Supplemental Digital Content is available in the text.
Keywords: eicosapentaenoic acid ethyl ester; fatty acids, omega-3; lipids; prevention and control; triglycerides; United States
Background:
Some trials have found that patients from the United States derive less benefit than patients enrolled outside the United States. This prespecified REDUCE-IT (Reduction of Cardiovascular Events with Icosapent Ethyl - Intervention Trial) subgroup analysis was conducted to determine the degree of benefit of icosapent ethyl in the United States.
Methods:
REDUCE-IT randomized 8179 statin-treated patients with qualifying triglycerides ≥135 and <500 mg/dL and low-density lipoprotein cholesterol >40 and ≤100 mg/dL and a history of atherosclerosis or diabetes mellitus to icosapent ethyl 4 g/d or placebo. The primary composite end point was cardiovascular death, nonfatal myocardial infarction, nonfatal stroke, coronary revascularization, or hospitalization for unstable angina. The key secondary composite end point was cardiovascular death, nonfatal myocardial infarction, or nonfatal stroke. A hierarchy was prespecified for examination of individual and composite end points.
Results:
A total of 3146 US patients (38.5% of the trial) were randomized and followed for a median of 4.9 years; 32.3% were women and 9.7% were Hispanic. The primary composite end point occurred in 24.7% of placebo-treated patients versus 18.2% of icosapent ethyl-treated patients (hazard ratio [HR], 0.69 [95% CI, 0.59–0.80]; P=0.000001); the key secondary composite end point occurred in 16.6% versus 12.1% (HR, 0.69 [95% CI, 0.57–0.83]; P=0.00008). All prespecified hierarchical end points were meaningfully and significantly reduced, including cardiovascular death (6.7% to 4.7%; HR, 0.66 [95% CI, 0.49–0.90]; P=0.007), myocardial infarction (8.8% to 6.7%; HR, 0.72 [95% CI, 0.56–0.93]; P=0.01), stroke (4.1% to 2.6%; HR, 0.63 [95% CI, 0.43–0.93]; P=0.02), and all-cause mortality (9.8% to 7.2%; HR, 0.70 [95% CI, 0.55–0.90]; P=0.004); for all-cause mortality in the US versus non-US patients, Pinteraction=0.02. Safety and tolerability findings were consistent with the full study cohort.
Conclusions:
Whereas the non-US subgroup showed significant reductions in the primary and key secondary end points, the US subgroup demonstrated particularly robust risk reductions across a variety of individual and composite end points, including all-cause mortality.
Clinical Trial Registration:
URL: https://www.clinicaltrials.gov. Unique identifier: NCT01492361.
Clinical Perspective.
What Is New?
REDUCE-IT (Reduction of Cardiovascular Events With Icosapent Ethyl–Intervention Trial) USA found that icosapent ethyl 4 g/d produced large and significant reductions in multiple ischemic end points, including cardiovascular death, myocardial infarction, stroke, coronary revascularization, and hospitalization for unstable angina.
REDUCE-IT USA demonstrated that icosapent ethyl provided a statistically significant 30% relative risk reduction and 2.6% absolute risk reduction in all-cause mortality.
The risk-benefit profile of icosapent ethyl was highly favorable, with an overall safety and tolerability profile virtually identical to that of placebo.
What Are the Clinical Implications?
Use of icosapent ethyl in eligible secondary and primary prevention patients would be expected to have a substantial benefit in reducing residual cardiovascular risk.
Several international guidelines have embraced the REDUCE-IT data, an independent analysis has found it to be highly cost-effective, and multiple registry analyses show the data are generalizable to a large number of patients in typical clinical practice.
Health care systems should implement the results of REDUCE-IT without delay in order to reduce the burden of initial and subsequent cardiovascular events that patients face with even modestly elevated triglycerides.
Triglyceride elevation is a potent marker of residual cardiovascular risk in patients with well-controlled low-density lipoprotein cholesterol (LDL-C) on statin therapy, as shown in both randomized statin trials and observational studies.1–3 REDUCE-IT (Reduction of Cardiovascular Events With Icosapent Ethyl–Intervention Trial) addressed this residual risk, finding a significant 25% relative risk reduction in first ischemic events with icosapent ethyl 4 g/d versus placebo in statin-treated patients with triglycerides >~135 mg/dL.4–7 This included a statistically significant 20% relative risk reduction in death attributable to cardiovascular causes, similar rates of noncardiovascular deaths, and therefore a trend towards lower all-cause mortality (13% relative risk reduction, P=0.09).4 Analysis of total ischemic events (first and subsequent events) found a significant 30% reduction, with consistent benefits across baseline and achieved triglyceride levels.5–9 In light of the strong results in REDUCE-IT, several national and international guidelines have incorporated icosapent ethyl into their recommendations.10–13 The results of REDUCE-IT appear to be generalizable to a sizable proportion of high-risk secondary and primary prevention populations.14 Trial-level analysis shows icosapent ethyl to be highly cost-effective.15
In the United States, there are at least 70 million patients with triglycerides ≥135 mg/dL, with >15 million of those on statins, and >7 million of those with LDL-C controlled to <100 mg/dL. It is estimated that there are almost 5 million US patients who fit the REDUCE-IT patient profile: statin-treated with diabetes mellitus or established cardiovascular disease who are ≥45 years of age with LDL-C <100 mg/dL.16,17 Thus, there is substantial residual risk in these patients that potentially could be addressed if they were identified and treated with icosapent ethyl.18
International trials of various pharmacotherapies have sometimes found discordant results in patients enrolled in the United States than seen in overall trial results.19–32 The reasons range from low numbers of patients enrolled in the United States to potential differences in background medical management or in patient risk profiles. We sought to explore the prespecified subgroup of patients from REDUCE-IT enrolled in the United States to determine the effects of icosapent ethyl in this subgroup.
Methods
The data that support the findings of this study may be made available from the corresponding author upon reasonable request. The details of the REDUCE-IT design have been published previously.4,5,33 Patients were randomized in a double-blind fashion to either icosapent ethyl 4 g/d (2 grams twice daily with meals) or matching placebo. The protocol was approved by health authorities, ethics committees, and institutional review boards; procedures were followed in accordance with institutional guidelines, and participants gave written informed consent. Patients were required to be either ≥45 years of age with established cardiovascular disease (secondary prevention stratum) or ≥50 years of age with diabetes mellitus and at least 1 additional cardiovascular risk factor (primary prevention stratum).
To qualify, patients were required to have fasting triglycerides of ≥135 mg/dL and <500 mg/dL and LDL-C >40 mg/dL and ≤100 mg/dL. Patients were required to be on stable statin therapy for at least 4 weeks with well-controlled LDL-C. Because of variability in fasting triglyceride levels, baseline was defined as an average of each patient’s qualifying triglyceride level and randomization day level; these baseline values ranged from 81 mg/dL to 1401 mg/dL.
The primary end point was the first occurrence of the composite of cardiovascular death, nonfatal myocardial infarction (MI), nonfatal stroke, coronary revascularization, or hospitalization for unstable angina. The key secondary end point was the composite of cardiovascular death, nonfatal MI, or nonfatal stroke. A hierarchical testing sequence of individual and composite end points was prespecified. All end points were adjudicated by an independent clinical end point committee blinded to treatment.
Statistical Analysis
For the present prespecified analysis, the effects of icosapent ethyl versus placebo were examined in patients randomized in the United States. Demographic and baseline characteristics were compared between treatment groups using the chi-square test for categorical variables and the Wilcoxon rank-sum test for continuous variables. Time to first occurrences of the primary and the secondary efficacy end points in the prespecified testing hierarchy were analyzed using a Kaplan-Meier analysis stratified by cardiovascular risk category and baseline ezetimibe use. Hazard ratios (HRs) and 95% confidence intervals (CIs) were generated from a corresponding stratified Cox proportional-hazards regression model. Heterogeneity of treatment effects between the US and non-US subgroups was examined by testing the interaction term of treatment by US versus non-US subgroup in the Cox regression model. Total (first and recurrent) cardiovascular events for the primary and key secondary composite end points were also analyzed using a negative binomial regression model to calculate rates and rate ratios, accounting for variability in each patient’s risk of events. Efficacy analyses were performed in accordance with the intention-to-treat principle, and all tests were based on a 2-sided significance level of 0.05. P values presented are nominal and exploratory with no adjustment for multiple comparisons. Statistical analyses were performed using SAS software, version 9.4 (SAS Institute, Cary, NC).
Results
Population Characteristics
A total of 6962 patients were screened in the United States. Of these, 3146 US patients (45% of those screened; 38.5% of the overall trial) were randomized and followed for a median of 4.9 years (maximum follow-up 6.2 years). Further details regarding the disposition of the patients are provided in Figure I in the online-only Data Supplement. Baseline characteristics in the United States were generally similar to the overall population (Table I in the online-only Data Supplement). Of the US patients, 92.5% were white, 32.3% were women, and 9.7% were Hispanic (Table). Patient characteristics were generally well-balanced between the 2 treatment groups. Table I in the online-only Data Supplement shows the US versus non-US subgroups. As expected, there were several baseline differences between them, such as greater age, higher proportion of women, higher body mass index, more diabetes mellitus, lower LDL-C, and lower eicosapentaenoic acid levels in the US subgroup. There were no significant between-group differences in baseline triglyceride levels. A higher proportion of US patients were in the primary prevention stratum; within the US subgroup, 58.8% of patients were randomized into the secondary prevention cohort and 41.2% into the primary prevention cohort. US placebo-treated patients had a higher primary end point event rate compared with the non-US group (67.4 versus 51.5 per 1000 patient-years, respectively; P=0.001). Overall, US placebo-treated patients in the secondary prevention cohort had a higher primary event rate compared with non-US placebo-treated patients (93.2 versus 58.7 per 1000 patient-years, respectively). Similarly, for the primary prevention cohort, primary end point event rates were higher in the US placebo-treated patients compared with the non-US placebo-treated patients (35.8 versus 28.9 per 1000 patient-years, respectively).
Table.
Patient Characteristics at Baseline
Efficacy End points
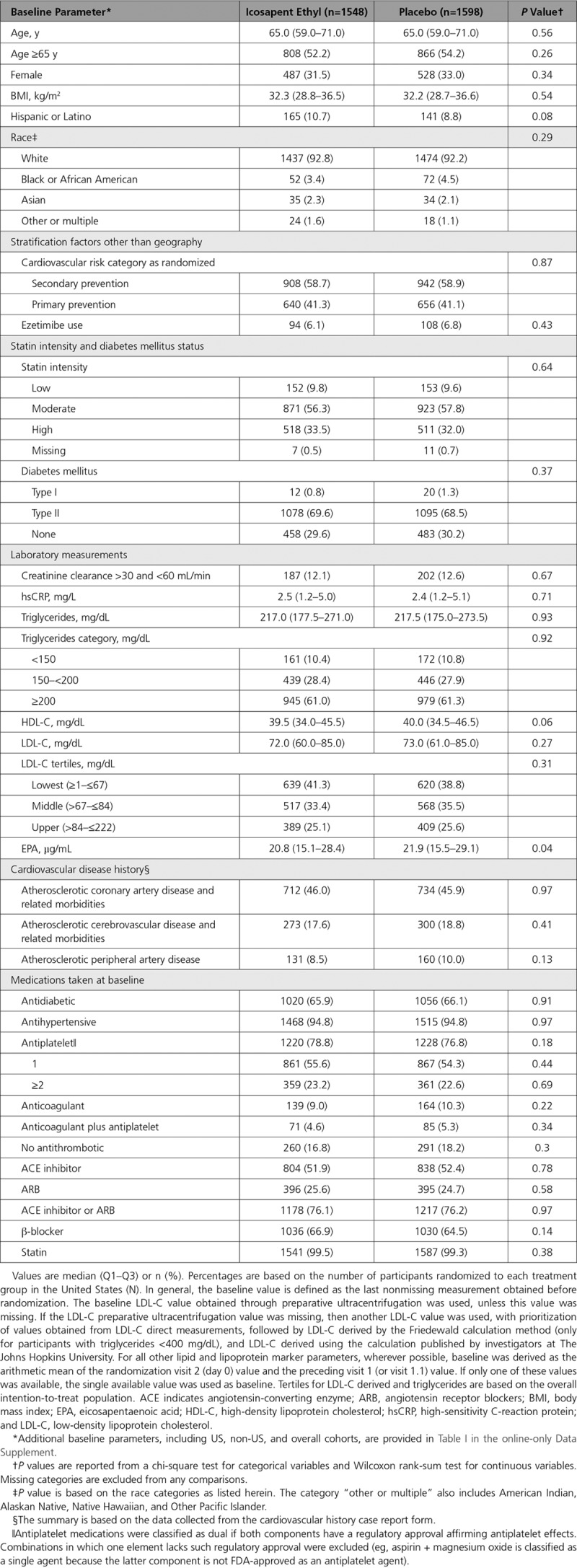
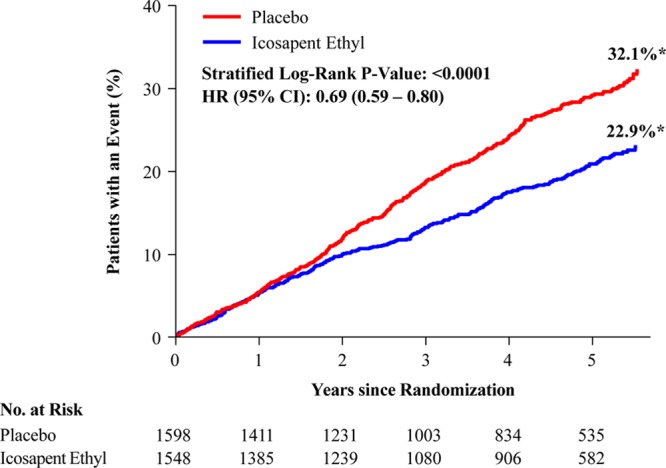
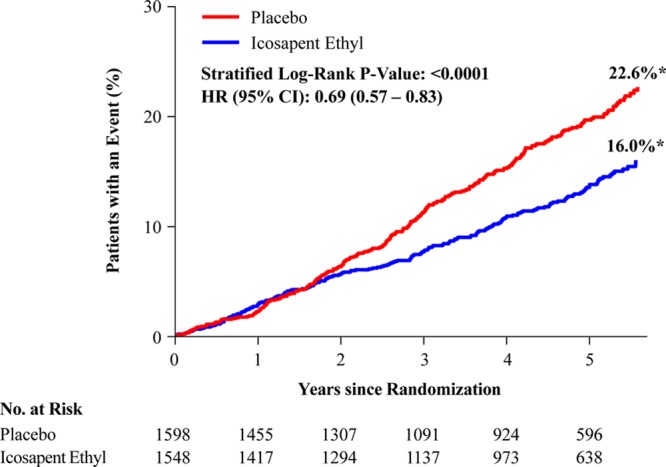
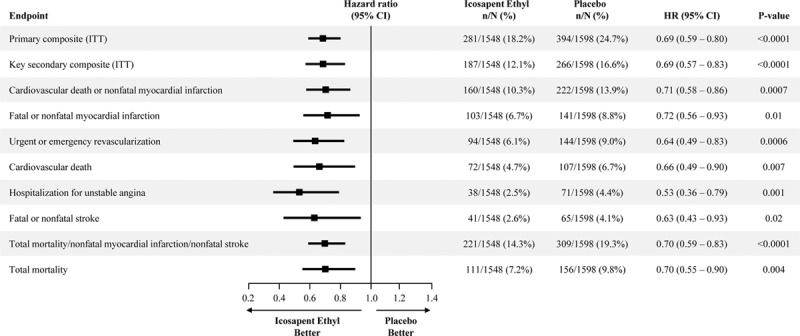
In the US cohort, the primary composite end point occurred in 24.7% of placebo-treated versus 18.2% of icosapent ethyl-treated patients (HR, 0.69 [95% CI, 0.59–0.80]; P=0.000001; number needed to treat [NNT], 15) (Figure 1). The key secondary composite end point occurred in 16.6% versus 12.1% (HR, 0.69 [95% CI, 0.57–0.83]; P=0.00008; NNT, 22) (Figure 2). All prespecified end points in the testing hierarchy were reduced significantly, including cardiovascular death (6.7% to 4.7%; HR, 0.66 [95% CI, 0.49–0.90]; P=0.007), MI (8.8% to 6.7%; HR, 0.72 [95% CI, 0.56–0.93]; P=0.01), stroke (4.1% to 2.6%; HR, 0.63 [95% CI, 0.43–0.93]; P=0.02), and all-cause mortality (9.8% to 7.2%; HR, 0.70 [95% CI, 0.55–0.90]; P=0.004) (Figures 3 and 4). The results for the US versus non-US subgroups in the above end points were generally consistent (Figure II in the online-only Data Supplement). For the primary efficacy end point, the HR outside the United States was 0.80 (95% CI, 0.71–0.91; P=0.0008; Pinteraction=0.14); for the key secondary efficacy end point, the HR outside the United States was 0.77 (95% CI, 0.66–0.91; P=0.001; Pinteraction=0.38). The interaction P value of all-cause mortality was 0.02.
Figure 1.
Kaplan-Meier curves for the primary composite end point in the US subgroup. The y axis represents the cumulative incidence rate. Primary composite end point events were cardiovascular death, nonfatal myocardial infarction, nonfatal stroke, coronary revascularization, or hospitalization for unstable angina. The curves were visually truncated at 5.7 years because a limited number of events occurred beyond that time point; all patient data were included in the analyses. *Estimated Kaplan-Meier event rate at ≈5.7 years. HR indicates hazard ratio; and no. at risk, number of patients at risk for an event.
Figure 2.
Kaplan-Meier curves for the key secondary composite end point in the US subgroup. The y axis represents the cumulative incidence rate. Key secondary composite end point events were cardiovascular death, nonfatal myocardial infarction, and nonfatal stroke. The curves were visually truncated at 5.7 years because a limited number of events occurred beyond that time point; all patient data were included in the analyses. *Estimated Kaplan-Meier event rate at ≈5.7 years. HR indicates hazard ratio; and no. at risk, number of patients at risk for an event.
Figure 3.
Hierarchical testing of end points in the US subgroup. The prespecified plan for hierarchical testing of end points for the US subgroup. The rates of all end points including total mortality were significantly lower in the icosapent ethyl group than in the placebo group. HR indicates hazard ratio; and ITT, intention to treat.
Figure 4.
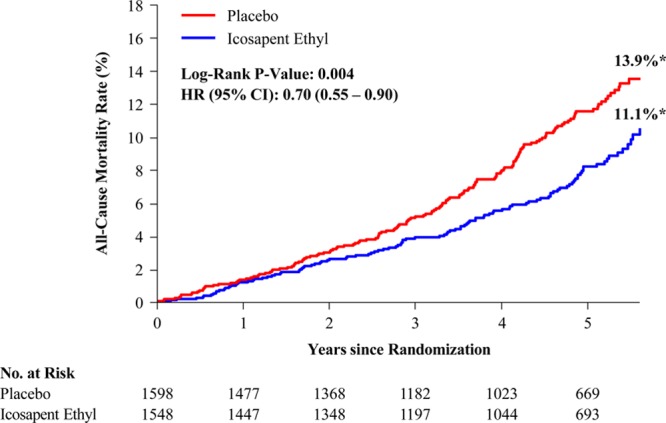
Total mortality in the US subgroup. Kaplan-Meier event curves for the end point of all-cause mortality in the US subgroup. The y axis represents the cumulative incidence rate. The curves were visually truncated at 5.7 years because a limited number of events occurred beyond that time point; all patient data were included in the analyses. *Estimated Kaplan-Meier event rate at ≈5.7 years. HR indicates hazard ratio; and no. at risk, number of patients at risk for an event.
The primary efficacy end point and key secondary efficacy end point results were generally consistent across the subgroups of US patients examined, including sex, race, and ethnicity (Figures III and IV in the online-only Data Supplement). The proportion of first (53% of total events) and subsequent ischemic events (47% of total events) is shown in Figure V in the online-only Data Supplement. Figures VI and VII in the online-only Data Supplement show the significant reductions in not only first, but also second, third, and total events. The Kaplan-Meier event curves are depicted for total (first and subsequent) ischemic events in Figures VIII and IX in the online-only Data Supplement, demonstrating large, statistically significant reductions in the primary (rate ratio, 0.68 [95% CI, 0.57–0.82]; P=0.00002) and key secondary (rate ratio, 0.68 [95% CI, 0.55–0.83]; P=0.0002) efficacy end points. Figure X in the online-only Data Supplement depicts the number of events per 1000 patients treated for 5 years with icosapent ethyl versus placebo, with 204 total primary composite end point events prevented, as well as 34 deaths of any cause.
Tolerability and Safety
Tolerability and safety findings in the US cohort were consistent with the full study cohort. There were no significant differences in the rates of adherence to double-blind treatment between icosapent ethyl-treated and placebo-treated patients. There were no significant differences between icosapent ethyl and placebo in overall treatment-emergent adverse event rates (Table II in the online-only Data Supplement). Serious treatment-emergent adverse events occurred in 34.4% of participants treated with icosapent ethyl versus 35.7% treated with placebo (P=0.46). Regarding overall treatment-emergent gastrointestinal disorders, there was no treatment difference (40.4% versus 42.6%; P=0.21); diarrhea was less frequent with icosapent ethyl versus placebo (10.3% versus 13.0%; P=0.02), constipation was more frequent (7.8% versus 5.1%; P=0.002), dysphagia was more frequent (2.2% versus 1.1%; P=0.02), and eructation was more frequent (1.2% versus 0.4%; P=0.008). Information regarding atrial fibrillation or flutter is presented in Table III in the online-only Data Supplement. Treatment-emergent atrial fibrillation/flutter adverse events (exclusive of those positively adjudicated as end points) occurred in 6.6% of the icosapent ethyl group and 4.5% of the placebo group (P=0.01); positively adjudicated end points of atrial fibrillation/flutter requiring hospitalization for 24 or more hours occurred in 3.6% versus 2.9% of participants (P=0.35). Bleeding results are summarized in Tables IV and V in the online-only Data Supplement. Bleeding treatment-emergent adverse events of any type (exclusive of positively adjudicated hemorrhagic stroke events which were accounted for as trial end points) occurred in 16.7% of the icosapent ethyl group versus 13.6% of the placebo group (P=0.02), with no significant difference in central nervous system or gastrointestinal bleeding. Serious bleeding adverse events occurred in 3.6% of the icosapent ethyl group versus 3.3% of the placebo group (P=0.77).
Discussion
In this prespecified analysis of the 3146 patients enrolled into REDUCE-IT in the United States, for the primary composite end point there was a 31% relative risk reduction and 6.5% absolute risk reduction in first ischemic events (NNT, 15). The key secondary composite end point of cardiovascular death, nonfatal MI, or nonfatal stroke was also reduced by 31%, with a 4.6% absolute risk reduction (NNT, 22). Significant reductions in all composite and individual end points in the prespecified testing hierarchy were also observed, including cardiovascular death, MI, stroke, coronary revascularization, or hospitalization for unstable angina, as was the case with the overall trial results. In the US subgroup, there was an important, significant (P=0.004) 30% relative and 2.6% absolute risk reduction in all-cause mortality (NNT, 39), with an interaction P value of 0.02 between the US and non-US subgroups.
Whereas the non-US subgroup also showed significant reductions in the primary and key secondary composite end points, the US subgroup demonstrated particularly robust risk reductions. Formal statistical testing did not clearly show heterogeneity between the US and non-US subgroups for most end points, and therefore the overall positive trial results apply to the entire population enrolled in REDUCE-IT, regardless of geography. However, it is reassuring that the results in the United States are at least as striking as the overall REDUCE-IT findings. Given prior positive randomized trials of various drugs and strategies that reported diminished efficacy or even lack of efficacy in the US subgroup, the results of the present analysis from REDUCE-IT provide confidence that US patients would derive at least as much benefit from icosapent ethyl as seen in the overall trial.
Tolerability and safety findings in the US subgroup were consistent with the full study population. The overall tolerability of icosapent ethyl was virtually identical to that of placebo, with no significant differences in the rates of treatment-emergent adverse events or in serious treatment-emergent adverse events. In the United States, there was an increase in any bleeding with icosapent ethyl, but no significant difference in serious adverse events related to bleeding. There was an increase in the overall treatment-emergent adverse event rate of atrial fibrillation or flutter, but not in the category of serious adverse events of atrial fibrillation or flutter or the adjudicated end point of hospitalization ≥24 hours for atrial fibrillation or flutter. Therefore, the risk-benefit profile of icosapent ethyl appears highly favorable. International guidelines have embraced the REDUCE-IT data10–13 and registry analyses show the data are generalizable to patients in typical clinical practice.14 An independent analysis using trial-level data found that icosapent ethyl was highly cost-effective.15 Thus, the remaining challenge, as is often the case with randomized clinical trial data, is implementation of the REDUCE-IT results in daily clinical practice.
Limitations of this analysis include that REDUCE-IT was not specifically powered to examine individual subgroups, including this prespecified subgroup of US patients. The statistical interaction terms for heterogeneity for the comparisons of US and non-US patients were not significant for most end points, and therefore differences in efficacy outcomes for the US patients are neither qualitative nor quantitative. Nevertheless, the data are informative and provide reassurance that the results seen in the United States are at least as strong as the results seen outside the United States and in the trial overall.
As seen in the overall REDUCE-IT population and in the non-US patients, icosapent ethyl 4 g/d significantly reduced the primary and key secondary efficacy end points within the prespecified subgroup of patients randomized within the United States. The benefits in the US subgroup included significant reductions in all ischemic end points tested within the prespecified hierarchical sequence, including all-cause mortality.
Acknowledgments
Editorial assistance under the direction of the authors, and limited to collation of author comments and formatting, was provided by Joy Bronson, MA, an employee of Amarin Pharma, Inc. The first draft of the article was written by Dr Bhatt. The authors thank the following Amarin employees who contributed to the success of the trial and the current analyses: Katelyn Diffin, MBA, and Angela Granger, BA for clinical operations support; and Ramakrishna Bhavanthula, MS; Richard H. Iroudayassamy, BS; James Jin, PhD; Dmitry Klevak, MS; Gang Liu, PhD; Hardik Panchal, MS; Jimmy Shi, MS; Robert Wang, PhD; and Shin-Ru Wang, MS; for data management and statistical support. The analyses were validated by the following employees of the Baim Institute for Clinical Research with funding from Amarin: Qi Gao, MS; Jane J. Lee, PhD; and Xiaohua Chen, MS. The authors also thank the investigators, the study coordinators, and the patients who participated in the REDUCE-IT trial, including the clinical site personnel and patients in the United States across more than 200 institutions in more than 40 states.
Sources of Funding
The study was funded by Amarin Pharma, Inc., which was involved in the study design; collection, analysis, and interpretation of data; and development and review of this article. The decision to submit the article for publication was made by the authors.
Disclosures
Dr Bhatt had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. All authors contributed to the conception and design of the study, data analysis/interpretation, and revision of the article for intellectual content and provided final approval of the article. Dr Bhatt discloses the following relationships: advisory board: Cardax, Cereno Scientific, Elsevier PracticeUpdate Cardiology, Medscape Cardiology, PhaseBio, Regado Biosciences; board of directors: Boston VA Research Institute, Society of Cardiovascular Patient Care, TobeSoft; Chair: American Heart Association Quality Oversight Committee; data monitoring committees: Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute, for the PORTICO trial, funded by St. Jude Medical, now Abbott), Cleveland Clinic (including for the ExCEED trial, funded by Edwards), Duke Clinical Research Institute, Mayo Clinic, Mount Sinai School of Medicine (for the ENVISAGE trial, funded by Daiichi Sankyo), Population Health Research Institute; honoraria: American College of Cardiology (Senior Associate Editor, Clinical Trials and News, ACC.org; Vice-Chair, ACC Accreditation Committee), Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute; RE-DUAL PCI clinical trial steering committee funded by Boehringer Ingelheim; AEGIS-II executive committee funded by CSL Behring), Belvoir Publications (Editor-in-Chief, Harvard Heart Letter), Duke Clinical Research Institute (clinical trial steering committees, including for the PRONOUNCE trial, funded by Ferring Pharmaceuticals), HMP Global (Editor-in-Chief, Journal of Invasive Cardiology), Journal of the American College of Cardiology (Guest Editor; Associate Editor), Medtelligence/ReachMD (CME steering committees), Population Health Research Institute (for the COMPASS operations committee, publications committee, steering committee, and US national coleader, funded by Bayer), Slack Publications (Chief Medical Editor, Cardiology Today’s Intervention), Society of Cardiovascular Patient Care (Secretary/Treasurer), WebMD (CME steering committees); other: Clinical Cardiology (Deputy Editor), NCDR-ACTION Registry Steering Committee (Chair), VA CART Research and Publications Committee (Chair); research funding: Abbott, Afimmune, Amarin, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Chiesi, CSL Behring, Eisai, Ethicon, Ferring Pharmaceuticals, Forest Laboratories, Fractyl, Idorsia, Ironwood, Ischemix, Lilly, Medtronic, PhaseBio, Pfizer, PLX Pharma, Regeneron, Roche, Sanofi Aventis, Synaptic, The Medicines Company; royalties: Elsevier (Editor, Cardiovascular Intervention: A Companion to Braunwald’s Heart Disease); site coinvestigator: Biotronik, Boston Scientific, CSI, St. Jude Medical (now Abbott), Svelte; trustee: American College of Cardiology; unfunded research: FlowCo, Merck, Novo Nordisk, Takeda. Dr Miller reports receiving consulting fees from Amarin and Akcea. Dr Brinton reports receiving fees as a speaker from Amarin, Amgen, Kowa, Regeneron, and Sanofi-Aventis, and consulting fees from Akcea, Amarin, Amgen, Esperion, Kowa, Medicure, PTS Diagnostics, Regeneron, and Sanofi-Aventis. Dr Jacobson reports receiving consulting fees from Amgen, Esperion, Novartis, Regeneron, and Sanofi. Dr Steg reports receiving research grant funding from Amarin, Bayer, Merck, Sanofi, and Servier; and speaking or consulting fees from Amarin, Amgen, AstraZeneca, Bayer/Janssen, Boehringer-Ingelheim, Bristol-Myers Squibb, Idorsia, Lilly, Merck, Novartis, Novo-Nordisk, Pfizer, Regeneron, Sanofi, and Servier. Dr Ketchum, R.T. Doyle, Dr Juliano, Dr Jiao, and Dr Granowitz report being employed by and being stock shareholders of Amarin Pharma. Dr Tardif reports receiving grant support from AstraZeneca, Esperion, and Ionis, grant support and consulting fees from DalCor, grant support and fees for serving as co-chairman of an executive committee from Pfizer, grant support and fees for serving on an executive committee from Sanofi, and grant support and consulting fees from Servier and holding a minor equity interest in DalCor and a patent (US 9,909,178 B2) on Dalcetrapib for Therapeutic Use. Dr Olshansky reports serving as Amarin DSMB, Boehringer Ingelheim GLORIA AF US co-coordinator, Sanofi consultant, Lundbeck speaker and consultant, and Respitonics consultant. Dr Chung served on the Amarin DSMB but has no relevant financial disclosures. Dr Gibson reports research grant support and consulting fees from Amarin. Dr Giugliano reports that his institution received research grant support from Amgen, Bristol-Myers Squibb, Merck, and The Medicines Company for clinical trials in lipid therapies, and honoraria for CME programs and/or consulting from Akcea, Amarin, Agmen, Bristol-Myers Squibb, CVS Caremark, Daiichi Sankyo, GlaxoSmithKline, Merck, and Pfizer. Dr Budoff has received grant support and is on the speaker’s bureau for Amarin Pharmaceuticals. Dr Ballantyne reports receiving consulting fees from Arrowhead, AstraZeneca, Eli Lilly, Matinas BioPharma, Merck, Boehringer Ingelheim, Novo Nordisk, Denka Seiken, and Gilead and grant support (paid to his institution) and consulting fees from Amarin, Amgen, Esperion, Novartis, Regeneron, Sanofi-Synthelabo, and Akcea. No other potential conflict of interest relevant to this article was reported.
Supplementary Material
Footnotes
A complete list of the REDUCE-IT trial investigators can be found in the online-only Data Supplement.
Sources of Funding, see page 374
Guest Editor for this article was Sripal Bangalore, MD, MHA.
The online-only Data Supplement is available with this article at https://www.ahajournals.org/doi/suppl/10.1161/CIRCULATIONAHA.119.044440.
References
- 1.Ganda OP, Bhatt DL, Mason RP, Miller M, Boden WE. Unmet need for adjunctive dyslipidemia therapy in hypertriglyceridemia management. J Am Coll Cardiol. 2018;72:330–343. doi: 10.1016/j.jacc.2018.04.061. doi: 10.1016/j.jacc.2018.04.061. [DOI] [PubMed] [Google Scholar]
- 2.Ference BA, Kastelein JJP, Ray KK, Ginsberg HN, Chapman MJ, Packard CJ, Laufs U, Oliver-Williams C, Wood AM, Butterworth AS, et al. Association of triglyceride-lowering LPL variants and LDL-C-lowering LDLR variants with risk of coronary heart disease. JAMA. 2019;321:364–373. doi: 10.1001/jama.2018.20045. doi: 10.1001/jama.2018.20045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Patel PN, Patel SM, Bhatt DL. Cardiovascular risk reduction with icosapent ethyl. Curr Opin Cardiol. 2019;34:721–727. doi: 10.1097/HCO.0000000000000678. doi: 10.1097/HCO.0000000000000678. [DOI] [PubMed] [Google Scholar]
- 4.Bhatt DL, Steg PG, Miller M, Brinton EA, Jacobson TA, Ketchum SB, Doyle RT, Jr, Juliano RA, Jiao L, Granowitz C, et al. REDUCE-IT Investigators. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med. 2019;380:11–22. doi: 10.1056/NEJMoa1812792. doi: 10.1056/NEJMoa1812792. [DOI] [PubMed] [Google Scholar]
- 5.Bhatt DL, Steg PG, Miller M, Brinton EA, Jacobson TA, Ketchum SB, Doyle RT, Jr, Juliano RA, Jiao L, Granowitz C, et al. REDUCE-IT Investigators. Effects of icosapent ethyl on total ischemic events: from REDUCE-IT. J Am Coll Cardiol. 2019;73:2791–2802. doi: 10.1016/j.jacc.2019.02.032. doi: 10.1016/j.jacc.2019.02.032. [DOI] [PubMed] [Google Scholar]
- 6.Bhatt DL, Steg PG, Miller M, Brinton EA, Jacobson TA, Jiao L, Tardif JC, Gregson J, Pocock SJ, Ballantyne CM REDUCE-IT Investigators. Reduction in first and total ischemic events with icosapent ethyl across baseline triglyceride tertiles. J Am Coll Cardiol. 2019;74:1159–1161. doi: 10.1016/j.jacc.2019.06.043. doi: 10.1016/j.jacc.2019.06.043. [DOI] [PubMed] [Google Scholar]
- 7.Bhatt DL, Steg PG, Miller M, Juliano RA, Ballantyne CM. Reply: Ischemic event reduction and triglycerides. J Am Coll Cardiol. 2019;74:1849–1850. doi: 10.1016/j.jacc.2019.08.007. doi: 10.1016/j.jacc.2019.08.007. [DOI] [PubMed] [Google Scholar]
- 8.Bhatt DL, Steg PG, Miller M. Cardiovascular risk reduction with icosapent ethyl: reply. N Engl J Med. 2019;380:1678. doi: 10.1056/NEJMc1902165. doi: 10.1056/NEJMc1902165. [DOI] [PubMed] [Google Scholar]
- 9.Granger CB, Nelson AJ, Pagidipati NJ. Risk of total events with icosapent ethyl: can we reduce it? J Am Coll Cardiol. 2019;73:2803–2805. doi: 10.1016/j.jacc.2019.03.492. doi: 10.1016/j.jacc.2019.03.492. [DOI] [PubMed] [Google Scholar]
- 10.American Diabetes Association. Cardiovascular disease and risk management: standards of medical care in diabetes. Diabetes Care. 2019;42:S103–S123. doi: 10.2337/dc19-S010. doi: 10.2337/dc19-S010. [DOI] [PubMed] [Google Scholar]
- 11.Skulas-Ray AC, Wilson PWF, Harris WS, Brinton EA, Kris-Etherton PM, Richter CK, Jacobson TA, Engler MB, Miller M, Robinson JG, et al. American Heart Association Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Lifestyle and Cardiometabolic Health; Council on Cardiovascular Disease in the Young; Council on Cardiovascular and Stroke Nursing; and Council on Clinical Cardiology. Omega-3 fatty acids for the management of hypertriglyceridemia: a science advisory from the American Heart Association. Circulation. 2019;140:e673–e691. doi: 10.1161/CIR.0000000000000709. doi: 10.1161/CIR.0000000000000709. [DOI] [PubMed] [Google Scholar]
- 12.Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, Chapman MJ, De Backer GG, Delgado V, Ference BA, et al. on behalf of the ESC Scientific Document Group. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk: the task force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS). Eur Heart J. 2019:ehz455. doi: 10.1093/eurheartj/ehz455. [Google Scholar]
- 13.Orringer CE, Jacobson TA, Maki KC. National Lipid Association scientific statement on the use of icosapent ethyl in statin-treated patients with elevated triglycerides and high- or very-high ASCVD risk. J Clin Lipidol. 2019 doi: 10.1016/j.jacl.2019.10.014. In press. Posted November 2, 2019. Accessed November 7, 2019. doi: 10.1016/j.jacl.2019.10.014. [DOI] [PubMed] [Google Scholar]
- 14.Picard F, Bhatt DL, Ducrocq G, Elbez Y, Ferrari R, Ford I, Tardif JC, Tendera M, Fox KM, Steg PG. Generalizability of the REDUCE-IT trial in patients with stable coronary artery disease. J Am Coll Cardiol. 2019;73:1362–1364. doi: 10.1016/j.jacc.2019.01.016. doi: 10.1016/j.jacc.2019.01.016. [DOI] [PubMed] [Google Scholar]
- 15.Institute for Clinical and Economic Review. Additive therapies for cardiovascular disease: effectiveness and value: final evidence report. https://icer-review.org/wp-content/uploads/2019/02/ICER_CVD_Final_Evidence_Report_101719.pdf.Published October 17, 2019. Accessed October 24, 2019.
- 16.Fan W, Philip S, Granowitz C, Toth PP, Wong ND. Hypertriglyceridemia in statin-treated US adults: the National Health and Nutrition Examination Survey. J Clin Lipidol. 2019;13:100–108. doi: 10.1016/j.jacl.2018.11.008. doi: 10.1016/j.jacl.2018.11.008. [DOI] [PubMed] [Google Scholar]
- 17.Fan W, Philip S, Toth PP, Granowitz C, Wong ND. Prevalence of United States adults with triglycerides ≥ 135 mg/dL: NHANES 2007–2014. Cardiol J. 2019;26 doi: 10.5603/CJ.2019.0099. doi: 10.5603/CJ.2019.0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bhatt DL. REDUCE-IT: Residual cardiovascular risk in statin-treated patients with elevated triglycerides: now we can REDUCE-IT! Eur Heart J. 2019;40:1174–1175. doi: 10.1093/eurheartj/ehz179. [Google Scholar]
- 19.Wallentin L, Becker RC, Budaj A, Cannon CP, Emanuelsson H, Held C, Horrow J, Husted S, James S, Katus H, et al. PLATO Investigators. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361:1045–1057. doi: 10.1056/NEJMoa0904327. doi: 10.1056/NEJMoa0904327. [DOI] [PubMed] [Google Scholar]
- 20.Mahaffey KW, Wojdyla DM, Carroll K, Becker RC, Storey RF, Angiolillo DJ, Held C, Cannon CP, James S, Pieper KS, et al. PLATO Investigators. Ticagrelor compared with clopidogrel by geographic region in the Platelet Inhibition and Patient Outcomes (PLATO) trial. Circulation. 2011;124:544–554. doi: 10.1161/CIRCULATIONAHA.111.047498. doi: 10.1161/CIRCULATIONAHA.111.047498. [DOI] [PubMed] [Google Scholar]
- 21.Vaduganathan M, Harrington RA, Stone GW, Steg PG, Gibson CM, Hamm CW, Price MJ, Prats J, Deliargyris EN, Mahaffey KW, et al. Variation in patient profiles and outcomes in US and non-US subgroups of the Cangrelor Versus Standard Therapy to Achieve Optimal Management of Platelet Inhibition (CHAMPION) PHOENIX Trial. Circ Cardiovasc Interv. 2016;9:e003612. doi: 10.1161/CIRCINTERVENTIONS.116.003612. doi: 10.1161/CIRCINTERVENTIONS.116.003612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O’Shea JC, Califf RM. Inter-regional differences in acute coronary syndrome trials. Eur Heart J. 2000;21:1397–1399. doi: 10.1053/euhj.2000.2121. doi: 10.1053/euhj.2000.2121. [DOI] [PubMed] [Google Scholar]
- 23.Bhatt DL, Cavender MA. Are all clinical trial sites created equal? J Am Coll Cardiol. 2013;61:580–581. doi: 10.1016/j.jacc.2012.10.024. doi: 10.1016/j.jacc.2012.10.024. [DOI] [PubMed] [Google Scholar]
- 24.Ruff CT, Giugliano RP, Antman EM, Murphy SA, Lotan C, Heuer H, Merkely B, Baracioli L, Schersten F, Seabro-Gomes R, et al. TRITON-TIMI 38 Investigators. Safety and efficacy of prasugrel compared with clopidogrel in different regions of the world. Int J Cardiol. 2012;155:424–429. doi: 10.1016/j.ijcard.2010.10.040. doi: 10.1016/j.ijcard.2010.10.040. [DOI] [PubMed] [Google Scholar]
- 25.Bernardez-Pereira S, Lopes RD, Carrion MJ, Santucci EV, Soares RM, de Oliveira Abreu M, Laranjeira LN, Ikeoka DT, Zazula AD, Moreira FR, et al. Methodological Evaluation of clinical TriAls Study Group. Prevalence, characteristics, and predictors of early termination of cardiovascular clinical trials due to low recruitment: insights from the ClinicalTrials.gov registry. Am Heart J. 2014;168:213–219.e1. doi: 10.1016/j.ahj.2014.04.013. doi: 10.1016/j.ahj.2014.04.013. [DOI] [PubMed] [Google Scholar]
- 26.Moses H, 3rd, Matheson DH, Cairns-Smith S, George BP, Palisch C, Dorsey ER. The anatomy of medical research: US and international comparisons. JAMA. 2015;313:174–189. doi: 10.1001/jama.2014.15939. doi: 10.1001/jama.2014.15939. [DOI] [PubMed] [Google Scholar]
- 27.Butler J, Subacius H, Vaduganathan M, Fonarow GC, Ambrosy AP, Konstam MA, Maggioni A, Mentz RJ, Swedberg K, Zannad F, et al. EVEREST Investigators. Relationship between clinical trial site enrollment with participant characteristics, protocol completion, and outcomes: insights from the EVEREST (Efficacy of Vasopressin Antagonism in Heart Failure: Outcome Study with Tolvaptan) trial. J Am Coll Cardiol. 2013;61:571–579. doi: 10.1016/j.jacc.2012.10.025. doi: 10.1016/j.jacc.2012.10.025. [DOI] [PubMed] [Google Scholar]
- 28.Tobbia P, Brodie BR, Stuckey T, McLaurin BT, Cox DA, Fahy M, Xu K, Mehran R, Stone GW. Are adverse events following an invasive strategy in patients with non-ST-segment elevation acute coronary syndromes more frequent at US sites versus non-US sites? Analysis from the ACUITY trial. Catheter Cardiovasc Interv. 2013;82:E365–E374. doi: 10.1002/ccd.24587. doi: 10.1002/ccd.24587. [DOI] [PubMed] [Google Scholar]
- 29.Tobbia P, Brodie BR, Witzenbichler B, Metzger C, Guagliumi G, Yu J, Kellett MA, Stuckey T, Fahy M, Mehran R, et al. Adverse event rates following primary PCI for STEMI at US and non-US hospitals: three-year analysis from the HORIZONS-AMI trial. EuroIntervention. 2013;8:1134–1142. doi: 10.4244/EIJV8I10A176. doi: 10.4244/EIJV8I10A176. [DOI] [PubMed] [Google Scholar]
- 30.Pocock S, Calvo G, Marrugat J, Prasad K, Tavazzi L, Wallentin L, Zannad F, Alonso Garcia A. International differences in treatment effect: do they really exist and why? Eur Heart J. 2013;34:1846–1852. doi: 10.1093/eurheartj/eht071. doi: 10.1093/eurheartj/eht071. [DOI] [PubMed] [Google Scholar]
- 31.Metra M, Mentz RJ, Hernandez AF, Heizer GM, Armstrong PW, Clausell N, Corbalan R, Costanzo MR, Dickstein K, Dunlap ME, et al. Geographic differences in patients in a global acute heart failure clinical trial (from the ASCEND-HF Trial). Am J Cardiol. 2016;117:1771–1778. doi: 10.1016/j.amjcard.2016.03.002. doi: 10.1016/j.amjcard.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 32.Pfeffer MA, Claggett B, Assmann SF, Boineau R, Anand IS, Clausell N, Desai AS, Diaz R, Fleg JL, Gordeev I, et al. Regional variation in patients and outcomes in the Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist (TOPCAT) trial. Circulation. 2015;131:34–42. doi: 10.1161/CIRCULATIONAHA.114.013255. doi: 10.1161/CIRCULATIONAHA.114.013255. [DOI] [PubMed] [Google Scholar]
- 33.Bhatt DL, Steg PG, Brinton EA, Jacobson TA, Miller M, Tardif JC, Ketchum SB, Doyle RT, Jr, Murphy SA, Soni PN, et al. REDUCE-IT Investigators. Rationale and design of REDUCE-IT: Reduction of Cardiovascular Events with Icosapent Ethyl–Intervention Trial. Clin Cardiol. 2017;40:138–148. doi: 10.1002/clc.22692. doi: 10.1002/clc.22692. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


