outcomes.18–20 We have previously developed and validated an imputed NIHSS score methodology using machine learning algorithms and predictive modeling that was based on features identified in claims and electronic health record databases.21,22
For comparison to clinical trial findings, specifically the ROCKET-AF trial in which stroke severity was an outcome measure, the objective of this study was to understand, in a real-world setting, the effects of rivaroxaban and warfarin on stroke outcomes, overall and by stroke severity, using imputed NIHSS scores.
Methods
Data for this study were available to the authors via third-party license from Optum, a commercial data provider in the United States, and Janssen Pharmaceuticals, which has a license for analysis of the de-identified Optum Clinformatics Extended Data Mart—Date of Death Database. As such, the authors cannot provide the raw data; however, other researchers may access the data by purchase through Optum, and the inclusion criteria specified in the methods would allow them to identify the same cohort of patients. Interested individuals may visit www.optum.com/contact.html for more information on accessing Optum Clinformatics Extended Data Mart—Date of Death Database.
Study Design
This retrospective cohort study was based on an intent-to-treat analysis of patients from July 1, 2011 to December 31, 2017, using the database comprising longitudinal claims data from United Healthcare fully insured patients, United Healthcare administrative services only, Medicaid, and legacy Medicare Choice membership and claims. Data available include integrated enrollment and medical and prescription claims data for ≈80 million unique de-identified members since 2000, most recently updated December 2017. The dataset complies with Health Insurance Portability and Accountability Act regulation and is managed according to Optum customer data use agreements.
Study Population
To ensure accurate identification of patients with NVAF, we required them to have ≥2 NVAF diagnoses that were ≥1 week apart during the period from January 1, 2012 through December 30, 2017. Patients also had continuous health plan enrollment (≥6 months before NVAF diagnosis) and were started on treatment with either rivaroxaban or warfarin as the first anticoagulant treatment within 30 days following the initial diagnosis of NVAF. Patients were also required to have a CHA2DS2-VASc score ≥2, and 2 consecutive prescriptions of either index treatment that were ≤45 days apart. Patients were excluded if they had a primary stroke diagnosis, had a diagnosis of transient AF, or were treated with an oral anticoagulant other than the index treatment before index date, defined as the initiation date of either rivaroxaban or warfarin treatment. The treatment period was defined as the period between index date and the earliest occurrence of either study outcome, the end of the study period, the end of healthcare enrollment, or the discontinuation or switch of the index treatment.
Patients were followed from index date until the earliest occurrence of a primary inpatient diagnosis of stroke, death, the end of health plan enrollment, or the end of the study. The first primary stroke diagnosis for ischemic or hemorrhagic stroke was identified using International Classification of Diseases (ICD)-9 and ICD-10 codes (ischemic: ICD-9: 433.01, 433.11, 433.21, 433.31, 433.81, 433.91, 434.01, 434.11, 434.91, 436; ICD-10: I63; transient ischemic attack: ICD-9: 435; ICD-10: G45.9; or hemorrhagic: ICD-9: 431; ICD-10, I61).
Although the NIHSS scoring measurement is widely accepted and is typically included as free text in physicians’ notes, NIHSS scores are not readily available in structured claims data.13 For the purposes of this study, NIHSS scores were imputed as described previously21,22 by machine learning algorithms using a random forest method among newly diagnosed patients with stroke. Briefly, 1505 patients from the de-identified Optum integrated databases of electronic health record and claims sources, who had an inpatient stroke diagnosis and NIHSS scores, were used to generate a predictive model based on a total of 1268 initial features derived from claims data. Subsequently, a final model was developed with the 127 most clinically relevant predictors to impute NIHSS scores for all patients with stroke.
The model for NIHSS imputation seems to be a valid proxy for stroke severity in patients with a primary stroke diagnosis. Sung et al23 recently also generated a predictive model for NIHSS using the Taiwan’s National Health Insurance claims database. We found that our predictive model achieved similar performance to theirs (Pearson correlation coefficient between imputed and true NIHSS scores; 0.76, Sung et al and 0.73, Kogan et al).21,22 Further, the Sung et al24 model was more recently externally validated using a second dataset.
In the present analysis, NIHSS scores were classified into 3 categories of severity: 1 to <5 (minor), 5 to <16 (moderate), 16 to 42 (severe). All-cause mortality was assessed at any time following treatment initiation, at any time poststroke, and within 30 days poststroke.
Statistical Analysis
The inverse probability of treatment weighting method25 was used to balance the difference in baseline characteristics between patients initiating rivaroxaban and those with warfarin treatment. Specifically, the propensity score (PS), representing treatment probability, was first estimated by regressing treatment assignment (ie, rivaroxaban) against baseline risk factors including age, sex, baseline Quan-Charlson comorbidity index, residence regions, health insurance type, health plan type, baseline CHA2DS2-VASc score, baseline HAS-BLED score, and presence of other baseline risk factors, including hypertension, diabetes mellitus, hyperlipidemia, obesity, coronary artery disease, and heart failure. Then, the Cox proportional hazard regression model was weighted by the inverse propensity score (rivaroxaban: 1/[propensity score]; warfarin: 1/[1–propensity score]), comparing risk for developing stroke (overall and by stroke severity) and all-cause mortality (at any time following treatment initiation, poststroke diagnosis, and within 30 days of stroke diagnosis) in patients who initiated rivaroxaban versus those who initiated warfarin (reference). Kaplan-Meier estimates were used to evaluate the timing of occurrence of stroke and all-cause mortality outcomes. Finally, subgroup analyses were performed by treatment duration (<9 and ≥9 months) and prior renal disease status.
Results
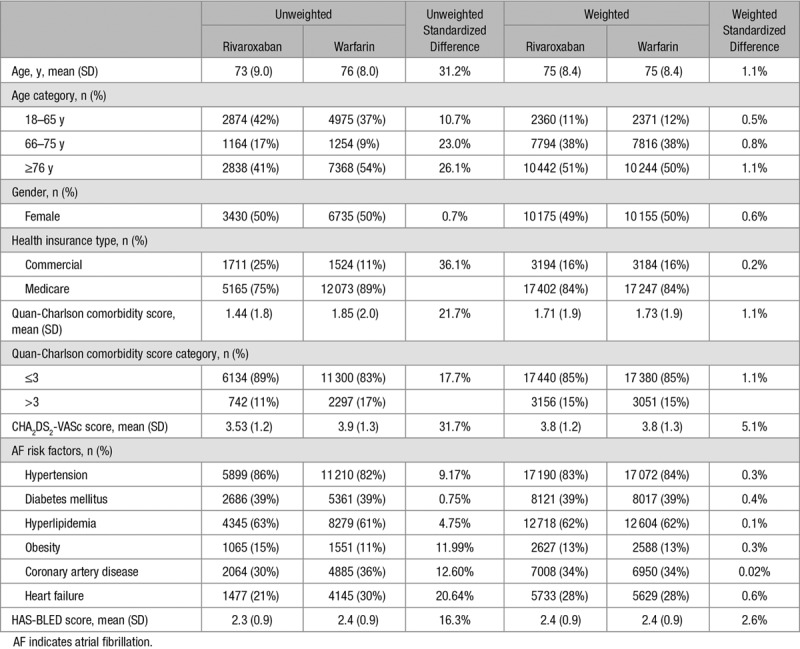
In total, 6876 rivaroxaban-initiated and 13 597 warfarin-initiated patients were included in the study. Following inverse probability of treatment weighting, the 2 groups were well balanced, with standardized differences for all baseline characteristics being ≤5%. Both weighted cohorts had an average age of 75 years, a CHA2DS2-VASc score of 3.8, a HAS-BLED score of 2.4, and had similar distributions of AF risk factors (Table 1).
Table 1.
Unweighted and Weighted Baseline Characteristics in Patients Treated With Rivaroxaban (N=6876) and Warfarin (N=13 597)
The rates for successful follow-up during the 6-month and 1-year periods were 83% and 64% for the rivaroxaban cohort and 88% and 74% for the warfarin cohort. During a mean (interquartile range) follow-up of 23 (9–36) months in the rivaroxaban cohort and 29 (8–46) months in the warfarin cohort, 175 (1.33/100 patient-years [PY]) rivaroxaban and 536 (1.66/100 PY) warfarin patients developed stroke (Figure). Compared with warfarin patients, rivaroxaban patients had a 19% risk reduction (hazard ratio [HR], 0.81 [95% CI, 0.73–0.91]) for stroke overall. Analysis by stroke severity revealed that rivaroxaban cohort was associated with a 48% lower risk (HR, 0.52 [95% CI, 0.33–0.82]) for severe stroke and 19% lower risk (HR, 0.81 [95% CI, 0.68–0.96]) for minor stroke (Figure). Risk for moderate stroke was not statistically different between treatment cohorts. Moreover, the Kaplan-Meier estimate showed that the percentages of stroke-free patients at 12, 36, and 48 months were 99%, 96%, and 95% in the rivaroxaban cohort and 98%, 95%, and 94% in the warfarin cohort (log-rank P<0.05; see the online-only Data Supplement).
Figure.
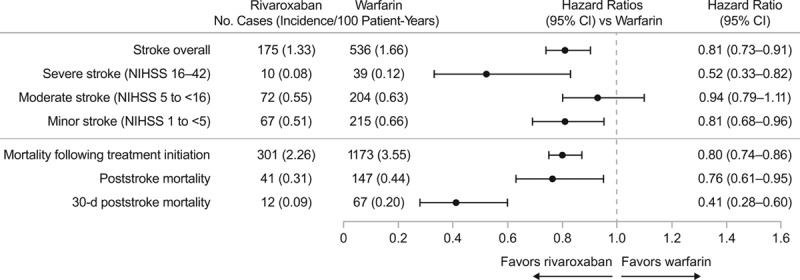
Comparative effectiveness of rivaroxaban vs warfarin. NIHSS indicates National Institutes of Health Stroke Scale.
A total of 301 (2.26/100 PY) and 1173 (3.55/100 PY) patients died during follow-up in the rivaroxaban and warfarin cohorts, respectively, representing a 20% reduction in risk of all-cause mortality following treatment initiation among rivaroxaban-treated patients (HR, 0.80 [95% CI, 0.74–0.86]; Figure). Following stroke diagnosis, a total of 41 (0.31/100 PY) patients died in the rivaroxaban cohort and a total of 147 (0.44/100 PY) died in the warfarin cohort, reflecting a 24% risk reduction (HR, 0.76 [95% CI, 0.61–0.95]) in rivaroxaban-treated patients. A total of 12 (0.09/100 PY) patients in the rivaroxaban cohort and a total of 67 (0.20/100 PY) patients in the warfarin cohort died within the first 30 days following stroke, which also reflects a significant risk reduction of 59% (HR, 0.41 [95% CI, 0.28–0.60]) in 30-day poststroke mortality for rivaroxaban relative to warfarin cohort (Figure). Additionally, according to the Kaplan-Meier estimates, the percentages of patients who remained alive at 12, 36, and 48 months were 98%, 94%, and 91% in the rivaroxaban cohort and 96%, 90%, and 87% in the warfarin cohort (log-rank P≤0.05; see the online-only Data Supplement).
We also conducted a sensitivity analysis by restricting patients with 1-year follow-up (mean, 10 months; interquartile range, 10–12 months). In rivaroxaban-treated patients as compared with warfarin-treated patients, we found similar risk reductions of 24% (HR, 0.76 [95% CI, 0.65–0.89]) for stroke overall and 35% (HR, 0.65 [95% CI, 0.57–0.74]) for all-cause mortality posttreatment initiation.
Subgroup Analysis by Treatment Duration
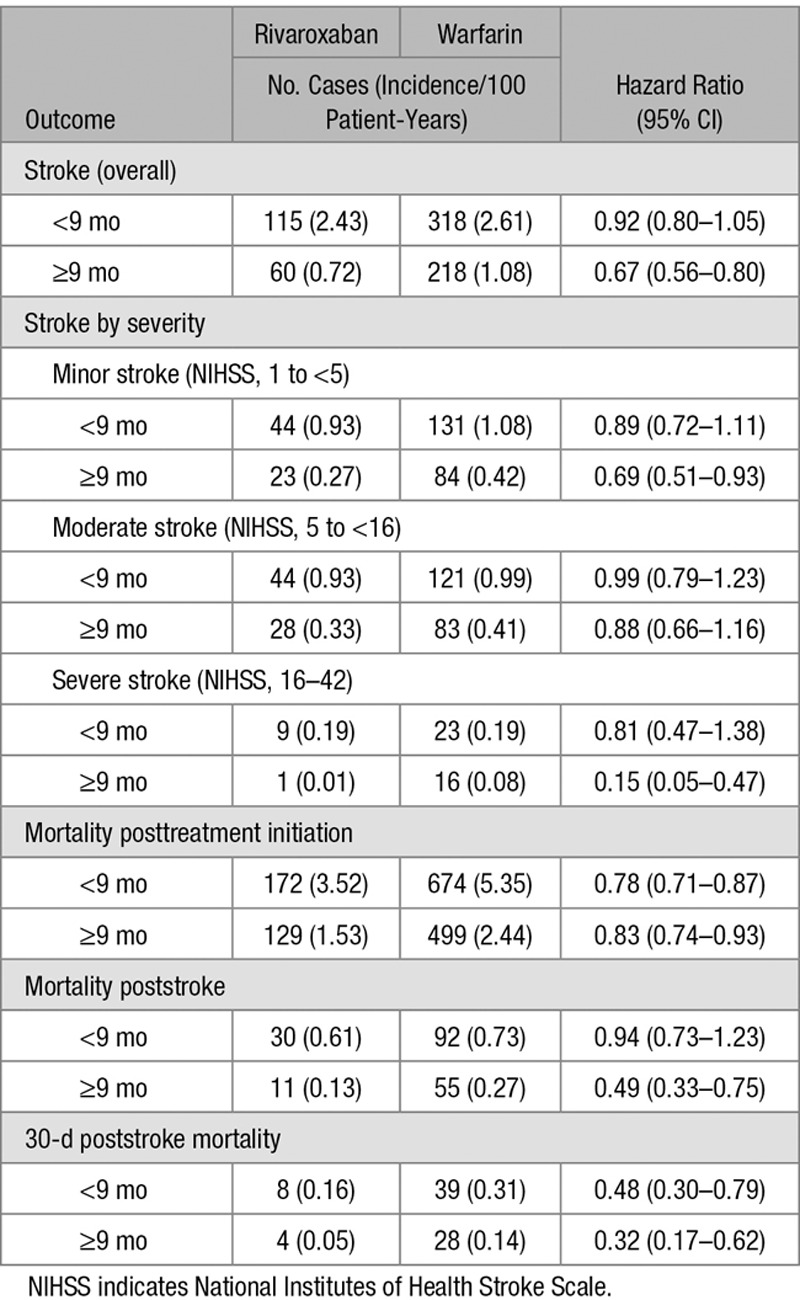
To assess if the duration of anticoagulation treatment had an impact on study outcomes, we performed a subgroup analysis of patients who were treated for less than or more than the median anticoagulation treatment duration of 9 months (Table 2). Although in both duration groups, the rivaroxaban cohort showed greater risk reductions compared with warfarin for most outcomes, the longer duration group showed the most pronounced risk reductions in the rivaroxaban cohort for severe stroke (HR, 0.15 [95% CI, 0.05–0.47]), poststroke mortality (HR, 0.49 [95% CI, 0.33–0.75]), and 30-day poststroke mortality (HR, 0.32 [95% CI, 0.17–0.62]).
Table 2.
Comparative Effectiveness of Rivaroxaban vs Warfarin (Reference Group), by Treatment Duration
Finally, risk reduction for overall stroke in the rivaroxaban cohort was 21% (HR, 0.79 [95% CI, 0.62–1.01]) among patients with prior renal disease and 18% (HR, 0.82 [95% CI, 0.73–0.93]) among those with no renal disease. Similarly, risk reduction for all-cause mortality in the rivaroxaban cohort was 31% (HR, 0.69 [95% CI, 0.59–0.81]) among those with prior renal disease and 14% among those without prior renal disease (HR, 0.86 [95% CI, 0.78–0.94]).
Discussion
This retrospective claims-based cohort study of patients with NVAF compared stroke-related risks associated with anticoagulant treatment with either rivaroxaban or warfarin. Our analysis found that, relative to warfarin-treated patients, patients who initiated rivaroxaban had significantly lower risks for stroke, specifically for minor and severe strokes. In addition, there was significant risk reduction in rivaroxaban-treated patients for death at any time following treatment initiation, at any time poststroke diagnosis, and within 30 days poststroke diagnosis. Most risk reductions were more pronounced for patients who received rivaroxaban for 9 months or longer.
Stroke and Stroke Severity Outcomes
In this study, rivaroxaban treatment in patients with NVAF was associated with ≈20% reduction in stroke risk. In agreement with our findings, several other observational studies have also shown the benefits of DOACs over warfarin in stroke prevention among patients with AF. A retrospective cohort study of Medicare population has shown that rivaroxaban-treated patients had 25% risk reduction for stroke/systemic embolism.26 Another claims-based study has reported ≈40% risk reduction with rivaroxaban use, relative to warfarin, for development of ischemic or intracranial hemorrhage.27 Similar results were also observed when other DOACs were compared with warfarin, showing ≈60% risk reduction of stroke/systemic embolism26 and intracranial hemorrhage.27 Moreover, a Canadian population-based study has supported risk reduction for the combined outcomes of stroke and mortality with the use of any DOACs (apixaban, rivaroxaban, and dabigatran) versus warfarin.28
Although warfarin treatment has been associated with less severe neurological deficits and favorable prognosis,14,29 less is known about the relationship between DOAC treatment and stroke severity. Our findings of ≈50% risk reduction for severe stroke with rivaroxaban relative to warfarin use are in line with results from the ROCKET-AF clinical trial30 demonstrating that stroke severity was favorably impacted by treatment with a DOAC (HR, 0.77 [95% CI, 0.52–1.14]).31 In contrast, the ENGAGE AF-TIMI 48 trial (Effective Anticoagulation with Factor Xa Next Generation in Atrial Fibrillation–Thrombolysis in Myocardial Infarction 48) failed to show the protective effects of edoxaban against disabling and fatal strokes (as defined by the modified Rankin Scale score ≥3), with annual rates of 0.71% for warfarin and 0.69% and 0.80% for high-dose and low-dose edoxaban, respectively.32 The impact of other DOACs (eg, apixaban) on stroke severity has not been reported. Limited evidence is available in the literature on the association of DOACs and stroke severity.
Mortality Outcomes
The current study found significant risk reductions in all-cause mortality. Specifically, our study has shown more risk reduction (≈60%) in early poststroke mortality, suggesting the protection of death is likely attributable to stroke-related death. The ROCKET-AF trial secondary end point of stroke-related mortality also suggested that the rivaroxaban cohort had a lower event rate for all-cause mortality (HR, 0.85 [95% CI, 0.70–1.02]) and stroke-related mortality (HR, 0.71 [95% CI, 0.49–1.03]).11 According to the ROCKET-AF trial, the majority of deaths (72%) were cardiovascular in nature, with only 6% classified as due to nonhemorrhagic stroke or systemic embolism.33 Several observational studies and clinical trials have compared other DOACs to warfarin for mortality risk. A Danish registry study of patients treated with warfarin and dabigatran, respectively, showed that mortality was significantly lower for dabigatran (P<0.05).34 A meta-analysis of 4 randomized trials, including DOACs (RE-LY [dabigatran], ROCKET-AF [rivaroxaban], ARISTOTLE [apixaban], and ENGAGE AF-TIMI 48 [edoxaban]), reported significantly lower mortality risk than warfarin. The 4 patient cohorts varied considerably in baseline risk factors of stroke, with ROCKET-AF having the higher mean CHA2DS2 score (3.5) versus RE-LY and ARISTOTLE (means of 2.1–2.2) and lowest median time in therapeutic range for warfarin (58% for ROCKET-AF versus 66% to 68% for other trials).35 The CHA2DS2-VASc in this study were 3.76 to 3.82, which is similar to what was observed in the ROCKET-AF trial. This highlights the importance of real-world evidence from nonselected patients in uncontrolled settings in determining the effectiveness of therapy received in typical clinical care.
Unlike most claims-based studies, the current study was able to assess risk for stroke severity within a population reflective of routine clinical practice. Historically, challenges in capturing stroke severity scores from claims databases have limited studies to analyze such relationships. By using a machine learning technique, we were able to impute NIHSS scores and compare stroke severity and outcomes for 2 treatments. To our knowledge, our study is one of the first to report stroke risk by severity using NIHSS cutoff imputed by a machine learning method. The current study’s observational design also allowed for a large sample population over a long follow-up time, representative of routine clinical practice. Additionally, our study went beyond overall mortality assessment by showing more protection of rivaroxaban relative to warfarin in early (ie, 30-day) poststroke mortality.
This study has several limitations. As with most database research, administrative claims data may not be an accurate comparison to a prospective study design. Our study also does not provide a measure of adherence and persistence to anticoagulant therapy, anticoagulant control (INR for warfarin), or other cardiovascular event prevention strategies. Nevertheless, inverse probability of treatment weighting modeling was used to balance the baseline characteristics between the 2 treatment cohorts to reduce the impact of confounding factors.
This study showed risk reduction with rivaroxaban treatment in stroke overall and across all but the moderate stroke category. A possible explanation for the nonsignificant risk reduction for moderate stroke is insufficient power, given that the occurrence of stroke is so rare in this study population of patients with NVAF taking anticoagulants.
With any anticoagulant use, unwanted bleeding rarely occurs. Although our study did not assess complications associated with organ-related bleeding as its focus was on stroke outcomes and not bleeding risk, it did include intracranial hemorrhage as a stroke outcome. Because too few intracranial hemorrhage events occurred to produce meaningful analysis (≈10% of total stroke events), intracranial hemorrhage events were combined with all other stroke types. Reasons for treatment discontinuation or switching are not directly available via claims data. However, neither treatment appeared to be stopped more often than the other, as the distributions for continuous treatment duration were similar for the 2 cohorts. Finally, findings from the current study apply only to prevention of a first stroke, thereby limiting the generalizability of findings to prevention of strokes other than first stroke.
In summary, this study showed risk reduction of stroke and mortality among patients with NVAF who were treated with rivaroxaban versus warfarin. Patients with AF impose a particularly high stroke-related disease burden relative to those without AF and are more likely to suffer severe strokes leading to greater disability and mortality.6 Therefore, understanding the importance of anticoagulant treatment choice in this population in a real-world setting is critical to optimizing patient access to the most effective treatments.
Conclusions
After the initial diagnosis of AF, patients treated with rivaroxaban versus warfarin had significant risk reduction for stroke, especially severe stroke, and all-cause mortality after a stroke. To our knowledge, this is the first study to show the association between anticoagulation treatments and stroke severity and mortality using a large administrative database. NVAF patients initiating anticoagulant treatment with rivaroxaban showed significant risk reduction relative to warfarin, both in stroke overall and in severe stroke, and lower mortality. Also, longer anticoagulation treatment with rivaroxaban showed significantly better protection from stroke and mortality relative to warfarin. This study’s findings may help healthcare providers choose more effective treatments for managing their patients with NVAF, which may improve stroke prevention, optimize functional outcomes secondary to risk reduction of severe stroke, and reduce mortality from stroke.
Acknowledgments
We thank Nancy Connolly, MPH, Janssen Scientific Affairs, LLC; Zhijie Ding, PhD, MS, Janssen Scientific Affairs, LLC; and Deepti Bisht, BS, of Mu Sigma Business Solutions Pvt. Ltd. (Bengaluru, India). Medical writing support was provided by Ashley O’Dunne, PhD, of MedErgy (Yardley, PA), which was funded by Janssen Scientific Affairs, LLC (Titusville, NJ).
Sources of Funding
This work was supported by Janssen Scientific Affairs, LLC.
Disclosures
Dr Alberts has not received compensation for this project but received consultancy fees in the past from Janssen Pharmaceuticals, LLC. Drs Chen, Lin, and Milentijevic are employees of Janssen Scientific Affairs, LLC. E. Kogan is an employee of Janssen Research & Development, LLC. Dr Twyman was an employee of Janssen Research & Development, LLC at the time of the analysis.
Supplementary Material
Footnotes
Presented in part at the European Society of Cardiology Congress, Munich, Germany, August 25–29, 2018; the American Heart Association's Scientific Sessions, Chicago, IL, November 10–12, 2018; the International Stroke Conference, Honolulu, HI, February 6–8, 2019; and the American College of Cardiology Annual Conference, New Orleans, LA, March 16–18, 2019.
The online-only Data Supplement is available with this article at https://www.ahajournals.org/doi/suppl/10.1161/STROKEAHA.119.025554.
References
- 1.Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics-2017 update: a report from the American Heart Association. Circulation. 2017;135:e146–e603. doi: 10.1161/CIR.0000000000000485. doi: 10.1161/CIR.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. Atrial fibrillation fact sheet. https://www.cdc.gov/dhdsp/data_statistics. Accessed May 4, 2018.
- 3.Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke. 1991;22:983–988. doi: 10.1161/01.str.22.8.983. doi: 10.1161/01.str.22.8.983. [DOI] [PubMed] [Google Scholar]
- 4.Meschia JF, Bushnell C, Boden-Albala B, Braun LT, Bravata DM, Chaturvedi S, et al. American Heart Association Stroke Council; Council on Cardiovascular and Stroke Nursing; Council on Clinical Cardiology; Council on Functional Genomics and Translational Biology; Council on Hypertension. Guidelines for the primary prevention of stroke: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45:3754–3832. doi: 10.1161/STR.0000000000000046. doi: 10.1161/STR.0000000000000046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.January CT, Wann LS, Calkins H, Chen LY, Cigarroa JE, Cleveland JC, Jr, et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2019;74:104–132. doi: 10.1016/j.jacc.2019.01.011. doi: 10.1016/j.jacc.2019.01.011. [DOI] [PubMed] [Google Scholar]
- 6.Ali AN, Abdelhafiz A. Clinical and economic implications of AF related stroke. J Atr Fibrillation. 2016;8:1279. doi: 10.4022/jafib.1279. doi: 10.4022/jafib.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Andrew NE, Thrift AG, Cadilhac DA. The prevalence, impact and economic implications of atrial fibrillation in stroke: what progress has been made? Neuroepidemiology. 2013;40:227–239. doi: 10.1159/000343667. doi: 10.1159/000343667. [DOI] [PubMed] [Google Scholar]
- 8.XARELTO® (rivaroxaban) Tablets, for Oral Use [package insert] Titusville, NJ: Janssen Pharmaceuticals, Inc; 2018. [Google Scholar]
- 9.Beyer-Westendorf J, Camm AJ, Coleman CI, Tamayo S. Rivaroxaban real-world evidence: validating safety and effectiveness in clinical practice. Thromb Haemost. 2016;116(suppl 2):S13–S23. doi: 10.1160/TH16-06-0485. doi: 10.1160/TH16-06-0485. [DOI] [PubMed] [Google Scholar]
- 10.Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, et al. ROCKET AF Investigators. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883–891. doi: 10.1056/NEJMoa1009638. doi: 10.1056/NEJMoa1009638. [DOI] [PubMed] [Google Scholar]
- 11.Johnson & Johnson Pharmaceutical Research & Development. Advisory committee briefing document. Rivaroxaban for the prevention of stroke and non-central nervous system systemic embolism in patients with atrial fibrillation. Aug 5, 2011.
- 12.Chan EW, Lau WC, Siu CW, Lip GY, Leung WK, Anand S, et al. Effect of suboptimal anticoagulation treatment with antiplatelet therapy and warfarin on clinical outcomes in patients with nonvalvular atrial fibrillation: a population-wide cohort study. Heart Rhythm. 2016;13:1581–1588. doi: 10.1016/j.hrthm.2016.03.049. doi: 10.1016/j.hrthm.2016.03.049. [DOI] [PubMed] [Google Scholar]
- 13.Xian Y, O’Brien EC, Liang L, Xu H, Schwamm LH, Fonarow GC, et al. Association of preceding antithrombotic treatment with acute ischemic stroke severity and in-hospital outcomes among patients with atrial fibrillation. JAMA. 2017;317:1057–1067. doi: 10.1001/jama.2017.1371. doi: 10.1001/jama.2017.1371. [DOI] [PubMed] [Google Scholar]
- 14.Hylek EM, Go AS, Chang Y, Jensvold NG, Henault LE, Selby JV, et al. Effect of intensity of oral anticoagulation on stroke severity and mortality in atrial fibrillation. N Engl J Med. 2003;349:1019–1026. doi: 10.1056/NEJMoa022913. doi: 10.1056/NEJMoa022913. [DOI] [PubMed] [Google Scholar]
- 15.Halperin JL, Dorian P. Trials of novel oral anticoagulants for stroke prevention in patients with non-valvular atrial fibrillation. Curr Cardiol Rev. 2014;10:297–302. doi: 10.2174/1573403X10666140513104523. doi: 10.2174/1573403x10666140513104523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adams HP, Jr, Davis PH, Leira EC, Chang KC, Bendixen BH, Clarke WR, et al. Baseline NIH stroke scale score strongly predicts outcome after stroke: a report of the Trial of Org 10172 in Acute Stroke Treatment (TOAST). Neurology. 1999;53:126–131. doi: 10.1212/wnl.53.1.126. doi: 10.1212/wnl.53.1.126. [DOI] [PubMed] [Google Scholar]
- 17.Alberts M, Chen YW, Lin JH, Deshmukh S, Kogan E, Twyman K, et al. Initial stroke severity is a significant predictor of all-cause mortality.. Poster presented at: International Stroke Conference; January 24–26, 2018; Los Angeles, CA. https://www.ahajournals.org/doi/10.1161/str.49.suppl_1.TP311. Accessed June 21, 2019. [Google Scholar]
- 18.Li X, Liu H, Du X, Zhang P, Hu G, Xie G, et al. Integrated machine learning approaches for predicting ischemic stroke and thromboembolism in atrial fibrillation. AMIA Annu Symp Proc. 2017;2016:799–807. [PMC free article] [PubMed] [Google Scholar]
- 19.Asadi H, Dowling R, Yan B, Mitchell P. Machine learning for outcome prediction of acute ischemic stroke post intra-arterial therapy. PLoS One. 2014;9:e88225. doi: 10.1371/journal.pone.0088225. doi: 10.1371/journal.pone.0088225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheng CA, Lin YC, Chiu HW. Prediction of the prognosis of ischemic stroke patients after intravenous thrombolysis using artificial neural networks. Stud Health Technol Inform. 2014;202:115–118. [PubMed] [Google Scholar]
- 21.Kogan E, Twyman K, Heap J, Milentijevic D, Lin JH, Chen YW, et al. Use of machine learning to determine stroke severity of patients diagnosed with stroke in claims data.. Poster presented at: European Society of Cardiology Congress; August 25–29, 2018; Munich, Germany. https://esc365.escardio.org/Congress/ESC-Congress-2018/Atrial-fibrillation-stroke-and-cardiovascular-risk/175888-use-of-machine-learning-to-determine-stroke-severity-of-patients-diagnosed-with-stroke-in-claims-data. Accessed June 21, 2019. [Google Scholar]
- 22.Kogan E, Twyman K, Heap J, Milentijevic D, Lin JH, Alberts MJ. Abstract 15849: Use of machine learning to determine stroke severity of patients diagnosed with stroke in integrated claims-medical records dataset. Circulation. 2017;136(suppl 1) [Google Scholar]
- 23.Sung SF, Hsieh CY, Kao Yang YH, Lin HJ, Chen CH, Chen YW, et al. Developing a stroke severity index based on administrative data was feasible using data mining techniques. J Clin Epidemiol. 2015;68:1292–1300. doi: 10.1016/j.jclinepi.2015.01.009. doi: 10.1016/j.jclinepi.2015.01.009. [DOI] [PubMed] [Google Scholar]
- 24.Hung LC, Sung SF, Hsieh CY, Hu YH, Lin HJ, Chen YW, et al. Validation of a novel claims-based stroke severity index in patients with intracerebral hemorrhage. J Epidemiol. 2017;27:24–29. doi: 10.1016/j.je.2016.08.003. doi: 10.1016/j.je.2016.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Austin PC, Stuart EA. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med. 2015;34:3661–3679. doi: 10.1002/sim.6607. doi: 10.1002/sim.6607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lip GYH, Keshishian A, Li X, Hamilton M, Masseria C, Gupta K, et al. Effectiveness and safety of oral anticoagulants among nonvalvular atrial fibrillation patients. Stroke. 2018;49:2933–2944. doi: 10.1161/STROKEAHA.118.020232. doi: 10.1161/STROKEAHA.118.020232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Coleman CI, Antz M, Bowrin K, Evers T, Simard EP, Bonnemeier H, et al. Real-world evidence of stroke prevention in patients with nonvalvular atrial fibrillation in the United States: the REVISIT-US Study. Curr Med Res Opin. 2016;32:2047–2053. doi: 10.1080/03007995.2016.1237937. doi: 10.1080/03007995.2016.1237937. [DOI] [PubMed] [Google Scholar]
- 28.Yu AYX, Malo S, Svenson LW, Wilton SB, Hill MD. Temporal trends in the use and comparative effectiveness of direct oral anticoagulant agents versus warfarin for nonvalvular atrial fibrillation: a Canadian population-based study. J Am Heart Assoc. 2017;6:e007129. doi: 10.1161/JAHA.117.007129. doi: 10.1161/JAHA.117.007129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim BJ, Kim HJ, Do Y, Lee JH, Park KY, Cha JK, et al. The impact of prior antithrombotic status on cerebral infarction in patients with atrial fibrillation. J Stroke Cerebrovasc Dis. 2014;23:2054–2059. doi: 10.1016/j.jstrokecerebrovasdis.2014.03.011. doi: 10.1016/j.jstrokecerebrovasdis.2014.03.011. [DOI] [PubMed] [Google Scholar]
- 30.Hankey GJ, Patel MR, Stevens SR, Becker RC, Breithardt G, Carolei A, et al. ROCKET AF Steering Committee Investigators. Rivaroxaban compared with warfarin in patients with atrial fibrillation and previous stroke or transient ischaemic attack: a subgroup analysis of ROCKET AF. Lancet Neurol. 2012;11:315–322. doi: 10.1016/S1474-4422(12)70042-X. doi: 10.1016/S1474-4422(12)70042-X. [DOI] [PubMed] [Google Scholar]
- 31.Daaboul Y, Korjian S, Plotnikov AN, Burton P, Braunwald E, Wiviott SD, et al. Rivaroxaban and post-stroke neurological outcomes in patients with acute coronary syndrome. J Am Coll Cardiol. 2018;71:1048–1049. doi: 10.1016/j.jacc.2017.12.045. doi: 10.1016/j.jacc.2017.12.045. [DOI] [PubMed] [Google Scholar]
- 32.Giugliano RP, Ruff CT, Braunwald E, Murphy SA, Wiviott SD, Halperin JL, et al. ENGAGE AF-TIMI 48 Investigators. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369:2093–2104. doi: 10.1056/NEJMoa1310907. doi: 10.1056/NEJMoa1310907. [DOI] [PubMed] [Google Scholar]
- 33.Pokorney SD, Piccini JP, Stevens SR, Patel MR, Pieper KS, Halperin JL, et al. ROCKET AF Steering Committee & Investigators; ROCKET AF Steering Committee Investigators. Cause of death and predictors of all-cause mortality in anticoagulated patients with nonvalvular atrial fibrillation: data from ROCKET AF. J Am Heart Assoc. 2016;5:e002197. doi: 10.1161/JAHA.115.002197. doi: 10.1161/JAHA.115.002197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Graham DJ, Reichman ME, Wernecke M, Zhang R, Southworth MR, Levenson M, et al. Cardiovascular, bleeding, and mortality risks in elderly Medicare patients treated with dabigatran or warfarin for nonvalvular atrial fibrillation. Circulation. 2015;131:157–164. doi: 10.1161/CIRCULATIONAHA.114.012061. doi: 10.1161/CIRCULATIONAHA.114.012061. [DOI] [PubMed] [Google Scholar]
- 35.Ruff CT, Giugliano RP, Braunwald E, Hoffman EB, Deenadayalu N, Ezekowitz MD, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014;383:955–962. doi: 10.1016/S0140-6736(13)62343-0. doi: 10.1016/S0140-6736(13)62343-0. [DOI] [PubMed] [Google Scholar]


