Purpose:
To describe the clinical, demographic, and etiological profile of patients of acquired ocular motor palsy presenting in a tertiary eye care center.
Design:
A retrospective hospital record-based study was conducted in patients of paralytic strabismus presenting from April 2016 to December 2017.
Methods:
Data included demographic and clinical details, diagnosis, underlying etiology, imaging, laboratory reports, and the outcome.
Results:
Mean age of presentation of 345 patients included in the study was 38.2 ± 19.5 years (range = 365 years). Pediatric patients (age: ≤16 years) constituted 9.5% of the entire cohort. Mean duration of complaints was 5.87 ± 2 months. Of the 372 eyes of 345 cases, 42.7% were sixth nerve palsy, 34.7% were third nerve palsy, 17.7% were fourth nerve palsy, and 4.8% had multiple ocular motor nerve involvement. Third and sixth nerve palsies were mostly due to ischemic event (58.1% and 69.8% cases, respectively), whereas fourth nerve palsies were commonly caused by trauma (63.6%). Amongst traumatic cases, road traffic accident was the most common mode of trauma. Systemic risk factors were preexistent in 18.2% cases (n = 63); in the remaining (40.8%; n = 141), they were diagnosed after presentation. Complete or partial recovery was noted in 69.7% cases in third nerve palsy, 67.9% cases in sixth nerve palsy, and 45% cases in fourth nerve palsy.
Conclusions:
Acquired cranial nerve palsy has younger onset in Indian scenario. Ischemia is the most common etiology raising concerns about the health issues of young Indians. Sixth nerve is most commonly involved in all age groups. Low recovery rate in fourth nerve palsy can be attributed to traumatic etiology.
Keywords: fourth nerve palsy, paralytic squint, sixth nerve palsy, third nerve palsy
Paralytic strabismus is a frequent occurrence and constitutes the most common cause of new-onset strabismus in adults.1–4 Disorders producing acquired ocular motor palsy include microvascular, trauma, intracranial neoplasms, infections, and inflammatory conditions. With the etiology ranging from minor anomaly to a life-threatening intracranial lesion, acquired ocular motor craniopathy may be the presenting sign suggestive of a potentially more serious condition involving the complex neuroanatomic pathways. Hence, it becomes important to be aware of the etiological profile of patients with acquired ocular motor craniopathy to enable an appropriate differential diagnosis. Although literature abounds in studies discussing the prevalence and etiological patterns of paralytic strabismus,5,6 most of them are based on Western population that may not be applicable to Indian scenario.
Therefore, this study describes the clinico-demographic profile, etiological spectrum, and outcome of acquired third, fourth, and sixth cranial nerve palsies presenting to a tertiary eye care center in Indian patient cohort.
METHODS
A retrospective hospital record-based study of acquired ocular motor palsy cases presenting to the specialty clinic at our center during the study period (April 2016 to December 2017) was conducted. The study was in accordance with the tenets of Declaration of Helsinki. Institute ethical clearance was sought. Inclusion criteria were cases of acquired isolated or combined third, fourth, sixth cranial nerve palsy diagnosed by a trained strabismologist at our center. Congenital ocular motor palsies, supranuclear palsies, and acquired strabismus due to other causes like myasthenia gravis, thyroid eye disease, and so on were excluded.
Relevant demographic details like age at presentation, sex, duration of complaints, presenting complaint, associated systemic or neurological disorders, and any prior investigation or treatment were noted. Clinical information, namely visual acuity, anterior and posterior segment examination were recorded. Results of squint workup were as follows: the type and amount of strabismus, ocular motility deficit, Lees charting, forced duction test (when indicated), cranial nerve involved (diagnosis), and underlying etiology. Quantification of strabismus was done using prism bar cover test in patients with visual acuity ≥20/30 with central fixation, Krimsky test whenever prism bar cover test was not possible, and Hirschberg test in young or uncooperative cases. Etiology was identified as ischemic, traumatic, compressive, iatrogenic, inflammatory, others, and idiopathic. Cases with vascular risk factors (like diabetes, hypertension, deranged lipid profile, and coronary artery disease) were identified as ischemic. In the absence of any underlying cause, it was designated as idiopathic. Additional investigations like blood tests (for levels of glucose, gylcosylated hemoglobin, lipid profile), blood pressure assessment, perimetry, and neuroimaging were noted wherever available. Complete recovery was defined as complete resolution of diplopia and angle of squint. Partial recovery was defined as partial improvement and no recovery was defined as no improvement in the above parameters.
STATISTICAL ANALYSIS
Data analysis was conducted using Statistical Package for Social Science (SPSS) version 12.1 (StataCorp LLC, Lakeway Drive, Texas, USA). Descriptive statistics were presented as percentages, mean, and standard deviation. Student t test, Wilcoxon signed rank test, and χ2 test were used for analysis. P value <0.05 was considered as significant.
RESULTS
Of the 41,836 new cases presenting to various specialty clinics at our center during the study period, 3112 cases (7.4%) were referred for squint evaluation. Of them, 345 patients were diagnosed with acquired ocular motor palsy (11.08%) and were included in the study. Mean age of onset was 38.2 ± 19.5 years (range = 3–65 years). Pediatric patients (age: ≤16 years) constituted 9.5% of the entire cohort (n = 33). Male predominance was seen in a ratio of 1.7:1. Mean duration of complaints was 5.87 ± 2 months. Diplopia was the presenting complaint seen in 75% cases. Left-sided involvement was seen in 55.6% (n = 192); 7.8% cases (n = 27) were bilateral.
Among 372 eyes of 345 cases, 42.7% (n = 159 eyes) had sixth nerve palsy, 34.7% had third nerve palsy (n = 129 eyes) (pupil sparing = 72, pupil involving = 57), 17.7% (n = 66 eyes) had fourth nerve palsy, and 4.8% (n = 18 eyes) had multiple ocular motor nerve involvement. Ischemic and traumatic were the most common etiologies seen in 59% (n = 204/345) and 23.5% (n = 81/345) cases, respectively. Table 1 enumerates the various causes of paralytic squint identified in our cohort. Road traffic accident was the most common mode of trauma accounting for 84% traumatic cases (n = 68). Systemic comorbidities like diabetes, hypertension, hyperlipidemia, and coronary artery disease were preexistent in 18.2% cases (n = 63) of the entire cohort; in another 40.8% (n = 141) cases, it was diagnosed after presentation. Ten cases with a mean age of 34.33 years had hyperhomocystinemia. At our center, 15% of the patients had been imaged before presentation. In 32.4% (n = 105) patients, neuroimaging was advised after presentation and was conclusive in over 50% (n = 54/105) cases. Mean follow-up of patients was 10.37 ± 2.3 months. Overall, of the 372 eyes, complete spontaneous recovery was seen in 129 eyes (34.7%), partial recovery in 102 eyes (27.4%), and no recovery in 141 cases (37.9%).
TABLE 1.
Etiology of Paralytic Strabismus in 345 Indian Patients
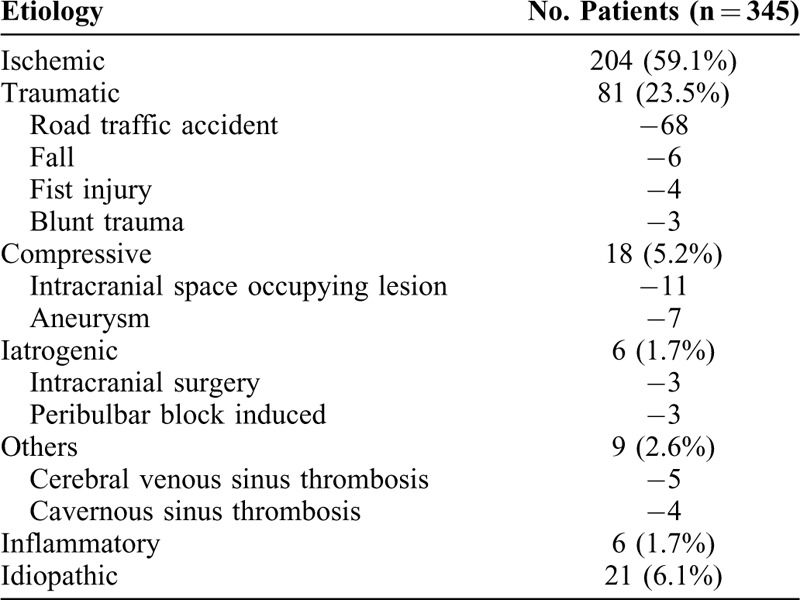
Subanalysis was performed to compare the two etiological groups: ischemic and traumatic/iatrogenic (Table 2). Mean age of onset was higher in the ischemic group. Diabetes (54.4%) and hypertension (48.5%) were the two most common vascular comorbidities seen in ischemic cases. Multiple comorbidities (≥2) were present in 34.8% cases (n = 71) (Table 3). Fundus findings suggestive of diabetic, hypertensive, or mixed retinopathy were present in 15% ischemic cases. Traumatic cases had a higher male preponderance. Signs of blunt trauma like traumatic optic neuropathy, Berlin's edema, choroidal rupture, and so on were present in 65% cases. Ninety percent cases (30/33) of traumatic third nerve palsy were pupil-involving, whereas in ischemic cases it was 36% (27/75). The recovery rates were significantly higher in the ischemic group (73.5%) than in traumatic group (38%) (P < 0.00).
TABLE 2.
Comparison of Ischemic and Traumatic Acquired Ocular Motor Palsy
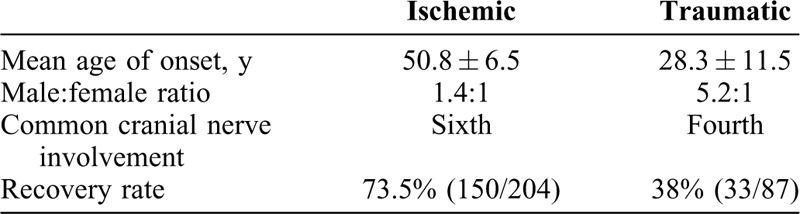
TABLE 3.
Comorbid Disease Analysis in Patients with Ischemic Etiology

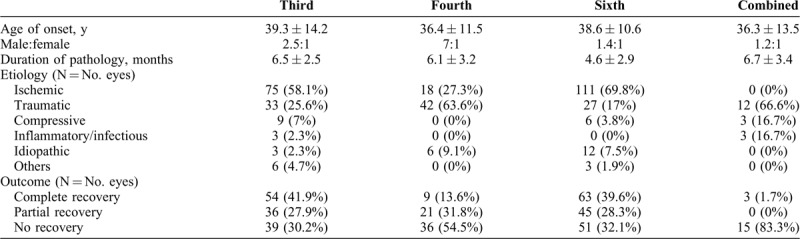
Third and sixth nerve palsies were mostly due to ischemic event as seen in 58.1% and 69.8% cases, respectively; whereas 63.63% cases of fourth nerve palsies were attributed to trauma. 3 cases of sixth nerve palsy aged 3, 9, and 34 years, respectively, were recurrent. Although no definite cause could be established in two cases, third nerve palsy was diagnosed with Tolosa-Hunt syndrome. Recovery was complete in all three cases. Complete or partial recovery was noted in 67.9% cases in sixth nerve palsy, 69.7% cases in third nerve palsy, and 45% cases in fourth nerve palsy. Table 4 gives demographic, etiological, and outcome comparison of cases of isolated and combined third, fourth, and sixth nerve palsies.
TABLE 4.
Comparison Between the Patients of Third, Fourth and Sixth Nerve Palsy
Univariate analysis was done to identify the factors that affect the outcome. Traumatic etiology (P = 0.02), fourth cranial nerve involvement (P = 0.04), and duration of complaints >3 months (P = 0.02) were significantly associated with no or incomplete recovery.
Pediatric cases were separately analyzed (n = 33). Abducent nerve affection was seen in 54.5% cases (n = 18). The causative etiologies were trauma (n = 15), inflammatory/infectious (n = 9), idiopathic (n = 6), and intracranial neoplasm (medulloblastoma) (n = 3). Spontaneous recovery (partial or complete) was noted in 36.4% (n = 12) cases. None of the cases with trauma and tumor showed improvement.
DISCUSSION
This study gives the current demographic and etiological trend of acquired ocular motor palsy of a tertiary center in Indian scenario. Unlike previous studies where fifth decade was the most affected age group (range = 48–58 years in several studies),5–8 our study reports a younger mean age of onset of 38 years. However, the etiological profile in our study remains somewhat similar to these studies where ischemia constitutes the commonest cause of acquired paralytic strabismus.5–8 Recent literature explains this changing demographic trend where an increasing prevalence of coronary artery disease and associated vascular comorbidities have been reported in young Indians (<40 y) in the last three decades.9,10 Changing lifestyle brought about by urbanization, stress, and lack of physical activity is among the possible risk factors11–13 predisposing to vascular diseases and its manifestations like coronary artery disease and cranial nerve palsies. This emphasizes the need to incorporate awareness programs related to the risk factors and the need for lifestyle modification in national health schemes.
Hypertension has been found to be the most significant risk factor predisposing to ocular motor palsy in Western literature.14,15 Conversely, our study identified diabetes as the major risk factor as was also reported in a study from Korea.16 Another population-based study of acquired sixth nerve palsy from the West reported an 8-fold increase in odds of having both diabetes and hypertension in cases over controls.17 With an upsurge in the number of diabetics in the country in the last few decades, diabetes has reached potentially epidemic levels in India.18,19 Resultantly, there is increased incidence of diabetes-related complications.
Road trauma constituted major cause (85%) of traumatic ocular motor palsies in this study. Corroborating with these results, we also have previously reported road traffic accidents as a leading cause of traumatic optic neuropathy in another study.20 A recent report has suggested that India tops the list of road-related fatalities. This is a reflection of the lack of adequate traffic management and enforcement of traffic safety norms.21,22 Considering that the road traffic-related injuries are preventable, well-coordinated efforts to ensure road safety is the need of the hour.
Undetermined etiology accounts for around 20% cases in various studies.5,7 In our study, we were able to identify the cause in 94% cases that hint towards a thorough diagnostic workup for the patients of acquired paralytic strabismus in our center.
Hyperhomocystinemia has been associated with increased risk of young-onset ocular vaso-occlusive disorders.23,24 We found its association in 10 young patients of ocular motor palsy. A case series from our subcontinent has reported isolated sixth cranial nerve palsy in four cases with a mean age of 25 years.25 Similar to the outcome of this report, all our patients with hyperhomocystinemia spontaneously recovered with medical management.
Sixth nerve was the commonly affected cranial nerve across all age groups (42.7%), which is in accordance with most previous studies done in adults.5–8 In pediatric population, clinic-based and population-based studies have shown varied results.26,27 Holmes et al (36%)26 and Olusanya et al (52%)27 identified fourth nerve palsy as the most frequently encountered diagnosis in children, whereas studies by Harley (51.2%)28 and Kodsi and Younge (55%),29 in keeping with our findings, have reported sixth nerve palsy as the commonest paralytic squint in pediatric cases. As fourth nerve palsy is mostly a congenital anomaly in children, exclusion of congenital cases from our study explains the difference in results.
Most common etiology in the pediatric age group was trauma as is also reported by other studies of acquired paralytic strabismus in children.27–29 With regard to the etiological profile of pediatric sixth nerve palsy, there are conflicting reports. In accordance with our results, most studies have reported trauma as a major cause of pediatric sixth nerve involvement,27–29 whereas Lee et al30 and Merino et al31 identified neoplasm in majority of their cases and advocated early neuroimaging in acquired sixth nerve palsy in children.
Neuroimaging was advised in 32% cases with diagnostic dilemma in this study. Although studies have emphasized the significance of imaging in all acute ocular motor nerve palsies,32,33 careful history taking and clinical evaluation can obviate the need for unnecessary investigations and bring down the cost of health care.34 Some important indications for consideration of neuroimaging in ocular motor palsies at our center are young-onset cases, associated neurological signs, multiple cranial nerve involvement, history of carcinoma, and nonresolution or progression of symptoms.34
Limitation that merit mention is retrospective case design of the study that is confounded by limited data availability. The follow-up in the study was restricted up to the period of observation. The type of surgical intervention in cases of partial or no recovery and its outcome have not been evaluated. Second, there is a referral bias of a tertiary eye care center. Hence, the etiological profile may not be truly representative of the general population.
CONCLUSIONS
In conclusion, acquired cranial nerve palsy has younger onset in Indian scenario. Sixth nerve is most commonly involved in all age groups. Ischemia followed by trauma is the common etiology raising concerns about the health issues of young Indian and road safety. Low recovery rates are associated with traumatic etiology, diagnosis of fourth nerve palsy, and longer duration of pathology. Despite its limitations, this study provides a baseline data related to the clinico-etiological profile of paralytic strabismus in Indian patients that will guide effective and timely management.
Footnotes
The authors have no funding or conflicts of interest to disclose.
REFERENCES
- 1.Martinez-Thompson JM, Diehl NN, Holmes JM, et al. Incidence, types, and lifetime risk of adult-onset strabismus. Ophthalmology 2014; 121:877–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scott WE, Kutschke PJ, Lee WR. 20th annual Frank Costenbader Lecture−adult strabismus. J Pediatr Ophthalmol Strabismus 1995; 32:348–352. [DOI] [PubMed] [Google Scholar]
- 3.Beauchamp GR, Black BC, Coats DK, et al. The management of strabismus in adults: I. Clinical characteristics and treatment. J AAPOS 2003; 7:233–240. [DOI] [PubMed] [Google Scholar]
- 4.Repka MX, Yu F, Coleman A. Strabismus among aged fee-for-service Medicare beneficiaries. J AAPOS 2012; 16:495–500. [DOI] [PubMed] [Google Scholar]
- 5.Park UC, Kim SJ, Hwang JM, et al. Clinical features and natural history of acquired third, fourth and sixth nerve palsy. Eye (Lond) 2008; 22:691–696. [DOI] [PubMed] [Google Scholar]
- 6.Akagi T, Miyamoto K, Kashii S, et al. Cause and prognosis of neurologically isolated third, fourth, or sixth cranial nerve dysfunction in cases of oculomotor palsy. Jpn J Ophthalmol 2008; 52:32–35. [DOI] [PubMed] [Google Scholar]
- 7.Ho TS, Lin HS, Lin MC, et al. Acquired paralytic strabismus in Southern Taiwan. J Chin Med Assoc 2013; 76:340–343. [DOI] [PubMed] [Google Scholar]
- 8.Berlit P. Isolated and combined pareses of cranial nerves III, IV, and VI. A retrospective study of 412 patients. J Neurol Sci 1991; 103:10–15. [DOI] [PubMed] [Google Scholar]
- 9.Dalal J, Hiremath MS, Das MK, et al. Vascular disease in young Indians (20–40 years): role of ischemic heart disease. J Clin Diagn Res 2016; 10: OE08-OE12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaveeshwar SA, Cornwall J. The current state of diabetes mellitus in India. Australas Med J 2014; 7:45–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Enas EA, Garg A, Davidson MA, et al. Coronary heart disease and its risk factors in first-generation immigrant Asian Indians to the United States of America. Indian Heart J 1996; 48:343–353. [PubMed] [Google Scholar]
- 12.Joshi P, Islam S, Pais P, et al. Risk factors for early myocardial infarction in South Asians compared with individuals in other countries. JAMA 2007; 297:286–294. [DOI] [PubMed] [Google Scholar]
- 13.Hasan A, Agarwal A, Parvez A, et al. Premature coronary artery disease and risk factors in India. Indian J Cardiol 2013; 16:5–11. [Google Scholar]
- 14.Rucker CW. The causes of paralysis of the third, fourth and sixth cranial nerves. Am J Ophthalmol 1966; 61:1293–1298. [DOI] [PubMed] [Google Scholar]
- 15.Rush JA, Younge BR. Paralysis of cranial nerves III, IV, and VI: cause and prognosis in 1,000 cases. Arch Ophthalmol 1981; 99:76–79. [DOI] [PubMed] [Google Scholar]
- 16.Jung JS, Kim DH. Risk Factors and prognosis of isolated ischemic third, fourth, or sixth cranial nerve palsies in the Korean population. J Neuroophthalmol 2015; 35:37–40. [DOI] [PubMed] [Google Scholar]
- 17.Patel SV, Holmes JM, Hodge DO, et al. Diabetes and hypertension in isolated sixth nerve palsy: a population-based study. Ophthalmology 2005; 112:760–763. [DOI] [PubMed] [Google Scholar]
- 18.Joshi SR, Parikh RM. India—diabetes capital of the world: now heading towards hypertension. J Assoc Physicians India 2007; 55:323–324. [PubMed] [Google Scholar]
- 19.Kumar A, Goel MK, Jain RB, et al. India towards diabetes control: key issues. Australas Med J 2013; 6:524–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dhiman R, Singh D, Gantayala SP, et al. Neuro-ophthalmology at a tertiary eye care centre in India. J Neuroophthalmol 2018; 38:308–311. [DOI] [PubMed] [Google Scholar]
- 21.Tiffin PA, MacEwen CJ, Craig EA, et al. Acquired palsy of the oculomotor, trochlear and abducens nerves. Eye (Lond) 1996; 10:377–384. [DOI] [PubMed] [Google Scholar]
- 22.Singh SK. Road traffic accidents in India: issues and challenges. Transp Res Procedia 2017; 25:4708–4719. [Google Scholar]
- 23.Pianka P, Almog Y, Man O, et al. Hyperhomocysteinemia in patients with nonarteritic anterior ischemic optic neuropathy, central retinal artery occlusion, and central retinal vein occlusion. Ophthalmology 2000; 107:1588–1592. [DOI] [PubMed] [Google Scholar]
- 24.Kawasaki A, Purvin VA, Burgett RA. Hyperhomocysteinemia in young patients with non-arteritic anterior ischaemic optic neuropathy. Br J Ophthalmol 1999; 83:1287–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sachdeva V, Mittal V, Pathengay A, et al. Isolated abducens nerve palsy with hyperhomocysteinemia: association and outcomes. Indian J Ophthalmol 2013; 61:598–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Holmes JM, Mutyala S, Maus TL, et al. Pediatric third, fourth, and sixth nerve palsies: a population-based study. Am J Ophthalmol 1999; 127:388–392. [DOI] [PubMed] [Google Scholar]
- 27.Olusanya B, Tinley C, Grotte R. Paralytic strabismus in South African Black and mixed race children: a 15-year clinic-based review. Ophthalmic Epidemiol 2012; 19:396–400. [DOI] [PubMed] [Google Scholar]
- 28.Harley RD. Paralytic strabismus in children: etiologic incidence and management of the third, fourth, and sixth nerve palsies. Ophthalmology 1980; 87:24–43. [DOI] [PubMed] [Google Scholar]
- 29.Kodsi SR, Younge BR. Acquired oculomotor, trochlear, and abducent cranial nerve palsies in pediatric patients. Am J Ophthalmol 1992; 114:568–574. [DOI] [PubMed] [Google Scholar]
- 30.Lee MS, Galetta SL, Volpe NJ, et al. Sixth nerve palsies in children. Pediatr Neurol 1999; 20:49–52. [DOI] [PubMed] [Google Scholar]
- 31.Merino P, Gómez de Liaño P, Villalobo JM, et al. Etiology and treatment of pediatric sixth nerve palsy. J AAPOS 2010; 14:502–505. [DOI] [PubMed] [Google Scholar]
- 32.Tamhankar MA, Volpe NJ. Management of acute cranial nerve 3, 4 and 6 palsies: role of neuroimaging. Curr Opin Ophthalmol 2015; 26:464–468. [DOI] [PubMed] [Google Scholar]
- 33.Chou KL, Galetta SL, Liu GT, et al. Acute ocular motor mononeuropathies: prospective study of the roles of neuroimaging and clinical assessment. J Neurol Sci 2004; 219:35–39. [DOI] [PubMed] [Google Scholar]
- 34.Murchison AP, Gilbert ME, Savino PJ. Neuroimaging and acute ocular motor mononeuropathies: a prospective study. Arch Ophthalmol 2011; 129:301–305. [DOI] [PubMed] [Google Scholar]


