Purpose:
The aim of this study was to describe the clinical presentations, management and factors determining outcomes of Aspergillus endophthalmitis.
Design:
Retrospective, interventional, multicentric case series.
Methods:
The study included 91 eyes of 91 patients with culture-proven Aspergillus endophthalmitis. Anterior chamber fluid and/or vitreous and/or intraocular lens were submitted for microbiological evaluation in all cases. The data analysis included patient demography, clinical presentations, interventions, and final treatment outcomes. The main outcome measures were the final visual acuity and the globe salvation. The factors determining better visual and/or anatomical outcomes were analyzed.
Results:
The mean age of the patients was 39.71 ± 20.16 years (median 40 years, range 3–76 years). By etiology, the primary event before the endophthalmitis was trauma (42; 46.15%) eyes, cataract surgery (acute-onset: 30; 32.96% and delayed-onset: 6; 6.59%) eyes, endogenous (10; 10.98%) eyes, and cornea surgery (3; 3.29%) eyes. The mean follow up was 5.78 ± 6.74 months (median 3, range 0.5–40 months). The odds of a favorable visual outcome were presenting vision > hand motions [odds ratio (OR) = 3.33, P = 0.02], absence of corneal infiltrate (OR = 5.4, P = 0.03), vitrectomy instead of a vitreous tap only (OR = 4.26, P = 0.03), administration of intravitreal voriconazole (OR = 3.63, P = 0.02), and absence of fungal elements on primary microscopy (OR = 3.42, P = 0.02).
Conclusions:
Early vitrectomy and intravitreal voriconazole yielded better anatomic and functional outcomes in Aspergillus endophthalmitis. Favorable visual outcome was achieved in a fifth of the eyes and globe was salvaged in two-thirds of the eyes.
Keywords: Amphotericin B, Aspergillus, endophthalmitis, treatment outcomes
Fungal endophthalmitis is a potentially devastating eye infection, which occurs due to either direct inoculation of fungus into the eye (exogenous) or a hematogenous spread (endogenous) to the eye secondary to systemic fungal infections.1–5 Trauma and intraocular surgery are the common causes. The common infecting fungi are Fusarium and Aspergillus species for exogenous endophthalmitis, and Candida species for endogenous endophthalmitis. Aspergillus is reported to have high ocular morbidity and poor outcome; in <50% of instances, the final vision is ≥20/400.6 Till date, the largest reported series is only 9 cases of Aspergillus endophthalmitis.7 In the current communication, we report a much larger series of Aspergillus endophthalmitis that has allowed us to identify the specific factors for the functional/anatomical outcome.
METHODS
Study Design
This was a retrospective 6-year (January 2010-December 2015) chart review of cases with culture-positive Aspergillus endophthalmitis, at 2 tertiary referral eye care centers in south India.
Methodology
Medical records of all Aspergillus endophthalmitis cases were identified from the institute medical record system and the microbiology laboratory records. Institutional Review board approval for the study was taken as appropriate. Details of history, clinical examination, clinical features at presentation, microbiological evaluation, and clinical response to therapy were noted. Clinical findings included presenting and final best corrected visual acuity, slit lamp findings of cornea, iris and pupil, presence and extent of hypopyon, presence of fundal glow, and status of the retinal vessels if visible. Whenever the fundus was not visible by the binocular indirect ophthalmoscope using the highest illumination, B-scan ultrasonography was done to determine the extent and location of vitreous involvement and also to detect any associated ocular disorders such as retinal detachment, choroidal thickening, choroidal detachment, or retained intraocular foreign body.
Intervention
As per the institute protocol, the surgical management of endophthalmitis consisted of vitreous tap with or without pars plana vitrectomy, microscopy and culture of undiluted vitreous, antimicrobial susceptibility testing of bacterial isolates, intravitreal antibiotics (vancomycin 1 mg/0.1 mL + ceftazidime 2.25 mg/0.1 mL) with or without dexamethasone (400 μg/0.1 mL). The medical treatment also included intensive topical antibiotics (ciprofloxacin 0.3% one hourly) and corticosteroid (prednisolone acetate 1% one hourly), and oral ciprofloxacin (750 mg 2 times per day for 7–10 days). Fungal infection was confirmed with positive microscopy and positive culture of the vitreous sample, and upon confirmation, intravitreal amphotericin B (5 μg/0.1 mL) or intravitreal voriconazole (50 μg/0.1 mL) injection was administered along with systemic ketoconazole/itraconazole (either one 200 mg twice a day for 15 days) as deemed appropriate by the treating physician. Additional procedures such as repeat intravitreal antifungals or vitreous surgery (vitrectomy/lavage) depend on the response to treatment and were left to the decision of the treating physicians. In cases with hazy view due to corneal involvement, a vitreous tap was performed instead of a vitrectomy. Undiluted vitreous, 1 to 1.5 mL, was collected using a vitreous cutter set at 5000 cuts/min attached to a 2-mL syringe with manual suction. We avoided vitreous aspiration to avoid possible peripheral vitreous traction. Depending on the presentation, other surgical procedures, beyond tap/vitrectomy, were combined as deemed appropriate.
Surgical Technique and Microbiologic Evaluation
The eyes were prepared with application of topical 5% povidone iodine in the conjunctival cul-de-sac. All vitrectomy procedures were transconjunctival suture-less procedures and were performed using 23G or 25G vitrectomy system. Undiluted vitreous samples were collected via a vitreous tap at the beginning of the surgery in all cases. Further handling and processing of the samples and final interpretation was done as per the institute protocol.8,9 The microbiological processing of the vitreous sample included direct microscopy and culture. Smears were examined after staining with 0.1% calcofluor white, Gram, and Gomori methenamine silver stains, and the culture media included 5% sheep blood agar, chocolate agar, thioglycollate broth, brain heart infusion, Sabouraud dextrose agar, and potato dextrose agar. All media were incubated aerobically at 37°C except Sabouraud dextrose agar and potato dextrose agar that were incubated at 27°C for 2 weeks. Chocolate agar was incubated in 5% CO2 at 37°C.9
Outcome Definition
The outcome at the last visit was considered for final analysis. A favorable anatomic outcome was defined as preservation of the globe, absence of hypotony (intraocular pressure ≤5mm Hg), attached retina, and absence of active inflammation. A favorable functional outcome was defined as an attached retina with a best-corrected vision of ≥20/400.
Statistical Analysis
The data were arranged on an Excel spread sheet and analyzed using the statistical software MedCalc ver 12.2.1.0 (Ostend, Belgium). Percentage confidence intervals were calculated using online statistical calculators (https://www.allto.co.uk/tools/statistic-calculators). OR with appropriate confidence intervals was computed for possible risk variables. A P value <0.05 was considered statistically significant. Comparison of continuous nondependent variable with a categorical dependent variable was done using logistic regression.
RESULTS
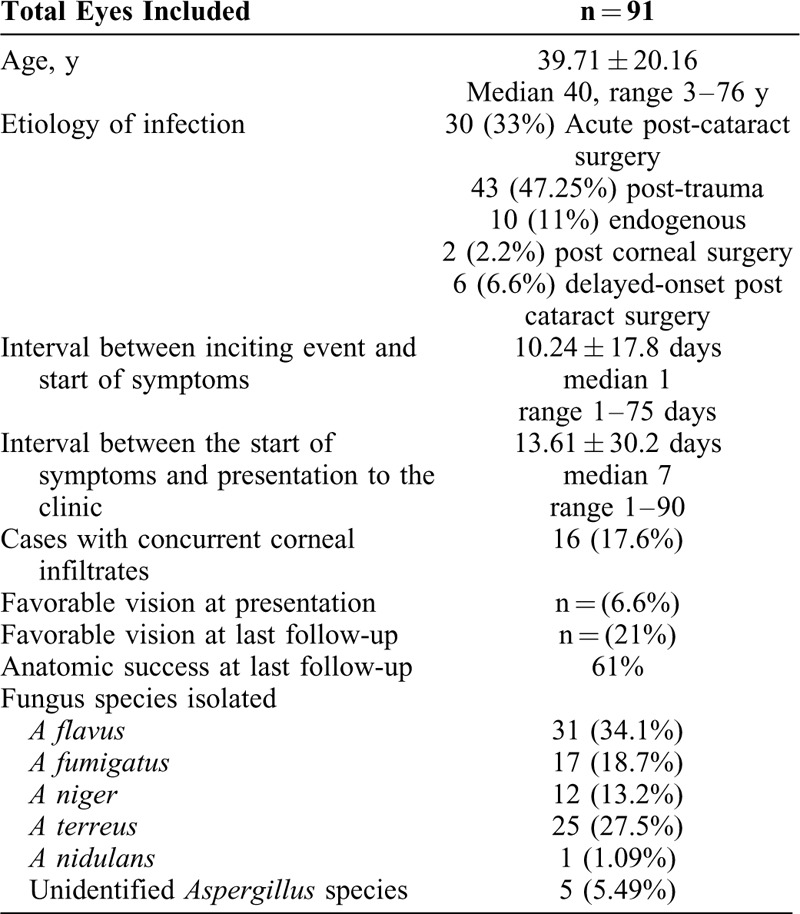
The study included 91 eyes of 91 patients with culture-proven Aspergillus endophthalmitis. This included 67 males (68.4%) and 25 females (31.6%). The mean age at presentation was 39.71 ± 20.16 years (median 40 years, range 3–76 years). Baseline demographic features of the cases are summarized in Table 1. 6 patients were diabetic at presentation but with well controlled blood sugars (mean fasting blood sugar of 110 mg%; range 92 mg% to 178 mg%, and HbA1c of 6.7; range 6.1–7.2 mg%). No patient was immunocompromised. The corrected vision was ≥20/400 at presentation in 6 eyes, and 16 eyes had associated keratitis. The mean duration of symptoms was 13.61 ± 30.2 days (median 7, range 1–90 days). Trauma (46.15%) was the common most cause of endophthalmitis and Aspergillus flavus (34.1%) was the commonest infecting species (Table 1).
TABLE 1.
Baseline Demographic and Clinical Features of Aspergillus Endophthalmitis
Thirty-two eyes (35.2%) received vitreous tap as the primary procedure and 59 eyes (64.8%) received a pars plana core vitrectomy as the primary procedure. Ten of 32 vitreous tap eyes subsequently received pars plana vitrectomy at a later date. Fifty-six eyes received intravitreal amphotericin B, 19 eyes received intravitreal voriconazole, and 16 eyes received both voriconazole and amphotericin B. The injections were repeated every 48 hours for amphotericin B and every 24 hours for voriconazole as decided by the treating physicians. The mean time interval between presentation and the first intravitreal antifungal antibiotic injection was 3.21 ± 1.92 days (range 1–13 days). The treating physicians also decided for further surgery, either vitrectomy after initial tap or vitreous lavage after initial vitrectomy. Finally, 91 eyes received 345 intravitreal injections (91 anti bacterial antibiotics at first stage and 254 antifungal antibiotic at later stages), 32 taps, 71 vitrectomy, and 21 vitreous lavage (Fig. 1); this amounted to mean of 3.8 intravitreal injection, and mean of 1.4 other surgical procedures per eye.
FIGURE 1.
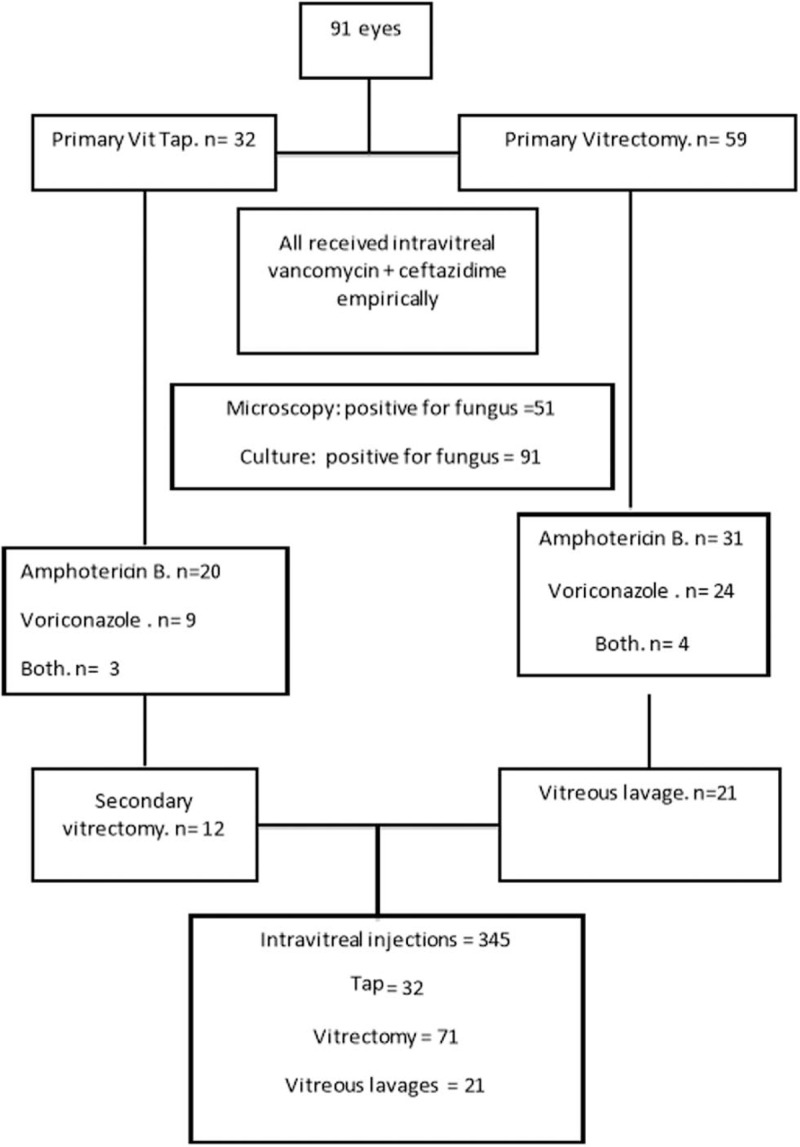
Management flow chart of cases in this study.
The mean follow-up was 5.78 ± 6.7 (median 3, range 0.5–40) months. The distribution of the pre and post operative visual acuity is shown in Figure 2. Adverse outcomes at the final follow-up included phthisis bulbi (15 eyes), vascularized corneal scar (15 eyes), recurrent retinal detachment (14 eyes), persistent inflammation (8 eyes) epiretinal membrane (4 eyes), and optic atrophy (4 eyes).
FIGURE 2.
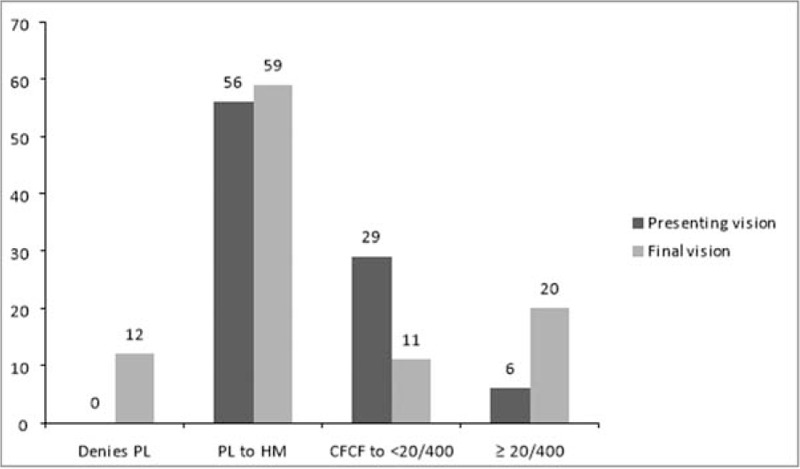
Comparative visual acuities at presentation and at the last follow up. CFCF indicates counting fingers close to face; HM, hand motions close to face; PL, perception of light. X axis - Distribution of visual acuities, Y axis - number of eyes.
The factors determining better visual outcomes included presenting vision greater than hand motions (OR 3.33; P = 0.01), absence of corneal infiltrate (OR 5.4; P = 0.03), vitrectomy instead of a vitreous tap alone (OR 4.26; P = 0.04), intravitreal voriconazole (OR 3.63; P = 0.79), and negative initial microscopy (OR 3.42; P = 0.03). The factors determining better anatomical outcomes included presenting vision greater than hand motions (OR 3.5; P = 0.01), vitrectomy instead of a vitreous tap alone (OR 2.52; P = 0.04), and negative initial microscopy (OR 2.74; P = 0.03). The absence of corneal infiltrate and the administration of voriconazole did not show any significant favorable odds for the anatomic outcome (Table 2). Better visual results were achieved in 19 of 91 (20.8%) eyes; 12 eyes regained vision ≥20/60, and 7 eyes regained vision ≥20/40 (Fig. 2). There was no difference between post cataract surgery and post trauma endophthalmitis (8/36, 22.2% vs 10/42, 23.8%, respectively) Age, sex, and presence of diabetes mellitus did not show any correlation to the final visual and anatomic outcome. Figure 3 shows the suggested intervention flow in such patients.
TABLE 2.
Factors Affecting Visual Outcome in Aspergillus Endophthalmitis
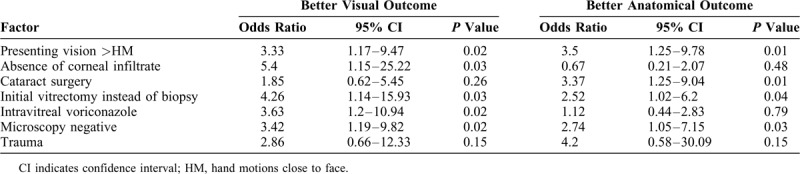
FIGURE 3.
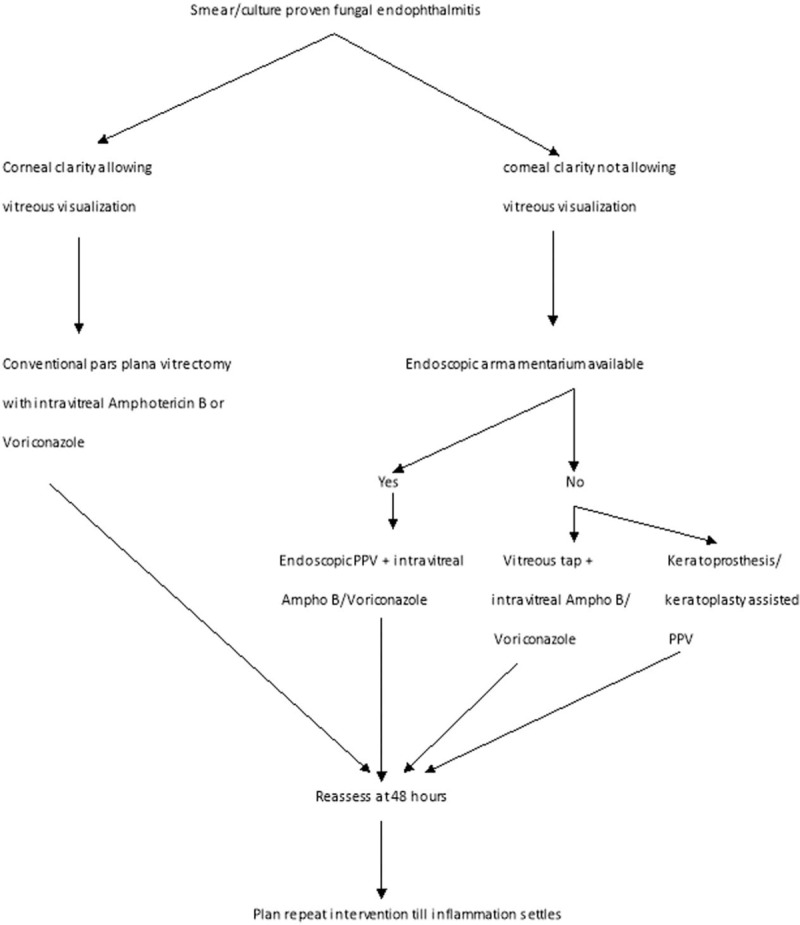
Standardized treatment protocol chart for fungal endophthalmitis. PPV indicates corneal clarity allowing visualization.
DISCUSSION
This is the largest study so far on Aspergillus endophthalmitis. We have also analyzed the factors predicting the visual and anatomical outcome. Factors associated with better visual outcome included presenting vision greater than hand motions, absence of corneal infiltrate, primary vitrectomy, administration of intravitreal voriconazole, and absence of fungal elements in initial microscopy.
Two important factors associated with better visual outcome in this series were early vitrectomy and use of intravitreal voriconazole. Others and we have shown the benefits of early vitrectomy in fungal endophthalmitis.10–12 The minimum inhibitory concentration (MIC) of voriconazole against various fungal isolates is lesser than amphotericin B, and voriconazole is reportedly less toxic to retina.13,14 But the half life of voriconazole in a vitrectomized eye is around 8 hours compared with >24 hours of amphotercin B and hence voriconazole requires frequent intravitreal injections. To overcome this disparity, one group of authors has suggested combination treatment of daily intravitreal voriconazole and alternate day amphotericin B in recalcitrant cases of fungal endophthalmitis.7 Two important factors associated with worse visual outcome in this series were corneal involvement and positive microscopy. Presence of fungal filaments on microscopy could mean either the microbiologist was more observant or the fungal load was large. The latter could also mean a gross infection.
The current study also has its own inherent limitations. The effect of various confounding factors could not be independently assessed due to the retrospective nature of the study. Despite the large number, the limited sample size did not allow us to reach a statistical significance of the impact of multiple variables. For variables where the P value was significant, it was observed that the 95% confidence intervals were wide. This indicates a variation in the results in the real world scenario. A wide confidence interval reflects a relatively lower sample size for that particular variable being tested. A larger study with more number of patients recruited would reduce the spread of the confidence interval and make the results more statistically significant.
In conclusion, Aspergillus is a common etiology of fungal endophthalmitis in the Indian subcontinent. An early vitrectomy is superior to vitreous tap alone and intravitreal voriconazole is superior to amphotericin B. But facts remain that despite the best possible treatment, most eyes are lost either anatomically or functionally.
Footnotes
Funding: This study was supported by the Hyderabad Eye Research Foundation.
Summary statement: Fungal endophthalmitis is a relatively rare cause of endophthalmitis around the world. In the Indian subcontinent, however, fungal etiology, especially Aspergillus is a common incriminate in endophthalmitis. With this background, we discuss the presentations management outcomes of Aspergillus endophthalmitis.
REFERENCES
- 1.Apt L, Isenberg SJ. Infectious endophthalmitis after cataract surgery. Br J Ophthalmol 1994; 78:948–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kresloff MS, Castellarin AA, Zarbin MA. Endophthalmitis. Surv Ophthalmol 1998; 43:193–224. [DOI] [PubMed] [Google Scholar]
- 3.Kunimoto DY, Das T, Sharma S, et al. Microbiologic spectrum and susceptibility of isolates: part I. Postoperative endophthalmitis. Endophthalmitis Research Group. Am J Ophthalmol 1999; 128:240–242. [DOI] [PubMed] [Google Scholar]
- 4.Gupta A, Gupta V, Gupta A, et al. Spectrum and clinical profile of post cataract surgery endophthalmitis in north India. Indian J Ophthalmol 2003; 51:139–145. [PubMed] [Google Scholar]
- 5.Narang S, Gupta A, Gupta V, et al. Fungal endophthalmitis following cataract surgery: clinical presentation, microbiological spectrum, and outcome. Am J Ophthalmol 2001; 132:609–617. [DOI] [PubMed] [Google Scholar]
- 6.Callanan D, Scott IU, Murray TG, Oxford KW, Bowman CB, Flynn HW. Early onset endophthalmitis caused by Aspergillus species following cataract surgery. Am J Ophthalmol 2006; 142:509–511. [DOI] [PubMed] [Google Scholar]
- 7.Mithal K, Pathengay A, Bawdekar A, et al. Filamentous fungal endophthalmitis: results of combination therapy with intravitreal amphotericin B and voriconazole. Clin Ophthalmol 2015; 9:649–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Das T, Choudhury K, Sharma S, Jalali S, Nuthethi R. the Endophthalmitis Research Group Clinical profile and outcome of Bacillus endophthalmitis 2001; 108:1819–1825. [DOI] [PubMed] [Google Scholar]
- 9.Sharma S, Jalali S, Adiraju MV, et al. Sensitivity and predictability of vitreous cytology, biopsy, and membrane filter culture in endophthalmitis. Retina 1996; 16:525–529. [DOI] [PubMed] [Google Scholar]
- 10.Wykoff CC, Flynn HW, Jr, Miller D, Scott IU, Alfonso EC. Exogenous fungal endophthalmitis: microbiology and clinical outcomes. Ophthalmology 2008; 115:1501–1507. [DOI] [PubMed] [Google Scholar]
- 11.Gao Y, Chen N, Dong XG, Yuan GQ, Yu B, Xie LX. Surgical management of fungal endophthalmitis resulting from fungal keratitis. Int J Ophthalmol 2016; 9:848–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Behera UC, Budhwani M, Das TP, et al. Role of early vitrectomy in the treatment of fungal endophthalmitis. Retina 2018; 38:1385–1392. [DOI] [PubMed] [Google Scholar]
- 13.Silva RA, Sridhar J, Miller D, et al. Exogenous fungal endophthalmitis: an analysis of isolates and susceptibilities to antifungal agents over a 20-year period (1990–2010). Am J Ophthalmol 2015; 159:257–264. [DOI] [PubMed] [Google Scholar]
- 14.Shen YC, Wang MY, Wang CY, et al. Clearance of intravitreal voriconazole. Invest Ophthalmol Vis Sci 2007; 48:2238–2241. [DOI] [PubMed] [Google Scholar]


