Abstract
Background:
Because Candida spp is a major cause of mortality and morbidity in preterm infants, fluconazole prophylaxis has been suggested by some experts and hospital policy. In our hospital, fluconazole prophylaxis was used in eligible preterm infants and set as the neonatal intensive care unit (NICU) practice in 2014.
Purpose:
This study focused on fungal bloodstream infections and aimed to evaluate the benefit and harm of fluconazole prophylaxis.
Methods/Search Strategy:
This retrospective, descriptive study involved medical record reviews in our hospital from April 2005 to October 2016. NICU patients were included if Candida species, yeast-like organisms, or Malassezia species were cultured from their venous catheter tips or blood cultures.
Findings/Results:
After fluconazole prophylaxis, cases of Candida spp decreased and those of Malassezia furfur emerged. We reviewed 19 cases of catheter-related M furfur colonization and 1 case of M furfur fungemia. The gestational age was 27.3 ± 2.0 weeks and birth weight was 959.2 ± 229.8 g. Hyperalimentation with lipid infusion was used in all cases. All of the neonates survived with antifungal agent use.
Implications for Practice:
This study highlights that prophylactic fluconazole may be an associated factor of Malassezia colonization; M furfur remains a potential concern for fungemia in the care of premature infants and thus requires our attention.
Implications for Research:
Future studies should further investigate the incidence and impact of noncandidal fungal infections with fluconazole prophylaxis use in premature infants.
Keywords: candidemia, fluconazole prophylaxis, Malassezia furfur, neonatal, prematurity
BACKGROUND AND SIGNIFICANCE
Preterm infants are more vulnerable to fungal infections than full-term infants because of their immature immune systems and poorly developed epithelial skin and mucosal barriers, as well as the high rate of invasive procedures such as central venous catheter insertion and intubation. Candida spp has emerged as a common cause of infections in preterm infants admitted to the neonatal intensive care unit (NICU). In very low birth-weight (VLBW) infants (below 1500 g), Candida albicans is the third most common cause of neonatal late-onset sepsis, which occurs after the first 72 hours of life.1 Invasive candidal infections are associated with a significant risk of death, neurodevelopmental impairment, and increased healthcare costs. Because Candida spp is a major cause of mortality and morbidity in preterm infants, prophylaxis with antifungal agents in premature infants or those with extremely or very low birth-weight (ELBW or VLBW) has been suggested by some experts and hospital policy. The Infectious Diseases Society of America guidelines recommended the use of intravenous or oral fluconazole prophylaxis 3 to 6 mg/kg twice weekly for 6 weeks in neonates with birth weights less than 1000 g in nurseries with high rates (>10%) of invasive candidiasis.2 The overall incidence of candidiasis in the NICU appears to decrease with the increased use of fluconazole prophylaxis in ELBW infants.3,4 Our institution has used fluconazole prophylaxis for premature infants since 2014.
Although fungal infections other than those caused by Candida species are uncommon, the incidence of noncandidal fungal infections, including Malassezia spp, appears to be increasing in preterm infants. Malassezia furfur is one species that mainly colonizes the skin and occasionally the respiratory tract and may cause neonatal infections.5,6 It requires exogenous long-chain fatty acids for growth, which may explain the associated risk of this infection in premature infants who receive intralipid emulsions.7Malassezia spp may cause fungemia, localized infections such as pneumonia, or severe systemic infections, especially in immunocompromised and premature infant hosts.8–12 Therefore, they may result in significant mortality and morbidity in preterm infants. In 1981, the first case of a neonatal Malassezia spp infection was reported in a preterm infant receiving intralipid emulsion therapy.13 Ilahi et al14 described a Malassezia spp outbreak in neonates that stopped after infection control measures, including the prohibition of a lipid-rich moisturizing hand cream used by the healthcare staff, were implemented.
Since 2014, several cases of catheter-related M furfur colonization have emerged in the NICU of Kaohsiung Chang Gung Memorial Hospital (KCGMH). During the same period, Malassezia spp did not emerge in other intensive care units of our hospital. The cluster has been consecutively investigated by the Department of Infection Control. In addition to hand hygiene measures, further surveillance involving environment, equipment, and medical staff was conducted. The cases were considered endogenous infections or colonization. According to literature review, risk factors related to M furfur colonization or infection have been well described, including prematurity, receipt of intralipid emulsions, and administration of broad-spectrum antibiotics. However, the risk factors remained the same as before but catheter-related M furfur colonization has emerged since 2014. As a result, we assumed that other factors related to clinical practice were associated with this kind of fungal outbreak in our NICU. This outbreak is worth paying attention because M furfur may cause invasive fungal infection as previously reported.8–10
Here we report our first clinical investigation of M furfur catheter-related colonization or infection and candidemia trends in our NICU over the past decade. This study focused on fungal bloodstream infections and aimed to evaluate the benefit and harm of fluconazole prophylaxis.
What This Study Adds
Prophylaxis with antifungal agents in premature infants effectively diminishes the invasive candidal infection, which is one of the major causes of mortality and morbidity in preterm infants.
Malassezia furfur remains a potential concern for fungemia in the care for premature infants and thus requires our attention.
METHODS
We collected data from the patient database of the NICU of KCGMH including those with bloodstream fungal infections or colonization via peripheral central venous catheter (pCVC) tip and blood cultures. The institutional review board (IRB) of the Chang Gung Medical Foundation approved this retrospective, descriptive study of all medical information with IRB approval. All medical records were anonymized and de-identified prior to the analysis. KCGMH is a medical center in southern Taiwan with a 30-bed NICU. The average number of NICU inpatients was 781 per year, which was the highest census of NICUs in southern Taiwan from 2005 to 2016. Prophylactic fluconazole (6 mg/kg per dose every 72 hours) has been routinely administered to the neonates in our NICU with a birth weight less than 1000 g or 1000-1250 g with pCVC since 2014 to prevent fungal infections. The clinical practice of our NICU requires that each pCVC removed from patients be subjected to tip culture, especially in cases of difficult removal, discharge, or erythematous changes at the insertion site. The blood sample is cultured in lipid-enriched fungal medium, and the BacT/Alert system is implemented by adding lipid substrates to increase the method sensitivity.
We conducted this retrospective, descriptive study of medical records from April 2005 to October 2016. During these years, patients were included if Candida species, yeast-like organisms, or Malassezia species were cultured from the pCVC tip or a blood culture. All cases were recorded with their clinical manifestations and demographic characteristics, including gestational age, birth weight, lipid infusion duration, prophylactic antifungal agent receipt, broad-spectrum antibiotic use, and hospitalization outcomes.
RESULTS
Eight cases of candidemia were reported between 2005 and 2013. Blood cultures revealed C albicans in 7 of the 8 cases and Candida parapsilosis in 1 case. The characteristics of the 8 cases of candidemia are shown in Table 1. The gestational age of the 8 cases was 28.3 ± 6.3 weeks (mean ± standard deviation) and birth weight was 1570.6 ± 1236.4 g. All had pCVC with broad-spectrum antibiotic treatment and lipid infusion supplementation. No neonates received prophylactic fluconazole. All had invasive candidal infections with sepsis; 2 infants died.
TABLE 1. Characteristics of 8 Cases of Candidemia.
| Sex | GA, wk | BBW, g | Duration of SMOF,a d | Broad-Spectrum Antibiotics | Prophylactic Fluconazoleb | Candida spp of the Blood Culture | Mortality | |
|---|---|---|---|---|---|---|---|---|
| 1 | F | 29 + 1 | 1260 | 12 | Yes | Nil | C albicans | Survival |
| 2 | M | 40 + 2 | 4000 | 8 | Yes | Nil | C albicans | Survival |
| 3 | F | 24 + 1 | 725 | 4 | Yes | Nil | C albicans | Expired |
| 4 | M | 23 + 3 | 670 | 23 | Yes | Nil | C albicans | Survival |
| 5 | M | 27 + 2 | 1150 | 32 | Yes | Nil | C parapsilosis | Survival |
| 6 | F | 37 | 3000 | 15 | Yes | Nil | C albicans | Survival |
| 7 | M | 25 + 3 | 910 | 4 | Yes | Nil | C albicans | Survival |
| 8 | F | 25 + 4 | 850 | 49 | Yes | Nil | C albicans | Expired |
Abbreviations: BBW, birth body weight; F, female; GA, gestational age; M, male; SMOF, soybean oil, medium-chain triglycerides, olive oil, and fish oil.
aLipid infusion routinely used at our neonatal intensive care unit.
bProphylactic fluconazole: 6 mg/kg every 72 hours.
We also obtained data including 19 cases of catheter-related M furfur colonization and 1 case of M furfur fungemia between 2014 and 2016. The characteristics of the 20 cases are described in Table 2. In the 19 cases of M furfur colonization, the male to female ratio was 9:10. The gestational age was 27.3 ± 2.0 weeks and birth weight was 959.2 ± 229.8 g. Twelve neonates were ELBW (<1000 g), whereas the other 7 were VLBW (1000-1500 g). Prophylactic fluconazole was administered to all but one patient, whose birth weight was 1140 g and who did not undergo central catheter placement immediately after birth. Lipid infusion was used in all neonates for a duration of 9 to 102 days. All underwent phototherapy for neonatal jaundice.
TABLE 2. Characteristics of 20 Cases of Catheter-Related Malassezia furfur Colonization or Fungemia.
| Sex | GA, wk | BBW, g | Duration of SMOF,a d | Broad-Spectrum Antibiotics | Prophylactic Fluconazoleb | Culture of M furfur | Outcome | Mortality | |
|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 27 | 880 | 18 | Yes | Yes | pCVC tip | Unaffected | Survival |
| 2 | M | 28 + 3 | 1080 | 38 | Yes | Yes | pCVC tip | Unaffected | Survival |
| 3 | M | 28 + 5 | 1140 | 9 | Yes | Nil | pCVC tip | Unaffected | Survival |
| 4 | M | 27 + 6 | 920 | 23 | Yes | Yes | pCVC tip | Unaffected | Survival |
| 5 | F | 27 + 5 | 710 | 30 | Yes | Yes | pCVC tip | Unaffected | Survival |
| 6 | F | 26 + 3 | 780 | 44 | Yes | Yes | pCVC tip | Unaffected | Survival |
| 7 | M | 28 | 770 | 48 | Yes | Yes | pCVC tip | Unaffected | Survival |
| 8 | M | 31 | 1420 | 28 | Yes | Yes | pCVC tip | Unaffected | Survival |
| 9 | F | 27 + 6 | 910 | 33 | Yes | Yes | pCVC tip | Unaffected | Survival |
| 10 | M | 25 + 3 | 760 | 32 | Yes | Yes | pCVC tip | Unaffected | Survival |
| 11 | M | 26 + 4 | 840 | 49 | Yes | Yes | pCVC tip | Unaffected | Survival |
| 12 | F | 29 + 1 | 1215 | 64 | Yes | Yes | pCVC tip | Unaffected | Survival |
| 13 | F | 26 + 3 | 990 | 27 | Yes | Yes | pCVC tip | Unaffected | Survival |
| 14 | F | 27 | 680 | 102 | Yes | Yes | pCVC tip | Unaffected | Survival |
| 15 | M | 24 + 5 | 660 | 62 | Yes | Yes | pCVC tip | Unaffected | Survival |
| 16 | M | 28 + 6 | 1170 | 48 | Yes | Yes | Blood | Affectedc | Survival |
| 17 | M | 29 + 2 | 1090 | 52 | Yes | Yes | pCVC tip | Unaffected | Survival |
| 18 | F | 29 + 3 | 1320 | 62 | Yes | Yes | pCVC tip | Unaffected | Survival |
| 19 | F | 31 + 1 | 1260 | 25 | Yes | Yes | pCVC tip | Unaffected | Survival |
| 20 | M | 24 + 3 | 800 | 47 | Yes | Yes | pCVC tip | Unaffected | Survival |
Abbreviations: BBW, birth body weight; F, GA, gestational age; female; M, male; pCVC, percutaneous central venous catheter; SMOF, soybean oil, medium-chain triglycerides, olive oil, and fish oil.
aLipid infusion routinely used at our neonatal intensive care unit.
bProphylactic fluconazole: 6 mg/kg every 72 hours.
cLonger stay with longer total parenteral nutrition support due to feeding intolerance during the period of invasive infection and subsequently with higher healthcare costs.
In all 20 cases, the only case of M furfur fungemia was treated with amphotericin B (1 mg/kg/d). For the other 19 neonates with positive tip cultures and negative blood culture, the initial management included pCVC removal. Eight of the 19 neonates remained on fluconazole at a prophylactic dose. In 7 of the 19 neonates, the prophylactic fluconazole dose was changed to a treatment dose (12 mg/kg/d). Four of the 19 neonates were administered amphotericin B (1 mg/kg/d).
Regarding the outcomes of the 19 cases with positive tip culture results, we reviewed the results of the blood cultures, manifestations of M furfur infection, and mortality rates. There was no fungemia or clinical manifestations of M furfur infection, including localized infection and fungemia-related symptoms observed in the 19 cases. The only case of M furfur fungemia presented some nonspecific symptoms including apnea, bradycardia, and thrombocytopenia. This case required a longer duration of total parenteral nutrition support due to feeding intolerance during the period of the invasive infection and subsequently had higher healthcare costs. Ultimately, all 20 neonates survived and were discharged.
In the investigation of the catheter-related M furfur colonization or infection in our NICU, some possibly related factors were also reviewed, including soybean oil, medium-chain triglycerides, olive oil, and fish oil (SMOF), which is used as the routine lipid infusion in our NICU, the evolution of the phototherapy, and the clinical practice of the prophylactic fluconazole (Figure 1). First, we found that intrafat, which contains soybean oil and medium-chain triglycerides, was used for the lipid infusions before May 2011 in our NICU. Since 2011-2012, the intrafat was replaced by a combination of oils (SMOF) that are good for brain and retina development in prematurity. However, SMOF also contains olive oil, which is an important growth factor for M furfur.15 Furthermore, SMOF is used as a lipid emulsion only in the NICU of our hospital. In contrast, a lipid emulsion without olive oil has been used in our adult ICU, in which no cases of M furfur colonization or infection have been observed. Figure 1 shows that M furfur colonization or infection emerged after SMOF was first used as routine lipid infusion at our NICU. Second, we found that the phototherapy light sources in our NICU had changed in recent years. Since 2014, light-emitting diodes (LEDs) have replaced fluorescent tubes for phototherapy since the narrower wavelength provides better treatment of neonatal jaundice. On the contrary, compared with the fluorescent tubes, the power consumption and heating caused by super LEDs are very low.16,17 Phototherapy using LEDs might reduce insensible water loss in neonates and preserve the environmental humidity. Malassezia furfur colonization or infection has emerged since LEDs were widely used (Figure 1). Third, both ELBW infants (birth weight <1000 g) and VLBW infants (1000-1250 g) who underwent central venous catheter placement were administered prophylactic fluconazole after 2014 in our NICU. Figure 1 shows that there have been no cases of candidemia in our NICU since 2014 and that the number of candidemia infections has reduced. In contrast, M furfur, which had never been previously identified in culture, has emerged since then.
FIGURE 1.
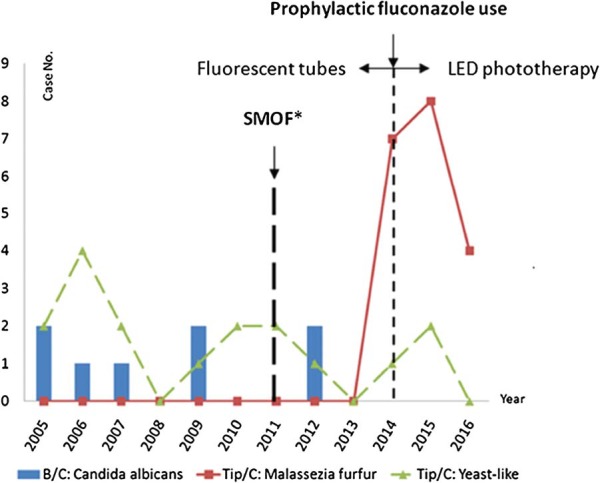
Investigation of catheter-related Malassezia furfur colonization in our neonatal intensive care unit. Our findings revealed that, since 2014, the incidence of candidal fungemia has decreased and that of M furfur colonization has increased. SMOF, soybean oil, medium-chain triglycerides, olive oil, and fish oil; LED, light-emitting diode; NICU, neonatal intensive care unit.
DISCUSSION
Candida species are important hospital-acquired pathogens in NICU patients. Because of the poor clinical outcomes of neonates with candidemia, we modified the recommendations of the Infectious Diseases Society of America and set our own guidelines according to the clinical conditions, although the rate of invasive candidiasis in our NICU did not exceed 10%. On the basis of our study and literature review, we concluded that the use of prophylactic fluconazole reduced the incidence of invasive candidal infection in ELBW infants who are at high risk of systemic fungal infection. However, concerns regarding the short- and long-term safety of antifungal prophylaxis remain unresolved.18 Data regarding the long-term outcomes of prophylactic fluconazole use are limited because of short follow-up periods and small case numbers.
Our study revealed that the use of prophylactic fluconazole for preventing invasive candidal infection in preterm infants may enhance selective pressure for other fungal growth, including Malassezia spp. Al-Sweih et al19 observed that the use of prophylactic fluconazole resulted in decreased fungal infection rates but increased rates of resistant Malassezia pachydermatis. Chen et al20 also described that there was a decrease in the total fungal infection rate after fluconazole prophylaxis for VLBW neonates but the infections of fluconazole-resistant M pachydermatis increased. Studies of antifungal susceptibility of Malassezia species found that high minimum inhibitory concentrations (MICs) for azole drugs tested were for M furfur. High MICs (≥8 μg/mL) and wide MIC ranges (≤0.125 to >64 μg/mL) were obtained with fluconazole, whereas ketoconazole, itraconazole, and voriconazole were the most active drugs against Malassezia species.21 Thus, the routine use of prophylactic fluconazole combined with the potential risk factors of prematurity might lead to resistant strains related to M furfur colonization.
Malassezia spp are lipophilic yeasts; M furfur is one of 14 species. They are associated with a wide spectrum of clinical manifestations ranging from benign skin conditions to invasive fungemia in immunocompromised hosts. All of our 20 neonate cases shared the same risk factors of M furfur colonization and infection, including prematurity, increased NICU length of stay, receipt of intralipid emulsions, central venous catheters, and administration of broad-spectrum antibiotics.7,22–27 According to these features of the Malassezia spp, we assumed that our clinical factors, SMOF rich in olive oil and LED phototherapy to preserve humidity, might be correlated with the phenomenon of M furfur emergence in our NICU (Figure 1). Limited studies describe the relationship between phototherapy and the incidence of M furfur. Further studies with longer study periods and more cases are needed.
Regarding the management of cases of M furfur colonization and infection, catheter-related Malassezia infections are usually treated with catheter removal, discontinuation of the lipid infusion, and the administration of antifungal therapy.28 However, there are reports of successful eradication of infection with antifungal therapy without catheter removal and with catheter removal without systemic antifungal treatment.29,30 Based on our study and the literature review,29 whether antifungal agents administered or not, catheter removal and discontinuation of intravenous lipids generally produce a favorable outcome in neonates with catheter-related M furfur colonization. For fungemia with M furfur, amphotericin B treatment is recommended because of the potential risk of mortality and morbidity.
The limitations of this study include its small case number and a short investigation period, which prevented further analysis of the factors contributing to the phenomenon of M furfur colonization, including antibiotic resistance. Moreover, selection and information bias might have been introduced by the study's retrospective nature.
In conclusion, here we observed that candidemia subsided after the routine use of prophylactic fluconazole; however, M furfur colonization gradually emerged. In addition to the risk of prematurity, SMOF supplementation, and broad-spectrum antibiotic use, prophylactic fluconazole may be correlated with Malassezia colonization. Future clinical practice studies should further investigate the incidence of fluconazole-resistant strains. Regardless of the use of antifungal treatment, the outcomes of catheter-related M furfur colonization were favorable after catheter removal. However, M furfur remains a potential concern for fungemia, which might cause mortality and morbidities in premature infants and thus requires our attention.
Summary of Recommendations for Practice and Research.
| What we know: |
|
| What needs to be studied: |
|
| What can we do today: |
|
Footnotes
The authors declare no conflicts of interest.
References
- 1.Stoll BJ, Hansen N, Fanaroff AA, et al. Late-onset sepsis in very low birth weight neonates: the experience of the NICHD Neonatal Research Network. Pediatrics. 2002;110:285–291. [DOI] [PubMed] [Google Scholar]
- 2.Pappas PG, Kauffman CA, Andes DR, et al. Clinical practice guideline for the management of candidiasis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis. 2016;62:e1–e50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chitnis AS, Magill SS, Edwards JR, Chiller TM, Fridkin SK, Lessa FC. Trends in Candida central line-associated bloodstream infections among NICUs, 1999-2009. Pediatrics. 2012;130:e46. [DOI] [PubMed] [Google Scholar]
- 4.Aliaga S, Clark RH, Laughon M, et al. Changes in the incidence of candidiasis in neonatal intensive care units. Pediatrics. 2014;133:236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ashbee HR, Leck AK, Puntis JW, Parsons WJ, Evans EG. Skin colonization by Malassezia in neonates and infants. Infect Control Hosp Epidemiol. 2002;23:212–216. [DOI] [PubMed] [Google Scholar]
- 6.Aschner JL, Punsalang A, Jr, Maniscalco WM, Menegus MA. Percutaneous central venous catheter colonization with Malassezia furfur: incidence and clinical significance. Pediatrics. 1987;80:535–539. [PubMed] [Google Scholar]
- 7.Gupta P, Chakrabarti A, Singhi S, Kumar P, Honnavar P, Rudramurthy SM. Skin colonization by Malassezia spp. in hospitalized neonates and infants in a tertiary care centre in North India. Mycopathologia. 2014;178:267–272. [DOI] [PubMed] [Google Scholar]
- 8.Marcon MJ, Powell DA. Epidemiology, diagnosis, and management of Malassezia furfur systemic infection. Diagn Microbiol Infect Dis. 1987;7:161–175. [DOI] [PubMed] [Google Scholar]
- 9.Gonzalez-Cuevas A, Alayeto J, Juncosa T, García-Fructuoso MT, Moreno J, Latorre C. Neonatal sepsis by Malassezia furfur. Rev Iberoam Micol. 1999;16:158–160. [PubMed] [Google Scholar]
- 10.Shek YH, Tucker MC, Viciana AL, Manz HJ, Connor DH. Malassezia furfur–disseminated infection in premature infants. Am J Clin Pathol. 1989;92:595–603. [DOI] [PubMed] [Google Scholar]
- 11.Mickelsen PA, Viano-Paulson MC, Stevens DA, Diaz PS. Clinical and microbiological features of infection with Malassezia pachydermatis in high-risk infants. J Infect Dis. 1988;157:1163–1168. [DOI] [PubMed] [Google Scholar]
- 12.Larocco M, Dorenbaum A, Robinson A, Pickering LK. Recovery of Malassezia pachydermatis from eight infants in a neonatal intensive care nursery: clinical and laboratory features. Pediatr Infect Dis J. 1988;7:398–401. [DOI] [PubMed] [Google Scholar]
- 13.Redline RW, Dahms BB. Malassezia pulmonary vasculitis in an infant on long-term intralipid therapy. N Engl J Med. 1981;305:1395–1398. [DOI] [PubMed] [Google Scholar]
- 14.Ilahi A, Hadrich I, Goudjil S, et al. Molecular epidemiology of a Malassezia pachydermatis neonatal unit outbreak. Med Mycol. 2018;56:69–77. [DOI] [PubMed] [Google Scholar]
- 15.Shparago NI, Bruno PP, Bennett J. Systemic Malassezia furfur infection in an adult receiving total parenteral nutrition. J Am Osteopath Assoc. 1995;95:375–377. [PubMed] [Google Scholar]
- 16.Sherbiny HS, Youssef DM, Sherbini AS, El-Behedy R, Sherief LM. High-intensity light-emitting diode vs fluorescent tubes for intensive phototherapy in neonates. Paediatr Int Child Health. 2016;6:1–7. [DOI] [PubMed] [Google Scholar]
- 17.Mohammadizadeh M, Eliadarani FK, Badiei Z. Is the light-emitting diode a better light source than fluorescent tube for phototherapy of neonatal jaundice in preterm infants? Adv Biomed Res. 2012;1:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patel RM. Does fluconazole prophylaxis reduce death or invasive Candida infection in extremely preterm infants? Acta Paediatr. 2017;106:844–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Al-Sweih N, Ahmad S, Joseph L, Khan S, Khan Z. Malassezia pachydermatis fungemia in a preterm neonate resistant to fluconazole and flucytosine. Med Mycol Case Rep. 2014;5:9–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen IL, Chiu NC, Chi H, et al. Changing of bloodstream infections in a medical center neonatal intensive care unit. J Microbiol Immunol Infect. 2017;50:514–520. [DOI] [PubMed] [Google Scholar]
- 21.Rojas FD, Sosa Mde L, Fernández MS, Cattana ME, Córdoba SB, Giusiano GE. Antifungal susceptibility of Malassezia furfur, Malassezia sympodialis, and Malassezia globosa to azole drugs and amphotericin B evaluated using a broth microdilution method. Med Mycol. 2014;52:641–646. [DOI] [PubMed] [Google Scholar]
- 22.Alexander P, Alladi A, Correa M, D'Cruz AJ. Neonatal colonic mucormycosis—a tropical perspective. J Trop Pediatr. 2005;51:54–59. [DOI] [PubMed] [Google Scholar]
- 23.Gupta AK, Kohli Y, Li A, Faergemann J, Summerbell RC. In vitro susceptibility of the seven Malassezia species to ketoconazole, voriconazole, itraconazole and terbinafine. Br J Dermatol. 2000;142:758–765. [DOI] [PubMed] [Google Scholar]
- 24.Papavassilis C, Mach KK, Mayser PA. Medium-chain triglycerides inhibit growth of Malassezia: implications for prevention of systemic infection. Crit Care Med. 1999;27:1781–1786. [DOI] [PubMed] [Google Scholar]
- 25.Chryssanthou E, Broberger U, Petrini B. Malassezia pachydermatis fungaemia in a neonatal intensive care unit. Acta Paediatr. 2001;90:323–327. [PubMed] [Google Scholar]
- 26.Ahtonen P, Lehtonen OP, Kero P, Tunnela E, Havu V. Malassezia furfur colonization of neonates in an intensive care unit. Mycoses. 1990;33:543–547. [DOI] [PubMed] [Google Scholar]
- 27.Richet HM, McNeil MM, Edwards MC, Jarvis WR. Cluster of Malassezia furfur pulmonary infections in infants in a neonatal intensive-care unit. J Clin Microbiol. 1989;27:1197–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Surmont I, Gavilanes A, Vandepitte J, Devlieger H, Eggermont E. Malassezia furfur fungaemia in infants receiving intravenous lipid emulsions. A rarity or just underestimated? Eur J Pediatr. 1989;148:435–438. [DOI] [PubMed] [Google Scholar]
- 29.Morrison VA, Weisdorf DJ. The spectrum of Malassezia infections in the bone marrow transplant population. Bone Marrow Transplant. 2000;26:645–648. [DOI] [PubMed] [Google Scholar]
- 30.Barber GR, Brown AE, Kiehn TE, Edwards FF, Armstrong D. Catheter-related Malassezia furfur fungemia in immunocompromised patients. Am J Med. 1993;95:365–370. [DOI] [PubMed] [Google Scholar]


