Abstract
Background.
Recently, chronic hepatitis E has been reported in solid organ transplant (SOT) recipients in European countries. Previously, we clarified the prevalence of hepatitis E virus (HEV) infection in Japanese liver transplant recipients and identified 2 chronic hepatitis E patients infected by blood transfusion. However, the rate of HEV infection in recipients of SOTs other than liver in Japan remains unclear, so we conducted a nationwide survey to clarify the prevalence of chronic HEV infection in Japanese heart and kidney transplant recipients.
Methods.
A total of 99 heart and 2526 kidney transplant recipients in 17 hospitals in Japan were examined for the presence of the IgG class of anti-HEV antibodies as well as for serum HEV RNA.
Results.
The prevalence of anti-HEV IgG among heart and kidney transplant recipients was 7.07% (7/99) and 4.08% (103/2526), respectively. One heart transplant patient (1.01%) and 11 kidney transplant patients (0.44%) were found to be positive for HEV RNA. The HEV isolates from all viremic patients were typed as genotype 3. Four patients developed chronic hepatitis E after transplantation. Three patients were treated with ribavirin; their liver enzymes normalized, and HEV RNA became negative immediately. Sustained virologic response was achieved in all cases.
Conclusions.
This is the first nationwide survey of HEV infection in Japanese heart and kidney transplant recipients. The prevalence of anti-HEV IgG and HEV RNA in heart and kidney transplant recipients in Japan was lower than that in European countries. Of note, 42% of viremic transplant patients developed chronic hepatitis.
INTRODUCTION
Hepatitis E is caused by infection with the hepatitis E virus (HEV), and the isolates that infect humans are currently categorized into 4 major genotypes (genotypes 1–4).1 Genotypes 1 and 2 are restricted to humans and are mainly spread by waterborne transmission in developing countries. In contrast, genotypes 3 and 4 are known to undergo zoonotic transmission via the consumption of uncooked or undercooked meat or the viscera of reservoir mammals, and autochthonous isolates cause sporadic infections in industrialized countries.2,3 Recently, there was a report of one case of a new genotype (genotype 7) that was isolated from camel meat and milk and that led to chronic HEV infection in a liver transplant recipient.4
Initially, HEV infection was recognized only as an acute, self-limiting liver disease requiring no specific therapy in healthy individuals,5 and HEV infection was known to occasionally cause fulminant hepatic failure in specific high-risk groups, that is, pregnant women and individuals with chronic liver diseases.6,7 However, since the first report of chronic HEV infection in solid-organ transplant (SOT) recipients,8 it has been recognized that HEV infection in immunocompromised patients leads to chronic hepatitis and liver cirrhosis.9 Furthermore, the first case of HEV-related hepatocellular carcinoma was recently reported.10
To date, various studies of HEV infection in SOT recipients have been reported.11 Previously, we reported the prevalence of anti-HEV antibodies and HEV RNA in liver transplant recipients in Japan and revealed transfusion-transmitted cases of chronic hepatitis E.12 To further assess the characteristics of HEV infection in SOT recipients in Japan, we conducted a nationwide survey to investigate the prevalence of HEV infection in heart and kidney transplant recipients.
MATERIALS AND METHODS
Human Subjects
Seventeen hospitals from all regions of Japan participated in this study. The following 3 hospitals that perform heart transplantation that participated (from north to south) are as follows: Tohoku University Hospital in the Tohoku area, University of Tokyo Hospital in the Kanto area, and Kyushu University Hospital in the Kyushu area. The following 14 hospitals that perform kidney transplantation that participated (from north to south) are as follows: Sapporo City General Hospital in Hokkaido; Akita University Hospital and Japan Community Health Care Organization Sendai Hospital in the Tohoku area; University of Tsukuba Hospital, Jichi Medical University Hospital, National Hospital Organization Chiba-East-Hospital, and Toho University Omori Medical Center in the Kanto area; Niigata University Medical and Dental Hospital and Nagoya Daini Red Cross Hospital in the Chubu area; Takatsuki General Hospital and Inoue Hospital in the Kinki area; Hiroshima University Hospital in the Chugoku area; Kochi Health Sciences Center in the Shikoku area; and Kyushu University Hospital in the Kyushu area. In Japan, heart and kidney transplantations are performed in 11 centers and 135 centers, respectively. Therefore, the percentages of centers in this study to the whole are 27.3% and 10.4% for heart and kidney transplantation, respectively. We selected the representative centers with more patients for inclusion in our study.
Between April 1, 2015, and December 31, 2017, blood samples were collected principally once from 2625 SOT recipients (including 99 heart transplant recipients and 2526 kidney transplant recipients), who received follow-up at the above-mentioned 17 hospitals after transplantation and agreed to participate in this study. All 2625 samples were tested for anti-HEV antibodies and HEV RNA at Division of Virology, Department of Infection and Immunity, Jichi Medical University School of Medicine. Only patients who were positive for HEV RNA received continuous follow-up testing for anti-HEV antibodies and HEV RNA retrospectively (if stored serum samples were available) and prospectively. The samples were stored at −80°C until the analysis. The clinical data of the recipients, including their medical history, medication profiles, and laboratory test results, were retrieved from their medical records. This study was approved by the institutional review board of each participating hospital and was conducted in accordance with the Declaration of Helsinki and ethical guidelines for clinical research. All SOT recipients gave their written informed consent to participate in this study.
Detection of Anti-HEV Antibodies and HEV RNA
To detect IgA, IgM, and IgG classes of anti-HEV antibodies, in-house ELISAs were performed using the purified recombinant ORF2 protein, as previously described.13 Our previous study indicated that (1) in solid-phase ELISA, the IgA anti-HEV assay is significantly more specific than the IgM anti-HEV assay with regard to the ability to diagnose hepatitis E; (2) IgA anti-HEV was useful as the first-choice marker for a diagnostic indicator of recent HEV infection; and (3) the diagnostic accuracy increases when positive results obtained by the IgA anti-HEV assay are confirmed by additional or simultaneous detection of IgM anti-HEV.14 Therefore, we checked for 3 (IgG, IgM, and IgA) anti-HEV antibodies.
The optical density of each sample was read at 450 nm. The cut-off values used for anti-HEV IgA, IgM, and IgG were 0.642, 0.440, and 0.175, respectively.14 Samples with optical density values for anti-HEV IgA, IgM, and IgG equal to or greater than the respective cut-off value were considered positive for the respective antibody. The specificity of the ELISA was validated based on absorption with the same recombinant ORF2 protein that was used as the antigen probe, as previously described.14
Total RNA was extracted from 100 µL of each serum or whole blood sample and subjected to nested reverse transcription-polymerase chain reaction (RT-PCR) for the detection of HEV RNA. Nested RT-PCR targeting of the 137-nucleotide (nt) ORF2/3 overlapping region was conducted to screen for HEV RNA.15 This method is highly sensitive and is able to detect 1–3 copies of HEV RNA in 100 µL of serum. For the samples that tested positive according to the ORF2/3–137 PCR, we performed additional nested RT-PCR targeting the 457-nt ORF2 region sequence to confirm the presence of HEV RNA.13 The HEV genotype was determined based on a phylogenetic analysis of the ORF2 nt sequence as previously described.16,17
Quantitation of HEV RNA was performed by real-time RT-PCR as previously described.18
RESULTS
Characteristics of the Subjects
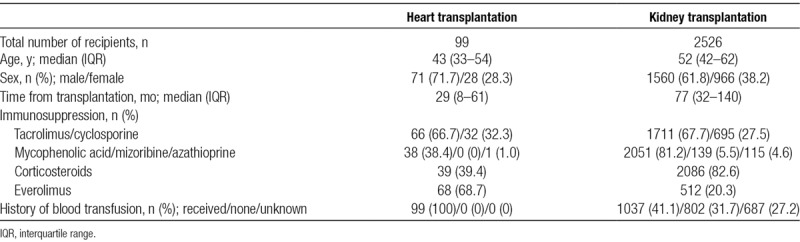
A total of 2625 patients participated in this study (99 heart transplant recipients and 2526 kidney transplant recipients). Their demographic characteristics and laboratory data are presented in Table 1. Among the heart transplant recipients, the median age was 43 years, and the median time between transplantation and serum sampling was 29 months; 71 (71.7%) of the heart transplant recipients were men. Among the kidney transplant recipients, the median age was 52 years, and the median time between transplantation and serum sampling was 77 months; 1560 (61.8%) of the kidney transplant recipients were men.
TABLE 1.
Characteristics of recipients
Prevalence of Anti-HEV Antibodies and HEV RNA
Among the heart transplant recipients, 7 patients were found to be positive for anti-HEV IgG, while none had detectable anti-HEV IgA or IgM (Table 2). In contrast, among the kidney transplant recipients, 103 patients were found to be positive for anti-HEV IgG. Among the patients who were positive for anti-HEV IgG, 11 were also positive for anti-HEV IgA and/or IgM.
TABLE 2.
Prevalence of anti-HEV antibodies and HEV RNA
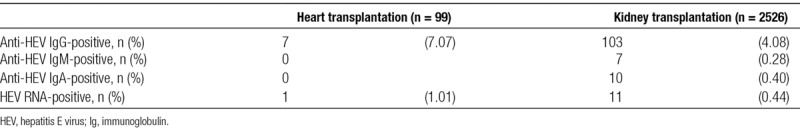
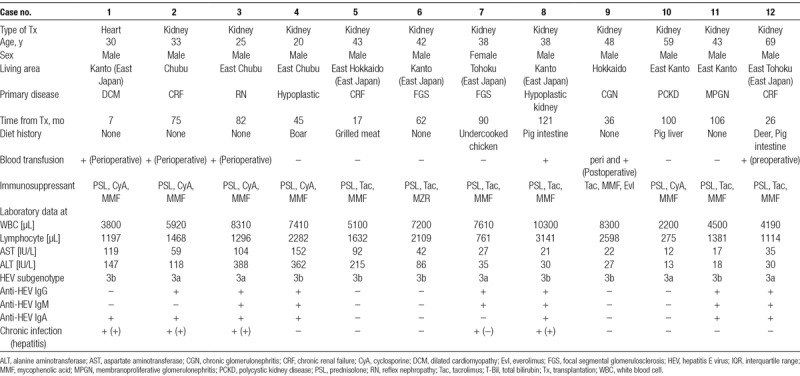
One of the 99 heart transplant recipients and 11 of the 2526 kidney transplant recipients were positive for HEV RNA. The prevalence rates of HEV RNA for heart and kidney transplant recipients were 1.01% (1/99) and 0.44% (11/2526), respectively (Table 2). Detailed clinical information for those 12 HEV RNA-positive patients is shown in Table 3. Four of the 12 viremic patients were found to be negative for all 3 classes of anti-HEV antibodies at sampling. All 12 of the viremic patients lived in east Japan, where HEV infection is prevalent.19 In the HEV-infected heart transplant patient, the time between heart transplantation and blood sampling was 7 months. In contrast, among the HEV-infected kidney transplant patients, the median time between kidney transplantation and blood sampling was 75 months (range: 17–121 months). The HEV isolates from all 12 viremic patients were typed as genotype 3 (subgenotype 3a or 3b) (Table 3).
TABLE 3.
Characteristics of HEV RNA-positive recipients
Chronic HEV Infection and Chronic Hepatitis E
Seven of the 12 HEV RNA-positive patients spontaneously became negative for HEV RNA, while the remaining 5 patients (including 1 heart and 4 kidney transplant recipients) progressed to chronic HEV infection, defined as HEV viremia exceeding 6 months (Table 3). They had no episodes of recent rejection, and the immunosuppressant use among these patients was similar to that in the others. The chronic rates of HEV infection for the heart and kidney transplant recipients were 100% (1/1) and 36.4% (4/11), respectively. Four of these 5 patients had liver injury during follow-up and were found to have developed chronic hepatitis E. The clinical course of these patients is shown in Figure 1.
FIGURE 1.
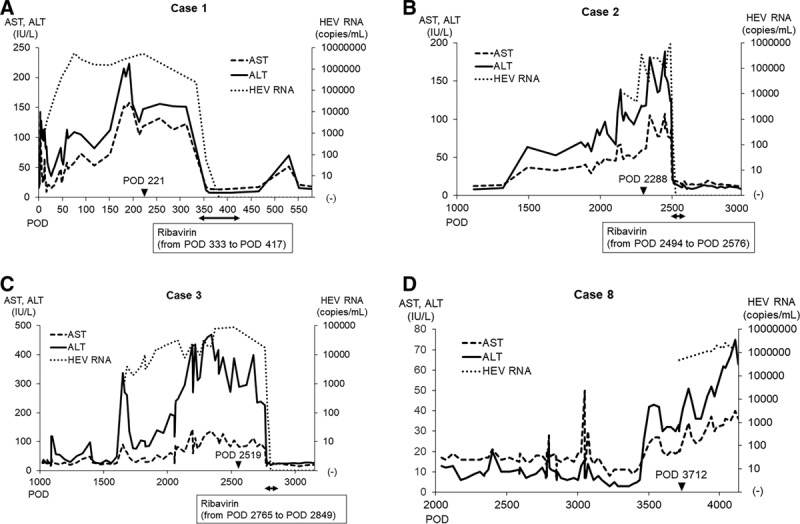
Clinical course in the chronic hepatitis cases. Postoperative transition of liver enzymes and HEV RNA titers in the chronic hepatitis cases: case 1 (A), case 2 (B), case 3 (C), case 8 (D). The closed arrowhead indicates the timing of sampling. The closed double arrow indicates the period of ribavirin administration. ALT, alanine aminotransferase; AST, aspartate aminotransferase; HEV, hepatitis E virus; POD, postoperative day.
Three patients who developed chronic hepatitis E were treated with ribavirin without reducing their immunosuppressant use at the judgment of each doctor in charge (cases 1, 2, and 3; Figure 1A–C). After the initiation of treatment, their liver enzyme levels became normal after 6–13 days, and HEV RNA became negative after 32–49 days. These patients remained negative for HEV RNA for more than 6 months after cessation of the treatment, indicating that a sustained virologic response had been achieved in all 3 cases. The remaining patient has continued to show HEV viremia and liver injury, and antiviral treatment for chronic hepatitis E is being considered (case 8; Figure 1D).
Elucidation of Possible Infectious Sources and Routes
Among the 12 viremic patients, 4 cases (1, 2, 3, and 9) were transfused during the perioperative period, and 1 (case 8) was transfused during the peri and postoperative period. In case 1, all pretransplant serum samples were negative for 3 classes of anti-HEV antibodies and HEV RNA. HEV RNA became positive on postoperative day (POD) 9 and remained positive until POD 354. Anti-HEV IgG converted from negative to positive on POD 76, and anti-HEV IgA and IgM converted from negative to positive on POD 333. Therefore, this patient may have been infected with HEV during or just after heart transplantation. Anti-HEV antibodies and HEV RNA in that patient were undetectable in the serum samples from the heart donor, and the recipient had no history of eating raw or undercooked meat or shellfish during the perioperative period. A total of 33 units of blood products, including red blood cells, fresh frozen plasma, and platelets, were transfused to this recipient between the day of the operation and POD 9. Samples of all of these blood products had been stored by the Japanese Red Cross Society and were tested for the presence of HEV RNA; however, HEV RNA was undetectable in all units. Unfortunately, the source of HEV infection could not be identified in this case.
In case 9, all pretransplant serum samples were negative for 3 classes of anti-HEV antibodies and HEV RNA and these remained negative until POD 356. HEV RNA became positive on POD 1119 and continued to be positive until POD 1196. Anti-HEV IgG was negative on POD 1119 and converted to positive on POD 1196. Therefore, it seems that there is no possibility that this patient was infected with HEV due to perioperative transfusion. In the other 3 patients (cases 2, 3, and 8), no serum samples had been preserved between transfusion and detection of HEV RNA. Therefore, the possibility of HEV infection due to transfusion was unclear.
Case 12 was transfused twice during hemodialysis treatment in a previous hospital. Since there were no instances of increased liver enzymes for 2 years after the transplantation, HEV infection due to the preoperative transfusion was unlikely in this case.
Seven of the 12 patients had a history of eating high-risk meals for HEV infection (undercooked pig liver, pig intestine, or deer and boar meat) (Table 3). Accordingly, there was a high possibility of foodborne transmission of HEV in those patients, although the source of HEV infection could not be definitively identified.
DISCUSSION
By 2016, 317 heart and over 36 000 kidney transplantation had been performed in Japan.20 Currently, approximately 1600 kidney transplantations are performed per year, and cases of heart transplantation have been increasing since the revised Japanese organ transplant law was enacted in 2010.21
Regarding HEV infection, the number of national notifications of acute hepatitis E has been increasing since anti-HEV IgA antibody measurement for the diagnosis began to be covered by the government insurance program in October 2011,22 and the isolation of autochthonous HEV as a causative agent of acute hepatitis has increased. Since 2016, the annual number of acute hepatitis E cases has exceeded that of acute hepatitis A cases in Japan. In our study, the overall prevalence rates of both anti-HEV IgG and HEV RNA in heart and kidney transplant recipients were higher than those in liver transplant recipients.12 Although it is difficult to clarify the underlying reason, this finding may suggest that the opportunity for exposure to HEV infection in heart and kidney transplant recipients is more frequent than in liver transplant recipients in Japan.
In recent years, the prevalence and clinical courses of hepatitis E in transplant recipients have been reported mainly in European countries.8,23-25 The prevalence rates of anti-HEV IgG and HEV RNA in heart transplant recipients in the present study were 7.07% and 1.01%, respectively, which were slightly lower than the reported rates in European countries, although the sample size of this study was smaller than in other studies (anti-HEV IgG, 11.3%; HEV RNA, mean 1.7%, range 1.5%–1.9%).11 Regarding kidney transplant recipients, the prevalence rates of anti-HEV IgG and HEV RNA in the current study were 4.08% and 0.44%, respectively, which were significantly lower than the reported rates in European countries (anti-HEV IgG, mean 11.7%, range 2.4%–14.5% and HEV RNA, mean 1.6%, range 0.2%–4.2%).11 This lower prevalence in heart and kidney transplant recipients in Japan can be attributed to the intrinsic lower prevalence of HEV in the general population in Japan than in European countries.6,26
The guidelines for both the European Association for the Study of the Liver (EASL) and British Transplantation Society (BTS) for hepatitis E recommend that HEV RNA can be used to diagnose HEV infection in transplant recipients.27,28 In our study, 4 of the 12 HEV RNA-positive recipients were found to be negative for all detectable anti-HEV antibodies at sampling, even though 2 patients (cases 5 and 6) had liver injury. In these 4 cases, HEV infection could not be diagnosed by measuring only anti-HEV antibodies. A reduction in anti-HEV antibody titers may be induced by immunosuppressive drugs.29 Therefore, given the results of the present study, the measurement of HEV RNA is highly recommended for ensuring a proper diagnosis in SOT recipients.
The main risks of HEV infection for immunosuppressed patients are exposure to raw or undercooked infected pork products and blood transfusion.30 Zoonotic HEV infections from pigs, wild boars, and deer have been shown to occur in Japan,2,3,7,13,17,19,31,32 similar to Europe. There is a risk of contracting HEV infection by consuming shellfish; however, this risk may be low in our transplant recipients because they are prohibited from eating sushi. There are few or no recognized hepatitis E cases caused by waterborne HEV infection (eg, via drinking well water) in Japan. In the present study, although the source of HEV infection could not be definitively identified, foodborne infection was suspected in 11 patients (except for case 1). Six of the 11 patients (cases 4, 5, 7, 8, 10, and 12) had a history of eating undercooked pig liver, pig intestine, and/or deer and boar meat; however, the remaining 5 patients (cases 2, 3, 6, 9, and 11) had no history of consuming such high-risk meals for HEV infection. Since 2 of these 5 patients (cases 6 and 11) also had no history of blood transfusion, they must have been infected with HEV via the oral route. There is a possibility of contracting HEV infection through the unwitting consumption of foods at high risk of contamination. Therefore, HEV markers should be examined when posttransplant recipients present with liver dysfunction of unknown cause even if they do not report consumption of food associated with a high risk of HEV infection.
Kamar et al33 suggested a management algorithm for treating HEV infection in posttransplant patients with chronic hepatitis. In patients with a low immunological risk, the reduction of immunosuppressants, especially calcineurin inhibitors, is recommended. In patients with a high immunological risk, antiviral therapy is suggested because the immunosuppression usually cannot be reduced. In the present study, since 3 patients who developed chronic hepatitis E (cases 1, 2, and 3) had a high immunological risk, they received antiviral therapy without reducing their immunosuppressant use, and sustained virologic response was achieved in all 3 cases. Because approximately 20% of patients who receive antiviral therapy experience recurrence of HEV infection,34 the BTS guideline recommends that HEV RNA can be measured for at least 6 months after the completion of ribavirin therapy.28
Recently, 11 European countries, including Denmark, France, Germany, Greece, Ireland, Italy, The Netherlands, Portugal, Spain, Switzerland, and United Kingdom, summarized the epidemiology of HEV infections among blood donors.30 The prevalence of HEV RNA in blood donor population in European countries ranges from 0.02% to 0.13%, which is generally higher than in Japan (Hokkaido area: 0.012%, outside Hokkaido area: 0.007%).35 Recently, however, Japanese Red Cross blood centers have reported that the prevalence of HEV RNA detectable by an individual HEV-nucleic acid amplification test screening in blood donor population in Tokyo, Japan, is 0.073%.36 This is nearly equal to the prevalence of European countries.30 Ireland, the Netherlands, and the UK have already implemented HEV RNA screening of blood donations.30 Germany and France are conducting screening for HEV RNA in several blood establishments or plasma donations intended for use for high-risk patients, respectively.30 In Japan, screening of donated blood for HEV has only been conducted in the Hokkaido area, where HEV infection is particularly prevalent.35 Recently, it has been argued that donated blood intended for administration to high-risk patients, such as SOT recipients, should be screened for HEV RNA. Furthermore, the implementation of nationwide HEV-nucleic acid amplification test screening system for blood donors is being considered in Japan.37
In conclusion, we conducted a nationwide multicenter survey of HEV infection in heart and kidney transplant recipients after having conducted a survey in liver transplant recipients. The results demonstrated that the prevalence of anti-HEV IgG and HEV RNA in heart and kidney transplant recipients was lower in Japan than in European countries. We should be aware of the possibility of HEV infection when posttransplant recipients present with liver dysfunction of unknown cause. We should measure HEV RNA to diagnose HEV infection in posttransplant recipients, as immunosuppressive drugs induce a reduction in anti-HEV antibody titers.
Footnotes
This work was supported by the Research Program on Hepatitis of the Japan Agency for Medical Research and Development, AMED (JP17fk0210301).
The authors declare no conflicts of interest.
Yohei Owada performed the literature search, study design, patients’ samples and data collection, data analysis, data interpretation, article writing, and table and figure development. Yukio Oshiro performed the literature search, study design, institution participation recruitment, and article writing. Y.I. performed the literature search, study design, and institution participation recruitment. H.H., N.F., N.K., T.Y., J.U., N.A., Y.I., K.S., Y.W., N.I., R.I., M.K., K.I., Y.S., Y.O., M.O., K.S., and A.S. performed the patients’ samples and data collection. K.Y. performed the study design and data interpretation. H.O., S.N., and M.T. performed the sample measurement and data interpretation. K.Y. performed the institution participation recruitment. H.O. performed the study design, sample measurement, data interpretation, and article editing. N.O. performed the study design, institution participation recruitment, and data interpretation. Yohei Owada and Yukio Oshiro contributed equally to this work, as first authors.
Clinical trial notation
Registry name: A nationwide survey of hepatitis E virus infection in heart and kidney transplant recipients in Japan
Institutional Review Board Registration number of University of Tsukuba Hospital: H27-048
REFERENCES
- 1.Okamoto H. Genetic variability and evolution of hepatitis E virus. Virus Res 2007127216–228 [DOI] [PubMed] [Google Scholar]
- 2.Takahashi M, Nishizawa T, Miyajima H, et al. Swine hepatitis E virus strains in Japan form four phylogenetic clusters comparable with those of japanese isolates of human hepatitis E virus. J Gen Virol 200384Pt 4851–862 [DOI] [PubMed] [Google Scholar]
- 3.Tei S, Kitajima N, Takahashi K, et al. Zoonotic transmission of hepatitis E virus from deer to human beings. Lancet 2003362371–373 [DOI] [PubMed] [Google Scholar]
- 4.Lee GH, Tan BH, Teo EC, et al. Chronic infection with camelid hepatitis E virus in a liver transplant recipient who regularly consumes camel meat and milk. Gastroenterology 2016150355–7.e3 [DOI] [PubMed] [Google Scholar]
- 5.Wedemeyer H, Pischke S, Manns MP. Pathogenesis and treatment of hepatitis e virus infection. Gastroenterology 20121421388–1397.e1 [DOI] [PubMed] [Google Scholar]
- 6.Kamar N, Bendall R, Legrand-Abravanel F, et al. Hepatitis E. Lancet 20123792477–2488 [DOI] [PubMed] [Google Scholar]
- 7.Suzuki K, Aikawa T, Okamoto H. Fulminant hepatitis E in Japan. N Engl J Med. 2002;347:1456. doi: 10.1056/NEJM200210313471819. [DOI] [PubMed] [Google Scholar]
- 8.Kamar N, Selves J, Mansuy JM, et al. Hepatitis E virus and chronic hepatitis in organ-transplant recipients. N Engl J Med 2008358811–817 [DOI] [PubMed] [Google Scholar]
- 9.Behrendt P, Steinmann E, Manns MP, et al. The impact of hepatitis E in the liver transplant setting. J Hepatol 2014611418–1429 [DOI] [PubMed] [Google Scholar]
- 10.Borentain P, Colson P, Bolon E, et al. Hepatocellular carcinoma complicating hepatitis E virus-related cirrhosis. Hepatology 201867446–448 [DOI] [PubMed] [Google Scholar]
- 11.Zhou X, de Man RA, de Knegt RJ, et al. Epidemiology and management of chronic hepatitis E infection in solid organ transplantation: a comprehensive literature review. Rev Med Virol 201323295–304 [DOI] [PubMed] [Google Scholar]
- 12.Inagaki Y, Oshiro Y, Tanaka T, et al. A nationwide survey of hepatitis E virus infection and chronic hepatitis E in liver transplant recipients in Japan. Ebiomedicine 201521607–1612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mizuo H, Suzuki K, Takikawa Y, et al. Polyphyletic strains of hepatitis E virus are responsible for sporadic cases of acute hepatitis in Japan. J Clin Microbiol 2002403209–3218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Takahashi M, Kusakai S, Mizuo H, et al. Simultaneous detection of immunoglobulin A (iga) and igm antibodies against hepatitis E virus (HEV) is highly specific for diagnosis of acute HEV infection. J Clin Microbiol 20054349–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Inoue J, Takahashi M, Yazaki Y, et al. Development and validation of an improved RT-PCR assay with nested universal primers for detection of hepatitis E virus strains with significant sequence divergence. J Virol Methods 2006137325–333 [DOI] [PubMed] [Google Scholar]
- 16.Smith DB, Simmonds P, Izopet J, et al. Proposed reference sequences for hepatitis E virus subtypes. J Gen Virol 201697537–542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamazaki Y, Naganuma A, Arai Y, et al. Clinical and virological features of acute hepatitis E in gunma prefecture, japan between 2004 and 2015. Hepatol Res 201747435–445 [DOI] [PubMed] [Google Scholar]
- 18.Takahashi M, Hoshino Y, Tanaka T, et al. Production of monoclonal antibodies against hepatitis E virus capsid protein and evaluation of their neutralizing activity in a cell culture system. Arch Virol 2008153657–666 [DOI] [PubMed] [Google Scholar]
- 19.Takahashi M, Okamoto H. Features of hepatitis E virus infection in humans and animals in Japan. Hepatol Res 20144443–58 [DOI] [PubMed] [Google Scholar]
- 20.Japanese Society for Clinical Renal Transplantation. The Japan Society for Transplantation. Annual progress report from the Japanese renal transplant registry: number of renal transplantations in 2016 and follow-up survey. Jpn J Transplant 201452113–133 [Google Scholar]
- 21.The Japanese Society for Heart Transplantation. Available at http://www.jsht.jp/. Accessed October 28, 2018.
- 22.The National Institute of Infectious Disease. Infectious disease weekly report (IDWR). Available at http://www.nih.go.jp/niid/ja/idwr.html. Accessed October 28, 2018.
- 23.Legrand-Abravanel F, Kamar N, Sandres-Saune K, et al. Hepatitis E virus infection without reactivation in solid-organ transplant recipients, France. Emerg Infect Dis 20111730–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pas SD, de Man RA, Mulders C, et al. Hepatitis E virus infection among solid organ transplant recipients, the Netherlands. Emerg Infect Dis 201218869–872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moal V, Legris T, Burtey S, et al. Infection with hepatitis E virus in kidney transplant recipients in southeastern France. J Med Virol 201385462–471 [DOI] [PubMed] [Google Scholar]
- 26.Takahashi M, Tamura K, Hoshino Y, et al. A nationwide survey of hepatitis E virus infection in the general population of Japan. J Med Virol 201082271–281 [DOI] [PubMed] [Google Scholar]
- 27.Dalton HR, Kamar N, Baylis SA, et al. EASL Clinical Practice Guidelines on hepatitis E virus infection. J Hepatol 2018681256–1271 [DOI] [PubMed] [Google Scholar]
- 28.McPherson S, Elsharkawy AM, Ankcorn M, et al. Summary of the british transplantation society UK guidelines for hepatitis E and solid organ transplantation. Transplantation 201810215–20 [DOI] [PubMed] [Google Scholar]
- 29.Sester M, Gärtner BC, Girndt M, et al. Vaccination of the solid organ transplant recipient. Transplant Rev 200822274–284 [DOI] [PubMed] [Google Scholar]
- 30.Domanović D, Tedder R, Blümel J, et al. Hepatitis E and blood donation safety in selected European countries: a shift to screening? Euro Surveill. 2017;22:30514. doi: 10.2807/1560-7917.ES.2017.22.16.30514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yazaki Y, Mizuo H, Takahashi M, et al. Sporadic acute or fulminant hepatitis E in Hokkaido, Japan, may be food-borne, as suggested by the presence of hepatitis E virus in pig liver as food. J Gen Virol 200384Pt 92351–2357 [DOI] [PubMed] [Google Scholar]
- 32.Sato Y, Sato H, Naka K, et al. A nationwide survey of hepatitis E virus (HEV) infection in wild boars in Japan: identification of boar HEV strains of genotypes 3 and 4 and unrecognized genotypes. Arch Virol 20111561345–1358 [DOI] [PubMed] [Google Scholar]
- 33.Kamar N, Lhomme S, Abravanel F, et al. Treatment of HEV infection in patients with a solid-organ transplant and chronic hepatitis. Viruses. 2016;8:222. doi: 10.3390/v8080222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kamar N, Izopet J, Tripon S, et al. Ribavirin for chronic hepatitis E virus infection in transplant recipients. N Engl J Med 20143701111–1120 [DOI] [PubMed] [Google Scholar]
- 35.Minagi T, Okamoto H, Ikegawa M, et al. Hepatitis E virus in donor plasma collected in Japan. Vox Sang 2016111242–246 [DOI] [PubMed] [Google Scholar]
- 36.The survey of HEV infection in Tokyo area. Available at https://www.mhlw.go.jp/file/05-Shingikai-11121000-Iyakushokuhinkyoku-Soumuka/0000138834.pdf. Accessed March 20, 2019.
- 37.About HEV infection. Available at http://www.jrc.or.jp/mr/news/pdf/yuketsuj_1803-158.pdf. Accessed March 20, 2019.


